Abstract
Classical biological control is a pest control tool involving the release of imported natural enemies. The Sterile Insect Technique (SIT) comprises releasing sexually sterile insects of a pest into the wild population for suppression or eradication. Both these approaches are environmentally friendly and their combination can result in a synergistic impact on pest populations and improve eradication. However, stringent regulation surrounding the introduction of biological control agents limits their use in eradication owing to the perceived risk of effects on non-target organisms. We investigated the irradiation biology of the egg parasitoid Trissolcus basalis to ascertain whether sterile parasitoids could mitigate the risk of potential sustained non-target impacts. Mated female T. basalis were gamma-irradiated at doses between 120 and 150 Gy and exposed to egg masses of their host Nezara viridula throughout their lifespans. This resulted in host mortality, despite a substantial reduction in developing parasitoid offspring, which followed a negative dose–response. There was no emergence of parasitoid offspring at 140 Gy and above. Irradiation did not affect oviposition behaviour but caused an increase in longevity. Consequently, sterile parasitoids could possibly alleviate concerns regarding the irreversibility of biological control release, which promotes further investigation of their potential role in eradication.
Subject terms: Agroecology, Behavioural ecology, Invasive species, Environmental impact, Animal behaviour, Entomology
Introduction
Classical biological control (CBC) involves the introduction of natural enemies to reduce the impact of target pests1, and is often a fundamental component of integrated pest management (IPM) programmes2,3. Unlike pesticides, natural enemies are self-sustaining, generally environmentally benign, and do not cause target pest resistance4,5. Despite the rarity of population-level impacts on non-target organisms, it remains a concern when considering the introduction of a CBC agent4. Consequently, some governments impose risk-attentive regulations to assess potential risk prior to the release of new CBC agents, which increases the expense of biological control release applications and limits their approval6–8.
Another effective and benign approach—the Sterile Insect Technique (SIT)—involves mass-rearing and repeatedly releasing sexually sterile conspecifics into the target pest population, at a ratio higher than the wild population. Generally, males are sterilised by gamma or X-ray radiation and released to mate with wild females, which inhibits offspring production and causes a reduction in the F1 generation9. The SIT is primarily utilised as a component of area-wide IPM, which involves managing a pest population within a defined area10, but is also used in eradication programmes9,11. If practicable, eradication is likely to be more beneficial than long-term management because it averts the accumulation of both ecological and economic impacts12,13,14. The SIT also poses virtually no non-target or environmental risks, and has increased efficacy at lower pest densities15.
The combination of biological control with SIT was first theorised by Knipling15 and Barclay16, who found that both techniques should interact synergistically, with each method exerting a greater impact on the target pest than if it were used alone. However, given the stringent regulations surrounding biosafety assessment of CBC agents and importation in many countries, and consequent length of time from application to approval6, it would be difficult to safely introduce an agent in time to be utilised in an eradication response13. The possibility of the CBC agent persisting on non-target organisms post-eradication would also restrict the implementation of biological control in such a programme.
These regulatory issues concerned with the irreversibility of CBC could potentially be mitigated by also applying SIT to a candidate CBC agent, such as parasitoids. Previous research has shown that irradiating female parasitoids can induce sterility, which does not affect their ability to kill the host, but the purpose of these studies was to investigate host immune response18–20. Theoretically, released sterile female parasitoids could therefore kill the target pest without producing viable offspring for their population to persist. However, there is evidence that inundative parasitoid releases may sometimes exert population-level non-target effects, though the impact is predictably local and transient4,21. Consequently, the release of sterile parasitoids as part of an eradication program may not be completely devoid of risk22. This novel approach has been termed the Kamikaze Wasp Technique (KWT), which was proposed recently as a potential synergistic component of insect eradication23.
The irradiation biology of the egg parasitoid Trissolcus basalis Wollaston (Hymenoptera: Scelionidae) was investigated by undertaking dose–response experiments to ascertain whether sterility could be induced, and whether this would affect the wasps’ ability to impact the survival and development of its host Nezara viridula Linnaeus (Hemiptera: Pentatomidae). Post-irradiation behaviour and fitness were also considered. This represents the first step required to examine the feasibility of the KWT. However, this constitutes a model system that lends support to the ongoing SIT work on Halyomorpha halys Stål (Hemiptera: Pentatomidae) as a potential means to eradicate this emerging pest24,25, as its parasitoid Trissolcus japonicus (congener to T. basalis) could similarly be employed for use in the KWT. Consequently, T. basalis is not being proposed as a candidate for the KWT but it serves as a model.
Methods
Source of insects
The N. viridula hosts and T. basalis parasitoids used for experiments were supplied from established colonies that were maintained in a controlled temperature room (25 ± 1 °C, 16:8 h L:D) at The New Zealand Institute for Plant and Food Research Limited, Auckland, New Zealand. The N. viridula colony was established from adults collected from tomato plants in Ruawai (36°06′06″ S, 174°00′05″ E) and from Cleome sp. at Kelmarna Gardens, Auckland (36°51′03.3″ S, 174°43′58.7″ E). The T. basalis colony was established from parasitised N. viridula egg masses (eggs turning black) collected from a number of host plants at Kelmarna Gardens. Host plants included beans, tomatoes26, and Cleome sp.27. The use of plants for insect collection complied with institutional, national and international guidelines.
Insect rearing
N. viridula
Egg masses were collected from the adult portion of the N. viridula colony and kept in 9 cm Petri dishes. When these eggs were visibly close to hatching, the emerging nymphs were provided with moist cotton wool as a water source. When these moulted into second instar nymphs, green beans were provided as a food source. Upon reaching the third instar, the nymphs were transferred to a plastic rearing cage (69 cm diameter × 19 cm height) containing filter paper on the bottom surface, on which moist cotton wool and green beans were provided. When nymphs reached the fourth instar, the conditions in the rearing cage remained identical except for the introduction of raw peanuts as a protein source. Fifth instar nymphs that had moulted into adults were then transferred into adult rearing cages (69 cm diameter × 20 cm height). Wax paper, folded into a fan, was introduced into each of these cages as an oviposition surface. Food, water, and filter paper were changed twice weekly for all rearing cages.
Trissolcus basalis
Parasitoids were reared within clear plastic vials (8.5 cm diameter × 6 cm height) inside a nylon mesh cage (69 cm × 48 cm × 48 cm), in the same location and under the same conditions as the N. viridula colony. A portion of the host egg masses from the N. viridula colony was collected daily to maintain the T. basalis colony. These were stored at 10 °C for up to a week if not immediately exposed to T. basalis, after which they were transferred to a freezer (− 20 °C). Frozen N. viridula egg masses were used to maintain the colony if fresh masses were not available at the time28,29. Upon emergence from the initial parasitised N. viridula egg masses, at least two days were given for females to mate. Every second to fourth day, three to five mated females, no older than 10 days, were removed into individual vials and each exposed to a single N. viridula egg mass (40–100 eggs) for 48 h. A drop of honey (~ 10 µL) was provided as a food source in every vial, which was replenished (~ 100 µL) upon the emergence of T. basalis offspring.
Irradiation dosimetry trials
Irradiation
Our methodology was adapted from that of Soller and Lanzrein18 and Tillinger et al.19, who sterilised female parasitoids to examine host immune response in the absence of developing parasitoid offspring. Male T. basalis, which eclose first30, were separated from the rearing vials before females began to emerge. A single female was then introduced into a vial containing a separated male, and left until mating was observed.
Five mated female T. basalis between 2 and 4 days old were placed into each of five vials (4 cm diameter × 5 cm height), which were then plugged with cotton. Four of the vials were then covered within a thick-walled plastic box, as cobalt60 radiation interacts with matter through the Compton effect, whereby electronic equilibrium of photons is not reached until a few millimetres of penetration31. The box therefore ensures that the set radiation dose is fulfilled before the beam reaches the insects. The vials were placed within the irradiation field whilst lowering the irradiator (Eldorado 78, Foss Therapy Services, North Hollywood) to give the maximum possible dose rate of 2.4 Gy/min. Vials were laid on their side to prevent inconsistency in received dosimetry through vertical movement of parasitoids under the radiation source. One vial of female parasitoids was removed when exposure reached each of 120 Gy, 130 Gy, 140 Gy and 150 Gy, which was based on timing. This was replicated twice, giving a total of 10 female T. basalis for each irradiation dose tested and for the non-irradiated positive control.
Upon completion of irradiation, parasitoids were returned to the laboratory (25 ± 1 °C, 16:8 h L:D) where irradiated females were individually separated into vials (8.5 cm diameter × 6 cm height), noting dosage, and rested for 24 h with a drop of honey (~ 10 µL). When possible, a single fresh N. viridula egg mass, no older than 24 h32, was placed into each vial and oviposition allowed for 24 h. However, to ensure an adequate supply, fresh N. viridula egg masses were stored at 10 °C for no longer than a week to halt their development prior to exposure to irradiated T. basalis females33,34. To ascertain whether induced sterility is permanent, these females were exposed to a new egg mass weekly until death. Any parasitoids that did not oviposit within 20 min were given a new egg mass, and re-observed. The mean number of eggs in masses exposed to parasitoids were 53, 51, 47, 47 and 54 for 120 Gy, 130 Gy, 140 Gy, 150 Gy and the non-irradiated controls, respectively.
Assessment of sterility
To evaluate parasitism for each host egg, nymphal development of N. viridula was assessed. This was necessary because it was possible that oviposition by irradiated females would not produce a developing parasitoid offspring (will not turn black), instead with host death occurring during oviposition, leaving no sign of either parasitoid or host development18,35. Eggs that became pink were recorded as containing a developing N. viridula nymph36. Any infertile eggs, which retained a pale colour with no change37, were not counted as parasitised, as it was not possible to determine the cause of the lack of N. viridula nymph development. Host eggs that did not either retain a pale colour or contain a developing host, or where a parasitoid offspring developed, were therefore recorded as parasitised, which also represents host death. Where these indicators of parasitism were seen without its occurrence during the observation period, oviposition was retrospectively confirmed and recorded as above.
The proportion of parasitised N. viridula eggs with developing parasitoids (i.e. eggs turn black), emerged parasitoids, and the sex ratio of any emerged offspring, were recorded for each parasitised egg mass to investigate the degree of induced sterility. Any egg containing a developing parasitoid that did not emerge within three weeks of oviposition by an irradiated mother was dissected to confirm its presence, which was recorded. The sex, level of development, and living status were also recorded. Eggs in which it was difficult to decipher whether a parasitoid or host had developed were also dissected to ensure that the result was recorded correctly. Any N. viridula offspring that successfully emerged was also recorded. Percentage F1 development and emergence were recorded as the proportion of offspring that either developed or emerged from eggs in which an irradiated T. basalis oviposited. This experiment was repeated with non-irradiated female T. basalis as a positive control. As a negative control, we left a fresh N. viridula egg mass to develop inside a vial without the presence of a parasitoid. This was replicated 21 times to ascertain natural host development rates and to confirm that host death was due to parasitism by irradiated T. basalis. To replicate the conditions that egg masses were subjected to before parasitoid exposure, they were collected at no older than 24 h and accumulated at 10 °C for up to a week. The time that each egg mass spent at 10 °C was also recorded.
Post-irradiation fitness and behaviour
Time spent searching for the host, drumming antennae on host eggs, ovipositing in first egg, and time taken to initiate the first oviposition were recorded for T. basalis females during each host exposure to ascertain potential impacts of irradiation on oviposition behaviour32. For drumming, recording of time continued if the parasitoid temporarily left the egg mass after locating it. The total number of host eggs attacked and longevity were recorded for T. basalis females as indicators of post-irradiation fitness20,38.
Statistical analysis
Binomial generalised linear models (GLM) (logit link) were used to assess the relationship between the irradiation dose for T. basalis females and both percentage F1 development and percentage F1 emergence. Binomial GLM’s (logit link) were also used to assess the potential difference in offspring sex ratio between irradiated and non-irradiated parasitoid mothers, as well as for the negative control. Infertile eggs were excluded from the analysis of the negative control data, as they were not considered for the confirmation of parasitism by irradiated parasitoids. Poisson GLM’s (log link) were used to assess the effect of irradiation dose on the lifetime sum of host eggs attacked and the change in the mean number of eggs parasitised over time, for each irradiation dose individually. Poisson GLM’s (log link) were also used to explore the relationship between mean longevity and irradiation dose, and for each component of the oviposition sequence compared with dose. Binomial (logit link) and Poisson (log link) GLM’s, for proportion and count data respectively, were used where comparisons were made between irradiated and non-irradiated parasitoids as distinct groups. Analysis of variance (ANOVA) was applied on all GLM’s. Statistics presented were generated in R (version 3.6.0; R Foundation for Statistical Computing, Australia) using the packages ggplot 239 for plots and lme440 for analyses.
Results
Irradiation dosimetry
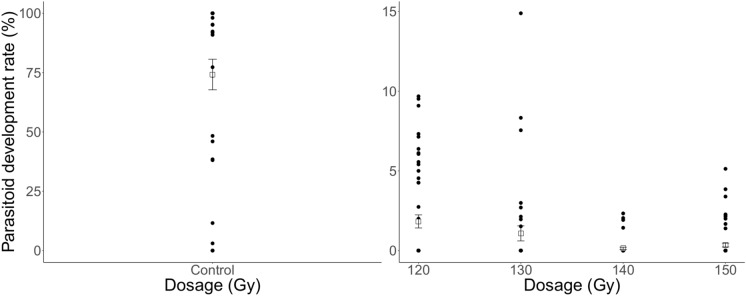
The percentage of host eggs attacked throughout the lifetime of T. basalis females, which resulted in developing T. basalis offspring, decreased with an increasing irradiation dose (F = 13.39, df = 3, P < 0.001) (Fig. 1). The means for 120 Gy, 130 Gy, 140 Gy, and 150 Gy were 1.83%, 1.08%, 0.14%, and 0.34%, respectively. However, there was considerable variation, most notably at 130 Gy where host egg exposures resulted in between 0 and 15% development of offspring. Conversely, the non-irradiated T. basalis females produced a substantially higher percentage of developing offspring, with a mean of 74.19% (F = 1528.80, df = 1, P < 0.001). However, there was also high variability in the non-irradiated control, which ranged between 0 and 100% development of offspring.
Figure 1.
Lifetime percentage of Nezara viridula eggs attacked by irradiated and non-irradiated female Trissolcus basalis, which resulted in developed offspring of the latter. Squares and error bars represent means and standard error, respectively.
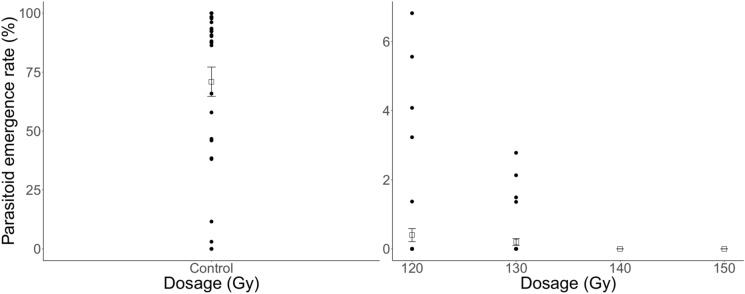
The percentage of eggs parasitised by irradiated female T. basalis that resulted in emerging offspring showed a similar relationship (F = 14.65, df = 3, P < 0.001) (Fig. 2). The means for 120 Gy and 130 Gy were 0.4% and 0.2% emergence, respectively, whereas 140 Gy and 150 Gy produced no successfully emerging offspring. Higher variability was observed at 120 Gy, with 6.82% emergence resulting from one host egg exposure. There was a significantly higher percentage of emerging offspring in the non-irradiated group, with a mean of 70.92% (F = 1976.20, df = 1, P < 0.001). There was also high variability in the non-irradiated control.
Figure 2.
Lifetime percentage of Nezara viridula eggs attacked by irradiated and non-irradiated female Trissolcus basalis, which resulted in emerged offspring of the latter. Squares and error bars represent means and standard error, respectively.
Of the non-emerged progeny from sterile female T. basalis irradiated at 140 Gy, all five were female, with two of these only partially developed. Amongst the offspring of sterile females irradiated at 150 Gy, that did not emerge, were seven females and one male, and three of unknown sex, with eight of these only partially developed. Of both the emerged and non-emerged progeny from the non-irradiated T. basalis females, 962 were female and 171 were male, all of which were fully developed. The sex ratio of developed offspring from sterile T. basalis mothers was similar to that for non-irradiated mothers (F = 0.05, df = 1, P = 0.827).
The negative control demonstrated that N. viridula egg masses stored at 10 °C for between one and seven days expressed an average of 96% development of N. viridula offspring (Table 1). There was no significant difference in the percentage of developing offspring between egg masses that had been stored at 10 °C for different time periods (F = 0.56, df = 6, P = 0.755).
Table 1.
Negative control showing the percentage of eggs that developed Nezara viridula nymphs after egg masses were kept at 10 °C for up to a week (N = 3 egg masses per day spent in refrigerator; 21 egg masses in total).
| Days in fridge | Average percentage development |
|---|---|
| 1 | 97.74 |
| 2 | 91.16 |
| 3 | 99.24 |
| 4 | 94.95 |
| 5 | 97.47 |
| 6 | 98.67 |
| 7 | 90.30 |
| Overall mean | 96.00 |
| Overall assessment | F = 0.56 P = 0.755 |
Post-irradiation fitness and behaviour
Oviposition behaviour was similar across all irradiation doses and the non-irradiated controls, with no discernible relationships between dose and mean time spent undertaking each step in the oviposition sequence (searching: F = 0.46, df = 4, P = 0.766; antennal drumming: F = 1.91, df = 4, P = 0.109; time taken to oviposit: F = 1.20, df = 4, P = 0.310; time spent ovipositing: F = 1.12, df = 4, P = 0.354) (Table 2). The irradiated group was also similar to the control group for each step in the oviposition sequence (searching: F = 1.28, df = 1, P = 0.260; antennal drumming: F = 0.01, df = 1, P = 0.924; time taken to oviposit: F = 0.20, df = 1, P = 0.652; time spent ovipositing: F = 0.10, df = 1, P = 0.755) (Table 2). High standard deviations demonstrate considerable variance in the time taken to complete each step in the oviposition sequence for both irradiated and control T. basalis females.
Table 2.
Mean time spent (seconds), by irradiated and control female Trissolcus basalis throughout their lifespans, undertaking each step in the oviposition sequence.
| Irradiation dose (Gy) | Step in oviposition sequence | |||||||
|---|---|---|---|---|---|---|---|---|
| Searching | SD | Antennal drumming | SD | Time to first oviposition | SD | Ovipositing in first egg | SD | |
| 120 | 92.88 | 68.05 | 171.46 | 165.08 | 264.54 | 165.03 | 243.37 | 113.17 |
| 130 | 94.47 | 51.64 | 189.14 | 257.43 | 283.50 | 277.72 | 260.34 | 139.99 |
| 140 | 99.16 | 101.81 | 107.10 | 83.67 | 210.18 | 133.51 | 212.24 | 114.83 |
| 150 | 90.18 | 71.52 | 143.94 | 153.48 | 234.12 | 165.02 | 223.65 | 109.71 |
| Control | 111.06 | 106.22 | 146.56 | 100.20 | 259.94 | 149.45 | 239.47 | 125.41 |
| Overall assessment |
F = 0.46 P = 0.766 |
F = 1.91 P = 0.109 |
F = 1.20 P = 0.310 |
F = 1.12 P = 0.354 |
||||
| Irradiated vs control |
F = 1.28 P = 0.260 |
F = 0.01 P = 0.924 |
F = 0.20 P = 0.652 |
F = 0.10 P = 0.755 |
||||
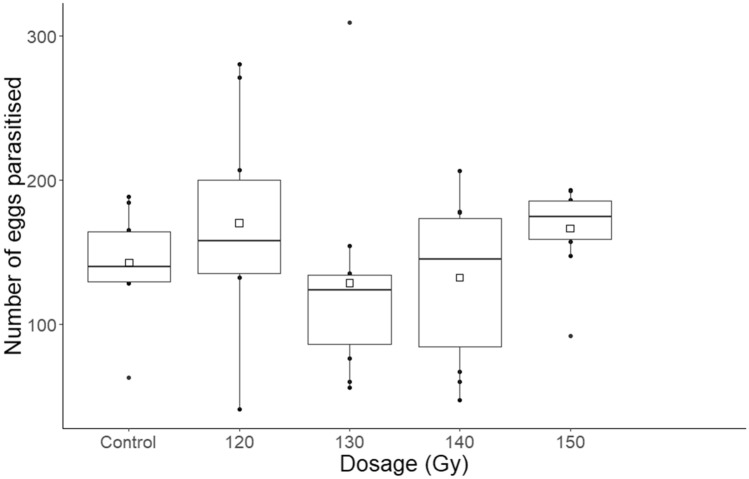
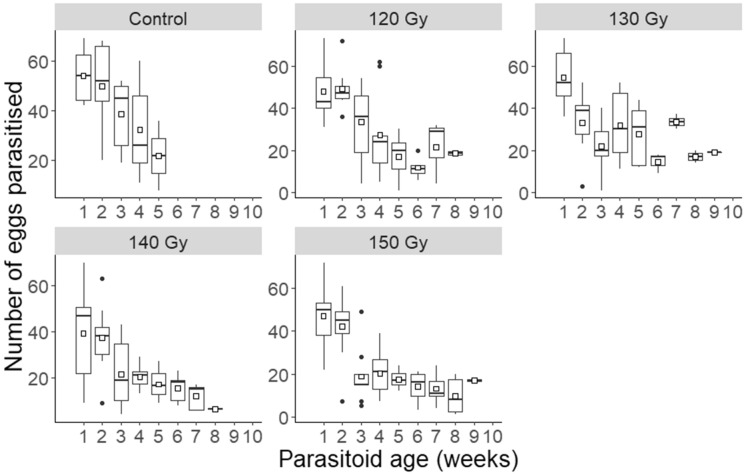
The total number of eggs parasitised and killed by T. basalis females over their lifespans was not related to irradiation dose (F = 1.17, df = 4, P = 0.337) (Fig. 3). Similarly, the lifetime number of eggs parasitised and killed did not vary strongly between irradiated and non-irradiated females (F = 0.11, df = 1, P = 0.742). Females irradiated at 120 Gy exhibited the highest mean number of eggs parasitised, at 170.10, whilst those irradiated at 130 Gy expressed the lowest, at 128.40. Irradiated parasitoids exhibited an abrupt decrease in the number of eggs parasitised at either the second or the third week of host egg exposure (Fig. 4). This decrease continued incrementally until either the eighth or the ninth week of exposure. Conversely, the non-irradiated T. basalis females demonstrated higher parasitism each week, but over a shorter period, with no oviposition occurring after week five. This negative relationship between parasitoid age and number of host eggs parasitised was statistically significant for all irradiation doses and the positive control (120 Gy: F = 6.47, df = 7, P < 0.001; 130 Gy: F = 3.85, df = 8, P = 0.003; 140 Gy: F = 6.55, df = 7, P < 0.001; 150 Gy: F = 11.31, df = 8, P < 0.001; control: F = 3.25, df = 4, P = 0.027). Irradiated parasitoids therefore parasitised and killed a similar number of hosts throughout their lifespans compared with non-irradiated parasitoids (Fig. 3), but over a longer period (Fig. 4).
Figure 3.
Boxplots of the total number of Nezara viridula eggs parasitised by non-irradiated female Trissolcus basalis, and by those subjected to increasing irradiation doses, throughout their lifespans. The box represents the interquartile range, and the whiskers are 1.5 times the interquartile range. Squares represent means.
Figure 4.
Boxplots of the number of Nezara viridula eggs parasitised by non-irradiated female Trissolcus basalis, and by those irradiated between 120–150 Gy, during each weekly host egg exposure. The box represents the interquartile range, and the whiskers are 1.5 times the interquartile range. Squares represent means.
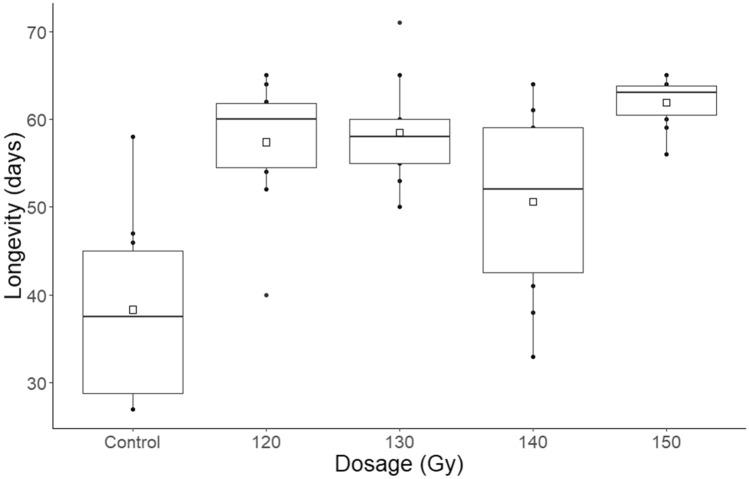
There was no discernible relationship between irradiation dose and mean longevity amongst the irradiated T. basalis females, where the lowest was 50.6 days at 140 Gy and the highest was 61.9 days at 150 Gy (F = 0.23, df = 1, P = 0.632) (Fig. 5). However, longevity for the non-irradiated group was substantially shorter than for the irradiated group with a mean of 38.30 days (F = 39.48, df = 1, P < 0.001).
Figure 5.
Boxplots of the longevity of irradiated and non-irradiated Trissolcus basalis. The box represents the interquartile range, and the whiskers are 1.5 times the interquartile range. Squares represent means.
Discussion
Sterility of T. basalis and the potential of the KWT
A substantial dose-dependent reduction in the proportion of developing offspring from irradiated female T. basalis was observed. This sterility is likely to have been caused by damage to the ovaries or oocytes, inhibiting the production of viable eggs41. Grosch and Sullivan42 confirmed this as the cause of irradiation-induced sterility in female Bracon hebetor Say (Hymenoptera: Braconidae). This is corroborated by more recent studies on irradiation-induced sterility in Chelonus inanitus Linnaeus (Hymenoptera: Braconidae)18 and Glyptapanteles liparidis Bouche (Hymenoptera: Braconidae)19, which resulted in the deposition of inviable eggs. These studies used irradiated parasitoids to assess host immune response in the absence of developing parasitoid offspring, rather than for the potential use of sterile parasitoids as part of a biosecurity response. However, Grosch and Sullivan42 noted that females successfully oviposited viable eggs produced prior to irradiation. This could explain the small number of female T. basalis amongst the developing F1 progeny from sterile females, indicating that some of the eggs produced before irradiation were successfully fertilised. It could also explain the lack of visual evidence for sterile T. basalis eggs inside parasitised host eggs, which was therefore an unsuitable method for confirming oviposition.
The presence of only one male amongst the developing offspring from sterile T. basalis mothers is unsurprising given the substantially higher ratio of female offspring in the non-irradiated control, which occurs in many hymenopteran parasitoids. This is due to a mating system with strong local mate competition where the mother produces only enough sons to mate with all of her daughters43.
The ability of irradiated T. basalis to kill host eggs, despite the absence of developing offspring, is supported by the findings of Soller and Lanzrein18, and Tillinger et al.19, who observed that irradiation does not affect the production and injection of venom and parasitoid-associated factors by parasitoid females. Irradiated females of the egg parasitoid Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae) also retain their ability to kill the host20. Scelionid parasitoids do not possess a venom gland35,44, but all non-venomous egg parasitoids kill their hosts during oviposition, even when offspring fails to develop, through mechanisms such as mechanical damage from ovipositor insertion, or the injection of growth arrestment factors44,45. Irradiated T. basalis females therefore retained their ability to kill the host. Furthermore, the negative control resulted in a high percentage of host development for N. viridula egg masses in the absence of a T. basalis female, indicating that parasitism by sterile parasitoids resulted in host death. This also validates a lack of host development as the methodological indicator for confirming parasitism.
Complete sterility was achieved with no F1 emergence from T. basalis females irradiated at 140 Gy and above. These results suggest that in a hypothetical field release, sterile T. basalis females could kill the target host, but it would not persist in the environment to potentially exert sustained non-target impacts after the pest is eradicated4,46. It is therefore possible that sterile ‘kamikaze wasps’ could be utilised synergistically in an eradication programme alongside SIT, or other tactics, to mitigate regulation associated with the perceived irreversible risk of exotic parasitoid introduction6–8. However, testing whether higher irradiation doses could eliminate any development of female offspring would be beneficial from a biosafety perspective, but may lead to reduced fitness19,47,48.
Regardless, the possible risk of ephemeral, but non-negligible, non-target impacts from inundatively released sterile parasitoids must be considered4,21. Well-known determinants of successful eradication include early detection of invasion and rapid response, with elimination generally viewed as impracticable if a pest has spread widely over a variety of habitats49–51. Eradication responses therefore tend to be highly targeted to specific urban locations, where incursions often begin49,52. This alleviates the concern of transient environmental risk from sterile parasitoids as they would not be released in large numbers across an array of non-target habitats. However, due to the complicated set of scientific, economic, and socio-political requirements, much of the scientific community questions the feasibility of eradication compared to long term area-wide management50,53.
Post-irradiation fitness and behaviour
To be effective in SIT programmes, released sterile individuals must retain fitness and normal reproductive behaviour54. The oviposition behaviour data suggest that the irradiation doses employed had no impact on any component of the T. basalis oviposition sequence. This is the first experimental assessment of the potential impact of irradiation on the oviposition behaviour of an egg parasitoid. However, Soller and Lanzrein18 mentioned that X-ray irradiation did not affect the oviposition behaviour of C. inanitus (without supporting data), which also attacks the eggs of its host55. For species from other insect orders, it is common for irradiated females to deposit substantially fewer eggs than control females, indicating a negative impact on oviposition behaviour56–58. Our results indicate that irradiation-induced sterility may not negatively influence oviposition behaviour in certain parasitoids. In the field, sterile wasps would need to retain the behaviours required for final host acceptance and oviposition in order to kill the host. This is analogous to the importance of sterile males retaining their competitive mating ability amongst the wild male population in a conventional SIT scenario54.
Fecundity of sterile individuals is a crucial measure of competitive fitness in SIT programmes54, as well as an indicator of parasitoid fitness59,60. For analysis, we used the number of host eggs parasitised, as opposed to percentage parasitism, because there was slight variability in the number of N. viridula eggs in each mass exposed to T. basalis females. Irradiation dose did not influence average lifetime parasitism in T. basalis females, suggesting that irradiation does not affect fecundity as defined by the number of host eggs attacked and killed. This also demonstrates that irradiation did not influence the lifetime number of hosts killed. Irradiated C. inanitus females also do not experience a reduction in oviposition behaviour18. However, Tillinger et al.19 found that G. liparidis irradiated at 48 Gy and 96 Gy exhibited a four-fold reduction in the number of deposited eggs compared with the non-irradiated control, which indicated a significant fitness impact, though the proportion of parasitised hosts that died was similar between these groups. The number of eggs laid by irradiated B. hebetor also decreased with an increasing dose42, but the number of oviposition attempts was not reported. It is therefore likely that the effect of irradiation on parasitism would vary amongst parasitoid species. Most species in other insect orders commonly targeted by SIT show a decrease in fecundity when irradiated47,48,58,61,62. Our fecundity results demonstrate the importance of investigating the potential impact of irradiation on parasitism when assessing the feasibility of the KWT scenario in relation to parasitoid fitness.
Despite this, irradiation clearly affected the decline in the number of host eggs parasitised and killed by T. basalis females during each weekly exposure. Irradiated females attacked fewer host eggs with each exposure but continued for a longer period than the non-irradiated controls. This is dissimilar to the effect of irradiation on G. liparidis, where non-irradiated females continued to oviposit for twice the amount of time as irradiated females19. Similar studies on species from different orders express variation in their findings. For example, Ali et al.48 found that female Mythimna separata Walker (Lepidoptera: Noctuidae) expressed a higher oviposition rate with an increase in irradiation dose, whereas other studies have observed the opposite effect47,63,64. The pattern observed in T. basalis is likely due to irradiation damage to the ovaries and/or oocytes, affecting egg production42,65, with Tillinger et al.19 suggesting this as the cause for a lower oviposition rate in irradiated G. liparidis females. This may constitute a negative impact of irradiation on female T. basalis fitness, and in the context of the KWT could affect their patch (i.e. single host egg mass) exploitation ability by limiting the number of hosts killed within each visited egg mass66. Female T. basalis exert more effort defending a patch from super-parasitism when they have laid more eggs66. However, parasitising a similar number of eggs over a longer period could be beneficial by improving parasitoid-host synchrony, which becomes an issue when parasitism does not temporally correspond with host availability/susceptibility67,68.
Observing a significantly longer lifespan for irradiated T. basalis was unexpected, given that it is generally reported that higher doses of irradiation decrease longevity in insects69. However, Tillinger et al.19 demonstrated that irradiation had no significant impact on the longevity of G. liparidis females. Several studies on insect pest orders have also found that irradiation-induced sterility stimulates either an increase in longevity, or has no significant impact70–72. In this case, a longer lifespan for irradiated female T. basalis was probably due to the lower number of host eggs parasitised per week, with this trade-off between fecundity and longevity being well studied in insects73–75. This occurs because an increase in gamete production is costly, and causes a compensatory decrease in longevity76. This is also known to occur in parasitoid wasps77,78. For example, females of the egg parasitoid Gryon pennsylvanicum Ashmead (Hymenoptera: Scelionidae) exhibit a reduction in fecundity when host-deprived, which in turn causes an increase in longevity77. For irradiated T. basalis females, this does not necessarily indicate a fitness gain, but rather an artificially induced trade-off in fitness between fecundity and longevity76. However, it could be beneficial for the KWT in terms of parasitoid-host synchrony67, though field trials would be required to assess this.
Potential application of KWT
The combination of biological control with SIT is well-researched for the suppression or eradication of insect pests, although the integration of biological control with pesticides, host plant resistance, and cultural techniques has also received attention13,79. This combination received early theoretical and modelling-based interest from Knipling16 and Barclay17, who demonstrated that it should have a synergistic impact on a target pest. This is because parasitoids generally target a different host life-stage than does SIT, which targets adults13. For example, sterile pest release would decrease the number of fertile eggs produced in the subsequent generation, resulting in a higher ratio of egg parasitoids to hosts and increasing the impact of the parasitoid on the pest. Similarly, the effect of the parasitoid population would result in a higher ratio of sterile to wild pests, increasing the impact of the SIT component80.
A few programmes have successfully combined biological control and SIT for pest eradication or suppression. A large-scale example is the eradication of Anastrepha spp. Schiner (Diptera: Tephritidae) fruit flies from the Mexican states of Chihuahua, Coahuila, and Sonora through the concurrent release of sterile males and their parasitoid Diachasmimorpha longicaudata Ashmead (Hymenoptera: Braconidae)81. An early example assessed the suppression of Ceratitis capitata (Diptera: Tephritidae) within a 13 km2 area on the island of Maui, Hawaii. Concurrent releases of sterile males and the parasitoid Diachasmimorpha tryoni Cameron (Hymenoptera: Braconidae) stimulated a significantly increased parasitism rate and decreased pest density compared with a control site82. Other studies have confirmed the efficacy of combining SIT with biological control through field cage assays using Cydia pomonella Linnaeus (Lepidoptera: Tortricidae) and Thaumatotibia leucotreta Meyrick (Lepidoptera: Tortricidae)57,83–85. Given that these studies relied on already present pest and parasitoid populations, they were not limited by stringent regulations surrounding the importation and release of natural enemies6. The scene was therefore set to explore whether the release of sterile parasitoids could enable their utilisation to improve an eradication attempt against an unfolding pest incursion, which is pertinent as the feasibility of eradication is often greatest at low target pest densities12,13.
Although we have used T. basalis and its host N. viridula to investigate the KWT, this predominantly promotes the potential for implementing the KWT on the globally emerging pest H. halys with its parasitoid T. japonicus. The former is not yet established in the Southern Hemisphere, with the exception of Chile86, and represents a serious biosecurity threat owing to its highly polyphagous feeding habits87. For example, in New Zealand, H. halys has been repeatedly intercepted at the border in recent years88,89. The Environmental Protection Authority has granted permission for the introduction of T. japonicus into New Zealand, and its release from containment to support an eradication response against H. halys90,91. Additionally, Welsh et al.24 ascertained the irradiation biology for male H. halys, showing that a gamma-irradiation dose of 16 Gy induced 97% egg sterility after mating with fertile females. This pest-parasitoid system therefore represents an appropriate case study to investigate the viability of the KWT as an eradication response, and the irradiation biology of T. japonicus should be investigated now that feasibility is confirmed for T. basalis, though field efficacy trials must first be completed. Overall, this study has demonstrated the potential for this novel concept to be developed further. However, the complicated set of scientific, economic, and socio-political limitations to the initiation of eradication programmes, including the need for early detection and preparedness of tools49,50, may limit the development and implementation of novel approaches such as the KWT.
It is pertinent to note that for pest management, the release of sterile parasitoid wasps would only be applicable to an eradication response. Once a pest has established and spread beyond the possibility of eradication13, self-sustaining parasitoid release would be optimal to provide inexpensive, long-term classical biological control1.
Conclusions and implications
This is the first experimental study to investigate the potential application of sterile CBC agent release. The successful permanent sterilisation of T. basalis, which retains its ability to kill host eggs, promotes further investigation into whether sterile parasitoids could be combined with SIT to improve eradication outcomes, with negligible risk of incurring irreversible non-target impacts after pest elimination. This could mitigate the need for extensive pre-release testing of parasitoids, which largely excludes them from eradication programmes owing to the need to act quickly whilst pest densities remain low. Further research required for the development of the KWT includes systematic fitness assessments of sterile parasitoids, population modelling, and efficacy trials for the KWT scenario, which would involve laboratory and field cage trials, as well as open field trials.
Acknowledgements
We are grateful to Anne Barrington (Plant and Food Research), who is responsible for maintaining the N. viridula colony used for this study, and to Darren Ward (National Arthropod Collection, Manaaki Whenua Landcare Research), who formally identified the T. basalis. We are also thankful to Professor Bill Wilson for facilitating our access to the Auckland Cancer Society Research Centre’s irradiation facilities (Faculty of Medical and Health Sciences, University of Auckland), and to Kate Richards and Ruth Butler (Plant and Food Research) for providing statistical consulting and support with data analysis.
Author contributions
D.M.S., K.J.H., G.A.A. and G.I.H. conceived and designed the study. K.J.H. and G.A.A. conducted experiments. G.A.A. contributed materials and tools. K.J.H. analysed data. K.J.H. wrote the manuscript. All authors read, edited and approved the manuscript.
Funding
KJH was supported by the University of Auckland Doctoral Scholarship and the New Zealand Plant Protection Society Research Scholarship, also receiving an international travel grant from Zespri International Limited (grant code BS20139), and a postgraduate scholarship from the Bragato Research Institute. GAA and DMS were supported by The New Zealand Institute for Plant and Food Research Limited, and the Better Border Biosecurity (B3) research collaboration. GIH and DMS were supported by the School of Biological Sciences, University of Auckland.
Data availability
The datasets generated and/or analysed during the current study are uploaded to Researchgate ([link to URL when uploaded]), and are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DeBach P, Rosen D. Biological Control by Natural Enemies. Cambridge University Press; 1991. [Google Scholar]
- 2.Naranjo SE, Ellsworth PC, Frisvold GB. Economic value of biological control in integrated pest management of managed plant systems. Annu. Rev. Entomol. 2015;60:621–645. doi: 10.1146/annurev-ento-010814-021005. [DOI] [PubMed] [Google Scholar]
- 3.Walker JTS, Suckling DM, Wearing CH. Past, present, and future of integrated control of apple pests: The New Zealand experience. Annu. Rev. Entomol. 2017;62:231–248. doi: 10.1146/annurev-ento-031616-035626. [DOI] [PubMed] [Google Scholar]
- 4.van Lenteren JC, Bale J, Bigler F, Hokkanen HMT, Loomans AJM. Assessing risks of releasing exotic biological control agents of arthropod pests. Annu. Rev. Entomol. 2006;51:609–634. doi: 10.1146/annurev.ento.51.110104.151129. [DOI] [PubMed] [Google Scholar]
- 5.Bale JS, van Lenteren JC, Bigler F. Biological control and sustainable food production. Phil. Trans. R. Soc. Lond. B. 2008;363:761–776. doi: 10.1098/rstb.2007.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheppard AW, et al. A global review of risk-benefit-cost analysis for the introduction of classical biological control agents against weeds: A crisis in the making? Biocontrol News Inf. 2003;24:91N–108N. [Google Scholar]
- 7.Barratt BIP, Blossey B, Hokkanen HM. Post-release evaluation of non-target effects of biological control agents. In: Bigler F, Babendreier D, Kuhlmann U, editors. Environmental Impact of Invertebrates for Biological Control of Arthropods: Methods and Risk Assessment. CABI Publishing; 2006. pp. 166–186. [Google Scholar]
- 8.Barratt BIP, Moeed A, Malone LA. Biosafety assessment protocols for new organisms in New Zealand: Can they apply internationally to emerging technologies? Environ. Impact Assess. Rev. 2006;26:339–358. doi: 10.1016/j.eiar.2005.11.008. [DOI] [Google Scholar]
- 9.Klassen W, Curtis CF. History of the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. Springer; 2021. pp. 3–38. [Google Scholar]
- 10.Hendrichs J, Kenmore P, Robinson AS, Vreyson MJB. Area-wide integrated pest management (AW-IPM): principles, practice and prospects. In: Vreysen MJB, Robinson AS, Hendrichs J, editors. Area-Wide Control of Insect Pests. Springer; 2007. pp. 3–34. [Google Scholar]
- 11.Knipling EF. Possibilities of insect control or eradication through the use of sexually sterile males. J. Econ. Entomol. 1955;48:459–462. doi: 10.1093/jee/48.4.459. [DOI] [Google Scholar]
- 12.Brockerhoff EG, Liebhold AM, Richardson B, Suckling DM. Eradication of invasive forest insects: Concepts, methods, costs and benefits. NZ J. For. Sci. 2010;40:S117–S135. [Google Scholar]
- 13.Suckling DM, Tobin PC, McCullough DG, Herms DA. Combining tactics to exploit Allee effects for eradication of alien insect populations. J. Econ. Entomol. 2012;105:1–13. doi: 10.1603/EC11293. [DOI] [PubMed] [Google Scholar]
- 14.Hendrichs J, Enkerlin WR, Pereira R. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. Springer; 2021. Invasive insect pests: challenges and the role of the sterile insect technique in their prevention, containment, and eradication; pp. 885–922. [Google Scholar]
- 15.Nagel P, Peveling R. Environment and the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. Springer; 2021. pp. 499–519. [Google Scholar]
- 16.Knipling EF. The Basic Principles of Insect Population Suppression and Management. U.S. Department of Agriculture; 1979. [Google Scholar]
- 17.Barclay HJ. Models for pest control: Complementary effects of periodic releases of sterile pests and parasitoids. Theor. Popul. Biol. 1987;32:76–89. doi: 10.1016/0040-5809(87)90041-4. [DOI] [Google Scholar]
- 18.Soller M, Lanzrein B. Polydnavirus and venom of the egg-larval parasitoid Chelonus inanitus (Braconidae) induce developmental arrest in the prepupa of its host Spodoptera littoralis (Noctuidae) J. Insect Physiol. 1996;42:471–481. doi: 10.1016/0022-1910(95)00132-8. [DOI] [Google Scholar]
- 19.Tillinger NA, Hoch G, Schopf A. Effects of parasitoid associated factors of the endoparasitoid Glyptapanteles liparidis (Hymenoptera: Braconidae) Eur. J. Entomol. 2004;101:243–249. doi: 10.14411/eje.2004.033. [DOI] [Google Scholar]
- 20.Tunçbilek AS, Canpolat U, Ayvaz A. Effects of gamma radiation on suitability of stored cereal pest eggs and the reproductive capability of the egg parasitoid Trichogramma evanescens (Trichogrammatidae: Hymenoptera) Biocontrol Sci. Techn. 2009;19:179–191. doi: 10.1080/09583150902790269. [DOI] [Google Scholar]
- 21.Lynch LD, et al. Insect biological control and non-target effects: a European perspective. In: Wajnberg E, Scott JK, Quimby PC, et al., editors. Evaluating Indirect Ecological Effects of Biological Control. Springer; 2001. pp. 99–126. [Google Scholar]
- 22.van Lenteren JCV, et al. Environmental risk assessment of exotic natural enemies used in inundative biological control. Biocontrol. 2003;48:3–38. doi: 10.1023/A:1021262931608. [DOI] [Google Scholar]
- 23.Horrocks KJ, Avila GA, Holwell GI, Suckling DM. Integrating sterile insect technique with the release of sterile classical biocontrol agents for eradication: Is the Kamikaze Wasp Technique feasible? Biocontrol. 2020;65:257–271. doi: 10.1007/s10526-020-09998-7. [DOI] [Google Scholar]
- 24.Welsh TJ, Stringer LD, Caldwell R, Carpenter JE, Suckling DM. Irradiation biology of male brown marmorated stink bugs: Is there scope for the sterile insect technique? Int. J. Radiat. Biol. 2017;93:1357–1363. doi: 10.1080/09553002.2017.1388547. [DOI] [PubMed] [Google Scholar]
- 25.Suckling DM, et al. The competitive mating of irradiated brown marmorated stink bugs, Halyomorpha halys, for the sterile insect technique. Insects. 2019;10:411. doi: 10.3390/insects10110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larivière M-C. Fauna of New Zealand. Manaaki Whenua Press; 1995. [Google Scholar]
- 27.Martin, N. A. Green vegetable bug - Nezara viridula. Interesting insects and other invertebrates. New Zealand arthropod factsheet number 47https://nzacfactsheets.landcareresearch.co.nz/factsheet/InterestingInsects/Green-vegetable-bug---Nezara-viridula.html (2018). Accessed 16 Sept 2020.
- 28.Powell JE, Shepard M. Biology of Australian and United States strains of Trissolcus basalis, a parasitoid of the green vegetable bug Nezara viridula. Austr. Ecol. 1982;7:181–186. doi: 10.1111/j.1442-9993.1982.tb01591.x. [DOI] [Google Scholar]
- 29.Cantón-Ramos JM, Callejón-Ferre ÁJ. Raising Trissolcus basalis for the biological control of Nezara viridula in greenhouses of Almería (Spain) Afr. J. Agric. Res. 2010;5:3207–3212. [Google Scholar]
- 30.Loch AD, Walter GH. Mating behavior of Trissolcus basalis (Wollaston) (Hymenoptera: Scelionidae): Potential for outbreeding in a predominantly inbreeding species. J. Insect Behav. 2002;11:2. [Google Scholar]
- 31.Johns HF, Cunningham JR. The Physics of Radiology. Charles C Thomas; 1983. The interaction of single beams of x and gamma rays with a scattering medium; pp. 349–350. [Google Scholar]
- 32.Bin F, Vinson SB, Strand MR, Colazza S, Jones WA. Source of an egg kairomone for Trissolcus basalis, a parasitoid of Nezara viridula. Physiol. Entomol. 1993;18:7–15. doi: 10.1111/j.1365-3032.1993.tb00443.x. [DOI] [Google Scholar]
- 33.Mahmoud AMA, Lim UT. Evaluation of cold-stored eggs of Dolycoris baccarum (Hemiptera: Pentatomidae) for parasitization by Trissolcus nigripedius (Hymenoptera: Scelionidae) Biol. Control. 2007;43:287–293. doi: 10.1016/j.biocontrol.2007.09.004. [DOI] [Google Scholar]
- 34.Haye T, et al. Fundamental host range of Trissolcus japonicus in Europe. J. Pest Sci. 2020;93:171–182. doi: 10.1007/s10340-019-01127-3. [DOI] [Google Scholar]
- 35.Cusumano A, et al. First extensive characterization of the venom gland from an egg parasitoid: Structure, transcriptome and functional role. J. Insect Physiol. 2018;107:68–80. doi: 10.1016/j.jinsphys.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Bundy CS, McPherson RM. Morphological examination of stink bug (Heteroptera: Pentatomidae) eggs on cotton and soybeans, with a key to genera. Ann. Entomol. Soc. Am. 2000;93:616–624. doi: 10.1603/0013-8746(2000)093[0616:MEOSBH]2.0.CO;2. [DOI] [Google Scholar]
- 37.Favetti BM, Butnariu AR, Doetzer AK. Storage of Euschistus heros eggs (Fabricius) (Hemiptera: Pentatomidae) in liquid nitrogen for parasitization by Telenomus podisi Ashmead (Hymenoptera: Platygastridae) Neotrop. Entomol. 2014;43:291–293. doi: 10.1007/s13744-014-0206-0. [DOI] [PubMed] [Google Scholar]
- 38.Kazmer DJ, Luck RF. Field tests of the size-fitness hypothesis in the egg parasitoid Trichogramma pretiosum. Ecology. 1995;76:412–425. doi: 10.2307/1941200. [DOI] [Google Scholar]
- 39.Wickham H. Ggplot2: Elegant Graphics for Data Analysis. Springer; 2016. [Google Scholar]
- 40.Bates D, Machler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 41.Chapman T, Miyatake T, Smith HK, Partridge L. Interactions of mating, egg production and death rates in females of the Mediterranean fruit fly, Ceratitis capitata. Proc. R. Soc. Lond. B. 1998;265:1879–1894. doi: 10.1098/rspb.1998.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grosch DS, Sullivan RL. The quantitative aspects of permanent and temporary sterility induced in female Habrobracon by x-rays and β radiation. Radiat. Res. 1954;1:294–320. doi: 10.2307/3570374. [DOI] [PubMed] [Google Scholar]
- 43.Colazza S, Wajnberg E. Effects of host egg mass size on sex ratio and oviposition sequence of Trissolcus basalis (Hymenoptera: Scelionidae) Environ. Entomol. 1998;27:329–336. doi: 10.1093/ee/27.2.329. [DOI] [Google Scholar]
- 44.Rosi MC, Isidoro N, Colazza S, Bin F. Source of the host marking pheromone in the egg parasitoid Trissolcus basalis (Hymenoptera: Scelionidae) J. Insect Physiol. 2001;47:989–995. doi: 10.1016/S0022-1910(01)00073-7. [DOI] [PubMed] [Google Scholar]
- 45.Abram PK, Brodeur J, Burte V, Boivin G. Parasitoid-induced host egg abortion: An underappreciated component of biological control services provided by egg parasitoids. Biol. Control. 2016;98:52–60. doi: 10.1016/j.biocontrol.2016.04.002. [DOI] [Google Scholar]
- 46.Kuske S, et al. Dispersal and persistence of mass released Trichogramma brassicae (Hymenoptera: Trichogrammatidae) in non-target habitats. Biol. Control. 2003;27:181–193. doi: 10.1016/S1049-9644(02)00191-3. [DOI] [Google Scholar]
- 47.Draz KA, Tabikha RM, El-Aw MA, Darwish HF. Impact of gamma radiation doses on sperm competitiveness, fecundity and morphometric characters of peach fruit fly Bactrocera zonata (Saunders) (Diptera: Tephiritidae) J. Radiat. Res. Appl. Sci. 2016;9:352–362. doi: 10.1016/j.jrras.2016.05.004. [DOI] [Google Scholar]
- 48.Ali A, Rashid MA, Huang QY, Lei C-L. Effect of UV-A radiation as an environmental stress on the development, longevity, and reproduction of the oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae) Environ. Sci. Pollut. Res. 2016;23:17002–17007. doi: 10.1007/s11356-016-6865-0. [DOI] [PubMed] [Google Scholar]
- 49.Liebhold AM, et al. Eradication of invading insect populations: From concepts to applications. Annu. Rev. Entomol. 2016;61:335–352. doi: 10.1146/annurev-ento-010715-023809. [DOI] [PubMed] [Google Scholar]
- 50.Tobin PC, et al. Determinants of successful arthropod eradication programs. Biol. Invasions. 2014;16:401–414. doi: 10.1007/s10530-013-0529-5. [DOI] [Google Scholar]
- 51.Pluess T, et al. Which factors affect the success or failure of eradication campaigns against alien species? PLoS ONE. 2012;7:e48157. doi: 10.1371/journal.pone.0048157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colunga-Garcia M, Magarey RA, Haack RA, Gage SH, Qi J. Enhancing early detection of exotic pests in agricultural and forest ecosystems using an urban-gradient framework. Ecol. Appl. 2010;20:303–310. doi: 10.1890/09-0193.1. [DOI] [PubMed] [Google Scholar]
- 53.Myers JH, Savoie A, van Randen E. Eradication and pest management. Annu. Rev. Entomol. 1998;43:471–491. doi: 10.1146/annurev.ento.43.1.471. [DOI] [PubMed] [Google Scholar]
- 54.Lance DR, McInnis DO. Biological basis of the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. Springer; 2021. pp. 69–94. [Google Scholar]
- 55.Godfray HCJ. Parasitoids: Behavioural and Evolutionary Ecology. Princeton University Press; 1994. Oviposition behaviour; pp. 83–150. [Google Scholar]
- 56.Ravuiwasa KT, Lu K-H, Shen T-C, Hwang S-Y. Effects of irradiation on Planococcus minor (Hemiptera: Pseudococcidae) J. Econ. Entomol. 2009;102:1774–1780. doi: 10.1603/029.102.0507. [DOI] [PubMed] [Google Scholar]
- 57.Bloem S, Bloem KA, Knight AL. Oviposition by sterile codling moths, Cydia pomonella (Lepidoptera: Tortricidae) and control of wild populations with combined releases of sterile moths and egg parasitoids. J. Entomol. Soc. 1998;95:99–109. [Google Scholar]
- 58.Hasaballah AI. Impact of gamma irradiation on the development and reproduction of Culex pipiens (Diptera; Culicidae) Int. J. Radiat. Biol. 2018;94:844–849. doi: 10.1080/09553002.2018.1490040. [DOI] [PubMed] [Google Scholar]
- 59.Sagarra LA, Vincent C, Stewart RK. Body size as an indicator of parasitoid quality in male and female Anagyrus kamali (Hymenoptera: Encyrtidae) Bull. Entomol. Res. 2001;91:363–367. doi: 10.1079/BER2001121. [DOI] [PubMed] [Google Scholar]
- 60.Bertin A, Pavinato VAC, Parra JRP. Effects of intraspecific hybridization on the fitness of the egg parasitoid Trichogramma galloi. Biocontrol. 2018;63:555–563. doi: 10.1007/s10526-018-9883-7. [DOI] [Google Scholar]
- 61.Bloem S, Bloem KA, Carpenter JE, Calkins CO. Inherited sterility in codling moth (Lepidoptera: Tortricidae): Effect of substerilizing doses of radiation on insect fecundity, fertility, and control. Ann. Entomol. Soc. Am. 1999;92:222–229. doi: 10.1093/aesa/92.2.222. [DOI] [Google Scholar]
- 62.Bloem S, Carpenter JE, Hofmeyr JH. Radiation biology and inherited sterility in false codling moth (Lepidoptera:Tortricidae) J. Econ. Entomol. 2003;96:1724–1731. doi: 10.1093/jee/96.6.1724. [DOI] [PubMed] [Google Scholar]
- 63.El-Kholy EMS. Biological and biochemical effects of vitamin ‘c’ on the normal and irradiated mediterranean fruit fly, Ceratitis capitata (wied) J. Radiat. Res. Appl. Sci. 2009;2:197–212. [Google Scholar]
- 64.Rempoulakis P, Castro R, Nemny-Lavy E, Nestel D. Effects of radiation on the fertility of the Ethiopian fruit fly, Dacus ciliatus. Entomol. Exp. Appl. 2015;155:117–122. doi: 10.1111/eea.12289. [DOI] [Google Scholar]
- 65.Würgler FE, Lütolf H-U. Radiosensitivity of oocytes of Drosophila I. sensitivity of class-a oocytes of triploid and diploid females. Int. J. Radiat. Biol. 1972;21:455–463. doi: 10.1080/09553007214550531. [DOI] [PubMed] [Google Scholar]
- 66.Field SA. Patch exploitation, patch-leaving and pre-emptive patch defence in the parasitoid wasp Trissolcus basalis (Insecta: Scelionidae) Ethology. 1998;104:323–338. doi: 10.1111/j.1439-0310.1998.tb00072.x. [DOI] [Google Scholar]
- 67.Sked SL, Calvin DD. Temporal synchrony between Macrocentrus cingulum (Hymenoptera: Braconidae) with its preferred host, Ostrinia nubilalis (Lepidoptera: Crambidae) Environ. Entomol. 2005;34:344–352. doi: 10.1603/0046-225X-34.2.344. [DOI] [Google Scholar]
- 68.Jiang N, Zhou G, Overholt WA, Muchugu E, Schulthess F. The temporal correlation and spatial synchrony in the stemborer and parasitoid system of Coast Kenya with climate effects. Ann. Soc. Entomol. Fr. 2006;42:381–387. doi: 10.1080/00379271.2006.10697470. [DOI] [Google Scholar]
- 69.Whitten M, Mahon R. Misconceptions and constraints. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. Springer; 2021. pp. 601–626. [Google Scholar]
- 70.Lee YJ, Ducoff HS. Radiation-enhanced resistance to oxygen: A possible relationship to radiation-enhanced longevity. Mech. Ageing Dev. 1984;27:101–109. doi: 10.1016/0047-6374(84)90087-3. [DOI] [PubMed] [Google Scholar]
- 71.Suckling DM, Wee SL, Pedley R. Assessing competitive fitness of irradiated painted apple moth Teia anartoides (Lepidoptera: Lymantriidae) N.Z. Plant Prot. 2004;57:171–176. [Google Scholar]
- 72.Wee SL, et al. Effects of substerilizing doses of gamma radiation on adult longevity and level of inherited sterility in Teia anartoides (Lepidoptera: Lymantriidae) J. Econ. Entomol. 2005;98:732–738. doi: 10.1603/0022-0493-98.3.732. [DOI] [PubMed] [Google Scholar]
- 73.Vilca Mallqui KS, Vieira JL, Guedes RNC, Gontijo LM. Azadirachtin-induced hormesis mediating shift in fecundity-longevity trade-off in the Mexican bean weevil (Chrysomelidae: Bruchinae) J. Econ. Entomol. 2014;107:860–866. doi: 10.1603/EC13526. [DOI] [PubMed] [Google Scholar]
- 74.Monroy Kuhn JM, Korb J. Editorial overview: Social insects: Aging and the re-shaping of the fecundity/longevity trade-off with sociality. Curr. Opin. Insect Sci. 2016;16:7–10. doi: 10.1016/j.cois.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Blacher P, Huggins TJ, Bourke AFG. Evolution of ageing, costs of reproduction and the fecundity–longevity trade-off in eusocial insects. Proc. R. Soc. B-Biol. Sci. 2017;284:20170380. doi: 10.1098/rspb.2017.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flatt T. Survival costs of reproduction in Drosophila. Exp. Gerontol. 2011;46:369–375. doi: 10.1016/j.exger.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 77.Vogt E, Nechols JR. The influence of host deprivation and host source on the reproductive biology and longevity of the squash bug egg parasitoid Gryon penssylvanicum (Ashmead) (Hymenoptera: Scelionidae) Biol. Control. 1993;3:148–154. doi: 10.1006/bcon.1993.1022. [DOI] [Google Scholar]
- 78.Ramesh B, Manickavasagam S. Tradeoff between longevity and fecundity in relation to host availability in a thelytokous oophagous parasitoid, Trichogramma brasiliensis Ashmead (Trichogrammatidae: Hymenoptera) Int. J. Trop. Insect Sci. 2003;23:207–210. doi: 10.1017/S1742758400023602. [DOI] [Google Scholar]
- 79.Gurr GM, Kvedaras OL. Synergizing biological control: scope for sterile insect technique, induced plant defences and cultural techniques to enhance natural enemy impact. Biol. Control. 2010;52:198–207. doi: 10.1016/j.biocontrol.2009.02.013. [DOI] [Google Scholar]
- 80.Knipling EF. Principles of Insect Parasitism Analyzed from New Perspectives: Practical Implications for Regulating Insect Populations by Biological Means. United States Department of Agriculture; 1992. [Google Scholar]
- 81.Orozco, D., Domínguez, J., Reyes, J., Villaseñor, A. & Gutiérrez, J. M. SIT and biological control of Anastrepha fruit flies in Mexico. in Proceedings of the 6th International Fruit Fly Symposium 245–249 (Isteg Scientific Publications, 2002).
- 82.Wong TTY, Ramadan MM, Herr JC, McInnis DO. Suppression of a Mediterranean fruit fly (Diptera: Tephritidae) population with concurrent parasitoid and sterile fly releases in Kula, Maui, Hawaii. J. Econ. Entomol. 1992;85:1671–1681. doi: 10.1093/jee/85.5.1671. [DOI] [Google Scholar]
- 83.Cossentine JE, Jensen LBM. Releases of Trichogramma platneri (Hymenoptera: Trichogrammatidae) in apple orchards under a sterile codling moth release program. Biol. Control. 2000;18:179–186. doi: 10.1006/bcon.2000.0828. [DOI] [Google Scholar]
- 84.Carpenter JE, Bloem S, Hofmeyr JH. Acceptability and suitability of eggs of false codling moth (Lepidoptera: Tortricidae) from irradiated parents to parasitism by Trichogrammatoidea cryptophlebiae (Hymenoptera: Trichogrammatidae) Biol. Control. 2004;30:351–359. doi: 10.1016/j.biocontrol.2003.10.006. [DOI] [Google Scholar]
- 85.Carpenter JE, Bloem S, Hofmeyr JH. Area-wide control tactics for the false codling moth Thaumatotibia leucotreta in South Africa: a potential invasive species. In: Vreysen MJB, Robinson AS, Hendrichs J, editors. Area-Wide Control of Insect Pests. Springer; 2007. pp. 351–359. [Google Scholar]
- 86.Faúndez EI, Rider DA. The brown marmorated stink bug Halyomorpha halys (Stål, 1855) (Heteroptera: Pentatomidae) in Chile. Arquivos Entomol. 2017;17:305–307. [Google Scholar]
- 87.Kriticos DJ, et al. The potential global distribution of the brown marmorated stink bug, Halyomorpha halys, a critical threat to plant biosecurity. J. Pest Sci. 2017;90:1033–1043. doi: 10.1007/s10340-017-0869-5. [DOI] [Google Scholar]
- 88.Kiwifruit Vine Health. KVH information sheet: BMSB risk update January 2019 (Kiwifruit Vine Health, 2019).
- 89.Vandervoet TF, Bellamy DE, Anderson D, MacLellan R. Trapping for early detection of the brown marmorated stink bug, Halyomorpha halys New Zealand. N.Z. Plant Prot. 2019;72:36–43. [Google Scholar]
- 90.Laing, K., Belton, D. & Taylor, J. Decision on releasing Trissolcus japonicus from containment. (Environmental Protection Authority, 2018).
- 91.Charles JG, et al. Experimental assessment of the biosafety of Trissolcus japonicus in New Zealand, prior to the anticipated arrival of the invasive pest Halyomorpha halys. Biocontrol. 2019;64:367–379. doi: 10.1007/s10526-019-09949-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are uploaded to Researchgate ([link to URL when uploaded]), and are available from the corresponding author on reasonable request.