Summary
Low temperatures can be fatal to insects, but many species have evolved the ability to cold acclimate, thereby increasing their cold tolerance. It has been previously shown that Drosophila melanogaster larvae perform cold-evoked behaviors under the control of noxious cold-sensing neurons (nociceptors), but it is unknown how the nervous system might participate in cold tolerance. Herein, we describe cold-nociceptive behavior among 11 drosophilid species; we find that the predominant cold-evoked larval response is a head-to-tail contraction behavior, which is likely inherited from a common ancestor, but is unlikely to be protective. We therefore tested the hypothesis that cold nociception functions to protect larvae by triggering cold acclimation. We found that Drosophilamelanogaster Class III nociceptors are sensitized by and critical to cold acclimation and that cold acclimation can be optogenetically evoked, sans cold. Collectively, these findings demonstrate that cold nociception constitutes a peripheral neural basis for Drosophila larval cold acclimation.
Subject areas: Biological sciences, Physiology, Neuroscience, Behavioral neuroscience, Cellular neuroscience, Sensory neuroscience
Graphical abstract
Highlights
-
•
Drosophila larvae respond to cold via behaviors which are not obviously protective
-
•
Genetically silencing cold nociceptors results in an inability to cold acclimate
-
•
Cold acclimation results in nociceptor hypersensitization
-
•
Cold tolerance can be improved by activating cold nociceptors sans cold
Biological sciences; Physiology; Neuroscience; Behavioral neuroscience; Cellular neuroscience; Sensory neuroscience
Introduction
Small ectotherms can be particularly susceptible to damage by chilling. For example, in Drosophila melanogaster, prolonged exposure to extreme cold can cause permanent defects in fertility, and even death, particularly among early life stages (Dillon et al., 2007; Mockett and Matsumoto, 2014). In response, many ectotherms have evolved the ability to acclimate to cold, both rapidly (rapid cold hardening; RCH) and in response to long-term, often seasonal, cooling (cold acclimation) (Bowler, 2005; Teets et al., 2020). Understanding these processes is important to understanding ectotherm ecology and evolution, but given that climate change is leading to both increased daily temperatures and increased severity of winter weather events, understanding thermal plasticity is also key to understanding how a changing climate might affect insect ecology (Cohen et al., 2018; Dillon et al., 2016; Sgrò et al., 2016).
A substantial and growing body of literature details how insect RCH and cold acclimation are mediated by a variety of physiological, genetic, and biochemical changes, many of which are tied to ionoregulatory balance (Andersen and Overgaard, 2020; Bayley et al., 2020; Des Marteaux et al., 2018a, 2018b; Gerber and Overgaard, 2018; MacMillan et al., 2016; Nadeau and Teets, 2020; Salehipour-shirazi et al., 2017; Toxopeus et al., 2019; Toxopeus and Sinclair, 2018). While there is evidence that aspects of RCH are mediated independently of the nervous system (Nadeau and Teets, 2020; Teets et al., 2013), there is also evidence that absolute temperature information encoded by cold sensors drives changes in sleep and wakefulness, and that shutdown in the central nervous system (via spreading depolarization) is coincident with cold acclimation (Alpert et al., 2020; Andersen et al., 2018). Yet, it remains unknown if the nervous system serves to proximally activate cold acclimation, or to what degree peripheral sensory neurons might function in this capacity—long-standing questions in the field of ectotherm biology (Bowler, 2005; Lagerspetz, 1974; Prosser and Nelson, 1981).
Given the suite of genetic tools available in Drosophila, and an emerging understanding of Drosophila larval cold nociception, the fruit fly larva constitutes a useful organism for investigating these unknowns. In response to noxious cold (≤10°C) D. melanogaster larvae primarily execute a highly stereotyped, bilateral contraction (CT) response, where the head and tail tuck toward the midline (Turner et al., 2016). This behavior is triggered by the activation of cold nociceptors innervating the barrier epidermis. In larvae, the primary cold nociceptors have been demonstrated to be Class III (CIII) dendritic arborization (da) peripheral sensory neurons, with Class II da (CII) and chordotonal (Ch) neurons also functioning in the noxious cold-sensing neural ensemble (Turner et al., 2016, 2018). In contrast, innocuous cool sensing takes place among Ch neurons and thermosensors in the dorsal and terminal organ ganglia; these sensors primarily inform thermotaxis, which is undoubtedly an important part of maintaining optimal temperature (Klein et al., 2015; Kwon et al., 2010; Ni et al., 2016).
Given that CT results in a reduced surface area to volume ratio—which is a common strategy for keeping warm (Canals et al., 1997; Contreras, 1984; Gilbert et al., 2006; Hayes et al., 1992; Vickery and Millar, 1984)—cold nociception appears, prima facie, to be protective. However, it is unknown how widespread cold nociception is among drosophilids, to what degree cold nociception protects against cold shocks, or how cold nociception might relate to cold acclimation.
Herein, we more completely assess cold nociceptive behavior among drosophilid larvae, describing cold-evoked behaviors in 11 species with known differences in their cold tolerance (Kellermann et al., 2012; MacLean et al., 2019; Strachan et al., 2011). We additionally test the hypotheses that cold nociceptors are necessary and sufficient to cold acclimation. Making use of cold-behavior and cold-shock assays, optogenetics, electrophysiology, and methods to genetically disrupt neural transmission, we demonstrate that: (1) Although behavioral programs differ between drosophilid species, cold nociception is broadly present and CT is the predominant cold-evoked behavior; (2) species within the repleta group perform a unique, highly stereotyped, cold-evoked behavior we termed the spiracle extension response (SER); (3) in D. melanogaster, silencing CIII nociceptors results in an inability to cold acclimate; (4) cold acclimation is coincident with nociceptor sensitization; and (5) optogenetic activation of CIII nociceptors, sans cold, is sufficient for driving cold acclimation.
Results
Cold nociception is widespread among drosophilid larvae
We have previously demonstrated that D. melanogaster larvae primarily respond to noxious cold by performing a bilateral CT along the head-to-tail axis (Turner et al., 2016). We first tested the hypothesis that CT behavior is a widespread, conserved trait among drosophilids by assessing cold-evoked behaviors (0-16°C) among 11 drosophilid species with known differences in distribution and cold tolerance (Figures 1A and 1B) (Kellermann et al., 2012; MacLean et al., 2019; Strachan et al., 2011).
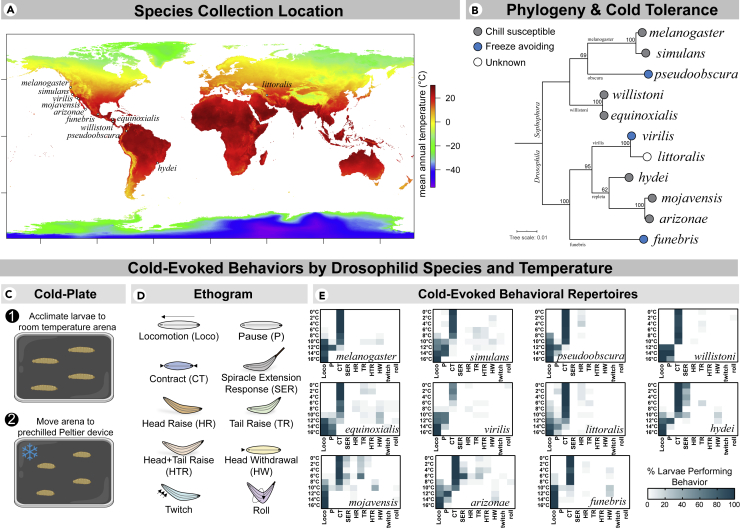
Figure 1.
Drosophila species native to a variety of thermal environments and with known differences in cold tolerance were chosen for behavioral analysis
(A) Map of species stock collection location (provided by the Drosophila Species Stock Center) mapped on mean annual temperature, as extracted from climate data retrieved from WorldClim (https://www.worldclim.org) (Fick and Hijmans, 2017).
(B) Maximum likelihood phylogeny with ultrafast bootstrapping (at nodes). This tree was generated using concatenated and aligned CoI, CoII, and Adh sequences (Table S1). Most species chosen are chill susceptible (die in response to sub-freezing temperatures), while 3 are freeze avoiding (able to survive to the supercooling point) (Sinclair et al., 2015; Strachan et al., 2011).
(C) Outline of the previously developed cold-plate behavioral assay (Patel and Cox, 2017; Turner et al., 2016). Subject behaviors were recorded for 30s after application of cold.
(D) Ethogram representations of full cold-evoked behavioral repertories.
(E) Heatmap representations of full cold-evoked behavioral repertoires by species and temperature. The predominant cold-evoked response among drosophilid larvae is a bilateral, head-and-tail contraction (CT) behavior. Among repleta group species, an additional, highly stereotyped behavior termed the spiracle extension response (SER) is present at low temperatures. CT was typically transient; locomotion resumed at higher temperatures (at and above approx. 8°C, depending on species), and lasting, flaccid paralysis occurred at lower temperatures (approx. 0-10°C, depending on species). Larvae performed a variety of other behaviors at relatively low frequencies, with no obvious patterns. N = 2,970, n = 30 for each condition, each referring to the number of larvae assayed.
Using the previously developed cold plate assay (Turner et al., 2016) (Figure 1C) we observed 9 distinct, cold-evoked behaviors, with each species exhibiting a slightly different behavioral repertoire (Figures 1D and 1E and Tables S2–S12). All species tested performed the CT response (Figure 1E), yet larvae typically failed to remain contracted for the 30 s behavioral analysis. Qualitatively, larvae either ceased CT and resumed locomotion (at higher temperatures; at and above ∼8°C, depending on species) or ceased CT and were paralyzed by the cold (at lower temperatures; ∼0-10°C, depending on species). It therefore appears unlikely that the CT behavior itself would protect larvae from long-term noxious cold exposure.
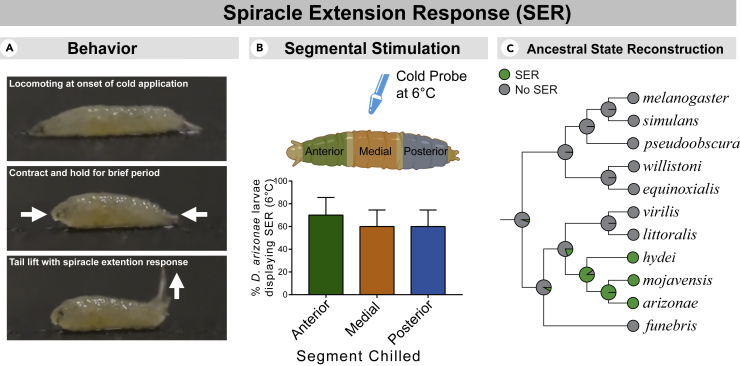
In addition to CT, we observed a behavior we termed the SER (Figure 2). In larvae, the posterior spiracles are part of a snorkel-like respiratory organ with the ability to extend and contract, allowing larvae to respirate while mostly submerged in semi-liquid media (Manning and Krasnow, 1993). In response to noxious cold, larvae from the repleta group performed a highly stereotyped behavior wherein the posterior stigmatophore—which contains the spiracular chamber—rapidly and greatly extended from the contracted state (Figure 2A). This behavior typically occurred post-CT and was accompanied by a robust tail-raise behavior. This behavior does not seem to be solely initiated by direct, chill-induced CTs (MacMillan et al., 2014) in the musculature controlling the stigmatophore, as segmental cold stimulation across the body also resulted in the SER (Figure 2B). SER likely emerged within the repleta group (supported by ancestral state reconstruction; Figure 2C).
Figure 2.
A robust spiracle extension response (SER) was performed by repleta species
(A) Description of cold-evoked SER, with images (subject, D. arizonae).
(B) % SER following segmental cold stimulation via cold-probe assay ±standard error of the proportion. N = 30, n = 10, each referring to the number of larvae assayed.
(C) Ancestral state reconstruction by single-rate continuous-time Markov chain model indicates that the SER behavior most likely evolved in a common repleta ancestor after the group split from other drosophilids.
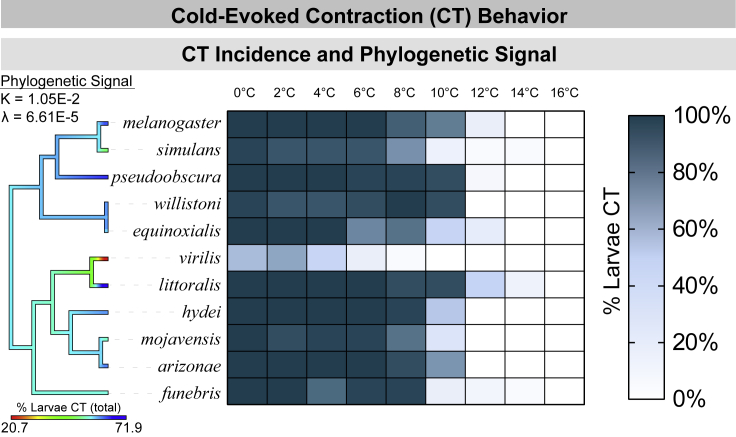
Given the widespread incidence of CT, the behavior is likely inherited from a common ancestor (Figure 3). We predicted that, if CT is adaptive, there might be evidence of trait lability and/or of thermal environment placing selective pressure on its evolution. We assessed this by measuring phylogenetic signal and investigating possible environmental correlates of CT behavior. Computed measures of phylogenetic signal—Blomberg's K (Blomberg et al., 2003) and Pagel's λ (Pagel, 1999)—show no evidence of phylogenetic signal for the incidence of CT (% CT in the entire intraspecies population tested; Figure 3). This is typically interpreted as trait lability, or that the trait is not evolving as a result of constant-rate genetic drift (Kamilar and Cooper, 2013; Münkemüller et al., 2012; Revell et al., 2008).
Figure 3.
The predominant cold-evoked behavior is CT, a behavior conserved from a common ancestor but with no obvious correlation to thermal environment
Heatmap representation of % CT by species and temperature. All species CT, but with slightly different cold sensitivities. N = 2,970, n = 30 for each condition, each referring to the number of larvae assayed. % of larvae which performed CT out of the total intraspecies population was color-mapped to the tree by maximum likelihood ancestral state estimation (color at tip indicates value for that species). Blomberg's K (Blomberg et al., 2003) and Pagel's λ (Pagel, 1999) show no evidence of phylogenetic signal.
We initially predicted that species from colder environments would be less cold sensitive. However, we found no correlations between the total percentage of subjects with CT across temperatures (total percentage CT) and a variety of cold-related climate variables (Table 1 and Figure S1), possibly indicating that the behavior is not evolving directly in response to differences in cold climate. We additionally looked for correlations between environmental variables and the temperature where at least 50% of subjects with CT (CT50; Table 1 and Figure S2), the highest temperature at which CT appears (CTinit; Table 1 and Figure S3), and the temperature where at least 50% of subjects ceased locomotion (L50; Table 1 and Figure S4). The only significant correlation we found was between L50 and mean diurnal range, indicating that larvae adapted to climates with rapid temperature swings can continue to locomote at lower temperatures.
Table 1.
Correlations of cold-evoked behavior and climate variables from WorldClim
| Variable | Value | Total % CT | CT50 | CTinit | L50 |
|---|---|---|---|---|---|
| Annual mean temperature | R2 | 0.032041 | 0.07076 | 0.36 | 0.0396 |
| p value | 0.598 | 0.43 | 0.051 | 0.558 | |
| BF₁₀ | 0.419 | 0.489 | 2.026 | 0.431 | |
| Mean diurnal range | R2 | 0.054756 | 0.08644 | 4 × 10−6 | 0.38069 |
| p value | 0.489 | 0.38 | 0.995 | 0.043∗ | |
| BF₁₀ | 0.458 | 0.523 | 0.369 | 2.289 | |
| Isothermality | R2 | 0.000289 | 0.0024 | 0.0169 | 0.00221 |
| p value | 0.96 | 0.885 | 0.702 | 0.891 | |
| BF₁₀ | 0.37 | 0.373 | 0.395 | 0.373 | |
| Min temperature of coldest month | R2 | 0.012321 | 0.01664 | 0.2043 | 0.06101 |
| p value | 0.745 | 0.705 | 0.163 | 0.464 | |
| BF₁₀ | 0.388 | 0.394 | 0.885 | 0.47 | |
| Temperature annual range | R2 | 0.000049 | 0.0024 | 0.02403 | 0.04623 |
| p value | 0.985 | 0.886 | 0.649 | 0.525 | |
| BF₁₀ | 0.369 | 0.373 | 0.406 | 0.443 | |
| Mean temperature of coldest quarter | R2 | 0.035344 | 0.04537 | 0.23329 | 0.01323 |
| p value | 0.579 | 0.53 | 0.132 | 0.737 | |
| BF₁₀ | 0.424 | 0.441 | 1.022 | 0.389 |
Total % CT is the total number of subjects which CT for each species, across all temperatures. CT50 is the temperature at which at least 50% of subjects CT. CTinit is the highest temperature in which CT was observed, at any frequency. L50 is the temperature at which at least 50% of subjects stop locomoting. The only significant correlation was between L50 and mean diurnal range.
Silencing CIII cold nociceptors inhibits cold acclimation
Given the widespread presence of cold nociception among drosophilids, we next questioned whether cold nociception plays a role in protecting larvae from noxious cold.
We first tested the hypothesis that cold nociception is a necessary component of cold acclimation. The GAL4-UAS system was used to drive the expression of the light chain of tetanus toxin (TNT) in targeted cold-sensing neural populations (CII, GAL437B02;CIII, GAL419−12; Ch, GAL4iav; CII + CIII, GAL41003.3; and CIII + Ch, GAL4nompC), thereby selectively silencing neural transmission. TNT expression in cold nociceptors has been previously shown to severely inhibit the cold-evoked CT behavioral response (Turner et al., 2016).
Transgenic D. melanogaster populations were raised at 24°C until third instars were present and were thereafter incubated for 48 hr at either 24°C or 10°C, the latter to drive cold acclimation. After incubation, only wandering third instar larvae were chosen for experimentation so as to developmentally match subjects. Wandering third instar larvae were cold-shocked at 0°C for 60 min by a modified cold-plate assay. Survival was assessed by the proportion of animals that eclosed as adults (Figure 4A). To control for any baseline differences in cold tolerance between transgenic strains, cold tolerance was also assessed in subjects expressing an inactive form of TNT (impaired TNT; TNTIMP) in the same GAL4 genetic backgrounds.
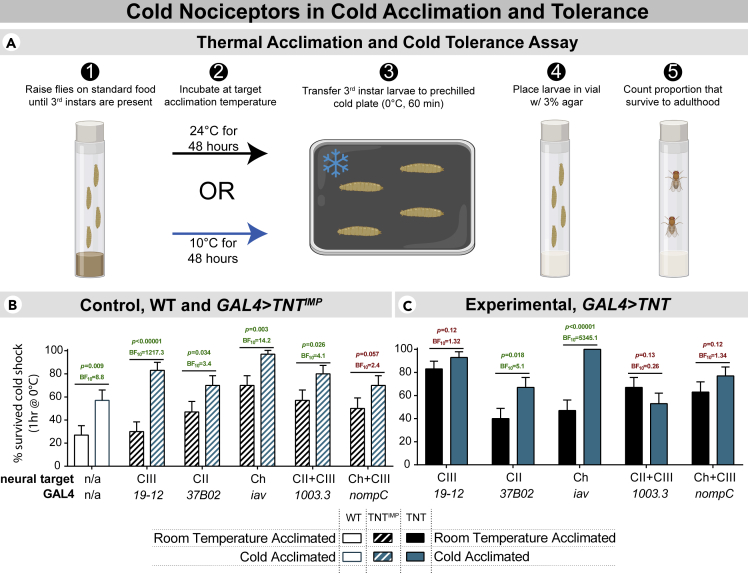
Figure 4.
Class III nociceptors are necessary for cold acclimation
(A) Outline of the approach used for thermal acclimation and cold tolerance assessment. D. melanogaster larvae were acclimated to either 24 or 10°C and subjected to 0°C cold shock for 60 min. The percentage of animals which successfully eclosed constituted the % survival rate.
(B) Control (w1118 and GAL4>TNTIMP) larvae showed some baseline differences in cold tolerance, but showed increased cold tolerance following cold acclimation, regardless. Bar charts show % animals which survived cold shock ±standard error of the proportion. N = 360, n = 30 in each condition, each referring to the number of larvae assayed, with experiments performed in triplicates of 10 larvae each. Differences were assessed by z-test and Bayesian A/B test.
(C) Silencing CIII neurons via active tetanus toxin (TNT) resulted in an inability to cold acclimate; cold acclimated larvae had similar cold tolerance to room temperature acclimated larvae. Silencing CII and Chordotonal (Ch) neurons independently had no effect on cold acclimating capacity. Silencing CIII neurons independently resulted in an increase in baseline cold tolerance, but this was not apparently present when combinatorially silencing CIII with CII/Ch. Bar charts show % animals which survived cold shock ±standard error of the proportion. N = 300; n = 30 in each condition with experiments performed in triplicates of 10 larvae each. Differences were assessed by z-test and Bayesian A/B test.
In accordance with previous studies (Colinet and Hoffmann, 2012; Hoffmann et al., 2003; MacMillan et al., 2015; Overgaard et al., 2011; Rako and Hoffmann, 2006; Ransberry et al., 2011), cold acclimated control animals (w1118 and TNTIMP) were more cold tolerant than counterparts raised at room temperature (Figure 4B). Although the cold-acclimated nompC control (CIII + Ch) condition was not significantly or substantially different from baseline, Bayesian statistics indicate that the hypothesis that they cold acclimate is ∼2.4 times more likely than the null hypothesis.
In contrast, silencing CIII neurons (via GAL419−12, GAL410033., and GAL4nompC) resulted in an inability to cold acclimate, indicating that CIII cold nociceptive neurons are necessary for cold acclimation (Figure 4C). Silencing CII (GAL437B02) and Ch (GAL4iav) alone did not inhibit the ability of larvae to cold acclimate. Surprisingly, silencing CIII neurons alone (GAL419−12) resulted in increased cold tolerance in room temperature-acclimated larvae, mimicking survival rates of cold-acclimated larvae. Combinatorially silencing CIII with CII or Ch neurons resulted in an inability to cold acclimate but did not obviously increase baseline cold tolerance.
Given that silencing CIII neurons via 3 different GAL4s (19-12, 1003.3, and nompC) resulted in an inability to cold acclimate, the preponderance of evidence suggests that CIII neurons are necessary for cold acclimation. Moreover, that single-class and CIII-combinatorial silencing of CII and Ch (via 1003.3 and nompC GAL4s) does not result in an obvious phenotype in room temperature acclimated animals, these results suggest that CII and Ch neurons are not necessary for cold acclimating capacity, but that they may play some role in modulating cold tolerance. This is consistent with CII and Ch neurons functioning in cold nociception but not in an independently necessary capacity (Turner et al., 2016, 2018).
Developmental exposure to cold results in nociceptive hypersensitization
We next asked how cold acclimation impacts CIII function by recording cold-evoked CIII activity following cold acclimation. Cold-evoked electrical activity was recorded from CIII neurons in live, filleted larvae; CIII neurons were identified by GAL4-UAS-mediated GFP labeling (GAL419−12>UAS-mCD8::GFP).
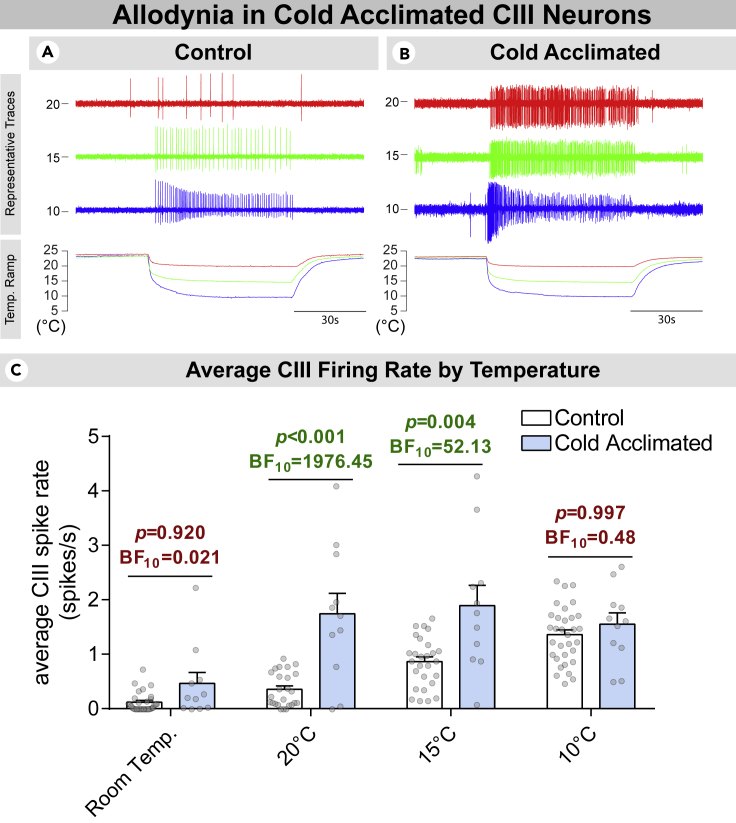
Electrophysiological recordings of CIII neurons reveal that cold acclimation results in CIII hypersensitization; on average, CIII neurons in cold acclimated larvae responded more strongly to temperature drops to 20°C and 15°C (Figure 5). Firing rates were increased and sustained at 20°C and 15°C, and although mean firing rate was not significantly or substantially increased at 10°C (Figure 5C), we observed an increased firing rate at the onset of chilling (first 10 s), with an activity quickly returning to control levels (Figure S5).
Figure 5.
Cold acclimation results in nociceptive sensitization
(A) Representative traces of extracellular CIII recordings in control larvae.
(B) Representative traces of extracellular CIII recordings in cold acclimated larvae. Neural activity was assessed at room temperature, and during/following ramps to 20, 15, and 10°C.
(C) Average spike frequency of CIII neurons by temperature. CIII neurons in cold acclimated larvae were more sensitive to temperature drops to 20 and 15°C. Neural activity was assessed at room temperature, and during/following ramps to 20, 15, and 10°C. Differences were assessed by frequentist and Bayesian ANOVA. Bar chart details mean spike rate ±standard error of the mean. N = 43; control, n = 32; cold acclimated, n = 11. N and n refer to the number of neurons recorded from.
Developmental optogenetic CIII activation increases cold tolerance
As CIII nociceptors were necessary for cold acclimation, we next tested the hypothesis that CIII nociceptors drive cold acclimation by optogenetically activating CIII neurons sans cold and assessing cold tolerance.
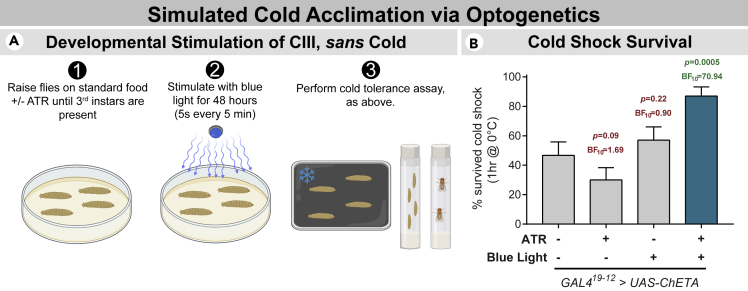
The GAL4-UAS system was used to drive expression of an engineered channelrhodopsin (ChETA)—a light-gated cation channel—in CIII neurons. Transgenic D. melanogaster were raised on food containing all-trans-retinol (ATR+, which is a necessary for the activity of ChETA) or standard food (ATR-, control condition). Larvae were housed in Petri dishes with 2.5mL of food spread extremely thin over the base. 48 hours prior to assessing cold tolerance, dishes were transferred to a custom built OptoBox which houses 12 independently operated chambers capable of delivering blue light at preset intervals. Blue light (∼470nm) was delivered for 5s every 5 min, a paradigm previously used for developmental activation of CIV nociceptors (Kaneko et al., 2017). After 48 hr, larvae were cold-shocked at 0°C for 60 min (Figure 6A).
Figure 6.
Developmental activation of CIII neurons is sufficient for increasing cold tolerance
(A) Outline of optogenetic acclimation and cold tolerance assay. Larvae expressing ChETA in CIII neurons were raised +/− ATR supplementation and +/− 48 hr of blue light exposure (5s every 5 min).
(B) Developmentally activating CIII neurons optogenetically, sans cold, resulted in larvae with higher cold tolerance. Bar charts show % animals which survived cold shock ±standard error of the proportion. N = 120; n = 30 in each condition, each referring to the number of larvae assayed, with experiments performed in triplicates of 10. Differences were assessed by z-test and Bayesian A/B test.
Developmental optogenetic activation of CIII neurons improved survival rates following cold shock, indicating that the activation of cold nociceptors is sufficient for driving cold acclimation (Figure 6B).
Discussion
Herein, we have found some conflicting evidence concerning the adaptive nature of the CT response. The CT response is widespread, occurs primarily at low, potentially harmful temperatures, results in a reduced surface-area-to-volume ratio, and is conserved from a common ancestor, collectively indicating that the behavior might be adaptive. The lack of phylogenetic signal we detected may also be consistent with this, as values close to 0 may indicate trait lability, perhaps suggesting that cold-evoked behavior has rapidly adapted as drosophilids have entered new thermal environments.
Several points are worth considering, however. Firstly, we found no evidence that CT incidence was correlated with any particular cold-climate variable. Secondly, low phylogenetic signal, despite being an indication of trait lability, does not give evidence of any specific type of selection. The absence of phylogenetic signal can therefore be interpreted several different ways; for example, it may indicate rapid adaptation into specific niches, stabilizing selection, and/or evolution under constant functional constraint (Kamilar and Cooper, 2013; Münkemüller et al., 2012; Revell et al., 2008). Thirdly, we observed that cold-evoked behaviors are highly transient—at low temperatures larvae eventually enter a state of flaccid paralysis; even if CT had been long-lasting, it seems unlikely that this change in shape would have significant impact on thermal inertia given the incredibly small size of larvae. Finally, we observed no obvious baseline collapse in cold-tolerance as a result of impairing cold nociception via TNT—in fact, one condition resulted in dramatically increased cold tolerance (Figure 4C; 19-12 GAL4).
With these considerations in mind, we do not believe there is a strong argument to be made that CT, or any noxious cold-evoked behavior, has long-term protective qualities in and of itself. Although further work is required to better clarify the evolutionary history and plasticity of insect cold nociceptive behavior, what remains clear is that drosophilid CT behavior is widespread and inherited from a common ancestor.
In contrast, cold nociception is necessary for cold acclimation; silencing cold nociceptors results in an inability to cold acclimate, and CIII activation appears sufficient for inducing cold acclimation, as activating CIII neurons optogenetically leads to increased cold tolerance.
Whether or not the nervous system plays a role in thermal acclimation has been a long-standing question in ectotherm biology (Bowler, 2005; Lagerspetz, 1974; Prosser and Nelson, 1981); here we have implicated the peripheral nervous system in cold acclimation, but it remains unknown what mechanisms might allow these neurons to interface with appropriate tissues and processes. Cold acclimation is the result of a substantial number of changes within an organism, including changes in the ionoregulatory capacity of the insect renal system (Overgaard and MacMillan, 2017). In many species, the renal system is modulated by endocrine factors produced by the nervous system; it therefore stands to reason that cold information encoded by cold nociceptors may pass to the central nervous system, engaging some central neuroendocrine circuitry with downstream effects on gene expression or cell signaling, thereby having effects on ion and water homeostasis. Several neuropeptides are involved in Drosophila renal ion homeostasis (Halberg et al., 2015; Talsma et al., 2012; Zandawala et al., 2018); one of these peptides, capa, has been shown to play a role in desiccation and cold tolerance (Terhzaz et al., 2015), making it one of many candidate mechanisms by which peripheral sensory information might be translated into physiological changes. Of course, not all mechanisms of cold acclimation need be nervous system dependent. As previously mentioned, there is evidence that non-neuronal cells can detect cold via rapid calcium signaling, thereby driving nervous system independent cold hardening (Nadeau and Teets, 2020; Teets et al., 2013).
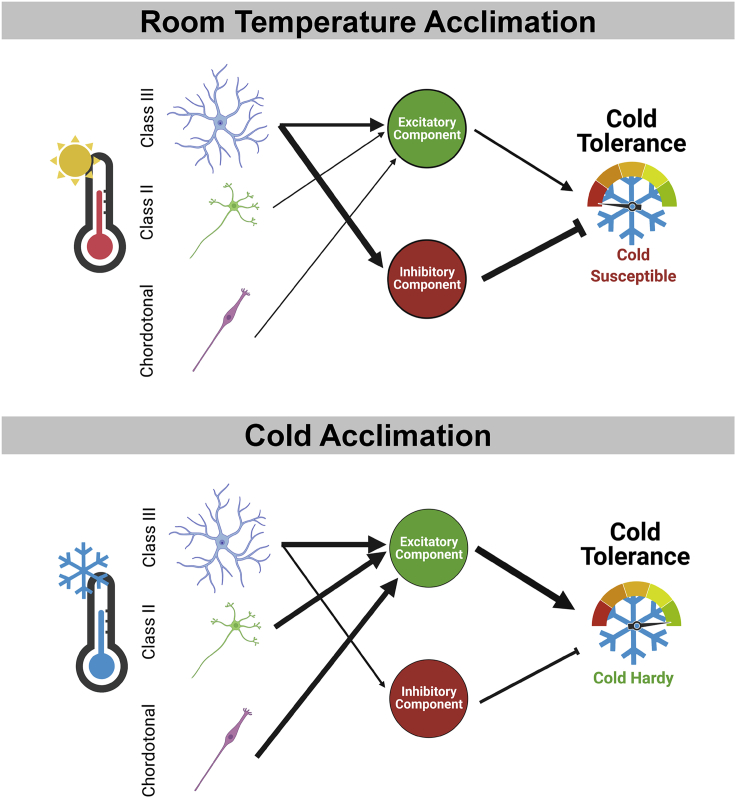
Curiously, these data indicate that silencing CIII neurons alone (via GAL419−12) may result in an increase in baseline cold tolerance. One speculative hypothesis is that CIII neurons contribute to both excitatory and inhibitory components, with the inhibitory component being relatively strong under optimal thermal conditions (Figure 7). Under cold conditions, the combined excitatory components of CIII, CII, and chordotonal neurons might overcome this inhibition, thereby driving cold acclimation. Under such a hypothesis, the targeted silencing of CIII neurons can result in a loss of inhibition, thereby driving cold acclimation under room temperature acclimation. Alternatively, increased baseline cold tolerance under the CIII-TNT condition may indicate some degree of homeostatic compensation (or overcompensation) in the cold-sensing circuit. Continued work on the downstream circuitry of these cold-sensing neurons may shed light on the veracity of these hypotheses.
Figure 7.
Hypothetical model of the interaction between cold nociceptive circuitry and cold acclimation
As silencing CIII neurons alone leads to increased cold tolerance, this may reflect a loss of inhibition under room temperature acclimation, whereas combinatorial silencing of CIII/CII and CIII/Ch leads to an inability to cold acclimation via an accumulated loss of activation/excitatory capacity.
Interestingly, cold acclimation also resulted in nociceptor sensitization, arguably a form of allodynia. One possible hypothesis is that cold acclimation induces a state of hyper-vigilance in response to chilling, therefore better preparing larvae for cold shocks. Given that cold acclimation is likely the result of long-term changes in physiology and gene expression (MacMillan et al., 2016; Toxopeus et al., 2019), it is unclear how hyper-vigilance might lead to increased cold tolerance, in and of itself. However, the presence of cold-induced hypersensitivity and the coincident increase in cold tolerance may be consistent with the hypothesis that nociceptive hypersensitization provides survival advantage, a broader interpretation of a hypothesis formulated by Crook et al., who observed that injury-induced nociceptor sensitization results in decreased predation risk in squid (Crook et al., 2014). Whether injury plays any role in cold acclimation is a subject which requires further study. Additional studies are also required to elucidate whether cold tolerance is necessarily tied to sensitization, or if nociceptive sensitization is simply a coincident phenomenon.
Further, as drosophilids are believed to overwinter under diapause as adults (Allen, 2007; Izquierdo, 1991), it's unclear what the ecological relevance of larval cold nociception and acclimation might be. Some environments see rapid changes in temperature from day to night (e.g. some deserts)—perhaps the ability to cold acclimate as larvae would be advantageous in species from such environments. Our finding that L50 correlates with mean diurnal range is perhaps consistent with this hypothesis.
In summary, we have shown that cold nociception is widespread among drosophilids and that D. melanogaster CIII neurons are necessary and sufficient components of cold acclimation. The results of these experiments suggest that insect cold acclimation has a peripheral neural basis in cold nociception.
Limitations of the study
This study made us of a relatively small sample of species, and only a single strain per species, which may lead to a degree of uncertainty in both our assessments of intraspecies behavior and phylogenetic signal. It remains commonplace and acceptable to use single strains in comparative Drosophila studies (Jezovit et al., 2020; Kellermann et al., 2012; Strachan et al., 2011; Watanabe et al., 2019); however some studies have shown substantial differences in a variety of metrics within species, across strains (Anderson et al., 2016; Colomb and Brembs, 2014; Qiu et al., 2017; Rahman et al., 2015). Our work here also evidences this, given that different GAL4 genetic backgrounds appeared to have different baseline cold tolerances (Figure 4B).
The class III-specific silencing experiments described herein should be interpreted with a degree of cation, as the high baseline cold tolerance in the GAL419−12>TNT condition likely makes it difficult or impossible to detect any statistically significant increase in cold tolerance in cold-reared GAL419−12>TNT animals. If our results reflect an inability to detect a change (rather than the lack of change), one would alternatively conclude that it is a combination of Class III, Class II, and Chordotonal neurons which are necessary for cold acclimation, and that silencing any single neural class is insufficient for disabling cold nociception.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| all trans-Retinal (ATR) | Sigma-Aldrich | R2500 |
| Experimental models: Organisms/strains | ||
| Drosophila melanogaster ORR | Available on request | N/A |
| D. melanogaster w1118 | Bloomington Drosophila Stock Center | 3605 |
| D. melanogaster GAL4-GMR37B02 | Available on request | N/A |
| D. melanogaster GAL4-iav | Available on request | N/A |
| D. melanogaster GAL4-1003.3 | Available on request | N/A |
| D. melanogaster GAL4-19-12 | Bloomington Drosophila Stock Center | 36369 |
| D. melanogaster GAL4-nompC | Bloomington Drosophila Stock Center | 36361 |
| D. melanogaster UAS-TeTxLC | Bloomington Drosophila Stock Center | 28837 |
| D. melanogaster UAS-TNTIMP | Bloomington Drosophila Stock Center | 28840 |
| D. melanogaster UAS-mCD8::GFP | Bloomington Drosophila Stock Center | 5130 |
| D. melanogaster UAS-ChETA::YFP | Bloomington Drosophila Stock Center | 36495 |
| D. arizonae | The National Drosophila Species Stock Center | 15081-1271.39 |
| D. equinoxialis | The National Drosophila Species Stock Center | 14030-0741.00 |
| D. fundebris | The National Drosophila Species Stock Center | 15120-1911.01 |
| D hydei | The National Drosophila Species Stock Center | 15085-1641.04 |
| D. willistoni | The National Drosophila Species Stock Center | 14030-0811.00 |
| D. virilis | The National Drosophila Species Stock Center | 15010-1051.00 |
| D. simulans | The National Drosophila Species Stock Center | 14021-0251.292 |
| D. pseudoobscura | The National Drosophila Species Stock Center | 14011-0121.35 |
| D. littoralis | The National Drosophila Species Stock Center | 15010-1001.03 |
| D. mojavensis | The National Drosophila Species Stock Center | 15081-1352.47 |
| Software and algorithms | ||
| JASP v0.14.0.0 | The JASP Team | https://jasp-stats.org/ |
| GraphPad Prism v7 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| Microsoft Excel | Microsoft | N/A |
| Adobe Illustrator 2020 | Adobe | N/A |
| MAFFT v7 | Katoh and Standley 2013 | https://mafft.cbrc.jp/alignment/software/ |
| IQ-Tree v1.6.10 | Nguyen et al., 2015 | http://www.iqtree.org/ |
| iTOL v5 | Letunic and Bork 2021 | https://itol.embl.de/ |
| phytools v0.7-70 | Revell 2012 | https://github.com/liamrevell/phytools |
| ape v5.5 | Paradis and Schliep 2019 | http://ape-package.ird.fr/ |
| Spike2 v8 | Cambridge Electronic Design Limited | http://ced.co.uk/products/spkovin |
| Other | ||
| Cold plate | TE Technology | CP-031 |
| Cold plate temperature controller | TE Technology | TC-48-20 |
| Cold plate power supply | TE Technology | PS-12-8.4A |
| Laminated aluminum shim (cut 7.5x11.5mm) | Global Equipment | WBB512969 |
| Cold probe apparatus | ProDev Engineering | N/A |
| Nikon DSLR Camera D5200 | Nikon | N/A |
| Husky multichamber storage bin | The Home Depot | N/A |
| Arduino Uno Rev3 microcontroller | Arduino | A000067 |
| Cree XP-E2 blue LED with 80° lens | RapidLED | XPEBBL-L1-0000-00301 |
| Patch-Clamp Amplifier | Molecular Devices | Multiclamp 700A |
| A/D Converter | Cambridge Electronic Design Limited | Micro1401 |
| Inline solution cooler | Warner Instruments | SC-20 |
| Temperature controller | Warner Instruments | CL-100 |
Resource availability
Lead contact
Information and requests for resources should be directed to and will be fulfilled by the lead contact, Daniel N. Cox (dcox18@gsu.edu).
Materials availability
All stable reagents generated in this study are available from the Cox Lab without restriction.
Data and code availability
The datasets generated during this study are available at Dryad (DOI: https://doi.org/10.5061/dryad.69p8cz91s).
Experimental model and subject details
Fly strains
All Drosophila stocks were maintained at 24°C, and all larval subjects were wandering 3rd instars. Sex was randomly proportioned by randomly selecting from a mixed population. The OregonR (ORR) and w1118 strains were used to assess wild-type (WT) behavior and cold tolerance in D. melanogaster, respectively. Transgenic D. melanogaster strains included: GAL4GMR37B02 (CII neurons); GAL419-12 (CIII neurons, BDSC #36369); GAL4iav (Ch neurons); GAL41003.3 (CII and CIII neurons); GAL4nompC (CIII and Ch neurons, BDSC #36361); UAS-TeTxLC (active tetanus toxin light chain, TNT, BDSC #28837); UAS-TNTIMP (impaired tetanus toxin light chain, BDSC #28840); UAS-mCD8::GFP (CD8-mediated membrane targeting of GFP, BDSC #5130); and UAS-ChETA::YFP (engineered derivative of Channelrhodopsin2, BDSC #36495).
Other drosophilid species were obtained from The National Drosophila Species Stock Center at Cornell University: Drosophila arizonae (#15081-1271.39); Drosophila equinoxialis (#14030-0741.00); Drosophila funebris (#15120-1911.01); Drosophila hydei (#15085-1641.04); Drosophila littoralis (#15010-1001.03); Drosophila willistoni (#14030-0811.00); Drosophila virilis (#15010-1051.00); Drosophila simulans (#14021-0251.292); Drosophila pseudoobscura (#14011-0121.35); Drosophila mojavensis (#15081-1352.47). Species distribution on map of annual mean temperature was generated in R using climate data retrieved from WorldClim (https://www.worldclim.org) (Fick and Hijmans, 2017) and location data provided by the Drosophila Species Stock Center.
Method details
Behavior assays
Cold-evoked behaviors were assessed using the previously developed cold-plate and cold-probe assays (Patel and Cox, 2017; Turner et al., 2016, 2017). For cold-plate, wandering 3rd instar larvae were acclimated to a room-temperature aluminum arena. The arena was then transferred to a prechilled Peltier device (TE Technologies, CP0031) under the control of a thermoelectric temperature controller (TE Technologies, TC-48-20). Behavior was recorded from above for 30 seconds and assessed qualitatively post hoc. For cold-probe, the cold probe apparatus (ProDev Engineering) was chilled to 6°C, and then placed at a 45° angle upon a posterior, medial, or anterior segment of the larva, and held for 5 seconds; behavior was scored qualitatively at the time of the assay.
Cold survival assay
Larval cold tolerance was assessed using a modified cold-plate assay. Drosophila populations were raised at 24°C until 3rd instar larvae were present. The entire population was then incubated for 48 hours at 24°C or 10°C. After the incubation, only wandering 3rd instar larvae were collected using a small paint brush, transferred to a room-temperature aluminum arena, and allowed to acclimate until locomotion resumed. To deliver cold shock, the arena was transferred to a Peltier device prechilled to 0°C. Larvae were cold-shocked for 60 minutes, then removed from the arena and placed in a new Drosophila vial. As wandering 3rd instar larvae are post-feeding, cold-shocked larvae were housed in vials containing 10mL of 3% agar instead of food (to prevent desiccation and confounds due to food molding). Larvae were cold-shocked immediately after incubation and the remaining populations were discarded. Survival was assessed by counting the eventual number of adults eclosed as compared to the number of larvae originally placed in the vial. Each experiment was performed in 3 replicates, with an independent sample of 10 larvae each (Figure S6).
Optogenetics
GAL419-12>UAS-ChETA::YFP larvae were raised in Petri dishes, in constant darkness, at 24°C. As channelrhodopsins require all-trans-retinol (ATR) to function, larvae with identical genotypes were either raised on food containing ATR (ATR+, 1.5mM) or standard food (ATR-, as control). When 3rd instar larvae were present in the dish, the dish was transferred to a custom-built OptoBox (Kaneko et al., 2017), where high intensity blue light was automatically delivered at a frequency of 5 seconds every 5 minutes, for 48 hours. The OptoBox consists of a plastic storage bin segmented into 12 independent test chambers, each equipped with a blue LED with an 80° lens (RapidLED, Cree XP-E2) under the control of an Arduino microcontroller (Arduino, Uno Rev3). After 48 hours, the dishes were then removed from the OptoBox and wandering 3rd instar larvae were used in the cold survival assay detailed above. Larvae were shocked immediately following removal from the OptoBox, and the remaining populations were discarded.
Phylogenetics
Amino acid sequences (Table S1) for Cytochome Oxidase I/II (mt:CoI/CoII) and alcohol dehydrogenase (Adh) were aligned via MAFFT (Katoh and Standley, 2013), using default settings. As the repleta group has two copies of Adh, we used Adh1 for these species. Phylogenetic trees were generated via IQ-Tree (Nguyen et al., 2015) by the maximum likelihood approach, using an LG+F+R2 substitution model (as determined by ModelFinder (Kalyaanamoorthy et al., 2017)). Branch support was calculated by ultrafast bootstrapping (UFboot (Hoang et al., 2018), 2000 replicates). Trees were visualized using iTOL (Letunic and Bork, 2021), R, and Adobe Illustrator.
All additional phylogenetic analyses were performed in R using the phytools package (Revell, 2012). SER ancestral state reconstruction was performed using a single-rate continuous-time Markov chain model; pie-charts at interior nodes of the presented cladogram represent empirical Bayesian posterior probabilities. % larvae CT (out of total population) ancestral states were mapped to an ultrametric phylogeny by maximum likelihood via the chronos and contMap functions in the ape package (Paradis and Schliep, 2019).
Electrophysiology
Demuscled fillet preparations were made from GAL419-12>UAS-mCD8::GFP larvae housed in Petri dishes lined with Sylgard 184 (Dow Corning), which were filled and constantly superfused with HL-3 saline. Extracellular recordings were made with a pipette (tip diameter, 5–10 μm) connected to the headstage of a patch-clamp amplifier (Multiclamp 700A, Molecular Devices). Gentle suction was applied to draw the soma and a small portion of neurite into the pipette. The amplifier was set to voltage-clamp mode to record neuronal spikes. The output signals from the amplifier were digitized at a sampling frequency of 10 kHz using a Micro1401 A/D converter (Cambridge Electronic Design) and acquired into a laptop computer running Windows 10 with Spike2 software v. 8 (Cambridge Electronic Design). To apply a low temperature stimulation, saline was passed through an SC-20 in-line solution cooler (Warner Instruments) connected to a CL-100 temperature controller (Warner Instruments). Average spike frequency was measured during baseline room-temperature conditions (60 seconds) and during superfusion of chilled saline (60 seconds at 20, 15, or 10°C).
Quantification and statistical analysis
Due to a growing call for statistical analyses that do not rely on p-values (Ioannidis, 2019; Kelter, 2020; Keysers et al., 2020; Matthews et al., 2017; Nuijten et al., 2016; van Doorn et al., 2020; Wasserstein and Lazar, 2016; Wasserstein et al., 2019; Wetzels et al., 2011), differences in mean and population proportions were analyzed using both traditional frequentist statistics and Bayesian alternatives. z-tests were performed in Microsoft Excel and all other analyses were performed in JASP (Bayesian analyses using default prior probability distributions) (Team, 2020). Population proportions are presented as % ± standard error of the proportion (SEP); differences in proportion were assessed by one-tailed z-test with the Benjamini-Hochberg procedure (false discovery rate at 0.05) and the Bayesian A/B Test. All other measures are presented as mean ± standard error of the mean (SEM); differences were assessed by frequentist ANOVA with a Scheffé correction for multiple comparisons, and the Bayesian ANOVA with multiple comparisons by the Westfall-Johnson-Utts method (Westfall et al., 1997).
For Bayesian analyses, degree of support for rejecting the null hypothesis was inferred by computed Bayes Factors (BF), via the method originally proposed by Jeffreys (1961): “no evidence” (BF10 < 1); “barely worth mentioning” (BF10: 1-3); “substantial” (BF10: 3-10); “strong” (BF10: 10-30); “very strong” (BF10: 30-100); and “decisive (BF10 > 100).
Histograms and heatmaps were generated using GraphPad PRISM (GraphPad Software, La Jolla, California, USA). In statistics bars and tables, green text indicates statistical significance (α=0.05, frequentist) or substantial evidence in favor of the alternative hypothesis (BF10 > 3, Bayesian). Red text indicates no statistical significance (frequentist) or less than substantial evidence in favor of the alternative hypothesis (Bayesian). Population (B) and sample (n) sizes are indicated in figure legends.
Acknowledgments
We thank Dr. Bing Ye (University of Michigan) for providing materials lists, wiring diagrams, and advice which contributed to the design and construction of the OptoBox, Dr. Gennady Cymbalyuk (Georgia State University) for use of electrophysiological equipment, and 2 anonymous reviewers for their constructive feedback (particularly for suggesting additional variables to use in the correlation analyses). We also thank and credit BioRender, which was used to generate many of the experimental outlines and other graphics.
This work is supported by NIH R01 NS115209-01 (to DNC). N.J.H. was supported by NIH F31 NS117087-01, a GSU Brains & Behavior Fellowship, and a Kenneth W. and Georganne F. Honeycutt Fellowship. J.M.L. was supported by a GSU Brains & Behavior Fellowship. T.R.G. and M.N.B. were supported by NIH R25GM109442-01A1 (to DNC). K.J.D. was supported by a GSU 2CI Neurogenomics Fellowship.
Author contributions
Conceptualization, N.J.H.; Methodology, N.J.H., J.M.L., A.S., K.J.D., and D.N.C.; Cold-plate assays, N.J.H., J.M.L., T.R.G., and M.N.B.; Survival assays, N.J.H.; OptoBox design and construction, N.J.H. and K.J.D.; Optogenetics, N.J.H.; Electrophysiology, A.S.; Photography, T.R.G.; Phylogenetics and associated analyses, N.J.H.; Statistics and other formal analyses, N.J.H.; Writing – Original Draft, N.J.H.; Writing – Review & Editing, N.J.H., J.M.L., A.S., T.R.G., M.N.B., K.J.D., and D.N.C.; Visualization, N.J.H. and A.S.; Supervision, D.N.C.; Funding Acquisition, N.J.H. and D.N.C.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. One or more of the authors of this paper self-identifies as a member of the LGBTQ + community. One or more of the authors of this paper received support from a program designed to increase minority representation in science.
Published: June 25, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102657.
Supplemental information
References
- Allen M.J. What makes a fly enter diapause? Fly. 2007;1:307–310. doi: 10.4161/fly.5532. [DOI] [PubMed] [Google Scholar]
- Alpert M.H., Frank D.D., Kaspi E., Flourakis M., Zaharieva E.E., Allada R., Para A., Gallio M. A circuit encoding absolute cold temperature in Drosophila. Curr. Biol. 2020;30:2275–2288.e5. doi: 10.1016/j.cub.2020.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen M.K., Jensen N.J.S., Robertson R.M., Overgaard J. Central nervous system shutdown underlies acute cold tolerance in tropical and temperate Drosophila species. J. Exp. Biol. 2018;221:jeb179598. doi: 10.1242/jeb.179598. [DOI] [PubMed] [Google Scholar]
- Andersen M.K., Overgaard J. Maintenance of hindgut reabsorption during cold exposure is a key adaptation for Drosophila cold tolerance. J. Exp. Biol. 2020;223:jeb213934. doi: 10.1242/jeb.213934. [DOI] [PubMed] [Google Scholar]
- Anderson B.B., Scott A., Dukas R. Social behavior and activity are decoupled in larval and adult fruit flies. Behav. Ecol. 2016;27:820–828. doi: 10.1093/beheco/arv225. [DOI] [Google Scholar]
- Bayley J.S., Sørensen J.G., Moos M., Koštál V., Overgaard J. Cold acclimation increases depolarization resistance and tolerance in muscle fibers from a chill-susceptible insect, Locusta migratoria. Am. J. Physiol. Regulat. Integr. Comp. Physiol. 2020;319:R439–R447. doi: 10.1152/ajpregu.00068.2020. [DOI] [PubMed] [Google Scholar]
- Blomberg S.P., Garland T., Jr., Ives A.R. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution. 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Bowler K. Acclimation, heat shock and hardening. J. Therm. Biol. 2005;30:125–130. doi: 10.1016/j.jtherbio.2004.09.001. [DOI] [Google Scholar]
- Canals M., Rosenmann M., Bozinovic F. Geometrical aspects of the energetic effectivenes of huddling in small mammals. Acta Theriolog. 1997;42:321–328. [Google Scholar]
- Cohen J., Pfeiffer K., Francis J.A. Warm Arctic episodes linked with increased frequency of extreme winter weather in the United States. Nat. Commun. 2018;9:869. doi: 10.1038/s41467-018-02992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet H., Hoffmann A.A. Comparing phenotypic effects and molecular correlates of developmental, gradual and rapid cold acclimation responses in Drosophila melanogaster. Funct. Ecol. 2012;26:84–93. doi: 10.1111/j.1365-2435.2011.01898.x. [DOI] [Google Scholar]
- Colomb J., Brembs B. Sub-strains of Drosophila Canton-S differ markedly in their locomotor behavior. F1000Res. 2014;3:176. doi: 10.12688/f1000research.4263.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras L.C. Bioenergetics of huddling: test of a psycho-physiological hypothesis. J. Mammalogy. 1984;65:256–262. doi: 10.2307/1381164. [DOI] [Google Scholar]
- Crook R.J., Dickson K., Hanlon R.T., Walters E.T. Nociceptive sensitization reduces predation risk. Curr. Biol. 2014;24:1121–1125. doi: 10.1016/j.cub.2014.03.043. [DOI] [PubMed] [Google Scholar]
- Des Marteaux L.E., Khazraeenia S., Yerushalmi G.Y., Donini A., Li N.G., Sinclair B.J. The effect of cold acclimation on active ion transport in cricket ionoregulatory tissues. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2018;216:28–33. doi: 10.1016/j.cbpa.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Des Marteaux L.E., Stinziano J.R., Sinclair B.J. Effects of cold acclimation on rectal macromorphology, ultrastructure, and cytoskeletal stability in Gryllus pennsylvanicus crickets. J. Insect Physiol. 2018;104:15–24. doi: 10.1016/j.jinsphys.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Dillon M.E., Cahn L.R.Y., Huey R.B. Life history consequences of temperature transients in Drosophila melanogaster. J. Exp. Biol. 2007;210:2897. doi: 10.1242/jeb.007591. [DOI] [PubMed] [Google Scholar]
- Dillon M.E., Woods H.A., Wang G., Fey S.B., Vasseur D.A., Telemeco R.S., Marshall K., Pincebourde S. Life in the frequency domain: the biological impacts of changes in climate variability at multiple time scales. Integr. Comp. Biol. 2016;56:14–30. doi: 10.1093/icb/icw024. [DOI] [PubMed] [Google Scholar]
- Fick S.E., Hijmans R.J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017;37:4302–4315. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- Gerber L., Overgaard J. Cold tolerance is linked to osmoregulatory function of the hindgut in Locusta migratoria. J. Exp. Biol. 2018;221:jeb173930. doi: 10.1242/jeb.173930. [DOI] [PubMed] [Google Scholar]
- Gilbert C., Robertson G., Le Maho Y., Naito Y., Ancel A. Huddling behavior in emperor penguins: dynamics of huddling. Physiol. Behav. 2006;88:479–488. doi: 10.1016/j.physbeh.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Halberg K.A., Terhzaz S., Cabrero P., Davies S.A., Dow J.A.T. Tracing the evolutionary origins of insect renal function. Nat. Commun. 2015;6:6800. doi: 10.1038/ncomms7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., Speakman J.R., Racey P.A. The contributions of local heating and reducing exposed surface area to the energetic benefits of huddling by short-tailed field voles (Microtus agrestis) Physiol. Zoolog. 1992;65:742–762. doi: 10.1086/physzool.65.4.30158537. [DOI] [Google Scholar]
- Hoang D.T., Chernomor O., von Haeseler A., Minh B.Q., Vinh L.S. UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A.A., Sørensen J.G., Loeschcke V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 2003;28:175–216. doi: 10.1016/S0306-4565(02)00057-8. [DOI] [Google Scholar]
- Ioannidis J.P.A. What have we (not) learnt from millions of scientific papers with P values? Am. Statist. 2019;73:20–25. doi: 10.1080/00031305.2018.1447512. [DOI] [Google Scholar]
- Izquierdo J.I. How does Drosophila melanogaster overwinter? Entomol. Exp. Appl. 1991;59:51–58. doi: 10.1111/j.1570-7458.1991.tb01485.x. [DOI] [Google Scholar]
- Jezovit J.A., Rooke R., Schneider J., Levine J.D. Behavioral and environmental contributions to drosophilid social networks. Proc. Natl. Acad. Sci. U S A. 2020;117:11573. doi: 10.1073/pnas.1920642117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., von Haeseler A., Jermiin L.S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamilar J.M., Cooper N. Phylogenetic signal in primate behaviour, ecology and life history. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20120341. doi: 10.1098/rstb.2012.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Macara A.M., Li R., Hu Y., Iwasaki K., Dunnings Z., Firestone E., Horvatic S., Guntur A., Shafer O.T. Serotonergic modulation enables pathway-specific plasticity in a developing sensory circuit in Drosophila. Neuron. 2017;95:623–638.e4. doi: 10.1016/j.neuron.2017.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann V., Loeschcke V., Hoffmann A.A., Kristensen T.N., Fløjgaard C., David J.R., Svenning J.-C., Overgaard J. Phylogenetic constraints in key functional traits behind species’ climate niches: patterns of desiccation and cold resistance across 95 Drosophila species. Evolution. 2012;66:3377–3389. doi: 10.1111/j.1558-5646.2012.01685.x. [DOI] [PubMed] [Google Scholar]
- Kelter R. Analysis of Bayesian posterior significance and effect size indices for the two-sample t-test to support reproducible medical research. BMC Med. Res. Methodol. 2020;20:88. doi: 10.1186/s12874-020-00968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C., Gazzola V., Wagenmakers E.-J. Using Bayes factor hypothesis testing in neuroscience to establish evidence of absence. Nat. Neurosci. 2020;23:788–799. doi: 10.1038/s41593-020-0660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Afonso B., Vonner A.J., Hernandez-Nunez L., Berck M., Tabone C.J., Kane E.A., Pieribone V.A., Nitabach M.N., Cardona A. Sensory determinants of behavioral dynamics in Drosophila thermotaxis. Proc. Natl. Acad. Sci. U S A. 2015;112:E220. doi: 10.1073/pnas.1416212112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y., Shen W.L., Shim H.-S., Montell C. Fine thermotactic discrimination between the optimal and slightly cooler temperatures via a TRPV channel in chordotonal neurons. J. Neurosci. 2010;30:10465. doi: 10.1523/JNEUROSCI.1631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerspetz K.Y.H. Temperature acclimation and the nervous system. Biol. Rev. 1974;49:477–514. doi: 10.1111/j.1469-185X.1974.tb01172.x. [DOI] [PubMed] [Google Scholar]
- Letunic I., Bork P. Interactive Tree of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021 doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean H.J., Sørensen J.G., Kristensen T.N., Loeschcke V., Beedholm K., Kellermann V., Overgaard J. Evolution and plasticity of thermal performance: an analysis of variation in thermal tolerance and fitness in 22 Drosophila species. Philos. Trans. R. Soc. B: Biol. Sci. 2019;374:20180548. doi: 10.1098/rstb.2018.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan H.A., Andersen J.L., Loeschcke V., Overgaard J. Sodium distribution predicts the chill tolerance of Drosophila melanogaster raised in different thermal conditions. Am. J. Physiology-Regulatory, Integr. Comp. Physiol. 2015;308:R823–R831. doi: 10.1152/ajpregu.00465.2014. [DOI] [PubMed] [Google Scholar]
- MacMillan H.A., Findsen A., Pedersen T.H., Overgaard J. Cold-induced depolarization of insect muscle: differing roles of extracellular K+ during acute and chronic chilling. J. Exp. Biol. 2014;217:2930. doi: 10.1242/jeb.107516. [DOI] [PubMed] [Google Scholar]
- MacMillan H.A., Knee J.M., Dennis A.B., Udaka H., Marshall K.E., Merritt T.J.S., Sinclair B.J. Cold acclimation wholly reorganizes the Drosophila melanogaster transcriptome and metabolome. Scient. Rep. 2016;6:28999. doi: 10.1038/srep28999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G., Krasnow M.A. Development of the Drosophila tracheal system. In: Bate M., editor. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; 1993. pp. 609–685. [Google Scholar]
- Matthews R., Wasserstein R., Spiegelhalter D. The ASA's p-value statement, one year on. Significance. 2017;14:38–41. doi: 10.1111/j.1740-9713.2017.01021.x. [DOI] [Google Scholar]
- Mockett R.J., Matsumoto Y. Effect of prolonged coldness on survival and fertility of Drosophila melanogaster. PLoS One. 2014;9:e92228. doi: 10.1371/journal.pone.0092228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münkemüller T., Lavergne S., Bzeznik B., Dray S., Jombart T., Schiffers K., Thuiller W. How to measure and test phylogenetic signal. Methods Ecol. Evol. 2012;3:743–756. doi: 10.1111/j.2041-210X.2012.00196.x. [DOI] [Google Scholar]
- Nadeau E.A.W., Teets N.M. Evidence for a rapid cold hardening response in cultured Drosophila S2 cells. J. Exp. Biol. 2020;223:jeb212613. doi: 10.1242/jeb.212613. [DOI] [PubMed] [Google Scholar]
- Nguyen L.-T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood Phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Klein M., Svec K.V., Budelli G., Chang E.C., Ferrer A.J., Benton R., Samuel A.D., Garrity P.A. The Ionotropic Receptors IR21a and IR25a mediate cool sensing in Drosophila. 2016;5 doi: 10.7554/eLife.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuijten M.B., Hartgerink C.H.J., van Assen M.A.L.M., Epskamp S., Wicherts J.M. The prevalence of statistical reporting errors in psychology (1985–2013) Behav. Res. Methods. 2016;48:1205–1226. doi: 10.3758/s13428-015-0664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard J., Kristensen T.N., Mitchell K.A., Hoffmann A.A. Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am. Natural. 2011;178:S80–S96. doi: 10.1086/661780. [DOI] [PubMed] [Google Scholar]
- Overgaard J., MacMillan H.A. The integrative physiology of insect chill tolerance. Annu. Rev. Physiol. 2017;79:187–208. doi: 10.1146/annurev-physiol-022516-034142. [DOI] [PubMed] [Google Scholar]
- Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- Paradis E., Schliep K. Ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2019;35:526–528. doi: 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- Patel A.A., Cox D.N. Behavioral and functional assays for investigating mechanisms of noxious cold detection and multimodal sensory processing in Drosophila larvae. Bio Protoc. 2017;7:e2388. doi: 10.21769/BioProtoc.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser C.L., Nelson D.O. The role of nervous systems in temperature adaptation of Poikilotherms. Annu. Rev. Physiol. 1981;43:281–300. doi: 10.1146/annurev.ph.43.030181.001433. [DOI] [PubMed] [Google Scholar]
- Qiu S., Xiao C., Meldrum Robertson R. Different age-dependent performance in Drosophila wild-type Canton-S and the white mutant w1118 flies. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017;206:17–23. doi: 10.1016/j.cbpa.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Rahman R., Chirn G.W., Kanodia A., Sytnikova Y.A., Brembs B., Bergman C.M., Lau N.C. Unique transposon landscapes are pervasive across Drosophila melanogaster genomes. Nucleic Acids Res. 2015;43:10655–10672. doi: 10.1093/nar/gkv1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rako L., Hoffmann A.A. Complexity of the cold acclimation response in Drosophila melanogaster. J. Insect Physiol. 2006;52:94–104. doi: 10.1016/j.jinsphys.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Ransberry V.E., MacMillan H.A., Sinclair B.J. The relationship between chill-coma onset and recovery at the extremes of the thermal window of Drosophila melanogaster. Physiol. Biochem. Zool. 2011;84:553–559. doi: 10.1086/662642. [DOI] [PubMed] [Google Scholar]
- Revell L.J. phytools: an R package for phylogenetic comparative biology (and other things) Methods Ecol. Evol. 2012;3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- Revell L.J., Harmon L.J., Collar D.C. Phylogenetic signal, evolutionary process, and rate. Syst. Biol. 2008;57:591–601. doi: 10.1080/10635150802302427. [DOI] [PubMed] [Google Scholar]
- Salehipour-shirazi G., Ferguson L.V., Sinclair B.J. Does cold activate the Drosophila melanogaster immune system? J. Insect Physiol. 2017;96:29–34. doi: 10.1016/j.jinsphys.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Sgrò C.M., Terblanche J.S., Hoffmann A.A. What can plasticity contribute to insect responses to climate change? Annu. Rev. Entomol. 2016;61:433–451. doi: 10.1146/annurev-ento-010715-023859. [DOI] [PubMed] [Google Scholar]
- Sinclair B.J., Coello Alvarado L.E., Ferguson L.V. An invitation to measure insect cold tolerance: methods, approaches, and workflow. J. Therm. Biol. 2015;53:180–197. doi: 10.1016/j.jtherbio.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Strachan L.A., Tarnowski-Garner H.E., Marshall K.E., Sinclair B.J. The evolution of cold tolerance in Drosophila larvae. Physiol. Biochem. Zool. 2011;84:43–53. doi: 10.1086/657147. [DOI] [PubMed] [Google Scholar]
- Talsma A.D., Christov C.P., Terriente-Felix A., Linneweber G.A., Perea D., Wayland M., Shafer O.T., Miguel-Aliaga I. Remote control of renal physiology by the intestinal neuropeptide pigment-dispersing factor in <em>Drosophila</em>. Proc. Natl. Acad. Sci. U S A. 2012;109:12177. doi: 10.1073/pnas.1200247109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team J. 2020. JASP (Version 0.14)[Computer Software] [Google Scholar]
- Teets N.M., Gantz J.D., Kawarasaki Y. Rapid cold hardening: ecological relevance, physiological mechanisms and new perspectives. J. Exp. Biol. 2020;223:jeb203448. doi: 10.1242/jeb.203448. [DOI] [PubMed] [Google Scholar]
- Teets N.M., Yi S.-X., Lee R.E., Denlinger D.L. Calcium signaling mediates cold sensing in insect tissues. Proc. Natl. Acad. Sci. U S A. 2013;110:9154. doi: 10.1073/pnas.1306705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhzaz S., Teets N.M., Cabrero P., Henderson L., Ritchie M.G., Nachman R.J., Dow J.A.T., Denlinger D.L., Davies S.-A. Insect capa neuropeptides impact desiccation and cold tolerance. Proc. Natl. Acad. Sci. U S A. 2015;112:2882. doi: 10.1073/pnas.1501518112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toxopeus J., Des Marteaux L.E., Sinclair B.J. How crickets become freeze tolerant: the transcriptomic underpinnings of acclimation in Gryllus veletis. Comp. Biochem. Physiol. D Genomics Proteomics. 2019;29:55–66. doi: 10.1016/j.cbd.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Toxopeus J., Sinclair B.J. Mechanisms underlying insect freeze tolerance. Biol. Rev. 2018;93:1891–1914. doi: 10.1111/brv.12425. [DOI] [PubMed] [Google Scholar]
- Turner H.N., Armengol K., Patel A.A., Himmel N.J., Sullivan L., Iyer S.C., Bhattacharya S., Iyer E.P.R., Landry C., Galko M.J., Cox D.N. The TRP channels Pkd2, NompC, and trpm act in cold-sensing neurons to mediate unique aversive behaviors to noxious cold in Drosophila. Curr. Biol. 2016;26:3116–3128. doi: 10.1016/j.cub.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner H.N., Landry C., Galko M.J. Novel assay for cold nociception in Drosophila larvae. JoVE. 2017:e55568. doi: 10.3791/55568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner H.N., Patel A.A., Cox D.N., Galko M.J. Injury-induced cold sensitization in Drosophila larvae involves behavioral shifts that require the TRP channel Brv1. PLoS One. 2018;13:e0209577. doi: 10.1371/journal.pone.0209577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn J., van den Bergh D., Böhm U., Dablander F., Derks K., Draws T., Etz A., Evans N.J., Gronau Q.F., Haaf J.M. The JASP guidelines for conducting and reporting a Bayesian analysis. Psychon. Bull. Rev. 2020 doi: 10.3758/s13423-020-01798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery W.L., Millar J.S. The energetics of huddling by endotherms. Oikos. 1984;43:88–93. doi: 10.2307/3544249. [DOI] [Google Scholar]
- Wasserstein R.L., Lazar N.A. The ASA statement on p-values: context, process, and purpose. Am. Statist. 2016;70:129–133. doi: 10.1080/00031305.2016.1154108. [DOI] [Google Scholar]
- Wasserstein R.L., Schirm A.L., Lazar N.A. Moving to a world beyond “p < 0.05”. Am. Statist. 2019;73:1–19. doi: 10.1080/00031305.2019.1583913. [DOI] [Google Scholar]
- Watanabe K., Kanaoka Y., Mizutani S., Uchiyama H., Yajima S., Watada M., Uemura T., Hattori Y. Interspecies comparative analyses reveal distinct carbohydrate-responsive systems among Drosophila species. Cell Rep. 2019;28:2594–2607.e7. doi: 10.1016/j.celrep.2019.08.030. [DOI] [PubMed] [Google Scholar]
- Westfall P.H., Johnson W.O., Utts J.M. A Bayesian perspective on the Bonferroni adjustment. Biometrika. 1997;84:419–427. doi: 10.1093/biomet/84.2.419. [DOI] [Google Scholar]
- Wetzels R., Matzke D., Lee M.D., Rouder J.N., Iverson G.J., Wagenmakers E.-J. Statistical evidence in experimental psychology: an empirical comparison using 855 t tests. Perspect. Psychol. Sci. 2011;6:291–298. doi: 10.1177/1745691611406923. [DOI] [PubMed] [Google Scholar]
- Zandawala M., Yurgel M.E., Texada M.J., Liao S., Rewitz K.F., Keene A.C., Nässel D.R. Modulation of Drosophila post-feeding physiology and behavior by the neuropeptide leucokinin. PLoS Genet. 2018;14:e1007767. doi: 10.1371/journal.pgen.1007767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during this study are available at Dryad (DOI: https://doi.org/10.5061/dryad.69p8cz91s).