Abstract
The inv(7)(p15q34) chromosomal abnormality which juxtaposes part of the HOXA gene cluster on 7p15 to the TCRβ locus on 7q34, has been described in a subset of cases of T-cell lymphoblastic leukemia, but its presence in cases of B-cell lymphoblastic leukemia is virtually unknown. Herewith, we report a case of a B-cell lymphoblastic leukemia with inv(7)(p15q34). The patient received standard induction chemotherapy, which failed to produce remission. After treatment with blinatumomab, a bispecific T-cell engager, the follow-up bone marrow biopsy showed no evidence of persistent/ relapsed B-cell lymphoblastic leukemia. The unique cytogenetics of this case may have contributed to its resistance of standard induction chemotherapy.
Introduction
B cell lymphoblastic leukemia /lymphoma (B-ALL/LBL) is a malignant neoplasm of precursor lymphoid cells (a.k.a. lymphoblasts) committed to the B cell lineage, which often involves blood, and bone marrow, and less frequently other tissues [1]. By flow cytometry, the lymphoblasts typically display a B-cell immunophenotype characterized by the expression of CD19, CD79a, and CD22 and in most cases TdT and CD10 [1]. B-ALL/LBL is often associated with various cytogenetic abnormalities including t(1;19)-E2A/PBX1,t(12;21)-ETV/ CBFα, and t(9;22)-BCR/ ABL [2].
We present a case of B-ALL/LBL with inv(7)(p15q34), a cytogenetic abnormality which is encountered in cases of T cell lymphoblastic leukemia (T-ALL/LBL), but is virtually unreported in B-cell ALL/LBL. The inv(7)(p15q34) gene inversion juxtaposes part of the HOXA gene cluster on 7p15 to the TCRβ locus on 7q34. These genes have been shown to have a critical association with embryonic development and their mutations can lead to various hematologic malignancies [3, 4]. The literature reports this mutation in 24 cases, 19 of which were T-ALL/LBL patients [5, 6]. In addition to lymphoblastic leukemia/lymphoma, this inversion has also been observed in a single case of chronic myelogenous leukemia in blast phase [7} and in tumors of the kidney and uterus [8, 9]. At present, a reference to a single case of B-ALL/LBL with this cytogenetic abnormality within a complex karyotype could be found included in the Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer [10]. The karyotype of this case, an 8 year old female patient with a CBC of 3.5 x× 103 /μL, showed: 46,XX,inv(7) (p15q34),+8,der(16)t(1;16)(q21;q12–13),−20, dup(21) (q11q22)/46, idem,dup(2)(q13q37) [11].
In this communication, we describe a case of B-ALL/LBL with inv(7)(p15q34) plus other abnormalities in a 19 year old patient. We describe the patient's clinical history, bone marrow microscopic findings, flow cytometric analysis, cytogenetic profile, and treatment response, and provide references to cases with inv(7)(p15q34) observed in other hematologic and non-hematologic malignancies.
Case report
A 19-year-old Hispanic female was admitted with fever of 39.0 °C, chills, and fatigue. She reported 2 weeks of severe fatigue, multiple bruises, petechial rash, bleeding gums, generalized jaundice, and severe bilateral leg pain. Past medical history was non-contributory, including no family history of hematologic diseases. Vital signs were all normal. Physical examination showed pallor, submaxillary lymphadenopathy, petechiae on soft palate and multiple ecchymosis on bilateral lower extremities, right arm, and lower back. The patient reported severe left leg pain with tenderness to touch.
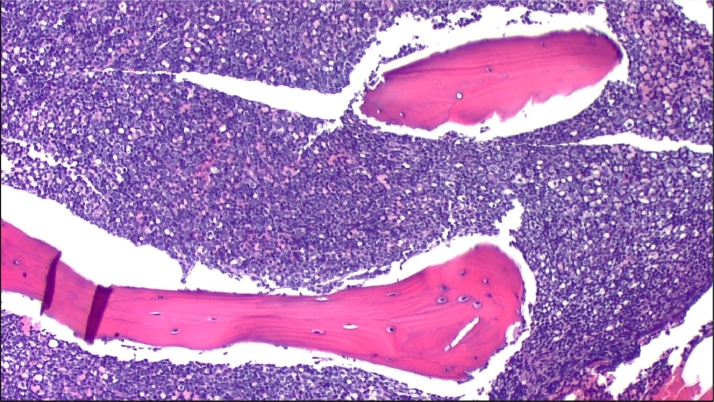
Laboratory studies on admission showed a leukocyte count of 54.51 × 103 /μL, a hemoglobin concentration of 7.5 g/dL, hematocrit of 22.8%, and a platelet count of 19 × 103 / μL. The leukocyte differential count on admission included 76% lymphocytes, 4% segmented neutrophils, 2% metamyelocytes, 3% monocytes and 15% blasts. Urea, creatinine and GFR were normal. AST and ALT were 39 units/L and 43 units/L, respectively. Pathology evaluation, flow cytometry, and cytogenetic studies A bone marrow biopsy was performed, showed a hypercellular (>90%) bone marrow occupied by >90% of medium to large sized blasts with dispersed chromatin, inconspicuous nucleoli, and scant cytoplasm (see Fig. 1, Fig. 2). Normal trilineage hematopoiesis was markedly decreased. Flow cytometry showed the blasts to be TdT (+), CD10 (+), CD13 (-), CD19 (+) CD33 (partial +) and CD34 (+), an immunophenotype which is consistent with a B-cell lymphoblastic leukemia.
Fig. 1.
The bone marrow biopsy shows hypercellularity (>90%) by mononuclear, immature cells. Flow cytometry demonstrated a B cell lymphoblastic leukemia.
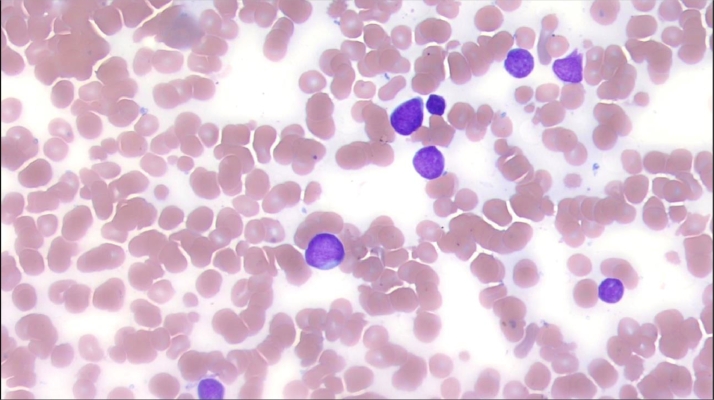
Fig. 2.
The bone marrow demonstrated medium to large sized blasts with dispersed chromatin, inconspicuous nucleoli, and scant cytoplasm.
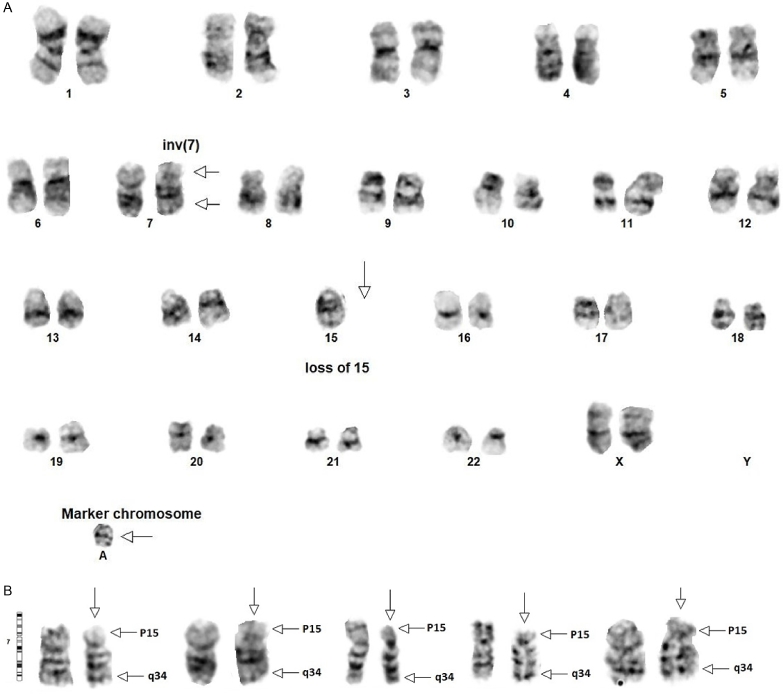
Cytogenetic analysis on bone marrow aspirate demonstrated 46~47,XX,inv(7)(p15q34),−15,+mar[cp8]/ 46,XX[12]. Overall, twenty metaphase cells were analyzed and eight (40%) were found to be abnormal. Clone 1 included eight of twenty (40%) metaphase cells and was comprised of two X chromosomes containing clonal and non-clonal alterations such that a composite karyotype was prepared. At least two cells contained the following alterations: 1) A pericentric inversion of chromosome 7 in which breakage and reunion have occurred at bands 7p15 and 7q34 (see Figs. 3A and 3B). 2) Monosomy of chromosome 15. 3) A marker chromosome of unknown origin. Clone 2 included the remaining twelve of twenty (60%) metaphase cells. It was comprised of two X chromosomes and showed a normal female karyotype.
Fig. 3.
A shows the karyotype of one clone with inv(7)(p15q34) and +mar[cp8]. B shows four separate chromosomes from different cells with the inv(7) and a normal chromosome for comparison.
Treatment and clinical follow up: The patient received induction chemotherapy with a Hyper-CVAD regimen with alternating courses of cyclophosphamide, vincristine, doxorubicin, and dexamethasone (course A) and methotrexate with cytarabine (course B). Bone marrow after 1st cycle continued to show 6% blasts which increased to 14% following cycle 2, part B. She then received blinatumomab, a monoclonal bi-specific T cell engager that is approved for use in refractory ALL cases [12]. Five weeks after blinatumomab administration, another bone marrow biopsy was performed showing trilineage hematopoiesis with no evidence of residual B-ALL/LBL. Blinatumomab was continued while she awaited an allogeneic stem cell transplant. Unfortunately, 9 months after achieving remission, she had a second relapse. She then received daunorubicin, vincristine, pegasparaginase, and dexamethasone and is still awaiting a stem cell transplant.
Discussion
The band q34 of chromosome 7 is the site of the T-cell receptor β gene, and mutations in this gene locus have been associated with various T-cell malignancies [13, 14]. In contrast, the band p15 of chromosome 7 contains the HOXA gene cluster, which plays a critical role in embryologic development [3, 15]. A pericentric inversion of chromosome 7, inv(7)(p15q34) juxtaposes part of the HOXA gene cluster on 7p15 to the TCRβ locus on 7q34, which has a strong association with T-cell lymphoblastic leukemia. Polymerase chain reaction (PCR) analysis has shown high levels of HOXA10 and HOXA11 expression with inv(7). Possibly, this is due to enhancers found in the TCRβ locus with strong evidence of involvement of HOXA genes in the development of T cell lymphoblastic leukemia [15, 16]. HOX homeobox genes are tightly linked to malignant transformation of hematopoiesis. The HOXA gene cluster on 7p15 has been shown to play a fundamental role in embryologic development and because of this, it also has a strong association with various hematologic malignancies [3, 4]. In cases of the chromosome 7 inversion, high levels of HOXA10 and HOXA11 are seen, possibly due to enhancers found in the TCRβ locus, establishing a connection between HOXA genes and the development of T-ALL/LBL [14, 15]. What is not well established is the extent to which this aberration leads to other malignancies, including B-ALL/LBL.
Various chromosomal aberrations have been identified in B-ALL/LBL and the World Health Organization Classification uses them to identify specific disease subgroups [1]. However, the presence of inv(7)(p15q34) in B-ALL/LBL is exceptionally rare. The Mitelman Database for Chromosomal Aberrations and Gene Fusions in Cancer [10] and the Atlas of Genetics and Cytogenetics in Hematology and Oncology [17] were searched for all known cases of inv(7)(p15q34) in hematologic malignancies. The only other case of B-ALL/LBL with this abnormality is listed in the former database [10]. The chromosome results and limited information for this patient identified as case 300, were included in an International Workshop database of 18 cytogenetics study groups published as supplementary information - see Table 1 [11]. All the other reported cases (19 of 24) of this chromosomal aberration seen in leukemias were T-ALL/LBL. An additional leukemic case was a chronic myeloid leukemia BCR-ABL1 positive in blast phase which showed the following karyotype: 46,XY,inv(7)(p15q34),t(9,22)(q34;q11) [7]. The 19 T-ALL/LBL cases included 11 males and 8 females ranging in age from 6 to 49. Other three cases of inv(7)(p15q34) included two carcinomas of the kidney and one of the uterus [8,9].
Unfavorable cytogenetics is known to frequently complicate treatment in various malignancies. The patient in our present case was unable to achieve remission with standard Hyper-CVAD treatment regimen. It is possible that the inv(7)(p15q34) aberration seen here may confer some resistance to standard first line treatment modalities in B-ALL/LBL patients. Blinatumomab helped the patient to achieve remission which lasted 9 months.
In summary, inv(7)(p15q34 is an exceptionally rare abnormality) in B-ALL/LBL. It may contribute to a poor response to standard induction chemotherapy but these patients may benefit from alternative treatment strategies. Additional cases of B-ALL/LBL with similar cytogenetics would be needed to elucidate the biological, clinical behavior, and treatment options of these rare neoplasms.
References
- 1.Swerdlow S.H., Campo E., Harris N.L. 4th edition. IARC Press; Lyon, France: 2017. (Eds.), WHO Classification of Tumours of Haematopoietic and Lymphoid tissues, Updated. [Google Scholar]
- 2.Mullighan C.G. Molecular genetics of b-precursor acute lymphoblastic leukemia. J. Clin. Invest. 2012;122(10):3407–3415. doi: 10.1172/JCI61203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach C., Buhl S., Mueller D. Leukemogenic transformation by hoxa cluster genes. Blood. 2010;115(14):2910–2918. doi: 10.1182/blood-2009-04-216606. Apr 8. [DOI] [PubMed] [Google Scholar]
- 4.Soulier J., Clappier E., Cayuela J.-.M. HOXA genes are included in genetic and biologic networks defining human acute t cell leukemia (t-all) Blood. 2005;106(1):274–286. doi: 10.1182/blood-2004-10-3900. Jul 1. [DOI] [PubMed] [Google Scholar]
- 5.Cauwelier B., Cavé H., Gervais C., Lessard M. Clinical, cytogenetic and molecular characteristics of 14 t-all patients carrying the tcrβ-hoxa rearrangement: a study of the groupe francophone de cytogénétique hématologique. Leuk. 2007;21(1):121–128. doi: 10.1038/sj.leu.2404410. Jan. [DOI] [PubMed] [Google Scholar]
- 6.Cauwelier B., Dastugue N., Cools J. Molecular cytogenetic study of 126 unselected t-all cases reveals high incidence of tcrbeta locus rearrangements and putative new t-cell oncogenes. Leuk. 2006;20(7):1238–1244. doi: 10.1038/sj.leu.2404243. [DOI] [PubMed] [Google Scholar]
- 7.Iwamasa K., Yasukawa M., Fujita S. A case of chronic myelogenous leukemia with tlymphoblastic and megakaryoblastic mixed crisis. Jpn. J. Med. 1989;28(1):89–95. doi: 10.2169/internalmedicine1962.28.89. [DOI] [PubMed] [Google Scholar]
- 8.Kovacs G., Brusa P., De Riese W. Tissue-specific expression of a constitutional 3;6 translocation: development of multiple bilateral renal-cell carcinomas. Int. J. Canc. 1989;43(3):422–427. doi: 10.1002/ijc.2910430313. [DOI] [PubMed] [Google Scholar]
- 9.Fan S.X., Sreekantaiah C., Berger C.S. Cytogenetic findings in nine leiomyomas of the uterus. Canc. Genet. Cytogenet. 1990;47(2):179–189. doi: 10.1016/0165-4608(90)90028-9. [DOI] [PubMed] [Google Scholar]
- 10.Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer Mitelmandatabase. isb-cgc.org. Cas. Cytogenet. Sear. Res.. [Online] 2021 [Accessed April 28, 2021] [Google Scholar]
- 11.Harrison C.J., Moorman A.V., Schwab C. Ponte di Legno International workshop in childhood acute lymphoblastic leukemia. an international study of intrachromosomal amplification of chromosome 21 (iamp21): cytogenetic characterization and outcome. Leuk. 2014;28(5):1015–1021. doi: 10.1038/leu.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J., Song Y., Liu D. Recent advances on blinatumomab for acute lymphoblastic leukemia. Exp. Hematol. Oncol. 2019;8:28. doi: 10.1186/s40164-019-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith S.D., Morgan R., Gemmell R. Clinical and biologic characterization of t cell neoplasias with rearrangements of chromosome 7 band q34. Blood. 1988;71(2):395–402. [PubMed] [Google Scholar]
- 14.Bhatlekar S., Fields J.Z., Boman B.M. Role of hox genes in stem cell differentiation and cancer. Stem Cell. Int. 2018 doi: 10.1155/2018/3569493. Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speleman F., Cauwelier B., Dastugue N. A new recurrent inversion, inv(7)(p15q34), leads to transcriptional activation of hoxa10 and hoxa11 in a subset of t-cell acute lymphoblastic leukemias. Leuk. 2005;19(3):358–366. doi: 10.1038/sj.leu.2403657. Mar. [DOI] [PubMed] [Google Scholar]
- 16.Van Vlierberghe P., van Grotel M., Tchinda J. The recurrent set-nup214 fusion as a new hoxa activation mechanism in pediatric t-cell acute lymphoblastic leukemia. Blood. 2008;111(9):4668–4680. doi: 10.1182/blood-2007-09-111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atlas of Genetics and Cytogenetics in Oncology and Haematology Atlasgeneticsoncology.org/anomalies. [Online] 2021 [Accessed April 28, 2021] [Google Scholar]