Abstract
Liver fibrosis is a significant health problem that can cause serious illness and death. Unfortunately, a standard treatment for liver fibrosis has not been approved yet due to its complicated pathogenesis. The current study aimed at assessing the anti-fibrotic effect of taurine against thioacetamide induced liver fibrosis in rats through the modulation of toll like receptor 4/nuclear factor kappa B signaling pathway. Both concomitant and late taurine treatment (100 mg/kg, IP, daily) significantly reduced the rise in serum ALT and AST activities and significantly reversed the decrease in serum albumin and total protein. These results were confirmed by histopathological examinations and immunehistochemical inspection of α-SMA, caspase-3 and NF-κB. The antioxidant potential of taurine was verified by a marked increase of GSH content and a reduction of MDA level in liver tissue. The anti-fibrotic effects of taurine were evaluated by investigating the expression of TLR4, NF-κB. The protein levels of IL-6, LPS, MyD88, MD2, CD14, TGF-β1 and TNF-α were determined. Docking studies were carried out to understand how taurine interacts inside TLR4-MD2 complex and it showed good binding with the hydrophobic binding site of MD2. We concluded that the anti-fibrotic effect of taurine was attributable to the modulation of the TLR4/NF-κB signaling.
Subject terms: Biochemistry, Drug discovery, Gastroenterology
Introduction
Liver fibrosis is a major health condition that can cause serious disease and death1. Liver fibrosis is initiated by the activation of immune cells that secrete cytokines and growth factors, leading to the activation of hepatic stellate cells (HSCs) and subsequent collagen production. Consequently, ECM accumulates in the liver and collagenolysis process continues2–5, eventually leading to cirrhosis with its complications of cancer and death6,7.
There is no FDA approved drug for liver fibrosis, although considerable efforts have been exerted to defeat liver fibrosis through the inhibition of common crucial pathways of the fibrogenesis process8. Many trials have been made with the aim of inhibiting the activation of hepatic stellate and Kupffer cells, because they act to propagate oxidative and inflammatory responses, and subsequently to stimulate many fibrogenic mediators9. When remedies are used for long periods, it is essential to protect against the development of fibrotic complications that are associated with hepatic injury. Many previous reports have shown that liver fibrosis can be reversed under certain conditions, with a restoration of near normal architecture10,11. It is hoped that therapeutic approaches to liver fibrosis and the management of cirrhosis could be developed by understanding the etiology of liver fibrosis and developing improved diagnostic tools.
Taurine, 2-aminoethane sulfonic acid, is the most abundant free amino acid in most animal tissues. Taurine is present in our daily foods, and also in anti-fatigue energy soft drinks and energizers for athletes12–14. It has a crucial role in many biological processes 15; stabilizing biological membranes and regulating calcium flux. It also has antioxidant and anti-inflammatory properties achieved by regulating the release of pro-inflammatory cytokines16–18. Many reports suggest a major role for taurine in the innate immune response, and recommend its use in the prevention and treatment of many infections and chronic inflammatory diseases19–21. Taurine has relatively low toxicity regarding the other active ingredients22,23.
Previous reports have shown that hepatic taurine levels are reduced in some liver diseases24. Taurine is released from pre-central hepatocytes, and therefore the plasma concentration of taurine is used as a potential marker of hepatic damage24,25. Taurine serves as a hepato-protective agent to prevent liver injury26, and has a major effect on the central nervous system (CNS)27,28. It may act as an osmoregulator and decrease oxidative stress in the CNS29–32.
Taurine is either ingested directly from foodstuffs or synthesized endogenously from cysteine and methionine. According to the European Food Safety Authority, a daily intake of 3–6 g of taurine showed no side effects. Absorbed taurine is accompanied by few plasma proteins, and reaches very high concentrations in tissues, in the mM range, and very low levels in the plasma (< µM). It therefore has a considerable concentration gradient across the cell membrane33. Previous studies have shown that exogenous taurine can prevent liver injury and the accumulation of ECM in the damaged liver, reversing liver fibrosis34–37. Taurine transporter knockout mice showed chronic liver disease accompanied by fibrosis, inflammation, and apoptosis of hepatocytes38. Taurine may also reduce oxidative stress and constrain the production of inflammatory and fibrogenic growth factors, and inhibit the activation of stellate cells36,37,39. Previous studies have explored the effect of taurine supplementation on the pathogenesis of hepatic fibrosis induced by infection with S. japonicum. Many clinical trials investigated the use of nutritional supplements and energy drinks containing taurine and to identify its therapeutic effects40,41.
Toll like receptors (TLRs) play a crucial role in triggering inflammatory responses against structural components of viruses, bacteria, and fungi42–44. The TLR cytoplasmic domain, Toll/IL-1-Receptor (TIR), interacts with other inflammatory markers that initiate signaling, and prompts the expression of many immune response genes by stimulating NF-κB 45,46. Taurine has been studied for its NF-κB inhibitory effect and subsequent regulation of inflammatory processes in vivo47. In our study, we explored the anti-fibrotic potential of taurine against liver fibrosis in rats through the modulation of the toll like receptor 4/nuclear factor kappa B signaling pathway.
Results
Biochemical assays
Leakage of the hepatic enzymes alanine transaminase (ALT) and aspartate aminotransferase (AST) from liver cells into the blood after the administration of thioacetamide (TAA) was identified by a significant elevation of their activities. There was a decrease in albumin and total protein concentrations in the TAA group compared to the control group. Concomitant (Con TAA + Tau) and late taurine (late TAA + Tau) treatment significantly decreased TAA induced elevation in the activity of AST and ALT, and reversed the decrease in total protein and albumin concentrations in the TAA group. The activity of AST was significantly decreased in the Con TAA + Tau groups compared to the late TAA + Tau groups, while total protein levels increased significantly in the Con TAA + Tau groups compared to the late TAA + Tau groups, as shown in Table 1.
Table 1.
Liver function analysis.
| Control group | Tau group | TAA group | Con TAA + Tau group | Late TAA + Tau group | |
|---|---|---|---|---|---|
| ALT (IU/L) | 45.1 ± 4.4 | 49.33 ± 3.3 | 113.4 ± 7.6* | 47.4 ± 3.3# | 56.1 ± 9.7# |
| AST (IU/L) | 102.8 ± 6.8 | 108.8 ± 10.8 | 226.8 ± 14.4* | 105 ± 7.4# | 131 ± 10.6#$ |
| Albumin (g/dl) | 4.263 ± 0.07 | 4.03 ± 0.09 | 2.99 ± 0.14* | 3.96 ± 0.08# | 3.73 ± 0.16# |
| Total protein (g/dl) | 7.93 ± 0.11 | 7.213 ± 0.23 | 6.80 ± 0.09* | 7.16 ± 0.12# | 7.035 ± 0.03#$ |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; Tau, taurine; TAA, thioacetamide.
*Significance against control group (P < 0.05), #Significance against TAA group (P < 0.05), $Significance against Con TAA + Tau group (P < 0.05).
A significant rise in malondialdehyde (MDA) levels was observed in the TAA group compared to the control group. This rise was accompanied by a significant depletion in hepatic GSH content, compared with the control group. Concomitant and late taurine treatment produced significant reductions in MDA level and a marked increase in hepatic GSH content compared to the TAA group. The Con TAA + Tau group had significantly decreased MDA content, and significantly increased GSH content compared to the late TAA + Tau group, as shown in Fig. 1.
Figure 1.
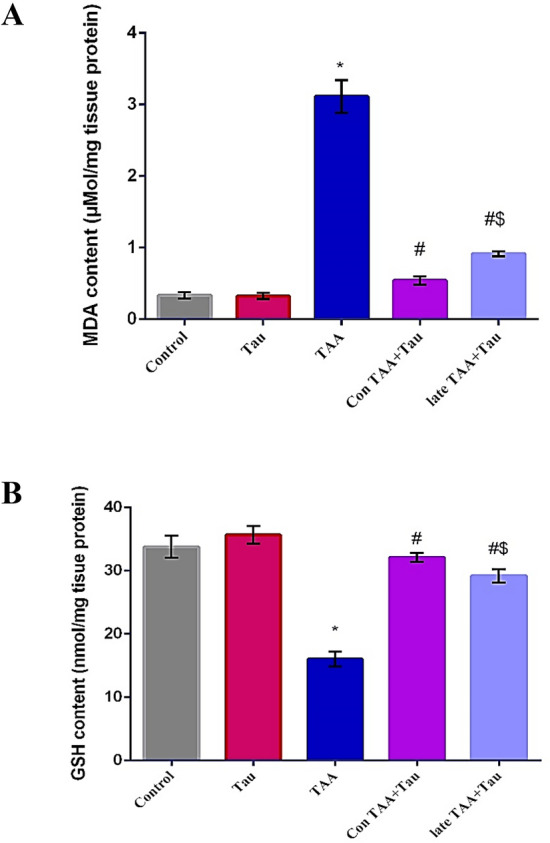
(A) MDA content in control, Tau, TAA, Con TAA + Tau and late TAA + Tau groups. (B) GSH content control, Tau, TAA, Con TAA + Tau and late TAA + Tau groups Values were expressed as mean ± SE. Number of rats in each group was 6. MDA: malondialdehyde, GSH: reduced glutathione, Tau: taurine, TAA: thioacetamide *: Significance against control group (P < 0.05). # Significance against TAA group (P < 0.05). $: Significance against Con TAA + Tau group (P < 0.05).
Protein level and gene expression
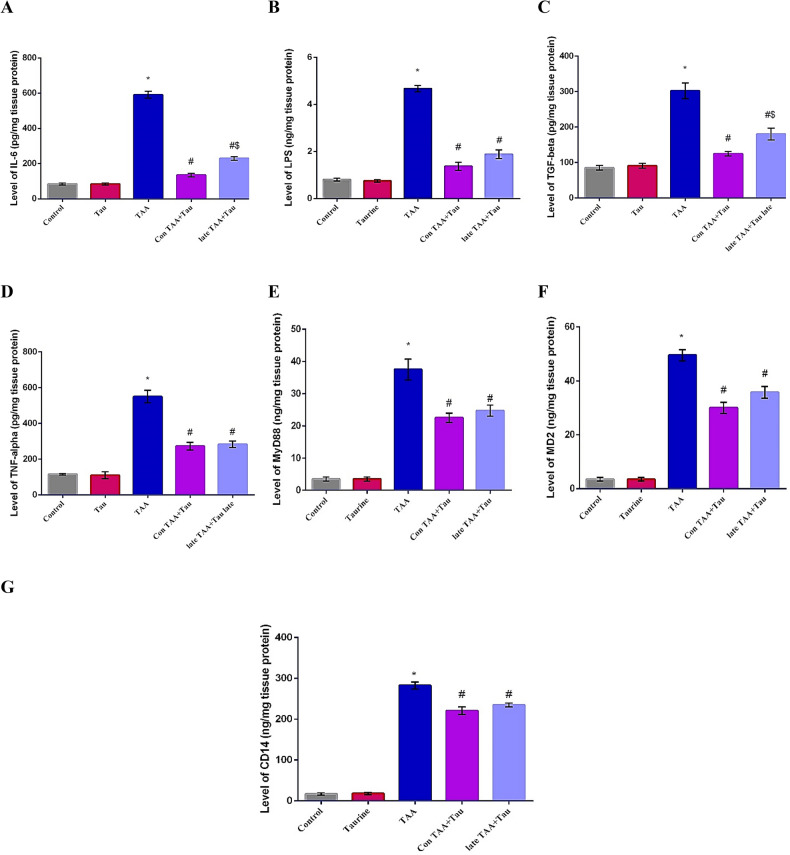
In the present work, the levels of IL-6, LPS, MyD88, MD2, CD14, TGF-β1 and TNFα were measured in liver homogenate of rats treated with TAA, some of which were treated with taurine either concomitantly or later groups. The relative expressions of hepatic TLR-4 and NFκB were evaluated using qRT-PCR. Significant increases in the levels of IL-6, LPS, MyD88, MD2, CD14, TGF-β1, and TNF-α were observed in the TAA group compared to the control group. Both taurine treated groups showed decreases in IL-6, LPS, MyD88, MD2, CD14, TGF-β1, and TNF-α levels compared to the TAA group. IL-6 and TGF-β1 levels decreased significantly in the Con TAA + Tau group compared to the late TAA + Tau group, as shown in Fig. 2.
Figure 2.
(A) Level of IL-6 in control, Tau, TAA, Con TAA + Tau and late TAA + Tau groups. (B) Level of LPS in control, Tau, TAA, Con TAA + Tau and late TAA + Tau groups. (C) Level of IL-6, TGF-beta in control, Tau, TAA, Con TAA + Tau and late TAA + Tau groups. (D) Level of TNF-alpha in control, Tau, TAA, Con TAA + Tau and late TAA + Tau groups. (E) Level of MyD88 in control, Tau, TAA, Con TAA + Tau and late TAA + Tau groups. (F) Level of MD2 in control, Tau, TAA, Con TAA + Tau and late TAA + Tau groups. (G) Level of CD14 in control, Tau, TAA, Con TAA + Tau and late TAA + Tau groups. Values were expressed as mean ± SE. Number of rats in each group was 6. IL-6: interleukin-6, LPS: lipopolysaccharides, TGF-beta: transforming growth factor-beta, TNF-alpha: tumor necrosis factor-alpha, CD14: Cluster of Differentiation 14, MyD88: Myeloid Differentiation primary response protein, MD2: myeloid differentiation protein 2. Tau: taurine, TAA: thioacetamide *: Significance against control group (P < 0.05), # Significance against TAA group (P < 0.05), $: Significance against Con TAA + Tau group (P < 0.05).
The levels of TLR-4 and NFκB were significantly upregulated in the TAA group compared to the control group. A significant downregulation of TLR-4 and NFκB levels was observed in both taurine treated groups in comparison with the TAA group. The relative expression of TLR-4 decreased significantly in the Con TAA + Tau group compared to the late TAA + Tau group, as shown in Fig. 3.
Figure 3.
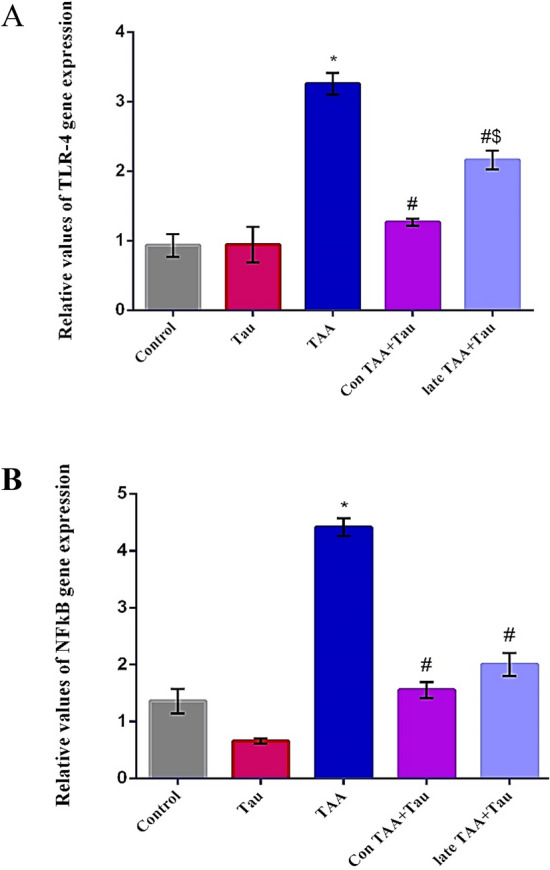
(A) Relative hepatic TLR-4 gene expression in control, Tau, TAA, Con TAA + Tau and late TAA + Tau groups. (B) Relative hepatic NFkB gene expression in control, Tau, TAA, Con TAA + Tau and late TAA + Tau groups. Values were expressed as mean ± SE. Number of rats in each group was 6. TLR-4: toll like receptor 4, NF-κB: nuclear factor-κB, Tau: taurine, TAA: thioacetamide. *: Significance against control group (P < 0.05), #: Significance against TAA group (P < 0.05), $ Significance against Con TAA + Tau group (P < 0.05).
Histological and immunohistochemical examination
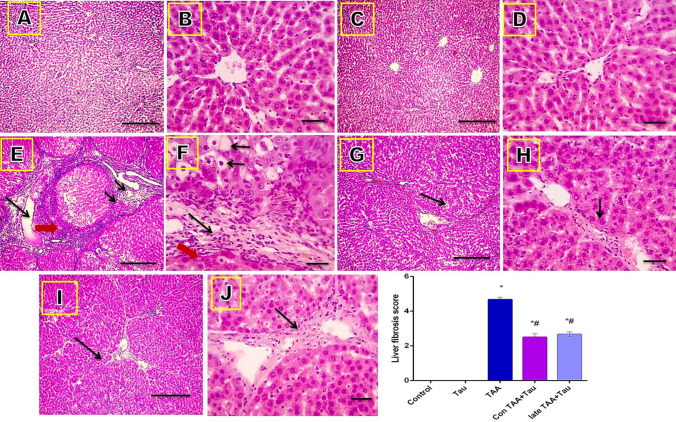
Microscope images of hematoxylin and eosin (H&E)-stained liver sections showed normal architecture of the hepatic lobules in the control and Tau groups (score 0). Liver sections of the TAA group showed disarrangement of the hepatic cords, central veins, and portal areas, and ballooning degeneration (short arrows), focal necrosis (red arrow), fibrous expansion of portal areas with infiltration of leukocytic cells (long arrows), and marked portal bridging, as well as portal-to-central bridging (score 5). Liver sections from the Con TAA + Tau and late TAA + Tau groups showed hepatic fibrosis (score 3) (long arrows), as shown in Fig. 4.
Figure 4.
Microscopic pictures of H&E stained liver sections. (A) control group. (B) Tau group. (C) TAA group. (D): Con TAA + Tau group. (E) late TAA + Tau group. (F) liver fibrosis score. (A,C,E,G,I) X: 100 bar 100. (B,D,F,H,J) X: 400 bar 50. *: Significance against control group (P < 0.05), #: Significance against TAA group (P < 0.05).
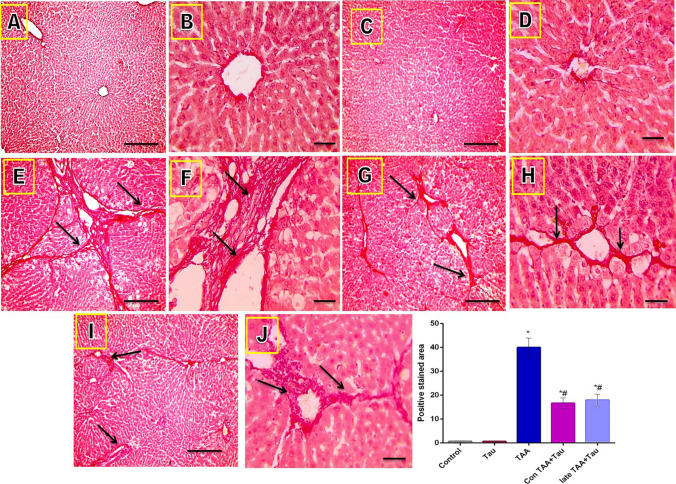
Microscope images of Sirius red stained liver sections showed significant collagens deposition in TAA induced liver fibrosis, which was significantly reduced by taurine treatment, as evidenced by the decrease of the positively stained area (long arrows point to fibrous tissue), as shown in Fig. 5.
Figure 5.
Microscopic pictures of Sirius red stained liver sections. (A) control group. (B): Tau group. (C) TAA group. (D) Con TAA + Tau group. (E) late TAA + Tau group. (F) positive stained area. (A,C,E.G,I) X: 100 bar 100. (B, D,F,H,J) X: 400 bar 50. *: Significance against control group (P < 0.05), #: Significance against TAA group (P < 0.05).
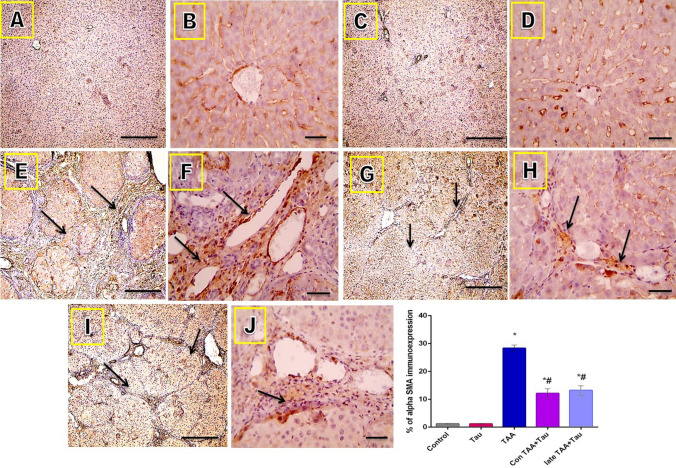
Microscope images of liver sections immunostained against α-SMA showed positive expression only in the smooth muscle layers surrounding the blood vessels in the control and Tau groups. The expression of α-SMA protein increased, as indicated by brown staining in the fibrotic areas in the TAA group. The positive staining was decreased in the Con TAA + Tau and late TAA + Tau groups (long arrows point to positively stained fibrous tissue). Immunohistochemistry (IHC) was counterstained with Mayer's hematoxylin, as shown in Fig. 6.
Figure 6.
Microscopic pictures of immunostained liver sections against α-SMA. (A) control group. (B) Tau group. (C) TAA group. (D) Con TAA + Tau group. (E) late TAA + Tau group. (F) % of α-SMA immunoexpression. α-SMA: alpha smooth muscle actin. (A,C,E,G,I): X: 100 bar 100., (B,D,F,H,J) X: 400 bar 50. *: Significance against control group (P < 0.05), #: Significance against TAA group (P < 0.05).
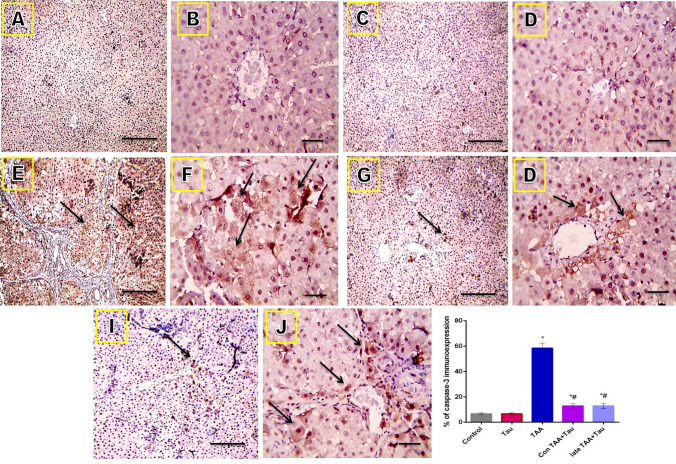
Microscope images of liver sections immunostained against caspase-3 showed very mild staining in the control and Tau groups, strong positive brown staining in the TAA group, and mild positive staining in the Con TAA + Tau and late TAA + Tau groups (long arrows point to positively stained hepatocytes). IHC was counterstained with Mayer's hematoxylin, as shown in Fig. 7.
Figure 7.
Microscopic pictures of immunostained liver sections against caspase-3. (A) control group. (B) Tau group. (C) TAA group. (D) Con TAA + Tau group. (E) late TAA + Tau group. (F) % of caspase-3 immunoexpression. (A,C,E.G,I) X: 100 bar 100. (B,D,F,H,J) X: 400 bar 50. *: Significance against control group (P < 0.05), #: Significance against TAA group (P < 0.05).
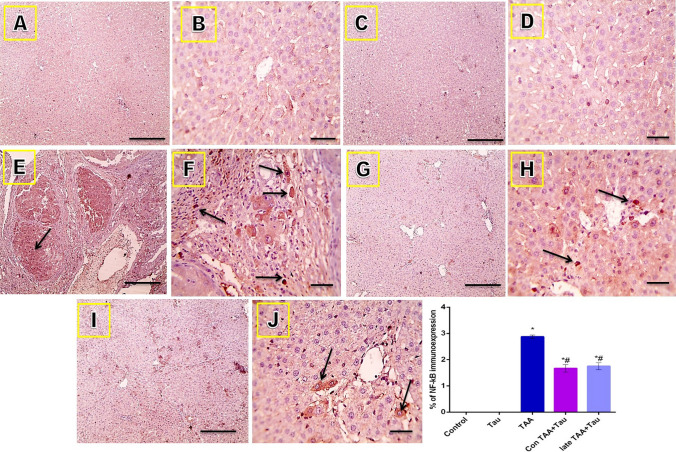
Microscope images of hepatic sections immunostained against NF-κB showed negative staining in the control and Tau groups and strong positive brown staining (nuclear reaction) in the TAA group. The positive brown reaction was markedly decreased in the Con TAA + Tau and late TAA + Tau groups (black arrows point to positive cells). IHC was counterstained with Mayer's hematoxylin, as shown in Fig. 8.
Figure 8.
Microscopic pictures of immunostained liver sections against nuclear factor kappa B. (A) control group. (B): Tau group. (C) TAA group. (D) Con TAA + Tau group. (E) late TAA + Tau group. (F) % of NF-kB immunoexpression. (A,C,E.G,I) X: 100 bar 100. (B,D,F,H,J): X: 400 bar 50. *: Significance against control group (P < 0.05), #: Significance against TAA group (P < 0.05).
Molecular docking study
Analysis of the docking results showed that taurine could be fitted into the hydrophobic binding pocket of MD2 within less than 0.3 Å RMSD where the docking score of best docked pose was 7.63 kcal mol−1, as shown in Fig. 9A. In order to have good results we performed a series of docking simulations using TLR4/MD2 complex (pdb code: 4G8A). We selected a large docking search area that contained the entire LPS binding pocket in MD2 which has a β-cup fold structure composed of two antiparallel β-sheets separated from each other forming a hydrophobic pocket. Looking closely at amino acid interactions between taurine and the pocket site, we found that the ligand was in contact with the phenyl rings of Tyr131 and Phe126, which move into this position upon binding. The polar interactions with the two amino acids in the binding site Tyr131 and Phe126 were at bond lengths of 2.82 and 3.53 A°, respectively. We also found hydrogen bond interaction with the basic amino acid Lys125 and another polar interaction with Ser127 of the binding site of MD2, as shown in Fig. 9B,C.
Figure 9.
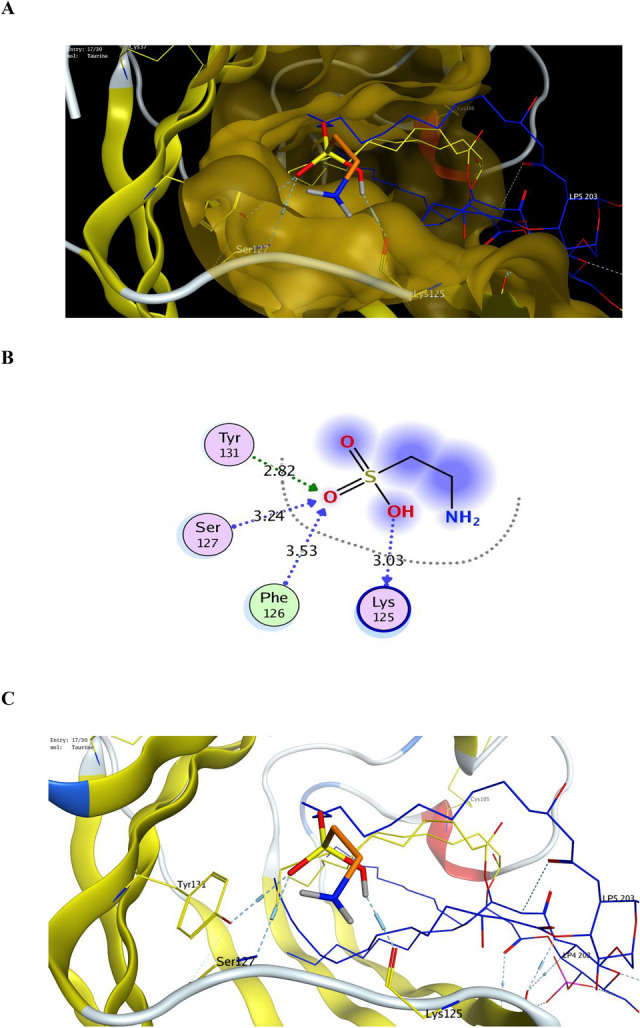
(A) Binding of Taurine (orange) inside the binding site of TLR4-MD2 complex (PDB code: 4G8A), (B) 2D binding mode of Taurine with binding site of TLR4-MD2 complex (PDB code: 4G8A), (C) 3D binding mode of Taurine with binding site of TLR4-MD2 complex (PDB code: 4G8A).
Discussion
The secretion of inflammatory cytokines from Kupffer cells was accompanied by the progression of liver fibrosis, which has an essential role in the pathogenesis of several liver diseases48. Liver fibrosis is characterized by alterations of the hepatic ECM. After the activation of quiescent HSCs they are differentiated into myofibroblast-like cells with increased proliferation, accumulation of ECM, and expression of α-SMA. Therefore, collagen accumulation in the liver is considered to be a mark of fibrosis49. α-SMA is the most widely used marker of activation of HSCs50.
Thioacetamide metabolism produces a hepatotoxin metabolite that triggers the overproduction of reactive oxygen species (ROS), leading to liver fibrosis and cirrhosis, and ending with HCC51,52. This metabolite is produced by cytochrome2E1 (CYP2E1)53, the principal P450 for the metabolism of many xenobiotics, such as TAA54–61. Excess formation of superoxide free radicals leads to an increase in lipid peroxidation, and consequent formation of MDA. MDA targets DNA and causes mutations. GSH usually counteracts the deleterious effects of oxidative stress. GSH also detoxifies many toxic compounds52. Our study found that concomitant and late treatment with 100 mg/kg taurine significantly conserved hepatocyte integrity, as indicated by the reduced serum activities of ALT, AST, and hepatic MDA. Taurine reversed the reduction of serum albumin, total protein concentration, and GSH. Histological results confirmed the protective effect of taurine against hepatic injury and fibrosis induced by TAA.
In this study, there was a significant increase in the levels of IL-6, LPS, MyD88, MD2, CD14, TGF-β1, and TNF-α in the TAA group compared to the control group. Previous reports have shown that collagen accumulation and the activation of fibroblasts are closely related to epidural fibrosis62, and that alpha-smooth muscle actin (α-SMA) plays an important role in fibrotic pathogenesis scars63,64. The overproduction of TGF-β is considered to be one of the underlying mechanisms by which fibrosis occurs65,66. TGF-β activates specific receptors, TGF-βRI and TGF-βRII, which leads to the activation of Smad2 and Smad3 phosphorylation, and then the formation of a complex with Smad4. The SMAD complex translocates into the nucleus and activates the transcription of collagens67, as confirmed by histological examination with H&E and Sirius red, and α-SMA immune-expression.
Elevated collagen expression stimulates the transdifferentiation of myofibroblasts, which secrete ECM that hinders the cellular capacity for ECM degradation, with the net result being fibrosis68. The sustained signaling by the TGF-β1 cascade promotes the proliferation of HSCs, which also produce ECM, resulting in fibrous scars69. TGF-β1 induces the differentiation of myofibroblasts through the PI3K-Akt pathway, resulting in liver fibrosis70.
The hepato-protective role of taurine was clarified by the reduction of either CYP2E1 metabolic activity or oxidative stress caused by hepatotoxin. The antioxidant and anti-inflammatory effects of taurine have previously been explained by the diminution of lipid peroxidation and neutrophil adhesion12–14 a suggestion that was confirmed, in the present study, by the significant decrease observed in IL-6, LPS, MyD88, MD2, CD14, TGF-β1 and TNF-α levels in the taurine treated groups compared to the TAA group.
TLRs include a highly conserved family of receptors that recognize pathogens and facilitate the host detection of microbial infection. Recent studies have indicated that TLR4 may be linked to inflammatory and fibrogenic response71–73. Previous studies indicated an important role for TLR4 in several signaling pathways involved in liver fibrogenesis. TLR4 polymorphism is strongly associated with fibrosis insult74. Signaling of TLRs is initiated when their ectodomains engage and complex with their respective ligands, and consequently enhances the recruitment of TLR adaptors, mainly through interaction of the TLRs with these adaptors. Known TLR signaling adaptors are MyD88, TRIF, TRAM, TIRAP, SARM, and BCAP75.
Inflammatory diseases, liver diseases and subsequent ROS overproduction can be the result of LPS induced hepatic injury by the resuscitation of dormant organisms which shed inflammatory molecules76,77. This process occurs by disruption of intestinal barrier and the release of a large amount of cell wall components, such as LPS, by intestinal flora passing the systemic and portal circulation and activating the release of inflammatory cytokines which further injure the intestinal mucosa78. The reduction of ROS signaling by the administration of antioxidant and anti-inflammatory agents such as taurine is beneficial, since it relieves such damage as indicated by previous studies79.
TLR4 is expressed in liver cells which are constantly confronted with gut-derived LPS. Normally, liver has relatively low expression of TLR4 and its adaptor molecules, MD2 and MyD88, and negatively regulates TLR4 signaling, a process known as “liver tolerance”. A breakdown of liver tolerance due to increased exposure and/or sensitivity of TLR4 to LPS may induce an inappropriate immune response72. Initially, the intestinal barrier is disrupted and a large amount of LPS is released by intestinal flora passing into the circulation80.
LPS induces excessive release of pro-inflammatory cytokines, including TNF-α and IL-6, and the production of ROS, by binding with toll like receptor 4 on the surface of Kupffer cells75. According to previous studies, the response of hepatocytes to LPS is complex, and requires cell–cell interaction between hepatocytes, Kupffer cells, sinusoidal endothelial cells, and stellate cells. The hepatocytes were assumed to have a direct response to LPS, similar to that of monocytes and macrophages. Hepatocytes have a rapid response, and therefore bypass the time needed for Kupffer cells, which are also considered to be highly responsive to LPS, to synthesize cytokines such as TNF-α, IL-1β, IL-6, IL-12, IL-18, IL-10, in addition to nitric oxide and oxygen radicals81. Because of the unique anatomical link between the liver and intestines, Kupffer cells are the first cells to encounter LPS and accordingly, Kupffer cells express TLR472. The complexity of Kupffer cell participation in hepatic toxicity is becoming more and more apparent, as some hepatic injury has been attributed to the deleterious effects of activated Kupffer cells82. Although there is no specific marker to identify hepatic Kupffer cells, Kupffer cells can be identified by their expression of CD14, CD16, CD68, CD68, and CD1683. The expression of CD14 is considered to be a marker of activation of Kuppfer cells in the liver, which is thought to cause inflammation and fibrosis84. Upregulation of CD14 in Kupffer cells has been implicated in the pathogenesis of several forms of liver injury. TNF-α production by Kupffer cells, a marker for Kupffer cell activation, increases in a dose-dependent manner with increasing concentrations of LPS. CD14 knockout mice and CD14 antibodies show significantly decreased production of TNF-α from Kupffer cells in response to LPS85.
The degree of liver injury correlates with the level of LPS, and with the level of Kupffer cell CD14 expression. Also, CD14 expression on Kupffer cells is low in normal human liver, but increases in different inflammatory liver diseases86.
Therefore, the secretion of chemokines is upregulated, and HSCs are sensitized to the action of TGF-β73. The TLR/NF-κB signaling pathway is the main pathway involved in the synthesis and secretion of inflammatory mediators during inflammation, in which TNF-α is the principal factor87. TNF-α regulates other inflammatory mediators, such as IL-1β, IL-6, and IL-816,88.
It has been reported that taurine decreases TNF-α, IL-6, and peroxide levels and, thus fibrogenic mediators and collagen accumulation were reduced during fibrogenesis36. Taurine reduces the elevation of TNF-α and modulates the inflammatory response through the TLRs/NF-κB signaling pathway89.
We used RT-PCR to assess the expression of mRNA for some factors involved in the LPS induced signaling pathway, such as TLR4 and NF-κB, because they are considered to be the main factors involved in these signaling pathways80. Taurine reduced the level of mRNA for TLR4 and NF-κB, consequently blocking the activity of the pathway, and decreasing the synthesis and release of inflammatory cytokines80.
Previous studies have shown that TLRs can regulate immune receptors and modulate the inflammatory process90. The stimulation of TLR4 by LPS is a complex process, which involves LBP, CD14, and MD2. LBP, a soluble protein, extracts LPS from the bacterial membrane and shuttles it to CD1472. CD14 is considered to be a receptor of LPS on the membrane surface of KC, which mediate LPS signal transduction80.
The CD14-LBP-LPS complex stimulates TLR4, a specific receptor of LPS, to trigger a KC signaling pathway involving the phosphorylation of IκB proteins, and subsequently activates the translocation of NF-κB. According to Wu et al., taurine can inhibit the LPS-KC signal pathway by downregulating the expression of CD14 and its combination with LPS80. This conclusion was supported by the results of the present study, as there was a significant decrease in TLR-4 and NFκB gene expression and IL-6, LPS, MyD88, MD2, CD14, TGF-β1, and TNF-α levels in both taurine treated groups compared to the TAA group. There was also a significant decrease in the NF-κB immune-positive brown staining in the taurine treated groups compared to the TAA group.
CD14 transfers LPS to MD291 which is considered to be a secondary associated protein which forms a complex with CD14-LPS. It is also considered as a co-receptor which physically associates with TLR4, and binds the lipid moiety of LPS through the central hydrophobic pocket92. Binding of LPS to MD2 induces a conformational change in MD2, thus achieving TLR4 homo-dimerization and signaling72, which in turn may trigger the KC signaling pathway. Consequently, NF-κB translocation is activated, leading to overexpression of pro-inflammatory cytokines80, including TNF-α, which provoke the release of ROS91. Therefore, docking studies were conducted to predict the binding mode of a ligand to its receptor, and also to explore the binding mode of taurine, to investigate the possible interactions, and consequently to understand the binding mode and key active site interactions.
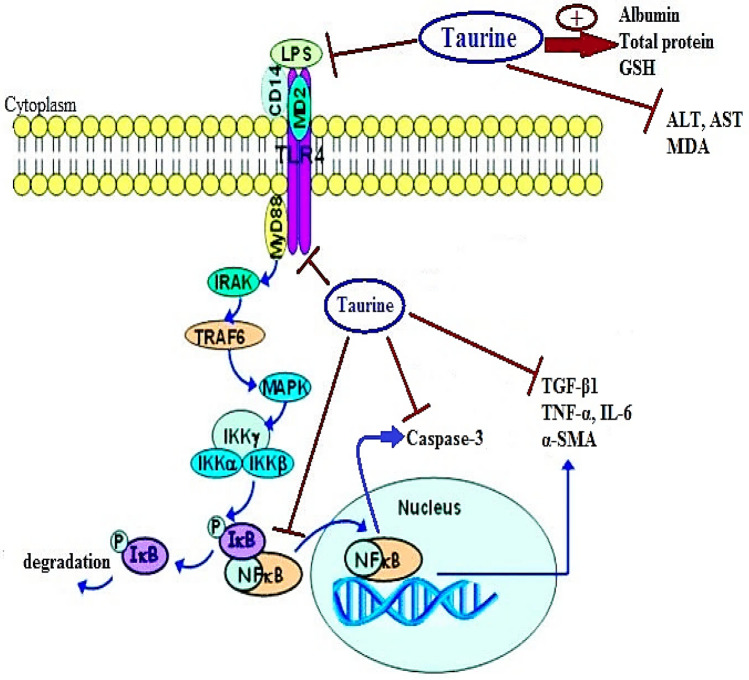
The damaging effects lipopolysaccharides take place via interactions with LBP and binding to TLR4 through CD14 and MD293. TLR4 activates both the MyD88- and TRIF-dependent (MyD88-independent pathway) signaling pathways. Early activation of NFκB is strongly related to the MyD88-dependent pathway, while the TRIF-dependent pathway is involved in late phase activation of NFκB. Activation of both pathways is important for the induction of downstream pro-inflammatory cytokine secretion in response to TLR4 stimulation75. MyD88 recruits IRAK4, TRAF6, and TAK1. Subsequently, degradation of the IκB kinase complex subunits occurs, and NF-κB is released. NF-κB is then translocated into the nucleus94, and as a result the transcription of IL-6, IL-12, and TNFα is induced95. Taurine administration could inhibit the binding and the expression of TLR4 to the CD14-LBP-LPS complex, thus decreasing the synthesis and release of cytokines that injure the hepatocytes80. It appears that taurine can alleviate liver injury and inhibit KC activation resulting from overproduction of LPS, TLR4, and NF-κB. The suggested mechanism for the effect of taurine on the TLR4/NF-κB signaling pathway is summarized in Fig. 10.
Figure 10.
Taurine modulates toll like receptors 4/nuclear factor kappa B signaling pathway. GSH: reduced glutathione, ALT: alanine aminotransferase, AST: aspartate aminotransferase, MDA: malondialdehyde, LPS: lipopolysaccharides, TLR4: toll like receptor 4, MD2: myeloid differentiation protein 2, MyD88: Myeloid Differentiation primary response protein, CD14: cluster of differentiation 14, IRAK: IL-1 receptor associated-kinase, TRAF6: tumor necrosis factor receptor-associated factor-6, MAPK: mitogen-activated protein kinase, IKK: I kappa B kinase complex, NF-kB: nuclear factor-kB, IL-6: interleukin-6, TGF-β1: transforming growth factor β1, TNF-α: tumor necrosis factor-α, α-SMA: alpha smooth muscle actin.
NF-κB, a key regulator of transcription of inflammatory genes96, functions as a transcription factor after its translocation to the nucleus42,97. The activation of NF-κB stimulates inflammatory responses, cell growth, and survival, during carcinogenesis98,99. Therefore, targeting NF-κB and its related pathways is considered to be a promising approach for the management of liver fibrosis100.
A previous study showed that taurine may modulate inflammatory injury induced by S. uberis in mammary tissue, through TLR-2 and TLR-4. Taurine treatment also markedly repressed NF-κB DNA binding activity89. These findings were supported by the results of the present study, as there was a significant decrease in the o NF-κB immune-positive brown staining and relative gene expression in the taurine treated groups compared to the TAA group. Another study, conducted in rheumatoid arthritis patients, found NF-κB activity and DNA binding were reduced16. TLR4 deficient mice resist hepatic fibrosis in multiple models73, TNF-α stimulates the proliferation of HSCs, and consequently inflammatory signaling101.
The exploration of the mechanisms of apoptosis has highlighted the involvement of NF-κB signaling in the regulation of apoptosis. However, apoptosis is dependent on caspase activation and the cleavage of specific death substrates within the cell, and therefore apoptosis may be viewed as a caspase-mediated form of cell death. Activated HSCs showed high levels of NF-κB and NF-κB-regulated anti-apoptotic proteins, such as IL-6102. There are two major pathways that link apoptosis: intrinsic (mitochondrial) and extrinsic. The extrinsic pathway of apoptosis is primarily initiated through caspase-8, which activates the downstream effector caspases-3, leading to apoptosis103. The intrinsic apoptotic pathway involves the disruption of the mitochondrial membrane and the release of apoptotic factors. Caspase-9 activation promotes the production of caspase-3, and consequently the morphological and biochemical changes associated with apoptosis104. In the present study, there was a significant decrease in caspases-3 immunostaining in the Con TAA + Tau and late TAA + Tau groups compared to the TAA group. The effect of taurine is based on its anti-oxidative and anti-apoptotic activities, which are consistent with previous murine models with respect to reduction in oxidation, apoptosis, and necrosis of liver cells105.
According to Marshall, et al.106, the TLR4/MD2 binding site is a known target for small molecule agonists activating TLR4. A recent study107 reported that the synthetic peptidomimetic ligand Neoseptin-3 causes dimerization of TLR4/MD2 and activation of TLR4 signaling, with a ligand-binding mode distinct from that of the native LPS molecule. The pocket containing Phe126 and stretching from residue 120 to residue 129 exhibits a backbone conformational change when bound to a ligand. Upon binding, the side chain of Phe126 flips, and is directed inside the binding pocket. Molecular docking studies showed good binding of taurine with the hydrophobic MD2 binding site, forming four polar interactions with the conservative amino acids Lys125, Phe126, Ser127, and Tyr131, which may be a reasonable interpretation for the results obtained from the biological experiments.
Methods
Chemicals
Thioacetamide 99% and taurine 99% pure were obtained from Sigma-Aldrich St. Louis, MO (USA).
Animal treatment outlines
Adult male Sprague Dawley rats weighing 180–200 g were obtained from the animal house of the Delta University Faculty of Pharmacy. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at the Delta University for Science and Technology (Approval Number: FPDV 9/2019). All experiments were carried out in accordance with relevant guidelines and regulations and in compliance with the ARRIVE guidelines108. Animals were retained under specific measured environmental settings: 22 ± 2 °C, 50 ± 10% humidity and a 12 h light/dark cycle, and were fed with standard pellets and free access to water was allowed.
Experimental design
After a one week adaptation period, 40 rats were assigned into five groups (n = 8) as follows: Control group (Control), Taurine group (Tau), TAA group, Concomitant Taurine (Con TAA + Tau); and Late Taurine (late TAA + Tau). The different groups received the doses shown in Table 2 as previously reported according to Yang et al. 2016109 and Furtado et al. 2012110 respectively.
Table 2.
Experimental animal design.
| Weeks | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control group | – | 0.2 ml PBS (10 mM, pH 7.4) IP, twice per week | ||||||||||
| Tau group | – | 100 mg/kg, IP, daily109 | ||||||||||
| TAA group | – | 200 mg/kg of thioacetamide (10 mM, pH 7.4 ) in PBS, IP, twice per week110 | ||||||||||
| (Con TAA + Tau) group | – | Rats were administered taurine (same dose as Tau group) two hours after administration of thioacetamide (same dose as TAA group) | ||||||||||
| (late TAA + Tau ) Group | Thioacetamide was injected (same dose as TAA group) | Taurine was injected (same dose as Tau group) for the subsequent 4 weeks | ||||||||||
Tau, taurine; TAA, thioacetamide; Con, concomitant.
Experimental design rationale
We aimed to investigate the indirect and direct effect of taurine on liver fibrosis induced by TAA, by measuring its antioxidant effects, anti-inflammatory effects, and anti-apoptotic effects, and also by performing docking studies.
Collection of samples
All rats fasted for at least eight hours at the end of the experiment. Blood samples were collected from the retro-orbital vein and were centrifuged for fifteen minutes to obtain serum, which was stored at − 80 °C. Rat livers from all groups were collected, weighed, and divided into three specific portions. For histological and immunohistochemical examination, the first portion was fixed in 10%formalin saline (El-Nasr Chemicals Co, Cairo, Egypt). To prepare liver homogenate, the second portion was homogenized in tenfold volume of sodium potassium phosphate buffer (0.01 M, pH 7.4) containing 1.15% KCl. To prevent protein hydrolysis, PMSF (protease inhibitor), EDTA (chelating agents) and DTT (reducing agents) were added to homogenizing solutions and then were centrifuged (5000 × g) for five minutes at 4 °C. The clear solution was stored at − 80 °C for further biochemical tests. For gene expression assessment by qRT-PCR, the last portion was immediately frozen in liquid nitrogen and was stored at − 80 °C.
Biochemical analysis
Serum alanine aminotransferase (ALT) (Cat. No: GOT111060), aspartate aminotransferase (AST) (Cat. No: GPT113100), total protein (Cat. No: TP116250), and serum albumin (Cat. No: ALB100250) were purchased from BioMed Diagnostics (Egy-Chem for lab technology Badr City, Egypt). ALT, AST, total protein, and albumin were quantified spectrophotometrically in serum, using the previously mentioned commercial kits, following the manufacturer’s instructions. The level of reduced glutathione (GSH) (Catalog Number. K464; from BioVesion, Milpitas, CA, USA) and the extent of lipid peroxidation (MDA) assay (Catalog Number. LIP39-K01; from Eagle Biosciences, Boston, MA, USA) were estimated colorimetrically in liver homogenate according to the methods described by the manufacturer.
Enzyme-linked immunosorbent assay (ELISA)
IL-6 ELISA Kits (Catalog Number. K4145) were acquired from BioVesion (Milpitas, CA, USA). Lipopolysaccharides (LPS) (Catalog Number. MBS704575), Myeloid Differentiation primary response protein (MyD88) (Catalog Number. MBS7204118), myeloid differentiation protein 2 (MD2) (Catalog Number. MBS3808316), Cluster of Differentiation 14 (CD14) (Catalog Number. MBS731954), transforming growth factor β1 (TGF-β1) (Catalog Number. MBS702305) and TNFα (Catalog No: MBS355371) ELISA kits were purchased from MyBioSource (San Diego, CA, USA). IL-6, LPS, MyD88, MD2, CD14, TGF-β1 and TNFα were measured in liver homogenates following the manufacturer’s protocols.
Real time PCR
Total RNA was extracted from the homogenized experimental tissues using total RNA purification kits purchased from Jena Bioscience (Munich, Germany). RNA was transformed into cDNA using archive kits (Applied Biosystems, Foster City, California, USA). qPCR was implemented using Go Taq PCR master mix (Promega Corporation, Madison, USA). The initial denaturation step was performed at 95 °C for 10 min followed by denaturing at 95 °C for 15 s (40 cycles), Annealing and extension were performed at 60 °C for one minute then 60 °C for 30 s, on a Step One Real Time PCR System (Applied Biosystems, Foster City, California, USA). The primers used are shown in Table 3.
Table 3.
Primer sequences used for the RT-PCR step.
| Primer | Sequence |
|---|---|
| TLR-4 (gene bank accession number NM053819.1) |
5′- AGACATCCAAAGGAATACTGCAA -3′(sense), 5′- GCCTTCATGTCTATAGGTGATGC -3′ (antisense) |
| NF-κB (gene bank accession number NM001276711.1) |
5′- CTGCGATACCTTAATGACAGCG -3′(sense), 5′- AATTTGGCTTCCTTTCTTGGCT -3′ (antisense) |
| β-actin (gene bank accession number NM001106409.1) as an internal control |
5′-GACGAGGCCCAGAGCAAGAGAGG-3′ (sense), 5′-GATCCACATCTGCTGGAAGGTGGAC-3′ (antisense) |
TLR-4, toll like receptor-4; NF-κB, nuclear factor-kB.
Histological and immunohistochemical techniques
Liver samples from different experimental groups were instantly fixed in 10% formalin saline then paraffin blocks were prepared and 5-μm thick sections were sliced and stained with hematoxylin–eosin (HE) for histopathological analysis to reveal the hepatic structural variations. The degree of liver fibrosis was evaluated and scored blindly as previously reported111. Where, portal tracts expansion and fibrosis was graded according to scoring system; (0): no fibrosis was detected, (1): some portal areas showed fibrous expansion with or without short fibrous septa detected, (2): most portal areas showed fibrous expansion with or without short fibrous septa, (3): most portal areas showed fibrous expansion with sporadic portal-to-portal (P-P) bridging, (4): portal areas showed fibrous expansion with obvious (P-P) as well as (P–C) bridging, (5): marked bridging (P-P and/or P–C) with infrequent nodules (incomplete cirrhosis), (6): probable or definite cirrhosis was identified. In addition; Sirius red staining was performed using standard protocols for morphometric analysis of collagen content indicating liver structural changes using image J program.
Immunohistochemical analysis for caspase-3, nuclear factor kappa B (NF-κB) and Alpha-smooth muscle actin (α-SMA) antibody was performed (Cat. No: 54-0017; from Genemed Biotecnologies, CA, USA) where, an antigen retrieval (EDTA solution, PH 8) was added to liver sections slides followed by hydrogen peroxide 0.3% and protein block, then incubation with either rat anti- Caspase-3 or anti-NF-κB or anti- α-SMA antibody (1: 100 dilution) was conducted. Incubation with anti-rat IgG secondary antibodies (HRP) was performed followed by visualization with (Liquid DAB + Substrate Chromogen System). Mayer's Hematoxylin was used as a counterstain. The positive staining results of liver tissue due to positive reaction was detected using Image J analysis software (National Institutes of Health, MD, USA) and the % of stained area (quantification of IHC staining) was expressed as mean ± SEM112.
Molecular docking study
TLR4 has a characteristic horseshoe-like shape. MD2 is bound to the side of the horseshoe ring and smoothly interfaces with the ligand. MD2 has a β-cup fold arrangement consisted of two antiparallel β-sheets, forming a large hydrophobic pocket for ligand binding.
The molecular docking study is an effective method for the prediction of the binding mode of a ligand to its receptor. To explore the binding mode of taurine, we simulated docking of the compound into the large hydrophobic binding pocket of MD2, to see the possible interactions, and consequently, to understand the binding mode and key active site interactions. We performed our molecular docking study using the Molecular Operating Environment software (MOE)113 on the crystal structure of human TLR4 in complex with MD2 and LPS (PDB code: 4G8A)114,115. The compound was built using MOE builder, energy minimized using the force field algorithm and the protein was prepared by adding missing hydrogens and 50 runs for the docking were performed with Triangle Matcher placement technique and energy of binding was scored according to London dG scoring function. In the present study, we tried to explore direct effect of taurine on TLR4 through docking study to understand how taurine interacts inside TLR4-MD2 complex.
Statistical analysis
The mean ± standard error was used for descriptive statistics of quantitative variables. One-way analysis of variance was used for comparisons between groups. Student t-tests were used to compare differences between two groups. Examination of non-parametric data (fibrosis score) was done using Kruskal–Wallis tests and Dunn’s post hoc test. GraphPad Prism 7 (GraphPad Software, San Diego, California USA, www.graphpad.com) was used for statistical analysis. Statistical significance was predefined as P < 0.05.
Conclusion
Both concomitant and late taurine treatment showed significant activity against TAA induced liver fibrosis in rats, with the concomitant treatment showing much more promising results.
The anti-fibrotic effect of taurine was attributable to its antioxidant (MDA, GSH), anti-inflammatory (LPS, MyD88, MD2, CD14, TLR4, NF-κB, IL-6, TGF-β1, TNF-α) and anti-apoptotic effects (caspase-3), mainly through modulation of the TLR4/NF-κB signaling pathway, indirectly by downregulation of LPS. The docking studies demonstrated good, direct binding of taurine inside the binding site of TLR4-MD2 complex with a binding energy of 7.63 kcal mol−1.
Author contributions
Conceptualization, H.A.M. and A.M.H.G.; Methodology, H.A.M., M.A.E. and A.M.H.G.; Software, N.S.Y. and M.A.E.; Validation, H.A.M., and A.M.H.G. and N.S.Y.; Formal Analysis, N.S.Y.., M.A.E.; Investigation, H.A.M.; Resources, A.M.H.G.; Data Curation, M.A.E..; Writing-Original Draft Preparation, H.A.M., and M.A.E.; Writing-Review & Editing, A.M.H.G.; Visualization, H.A.M.; Supervision, H.A.M., N.S.Y. and A.M.H.G.
Competing interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: 10.1038/s41598-023-35116-5
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/30/2023
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41598-023-35116-5
References
- 1.Sanchez-Valle V, Chavez-Tapia NC, Uribe M, Mendez-Sanchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr. Med. Chem. 2012;19:4850–4860. doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- 2.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 3.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003;112:1776–1784. doi: 10.1172/jci20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett. 2004;559:107–110. doi: 10.1016/s0014-5793(04)00037-7. [DOI] [PubMed] [Google Scholar]
- 5.Forbes SJ, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–963. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am. Family Phys. 2006;74:756–762. [PubMed] [Google Scholar]
- 7.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Bataller R, Brenner DA. Liver fibrosis. J. Clin. Investig. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tacke F, Weiskirchen R. Update on hepatic stellate cells: pathogenic role in liver fibrosis and novel isolation techniques. Expert Rev. Gastroenterol. Hepatol. 2012;6:67–80. doi: 10.1586/egh.11.92. [DOI] [PubMed] [Google Scholar]
- 10.Desmet VJ, Roskams T. Cirrhosis reversal: a duel between dogma and myth. J. Hepatol. 2004;40:860–867. doi: 10.1016/j.jhep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Iredale JP, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Investig. 1998;102:538–549. doi: 10.1172/jci1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ripps H, Shen W. Review: taurine: a "very essential" amino acid. Mol. Vis. 2012;18:2673–2686. [PMC free article] [PubMed] [Google Scholar]
- 13.De Luca A, Pierno S, Camerino DC. Taurine: the appeal of a safe amino acid for skeletal muscle disorders. J. Transl. Med. 2015;13:243. doi: 10.1186/s12967-015-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lombardini JB. Effects of ATP and taurine on calcium uptake by membrane preparations of the rat retina. J. Neurochem. 1983;40:402–406. doi: 10.1111/j.1471-4159.1983.tb11296.x. [DOI] [PubMed] [Google Scholar]
- 15.Grimble RF. The effects of sulfur amino acid intake on immune function in humans. J. Nutr. 2006;136:1660s–1665s. doi: 10.1093/jn/136.6.1660S. [DOI] [PubMed] [Google Scholar]
- 16.Kontny E, et al. The mechanism of taurine chloramine inhibition of cytokine (interleukin-6, interleukin-8) production by rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheum. 2000;43:2169–2177. doi: 10.1002/1529-0131(200010)43:10<2169::aid-anr4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Grimble RF. Sulphur amino acids and the metabolic response to cytokines. Adv. Exp. Med. Biol. 1994;359:41–49. doi: 10.1007/978-1-4899-1471-2_5. [DOI] [PubMed] [Google Scholar]
- 18.Huxtable RJT. Past, present, and future. Adv. Exp. Med. Biol. 1996;403:641–650. doi: 10.1007/978-1-4899-0182-8_71. [DOI] [PubMed] [Google Scholar]
- 19.Erdem A, et al. The effect of taurine on mesenteric blood flow and organ injury in sepsis. Amino Acids. 2008;35:403–410. doi: 10.1007/s00726-007-0622-0. [DOI] [PubMed] [Google Scholar]
- 20.Nagl M, Hess MW, Pfaller K, Hengster P, Gottardi W. Bactericidal activity of micromolar N-chlorotaurine: evidence for its antimicrobial function in the human defense system. Antimicrob. Agents Chemother. 2000;44:2507–2513. doi: 10.1128/aac.44.9.2507-2513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verdrengh M, Tarkowski A. Inhibition of septic arthritis by local administration of taurine chloramine, a product of activated neutrophils. J. Rheumatol. 2005;32:1513–1517. [PubMed] [Google Scholar]
- 22.Ebrahim AS, Babu E, Thirunavukkarasu C, Sakthisekaran D. Protective role of vitamin E, 2-deoxy-D-glucose, and taurine on perchloroethylene induced alterations in ATPases. Drug Chem. Toxicol. 2001;24:429–437. doi: 10.1081/dct-100106267. [DOI] [PubMed] [Google Scholar]
- 23.Zeidan-Chulia F, et al. Major components of energy drinks (caffeine, taurine, and guarana) exert cytotoxic effects on human neuronal SH-SY5Y cells by decreasing reactive oxygen species production. Oxid. Med. Cell. Longev. 2013;2013:791795. doi: 10.1155/2013/791795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huxtable RJ, editor. Taurine 2: Basic and Clinical Aspects. Springer US; 1996. [Google Scholar]
- 25.Ghandforoush-Sattari M, Mashayekhi S. Evaluation of taurine as a biomarker of liver damage in paracetamol poisoning. Eur. J. Pharmacol. 2008;581:171–176. doi: 10.1016/j.ejphar.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki T, Matsuzaki Y. Taurine and liver diseases: a focus on the heterogeneous protective properties of taurine. Amino Acids. 2014;46:101–110. doi: 10.1007/s00726-012-1381-0. [DOI] [PubMed] [Google Scholar]
- 27.Menzie J, Pan C, Prentice H, Wu J-Y. Taurine and central nervous system disorders. Amino Acids. 2014;46:31–46. doi: 10.1007/s00726-012-1382-z. [DOI] [PubMed] [Google Scholar]
- 28.Huxtable RJ. Taurine in the central nervous system and the mammalian actions of taurine. Prog. Neurobiol. 1989;32:471–533. doi: 10.1016/0301-0082(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 29.Foos TM, Wu JY. The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem. Res. 2002;27:21–26. doi: 10.1023/a:1014890219513. [DOI] [PubMed] [Google Scholar]
- 30.Wu J-Y, Prentice H. Role of taurine in the central nervous system. J. Biomed. Sci. 2010;17:S1. doi: 10.1186/1423-0127-17-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saransaari P, Oja SS. Taurine and neural cell damage. Amino Acids. 2000;19:509–526. doi: 10.1007/s007260070003. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J, et al. Protective role of taurine against morphine-induced neurotoxicity in C6 cells via inhibition of oxidative stress. Neurotox. Res. 2011;20:334–342. doi: 10.1007/s12640-011-9247-x. [DOI] [PubMed] [Google Scholar]
- 33.Schaffer SW, et al. Effect of taurine and potential interactions with caffeine on cardiovascular function. Amino Acids. 2014;46:1147–1157. doi: 10.1007/s00726-014-1708-0. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki T, et al. Taurine inhibits oxidative damage and prevents fibrosis in carbon tetrachloride-induced hepatic fibrosis. J. Hepatol. 2005;43:117–125. doi: 10.1016/j.jhep.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 35.Devi SL, Viswanathan P, Anuradha CV. Taurine enhances the metabolism and detoxification of ethanol and prevents hepatic fibrosis in rats treated with iron and alcohol. Environ. Toxicol. Pharmacol. 2009;27:120–126. doi: 10.1016/j.etap.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Devi SL, Viswanathan P, Anuradha CV. Regression of liver fibrosis by taurine in rats fed alcohol: effects on collagen accumulation, selected cytokines and stellate cell activation. Eur. J. Pharmacol. 2010;647:161–170. doi: 10.1016/j.ejphar.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Gentile CL, et al. Experimental evidence for therapeutic potential of taurine in the treatment of nonalcoholic fatty liver disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R1710–1722. doi: 10.1152/ajpregu.00677.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warskulat U, et al. Chronic liver disease is triggered by taurine transporter knockout in the mouse. FASEB J. 2006;20:574–576. doi: 10.1096/fj.05-5016fje. [DOI] [PubMed] [Google Scholar]
- 39.Erman F, Balkan J, Cevikbas U, Kocak-Toker N, Uysal M. Betaine or taurine administration prevents fibrosis and lipid peroxidation induced by rat liver by ethanol plus carbon tetrachloride intoxication. Amino Acids. 2004;27:199–205. doi: 10.1007/s00726-004-0105-5. [DOI] [PubMed] [Google Scholar]
- 40.Ginguay A, De Bandt JP, Cynober L. Indications and contraindications for infusing specific amino acids (leucine, glutamine, arginine, citrulline, and taurine) in critical illness. Curr. Opin. Clin. Nutr. Metab. Care. 2016;19:161–169. doi: 10.1097/mco.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 41.McCarty MF. Nutraceutical strategies for ameliorating the toxic effects of alcohol. Med. Hypotheses. 2013;80:456–462. doi: 10.1016/j.mehy.2012.12.040. [DOI] [PubMed] [Google Scholar]
- 42.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 44.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 46.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Tokunaga S, Kanayama A, Miyamoto Y. Modification of IkappaBalpha by taurine bromamine inhibits tumor necrosis factor alpha-induced NF-kappaB activation. Inflamm. Res. 2007;56:479–486. doi: 10.1007/s00011-007-7016-3. [DOI] [PubMed] [Google Scholar]
- 48.Olaso E, Friedman SL. Molecular regulation of hepatic fibrogenesis. J. Hepatol. 1998;29:836–847. doi: 10.1016/s0168-8278(98)80269-9. [DOI] [PubMed] [Google Scholar]
- 49.Algandaby MM. Antifibrotic effects of crocin on thioacetamide-induced liver fibrosis in mice. Saudi J. Biol. Sci. 2018;25:747–754. doi: 10.1016/j.sjbs.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodhoo A, et al. Human antigen R contributes to hepatic stellate cell activation and liver fibrosis. Hepatology. 2012;56:1870–1882. doi: 10.1002/hep.25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat. Res. 2005;569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 52.Metwaly HA, El-Gayar AM, El-Shishtawy MM. Inhibition of the signaling pathway of syndecan-1 by synstatin: a promising anti-integrin inhibitor of angiogenesis and proliferation in HCC in rats. Arch. Biochem. Biophys. 2018;652:50–58. doi: 10.1016/j.abb.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Bieche I, et al. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet. Genom. 2007;17:731–742. doi: 10.1097/FPC.0b013e32810f2e58. [DOI] [PubMed] [Google Scholar]
- 54.Delaney J, Timbrell JA. Role of cytochrome P450 in hydrazine toxicity in isolated hepatocytes in vitro. Xenobiotica. 1995;25:1399–1410. doi: 10.3109/00498259509061927. [DOI] [PubMed] [Google Scholar]
- 55.Guengerich FP, Kim DH, Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem. Res. Toxicol. 1991;4:168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- 56.Kang JS, et al. Role of CYP2E1 in thioacetamide-induced mouse hepatotoxicity. Toxicol. Appl. Pharmacol. 2008;228:295–300. doi: 10.1016/j.taap.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Konishi M, Ishii H. Role of microsomal enzymes in development of alcoholic liver diseases. J. Gastroenterol. Hepatol. 2007;22(Suppl 1):S7–10. doi: 10.1111/j.1440-1746.2006.04638.x. [DOI] [PubMed] [Google Scholar]
- 58.Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J. Biol. Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 59.Slater TF, Cheeseman KH, Ingold KU. Carbon tetrachloride toxicity as a model for studying free-radical mediated liver injury. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1985;311:633–645. doi: 10.1098/rstb.1985.0169. [DOI] [PubMed] [Google Scholar]
- 60.Sohn OS, Ishizaki H, Yang CS, Fiala ES. Metabolism of azoxymethane, methylazoxymethanol and N-nitrosodimethylamine by cytochrome P450IIE1. Carcinogenesis. 1991;12:127–131. doi: 10.1093/carcin/12.1.127. [DOI] [PubMed] [Google Scholar]
- 61.Wilson AS, et al. Characterisation of the toxic metabolite(s) of naphthalene. Toxicology. 1996;114:233–242. doi: 10.1016/s0300-483x(96)03515-9. [DOI] [PubMed] [Google Scholar]
- 62.Pascu EI, et al. Heterogeneity of collagen secreting cells in gingival fibrosis—an immunohistochemical assessment and a review of the literature. Roman. J. Morphol. Embryol. 2015;56:49–61. [PubMed] [Google Scholar]
- 63.Song HY, et al. Synovial fluid of patients with rheumatoid arthritis induces alpha-smooth muscle actin in human adipose tissue-derived mesenchymal stem cells through a TGF-beta1-dependent mechanism. Exp. Mol. Med. 2010;42:565–573. doi: 10.3858/emm.2010.42.8.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hong IH, et al. JNK1 and JNK2 regulate alpha-SMA in hepatic stellate cells during CCl4 -induced fibrosis in the rat liver. Pathol. Int. 2013;63:483–491. doi: 10.1111/pin.12094. [DOI] [PubMed] [Google Scholar]
- 65.Wang R, et al. Hypertrophic scar tissues and fibroblasts produce more transforming growth factor-beta1 mRNA and protein than normal skin and cells. Wound Repair Regen. 2000;8:128–137. doi: 10.1046/j.1524-475x.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- 66.Liu J, et al. Wnt/beta-catenin pathway forms a negative feedback loop during TGF-beta1 induced human normal skin fibroblast-to-myofibroblast transition. J. Dermatol. Sci. 2012;65:38–49. doi: 10.1016/j.jdermsci.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 67.Borthwick LA, Gardner A, De Soyza A, Mann DA, Fisher AJ. Transforming growth factor-beta1 (TGF-beta1) driven epithelial to mesenchymal transition (EMT) is accentuated by tumour necrosis factor alpha (TNFalpha) via crosstalk between the SMAD and NF-kappaB pathways. Cancer Microenviron. 2012;5:45–57. doi: 10.1007/s12307-011-0080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang J, et al. NDRG2 ameliorates hepatic fibrosis by inhibiting the TGF-beta1/Smad pathway and altering the MMP2/TIMP2 ratio in rats. PLoS ONE. 2011;6:e27710. doi: 10.1371/journal.pone.0027710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brenner DA. Molecular pathogenesis of liver fibrosis. Trans. Am. Clin. Climatol. Assoc. 2009;120:361–368. [PMC free article] [PubMed] [Google Scholar]
- 70.Kulkarni AA, et al. PPAR-gamma ligands repress TGFbeta-induced myofibroblast differentiation by targeting the PI3K/Akt pathway: implications for therapy of fibrosis. PLoS ONE. 2011;6:e15909. doi: 10.1371/journal.pone.0015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paik YH, et al. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 72.Soares JB, Pimentel-Nunes P, Roncon-Albuquerque R, Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hep. Intl. 2010;4:659–672. doi: 10.1007/s12072-010-9219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seki E, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat. Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 74.Kluwe J, Mencin A, Schwabe RF. Toll-like receptors, wound healing, and carcinogenesis. J. Mol. Med. (Berl) 2009;87:125–138. doi: 10.1007/s00109-008-0426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Troutman TD, Bazan JF, Pasare C. Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle. 2012;11:3559–3567. doi: 10.4161/cc.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kell DB, Pretorius E. On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: the central roles of LPS and LPS-induced cell death. Integr. Biol. 2015;7:1339–1377. doi: 10.1039/c5ib00158g. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y, et al. Taurine alleviates lipopolysaccharide-induced liver injury by anti-inflammation and antioxidants in rats. Mol. Med. Rep. 2017;16:6512–6517. doi: 10.3892/mmr.2017.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu G., et al. Taurine 10. Springer; 2017. [Google Scholar]
- 79.Marcinkiewicz J, Kontny E. Taurine and inflammatory diseases. Amino Acids. 2014;46:7–20. doi: 10.1007/s00726-012-1361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu G, et al. Taurine inhibits Kupffer cells activation induced by lipopolysaccharide in alcoholic liver damaged rats. Adv. Exp. Med. Biol. 2017;975(Pt 2):789–800. doi: 10.1007/978-94-024-1079-2_61. [DOI] [PubMed] [Google Scholar]
- 81.Liu S, et al. Role of toll-like receptors in changes in gene expression and NF-kappa B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect. Immun. 2002;70:3433–3442. doi: 10.1128/iai.70.7.3433-3442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cattley R. C., Popp J. A. Handbook of Toxicologic Pathology (Second Edition) Academic Press; 2002. [Google Scholar]
- 83.Kawada N, Parola M. Stellate Cells in Health and Disease . Academic Press; 2015. [Google Scholar]
- 84.Kessoku T, et al. Resveratrol ameliorates fibrosis and inflammation in a mouse model of nonalcoholic steatohepatitis. Sci. Rep. 2016;6:22251. doi: 10.1038/srep22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Su Grace, et al. Activation of human and mouse Kupffer cells by lipopolysaccharide is mediated by CD14. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G640–G645. doi: 10.1152/ajpgi.00253.2001. [DOI] [PubMed] [Google Scholar]
- 86.Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am. J. Physiol. Gastrointestinal Liver Physiol. 2002;283:G256–265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 87.Schmitz S, Pfaffl MW, Meyer HH, Bruckmaier RM. Short-term changes of mRNA expression of various inflammatory factors and milk proteins in mammary tissue during LPS-induced mastitis. Domest. Anim. Endocrinol. 2004;26:111–126. doi: 10.1016/j.domaniend.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 88.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miao J, et al. The effect of taurine on the toll-like receptors/nuclear factor kappa B (TLRs/NF-kappaB) signaling pathway in Streptococcus uberis-induced mastitis in rats. Int. Immunopharmacol. 2011;11:1740–1746. doi: 10.1016/j.intimp.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 90.Kawasaki K, et al. Mouse toll-like receptor 4MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J. Biol. Chem. 2000;275:2251–2254. doi: 10.1074/jbc.275.4.2251. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y, et al. Taurine alleviates lipopolysaccharideinduced liver injury by antiinflammation and antioxidants in rats. Mol. Med. Rep. 2017;16:6512–6517. doi: 10.3892/mmr.2017.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kutikhin AG. Impact of Toll-like receptor 4 polymorphisms on risk of cancer. Hum. Immunol. 2011;72:193–206. doi: 10.1016/j.humimm.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 93.da Silva Correia J, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex transfer from CD14 to TLR4 and MD-2. J. Biol. Chem. 2001;276:21129–21135. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- 94.Luedde T, Schwabe RF. NF-kappaB in the liver-linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tacke F, Luedde T, Trautwein C. Inflammatory pathways in liver homeostasis and liver injury. Clin. Rev. Allergy Immunol. 2009;36:4–12. doi: 10.1007/s12016-008-8091-0. [DOI] [PubMed] [Google Scholar]
- 96.Wang X, Bi Z, Wang Y, Wang Y. Increased MAPK and NF-kappaB expression of Langerhans cells is dependent on TLR2 and TLR4, and increased IRF-3 expression is partially dependent on TLR4 following UV exposure. Mol. Med. Rep. 2011;4:541–546. doi: 10.3892/mmr.2011.450. [DOI] [PubMed] [Google Scholar]
- 97.Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Greten FR, Karin M. The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 2004;206:193–199. doi: 10.1016/j.canlet.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 99.Li X, Benjamin IS, Alexander B. Reproducible production of thioacetamide-induced macronodular cirrhosis in the rat with no mortality. J. Hepatol. 2002;36:488–493. doi: 10.1016/s0168-8278(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 100.Cao X, Zhou M, Wang C, Hou L, Zeng B. Lectin purified from Musca domestica pupa up-regulates NO and iNOS production via TLR4/NF-kappaB signaling pathway in macrophages. Int. Immunopharmacol. 2011;11:399–405. doi: 10.1016/j.intimp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 101.Friedman SL. Evolving challenges in hepatic fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 102.Chakraborty JB, Oakley F, Walsh MJ. Mechanisms and biomarkers of apoptosis in liver disease and fibrosis. Int. J. Hepatol. 2012;2012:648915. doi: 10.1155/2012/648915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 104.Kroemer G. B709 mitochondrial control of cell death. Sci. World J. 2001;1:48–48. doi: 10.1100/tsw.2001.23.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gabr SA, Gabr NS, Elsaed WM. Protective activity of taurine and molecular fibrogenesis in iron overloaded hepatic tissues. Int. J. Pharmacol. 2019;15:418–427. doi: 10.3923/ijp.2019.418.427. [DOI] [Google Scholar]
- 106.Marshall JD, et al. A novel class of small molecule agonists with preference for human over mouse TLR4 activation. PLoS ONE. 2016;11:e0164632. doi: 10.1371/journal.pone.0164632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y, et al. TLR4/MD-2 activation by a synthetic agonist with no similarity to LPS. Proc. Natl. Acad. Sci. 2016;113:E884–E893. doi: 10.1073/pnas.1525639113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Percie du Sert N, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18:3000411. doi: 10.1371/journal.pbio.3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang L, et al. Taurine reduced epidural fibrosis in rat models after laminectomy via downregulating EGR1. Cell Physiol. Biochem. 2016;38:2261–2271. doi: 10.1159/000445581. [DOI] [PubMed] [Google Scholar]
- 110.Furtado KS, et al. Coffee and caffeine protect against liver injury induced by thioacetamide in male. Wistar Rats. 2012;111:339–347. doi: 10.1111/j.1742-7843.2012.00903.x. [DOI] [PubMed] [Google Scholar]
- 111.Ishak K, et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 112.Klein S., et al. Novel rat model of repetitive portal venous embolization mimicking human non-cirrhotic idiopathic portal hypertension. PLoS One. 2016;11:e0162144. doi: 10.1371/journal.pone.0162144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Molecular Operating Environment (MOE), 2019.01; Chemical Computing Group ULC, 1010 Sherbrooke St. West, Suite #910, Montreal, QC, Canada, (2019) H3A 2R7.
- 114.Ohto U, Yamakawa N, Akashi-Takamura S, Miyake K, Shimizu T. Structural analyses of human Toll-like receptor 4 polymorphisms D299G and T399I. J. Biol. Chem. 2012;287:40611–40617. doi: 10.1074/jbc.M112.404608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cuadrado I, Amesty A, Cedron JC, Oberti JC, Estevez-Braun A. Semisynthesis and inhibitory effects of solidagenone derivatives on tlr-mediated inflammatory responses. Molecules (Basel, Switzerland) 2018 doi: 10.3390/molecules23123197. [DOI] [PMC free article] [PubMed] [Google Scholar]