Summary
Central gain compensation for reduced auditory nerve output has been hypothesized as a mechanism for tinnitus with a normal audiogram. Here, we investigate if gain compensation occurs with aging. For 94 people (aged 12–68 years, 64 women, 7 tinnitus) with normal or close-to-normal audiograms, the amplitude of wave I of the auditory brainstem response decreased with increasing age but was not correlated with wave V amplitude after accounting for age-related subclinical hearing loss and cochlear damage, a result indicative of age-related gain compensation. The correlations between age and wave I/III or III/V amplitude ratios suggested that compensation occurs at the wave III generator site. For each one of the seven participants with non-pulsatile tinnitus, the amplitude of wave I, wave V, and the wave I/V amplitude ratio were well within the confidence limits of the non-tinnitus participants. We conclude that increased central gain occurs with aging and is not specific to tinnitus.
Subject areas: biological sciences, neuroscience, sensory neuroscience
Graphical abstract

Highlights
-
•
For people with a normal audiogram, the auditory nerve response decreases with age
-
•
The decrease is compensated by elevated gain in the central auditory system
-
•
Gain compensation likely occurs in the cochlear nuclei
-
•
Elevated central gain can occur in people without tinnitus
Biological sciences; Neuroscience; Sensory neuroscience
Introduction
Tinnitus, an auditory sensation in the absence of an external sound, is often associated with hearing loss. Reduced peripheral activation due to hearing loss is thought to produce plastic readjustments in the central auditory system that lead to a tinnitus percept (Kaltenbach, 2011; Noreña, 2011). Although less frequently, tinnitus also occurs in otherwise audiologically normal people. For these people, some studies have shown that tinnitus is associated with reduced amplitudes of wave I of the auditory brainstem response (ABR) but normal wave V amplitudes, hence with increased wave V/I amplitude ratios (Kehrle et al., 2008; Schaette and McAlpine, 2011; Gu et al., 2012; Bramhall et al., 2018; Valderrama et al., 2018). This has been interpreted as indicating that mechanisms central to the auditory nerve amplify the reduced nerve output (wave I) to give normal wave V amplitudes (Chambers et al., 2016) and that the increased central gain could amplify spontaneous nerve activity to a level that produces a tinnitus sensation (Noreña, 2011; Schaette and McAlpine, 2011).
For people with a normal audiogram, aging is associated with reduced auditory nerve output (Makary et al., 2011; Sergeyenko et al., 2013; Viana et al., 2015; Wu et al., 2018; Johannesen et al., 2019). This, together with the hypothesized mechanism just described that links tinnitus with increased central gain, raises the following questions: does central gain increase with aging? If it does, where does this increase occur? And is this enhanced central gain sufficient to produce tinnitus?
The evidence in support of central gain compensation in aging remains controversial. While some animal studies have reported reduced wave 1 with normal wave 5 amplitude associated with aging (Sergeyenko et al., 2013; Hickox and Liberman, 2014; Cai et al., 2018; Muniak et al., 2018; Parthasarathy and Kujawa, 2018), others have not (Rüttiger et al., 2013; Möhrle et al., 2016; Lai et al., 2017). Similarly, some human studies have reported aging to be associated with a greater reduction in the amplitude of wave I than wave V (Costa et al., 1990; Burkard and Sims, 2001; Moosavi et al., 2016), but few studies have explicitly shown that the wave V/I amplitude ratio increases with increasing age (Psatta and Matei, 1988; Sand, 1991).
An important shortcoming of most existing studies about the effect of age on ABR wave amplitudes and/or amplitude ratios for normal-hearing humans (or animals) is that they did not control for the potential confounding effects of subclinical hearing loss (Psatta and Matei, 1988; Costa et al., 1990; Sand, 1991; Burkard and Sims, 2001; Moosavi et al., 2016). Audiometric thresholds often increase with increasing age, even when they remain within normal limits, and the increase is typically greater at higher frequencies than that at lower frequencies (e.g., Johannesen et al., 2019). Wave I likely reflects neural responses to mid-high stimulus frequencies (2–12 kHz), while wave V reflects responses to low-mid frequencies (0.4–2 kHz) (Don and Eggermont, 1978; Eggermont and Don, 1980). Therefore, subclinical hearing loss, rather than central gain compensation, might explain the increased wave V/I amplitude ratios in audiometrically normal older listeners (Musiek et al., 1984). Indeed, wave V/I amplitude ratios inferred from Konrad-Martin et al. (2012), who did control statistically for age-related increases in thresholds (all within the normal range) do not support the central gain compensation hypothesis, while the ABRs from previous studies that did not control for threshold are indicative of central gain compensation (Psatta and Matei, 1988; Sand, 1991).
Here, we searched for age-related central gain compensation while statistically accounting for the effects of subclinical hearing loss and cochlear mechanical deficits on ABRs. We analyzed the amplitude of ABR waves I, III and V for 94 people (aged 12–68 years; 64 women) with normal audiograms or very mild hearing losses (Figure S1), seven of whom incidentally had chronic tinnitus (Figures S2 and S3). Because otoacoustic emissions are likely more sensitive than hearing thresholds to mild cochlear mechanical damage (Attias et al., 1995; LePage, 1998; Seixas et al., 2005; Lapsley Miller et al., 2006), we used both the across-frequency mean hearing threshold and distortion product otoacoustic emissions (DPOAEs) level to partial out the effects of subclinical hearing loss on the amplitude of wave I, III, and V before analyzing the (adjusted) ABR amplitudes.
We show that central gain compensation (i.e., decreased wave I/V ratio, see below) occurs with aging in people without tinnitus. Further, we show that central gain compensation in aging likely occurs at the wave III generation site. Lastly, we show that for each one of the seven participants with tinnitus, the amplitude of wave I, wave V, and the wave I/V amplitude ratio were within the corresponding distribution of values for the non-tinnitus participants. We conclude that increased central gain occurs with aging and is not specific to tinnitus.
Results
The study was part of a larger study aimed at investigating the presence of cochlear synaptopathy in audiometrically normal human listeners and its impact on auditory perception. For this reason, part of the data reported here has already been reported elsewhere, where further details can be found (Johannesen et al., 2019). This includes audiometric thresholds (0.25–8 kHz), high-frequency thresholds (12 kHz), DPOAEs (0.5–4 kHz), and ABR wave I responses. Here, for the same participants, we additionally report if they had chronic tinnitus and analyze their ABR wave III and V responses.
Relation between ABR wave I amplitude and age
Because reduced auditory nerve output is a prerequisite for any potential gain compensation along the auditory pathway to occur, we first investigated if aging is associated with reduced auditory nerve output. Because age affects many aspects of auditory function, particularly hearing thresholds and/or cochlear function, we applied partial regression to partial out the effects of subclinical hearing loss and cochlear mechanical deficits on wave I amplitude before assessing the correlation of the (adjusted) wave I amplitude with age. Potential differences in ABR characteristics across sexes (Jerger and Hall, 1980; Trune et al., 1988; Mitchell et al., 1989) were accounted for by conducting separate analyses for men and women. Unless otherwise stated, in what follows ABR wave amplitudes refer to the mean value across stimulus levels from 100 to 110 dB ppeSPL.
Wave I amplitude decreased significantly with increasing age for both men and women (Figure 1A) and the correlations remained statistically significant after partialling out the effects of hearing thresholds and DPOAEs on wave I amplitude (Figure 1B). This suggests that age-related subclinical hearing loss (Figure S4) and cochlear mechanical deficits (Figure S5) are insufficient to explain the decreasing wave I amplitude with increasing age (Figure 1A), although the shallower regression line slopes in Figure 1B than Figure 1A (i.e., the smaller R2 values) indicate that cochlear deficits explained some of the age-related decline in wave I amplitude. Overall, these results are consistent with reduced output from the auditory nerve with increasing age independent of hearing loss and cochlear mechanical damage.
Figure 1.
Relation between ABR wave I amplitude and age
(A) ABR wave I amplitude (mean across stimulus levels from 100 to 110 dB ppeSPL) as a function of age for women and men. The insets show the proportion of variance explained (R2) and the probability of a non-zero regression line slope occurring by chance (p).
(B) As panel A, but with wave I amplitude adjusted for the effect of hearing thresholds and DPOAEs. Open and filled symbols depict data for non-tinnitus and tinnitus participants, respectively.
Relation between ABR wave V amplitude and wave I amplitude or age
Second, we investigated if a central gain mechanism compensated for reduced auditory nerve output as measured by the ABR wave I amplitude or as indicated by age. To do this, we assessed the correlation of wave V amplitude with both wave I amplitude and age (Figure 2). If a central mechanism compensated perfectly for reduced output from the wave I generator site to preserve wave V amplitude at normal (constant) levels, the amplitude of wave V should be independent of wave I amplitude or age. To test if wave V amplitudes for men and women could be analyzed jointly, the wave V amplitudes of the non-tinnitus participants were subjected to a repeated-measures analysis of variance (RMANOVA) with sex as a between-subjects factor and stimulus level as a within-subjects factor. The test revealed that wave V amplitudes were larger for women than for men [F(1,84) = 15.7, p = 1.5·10−4] and for this reason the correlation between wave V amplitude and wave I amplitude was calculated separately for women and men (Figure 2).
Figure 2.
Relation between ABR wave V amplitude and wave I amplitude or age
(A and B) ABR wave V amplitude as a function of wave I amplitude (A) and age (B) for women and men. The amplitudes of wave I and V are the mean across stimulus levels from 100 to 110 dB ppeSPL. The insets show the proportion of variance explained (R2) and the probability of a non-zero regression line slope occurring by chance (p).
(C and D) As panels A and B, but with wave I and V amplitudes adjusted for the effects of hearing thresholds and DPOAEs. Open and filled symbols depict data for non-tinnitus and tinnitus participants, respectively.
Wave V amplitude tended to increase with increasing wave I amplitude for both men and women, although the trend was statistically significant only for women (Figure 2A). Further, wave V amplitude decreased with increasing age at roughly the same rate for men and women although the decrease was significant only for women (Figure 2B). Overall, the observed relationships between wave V amplitude and wave I amplitude or age appeared to be inconsistent with the presence of a perfect compensatory mechanism for age-related reduced auditory nerve output.
These relationships, however, are likely to be misleading as the amplitudes of waves I and V were correlated with both hearing threshold (Table 1) and overall DPOAE level (Table 2) at one or more test frequencies. Furthermore, across-frequency mean thresholds and DPOAE levels were both predictors of wave V amplitude [mean thresholds, partial R2 = 0.055, p = 0.0093; DPOAEs, partial R2 = 0.050, p = 0.0131]. For this reason, the analyses were repeated after adjusting the amplitudes of waves I and wave V for the effects of both hearing thresholds and DPOAEs.
Table 1.
Effect of hearing loss on ABR wave I and V amplitude
| Frequency (kHz) |
|||||||
|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | 12 | ||
| Wave I | R | −0.14 | −0.13 | −0.24 | −0.32 | −0.28 | −0.43 |
| p | 0.177 | 0.201 | 0.021 | 1.64·10-3 | 6.30·10-3 | 1.23·10-5 | |
| Wave V | R | −0.23 | −0.06 | −0.11 | −0.23 | −0.17 | −0.37 |
| p | 0.024 | 0.570 | 0.273 | 0.029 | 0.098 | 2.6·10−4 | |
Pearson correlations (R) between hearing threshold (N = 94) and ABR wave I or V amplitude (mean across click levels 100, 105, and 110 dB ppeSPL). The probabilities for significant correlations (p) were not corrected for multiple comparisons.
Table 2.
Effect of DPOAE response level on ABR wave I and V amplitude
| Test frequency, f2 (kHz) |
||||||
|---|---|---|---|---|---|---|
| 0.5 | 1 | 1.5 | 2 | 4 | ||
| Wave I | R | 0.09 | 0.07 | 0.11 | 0.17 | 0.26 |
| p | 0.409 | 0.520 | 0.277 | 0.106 | 0.011 | |
| Wave V | R | 0.26 | 0.30 | 0.33 | 0.36 | 0.43 |
| p | 0.014 | 3.50·10-3 | 1.14·10-3 | 4.39·10-4 | 1.60·10−5 | |
As Table 1 but for Pearson correlations between overall DPOAE levels (N = 94) and ABR wave I or V amplitude.
The reanalysis revealed that the adjusted wave V amplitude was not correlated with the adjusted wave I amplitude for women or men (Figure 2C) (note that the adjusted wave V amplitude for men tended to be correlated with the adjusted wave I amplitude because of two high-leverage data points, i.e., two outliers around adjusted wave I amplitudes of 0.7 and 0.88 μV). Likewise, the adjusted wave V amplitude was not correlated with age for women or men (Figure 2D). This suggests that the significant correlations between the unadjusted wave V and I amplitudes (Figure 2A) or age (Figure 2B) in women were due to the effect of subclinical cochlear mechanical deficits on ABR wave amplitudes. Further, the lack of dependence of adjusted wave V amplitude on age supports the presence of central compensation for reduced auditory output with aging (Figure 2D).
Wave I/V amplitude ratio decreases with increasing age
To further investigate central gain compensation while reducing individual variability in ABR amplitude, we assessed the correlation of the wave I/V amplitude ratio (the inverse of the central gain) with age. We chose to use this ratio over the more conventional wave V/I ratio because the latter can result in extremely large values when the amplitude of wave I approaches zero. Figure 3 presents the wave I/V ratio as a function of age and separately for women and men. One outlier (man, wave I/V ratio = 2.5) was omitted here (Figure 3) and in subsequent analyses because his ratio was outside 2.5 times the interquartile range. The wave I/V ratio (Figure 3A) decreased significantly with increasing age for both women (Pearson R = −0.40, p = 0.0011) and men (Pearson R = −0.37, p = 0.048), consistent with central gain compensation for reduced output from the wave I generator site.
Figure 3.
ABR wave I/V amplitude ratio as a function of age
(A) For women and men separately. The insets show the proportion of variance explained (R2) and the probability of a non-zero regression line slope occurring by chance (p).
(B) As panel A, but for ABR wave I/V amplitude ratio adjusted for effects of absolute thresholds and DPOAEs, and pooling data for men and women. In all panels, wave amplitudes were the mean of the responses to stimulus levels from 100 to 110 dB ppeSPL. Open and filled symbols depict data for non-tinnitus and tinnitus participants, respectively.
The decreasing ABR wave I/V ratio with increasing age (Figure 3A) may have been potentially influenced by increasing cochlear hearing loss with aging. We investigated this possibility because (1) thresholds did increase with increasing age (Figure S4; see also Johannesen et al., 2019); (2) DPOAEs decreased with age (Figure S5); and (3) wave V amplitudes were correlated with thresholds (Table 1) and DPOAEs (Table 2). We adjusted the wave I/V ratio for the effects of hearing thresholds and DPOAEs using partial regression methods. Because the age-related effect on wave I/V ratio was similar for men and women (Figure 3A), data for men and women were analyzed jointly. Partial regression showed (Figure 3B) that adjusted wave I/V ratios decreased significantly with increasing age (partial R = −0.30, p = 7.8·10-5, N=93). This suggests that age-related cochlear mechanical deficits are insufficient to explain the decreasing wave I/V ratio with increasing age (Figure 3A), although the shallower regression line slopes in Figure 3B than Figure 3A (i.e., the smaller R2 values) indicate that cochlear deficits explained some of the age-related decline in wave I/V ratio.
Origin of compensatory mechanism
The wave I/V amplitude ratio decreased with increasing age (Figure 3), consistent with the presence of a central gain compensation mechanism. We investigated where along the auditory neural pathway this compensation occurred by analyzing the wave amplitude ratios I/III and III/V, while using partial regression to adjust for effects of hearing thresholds and DPOAEs on wave I, III, and V amplitude (Figure 4). If compensation occurs at the cochlear nuclei, where wave III is generated (Moller, 2006), we would expect normal wave III and reduced wave I with increasing age and hence the wave I/III ratio should decrease with increasing age while wave III/V ratio should be constant with age. Conversely, if gain compensation occurs in the inferior colliculus, where wave V is generated (Moller, 2006), we would expect no correlation between age and wave I/III ratio but a decreasing wave III/V ratio with increasing age.
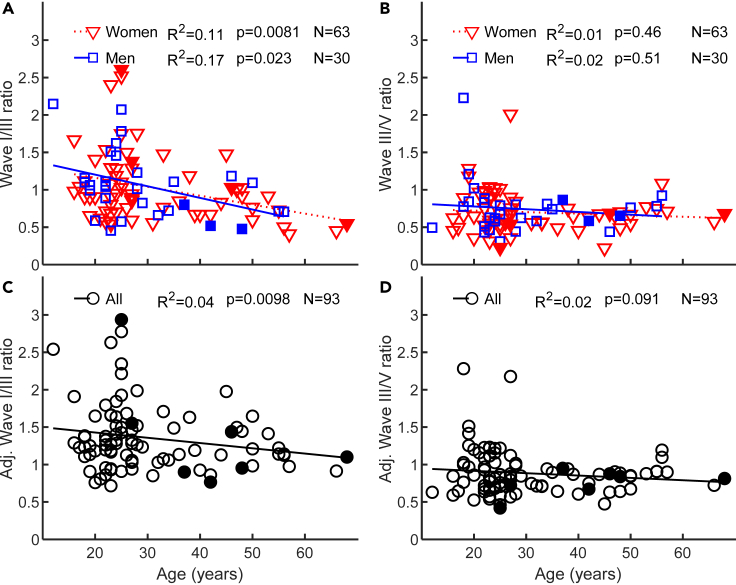
Figure 4.
Relation between age and ABR wave I/III or III/V amplitude ratios
(A) ABR wave I/III amplitude ratios for men and women. The insets show the proportion of variance explained (R2) and the probability of a non-zero regression line slope occurring by chance (p).
(B) As panel A, but for wave III/V amplitude ratios.
(C) As panel A, but for wave I/III amplitude ratios adjusted for effects of hearing thresholds and DPOAEs, and pooling data for men and women.
(D) As panel C, but for wave III/V amplitude ratios. In all panels, wave amplitudes were the mean of the responses to stimulus levels from 100 to 110 dB ppeSPL. Open and filled symbols depict data for non-tinnitus and tinnitus participants, respectively.
The wave I/III ratio decreased significantly with increasing age (Figure 4A) for both women (Pearson correlation, R = -0.33, p = 0.0081, N = 63) and men (Pearson correlation, R = -0.41, p = 0.023, N = 30) while the wave III/V ratio was uncorrelated with age (p≥0.46, Figure 4B). This suggests that gain compensation occurs at the cochlear nuclei. Aging, however, is also associated with cochlear deficits and because some reports have suggested that thresholds and/or cochlear mechanical deficits may affect waves I, III, and V differently (Don and Eggermont, 1978; Eggermont and Don, 1980; Verhulst et al., 2016), we reassessed age-related effects on wave amplitude ratios after adjusting for the effects of hearing thresholds and DPOAEs (Figures 4C and 4D). Because unadjusted wave I/III and III/V ratios were similar for women and men, adjusted wave amplitude ratios for men and women were analyzed together. Partial regression analysis showed that wave I/III ratio adjusted for mean threshold and DPOAEs decreased significantly with increasing age (Figure 4C). The regression line for the wave I/III ratio after adjusting for cochlear deficits (Figure 4C) was shallower than for the unadjusted wave I/III ratio, which shows that part of the age effect on (unadjusted) wave I/III ratio (Figure 4A) was due to age-related cochlear deficits. Wave III/V ratio was uncorrelated with age, regardless of whether or not the ratio was adjusted for cochlear deficits (Figures 4D and 4B). Overall, the present results are consistent with a gain compensation mechanism located in the cochlear nuclei.
Effect of tinnitus on ABRs
Seven out of the 94 participants reported having chronic tinnitus either bilaterally (N = 4) or unilaterally (N = 3) in the test ear (Figure S2). Based on their scores in two tinnitus severeness questionnaires (Figure S3), the tinnitus was mild for all participants except one whose tinnitus was moderate. We tested if the presence of tinnitus for these participants was associated with alterations of wave I and/or V responses, particularly with reduced wave I amplitudes together with normal wave V amplitudes (Schaette and McAlpine, 2011; Gu et al., 2012). For this purpose, wave V amplitudes were plotted as a function of wave I amplitudes for each participant. Separate plots were produced for each stimulus level, for men and women, and for tinnitus and non-tinnitus participants (Figure 5).
Figure 5.
Relation between ABR wave V and I amplitudes for the different stimulus levels
ABR wave V amplitudes as a function of wave I amplitudes for each of the ABR click stimulus levels, as indicated at the top of each panel. The upper and lower rows show data for non-tinnitus and tinnitus participants, respectively. Shaded areas in the lower row indicate 95% confidence intervals (mean ± 2 standard deviations) for wave I and V amplitudes based on the response amplitudes of the non-tinnitus participants (from upper row).
For two female participants, their ABR wave I amplitudes to 90 dB ppeSPL clicks were small and below the noise floor and hence their data are omitted in Figure 5F. The sample size for the tinnitus participants (women: N = 4; men: N = 3) was too small for a sufficiently powerful group comparison of wave amplitudes between the tinnitus and non-tinnitus groups. Instead, to assess if the ABR wave I and V responses for any individual of the tinnitus participants were statistically different from those for the non-tinnitus group, we calculated the 95% confidence intervals for wave I and V for one single new observation (mean +/- two standard deviations) based on the non-tinnitus data (Figures 5A–5E) and superimposed the confidence intervals on the ABR wave I and V responses for the tinnitus participants, separately for men and women (Figures 5F–5J: blue and red shaded areas, respectively). Wave I and V amplitudes were within the 95% confidence intervals for all individual tinnitus participants and stimulus levels, except for one male participant at the lowest stimulus level (Figure 5F). This suggests that neither the wave I nor wave V amplitudes of any individual tinnitus participant were statistically significantly different from those of the non-tinnitus group.
We also assessed the amount of central gain directly by calculating the wave I/V amplitude ratio for each of the stimulus levels, and separately for the tinnitus and non-tinnitus participants and for each sex. Results are shown in Figure 6, where error bars indicate one standard deviation for the non-tinnitus group and symbols illustrate individual wave I/V ratios for the tinnitus participants. The wave I/V ratio of every tinnitus participant was within or close to the +/- one standard deviation range (Figure 6), indicating that that the wave I/V ratio of every tinnitus participant in our sample was well within the ratio distribution for the non-tinnitus group.
Figure 6.
ABR wave I/V amplitude ratio as a function of ABR click stimulus level
Lines and error bars illustrate mean +/− one standard deviation for the non-tinnitus participants. Symbols illustrate individual wave I/V ratios for the tinnitus participants. Data are shown separately for men and women.
Discussion
In summary, we found ABR wave V amplitude not to be correlated with age or with wave I amplitude after correction for effects of subclinical hearing loss and cochlear mechanical deficits. Assuming that smaller wave I amplitudes and/or increasing age are indicative of reduced peripheral output, this suggests that a central gain mechanism along the auditory neural pathway amplifies peripheral responses to normal levels at the wave V generation site. In addition, we found the ABR wave I/V amplitude ratio to be negatively correlated with age, indicative of comparatively larger wave V than wave I amplitudes with increasing age. This is also consistent with a central gain mechanism amplifying wave V relative to wave I. Our findings survived rigorous controls for potentially confounding effects of small threshold increases and reduced DPOAEs that accompany age. Further, we found evidence that the gain compensatory mechanism is likely located at the wave III generator site (the cochlear nuclei). Lastly, we found no differences in wave I or V amplitudes for seven individual tinnitus participants compared to the distribution of the wave amplitudes for the non-tinnitus participants. Overall, our findings suggest that increased central gain occurs with aging for people with normal audiograms and that central gain compensation is not specific to tinnitus.
On the contribution of cochlear mechanical deficits to wave I and V amplitudes and their ratio
The impact of small cochlear mechanical deficits on ABR amplitudes may have contributed to the discrepant findings across earlier studies of central gain compensation (see the introduction). The present study illustrates the importance of partialling out cochlear mechanical deficits in that the significant correlation between wave V amplitude and age (Figure 2B) and between the amplitudes of wave V and wave I for women (Figure 2A) became not significant after adjusting for hearing threshold and DPOAEs (Figures 2C and 2D), most likely because of differential contributions of small hearing losses to wave I and wave V (Don and Eggermont, 1978; Eggermont and Don, 1980).
Adjusting for small hearing loss and cochlear mechanical deficits is also required to get precise estimates of neural gain. For example, the wave I/V amplitude ratio decreased at a rate of 0.0083 per year (Figure 3A), which was reduced to 0.0056 per year (Figure 3B) after adjusting for cochlear deficits. DPOAEs and hearing thresholds together explained more variance in wave V amplitude than thresholds alone, which suggests that OAEs may be sensitive to small cochlear deficits not (yet) reflected as a threshold elevation. This is consistent with studies that have reported noise-exposure to reduce OAEs before thresholds are affected (Attias et al., 1995; LePage, 1998; Seixas et al., 2005; Lapsley Miller et al., 2006) but inconsistent with others that found age-related declines in DPOAEs and transient-evoked OAEs to be inversely correlated with threshold increases (e.g., Hoth et al., 2010). In summary, cochlear mechanical deficits contribute significantly to ABR amplitudes and it seems appropriate to account for their effect using both hearing thresholds and OAEs.
The cause of the age-related decrease in auditory nerve output
We found the amplitude of wave I to decrease with increasing age even after adjusting the amplitude of wave I for subclinical threshold elevations and cochlear mechanical deficits. This indicates that auditory nerve responses decrease with increasing age probably because of inner hair cell and/or auditory nerve deficits. One likely cause is age-related cochlear synaptopathy. Two arguments support this view. First, in human temporal bones, the number of synapses decreases with increasing age (Makary et al., 2011; Viana et al., 2015; Wu et al., 2018). Second, animal research has demonstrated a link between reduced wave 1 amplitude and age-related reduction in number of synapses (Sergeyenko et al., 2013).
Evidence consistent with increased central gain with increasing age
The present evidence for central gain compensation for an age-related reduction of auditory nerve output appears inconsistent with animal research that found that the aged brain does not compensate for reduced peripheral output (e.g., Rüttiger et al., 2013; Möhrle et al., 2016). On the other hand, our finding is consistent with studies that reported decreasing wave I/V ratios with aging without controlling for age-related cochlear deficits (Psatta and Matei, 1988; Costa et al., 1990; Sand, 1991; Burkard and Sims, 2001; Moosavi et al., 2016). Konrad-Martin et al. (2012) did control for age-related cochlear deficits and reported the amplitude of wave I to be reduced more than that of wave V with increasing age. Their model equation, however, suggests that the wave I/V amplitude ratio varied only from 0.56 to 0.52 over a 40-year age range (i.e., -0.0010 per year). We found the ratio to decrease at a rate of 0.0056 per year, which suggests much larger gain compensation. The reason for the discrepancy across the two studies is unclear. One difference is that Konrad-Martin et al. (2012) included mostly male veterans (79%) and relatively few younger participants (N = 9; age < 40 years), while we included mostly women (68%) and relatively more young participants (Figure S2).
Origin of the central gain mechanism
We found that the wave I/III ratio decreases with aging while the wave III/V ratio does not (Figure 4), which suggests that most of the compensation for reduced peripheral output occurs at the wave III generation site. Previous studies of humans have reported age-related gain compensation to occur mostly at the wave III generator site and to a smaller extent at the wave V generator site (Costa et al., 1990), equally at the wave III and V generator sites (Sand, 1991), or mostly at the wave V generator site (Psatta and Matei, 1988). One study reported wave I/III and III/V amplitude ratios to be constant with age (Moosavi et al., 2016). The discrepancy across studies might be due to the fact that cochlear deficits predominantly affect peripherally generated ABR waves (Don and Eggermont, 1978; Eggermont and Don, 1980; Verhulst et al., 2016), and previous studies did not compensate for cochlear deficits. We have statistically compensated wave I/III and wave III/V ratios for thresholds and DPOAEs, so our results are unlikely to be affected by those cochlear deficits.
Reduced wave I with normal wave III amplitudes has been predicted based on computational models (Schaette and Kempter, 2009) and reported after noise-exposure (Bramhall et al., 2017) as well as for tinnitus patients with unknown noise-exposure history (Gu et al., 2012). Like ours, those studies controlled for the confounding effects of thresholds and their results are consistent with our findings.
No alterations of ABR responses in tinnitus
Participants were recruited opportunistically and so the number of participants who incidentally had tinnitus (N = 7) was insufficient for a powerful group-level comparison with the non-tinnitus group (N = 87). Nonetheless, except for one man at 90 dB ppeSPL (Figure 5F), in no other condition did any of the tinnitus individuals in our sample have ABR wave I and V amplitudes (Figure 5) or wave I/V ratios (Figure 6) outside the 95% confidence intervals of the non-tinnitus group. This finding is consistent with most studies (Barnea et al., 1990; Attias et al., 1993; Attias et al., 1996; Gilles et al., 2016; Guest et al., 2017; Shim et al., 2017; see also review by Milloy et al., 2017), but inconsistent with two studies that reported reduced wave I with normal wave V amplitudes for tinnitus subjects (Schaette and McAlpine, 2011; Gu et al., 2012). The reason for the discrepant findings is uncertain but might be related to differences in age, degree of subclinical hearing loss, and/or severeness of tinnitus across the participants used in the different studies.
Implications for the neural basis of tinnitus
We have presented evidence for increased central gain in older participants with reduced nerve output. If increased central gain was the only required condition to produce a tinnitus percept, it would not be expected that only a small fraction (8%) of the present participants reported having tinnitus. Perhaps, our older participants did not (yet) suffer from sufficient synaptopathy and their central gain was not (yet) increased enough to produce tinnitus. This explanation, however, seems unlikely because Schaette and McAlpine (2011) showed a relative increase in gain of 1.2 to 1.27 on average (or a 20% to 27% reduction in wave I/V ratio) for the tinnitus compared to their control group. In comparison, the present older participants had on average 20% smaller wave I/V ratios than the younger participants (Figure 3) and hence a larger proportion of them would be expected to have tinnitus than was the case. Further, except for one, all of the present tinnitus participants were younger than 50 years and hence not among those with the largest gain compensation. In summary, our results suggest that increased central gain is not specific to tinnitus.
Limitations of study
-
•
We have used the term “age-related” to refer to changes across a cohort of participants with different ages. This should not be taken as indicating that the same changes occur necessarily along the life of every individual, i.e., as a result of age per se. It is conceivable that reduced auditory nerve responses occur as a result of specific events in life, and the probability of undergoing one or more such events increases with increasing age.
-
•
Our analyses were restricted to ABRs wave I, III and V, and thus the present results do not exclude further gain compensation from occurring at sites central to the inferior colliculus (e.g. reviewed by Parthasarathy et al., 2019).
-
•
The present analyses and findings were restricted to click-evoked ABRs. It is uncertain if the age-related gain compensation reported here would also apply to sustained stimuli. Some animal studies suggest that gain compensation occurs more peripherally for transient stimuli and more centrally for sustained stimuli (Lai et al., 2017).
-
•
Most of our older participants did not have tinnitus despite presenting elevated central gain. This shows that increased central gain is not sufficient for tinnitus to occur but does not exclude elevated central gain being required for tinnitus to occur for people with a normal audiogram.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| Matlab R2014a | Mathworks, Inc. | RRID: SCR_001622 |
| Intelligent Hearing Systems SmartOAE custom v5.10 (DPOAEs) | Intelligent Hearing Systems Corp. | http://www.ihsys.com/site/ |
| Intelligent Hearing Systems SmartEP v5.10 (ABRs) | Intelligent Hearing Systems Corp. | http://www.ihsys.com/site/ |
| Custom Matlab software (12 kHz threshold and statistical analysis) | This study | N/A |
| Other | ||
| Interacoustics audiometer AD229e (thresholds) | Interacoustics A/S, Assens, Denmark | www.interacoustics.com |
| Interacoustics tympanometer AT235H (middle ear test) | Interacoustics A/S, Assens, Denmark | www.interacoustics.com |
| Intelligent Hearing Systems Smart device (ABRs) | Intelligent Hearing Systems Corp. | http://www.ihsys.com/site/ |
| Etymotic ER-3A insert earphones (ABRs) | Etymotic Research; Elk Grove Village,IL, USA | www.etymotic.com |
| Intelligent Hearing Systems Smart device (DPOAEs) | Intelligent Hearing Systems Corp. | http://www.ihsys.com/site/ |
| Etymotic ER-10D probe (DPOAEs) | Etymotic Research; Elk Grove Village,IL, USA | www.etymotic.com |
| RME Fireface 400 sound card (12 kHz thresholds) | RME audio, Haimhausen, Germany | www.rme-audio.de |
| Etymotic ER-2 insert earphones (12 kHz thresholds) | Etymotic Research; Elk Grove Village,IL, USA | www.etymotic.com |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Enrique A. Lopez-Poveda (ealopezpoveda@usal.es).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The raw data sets supporting the current study are available from the lead contact on request.
Experimental model and subject details
The study was approved by the Ethics Review Board of the University of Salamanca. Participants were volunteers and not paid for their services. They all signed an informed consent before they were admitted to the study.
Participants were required to have hearing thresholds ≤20 dB HL at audiometric frequencies between 0.5 to 4 kHz and ≤30 dB HL at 6 and 8 kHz (Figure S1), and normal tympanometry in the test ear. Participants were tested in the ear with lowest mean threshold across the frequencies 0.5 to 8 kHz (53 left ears, and 41 right ears). Participants were excluded if they reported suffering from, or having suffered from any audiological or neurological disorders other than chronic tinnitus or had been diagnosed with memory or attention disorders. The age distribution of the non-tinnitus participants was similar for men and women (Figure S2A). The ages of the tinnitus participants ranged from 37 to 48 years for men (N=3) and from 25 to 68 years for women (N=4) (Figure S2B).
Method details
Hearing thresholds
Air-conduction hearing thresholds at the audiometric frequencies (0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz) (ANSI, 2004) were measured using a clinical audiometer (Interacoustics AD229e) equipped with TDH-39 headphones. Stimuli were warble tones. Thresholds were measured using a 10-dB-down, 5-dB-up rule and threshold was defined as the level at which the participant detected the tone at least 50% of the times that it was presented during the ascents. Thresholds at 12 kHz were measured using custom-made Matlab (R2014a) software. Stimuli were pure tones with a duration of 500 ms (including 1-ms cosine-squared onset and offset ramps) and were presented to the participants via an RME Fireface sound card connected to Etymotic ER-2 insert phones. 12-kHz thresholds were measured using a three-interval, three-alternative, forced choice adaptive procedure with feedback to track the sound pressure level at 71% correct tone detection in the psychometric function. The initial step size was 4 dB, which was decreased to 2 dB after three reversals in level occurred. The adaptive procedure continued until 12 reversals in level were measured. Threshold was calculated as the mean tone level at the last 10 reversals. A measurement was discarded if the standard deviation of the levels at the last 10 reversals exceeded 4 dB. Three threshold estimates were obtained in this way and their mean was taken as the threshold.
Distortion product otoacoustic emissions
DPOAE input/output (I/O) curves were measured to assess cochlear outer hair cell function. Test frequencies (f2) were 0.5, 1, 1.5, 2 and 4 kHz (f1=f2/1.2). Stimulus levels (L2) ranged from 35 dB SPL to 70 dB SPL in 5-dB steps. The level of the f1 tone (L1) was set equal to L1 = 34 + 0.6·L2. A third tone (with a frequency f3≈2·f1-f2-50 Hz) was presented to suppress the contribution of the reflection source to the ear canal DPOAE. The level (L3) of the third tone (f3) was set equal to L3 = 37+0.33·L2 for f2 = 0.5, 1, 1.5 and 2 kHz and to L3 = 32+0.42·L2 for 4 kHz.
DPOAE responses were regarded as present when they exceeded by at least 6 dB the participant’s noise level and the artifactual response from the measurement equipment. Absent DPOAEs need not be a sign of outer hair cell lesions. Here, most of the absent DPOAEs were due to high levels of physiological noise at 0.5 and 1 kHz (Lonsbury-Martin and Martin, 2008) and/or high levels of artifactual responses produced by the measurement equipment. Given that there is no consensus on normal DPOAE I/O curve responses, the participants’ DPOAE responses seemed typical for a study population with audiometric thresholds within the range required here but yet low DPOAE responses might indicate small sub-clinical mechanical damage (Figure S5) that should be compensated for when analyzing the effect of age on ABR amplitudes.
To be able to calculate the correlation of DPOAE levels with ABR wave amplitudes, the DPOAE levels recorded at multiple (L2) levels and test frequencies (f2) were collapsed into a single value as follows. First, an overall DPOAE level for each test frequency (f2) was calculated as the area underneath of the measured DPOAE I/O curve with the base of the area arbitrarily set at -15 dB SPL. Second, the overall DPOAE levels at the various test frequencies were averaged.
ABRs
ABR responses were obtained using rarefaction click stimuli (duration 100 μs) with levels from 110 dB peak-peak equivalent sound pressure level (dB ppeSPL) down to 90 dB ppeSPL in steps of 5 dB. The clicks were delivered at a rate of 11 per s through Etymotic ER-3A insert phones. The electrodes were positioned on the mastoid (active), high forehead (reference) and the very high forehead (ground). Responses were amplified 100,000 times and bandpass filtered from 100 to 3000 Hz. Noisy epochs with peak amplitude exceeding ±31 mV were eliminated. The number of averaged responses increased with decreasing stimulus level from 2048 at 110 ppeSPL to 8196 at 90 ppeSPL. The ABR wave amplitudes were calculated from the wave peak to the next trough. Unless otherwise stated, the amplitudes of waves I, III and V were quantified as the mean across stimulus levels of 100, 105 and 110 dB ppeSPL, in an attempt to reduce variability.
Tinnitus assessment
A participant was classified as having tinnitus if they reported (1) perceiving tinnitus in either ear; (2) the percept was non-pulsatile; and (3) the percept was constantly present except for one participant who had several episodes of tinnitus each with a duration of at least 3 days. Four participants had bilateral tinnitus and three had lateralized tinnitus in the test ear. All but one of the tinnitus participants completed the tinnitus functional index (TFI) questionnaire (Meikle et al., 2012) and the tinnitus handicap inventory (THI) (Kuk et al., 1990; Newman et al., 1996). A summary of the questionnaire scores is shown in Figure S3.
Quantification and statistical analysis
Statistical analyses were performed using the Statistics Toolbox of MATLAB (R2014a). Pearson correlation was used to calculate the correlations presented in the upper row of Figures 1, 2, 3, and 4, in Tables 1 and 2, and in Figures S4 and S5. Semi-partial regression analysis was used in the lower rows of Figures 1, 2, 3, and 4 to adjust the dependent variable for the effect of a potential covarying variable and test for the possible additional effects of the independent variable. As an example, in Figure 1, ABR wave I amplitude was adjusted for the effects of increasing threshold with age before assessing the potential correlation with age. Repeated-measures analysis of variance was used to test if ABR wave V amplitudes were different across sexes. Statistical significance was defined as rejection of the null hypotheses with 95% confidence (p < 0.05).
Acknowledgments
We thank Byanka C. Buzo for help with data collection. We thank Brian C. J. Moore and three anonymous reviewers for thoughtful comments on earlier versions of this paper. Work supported by the Oticon Foundation (grant 15-3571), Junta de Castilla y León (grant SA252P20), Ministerio de Ciencia e Innovación (grant PID2019-108985GB-I00), and the European Regional Development Fund.
Author contributions
Conceptualization, P.T.J. and E.A.L.-P.; Methodology, P.T.J. and E.A.L.-P.; Investigation, P.T.J. Writing-Original draft, P.T.J.; Writing-Review & Editing, P.T.J. and E.A.L.-P.; Funding Acquisition, E.A.L.-P.; Supervision, E.A.L.-P.
Declaration of interests
The authors declare no competing interests.
Published: June 25, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102658.
Supplemental information
References
- ANSI . American National Standards Institute; 2004. S3.6 Specification for Audiometers. [Google Scholar]
- Attias J., Furst M., Furman V., Reshef I., Horowitz G., Bresloff I. Noise-induced otoacoustic emission loss with or without hearing loss. Ear Hear. 1995;16:612–618. doi: 10.1097/00003446-199512000-00007. [DOI] [PubMed] [Google Scholar]
- Attias J., Pratt H., Reshef I., Bresloff I., Horowitz G., Polyakov A., Shemesh Z. Detailed analysis of auditory brainstem responses in patients with noise-induced tinnitus. Audiology. 1996;35:259–270. doi: 10.3109/00206099609071946. [DOI] [PubMed] [Google Scholar]
- Attias J., Urbach D., Gold S., Shemesh Z. Auditory event related potentials in chronic tinnitus patients with noise induced hearing loss. Hear. Res. 1993;71:106–113. doi: 10.1016/0378-5955(93)90026-w. [DOI] [PubMed] [Google Scholar]
- Barnea G., Attias J., Gold S., Shahar A. Tinnitus with normal hearing sensitivity: extended high-frequency audiometry and auditory-nerve brainstem-evoked responses. Audiology. 1990;29:36–45. doi: 10.3109/00206099009081644. [DOI] [PubMed] [Google Scholar]
- Bramhall N., Konrad-Martin D., McMillan G., Griest S. Auditory brainstem response altered in humans with noise exposure despite normal outer hair cell function. Ear Hear. 2017;38:e1–e12. doi: 10.1097/AUD.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall N.F., Konrad-Martin D., McMillan G.P. Tinnitus and auditory perception after a history of noise exposure: relationship to auditory brainstem response measures. Ear Hear. 2018;39:881–894. doi: 10.1097/AUD.0000000000000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard R.F., Sims D. The human auditory brainstem response to high click rates: aging effects. Am. J. Audiol. 2001;10:53–61. doi: 10.1044/1059-0889(2001/008). [DOI] [PubMed] [Google Scholar]
- Cai R., Montgomery S.C., Graves K.A., Caspary D.M., Cox B.C. The FBN rat model of aging: Investigation of ABR waveforms and ribbon synapse changes. Neurobiol. Aging. 2018;62:53–63. doi: 10.1016/j.neurobiolaging.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers A.R., Resnik J., Yuan Y., Whitton J.P., Edge A.S., Liberman M.C., Polley D.B. Central gain restores auditory processing following near-complete cochlear denervation. Neuron. 2016;89:867–879. doi: 10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P., Benna P., Bianco C., Ferrero P., Bergamasco B. Aging effects on brainstem auditory evoked potentials. Electromyogr. Clin. Neurophysiol. 1990;30:495–500. [PubMed] [Google Scholar]
- Don M., Eggermont J.J. Analysis of the click-evoked brainstem potentials in man using high-pass noise masking. J. Acoust. Soc. Am. 1978;63:1084–1092. doi: 10.1121/1.381816. [DOI] [PubMed] [Google Scholar]
- Eggermont J.J., Don M. Analysis of the click-evoked brainstem potentials in humans using high-pass noise masking. II. Effect of click intensity. J. Acoust. Soc. Am. 1980;68:1671–1675. doi: 10.1121/1.385199. [DOI] [PubMed] [Google Scholar]
- Gilles A., Schlee W., Rabau S., Wouters K., Fransen E., Van de Heyning P. Decreased speech-in-noise understanding in young adults with tinnitus. Front. Neurosci. 2016;10:288. doi: 10.3389/fnins.2016.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J.W., Herrmann B.S., Levine R.A., Melcher J.R. Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. J. Assoc. Res. Otolaryngol. 2012;13:819–833. doi: 10.1007/s10162-012-0344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest H., Munro K.J., Prendergast G., Howe S., Plack C.J. Tinnitus with a normal audiogram: relation to noise exposure but no evidence for cochlear synaptopathy. Hear. Res. 2017;344:265–274. doi: 10.1016/j.heares.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox A.E., Liberman M.C. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J. Neurophysiol. 2014;111:552–564. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth S., Gudmundsdottir K., Plinkert P. Age dependence of otoacoustic emissions: the loss of amplitude is primarily caused by age-related hearing loss and not by aging alone. Eur. Arch. Otorhinolaryngol. 2010;267:679–690. doi: 10.1007/s00405-009-1106-5. [DOI] [PubMed] [Google Scholar]
- Jerger J., Hall J. Effects of age and sex on auditory brainstem response. Arch. Otolaryngol. 1980;106:387–391. doi: 10.1001/archotol.1980.00790310011003. [DOI] [PubMed] [Google Scholar]
- Johannesen P.T., Buzo B.C., Lopez-Poveda E.A. Evidence for age-related cochlear synaptopathy in humans unconnected to speech-in-noise intelligibility deficits. Hear. Res. 2019;374:35–48. doi: 10.1016/j.heares.2019.01.017. [DOI] [PubMed] [Google Scholar]
- Kaltenbach J.A. Tinnitus: models and mechanisms. Hear. Res. 2011;276:52–60. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrle H.M., Granjeiro R.C., Sampaio A.L.L., Bezerra R., Almeida V.F., Oliveira C.A. Comparison of auditory brainstem response results in normal-hearing patients with and without tinnitus. Arch. Otolaryngol. Head Neck Surg. 2008;134:647–651. doi: 10.1001/archotol.134.6.647. [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D., Dille M.F., McMillan G., Griest S., McDermott D., Fausti S.A., Austin D.F. Age-related changes in the auditory brainstem response. J. Am. Acad. Audiol. 2012;23:18–35. doi: 10.3766/jaaa.23.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk F., Tyler R.S., Russel D., Jordan H. The psychometric properties of a tinnitus handicap questionnaire. Ear Hear. 1990;11:434–442. doi: 10.1097/00003446-199012000-00005. [DOI] [PubMed] [Google Scholar]
- Lai J., Sommer A.L., Bartlett E.L. Age-related changes in envelope-following responses at equalized peripheral or central activation. Neurobiol. Aging. 2017;58:191–200. doi: 10.1016/j.neurobiolaging.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapsley Miller J.A., Marshall L., Heller L.M., Hughes L.M. Low-level otoacoustic emissions may predict susceptibility to noise-induced hearing loss. J. Acoust. Soc. Am. 2006;120:280–296. doi: 10.1121/1.2204437. [DOI] [PubMed] [Google Scholar]
- LePage E. Occupational noise-induced hearing loss: origin, characterization and prevention. Acoust. Aust. 1998;26:2–57. [Google Scholar]
- Lonsbury-Martin B.L., Martin G.K. Mammalian models of otoacoustic emissions. In: Manley G.A., Fay R.R., Popper A.N., editors. Active Processes and Otoacoustic Emissions. Springer Science); 2008. pp. 261–303. [Google Scholar]
- Makary C.A., Shin J., Kujawa S.G., Liberman M., Merchant S.N. Age-related primary cochlear neuronal degeneration in human temporal bones. J. Assoc. Res. Otolaryngol. 2011;12:711–717. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle M.B., Henry J.A., Griest S.A., Stewartm B.J., Abrams H.B., McArdle R., Myers P.J., Newman C.W., Sandridge S., Turk D.C. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012;33:153–176. doi: 10.1097/AUD.0b013e31822f67c0. [DOI] [PubMed] [Google Scholar]
- Milloy V., Fournier P., Benoit D., Noreña A., Koravand A. Auditory brainstem responses in tinnitus: a review of who, how, and what? Front. Aging Neurosci. 2017;9:237. doi: 10.3389/fnagi.2017.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C., Phillips D.S., Trune D.R. Variables affecting the auditory brainstem response: audiogram, age, gender and head size. Hear. Res. 1989;40:75–85. doi: 10.1016/0378-5955(89)90101-9. [DOI] [PubMed] [Google Scholar]
- Moller A.R. Neural generators for auditory brainstem evoked potentials. In: Burkard R.F., Don M., Eggermont J.J., editors. Auditory Evoked Potentials: Basic Principles and Clinical Applications. Lippincott, Williams and Wilkins; 2006. pp. 336–354. [Google Scholar]
- Moosavi A., Nazeri A.R., Lotfi Y., Bakhshi E. Comparison of auditory evoked potentials between younger and older adults. J. Hear. Sci. Otolaryng. 2016;2:29–36. [Google Scholar]
- Muniak M.A., Ayeni F.E., Ryugo D.K. Hidden hearing loss and endbulbs of Held: evidence for central pathology before detection of ABR threshold increases. Hear. Res. 2018;364:104–117. doi: 10.1016/j.heares.2018.03.021. [DOI] [PubMed] [Google Scholar]
- Musiek F.E., Kibbe K., Rackliffe L., Weider D.J. The auditory brain stem response I-V amplitude ratio in normal, cochlear, and retrocochlear ears. Ear Hear. 1984;5:52–55. doi: 10.1097/00003446-198401000-00011. [DOI] [PubMed] [Google Scholar]
- Möhrle D., Ni K., Varakina K., Bing D., Lee S.C., Zimmermann U., Knipper M., Rüttiger L. Loss of auditory sensitivity from inner hair cell synaptopathy can be centrally compensated in the young but not old brain. Neurobiol. Aging. 2016;44:173–184. doi: 10.1016/j.neurobiolaging.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Newman C.W., Jacobson G.P., Spitzer J.B. Development of the tinnitus handicap inventory. Arch. Otolaryngol. Head Neck Surg. 1996;122:143–148. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- Noreña A.J. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci. Biobehav. Rev. 2011;35:1089–1109. doi: 10.1016/j.neubiorev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A., Kujawa S.G. Synaptopathy in the aging cochlea: characterizing early-neural deficits in auditory temporal envelope processing. J. Neurosci. 2018;38:7108–7119. doi: 10.1523/JNEUROSCI.3240-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A., Bartlett E.L., Kujawa S.G. Age-related changes in neural coding of envelope cues: peripheral declines and central compensation. Neuroscience. 2019;407:21–31. doi: 10.1016/j.neuroscience.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psatta D.M., Matei M. Age-dependent amplitude variation of brain-stem auditory evoked potentials. Electroencephalogr. Clin. Neurophysiol. 1988;71:27–32. doi: 10.1016/0168-5597(88)90016-0. [DOI] [PubMed] [Google Scholar]
- Rüttiger L., Singer W., Panford-Walsh R., Matsumoto M., Lee S.C., Zuccotti A., Zimmermann U., Jaumann M., Rohbock K., Xiong H. The reduced cochlear output and the failure to adapt the central auditory response causes tinnitus in noise exposed rats. PLoS One. 2013;8:e57247. doi: 10.1371/journal.pone.0057247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand T. BAEP amplitudes and amplitude ratios: relation to click polarity, rate, age and sex. Electroencephalogr. Clin. Neurophysiol. 1991;78:291–296. doi: 10.1016/0013-4694(91)90183-5. [DOI] [PubMed] [Google Scholar]
- Schaette R., Kempter R. Predicting tinnitus pitch from patients’ audiograms with a computational model for the development of neuronal hyperactivity. J. Neurophysiol. 2009;101:3042–3052. doi: 10.1152/jn.91256.2008. [DOI] [PubMed] [Google Scholar]
- Schaette R., McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J. Neurosci. 2011;31:13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seixas N.S., Goldman B., Sheppard L., Neitzel R., Norton S.J., Kujawa S.G. Prospective noise induced changes to hearing among construction industry apprentices. Occup. Environ. Med. 2005;62:309–317. doi: 10.1136/oem.2004.018143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyenko Y., Lall K., Liberman M.C., Kujawa S.G. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J. Neurosci. 2013;33:13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H.J., An Y.-H., Kim D.H., Yoon J.E., Yoon J.H. Comparisons of auditory brainstem response and sound level tolerance in tinnitus ears and non-tinnitus ears in unilateral tinnitus patients with normal audiograms. PLoS one. 2017;12:e0189157. doi: 10.1371/journal.pone.0189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trune D.R., Mitchell C., Phillips D.S. The relative importance of head size, gender and age on the auditory brainstem response. Hear. Res. 1988;32:165–174. doi: 10.1016/0378-5955(88)90088-3. [DOI] [PubMed] [Google Scholar]
- Valderrama J.T., Beach E.F., Yeend I., Sharma M., Van Dun B., Dillon H. Effects of lifetime noise exposure on the middle-age human auditory brainstem response, tinnitus and speech-in-noise intelligibility. Hear. Res. 2018;365:36–48. doi: 10.1016/j.heares.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Verhulst S., Jagadeesh A., Mauermann M., Ernst F. Individual differences in auditory brainstem response wave characteristics: relations to different aspects of peripheral hearing loss. Trends Hear. 2016;20:1–20. doi: 10.1177/2331216516672186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana L.M., O'Malley J.T., Burgess B.J., Jones D.D., Oliveira C.A., Santos F., Merchant S.N., Liberman L.D., Liberman M.C. Cochlear neuropathy in human presbycusis: confocal analysis of hidden hearing loss in post-mortem tissue. Hear. Res. 2015;327:78–88. doi: 10.1016/j.heares.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P.Z., Liberman L.D., Bennett K., de Gruttola V., O’Malley J.T., Liberman M.C. Primary neural degeneration in the human cochlea: evidence for hidden hearing loss in the aging ear. Neuroscience. 2018;407:8–20. doi: 10.1016/j.neuroscience.2018.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data sets supporting the current study are available from the lead contact on request.