Abstract
Chemosensory signals allow vertebrates and invertebrates not only to orient in its environment toward energy-rich food sources to maintain nutrition but also to avoid unpleasant or even poisonous substrates. Ethanol is a substance found in the natural environment of Drosophila melanogaster. Accordingly, D. melanogaster has evolved specific sensory systems, physiological adaptations, and associated behaviors at its larval and adult stage to perceive and process ethanol. To systematically analyze how D. melanogaster larvae respond to naturally occurring ethanol, we examined ethanol-induced behavior in great detail by reevaluating existing approaches and comparing them with new experiments. Using behavioral assays, we confirm that larvae are attracted to different concentrations of ethanol in their environment. This behavior is controlled by olfactory and other environmental cues. It is independent of previous exposure to ethanol in their food. Moreover, moderate, naturally occurring ethanol concentration of 4% results in increased larval fitness. On the contrary, higher concentrations of 10% and 20% ethanol, which rarely or never appear in nature, increase larval mortality. Finally, ethanol also serves as a positive teaching signal in learning and memory and updates valence associated with simultaneously processed odor information. Since information on how larvae perceive and process ethanol at the genetic and neuronal level is limited, the establishment of standardized assays described here is an important step towards their discovery.
Subject terms: Genetics, Neuroscience
Introduction
Communication with the environment through chemical signals is an essential process for the survival of most if not all organisms. Specialized signal transduction pathways are used to detect chemical cues and convert information into neuronal activity that induces appropriate behavioral output1. Important insights into principals of chemosensory perception and information processing are provided by genetically modifiable organisms such as the fruit fly D. melanogaster2–5. This includes also the larval central nervous system with its simpler structure consisting of only about 10,000 neurons. Larvae provide access to combinations of genetic tools, robust behavioral assays, the possibility of transgenic single-cell manipulation, and even connectome data of the central nervous system6–14. Various studies have identified chemosensory stimuli that larvae perceive from their environment. Most odorants, are attractive to larvae in a dose-dependent manner15–17. Likewise, larvae show dose-dependent responses to gustatory cues18–22. Even the characteristics of the substrate seem to influence larval chemosensory responses23,24. However, to understand how larvae orient in their complex chemosensory environment, further studies are needed on additional environmental occurring stimuli, such as ethanol.
Most Drosophila species are saprophagous and feed on decaying sweet substrates like rotting fruits, which contain ethanol produced by natural fermentation25. Adult flies exhibit acute ethanol responses similar to those of mammals: as ethanol concentration increases, flies exhibit locomotor stimulation, loss of postural control, and eventually sedation26. With repeated exposure, adults develop tolerance to the effects of ethanol27. It is even assumed that D. melanogaster has altered its ecological niche to benefit from food sources characterized by a higher alcohol concentration28. D. melanogaster has an unusually high alcohol dehydrogenase (Adh) activity within its genus, which allows to deal with higher ethanol levels to occupy microhabitats that are not accessible to other species such as Drosophila simulans, which have a lower Adh activity29. In addition, larvae are even able to perceive and selectively consume food containing ethanol when they are infected by parasitic wasps30. A higher ethanol in the larval hemocoel due to increased consumption of ethanol enriched food leads to enhanced death of the growing parasites and thus increases the larval survival rate. This means that D. melanogaster larvae can adjust their alcohol consumption depending on the particular situation, and use it as a kind of medical treatment. Accordingly, ethanol is an important ecological parameter, which has selected to the establishment of specific larval sensory systems, physiological adaptations and related behaviors.
Therefore, it is plausible that several studies have been able to show that D. melanogaster larvae preferentially migrate to substrates that contain ethanol. For instance, different wild-type strains derived from Australian populations revealed strong preferences for 6% ethanol31–33. Even 17% ethanol was attractive for two larval strains that were either homozygous for the AdhF or the AdhS allele34. AdhF and AdhS describe two functional allozymes that exist in natural populations of Drosophila species on several continents35,36. The polymorphism is maintained by natural selection; while AdhF is showing a higher enzymatic activity for ethanol, AdhS is more resistant to higher environmental temperatures37. However, there are also studies in which ethanol is not attractive to larvae15,38,39, which complicates the interpretation of the various results. Therefore, we analyzed ethanol guided behavior by using a set of simple and robust experimental designs.
In recent years, a set of well-defined larval behavioral assays was established to investigate substrate choice, feeding, survival, and associative learning and memory14,18,19. It is therefore possible to analyze how larvae react to individual chemical components of its environment. Fructose, for example, provides nutrition and acts as a positive teaching signal during learning20,21. On the other hand, many chemicals that humans categorize as bitter are also repulsive to the larvae (for example among others caffeine, denatonium, or quinine) and can act as a negative teaching signal (caffeine, quinine)18,19,22,40. Sodium chloride even proved to be dichotomous, being attractive and a positive teaching signal at low concentrations and repellent and a negative teaching signal at high concentrations41,42. Using a set of standardized assays, we have now addressed the effect of ethanol on larval behavior. Our results support previous studies suggesting that the odorant ethanol is attractive to larvae in a dose dependent manner at moderate naturally occurring concentrations and has a positive effect on larval survival. The larval ethanol choice is based on smell and taste. Finally, ethanol can be used in associative olfactory learning paradigms to establish appetitive memories as it provides a positive teaching signal.
Results
Drosophila melanogaster larvae are attracted to ethanol
Several studies have repeatedly shown that D. melanogaster larvae are attracted to ethanol31–34. However, in almost all studies there are slight differences in the applied assays, used substrates and mode of ethanol presentation. This might influence the observed results. In some cases ethanol is attractive and in others not15,38,39. Therefore, we first reanalyzed the attractiveness of ethanol in a widely applied standardized assay. We analyzed the preference of wild-type Canton-S to ethanol by observing their approach behavior to a substrate that contains different concentrations of ethanol (Fig. 1). The ethanol concentration ranged from 1 to 50%. Ethanol concentrations above 50% prevent the agarose plates from solidifying and thus could not be analyzed. We found that Canton-S and w1118 larvae are attracted to ethanol in a dose-dependent manner (Fig. 1b). The attractiveness of ethanol peaked at around 4–10% ethanol for Canton-S. For 10% and 20% ethanol Canton-S-larvae showed higher preferences than w1118 larvae. Additionally, we observed the attractiveness to 8% ethanol of the Canton-S larvae for 120 min (Fig. 1c). The attraction to ethanol remained equally stable and no sedative effect on larval locomotion was seen during this prolonged ethanol exposure. However, larvae respond differently over time to 20% ethanol. The initial attractiveness turns into an aversion after about 15 min of ethanol exposure (Fig. 1c). To investigate the influence of ethanol pre-exposure on the substrate choice of larvae we compared how animals reared on standard food (containing 1% ethanol) versus animals reared for one or two generations on ethanol-free food respond to 8% ethanol (Fig. 1d). Canton-S larvae grown for one or two generations on ethanol-free food preferred the ethanol containing site similar to larvae raised on standard food. These results show that D. melanogaster larvae are attracted to ethanol in a concentration-dependent manner.
Figure 1.
Ethanol attraction of Drosophila melanogaster larvae. (a) Scheme of the experimental procedure. Larvae were allowed to choose within 5 min between control agarose and a substrate containing different concentrations of ethanol ranging from 1 to 50%. (b) Wild-type Canton-S (red) and mutant w1118 (white) larvae are attracted to ethanol following a Gaussian-shape like dose response curve. The highest behavioral response (preference index (Pref)) was seen at 8% ethanol for both strains (PrefCanton-S = 0.62, ci = 0.53–0.68, Prefw1118 = 0.60, ci = 0.51–0.74). All groups are significant different from zero, except for 1% w1118 (one-sample t test, p < 0.17). Multiple comparison indicates a significant difference between the two genotypes for 10% and 20% ethanol (Wilcoxon-Rank, p10% < 0.001, p20% < 0.001). Letters above each box-plot indicate differences between only Canton-S (red) and mutant w1118 (white) larvae, respectively. (c) Larval 8% and 20% ethanol substrate choice over a test period of 120 min. Canton-S larvae did not vary in their attraction within 120 min to 8% ethanol (Kruskal Wallis, p < 0.032). (Pref5min = 0.55, ci = 0.47–0.6, Pref10min = 0.65, ci = 0.53–0.69, Pref15min = 0.63, ci = 0.6–0.73, Pref30min = 0.53, ci = 0.5–0.67, Pref60min = 0.5, ci = 0.45–0.61, Pref120min = 0.57, ci = 0.39–0.59). However, the initial attraction for 20% ethanol turns into an avoidance after about 15 min of ethanol presentation (Pref5min = 0.15, ci = 0.03–0.25, Pref10min = 0.1, ci = − 0.04 to 0.25, Pref15min = − 0.16, ci = − 0.38 to 0.1, Pref30min = − 0.33, ci = − 0.52 to − 0.05, Pref60min = − 0.46, ci = − 0.64 to − 0.27, Pref120min = − 0.62, ci = − 0.5 to − 0.69). Indicated values show medians; error bars represent standard errors. (d) Canton-S larvae raised on standard food that contains 1% ethanol (ethanol +) shows a substrate choice behavior that was not different from larvae that were raised for one (ethanol—[1]) or two generations (ethanol—[2]) on ethanol free food (TukeyHSD, pstandard-w/o < 0.202, pstandarad-w/o2 < 0.639). Note a slight increase in ethanol substrate choice with increasing generations of ethanol food free raised flies (TukeyHSD, p < 0.03). Differences against zero are indicated in red and black at the bottom of each panel. Sample size for each box plot is n = 16. Significant differences between the two groups are given with letters. Preference scores and statistical tests underlying the different indices are documented in the Supplementary material.
Ethanol attraction changes during post-embryonic development
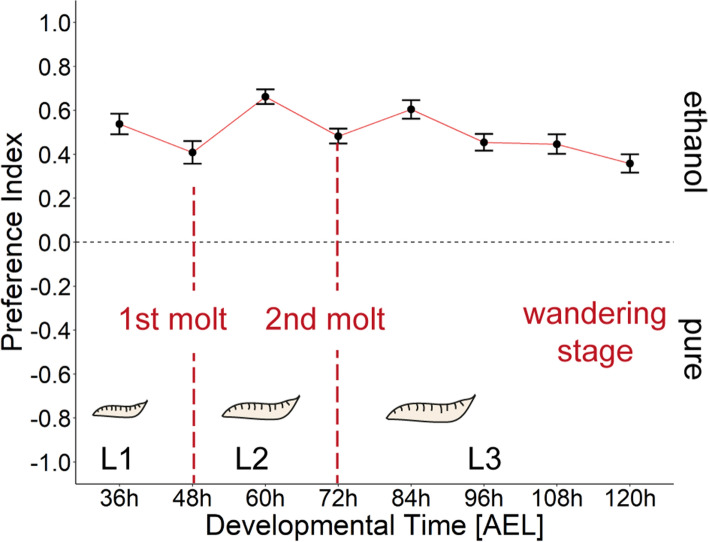
The life cycle of D. melanogaster comprises three larval stages (L1, L2 and L3), clearly separated by two molting stages, which occur approximately 48 and 72 h after egg laying (AEL). To understand whether ethanol choice changes during larval development, we analyzed eight groups of Canton-S larvae from 36 – 120 h AEL every 12 h for their 8% ethanol attraction (Fig. 2). We chose this concentration because it was most preferred by the larvae in the first experiment (Fig. 1). Each of the tested groups showed an attraction to ethanol (Table 1). However, during the first and second molt, the preferences are reduced (Fig. 2, Table 1). Furthermore, in L3 larvae the preference decreases with age.
Figure 2.
Ethanol substrate choice during larval development. Canton-S larvae are attracted to 8% ethanol throughout the post-embryonal development. Data points represent the median behavioral response (substrate choice) according to the time after egg laying (AEL). Note a decrease of the behavioral response to some time points (48 h AEL, 72 h AEL, 96 h–120 h AEL) which correlates to larval molting events. Statistics are presented in Table 1.
Table 1.
Statistical analysis of substrate choice throughout the post-embryonal development of D. melanogaster.
| Developmental time points (AEL) | N | Preference index | Statistics Dunn’s multiple comparison | Event | CI |
|---|---|---|---|---|---|
| 36 h | 32 | 0.57 | *p < 0.001 | 1st molt | 0.44–0.63 |
| 48 h | 24 | 0.33 | 0.30–0.52 | ||
| 60 h | 31 | 0.77 | *p < 0.002 | 2nd molt | 0.59–0.73 |
| 72 h | 33 | 0.53 | 0.41–0.55 | ||
| 84 h | 16 | 0.65 | *p < 0.001 | Feeding L3 | 0.51–0.69 |
| 96 h | 32 | 0.47 | 0.38–0.53 | ||
| 108 h | 16 | 0.43 |
*Comparison 84 h AEL p < 0.017 |
Wandering L3 | 0.35–0.54 |
| 120 h | 16 | 0.35 |
*Comparison 84 h AEL p < 0.001 |
0.27–0.45 |
Ethanol has nutritional benefit for the larva
Ethanol can be used as energy source (reviewed in43). However, the nutrient gain for the larva appears to be lower, as survival rates on ethanol substrates are lower than those of adult Drosophila44,45 and also than those of larvae on sugar diets20. To complicate matters, ethanol is pharmacologically active and in higher concentrations toxic and needs to be neutralized46–48. These diametrically opposed effects complicate the interpretation of ethanol-dependent survival studies. To resolve and better interpret these effects, we analyzed ethanol dependent survival under standard conditions applied for other environmental cues18,20,23. We put L2 Canton-S larvae in food vials (to ease comparison with previous studies18–20) containing 1% agarose and either no ethanol, 4%, 8%, 12%, or 20% (Fig. 3). We calculated a survival rate by counting the number of living larvae and pupae for eight days in a 24 h cycle (Fig. 3b,c). The results show two effects: at 4% ethanol, more larvae survive than on control agarose until pupation (73.8% versus 35.8%; Fig. 3b,c, light blue line) and at higher concentration of 12% and 20% only few animals survive until pupation (21.40% and 12.40%, respectively; Fig. 3b,c, black line for 12% and dark blue line for 20%). The larval survival rates on control agarose and 8% ethanol are right in between (Fig. 3b, 44.9% versus 60.9%, green versus blue line). However, of these animals less larvae pupated on 8% ethanol in comparison to control agarose condition (Fig. 3c).
Figure 3.
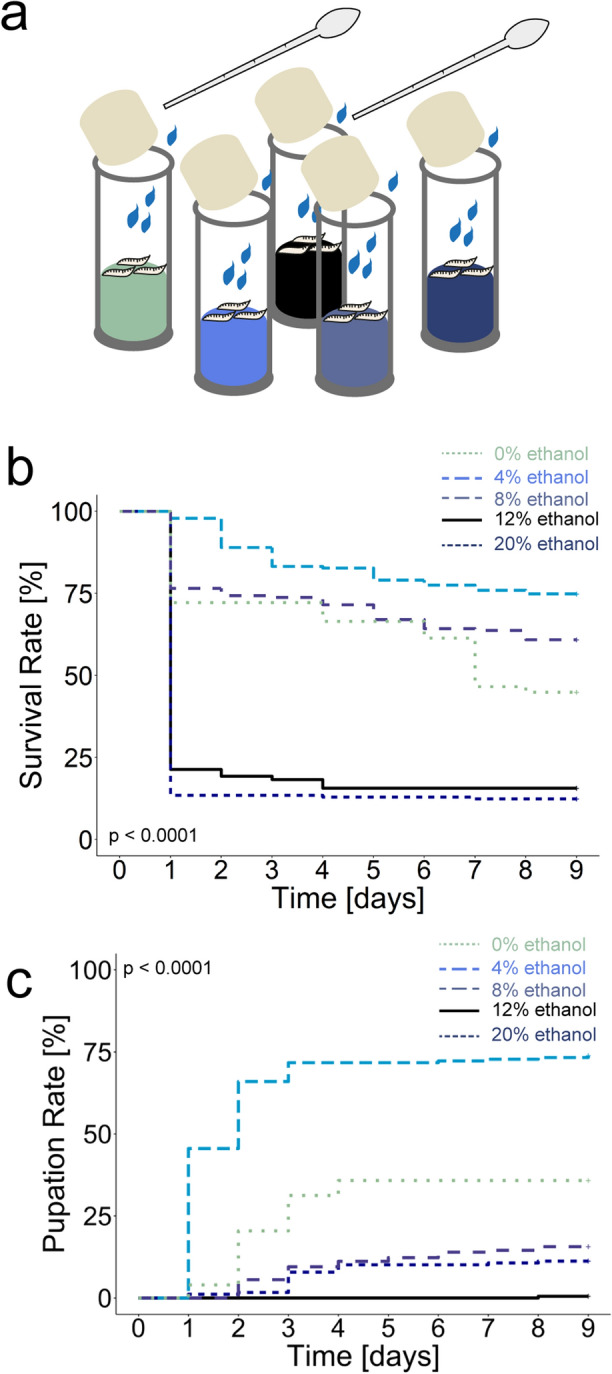
Larval survival on diets containing different concentrations of ethanol. (a) Scheme of the experimental procedure. Independent groups of 12 second instar Canton-S larvae were put into food vials that contained an agarose substrate plus different concentration of ethanol. Surviving of larvae and pupae were counted every 24 h. Water was added every 24 h to avoid dehydration. Pupation was used as measure of survival. (b) Diagram shows Kaplan–Meier survival curves of larvae. Larvae were put either on 0% (green), 4% (light blue), 8% (blue), 12% (black), or 20% ethanol (dark blue) diet for eight days. 12% and 20% ethanol significantly reduced larval survival as most of the animals died within one day (survival rate12%Ethanol = 21.40%, survival rate20%Ethanol = 12.40%). Larvae reared on 0% and 8% ethanol showed a nearly similar survival (Log-Rank-test, p < 0.001, survival rate0%Ethanol = 44.90%, survival rate8%Ethanol = 60.90%). In contrast 4% ethanol diet significantly increased larval survival compared to the 0% ethanol control diet (Log-Rank-test, p < 0.001, survival rate4%Ethanol = 74.90%). (c) Diagram shows Kaplan–Meier survival curves of the related pupation rates of the surviving animals shown above raised at 0% (green), 4% (light blue), 8% (blue), 12% (black), or 20% ethanol (dark blue) diet. 4% ethanol diet significantly increased the pupation rate compared to larvae reared at 0% ethanol diet (Log-Rank-test, p < 0.001, pupation rate0%Ethanol = 35.8%, pupation rate4%Ethanol = 73.80%). In contrast 8%, 12%, and 20% ethanol diet significantly reduced the pupation rate compared to larvae reared at 0% ethanol diet (Log-Rank-test, p0%-8%Ethanol < 0.001, p0%-12%Ethanol < 0.001, p0%-20%Ethanol < 0.001, pupation rate8%Ethanol = 15.6%, pupation rate12%Ethanol = 0.5%, pupation rate20%Ethanol = 12.2%). Single scores and statistical tests underlying the different survival curves are documented in the Supplementary material. Sample size for each Kapplan-Meier survival curve is n = 16.
Larvae perceive ethanol as an olfactory cue
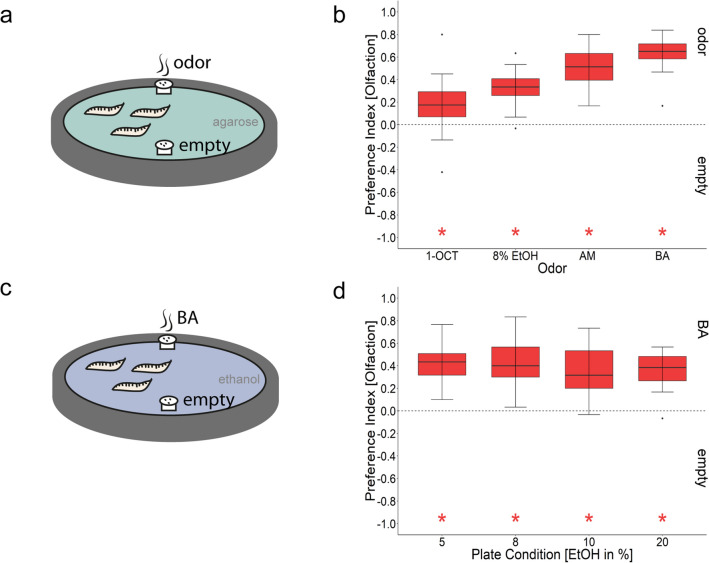
Initially we performed substrate choice experiments that allowed larvae to get into direct contact with ethanol in addition to smelling the odorant source (Figs. 1, 2). To disentangle the olfactory from contact cues we refined our behavioral approach by presenting ethanol in custom-made Teflon containers with perforated lids. This eliminates a physical contact with the substrate to specifically address the olfactory response of larvae (Fig. 4). We compared the attraction of 8% ethanol to three well-known attractive odorants: 1-octanol (1-OCT), amyl acetate (AM), and benzaldehyde (BA)15–17. Canton-S larvae showed olfactory preference for all four odorants (Fig. 4b). Thus, D. melanogaster larvae perceive ethanol as an attractive olfactory stimulus. In the next step we analyzed whether the larva can detect other odorants in a homogeneous ethanol background, which is a basic requirement for further odorant-ethanol learning experiments (Fig. 4d). Since BA triggered the greatest behavioral response, we used this stimulus to test whether larvae can still perceive it on 5%, 8%, 10% and 20% ethanol containing agarose plates (Fig. 4d). For all four ethanol concentrations wild-type Canton-S larvae were similarly attracted to BA (Fig. 4d). Therefore, in the assay D. melanogaster larvae could distinguish BA from a week or high concentrated ethanol background.
Figure 4.
The effects of ethanol on larval olfactory attraction. (a) Scheme of the experimental procedure. The olfactory stimuli 1-OCT, AM, BA, and 8% ethanol were presented on a 2.5% agarose plate in custom made Teflon containers to prevent direct contact of the animals with the chemical. Larvae were allowed to crawl for 5 min. (b) Canton-S larvae preferred all odors including 8% ethanol over empty containers (n1-Octanol = 19, Pref1-Octanol = 0.17, ci = 0.04–0.29, nAM = 8, PrefAM = 0.51, ci = 0.3–0.69, n8%Ethanol = 16, Pref8%Ethanol = 0.33, ci = 0.24–0.41, nBA = 16, PrefBA = 0.65, ci = 0.55–0.72). All groups were significantly different to zero (one-sample t test, p1-octanol < 0.011, pAM < 0.001, p8%Ethanol < 0.001, pBA < 0.001). (c) Scheme of the experimental procedure. Test plates contained 2.5% agarose mixed with either 5%, 8%, 10%, or 20% ethanol. BA was presented in Teflon containers. Larvae were allowed to crawl for 5 min. (d) Canton-S larvae showed an olfactory preference for BA irrespective of the ethanol concentrations in the test plate. All groups were significantly different to zero (one-sample t test, pBA-5%Ethanol < 0.001, pBA-8%Ethanol < 0.001, pBA-10%Ethanol < 0.001, pBA-20%Ethanol < 0.001), but not different from each other (ANOVA, p < 0.697). Sample size for each box plot is n = 16. Differences against zero are indicated in red at the bottom of each panel. Significant differences of two groups are specifically indicated with an asterisk. Non-significant results are not indicated. Preference scores and statistical tests underlying the different indices are documented in the Supplementary material.
Ethanol provides a teaching signal for larval olfactory learning
Larvae of D. melanogaster are able to associate odorants (conditioned stimulus) with cues of different sensory modalities (unconditioned stimulus) to establish appetitive or aversive olfactory memories. So far, tastants, temperature, vibration, electric shock, and light have been identified as teaching signals (reviewed in14). In contrast to adult flies, ethanol has not yet been tested as reinforcer. Therefore, we performed standardized learning experiments to validate the potential of ethanol in differential conditioning. In this assay larvae are trained three times with a given concentration of ethanol (2.5%, 8%, or 20% as a teaching signal) and then tested on an agarose plate for their odorant preference between the previously ethanol paired and the non-paired odorant (Fig. 5). Higher ethanol concentrations of 8% and 20%, in contrast to a lower concentration of 2.5%, provide an appetitive teaching signal for larval olfactory learning (Fig. 5b). For appetitive olfactory learning with fructose, it has been reported that larvae show no memory when tested in the presence of the unconditioned stimulus. This is probably because in the presence of food the larvae do not have to search for food41,49. This also seems to be the case for ethanol, since larvae trained with 8% ethanol only recall odor-ethanol memory on control agarose, but not on a test plate containing 8% ethanol (Fig. 5c). Ethanol memory also appears to be somehow similar to fructose memory, as even 2 M fructose added to the test plate prevents the recall of the odor-ethanol memory.
Figure 5.
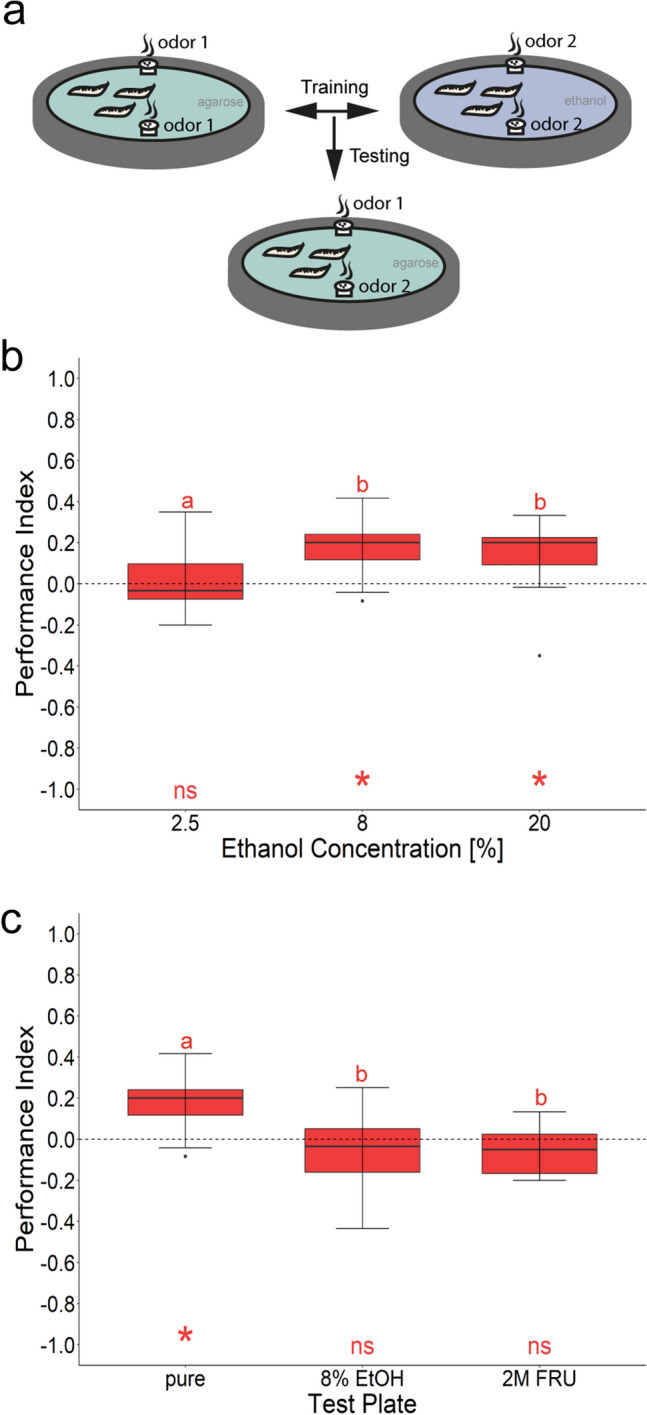
Larval olfactory learning reinforced by three different ethanol concentrations. (a) Scheme of the experimental procedure. Canton-S were trained three times with two odorants (AM and BA) and either 2.5%, 8%, or 20% ethanol as reinforcer. Olfactory memory is quantified by the performance index (PI). (b) Larvae trained with 8% and 20% ethanol concentration show a significant appetitive memory (PI8%Ethanol = 0.20, ci = 0.11–0.26, PI20%Ethanol = 0.20, ci = 0.05–0.24, one-sample t test, p8% < 0.001, p20% < 0.005). When 2.5% ethanol was used as a teaching signal no memory was detectable (PI2.5%Ethanol = 0.83, ci = − 0.07 to 0.08, one-sample t test, p2.5% < 0.828). Accordingly, larvae trained with 8% and 20% ethanol behaved differently from larvae trained with 2.5% ethanol (Dunn’s Multiple Comparison test, p < 0.001 and p < 0.003, respectively). (c) Larvae trained with 8% ethanol concentration as teaching signal and tested on control agarose showed a significant appetitive memory (PI = 0.20, ci = 0.11–0.26, one-sample t test, p < 0.001); if tested in the presence of the teaching signal of 8% ethanol or 2 M fructose (2 M FRU), appetitive associative memory is not expressed (PI8%Ethanol = − 0.03, ci = − 0.17 to 0.05, PI2MFru = − 0.05, ci = − 0.11 to 0.01) as it is not significantly different from zero (one-sample t test, p8%Ethanol < 0.260, p2MFru < 0.080) and not significantly different from each other (paired t-test, p < 0.902). Differences against zero are indicated in red at the bottom of each panel. Sample size for each box plot is n = 15. Significant differences between the groups are indicated with letters; Preference scores and statistical tests underlying the different indices are documented in the Supplementary material.
Ethanol does not alter appetitive and aversive olfactory memory
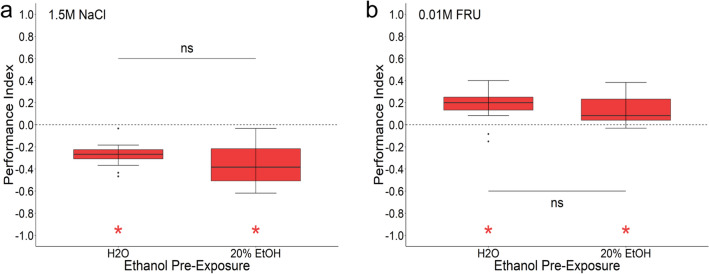
Treating larvae with 20% ethanol for 20 min impairs aversive odorant-heat shock memory after one-odor conditioning at 35 °C50. The effect of ethanol was absent, when the odorant was paired with a higher temperature heat-shock of 41 °C, which likely forms a stronger memory unsusceptible for ethanol treatment. Therefore, we similarly investigated whether the 20 min incubation with 20% ethanol interferes with larval olfactory memory formation when the odorant is paired with fructose or salt (Fig. 6). We found that both, aversive salt memory (1.5 M NaCl; Fig. 6a) and appetitive sugar memory (0.01 M fructose; Fig. 6b), showed no reduction after ethanol treatment in comparison to H2O treated larvae. Therefore, the olfactory memory reinforced by salt or fructose was not altered by ethanol treatment.
Figure 6.
Pre-exposure to ethanol does not change aversive and appetitive olfactory learning. Before the larvae were conditioned, they were separated from their food and treated for 20 min with either water or 20% ethanol. They were then washed and trained with the odors AM and BA and either 1.5 M NaCl or 0.01 M fructose as a teaching signal. (a) Larvae treated with water and 20% ethanol both showed an aversive odor-salt memory (one-sample t test, pwater < 0.001, ppre-EtOH < 0.001) that was not different from each other (paired t-test, p < 0.112). Sample size for each box plot is n = 15. (b) Larvae treated with water and 20% ethanol both showed an appetitive odor-fructose memory (one-sample t test, pwater < 0.001, ppre-EtOH < 0.001) that was not different from each other (paired t-test, p < 0.484). Sample size for each box plot is n = 15. Differences against zero are indicated in red at the bottom of each panel. Significant differences of two groups are specifically indicated with an asterisk. Non-significant results are not indicated. Preference scores and statistical tests underlying the different indices are documented in the Supplementary material.
Discussion
Due to the numerically relatively simple anatomy of the larva, it has an enticing analytical power based on the combination of genetic tractability, the availability of robust behavioral assays, the possibility for transgenic single cell manipulation, and an emerging synaptic connectome of the complete central nervous system. The focus of this work was on the behavioral description of ethanol driven larval behavior in order to provide a basis for subsequent genetic, molecular and cellular studies.
Ethanol is an important stimulus in the environment of adult and larval D. melanogaster (reviewed in51,52). The concentration of ethanol in the natural habitat of this species vary between 0.6% in ripe hanging fruits and up to 4.5% in rotting ones53. In some man-made environments, such as wine cellars, the ethanol concentration can reach more than 10%53–55. Given that adult D. melanogaster often carry yeast to the egg laying sites and inoculate ripening fruits, the induced fermentation and associated ethanol production is likely to result in higher concentrations at the larval stage56. Therefore, the naturally occurring range of ethanol concentrations experienced by a larva is in agreement with most of the concentrations tested in our behavioral analysis (Figs. 1, 3, and 5). Only the highest concentration tested (20%, 30%, and 50% ethanol) do not occur in the natural habitat of the larva. Of course, one has to keep in mind that our experiments were performed under laboratory conditions using a substrate of agarose mixed with ethanol. Due to the evaporation of ethanol, which is indeed volatile, especially higher ethanol concentrations could have been lower in reality. However, these effects are likely to be small, as studies have shown that within the first few hours, ethanol levels drop only slightly in a 13% ethanol containing Petri dish55. This corresponds approximately to the period of preparation of the plates and the conduction of the ethanol attraction and learning experiments. Similarly, evaporation seems to be of little relevance in the survival experiments, where the experimental vials were changed every day. It was shown that the ethanol concentration in vials closed with polyurethane bungs remains very stable over several days (in bottles with 6% ethanol in the medium the fall is to 3.5% after six days)55.
The ethanol attraction is based on various sensory modalities
We show that D. melanogaster larvae preferentially migrate to substrates containing ethanol, regardless of whether they have been previously exposed to ethanol or not. In contrast to adult Drosophila 57, exposure over several days does not increase the attractiveness of ethanol to larvae (Fig. 1d). Larval attraction appears to follow a Gaussian-shape like dose response curve reaching a plateau between 4 and 10% (Figs.1 and 2). Our results are in agreement with several published findings that have shown that larvae prefer 1%58, 2.5%59, 5%59, 6%31–33, 10%58,59, 17%34, 20%59 and even pure ethanol58. Thus, our standardized experimental design provides a robust behavioral assay that allows to identify the neural and molecular basis of larval ethanol substrate choice. However, it must be mentioned that there are some studies on larval ethanol substrate choice, where the tested animals show no or only weak attraction to ethanol concentrations from 2 to 6%38,39,60 and even aversion at higher concentrations39,60. However, it is not clear whether, in addition to methodological matters such as a low volume of applied ethanol38, other factors such as the enzymatic activity of Adh, the surrounding temperature, specific pre-treatments and the precise experimental procedure are responsible for this32–34,39,59. Likewise, there are changes across larval development. During first and second molting, the attractiveness of ethanol is reduced (Fig. 2). During these developmental stages, larval locomotion is generally reduced as they renew their exoskeleton on which all external sensory organs are also located61. Our results also suggest that certain mutations may alter the attraction to ethanol, as w1118 mutants showed a reduced attraction to higher ethanol concentrations compared to the wild-type (Fig. 1). The white gene encodes for an ABC transporter a key player of the eye pigmentation pathway62. In addition, white mutant flies also possess abnormally low levels of the biogenic amines serotonin, dopamine, and histamine63,64. Whether this plays a role in the lower attractiveness of these mutants towards 10% and 20% ethanol, however, remains to be investigated.
Substrate attraction tests (Figs. 1 and 2) allow larvae to come into direct contact with ethanol. Therefore, larvae can potentially be guided by different inputs: the sense of smell or taste, but also by a caloric gain or a pharmacological effect. Examples for such cases are reported for adult D. melanogaster (reviewed in26,51,65). In this work, we have separated gustatory and olfactory inputs from each other. (Please note that the pharmacological effect can only be analyzed by behavioral changes and measurement of endogenous ethanol concentration in the larvae, which were not done in our study). To investigate whether larvae specifically perceive and behaviorally respond to ethanol as an odorant, we have filled the stimulus in containers that allow ethanol to evaporate, but prevent the larvae from directly contacting it and thus excludes feeding or ingesting it (Fig. 4). This has not been studied so far. Larval ethanol attraction for the odorant stimulation was clearly evident (Fig. 4), but reduced compared to the substrate attraction of ethanol (Fig. 1). The behavioral response is almost halved from a median of 0.61 (Fig. 1) to 0.33 (Fig. 4). This means that (1) larvae indeed do perceive ethanol via the olfactory signaling pathway and (2) larvae can perceive ethanol not only as an odor.
Ethanol is both nutritious and toxic for the larva
Survival experiments, as we have shown in Fig. 3, are often used to analyze a positive life-prolonging effect (e.g. based on additional calories of sugar in a minimal diet), or to identify harmful effects that lead to an increased mortality of larvae (e.g. by adding bitter compounds to the food)18–20. Our results suggest that ethanol performs two opposing functions. Below 8% the positive—most likely nutritional effect—increases larval survival on control agarose; at high concentrations above 8% ethanol toxicity prevails (Fig. 3). Many early studies focused on the effects of ethanol on larval survival and increases were referred to as tolerance (reviewed in27). Nowadays tolerance usually stands for an individual's resistance to the intoxicating effects of ethanol, according to its importance for humans27. Similar to our results (Fig. 3), previous work showed that ethanol consumption of up to 4% leads to increased larval fitness47,48,66,67. Parson and colleagues showed that ethanol concentrations of 0.5%–3% allow larvae to develop to third instar larvae. At a concentration of 6% or higher this was not the case48. Quite similar, McKechnie and Geer showed that wild-type Canton-S larvae transferred to ethanol concentrations of up to 4.5% partially survived into pupae47. Thus, in agreement with published results, our data suggest that Drosophila larvae benefit from metabolizing ethanol. Because ethanol is one of the primary alcohols, it is degraded to aldehydes, which then act as acetyl-CoA in metabolic processes such as the Krebs cycle (reviewed in43). Thus, ethanol contributes to the formation of adenosine triphosphate (ATP), which can be used directly or indirectly as an energy source. Studies in larvae have also shown that the carbons of ethanol serve as backbones for triacylglycerols and phospholipids66,68. Consequently, larvae prefer ethanol and associate it with a rewarding function, as they benefit from metabolizing the amount of ethanol usually found in their natural environment.
At higher ethanol concentrations the Adh dependent detoxification reaches its limits. In most studies, reduced survival rates occur at concentrations above 9% or 16% of ethanol28,46,48 (but see also for lower concentrations47). Increasing developmental delay and reduced body size in adulthood have also been described for ethanol-containing diets of 5%, 10%, and 12%69. Our results are therefore comparable with most of the published work, as we show that larvae do not display increased mortality at 8% ethanol, but many of them die quickly at ethanol concentrations of 12% and 20% (Fig. 3). It was shown that high intracellular concentrations of ethanol limits the capacities to synthesis and store glycogen, lipid, protein in the larval fat body cells66, and reduces cell division rates in the developing larval central nervous system69. Even structural changes within cells of the endoplasmatic reticulum and mitochondria were visible66. Therefore, higher doses of ethanol have a deleterious effect at both the cellular and organism levels.
Surprisingly, larvae still seek out these deleterious concentration and process it as rewarding in learning experiments (Figs. 1, 3, 5). Thus, it is tempting to speculate that the answer is based on the organization of the larval microhabitats. Ethanol occurs most often as a gradient in food sources. Therefore, it might be advantageous for the larva to seek out even high ethanol sources in the first step to then consume lower substrate concentrations on site in the second step. In general, however, it appears that larvae respond differently to a given concentration of a chemosensory stimulus depending on their behavioral response. 0.3 M sodium chloride is usually repellent to larvae, but can be used as a positive teaching signal in olfactory learning experiments42. 2 M arabinose, a sugar that is sweet but offers no nutritional benefit, is harmful for the larva, but also highly attractive and triggers a reward function during learning20. It appears that this is a general function for many chemosensory components, which may be based on mutual neuronal and genetic mechanisms.
Ethanol provides a positive teaching signal
To our knowledge it has not yet been tested whether larvae can utilize ethanol as a positive teaching signal (unconditioned stimulus) in learning and memory assays. Our results suggest that this is the case for concentrations of 8% and 20% ethanol (Fig. 5). Therefore, not only adult flies can use ethanol as a teaching signal70,71, but also larvae. The initial memory of adult flies, however, is aversive and only changes after 24 h into a positive long-term memory that lasts several days70. However, these results are based on multiple training stimuli that are separated in time (spaced training). Adult training protocols more similar to the larval procedure used here are also able to induce appetitive memories71. Thus, the adult behavioral response is much more similar to the larval one than suspected at first glance. In addition, we gain increasing insight to understand what stimulation the larva classifies as rewarding. Chemosensory stimuli include ethanol, low salt concentrations, amino acids, ribonucleosides and various sugars (e.g., sucrose, glucose, maltodextrin, sorbitol, ribose), even though some cannot be metabolized (arabinose, xylose)20,21,42,49,72,73. Without a direct comparison, it is of course difficult to assess how rewarding ethanol is for the larva. However, a comparison of published values suggests that fructose and a low salt concentration, which are used in most studies, lead to reward memories that are about twice as strong.
We also do not yet have any insights into the neuronal and molecular organization of larval ethanol learning and memory. However, the assay described here allows their analysis. It is worth noting that ethanol memory cannot be retrieved on a fructose test plate (Fig. 5). According to Schleyer and colleagues D. melanogaster larvae search for food after the conditioning phase based on their acquired experience. Thus, the odorant paired with ethanol predicts a certain gain. At the moment of testing, the animals expect such a gain and compare it with their current environmental input. It seems that the larvae do not only expect a positive gain in this situation but also its specific quality. This was shown as sugar memory could be recalled on a positive aspartic acid containing test plate and vice versa74. Following the same logic, the absence of ethanol memory recall on a fructose plate thus means that larvae do not distinguish between ethanol and fructose quality. Therefore, it is possible that fructose and ethanol processing circuits overlap in the larval brain. In adult flies food reward and ethanol circuits overlap in context of regulating ethanol preference. Thus is tempting to speculate that this is the case in larvae as well. However, to exclude that sugar presence just distracts larvae from odorant attraction in our experiments further work has to be done.
Outlook
Which cells and molecules might be involved in the perception of ethanol in larvae? Based on studies on olfactory ethanol sensing in adult flies, the octopaminergic system and different olfactory receptors can be considered as an analysis entry point75,76. Using the Flywalk77 or a two odor vial assay78 to measure naive adult olfactory output, it was shown that the olfactory co-receptor Orco plays an essential role in both assays when ethanol was applied. For the Flywalk analysis the effect could even be refined to the olfactory receptor genes Or42b and Or59b76. At the larval stage, only Or42b is expressed in a single ORN of the dorsal organ, the main larval olfactory sense organ15. Or42b responds to ethyl acetate, ethyl butyrate, propyl acetate and pentyl acetate; chemicals that are all highly attractive to larvae16,17. It is tempting to expect that Or42b serves a similar function at the larval stage. However, ethanol has not yet been tested, and we lack any molecular and neuronal information on its perception in the larva. With respect to learning and memory, we have recently obtained a cellular understanding of the involved neuronal pathways of the larval brain. Four dopaminergic neurons of the primary protocerebral anterior medial (pPAM) cluster encode fructose dependent teaching signals and these neurons are directly connected to the mushroom body, the larval memory center9,79–82. Hence, it is now possible to test whether aspects of the ethanol reward learning and memory are also processed by the same neuronal mushroom body network. This would also reveal a conservation of the functional patterns throughout development, since adult appetitive ethanol memory also requires dopaminergic cells and the mushroom body70.
Methods
Fly strains
Fly strains were kept on standardized cornmeal medium containing 1% ethanol at 25 °C and 65% humidity under a 14:10 h light:dark cycle. Adult flies were transferred to new food vials every 72 h. Larvae were taken from food vials and briefly washed in tap water to remove food residues. Larvae were then collected with a small brush and transferred to a small drop of tap water in groups of 30 or 12 larvae, respectively. Two fly strains wild-typeCS and mutant w1118 were used in the experiments. For experiments at specific time points during larval development, larvae with defined age were used. If not otherwise mentioned larval of different age were used, except for the wandering larval three. For staging larvae, adult flies were allowed to lay eggs for six hours; larvae were then collected in a 12 h cycle starting 36 h after egg laying (AEL).
Substrate choice
For gustatory preference tests, Petri dishes (85 mm diameter; Greiner) were filled with 2.5% agarose substrate (VWR Life Science; type number: 97062-250). Agarose and ddH2O were mixed and boiled in a microwave oven. A thin layer of the hot mixture was filled into Petri dishes. After cooling, agarose was removed from half of the plate and re-filled with 2.5% agarose substrate containing different concentration of 99.8% ethanol (1%, 2%, 4%, 8%, 10%, 20%,30%, or 50% ethanol; CHEMSOLUTE®; type number: 2273.1000). In all experiment the final volume was kept the same. No obvious change of the texture of the plates was seen with increasing ethanol concentrations up to 50%. Therefore, we currently have no evidence that our results are affected by texture effects, although some are described in the literature23,24. To keep an evaporation effect as low as possible, groups of 30 larvae of different ages were placed immediately after plate preparation (in the order of maximum 45 min) in the middle of the Petri dish and allowed to crawl for 5 min (or up to 120 min) at room temperature (RT)55. Then, larvae were counted on each side of the Petri dish. A preference index (Pref) was calculated by subtracting the number of larvae on the control agarose side (#nS) from the number of larvae on the side with a stimuli (#S), divided by the total number of larvae (#total): Pref = (#S − #nS)/#total. A positive Pref indicates attraction and a negative aversion to the presented stimuli.
Olfactory attraction
To test for olfactory attraction, Petri dishes (85 mm diameter; Greiner) were filled 2.5% agarose substrate or with 2.5% agarose substrate with different concentration of ethanol (5%, 8%, 10% and 20%). Agarose and ddH2O were mixed and boiled in a microwave oven. A thin layer of the hot mixture was filled into Petri dishes. Closed Petri dishes were kept at room temperature and used on the same day. To test the olfactory stimuli of ethanol, either 8% ethanol, benzaldehyde (BA; Sigma-Aldrich, type number: 102213897; undiluted), amyl acetate (AM; Sigma-Aldrich, type number: 102172386); diluted 1:250 in paraffin oil), 1-octanol (1-Oct; Sigma-Aldrich, type number: 101858766 undiluted) or distilled water was filled into custom-made Teflon containers (4.5 mm diameter) with perforated lids and placed on each side of the plate. Immediately after plate preparation, groups of 30 larvae were placed in the middle of the Petri dish and allowed to crawl for 5 min at RT. Then, larvae were counted on each side of the Petri dish. A preference index (Pref) was calculated by subtracting the number of larvae on the side without an odor (#nO) from the number of larvae on the side with an odor (#O), divided by the total number of larvae (#total): Pref = (#O − #nO)/#total. A positive Pref indicates an attractiveness, a negative Pref represents an avoidance.
Olfactory ethanol learning and memory
All experiments were performed on Petri dishes filled with either 2.5% control agarose or 2.5% agarose containing different concentrations of ethanol (8% or 20%), 0.01 M fructose (Carl Roth®; type number: 4981.2) or 1.5 M sodium chloride (Carl Roth®; type number: 3957.2). Agarose and ddH2O were mixed and boiled in a microwave oven. A thin layer of the hot mixture was filled into Petri dishes. Closed Petri dishes were kept at room temperature and used on the same day. As olfactory stimuli, we filled 10 µl AM, and 10 µl BA, in custom-made Teflon containers (4.5 mm diameter) with perforated lids. Immediately after plate and container preparation, groups of 30 larvae were placed in the middle of the Petri dish containing a control agarose substrate. All experiments were conducted at RT. Larvae were exposed to AM and allowed to crawl for 5 min. Then, larvae were transferred with a small brush to a new Petri dish containing a positive (ethanol, fructose) or negative (NaCl) reinforcer and exposed to BA for 5 min. Each transfer usually takes about one minute. After three training cycles, larvae were placed on a new Petri dish containing control agarose substrate or agarose substrate containing 8% ethanol, 0.01 M fructose, or 1.5 M sodium chloride and exposed to AM and BA on opposite sides for 5 min. Then, larvae were counted on each side of the Petri dish. A second group of larvae was trained via a reciprocal training regime. For each group an independent olfactory preference index was calculated as described above. A performance index (PI) was calculated by adding the Pref of the first training group (Pref1) to the Pref of the second training group (Pref2) and dividing them by the number of experimental groups (#2): PI = (Pref1 + Pref2)/#2. A positive PI indicates an attractiveness; a negative PI represents an avoidance.
Larval survival on ethanol
To investigate larval survival in the presence of ethanol, 12 wild-type L2 stage larvae were placed in vials containing either 1% control agarose substrate or 1% agarose substrate plus different concentrations of ethanol of 4%, 8% or 20%. Three drops of tap water were daily added to prevent larvae from dehydrating. Larvae that were alive and later the pupal stage were counted from day 1 to day 9. The data are shown as Kaplan–Meier survival curves. The percentage of survival and pupation were calculated as follows: Percentage = (number of living larvae or pupae / total number of larvae or pupae) × 100. For each condition 16 independent experimental groups were analyzed (n = 16).
Data analysis and visualization
Groups that did not violate the assumption of normal distribution (Shapiro–Wilk test) and homogeneity of variance (Bartlett's test) were analyzed with parametric statistics: paired t-test (comparison between two groups) or Oneway ANOVA followed by planned pairwise comparisons between the relevant groups with a Tukey honestly significant difference HSD post hoc test (comparisons between groups larger than two). Experiments with data that were significantly different from the assumptions above were analyzed with non-parametric tests, such as Wilcoxon signed rank test (comparison between two groups) or Kruskal–Wallis test followed by Dunn's multiple pairwise comparison (comparisons between groups larger than two). To compare single genotypes against chance level, we used one-sample t test. All statistical analyses and data visualization were done with R (V 3.5.1) in RStudio by using stats, dunn.test and ggplot2 package83. Figure panels were edited with Adobe Illustrator CS5 (San Jose, CA, USA). The significance level of statistical tests was set to 0.05; the shown confidence interval (ci) is the 95% confidence interval of the data plots. Data are displayed as box plots with the median as the middle line, the box boundaries as 25% and 75% quantiles and the whiskers as 1.5 times the interquartile range. Outliers are shown as dots directly above or below the box plots. Larval and pupal survival rates are shown as Kaplan–Meier curves. Further details are documented in the Supplemental Data files.
Supplementary Information
Acknowledgements
The authors thank Wolf Huetteroth, Tilman Triphan, Bert Klagges and Astrid Rohwedder for fruitful discussions and comments on the paper. This work was supported by grants from the German Research Foundation (DFG) to AST (TH1584/3-1, TH1584/6-1 and TH1584/7-1) and HS (HS675/10-1).
Author contributions
I.S. and A.S.T. outlined the manuscript. I.S., M.B., H.S. and A.S.T. developed the design of the methodology; I.S., M.B., N.N., Y.S. and J.S. conducted the experiments; I.S. prepared and created the analysis and visualization of the data; I.S., M.B., H.S. and A.S.T. wrote and finalized the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91677-3.
References
- 1.Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. Principles of Neural Science. McGraw-Hill Education Ltd; 2012. [Google Scholar]
- 2.Gerber B, Stocker RF, Tanimura T, Thum AS. Smelling, tasting, learning: Drosophila as a study case. Results Probl. Cell Differ. 2009;47:139–185. doi: 10.1007/400_2008_9. [DOI] [PubMed] [Google Scholar]
- 3.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 4.Liman ER, Zhang YV, Montell C. Peripheral coding of taste. Neuron. 2014;81:984–1000. doi: 10.1016/j.neuron.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahanukar A, Hallem EA, Carlson JR. Insect chemoreception. Curr. Opin. Neurobiol. 2005;15:423–430. doi: 10.1016/j.conb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Li HH, et al. A GAL4 driver resource for developmental and behavioral studies on the larval CNS of Drosophila. Cell. Rep. 2014;8:897–908. doi: 10.1016/j.celrep.2014.06.065. [DOI] [PubMed] [Google Scholar]
- 7.Ohyama T, et al. A multilevel multimodal circuit enhances action selection in Drosophila. Nature. 2015;520:633–639. doi: 10.1038/nature14297. [DOI] [PubMed] [Google Scholar]
- 8.Berck ME, et al. The wiring diagram of a glomerular olfactory system. Elife. 2016 doi: 10.7554/eLife.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eichler K, et al. The complete connectome of a learning and memory centre in an insect brain. Nature. 2017;548:175–182. doi: 10.1038/nature23455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miroschnikow A, et al. Convergence of monosynaptic and polysynaptic sensory paths onto common motor outputs in a Drosophila feeding connectome. Elife. 2018 doi: 10.7554/eLife.40247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeiffer BD, et al. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diegelmann S, Klagges B, Michels B, Schleyer M, Gerber B. Maggot learning and synapsin function. J. Exp. Biol. 2013;216:939–951. doi: 10.1242/jeb.076208. [DOI] [PubMed] [Google Scholar]
- 13.Gerber B, Stocker RF. The Drosophila larva as a model for studying chemosensation and chemosensory learning: A review. Chem. Senses. 2007;32:65–89. doi: 10.1093/chemse/bjl030. [DOI] [PubMed] [Google Scholar]
- 14.Widmann A, Eichler K, Selcho M, Thum AS, Pauls D. Odor-taste learning in Drosophila larvae. J. Insect Physiol. 2018;106:47–54. doi: 10.1016/j.jinsphys.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Fishilevich E, et al. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr. Biol. 2005;15:2086–2096. doi: 10.1016/j.cub.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Hoare DJ, et al. Modeling peripheral olfactory coding in Drosophila larvae. PLoS ONE. 2011;6:e22996. doi: 10.1371/journal.pone.0022996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreher SA, Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59:110–124. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apostolopoulou AA, et al. Caffeine taste signaling in Drosophila larvae. Front. Cell Neurosci. 2016;10:193. doi: 10.3389/fncel.2016.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apostolopoulou AA, Mazija L, Wust A, Thum AS. The neuronal and molecular basis of quinine-dependent bitter taste signaling in Drosophila larvae. Front. Behav. Neurosci. 2014;8:6. doi: 10.3389/fnbeh.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohwedder A, et al. Nutritional value-dependent and nutritional value-independent effects on Drosophila melanogaster larval behavior. Chem. Senses. 2012;37:711–721. doi: 10.1093/chemse/bjs055. [DOI] [PubMed] [Google Scholar]
- 21.Schipanski A, Yarali A, Niewalda T, Gerber B. Behavioral analyses of sugar processing in choice, feeding, and learning in larval Drosophila. Chem. Senses. 2008;33:563–573. doi: 10.1093/chemse/bjn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H, Choi MS, Kang K, Kwon JY. Behavioral analysis of bitter taste perception in Drosophila larvae. Chem. Senses. 2016;41:85–94. doi: 10.1093/chemse/bjv061. [DOI] [PubMed] [Google Scholar]
- 23.Apostolopoulou AA, et al. Composition of agarose substrate affects behavioral output of Drosophila larvae. Front. Behav. Neurosci. 2014;8:11. doi: 10.3389/fnbeh.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudow N, Kamikouchi A, Tanimura T. Softness sensing and learning in Drosophila larvae. J. Exp. Biol. 2019 doi: 10.1242/jeb.196329. [DOI] [PubMed] [Google Scholar]
- 25.Parsons PA. The Evolutionary Biology of Colonizing Species. Cambridge Univ; 1983. [Google Scholar]
- 26.Kaun KR, Devineni AV, Heberlein U. Drosophila melanogaster as a model to study drug addiction. Hum. Genet. 2012;131:959–975. doi: 10.1007/s00439-012-1146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron. 2000;28:261–271. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 28.David JR, Bocquet C. Similarities and differences in latitudinal adaptation of two Drosophila sibling species. Nature. 1975;257:588–590. doi: 10.1038/257588a0. [DOI] [PubMed] [Google Scholar]
- 29.McDonald JF, Avise JC. Evidence for the adaptive significance of enzyme activity levels: Interspecific variation in alpha-GPDH and ADH in Drosophila. Biochem. Genet. 1976;14:347–355. doi: 10.1007/BF00484773. [DOI] [PubMed] [Google Scholar]
- 30.Milan NF, Kacsoh BZ, Schlenke TA. Alcohol consumption as self-medication against blood-borne parasites in the fruit fly. Curr. Biol. 2012;22:488–493. doi: 10.1016/j.cub.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsons PA. Larval reaction to alcohol as an indicator of resource utilization differences between Drosophila melanogaster and Drosophila simulans. Oecologia. 1977;30:141–146. doi: 10.1007/BF00345417. [DOI] [PubMed] [Google Scholar]
- 32.Parsons PA, King SB. Ethanol larval discrimination between two Drosophila sibling species. Experientia. 1977;33:898–899. doi: 10.1007/BF01951269. [DOI] [PubMed] [Google Scholar]
- 33.Parsons PA. Larval responses to environmental ethanol in Drosophila melanogaster: Variation within and among populations. Behav. Genet. 1980;10:183–190. doi: 10.1007/BF01066268. [DOI] [PubMed] [Google Scholar]
- 34.Cavener D. Preference for ethanol in Drosophila melanogaster associated with the alcohol dehydrogenase polymorphism. Behav. Genet. 1979;9:359–365. doi: 10.1007/BF01066974. [DOI] [PubMed] [Google Scholar]
- 35.Oakeshott JG, et al. Alcohol dehydrogenase and glycerol-3-phosphate dehydrogenase clines in Drosophila melanogaster on different continents. Evolution. 1982;36:86–96. doi: 10.1111/j.1558-5646.1982.tb05013.x. [DOI] [PubMed] [Google Scholar]
- 36.David J, Mercot H, Capy P, McEvey S, Van Herrewege J. Alcohol tolerance and Adh gene frequencies in European and African populations of Drosophila melanogaster. Genet. Sel. Evol. 1986;18:405–416. doi: 10.1186/1297-9686-18-4-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKechnie SW, McKenzie JA. Polymorphism of alcohol dehydrogenase (Adh) in a winery cellar population of Drosophila melanogaster: Gene frequency association with temperature and genotypic differences in progeny production. Evolution. 1983;37:850–853. doi: 10.1111/j.1558-5646.1983.tb05606.x. [DOI] [PubMed] [Google Scholar]
- 38.Cobb M, Bruneau S, Jallon JM. Genetic and developmental factors in the olfactory response of Drosophila melanogaster larvae to alcohols. Proc. Biol. Sci. 1992;248:103–109. doi: 10.1098/rspb.1992.0048. [DOI] [PubMed] [Google Scholar]
- 39.Gelfand LJ, McDonald JF. Relationship between ADH activity and behavioral response to environmental alcohol in Drosophila. Behav. Genet. 1980;10:237–249. doi: 10.1007/BF01067770. [DOI] [PubMed] [Google Scholar]
- 40.El-Keredy A, Schleyer M, Konig C, Ekim A, Gerber B. Behavioural analyses of quinine processing in choice, feeding and learning of larval Drosophila. PLoS ONE. 2012;7:e40525. doi: 10.1371/journal.pone.0040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schleyer M, et al. A behavior-based circuit model of how outcome expectations organize learned behavior in larval Drosophila. Learn. Mem. 2011;18:639–653. doi: 10.1101/lm.2163411. [DOI] [PubMed] [Google Scholar]
- 42.Niewalda T, et al. Salt processing in larval Drosophila: Choice, feeding, and learning shift from appetitive to aversive in a concentration-dependent way. Chem. Senses. 2008;33:685–692. doi: 10.1093/chemse/bjn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinstra PWH. Evolutionary genetics of the Drosophila alcohol dehydrogenase gene-enzyme system. Genetica. 1993;92:1–22. doi: 10.1007/BF00057503. [DOI] [PubMed] [Google Scholar]
- 44.Libion-Mannaert M, Delcour J, Deltmobe-Lietaert MC, Lenelle-Montfort N, Elens A. Ethanol as a "Food" for Drosophila melanogaster: Influence of the ebony gene. Experientia. 1976;32:22–24. doi: 10.1007/BF01932603. [DOI] [Google Scholar]
- 45.Pecsenye K, Lefkovitch LP, Giles BE, Saura A. Does Drosophila melanogaster use ethanol as an energy source during starvation? Hereditas. 1994;121:225–236. doi: 10.1111/j.1601-5223.1994.t01-1-00225.x. [DOI] [PubMed] [Google Scholar]
- 46.Fry JD. Direct and correlated responses to selection for larval ethanol tolerance in Drosophila melanogaster. J. Evol. Biol. 2001;14:296–309. doi: 10.1046/j.1420-9101.2001.00271.x. [DOI] [Google Scholar]
- 47.McKechnie SW, Geer BW. Regulation of alcohol dehydrogenase in Drosophila melanogaster by dietary alcohol and carbohydrate. Insect Biochem. 1984;14:231–242. doi: 10.1016/0020-1790(84)90034-9. [DOI] [Google Scholar]
- 48.Parson PA, Stanley SM, Spence GE. Environmental ethanol at low concentrations: Longevity and development in the sibling species Drosophilu melanogaster and D. simuluns. Aust. J. Zool. 1979;27:747–754. doi: 10.1071/ZO9790747. [DOI] [Google Scholar]
- 49.Gerber B, Hendel T. Outcome expectations drive learned behaviour in larval Drosophila. Proc. Biol. Sci. 2006;273:2965–2968. doi: 10.1098/rspb.2006.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson BG, et al. A low concentration of ethanol impairs learning but not motor and sensory behavior in Drosophila larvae. PLoS ONE. 2012;7:e37394. doi: 10.1371/journal.pone.0037394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devineni AV, Heberlein U. The evolution of Drosophila melanogaster as a model for alcohol research. Annu. Rev. Neurosci. 2013;36:121–138. doi: 10.1146/annurev-neuro-062012-170256. [DOI] [PubMed] [Google Scholar]
- 52.Guarnieri DJ, Heberlein U. Drosophila melanogaster, a genetic model system for alcohol research. Int. Rev. Neurobiol. 2003;54:199–228. doi: 10.1016/s0074-7742(03)54006-5. [DOI] [PubMed] [Google Scholar]
- 53.Dudley R. Ethanol, fruit ripening, and the historical origins of human alcoholism in primate frugivory. Integr. Comp. Biol. 2004;44:315–323. doi: 10.1093/icb/44.4.315. [DOI] [PubMed] [Google Scholar]
- 54.McKenzie JA, McKechnie SW. A comparative study of resource utilization in natural populations of Drosophila melanogaster and D. simulans. Oecologia. 1979;40:299–309. doi: 10.1007/BF00345326. [DOI] [PubMed] [Google Scholar]
- 55.Gibson JB, May TW, Wilks AV. Genetic variation at the alcohol dehydrogenase locus in Drosophila melanogaster in relation to environmental variation: Ethanol levels in breeding sites and allozyme frequencies. Oecologia. 1981;51:191–198. doi: 10.1007/BF00540600. [DOI] [PubMed] [Google Scholar]
- 56.Stamps JA, Yang LH, Morales VM, Boundy-Mills KL. Drosophila regulate yeast density and increase yeast community similarity in a natural substrate. PLoS ONE. 2012;7:e42238. doi: 10.1371/journal.pone.0042238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devineni AV, Heberlein U. Preferential ethanol consumption in Drosophila models features of addiction. Curr. Biol. 2009;19:2126–2132. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodrigues V. Olfactory behavior of Drosophila melanogaster. Basic Life Sci. 1980;16:361–371. doi: 10.1007/978-1-4684-7968-3_26. [DOI] [PubMed] [Google Scholar]
- 59.Depiereux E, et al. Larval behavioral response to environmental ethanol in relation to alcohol dehydrogenase activity level in Drosophila melanogaster. Behav. Genet. 1985;15:181–188. doi: 10.1007/BF01065898. [DOI] [PubMed] [Google Scholar]
- 60.Gelfand LJ, McDonald JF. Relationship between alcohol dehydrogenase (ADH) activity and behavioral resonse to environmental alcohol in five Drosophila species. Behav. Genet. 1983;13:281–293. doi: 10.1007/BF01071873. [DOI] [PubMed] [Google Scholar]
- 61.Schumann I, Triphan T. The PEDtracker: An automatic staging approach for Drosophila melanogaster larvae. Front. Behav. Neurosci. 2020;14:612313. doi: 10.3389/fnbeh.2020.612313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sullivan DT, Grillo SL, Kitos RJ. Subcellular localization of the first three enzymes of the ommochrome synthetic pathway in Drosophila melanogaster. J. Exp. Zool. 1974;188:225–233. doi: 10.1002/jez.1401880210. [DOI] [PubMed] [Google Scholar]
- 63.Borycz J, Borycz JA, Kubow A, Lloyd V, Meinertzhagen IA. Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J. Exp. Biol. 2008;211:3454–3466. doi: 10.1242/jeb.021162. [DOI] [PubMed] [Google Scholar]
- 64.Sitaraman D, et al. Serotonin is necessary for place memory in Drosophila. Proc. Natl. Acad. Sci. U S A. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Das G, Lin S, Waddell S. Remembering components of food in Drosophila. Front. Integr. Neurosci. 2016;10:4. doi: 10.3389/fnint.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geer BW, Dybas LK, Shanner LJ. Alcohol dehydrogenase and ethanol tolerance at the cellular level in Drosophila melanogaster. J. Exp. Zool. 1989;250:22–39. doi: 10.1002/jez.1402500105. [DOI] [PubMed] [Google Scholar]
- 67.McKenzie JA, Parsons PA. Alcohol tolerance: An ecological parameter in the relative success of Drosophila melanogaster and Drosophila simulans. Oecologia. 1972;10:373–388. doi: 10.1007/BF00345738. [DOI] [PubMed] [Google Scholar]
- 68.Geer BW, Langevin ML, McKechnie SW. Dietary ethanol and lipid synthesis in Drosphila melanogaster. Biochem. Genet. 1985;23:607–622. doi: 10.1007/BF00504295. [DOI] [PubMed] [Google Scholar]
- 69.McClure KD, French RL, Heberlein U. A Drosophila model for fetal alcohol syndrome disorders: Role for the insulin pathway. Dis. Model Mech. 2011;4:335–346. doi: 10.1242/dmm.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. A Drosophila model for alcohol reward. Nat. Neurosci. 2011;14:612–619. doi: 10.1038/nn.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nunez KM, Azanchi R, Kaun KR. Cue-induced ethanol seeking in Drosophila melanogaster is dose-dependent. Front. Physiol. 2018;9:438. doi: 10.3389/fphys.2018.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mishra D, Thorne N, Miyamoto C, Jagge C, Amrein H. The taste of ribonucleosides: Novel macronutrients essential for larval growth are sensed by Drosophila gustatory receptor proteins. PLoS Biol. 2018;16:e2005570. doi: 10.1371/journal.pbio.2005570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toshima N, Kantar Weigelt M, Weiglein A, Boetzl FA, Gerber B. An amino-acid mixture can be both rewarding and punishing to larval Drosophila melanogaster. J. Exp. Biol. 2019 doi: 10.1242/jeb.209486. [DOI] [PubMed] [Google Scholar]
- 74.Schleyer M, Miura D, Tanimura T, Gerber B. Learning the specific quality of taste reinforcement in larval Drosophila. Elife. 2015 doi: 10.7554/eLife.04711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schneider A, et al. Neuronal basis of innate olfactory attraction to ethanol in Drosophila. PLoS ONE. 2012;7:e52007. doi: 10.1371/journal.pone.0052007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keesey IW, et al. Alcohol boosts pheromone production in male flies and makes them sexier. bioRxiv. 2020 doi: 10.1101/2020.08.09.242784. [DOI] [Google Scholar]
- 77.Steck K, et al. A high-throughput behavioral paradigm for Drosophila olfaction: The Flywalk. Sci. Rep. 2012;2:361. doi: 10.1038/srep00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogueta M, Cibik O, Eltrop R, Schneider A, Scholz H. The influence of Adh function on ethanol preference and tolerance in adult Drosophila melanogaster. Chem. Senses. 2010;35:813–822. doi: 10.1093/chemse/bjq084. [DOI] [PubMed] [Google Scholar]
- 79.Selcho M, Pauls D, Han KA, Stocker RF, Thum AS. The role of dopamine in Drosophila larval classical olfactory conditioning. PLoS ONE. 2009;4:e5897. doi: 10.1371/journal.pone.0005897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rohwedder A, et al. Four individually identified paired dopamine neurons signal reward in larval Drosophila. Curr. Biol. 2016;26:661–669. doi: 10.1016/j.cub.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 81.Saumweber T, et al. Functional architecture of reward learning in mushroom body extrinsic neurons of larval Drosophila. Nat. Commun. 2018;9:1104. doi: 10.1038/s41467-018-03130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lyutova R, et al. Reward signaling in a recurrent circuit of dopaminergic neurons and peptidergic Kenyon cells. Nat. Commun. 2019;10:3097. doi: 10.1038/s41467-019-11092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.