Abstract
Flavonoids are natural phytochemicals known for their antiviral activity. The flavonoids acts at different stages of viral infection, such as viral entrance, replication and translation of proteins. Viruses cause various diseases such as SARS, Hepatitis, AIDS, Flu, Herpes, etc. These, and many more viral diseases, are prevalent in the world, and some (i.e. SARS-CoV-2) are causing global chaos. Despite much struggle, effective treatments for these viral diseases are not available. The flavonoid class of phytochemicals has a vast number of medicinally active compounds, many of which are studied for their potential antiviral activity against different DNA and RNA viruses. Here, we reviewed many flavonoids that showed antiviral activities in different testing environments such as in vitro, in vivo (mice model) and in silico. Some flavonoids had stronger inhibitory activities, showed no toxicity & the cell proliferation at the tested doses are not affected. Some of the flavonoids used in the in vivo studies also protected the tested mice prophylactically from lethal doses of virus, and effectively prevented viral infection. The glycosides of some of the flavonoids increased the solubility of some flavonoids, and therefore showed increased antiviral activity as compared to the non-glycoside form of that flavonoid. These phytochemicals are active against different disease-causing viruses, and inhibited the viruses by targeting the viral infections at multiple stages. Some of the flavonoids showed more potent antiviral activity than the market available drugs used to treat viral infections.
Keywords: Natural products, Flavonoids, Viruses, Replication, Inhibitors
Graphical Abstract
1. Introduction
Flavonoids class of compounds are naturally occurring poly-phenolic phytochemicals that are broadly found in plants and are responsible for a variety of biological processes [1]. They are associated with a group of secondary metabolites having poly-phenolic structure in plants. [2]. These phytochemicals are broadly found in fruits and vegetables [3]. Many flavonoids are easily identified as flower pigments but they are not restricted to flowers, and are found in all parts of various plants [4].
Flavonoids have many sub-classes like flavones, flavanols, flavanonols, flavanones, isoflavones, and anthocyanidins, and researchers have identified more than 5000 flavonoids. Their ring structure comprises a basic C6-C3-C6 skeleton structure. It has an impressive biochemical as well as antioxidant effects closely related to numerous ailments, for instance cancer, Alzheimer's disease & Coronary artery diseases [4], [5], [6]. Flavonoids have additional pharmacological activities such as antitumor, prevention of cardiovascular diseases [7], antibacterial [8] antifungal, anti-allergic, estrogenic, anti-inflammatory [9], [10], [11], and antiviral activities [2], [12].
Phytochemicals such as flavonoids are beneficial in antiviral chemotherapy. Existing antiviral drugs, however, have a narrow antiviral activities and show varying toxicity towards patients, and thus are limited in their usefulness. Alternatively, phytochemicals like Flavonoids have a broad variety of biological roles, and can be used as medicines directly. Another alternative is the synthesis of new drugs based on this class of natural products. During the last few years, many researches have made efforts to synthesize more compounds with antiviral activity [13], [14].
2. Flavonoids and their anti-viral mode of action
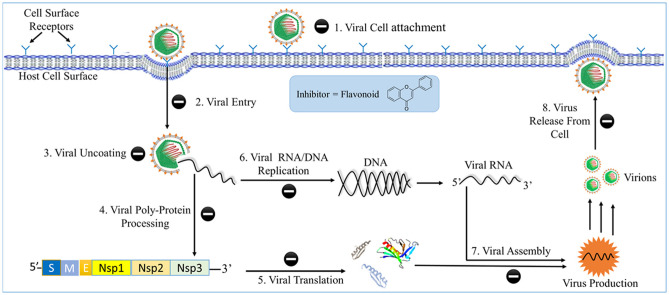
Viruses have DNA or RNA genome and some of them have protein envelopes. They rely on the metabolism of the hosts and its surroundings to reproduce and continue to survive. They abuse and take over the cellular machinery of the host and spread throughout them [14].
There are several mechanisms by which flavonoids phytochemicals inhibit and act on the viruses. They can obstruct the attachment and entrance of viruses into the cells, Hamper with different phases of viral DNA replication, Protein Translation and poly-protein processing. They can also inhibit the viruses from being released to invade other healthy host cells [15].
Roschek et al. [16] described that Flavonoids can attach itself to the surface proteins of viruses, Prohibiting the virus from entering the host cells. Some flavonoids act as a transcription blocker and affect the replication process while others hinder the late stages of viral assembly, packaging and release. Flavonoids can also modulate the immune system and reduce viral load [16].
The antiviral role of flavonoids against several viruses in this review will be examined and discussed.
3. Flavonoids against influenza A, B virus
Influenza A, B viruses belong to the viral genus of alpha-influenzavirus and beta-influenzavirus of the Ortho-myxoviridae family, which causes influenza. They are RNA viruses and their genetic material is enveloped.
3.1. Green tea extracts (GTE), and their constituent flavonoid epigallocatechin (-) EGC
Imanishi et al. [18] tested GTE for its Inhibitory effect in the Madin-Darby canine kidney (MDCK) on influenza virus strains, and the influenza virus strains used were PR8 (H1N1 subtype), H3N2 Aichi strain, and Sing influenza virus-strain. These strains of influenza viruses were propagated in MDCK cell culture with a 5-PFU infection multiplicity per cell. The cytotoxicity of GTE determined by MTT assay [17] was a 1:20 dilution, and no cytotoxic effect on MDCK cells is seen. When GTE was added at different dilutions from 0-hour post-infection (p.i.) to 10-hour p.i. in a dose - dependent manner, virus growth slowed logarithmically, about 3 log, 2 log, and 1 log reductions for 1:20, 1:40 & above 1:80 dilution, respectively in proportion to the vanishing of acidified ELS (endosomes and lysosomes).
GTE was then further tested to see if it can inhibit the early stage of the influenza virus. GTE was added within 30 min (p.i.), and the virus yield decreased only when the GTE was added 5–15 min post infection, demonstrating that GTE affected the early viral infection stages.
GTE has many flavonoids, among which the epigallocatechin (-) EGC is present in ~44% of green tea extracts. As a main constituent of GTE, EGC was then tested for its anti-influenza-A, B activity. The CC50 value of (-) EGC calculated was 400 μg/ml for MDCK cells. When MDCK cells that were exposed to the virus were treated with 400 μg/ml of Epigallocatechin, the same result was obtained as when MDCK cells were treated with GTE (i.e. an inhibitory effect). This result confirms that flavonoid epigallocatechin (-) EGC is the main antiviral agent, which inhibits the early viral infection, phase of the influenza-A, B virus multiple strains [18].
3.2. Elderberry extracts and their constituent flavonoids ( ± ) Dihydromyricetin and 5, 7, 30, 40-tetra-O-methylquercetin
Roschek et al. [16] used elderberry extract on influenza H1N1 virus in MDCK cells. Elderberry extract has many active flavonoids which inhibit the influenza virus; some of the active compounds found in elderberry are ( ± ) Dihydromyricetin and 5, 7, 30, 40-tetra-O-methylquercetin. They used a direct binding assay and determined that the IC50 value of ( ± ) Dihydromyricetin is 2.8 μg/ml (8.7 μM), thus showing strong inhibition of H1N1 viral infection. 5, 7, 30, 40-tetra-O-methylquercetin flavonoid achieved an IC50 of 0.13 μg/ml (0.36 μM) against H1N1 and efficiently inhibited the virus. The efficiency of the 5, 7, 30, 40-tetra-O-methylquercetin flavonoid was nearly the same as that of Oseltamivir, a drug used against influenza infection. 5, 7, 30, 40-tetra-O-methylquercetin flavonoid also acts at a much lower concentration (0.36 μM) like Oseltamivir, whose concentration is (0.32 μM). These flavonoids were found to be bound to the surface of H1N1 and prevented the viral attachment to the host cell [16].
3.3. Quercetin 3-rhamnoside (Q3R) flavonoid
Choi et al. [19] used influenza virus A/WS/33 strain & (MDCK) cells was used for its propagation. They treated it with flavonoid quercetin 3-rhamnoside (Q3R), a quercetin glycoside derivative. The antiviral assays showed that at 100 μg/ml, Q3R had 86% inhibitory effect on the influenza virus. At 10 μg/ml concentration, Q3R antiviral activity was noted to be 66% on influenza virus. Furthermore, the addition of Q3R to the infected MDCK cells prevented a noticeable cytopathic effect (CPE) from developing. Q3R inhibited the early stage of influenza virus replication [19].
3.4. Quercetin flavonoid
Wu et al. [20] used MDCK cells, and it was then propagated with distinct influenza-A viral strains, and studied the antiviral effect of quercetin on the infected MDCK cells. Quercetin interacted with the envelop protein Hemagglutinin (HA) of the influenza-A virus. Hemagglutinin (HA) is a vital glycoprotein (GP) on the surface membrane of influenza viruses and is responsible for fusion and entry of the influenza-A virus into the host cells. Quercetin inhibited this HA-protein and reduced its mRNA transcription. Quercetin showed strong inhibitory effect on different viral strains of Influenza-A Virus. The IC50 values of quercetin against (H1N1) A/Puerto Rico/8/34 strain, (H1N1) A/FM-1/47/1, and (H3N2) A/Aichi/2/68 influenza -A strains were 7.756 ± 1.097, 6.225 ± 0.467, and 2.738 ± 1.931 μg/ml, respectively. Moreover, quercetin also interfered with Hemagglutinin (HA) of H5N1 influenza Virus. At concentrations as high as 250 μg/ml, Quercetin demonstrated no cytotoxic activity in MDCK cells [20].
4. Baicalein flavonoid against the Japanese encephalitis virus (JEV)
JEV is an enveloped virus with a positive-sense single-stranded RNA genome. The length of its genome is 11 kb. JEV is an arthropod-borne virus in the genus Flaviviridae [21]. JEV is a causative agent for virally caused encephalitis in humans, and can induce signs ranging from febrile to Mortal illnesses, particularly with 30,000–50,000 worldwide cases annually in children. In East and South Asian countries, JEV infection is prevalent. The death rate of JEV infection is estimated to be around 30%, and life-long neurological impairment among half of the survivors.
Johari et al. [22] determined the cytotoxicity of baicalein by MTT assay for Vero cells. The cytotoxic concentration for Vero cells or its CC50 was 115.2 ± 0.2 μg/ml. Baicalein was then treated with the JEV infected Vero cells, and at different stages, the inhibitory effect of these flavonoids were noted. Baicalein prevented the adsorption activity of JEV at a concentration of IC50 = 7.27 ± 1.08 μg/ml. The number of the JEV RNA copies were also inhibited by baicalein, and decreased by 77.3% ± 2.4% whenVero cells infected with the virus were added at a dosage of 25 μg/ml. When bacalein was added to cells infected with JEV, it powerfully repressed the JEV viral RNA to 68.46% ± 1.19% at a 25 μg/ ml concentration. The IC50 value of baicalein was 5.8 ± 1.09 μg/ml against this stage. Direct virucidal activity with IC50 = 3.44 ± 1.04 μg/ml against JEV Virus was also shown by Baicalein. These findings indicate that baicalein has strong antiviral activity against the various stages of replication of in-vitro of JEV [22].
5. Flavonoids against dengue virus type-2 (DENV-2)
The Dengue virus (DENV) belongs to the Flavivirus class of the family Flaviviridae. Its genome is a single positive-stranded RNA-genome and causes various human illnesses like dengue and dengue hemorrhagic fever (DHF) & (DF). DENV-1, DENV-2, DENV-3 and DENV-4 are its 4 genotypes. Two species of mosquitoes, Aedes agypti and Aedes albopictus transmits these viruses to humans [23].
5.1. Quercetin flavonoid
Zandi et al. used Vero cells and infected it with DENV-2 New Guinea C strain (NGC), and added different concentrations of quercetin flavonoid to the Vero cells. The CC50 value for quercetin against culture cells were 252.6 ± 0.17 μg/ml. Quercetin was used against DENV-2 in infected cells at a concentration of IC50 = 35.7 μg/ml, and quercetin showed strong antiviral activity. The production of RNA copies of DENV-2 was decreased by 67% when treated with 50 μg/ml of quercetin. Quercetin did not have a major virucidal effect on DENV-2 but when added to cell culture between 5 h and up to 4 days Pre-infection with the virus at a concentration of IC50 = 28.9 μg/ml, quercetin exhibited inhibitory effect against DENV-2. When the concentration of quercetin was increased to 50 μg/ml of quercetin 5 h before virus infection, & up to 4 days after infection, the DENV-2 RNA declined by more than 75.7% ± 1.57. No extracellular inhibitory activity of quercetin was observed against DENV-2.
These results show that Quercetin has substantial anti-DENV-2 replication properties and also influences intracellular DENV virus replication, but not the DENV entrance & attachment into the host cell [24].
5.2. Baicalin flavonoid
Moghaddam et al. [25] used the same DENV-2 virus and propagated it in the Vero cells and used another flavonoid, baicalin (5, 6, 7-trihydroxyflavone), and then noted its anti-DENV-2 activity. The CC50 value determined for Baicalin was 823.53 μg/ml. At a concentration of about 62.5 mg/ml, the cell viability of Baicalin treated Vero cells was greater than 90%. When baicalin was studied for antiviral activities against DENV-2 at the concentration of IC50 = 14.9 μg/ml, it potently inhibited the intracellular stage of DENV-2. It targeted DENV-2 replicons Nsps (non-structural proteins). Further investigations showed that baicalin at 50 μg/ml likewise repressed DENV-2 replication more than 99% with IC50 = 13.50 μg/ml [25].
6. Flavonoids against the Chikungunya virus (CHIKV)
Chikungunya virus (CHIKV) belongs to a viral genus Alphavirus of the Togaviridae family. Its genome is a positive sense single stranded RNA genome and is an enveloped virus and its genome size is ~11.8 kb. This virus is transmitted to its human hosts by two mosquitoes, Aedes aegypti, & Aedes albopictus. Various outbreaks of CHIKV have affected more than two million people and caused hundreds of deaths worldwide [26].
6.1. Baicalein, fisetin and quercetagetin flavonoids
Lani et al. used the CHIKV virus and propagated it in BHK-21 cells & Vero cells. Then, they treated the cells with baicalein, fisetin and quercetagetin. The cytotoxic values were determined for all the 3 flavonoids for Vero and BHK-21 cells. The CC50 values were 356.65 μg/ml and 474.5 μg/ml for baicalein, 194.45 μg/ml and 212.35 μg/ml for fisetin, and 226.75 μg/ml and 52.42 μg/ml for quercetagetin, for the Vero and BHK-21 cells respectively.
Its antiviral activity was noted against CHIKV replicon. Baicalein repressed the activity of virus with an IC50 value of 3.243 µg/ml (12 µM), whereas quercetagetin had potent inhibitory effects on CHIKV replicon at the concentration of IC50 of 13.53 µg/ml (42.52 µM). Baicalein was the strongest inhibitor of the two flavonoids against the CHIKV replicon. When the optimum time for inhibition of CHIKV infection for baicalein, quercetagetin, and fisetin were checked at 100 µg/ml these 3 flavonoids proficiently diminished CHIKV RNA creation at the early hour of the treatment and showed effective inhibition. Nevertheless, when fisetin was treated at any time of CHIKV infection, it inhibited CHIKV very strongly. On the extracellular stage of CHIKV, baicalein and quercetagetin exerted strong dose dependent activity on Chikungunya virus. The IC50 concentrations for the inhibition of extracellular stage noted was 9.934 µg/ml or (31.21 µM), and 11.64 µg/ml or (43.07 µM) for quercetagetin flavonoid & baicalein flavonoid respectively.
Baicalein and quercetagetin also exerted dose-dependent inhibition of the entry of CHIKV into Vero cells. IC50 values for this stage of inhibition were 28.04 µg/ml (103.76 µM) and 8.050 µg/ml (25.3 µM), for baicalein and quercetagetin, respectively.
Baicalein with an IC50 concentration of 1.891 µg/ml (6.997 µM) had more impressive intracellular anti-CHIKV activity than quercetagetin flavonoid, whose IC50 value was 13.85 µg/ml or (43.52 µM); and fisetin, flavonoid whose IC50 value was 8.444 µg/ml (29.5 µM). Bacalein targeted CHIKV replication process. In comparison to the IC50 value of ribavirin, a positive control with a defined anti- Chikungunya virus activity (11.07 µg/ml) it could be concluded form these observations that the tested flavonoid compounds has stronger intracellular anti-CHIKV activity [27].
7. Flavonoids against hepatitis C virus (HCV)
Hepatitis C virus (HCV) infects more than 3% of the population worldwide & is the main causative agent of chronic liver-diseases, and it can cause both acute and chronic hepatitis, ranging in severity from a mild illness lasting a few weeks to a serious, lifelong illness [99]. It is the major cause of liver cancers. HCV is a blood borne virus; the most common modes of transmission of HCV is exposure to small quantities of infected blood. This may occur through injection drug use or through un-safe injection practices, unscreened transfusion of blood and blood products, while unsafe sexual practices can also lead to exposure to blood causing the infection [28].
Many researchers have found that HCV secretion is associated with lipid metabolism and that it attaches itself with various lipoproteins like (LDL), (VLDL) and apolipoprotein AII (ApoAII) and spreads in the host.
7.1. Naringenin flavonoid
Nahmias et al. [29] studied HCV and ApoB and VLDL relationship in Huh7.5.1 cells a plasmid that has the JFH-1 which has (HCV) genome. Then the JFH-1 RNA was inserted or in other words transcribed into Huh7.5.1 cells by using the liposome-mediated transfection method. Naringenin flavonoid is known to repress cholesterol levels. It is accepted that naringenin represses ApoB emission by decreasing the action and of MTP protein gene expression and as well the ACAT (acyl-coenzyme A: cholesterol acyltransferase) gene expression, the HCV propagated Huh7.5.1 cells were treated for 24 h with naringenin flavonoid. Naringenin repressed the release of HCV core & its positive strand RNA dose-dependently. At 200 µM concentration Naringenin flavonoid efficiently repressed HCV secretion by 80% whereas the inside cell levels of HCV positive strand RNA and intracellular HCV core protein expression remain unchanged. When HCV was pre-treated with naringenin to check if it can infect the cells when pre-treated with naringenin, the infectiousness of the cell supernatant was potently repressed following prior treatment with naringenin by 79% ± 10%. At 200 µM concentration the activity of MTP was decreased by 58%. Naringenin brought changes in hepatic gene transcription and reduced it by 57%, whereas the transcription of ACAT2 (acyl-coenzyme A: cholesterol acyltransferase) was reduced by 55%. This means that naringenin can inhibit the secretion of the HCV by inhibiting the apoB and VLDL lipoproteins secretion [29].
7.2. Silymarin
Ferenci et al. [30] used silymarin, which is a polyphenol complex also known as flavo-lignans, and is a blend of silybin-A and silybin-B (50:50 mixture). They tested these two compounds on chronic HCV infections. The treatment of chronic patients with the silymarin were started and they received a dosage of 5, 10, 15, or 20 mg/kg infusions over 4 h for 14 days consecutively. The HCV replicons numbers decreased in the patients, but when the patients were not infused with the silymarin, the HCV replicons number again increased. This shows that silymarin can effectively control the HCV infection [30].
7.3. Apigenin flavonoid
Shibata et al. [31] used Huh7-cells and infected it with HCV and examined Apigenin activity against HCV. Apigenin inhibited the activity of HCV by targeting its replication process. It was found that this flavonoid targeted the TRBP (transactivation response element RNA-binding protein) of mature miRNA122 (MicroRNA) and at a concentration of 5 μM Apeginin decreased the expression of mature miRNA122 (MicroRNA) by down-regulation of its miRNA 122 RNA gene levels without considerably upsetting the cell growth [31].
7.4. Quercetin flavonoid
Bachmetov et al. [32] used quercetin flavonoid to inhibit HCV virus by targeting the NS3 protease. They used the Huh-7 cells and transfected it with a Plasmid EGFP-NS5A/B-DsRed-NLSX3, which has the NS3 protease gene. Quercetin showed no cytotoxicity towards the Huh-7 cells at a concentration of 10 μg/ml. When the NS3 protease expressing cells was treated with quercetin at a concentration of 10 μg/ml, its expression levels were reduced by up to 90%. It can be inferred from these results the HCV virus can be mediated by Quercetin by inhibiting the NS3 protease. The same concentration of quercetin (10 μg/ml) was tested to see if quercetin can inhibit the HCV HJ3–5 chimeric virus. Quercetin inhibited the production of HCV virus by 95%. Quercetin targeted and reduced the HCV RNA efficiently, resulting in the inhibition of the HCV virus [32].
7.5. Ladanein (BJ486K) flavonoid
Haid et al. used a flavonoid extracted from Marrubium peregrinum L (Lamiaceae) and used it against various strains of HCV viruses. First, the flavonoid ladanein (BJ486K) was tested against the HCV JcR-2a reporter virus in Huh7-Lunet and CD81 cell lines. The flavonoid ladanein (BJ486K) was then added into the virus infected cultured cells at different times from 10 to 60 min post infection. It was noted that the addition of the ladanein during the inoculation with virus results in 90% inhibition, and after 20 min of the infection, 80% inhibition of the HCV virus was achieved. When added later, no inhibition was noted. When the mode of action of ladanein was studied it was noted that the flavonoid inhibited the entrance of the HCV virus to the cells. The virus is found attached to the cells surface, but its entry was inhibited by ladanein at IC50 = 2.5μmol/L; the cytotoxic concentration for Huh7-Lunet/CD81 cells of ladanein was 98.04 μmol/L.
When ladanein was tested against other HCV genotype strains like 1a, 1b&2b, 3a and 4a, as well 5a, and 6a chimeric-reporter HCV viruses, ladanein potently inhibited the entry of these different HCV viral strains into Huh7-Lunet & CD81 cells at a concentration of 4.2 μmol/L. These results confirm that ladanein inhibits the post attachment entry of almost all genotypes of HCV viruses efficiently [33].
7.6. Sorbifolin and pedalitin
Shimizu et al. [34] wanted to measure the antiviral activity of these 2 flavonoids sorbifolin and pedalitin, they are found in Pterogyne nitens. It was tested on the replication cycle of HCV. These two flavonoids were studied on genotype 2a JFH-1 sub-genomic replicons and infectious viral systems. Sorbifolin had virucidal effects, and repressed virus entrance up to 45.0% with no cytotoxicity towards host cells. Pedalitin, on the other hand, directly acted upon the virus and interfered on the host cell, and it inhibited virus entry up to 78.7%. Neither flavonoid inhibited HCV release or replication. Nevertheless, the results of this study demonstrate the potent effect of flavonoids as antiviral agents [34].
8. Epigallocatechin gallate (EGCG), a flavonoid against HBV
Hepatitis B virus disease remains one of the main world health problem, in spite of the accessibility of a viable vaccine. Roughly 350 million individuals are constantly affected by HBV around the world. It has a 3.2 kb (RC-DNA) genome. When the disease begins, this viral genome is discharged into the cytoplasm and changed into covalently closed circular (cccDNA) in the cell nucleus of host cell. cccDNA is template for viral RNAs creation. Amongst the HBV RNAs, the pregenomic RNA (pgRNA) is packed into a new capsid and works as a template for HBV DNA creation.
He et al. used HepG2.117, which is an HBV-replicating cell line and treated it with EGCG. The cytotoxic impacts were invisible when the concentration of EGCG was lower than 100 μmol/L. When EGCG treatment was started, the hepatitis B virus e antigen (HBeAg) expression was hindered by EGCG with an IC50 of 39.4 μmol/L dose-dependently. While, the hepatitis B virus surface antigen (HBsAg) expression level remained primarily the same. These results suggests that EGCG represses HBeAg levels while HBsAg levels remain the same in HepG2.117 cells.
Upon treatment with EGCG of HepG2.117 cells for 3-days, the complete RNAs of the HBV were separated and then subjected to semi-quantitative RT-PCR investigation utilizing the specific primers of precore mRNA. The amount of 563 bp precore mRNA of HBV decreased when the doses of the EGCG were increased from 0 to 100 μmol/L, which means inhibition of the HBV precore mRNA is dose dependent. These outcomes demonstrate that after EGCG treatment, precore mRNA level was repressed in HepG2.117 cells, and the lower precore mRNA level represented the diminished production of HBeAg.
The development of HBV cccDNA and RI-DNA was likewise restrained by EGCG in dose-dependent manner. The rates of inhibition of cccDNA production were 36.3%, 63%, and 72.4%, when the EGCG concentration was 25, 50, and 100 μmol/L respectively. These results confirm that EGCG had a potent inhibitory effect on the HBV Virus in vitro in HepG2.117 cells [35].
9. Baicalin (BA) flavonoid against HIV-1 virus
Human-immunodeficiency (HIV) viruses are two types of Lentivirus (a subgroup of retrovirus) that causes diseases in people. After some time they cause AIDS, an immunodeficiency syndrome, a condition where the immune system gradually fails and allows fatal opportunistic infections and cancers to flourish and worsen the situation. It has a single-stranded, positive-sense genome and is an enveloped RNA virus. Upon entrance to the host cell, the viral RNA genome is converted into dsDNA in the host cell by an enzyme known as Reverse transcriptase [36].
Li et al. used Human osteosarcoma cells co-expressing CD4/CR5 for infection with HIV-1 virus. The effect of Baicalin (BA) on HIV-1 Env-mediated fusion was studied by using quantitative colorimetric technique which is an assay technique. In a dose dependent manner, baicalin inhibited both the X4 and R5 HIV-1 Envelop-mediated fusion. The presence of 4 μM baicalin in fusion assays generated 50% repression and as the BA concentration increased from 40 to 400 μM, about 80% inhibition of HIV-1 was noted. The effect of baicalin (BA) was extensive since this compound inhibited the cell fusion mediated by both X4 and R5 HIV-1 Env.
X4 HIV-1 Env-mediated cell attachment or fusion will stimulate the formation of syncytia (a single cell or a cytoplasmic mass which has many nuclei, created by the combination of cells or by nuclei division) in infected cells. BA was then tested on the syncytia (fusion of cells caused by HIV-1 infection). The X4 HIV-1 virus infected cell resulted in the formation of multinucleated giant cells, interestingly, the presence of baicalin (BA) completely disposed of the development of syncytial mass in the diseased host cells.
As noted above, that BA eliminated the HIV-1 Env-mediated cell fusion, BA was further tested to see if it inhibits the formation of HIV-1 ss-DNA. When the infected cells were treated with BA, HIV-1 strong stop DNA was not detected in HIV-1 infected host cells. The inability to detect HIV-1 ss-DNA in the infected cells supports the opinion that baicalin represses HIV-1 infection at the stage of entrance to cells [37].
10. Flavonoids against enterovirus A71
Enterovirus EV-A71 is a member of Picornaviridae family and is a RNA virus. Its genome is 7.4 kb which codes four viral proteins VP1 to VP4 (structural) and seven NSPs. It is known to cause encephalitis, and it is the principal cause of hand, foot, and mouth disease (HFMD), a worldwide disease in childrens. It is dominating in Asia, with yearly surges going on causing millions of cases. EV-A71 is one of the principle etiological cause of HFMD, common in smaller childrens under 6 years age [38].
10.1. Apigenin flavonoid
Apigenin is a Flavone flavonoid. Zhang et al. used Vero cells and RD cells. The viral strains of Enterovirus BrCr and Fuyang types were propagated in these cultured cells. The cells were then treated with apigenin flavonoid, and its antiviral activity was then noted. Apigenin was capable of inhibiting and reduction of EV-A71 ailment by the suppression of the internal ribosome entry site (IRES) operation of the Enterovirus at a concentration of EC50 of 10.3 μM. Apigenin also reduced the cytopathic effect (CPE) induced by Enterovirus when treated at a concentration of 30 μM apigenin. It also disrupted the viral RNA & the hnRNP A1 and A2 association. The CC50 of Apigenin was 79.0 μM [39].
10.2. Apigenin flavonoid in-vivo
Dai et al. [40] also studied the antiviral effect of apigenin in-vivo. It was tested to see if it has any prophylactic activity that can defend newly born BALB/c mice from the intra-cranial lethal challenge of enterovirus. Apigenin provided protection to the mice from the virus at the dosage of 50 mg/kg with an 88.89% survivability and an enhancement was witnessed in the treated mice when they were compared to the control group [40].
10.3. Baicalin flavonoid
Li et al. [41] used baicalin flavonoid to study its antiviral activity EV-A71. They used (human embryonal rhabdomyosarcoma) RD cells and used the BrCr-Tr strain of EV-A71. They prepared the RD cells culture, infected it with the enterovirus, and then treated it with baicalin and noted its antiviral activity. The cytotoxicity values of baicalin was CC50 = 823.53 μg/ml against RD cells. Baicalin inhibited viral activity by targeting the polymerase and protein translation of the enterovirus. At about IC50 of 4.96 μg/ml of baicalin, the viral infection was reduced by 75% at 8 hr p.i. Baicalin inhibited the virus at the early stages. It was also noted that the said flavonoid also decreased the level of expression of Fas1 and caspase-3. Baicalin also inhibited the inflammatory compounds cytokines by targeting the NF-κB p65, and decreasing its expression [41].
10.4. Kaempferol flavonoid
Tsai et al. [42] used RD cells, infected it with EV-A71, and used another flavonoid, kaempferol and noted its antiviral activity. When treated at a concentration of 35 μM of kaempferol for 24 h, the activity of the virus decreased by 80%. Kaempferol interfered with viral replication and also inhibited the activity of IRES (internal ribosome entry site), thus limiting the viral infection [42].
11. Flavonoids against Poliovirus
Poliovirus, is the main agent of polio (poliomyelitis), PV is a serotype of Enterovirus C. It is a member of Picornaviridae family. Poliovirus has RNA genome and it is single-stranded positive-sense. It has a capsid made up of protein and its genome length is about 7.5 kb. PV infects humans by attaching to CD155 receptor found on the exterior of the cells [43].
11.1. 3-methyl quercetin (3-MQ) and Ro-090179 flavonoids
Gonzalez et al. used HeLa cells and propagated Poliovirus type 1 (Mahoney strain) in HeLa cells. Then, the cells were treated with Ro-090179 and 3-MQ flavonoids, and their antiviral activity was noted. They treated these polioviruses infected cells with different concentrations of the above said flavonoids. When Ro-090179 flavonoid was added one-hour post infection with poliovirus, this flavonoid, at a very low concentration of 1.5 μM prohibited the appearance of the poliovirus proteins. When the poliovirus cultured HeLa cells were treated with 3-MQ flavonoid, they saw the same inhibitory effect on protein synthesis at a concentration of 5 μM.
The mode of action of 3-MQ was analyzed against poliovirus. 3-MQ selectively inhibited the poliovirus transcription (RNA synthesis) process. At a concentration of 5 μg/ml 3-MQ inhibited uridine incorporation in poliovirus-infected cells by 90%. When analyzed, the viral RNA produced in the presence of this flavone (3-MQ) showed that the production of single-stranded RNA was totally blocked, whereas the synthesis of double-stranded RNA was detected. These results confirm that 3-Methyl Quercetin selectively and specially repressed the synthesis of stranded RNA of the Polio Virus type 1 (Mahoney strain) [44].
11.2. 6-chloro-4′-oxazolinyl and 6-chloro-3-hydroxyflavone-4′-carboxylic acid flavonoids
Desideri et al. [45] used Hela cells and infected it with poliovirus type-2 (PV-2). Various flavonoids were tested against the PV-2, Among them, two flavonoids showed strong inhibition of PV-2. The flavanone 6-chloro-4′-oxazolinyl, and the flavone (6-chloro-3-hydroxyflavone-4′-carboxylic acid) at IC50 values of 2.791 μM and 1.91 μM respectively, showed strong inhibition of the PV-2. Both of these flavonoids showed no cytotoxicity at a concentration of greater than 12.5 μM towards Hela cells [45].
12. Kaempferol and juglanin flavonoids against SARS-CoV-1
The 2002–2004 SARS outbreak was caused by SARS-CoV-1. It is a single-stranded, enveloped, positive-sense RNA virus that infects the epithelial cells in the lungs. By attaching to ACE-2 receptor, the virus enters the host cell. It infects mostly humans and bats [46]. Researchers have shown the viral protein coded by ORF-3a of SARS-1 coronavirus creates a cation-selective ion-channel. These channels are then expressed in the infected host cells and are used by the virus to release its progeny from the host cell and infect other host cells. If drugs inhibit these viral coded channels, It will block the discharge of the virus from infected cells and the virus then would not be able to invade and infect other host cells.
Xenopus oocyte cells isolated from females of the clawed toad Xenopus laevis was used for expression of the virus, and then treated with kaempferol and juglanin. Then, its effects against 3a protein of SARS-CoV-1 were analyzed by voltage-clamp techniques. Kaempferol and its glycoside, Juglanin has been investigated for its efficacy in inhibiting the Ba2 + sensitive current created by the 3a virus ion channel. At a concentration of about 20 μM of kaempferol, it reduced the Ba2+sensitive endogenous current in comparison to control group oocytes the current was 3–5 factors larger. Owing to the low water solubility of kaempferol, further testing was not allowed at higher concentration to evaluate its IC50 value. The water-soluble glycoside of kaempferol (juglanin) was then analyzed for its inhibitory effect. When tested, juglanin completely and very potently inhibited the 3a-ion channel produced current at a concentration of 20 μM while at a low concentration of 10 μM juglanin nearly obtained complete inhibition. Juglanin at IC50 = 2.3 μM concentration showed strong inhibition of the viral Ion channel.
As discussed above, the 3a ion channel protein is very necessary for the virus to be released from the infected cell. This study shows that the ion channel was successfully inhibited by kaempferol and its glycoside derivative, juglanin, which carries an arabinose residue [47].
13. Genistein flavonoid against HIV virus vpu-ion channel
Viruses has genes that code for ion-selective channels which are then integrated into the membrane of the infected host cells. The activation of such channels is part of the virus release process from infected cells. Inhibition of these viral ion channels will counter virus release. HIV-1 encodes for a viral ion channel called viral-protein-U (Vpu) that exhibits various roles, including virus release.
Sauter et al. used the flavonoid genistein to block this ion-channel. Xenopus oocyte cells isolated from females of the clawed toad Xenopus laevis were used to express HIV associated Vpu of the virus, and were injected with 5–40 ng cRNA one to three days before adding genistein. Then, its effects against viral-protein U (Vpu) of HIV-1 were analyzed by voltage-clamp techniques.
Apart from genistein, other flavonoids like quercetin, kaempferol, and (-) EGC were first used against this ion-channel but they only inhibited the Vpu-Mediated current by only 10% at a concentration of 20 μM. When the treatment with genistein began, this isoflavone inhibited the Vpu-mediated current. At a concentration of 80 μM, 50% inhibition of the ion-channel was observed. These results show promise that synthesizing a derivative of genistein could be more active and have more bioavailability than genistein itself. The glucoside derivative of genistein, for example, had the same inhibitory effect as genistein.
Vpu-channel activity is necessary for the release of the HIV-1 and its inhibition would block the virus from being released to infect other host cells [48].
14. Sodium rutin sulfate (SRS) flavonoid against HIV
HIV has been placed in Lentivirus (a retrovirus subgroup) which causes serious illnesses in humans. Over the time, they cause acquired immunodeficiency syndrome (AIDS). It has a positive-sense genome, that is single-stranded, and is an enveloped RNA virus. Tao et al. [49] used the HIV-1 virus and propagated it in HeLa cells, C8166cells, PBMC cells, and Vero cells. Various strains of HIV were used in this study. When these infected cultured cells were treated with sodium rutin sulfate (SRS), effective inhibition of these viruses was noted. SRS effectively inhibited the HIV viral strains HIV-1 × 4 virus IIIB, Ada-M, Ba-L HIV strain, and HIV-1 R5 (isolated from a patient in Yunnan), at a concentration of IC50 = 2.3 ± 0.2, 4.5 ± 2.0,8.5 ± 3.8 μM and 13.1 ± 5.5 μM, respectively. Only the sulfated rutin flavonoid showed antiviral activity, the non-sulfated rutin flavonoid did not show any inhibitory effect on the HIV-1. Upon further analysis of the mode of action of SRS, it was noted that SRS inhibited the entrance process of the HIV-1 into the cell by targeting the glycoproteins of the HIV-1 envelop [49].
15. Quercetin against cardio virus infection in mice model
Cardio Viruses belong to the Picornaviridae family; there are several types of this virus and have a single-stranded RNA genome, and is non-enveloped. Its various types can cause severe illnesses like encephalomyelitis, acute gastroenteritis in humans, and it infects mice and causes neurological diseases in them [50].
Veckenstedt et al. performed in vivo studies on various types of mice models in different strains of this virus. These strains were MengoM, encephalomyocarditis (EMC) viral strain, Col. SK and MM viral strain. Mice of various kinds like Male Type-ABD2F1 or (AB/Jena X DBA 2/Jena) type hybrids were used. At a dose of 80–240 mg per kg of body weight, mice were given quercetin, and no toxicity was observed at this dose in either of the mice types tested.
Quercetin flavonoid treatment was started, and its antiviral activity was checked for mice type ABD2F 1/Jena and nude mutant mice type. Quercetin protected the mice from lethal infection of various strains of cardiovirus; this protective effect was only noted when the treatment with quercetin was started 3-days prior to the lethal infection of the viral strains via intraperitoneal (i.p) route injection.
Nevertheless, when the cardiovirus viral strain MengoM-virus was given to AB/Jena and Lati: CFLP mice types at a lethal dose via intra-cerebral (i.c) injection, quercetin failed to inhibit this cardio virus strain and showed no protective effect. Therefore, these mice died. Only ABD2F 1/Jena and nude mutant mice type were protected by quercetin flavonoid.
When in vitro studies were performed in L-929 cells infected with cardiovirus it was noted that quercetin had no in vitro effect on the cardiovirus, and showed protective effect against cardiovirus only in vivo [51].
16. Flavonoids against rhinovirus (RV)
Rhinovirus (RV) belongs to the family of viruses known as Picornaviridae, which have a single-stranded RNA genome and are non-enveloped viruses. Its genome is a positive-sense single-stranded RNA genome and is about 7.1–7.2 kb. This virus causes URTIs (upper respiratory tract infections) in humans. Researchers observed that in patients, of asthma and chronic obstructive pulmonary disease (COPD) it further increases the severity of the disease [52].
16.1. Quercetin flavonoid
BEAS-2B cells (immortalized human bronchial epithelial cells) were used for the propagation of RV. These cells were then cultured with two viral strains of RV (RV1B and RV39 viral strains).
When the treatment of BEAS-2B cells with quercetin was started at a concentration of 5, 10 and 25 μM, quercetin decreased the levels of RV virus various strains by up to 75–85% in the BEAS-2B cells.
RV requires a specific cell enzyme activation, to enter the host cell and infect it. This enzyme is PI-3-kinase (used for cell growth and cell proliferation); quercetin is known to inhibit this enzyme. When quercetin was analyzed to see if it can stop the endocytosis of the RV virus strains into the cultured BEAS-2B cells. At a concentration of about 10 μM of quercetin pretreatment of the cultured cells the virus endocytosis process was inhibited. When RV viral replication and transcription process occurs in host cell, RV negative-strand RNA is produced. After using PCR technique, it was observed that the number of the (-) strand RNA in the BEAS-2B cells was at high levels in these cells (non-infected cells). But when the quercetin treated BEAS-2B cells which was infected with RV was checked, the number of the negative-strand RNA of the Rhinovirus is not spotted, which suggests that quercetin effectively inhibited the replication of the Rhinovirus at the transcriptional stage [53].
16.2. 6-chloro-3-methoxy flavone-4′-carboxylic acid and 6-chloro-3-methoxy-4′-oxazolinyl flavone and 6-chloro-4′-oxazolinyl flavonoids
Desideri et al. [45] used HeLa cells and infected it with the Human Rhino Virus (HRV-1B). Various flavonoids were tested against the HRV-1B. Among them three flavonoids showed strong inhibition of the HRV-1B. These three flavonoids were 6-chloro-3-Methoxyflavone-4′-carboxylic acid, 6-chloro-4′-oxazolinyl flavonoids, and 6-Chloro-3-methoxy-4′-oxazolinyl flavone. Treatment with these three flavonoids effectively inhibited the HRV-1B at IC50 values of 3.82 μM, 5.161 μM and 4.47 μM respectively. Both 6-chloro-3-Methoxy flavone-4′-carboxylic acid and 6-Chloro-3-methoxy-4′-oxazolinyl flavone showed no cytotoxicity towards HeLa cells at a concentration greater than 25 μM, and 6-chloro-4′-oxazolinyl flavonoid also showed no cytotoxicity towards HeLa cells at a concentration greater than 12.5 μM [45].
17. Flavonoids against Zika virus
The Zika virus (ZIKV) belongs to the Flavivirus class and the Flaviviridaee viral family. Its genome size is 10.7 kb. It has a genome of single-stranded, positive-sense RNA and it is an enveloped virus [100], [101], [102]. This virus is spread by two Aedes mosquitoes' species, A. aegypti and A. albopictus. It causes microcephaly and other brain associated defects in newborn babies [105]. Various outbreaks of ZIKV have been reported worldwide [54], [96].
17.1. Epigallocatechin gallate (EGCG)
Aedes albopictus derived cells clone C6/36 cells were used to propagate Zika virus strain ZIKVBR. EGCG was first evaluated if it can have any virucidal activity against the ZIKBVR strains of the ZIKV. EGCG treatment was started by incubating EGCG at several different concentrations. When the virus activity was checked after treatment with EGCG, it was observed that 1-Log or more than 90% of the virus activity was inhibited when a concentration of about 200 μM of EGCG flavonoid was pre-incubated with ZIKV for 1 h. This suggests that this flavonoid has potent virucidal effect on the virus. The EC50 value of EGGC was 21.4 μM. Another strain of zika virus was also tested. The zika virus MR766 strain infected cells treated with EGCG (25 μM) showed ~85% inhibition. The mode of action of EGCG was inhibition of the entry of the viral strains of the ZIKV into the host cells. The results confirm that EGCG effectively achieved inhibition of various ZIKV strains at an inhibition rate of 85–90% [55].
17.2. Isoquercitrin flavonoid
Gaudri et al. [56] examined the antiviral effect of Isoquercitrin against the zika virus in different cell lines. Isoquercitrin showed strong inhibition of the zika virus by interfering with the entrance process of the ZIKV and blocked its internalization into host cells. In the SH-SY5Y cell line, isoquercitrin showed strong inhibitory action with an IC50 = 9.7 ± 1.2 μM. The CC50 value of isoquercitrin for SH-SY5Y cell line was 582.2 ± 41.4 μM [56].
17.3. Myricetin and quercetin flavonoids
Zou et al. [57] researched the antiviral function of different flavonoids on Zika virus in Vero cells. Myricetin and quercetin were analyzed for their anti-ZIKV activity, and both of them showed potent anti-ZIKV activity by targeting the replication process of the virus. These two flavonoids nearly obtained complete inhibition on the process of zika virus viral RNA production. The IC50 value for myricetin was 0.58 ± 0.17 μM, and IC50 value for quercetin was 2.30 ± 0.50 μM. The CC50 values for Vero cells of both of the flavonoids were >500 μM [57].
17.4. Naringenin flavonoid
Cataneo et al. [58] studied the antiviral effect of naringenin against several strains of the ZIKV in A549 cell line, which had Asian and African origins. In A549 cell line. Naringenin impaired the zika virus infection in the A549 cells with an IC50 = 58.79 μM. Researchers suggest that collective inhibition and impairment of the viral NS2B-NS3 protease is the mode of action of this flavonoid, its replication process, and impairment of virion assembly of ZIKV. The CC50 value of naringenin was 693.6 μM against A549 cells [58].
18. Silymarin (flavonolignan complex) against influenza-A virus
Influenza A, B viruses belong to the viral genus of alphainfluenzavirus and betainfluenzavirus of the Orthomyxoviridae family, which causes influenza Flu. They are RNA viruses and their genetic material is enveloped. Influenza-A viruses are respiratory pathogens that can cause severe illness in humans such as acute respiratory illnesses, like fever, cough etc.This virus has caused many outbreaks and pandemics since 1918. These annual epidemics are expected to result in approximately 3–5 million cases of acute disease and 290,000–650,000 cases of respiratory deaths worldwide according to World Health Organziation.
Song and Choi [59] propagated influenza-A viral strain Influenza A/PR/8/34 in the MDCK cells. Upon treatment of virus infected cultured cells with silymarin, Song and Choi (2011) started to analyze silymarin antiviral activity. The antiviral assay revealed that silymarin had good antiviral activity against the influenza A/PR/8/34 virus. At about 100 μg/ml concentration silymarin possessed a remarkable antiviral activity of 98% against the viral infection. Further analyses showed that at a lower concentration of silymarin of about 10 μg/ml, 45% of the viral infection was inhibited. Oseltamivir a drug used to treat influenza-A viral infection, at 100 μg/ml concentration only inhibited the viral infection by 52%. Its 10 μg/ml concentration had a very weak 40% inhibitory effect on the viral strain of influenza A/PR/8/34. Silymarin inhibited the viral RNA synthesis process. Moreover, Silymarin at 100 μg/ml also strongly prevented the severe cytopathic effect (CPE) caused by influenza virus when added to the cultured cells after 2 days (p.i) while the control Drug Oseltamivir failed in preventing the CPE at 100 μg/ml concentration. These results confirm that the virus infection was prevented by silymarin more potently than the market available drug Oseltamivir [59].
19. Baicalein and genistein flavonoids against HCMV
Cytomegalovirus belongs to the Herpesvirales genus in Herpesviridae family. It is a beta herpes virus. Its genome is double stranded DNA, and its genome size is about 230 kbp. HCMV is an enveloped virus. It is an opportunistic virus, and infects immune-compromised patients such as AIDS patients and childrens with various illnesses in them [60]. HEL 299 human embryonic lung fibroblasts cells cultures were prepared and infected with the HCMV viral strain RC256 and then treated with both the flavonoids (baicalein and genistein and their inhibitory effect was noted. Baicalein and genistein showed no cytotoxicity to (HEL 299) human embryonic lung fibroblasts cells at a concentration of CC50 = 100 μM. Through the use of colorimetric assay, the inhibitory effect of these flavonoids was noted. When analyzed for its effect on the replication of HCMV, at a concentration of IC50 = 0.4 ± 0.04 μM baicalein strongly inhibited the replication process of the HCMV virus at a concentration of IC50 = 0.4 ± 0.04 μM. HCMV Replication was inhibited by genistein at IC50 = 38 μM. When the mode of action against replication of the HCMV by the flavonoids was studied, these flavonoids targeted and decreased the HCMV immediate early proteins IE1–72, IE2–86; these proteins are essential in the replication process of the HCMV. Baicalein at 20 μM (5.4 μg/ml) completely reduced the levels of these essential proteins, and genistein at 50 μM (13.5 μg/ml) reduced IE1–72 proteins to slightly lower levels after 24-hour post infection (hpi), while the levels of the IE2–86 essential protein were reduced up to 65%. The UL84, UL94 proteins (called late proteins regulated by immediate early proteins IE1–72, IE2–86) were completely inhibited by genistein at 48 and 72-hour post infection (hpi).
When DNA synthesis of HCMV was checked, genistein showed about 95% of inhibition on the HCMV DNA synthesis, Baicalein also inhibited the entry process of HCMV by blocking the kinase activity of the EGFR gene [61].
20. Flavonoids against coronavirus 3CLprotease
Coronaviruses (CoVs) are enveloped, they carry positive-sense, single-stranded RNA viral genome. Its genome size varies in size from 27 to 30 kbp. These viruses mainly infect the epithelial cells of lungs by binding to angiotensin-converting enzyme 2, (ACE-2). It infects humans, pigs, birds and bats, and other hosts.
CoVs are the main etiological agents for many viral infections, pandemics and epidemics like MERS, SARS-1, and SARS-CoV-2 [103], [104].
20.1. Pectolinarin, herbacetin and rhoifolin flavonoids
In E. coli, the SARS-CoV-2 3CLprotease gene was expressed and the protease enzyme was then extracted and FRET assay was performed with these three flavonoids: rhoifolin (apigenin-7-O-rhamnoglucoside), pectolinarin (5, 7-dihydroxy 40, 6-dimethoxyflavone 7-rutinoside) and herbacetin (3, 40, 5, 7, 8-pentahydroxyflavone). When these compounds were checked for their inhibitory effect on the 3CL-protease of the coronavirus, these three flavonoids inhibited this enzyme at dose dependent inhibitory concentrations. Rhoifolin inhibited the CoV-3CL-protease enzyme at a concentration of IC50 = 27.24 μM, whereas berbacetin and pectolinarin had IC50 = 33.17 μM and IC50 = 37.78 μM concentrations, respectively, against the inhibition of the SARS-CoV-2 3CLprotease. These three flavonoids showed strong inhibition towards SARS-CoV-2 3CL protease [62].
20.2. Amentoflavone
Ryu et al. [63] studied various flavonoids extracted from Torreya nucifera plant against the SARS-CoV-2 3CL protease. It is a vital protease of SARS-CoV-2 and main protease enzyme used by SARS-CoV-2 in the processing of the viral poly-proteins, and is also used in replicase complex activity. From the plant extracts they identified that the Amentoflavone biflavonoid present in the plant extracts strongly inhibited the SARS-CoV-2 3CL protease. They used FRET enzyme assay for the determination of the antiviral activity of Amentoflavone against SARS-CoV-2 3CL protease. Amentoflavone exhibited strong inhibitory effect on the SARS-CoV-2 3CL protease with an IC50 = 8.3 μM [63].
21. Flavonoids against herpes simplex virus-1 and 2 (HSV-1, HSV-2)
Herpes simplex virus 1 (HSV-1) is a linear DNA virus that is double-stranded or dsDNA. The size of its genome is around 152 kbp, it belongs to the human Herpesviridae family. The principal cause of cold sores or oral herpes is HSV-1, but it can also cause genital herpes according to WHO.
21.1. Novel prenylated flavonoid leachianone G
HSV-1 virus was propagated in Vero cells, and then it was treated with Leachianone-G, a novel flavonoid. For Vero cells, the Leachianone-G flavonoid CC50 was 15.5 μg/ml. When the anti-viral activity of leachianone-G was analyzed, a concentration of IC50 = 1.6 μg/ml of Leachianone-G displayed a strong Herpes simplex virus-1 inhibition [64].
21.2. (SRS) sodium rutin sulfate flavonoid
Herpes simplex virus –1 (HSV-1) was analyzed to see if it can be inhibited by SRS flavonoid. HSV-1 was propagated in Vero cells, and then the virus infected cell culture was treated with SRS. It was noted that at a concentration of IC50 = 88.3 ± 0.1 μM of SRS, HSV virus was inhibited. The non-sulfated rutin flavonoid could not hinder the viral infection of HSV-1, only the sulfated-rutin flavonoid inhibited the HSV-1 infection. The non-sulfated rutin had no antiviral activity against the viral infection [49].
21.3. Galangin, epicatechin (EC), epigallocatechin (ECG), quercetin and genistein
Lyu et al. [65] used various flavonoids for the inhibition of HSV-1 Herpes simplex viruses. They used Vero cells, and propagated the virus in these cells and then treated it with different flavonoids. Flavonoids tested against the HSV-1 showed strong inhibitory effects and reduced the CPE (cytopathic effect) in the virus infected cells. Galangin at EC50 = 0.64 μg/ml, epicatechin (EC) at EC50 = 0.725 μg/ml, epicatechin gallate (ECG) at EC50 = 0.725 μg/ml, quercetin at EC50 = 1.69 μg/ml and genistein at EC50 = 1.35 μg/ml showed strong inhibition by reduction of the CPE in cultured cells infected with the HSV-1. With the exception of the galangin flavonoid, all the above flavonoids showed potent inhibitory effect on the HSV-1 by reducing its CPE in infected Vero cells. All of the flavonoids tested showed no cytotoxicity towards the Vero cells up to a concentration of 100 μM [65].
21.4. (-) Epigallocatechin gallate (EGCG) flavonoid
Isaacs et al. [66] used Vero and CV-1 cells and infected them with separate strains of various clinical isolates of the Herpes Simplex virus (both HSV-1 and HSV-2 strains). They studied the antiviral effect of a catechins molecule found in green tea, epigallocatechin gallate (EGCG). It was noted that both viral strains of the HSV were strongly inhibited by the EGCG Flavonoid. It was found that the EGCG flavonoid interacts with and inhibits the gB and gD envelop proteins of HSV. In the fusion and entry of HSV into the host cells, these gB and gD proteins play essential roles. Quercetin IC50 values for HSV-1 and its different clinically isolated strains ranged from 12 to 25 μM, while IC50 of quercetin for HSV-2 and its different clinically isolated strains ranged from 6 to 12.5 μM. All of the HSV-1 and HSV-2 strains were 100% inhibited at a concentration of 100 μM of quercetin. Quercetin showed no cytotoxicity to both Vero and CV-1 cells at concentration of 100 μM [66].
21.5. (-)Gallocatechin gallate (GCG) flavonoid
Isaacs et al. [66] used Vero and CV-1 cells and infected it with different HSV1 and HSV-2 strains. The antiviral activity of Gallocatechin gallate (GCG) was noted. The GCG flavonoid is an epimer of the epigallocatechin gallate (EGCG) flavonoid. GCG inhibited the gB and gD Envelop proteins of HSV-1&2. Strong antiviral activity of GCG was noted against the HSV different strains. At 100 μM, GCG reduced HSV-1 and HSV-2 titers by 3.5 log and 4.0 log, respectively. GCG showed no cytotoxicity to both Vero and CV-1 cells at a 100 μM concentration [66].
22. Pinocembrin flavonoid against ZIKV replication and protein synthesis
The Zika virus (ZIKV) belongs to the Flavivirus class and the Flaviviridaee virus family. Its genome size is 10.7 kb. It has a genome of single-stranded, positive-sense RNA and it is an enveloped virus. This virus is spread by two Aedes mosquitoes' species, A. aegypti and A. albopictus. It causes microcephaly and other brain associated defects in newborn babies. Various outbreaks of ZIKV have been reported worldwide.
For the ZIKV inhibition, Lee et al. used pinocembrin flavonoid. The CC50 concentration of pinocembrin against JEG-3 cells was 251 μM. When the treatment of the pinocembrin flavonoid was started to see if pinocembrin can inhibit the ZIKV cultured in JEG-3 cells, it was noted that at a concentration of IC50 = 17.4 μM of pinocembrin, inhibition of ZIKV was observed. When the treatment was continued, pinocembrin produced more effective inhibition at 40 µg/ml concentration.
When the mode of action of pinocembrin was checked against the JEG-3 cells infected with the ZIKV, it was observed that at 156 μM concentration of pinocembrin treatment with ZIKV infected cells at 8 and 14 (hpi), the positive and negative-sense ZIKV RNA was decreased when checked by qRT-PCR. Another technique, Western blot, confirmed that the viral protein synthesis of ZIKV was also decreased by treating JEG-3 cells with pinocembrin at a concentration of 156 μM.
These results confirm that pinocembrin flavonoid inhibits the ZIKV by targeting its replication process and protein synthesis [67].
23. Flavonoids against Ebola virus
The Ebola virus caused the Ebola heamorrhagic disease. It is also known as the Zaire Ebola virus. Its name Ebola is derived from the Ebola River in DR Congo. Its genome size is 18,898 nucleotides and it has a single negative strand RNA genome [97]. It belongs to the genus Ebola virus and filoviridae family of viruses [47]. Various outbreaks of Ebola virus have been reported since 1976 and have caused more than 11,000 deaths across different countries according to WHO.
23.1. Q3G (Quercetin-3 ß-O-D-glucoside)
Vero E6 epithelial cells were infected with different strains of Ebola viruses, EBOV-Kikwit and EBOV-Makona strains, and were then treated with Q3G flavonoid. The cytotoxic concentration of Q3G for Vero E6 cells was determined, and up to 100 μM of Q3G showed no toxicity to the Vero E6 cells. Q3G was able to inhibit the virus in vitro, and determined EC50 value for Q3G was 5.3 μM. Q3G inhibited the viral replication of Ebola virus. When Q3G was tested against other types of Ebola viruses such as SUDV (an Ebola virus strain from Sudan), and wild type Ebola viruses, it showed potent inhibition and targeted the viral entry of these viruses.
Antiviral activity against the mouse-adapted Ebola virus (MA-EBOV) strain was also seen in Q3G in a mice model. A group of 10 C57BL/6 mice was tested, and given a dosage of 50 mg/kg of Q3G for two weeks, and were then given a lethal dose (1000 x LD50) of the mouse adapted Ebola virus. The mice survived the lethal dose of the virus. The treatment of Q3G was continued 48 h after infection. Only minimal weight loss and mild signs of the disease were seen in the mice, and all the mice survived. Q3G was tested if it can give prophylactic protection to mice. Doses of 12.5 and 25 kg/ml resulted in 3/10 and 2/10 survival of the mice and protected the mice prophylactically [68].
23.2. Cyanidin-3-(p-coumaroyl)-rutinoside-5-glucoside, myrciatrin V & 7-O (6-feruoylglucosyl) isoorientin flavonoids in silico
Putra et al. [69] used many flavonoids in silico via docking and simulation against a Sudan Ebolavirus SEBOV strain glycoprotein (GP). They found that three flavonoids showed low ∆G-binding values towards the GP protein of SEBOV. The three flavonoids, cyanidin-3-(p-coumaroyl)-rutinoside-5-glucoside flavonoid, Myrciatrin-V and 7-O-(6-feruoylglucosyl) isoorientin showed higher molecular interaction with various amino acids residues of the GP-protein of the Ebola virus. Cyanidin-3-(p-coumaroyl)-rutinoside-5-glucoside flavonoid showed the lowest ∆G-binding value −9.7 kcal/mol of all the other flavonoids. Myrciatrin-V and 7-O-(6-feruoylglucosyl) isoorientin had ∆G-binding values of −9.2 kcal/mol and −9.0 kcal/mol respectively. These three flavonoids had the highest binding affinities towards the GP-Protein of the Sudan Ebola virus. Based on root means square deviation (RMSD) score values, which determine if the ligand pose created by the chemical during the docking simulation is acceptable and can be replicated during the actual interaction. In computational studies of the drug (flavonoids) against the test subject (i.e. Ebola virus), only 2 of the 3 flavonoids had lower RMSD values. The recommended RMSD value of docking pose is below 2.0 Å. Among the tested flavonoids, Myrciatrin-V had RMSD value = 1.84 Å and the other flavonoid 7-O-(6-feruoylglucosyl) isoorientin had RMSD value= 1.9 Å, which is below the 2.0 Å recommended value of RMSD, which means that these two flavonoids can inhibit the Ebola virus by Targeting its GP [69].
23.3. Gossypetin and taxifolin flavonoids in silico
Raj et al. studied the interaction of two flavonoids, gossypetin and taxifolin with the Ebola virus viral proteins, VP35 and VP24 via docking and simulation. These VP24 and 35 viral proteins are essential for the Ebola virus function. these proteins suppress the host immune system by inhibiting the production of the anti-viral cytokines such as interferons (IFN), which induce innate antiviral resistance and contribute to adaptive immunity. They performed docking simulation of the Viral VP-24 and 35 with the gossypetin and taxifolin flavonoids. It was found that gossypetin binds to the VP24 protein with the highest docking value of -8.252 kcal/mol and the binding free energy of −34.633 kcal/mol and forms four hydrogen bonds with the VP24 protein side chain. Gossypetin also strongly interacts with VP35; its docking score with VP35 was −5.395, and the binding energy was 31.628 kcal/mol.
Taxifolin, the second flavonoid, strongly interacted with the VP35 protein of Ebola virus and formed various H-bonds with it. The docking score and binding free energy values for taxifolin with VP35 protein were -5.433 kcal/mol and −37.433 kcal/mol, respectively. And for VP24, its docking energy was −7.328 and binding free energy was - 47.572 Kcal/mol. VP24 and VP35 viral proteins perform other vital viral functions such as forming a replication complex with the VP-40 viral protein of Ebola virus. This in silico study confirms that these two flavonoids can target these viral proteins, resulting in the inhibition of Ebola Virus [70].
23.4. Quercetin flavonoid
Fanunza et al. [71] used HEK293T cells and propagated EBOV in this cell culture. They used the quercetin flavonoid to inhibit the EBOV by targeting the VP24 viral protein. This VP24 viral protein suppresses the interferon production of the infected host, thereby evading the host’s immune system. Quercetin potently inhibited the VP24 protein of the EBOV, Which resulted in the IFN-I signaling cascade being restored which was blocked by the VP24. Quercetin restored the function of the IFN genes and effectively blocked the viral infection. Quercetin had an IC50 value of 7.4 μM against EBOV. At concentrations between 3 μM and 30 μM, quercetin exhibited no cytotoxicity, its CC50 was greater than 100 μM [71].
24. Flavonoids against coxsackievirus
Coxsackieviruses are a few related enteroviruses that belong to the non-enveloped, linear, positive-sense single-stranded RNA virus family of Picornaviridae. Its genome size is around 7.4 kb and in children it causes hand, foot, and mouth disease (HFMD) [72].
24.1. Dihydroquercetin (DHQ)
Galochkina et al. [73] studied the antiviral activity of Dihydroquercetin (DHQ) using a mice model. They infected the mice with the Coxsackievirus B4 (CVB4). Then, DHQ treatment was started via intraperitoneal route at a concentration of 75 and 150 mg/kg per day for 5-days post-infection. It was noted that DHQ treatment caused dose dependent inhibition, and decreased the viral titer in the pancreatic tissue of the mice. When treated with the highest dose of DHQ, a 2.4 log decrease was noted in the virus titer on day-5 (p.i). When the antiviral activity was compared with the positive control ribavarin, dihydroquercetin exceeded the antiviral activity of ribavarin, and effectively inhibited the Coxsackievirus B4 (CVB4). The mechanism of action of DHQ against this virus was to suppress the process of its replication [73].
24.2. Luteolin flavonoid
Xu et al. [74] used RDS cells and infected it with coxsackievirus A16 (CA-16) and studied the effect of luteolin flavonoid on this CA16 infected cultured cells. It was noted that Luteolin effectively inhibited the CA16 with EC50 = 10.52 μM by targeting and inhibiting CA16 viral-RNA replication, luteolin inhibited the post-attachment stage of CA16. The CC50 value of luteolin towards the cultured cells was 148.02 μM. Peak inhibition of coxsackievirus A16 by luteolin was noted at a concentration range of between 25 and 100 μM [74].
24.3. Selaginella moellendorffii Hieron total flavonoid extracts (TFE) and Amentoflavone flavonoid
Yin et al. [75] used HEp-2 cells and infected it with coxsackievirus B3 (CVB3), and then observed the antiviral effect of Selaginella moellendorffii Hieron total Flavonoid Extract (TFE), and its main flavonoid constituent Amentoflavone. TFE and Amentoflavone at IC50 = 19 ± 1.6–41 ± 1.2 μg/ml and 25 ± 1.2–52 ± 0.8 μg/ml, respectively, showed inhibitory effect on the Coxsackievirus B3. The CC50 values for TFE and Amentoflavone were 85 ± 1.7 μg/ml and 53 ± 0.9 μg/ml respectively. Both TFE from Selaginella moellendorffii Hieron and Amentoflavone showed significant virucidal effect on coxsackievirus B3 (CVB3) [75].
25. Quercetin 7-rhamnoside against porcine epidemic diarrhea virus (PEDV)
The Porcine Outbreak Diarrhea Virus (PEDV) belongs to the Alphacoronavirus class and to the Coronaviridae family of viruses. It is an enveloped virus with a genome of single-stranded, positive-sense RNA and is 30 kb in size. Acute diarrhea and/or vomiting, dehydration and high mortality in pigs are caused by PEDV virus [76].
Choi et al. [77] used PEDV, and propagated it in Vero cells and studied the antiviral activity of quercetin 7-rhamnoside. Quercetin 7-rhamnoside strongly inhibited the replication process of PEDV. It inhibited the early phase of PEDV replication. The IC50 of quercetin 7-rhamnoside was 0.014 μg /ml. Its CC50 value against Vero cells was 100 μg/ml [77].
26. Flavonoids against coronavirus SARS-CoV-2
Coronaviruses are Picornaviruses. They have a positive-sense RNA genome, and they are widely found in bats and also infect other organisms like humans, birds, and other mammals. It causes various illnesses; the most common ones are respiratory diseases [78]. Several strains of coronaviruses are responsible for epidemics like (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) [79]. SARS-CoV-2 also belong to this family of viruses but it is more infectious, since its first case in 2019 in Wuhan, China. It has infected 90 million persons and caused over 1.8 million deaths and counting. There is still no potent drug available to remedy the effect of this virus.
26.1. EGCG and theaflavin flavonoids
Jang et al. [80] performed an in vitro study to analyze the effects of tea flavonoids against the SARS-CoV-2 3CL-Protease. Theaflavin & EGCG flavonoids firmly repressed the activity of the SARS-CoV-2 3CL-Protease dose dependently. EGCG and theaflavin with the IC50 values of 7.58 μg/ml for EGCG and 8.44 μg/ml of theaflavin effectively decreased the activity of the 3CL-Protease. Both of these flavonoids showed no cytotoxicity towards the HEK293T cells up to 40 μg/ml concentration. This study indicates that these flavonoids can be useful in SARS-CoV-2 treatment [80].
26.2. Myricitrin & Taiwan homoflavone A flavonoids
Joshi et al. [81] screened various different classes of compounds via docking and simulation against the Main-Protease of SARS-CoV-2. They identified that myricitrin flavonoid has high binding affinity with a binding energy score of −8.9 kcal/mol with the Main-Protease enzyme of SARS-CoV-2. Myricitrin showed strong interaction with the amino acid of the active site of the Mpro enzyme. Pi-Alkyl, Pi-Sulfur interactions and six Hydrogen bond interactions were observed with Mpro enzyme. In the same study, they identified another flavonoid isolated from Cephalotaxus wilsoniana plant, the flavonoid Taiwan homoflavone A, which had a strong binding score of −9.6 kcal/mol with the Mpro of the SARS-2. Apart from this the Taiwan homo-flavone, a flavonoid also demonstrated good binding interactions with the other RNA-polymerase (RdRp) essential enzymes. These findings indicate that these flavonoids could be strong candidates for SARS-CoV-2 drug discovery [81].
26.3. Rutin flavonoid
Abd El-Mordy et al. [82] also screened various flavonoids against the Mpro enzyme of SARS-CoV-2 in in-silico studies. They found several flavonoids which showed strong binding interaction with the Mpro enzyme of SARS-CoV-2, and among these flavonoids, rutin showed highest binding energy score of −8.2 kcal/mol with the Main-Protease (Mpro) of COVID-19 [82].
26.4. Fisetin & quercetin flavonoids
Pandey et al. [83] studied various flavonoids against the spike protein via an in silico docking simulation, and identified two flavonoids that showed strong affinity towards the S2-domain of the spike protein of SARS-CoV-2. These two flavonoids, fisetin & quercetin, both had a binding affinity score of −8.5 Kcal/mol toward the S2 domain of the spike protein. Further molecular dynamics studies showed that quercetin has the strongest binding energy score of ΔGbind = −22.17 ± 3.04 kcal/mol towards the hACE2-S protein complex of SARS-CoV-2, indicating that quercetin could be a good drug candidate against SARS-CoV-2 [83].
26.5. Biochanin A and silymarin bioflavonoids
Gorla et al. [84] performed molecular docking studies of two flavonoids biochanin A and silymarin with the host cells ACE-2 protease domain (PD-ACE-2) and SARS-CoV-2 S-protein receptor binding domain (RBD-S), and found that these two flavonoids has strong binding affinities with PD-ACE-2 and RBD-S. The molecular docking score of biochanin A and silymarin with the PD-ACE-2 & RBD-S were −78.41and −121.28 kcal/mol respectively. These results confirm that these bioflavonoids can be good inhibitors of the SARS-CoV-2 if further pursued in the in-vivo and in-vitro studies [84].
26.6. Quercetin flavonoid
Vijaya kumar et al. [85] identified another flavonoid, quercetin, via in silico studies that strongly interacted with the active site residues of the Mpro of SARS-CoV-2. The binding energy affinity scores for quercetin with the SARS-CoV-2 Main protease was −9.2 kcal/mol [85].
26.7. Narcissin & kaempferol-3-O-rutinoside flavonoid
Owis et al. [86] also performed docking studies of several flavonoids against the main protease (Mpro) of SARS-CoV-2, and found two flavonoids that have strong binding affinities with the main protease (Mpro) enzyme. The flavonoids narcissin and kaempferol-3-O-rutinoside showed strong affinities toward the active site of the Mpro enzyme. The Docking interaction energies for the Narcissin & kaempferol-3-O-rutinoside with the substrate binding unit of the Mpro of SARS-CoV-2 were −8.25 and −8.12 kcal/mol respectively [86]. Table 1.
Table 1.
Antiviral activities of flavonoids.
| S/N | Flavonoid | Structure | Virus | Experiment Model | Inhibitory Concentration IC50/EC50 | Cytotoxic Concentration CC50 | Inhibition Stage | Mode of inhibitory action | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Taxifolin |  |
Ebola Virus | In-silico | Glide (binding) energy of - 47.57 kcal/mol with VP24 and − 37.433 kcal/mol for VP35 | – | VP24 & VP35 Viral surface Protein inhibition | Blocking the active site of VP24&35 which suppresses host interferon production. | [70] |
| 2 | Sodium Rutin Sulfate |  |
Human Immune Virus-1 | In-vitro | >3048.1 μM for Hela cells. | Viral Entry | Inhibition of Glycoproteins of HIV-1 Envelop. | [49] | |
| Strain 1: HIV-1×4 virus IIIB | IC50 = 2.3 ± 0.2 μM | >2589.5 μM for PBMC cells. | |||||||
| strain 2 HIV-1Ada-M | IC50 = 8.5 ± 3.8 μM | >1481.4 ± 355.6 for C8166 cells. | |||||||
| Strain 3: HIV-1 R5 | IC50 = 13.1 ± 5.5 μM | ||||||||
| HSV-1 | In-vitro | IC50 = 88.3 ± 0.1 μM | > 3.0 mM | Not reported | Not reported | [49] | |||
| 3 | Ro-090179 |  |
Polio Virus PV-1 | In-vitro | 1.5 μM Prevented appearance of viral proteins (IC50 not reported) | Not reported | Viral RNA Replication | strong blockade of poliovirus plus-stranded viral RNA synthesis | [44] |
| 4 | Rhoifolin |  |
SARS-CoV-1 | In-vitro | IC50 = 27.24 μM | Not reported | Inhibition of SARS-CoV 3CLprotease enzyme. | Blocking of active sites of Protease enzyme | [62] |
| 5 | Quercetin-3 ß-O-D-glucoside |  |
Ebola virus Strain:1 EBOV Makona | In-vitro | EC50 = 5.3 μM | 100 μM showed no toxicity to Vero E6 cells | Entry and Replication | Inhibited Envelop proteins of Ebola virus and its Replication. | [68] |
| Strain:2 MA-EBOV | In-vivo | 50 mg/kg | Less than 100 mg/kg Mice model | ||||||
| 6 | (-) Gallocatechin gallate (GCG) |  |
HSV-1 | In-vitro | 3.5 log reduction | No cytotoxicity at 100 μM to both Vero and CV-1 cells | Entry | Inhibition of gB and gD viral Envelop proteins of HSV-1,2 | [66] |
| HSV-2 | 4.0 log reduction | ||||||||
| 7 | Quercetin 7-rhamnoside | 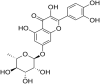 |
(PEDV) Porcine epidemic diarrhea virus | In-vitro | IC50 = 0.014 μg /ml | CC50 = 100 μg /ml against Vero cells | Replication | Early stage of Viral Replication | [77] |
| 8 | Quercetin 3-rhamnoside | 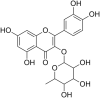 |
Influenza virus strain-A/WS/33 | In-vitro | Not reported | Not reported | Replication | Early stage of Viral Replication | [19] |
| 9 | Quercetagetin | 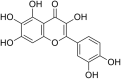 |
(CHIKV)Chikungunya virus | In-vitro | IC50 = 13.53 µg/ml | CC50 = 52.42 μg/ml for BHK-21 cells & CC50 = 52.42 μg/ml for the Vero | Replication | Multi-stage inhibition. Extracellular inhibition of CHIKV, Blocking its attachment to host cells, inhibition of CHIKV replicon system. | [27] |
| IC50 = 9.934 µg/ml | Extracellular stage | ||||||||
| IC50 = 8.050 µg/ml | Entry | ||||||||
| 10 | Pinocembrin | 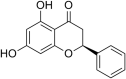 |
Zika virus (ZIKV) | In-vitro | IC50 = 17.4 μM | CC50 = 251 μM for JEG-3 cells | Replication | Inhibition of positive+ and negative-sense ZIKV RNA synthesis | [67] |
| 11 | Pectolinarin | 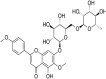 |
SARS-CoV-1 | In-vitro | IC50 = 27.24 μM | Not reported | Poly-protein processing. | Inhibition of SARS-CoV 3CLprotease which is main protease enzyme in SARS-CoV-1 | [62] |
| 12 | Naringenin | 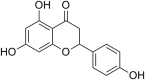 |
Hepatitis C virus (HCV) | In-vitro | 80% inhibition at a concentration of 200 μM. IC50 is not reported. | More than 200 μM against Huh7.5.1 cells | Secretion from infected cells | Inhibition of HCV secretion from infected host cells by host factor modulation. | [29] |
| 13 | Myrciatrin V | 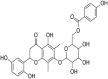 |
Ebola virus | In-silico | ∆G-binding values of −9,1728 kcal/mol | – | Entry and attachment | In-silico simulation showed strong interaction and attachment of Myrciatrin V with the envelop glyco-proteins (GP) of Ebola virus. | [69] |
| 14 | Luteolin | 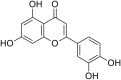 |
Coxsackieviruses (CA-16) | In-vitro | EC50 = 10.52 μM | CC50 = 148.02 μM Against RDS cells | Replication | Inhibition of post entry stage by targeting the Viral RNA replication. | [74] |
| 15 | Leachianone G | 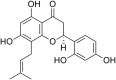 |
HSV-1 Herpes simplex virus-1 | In-vitro | IC50 = 1.6 μg/ml | CC50 = 15.5 μ g/ml against Vero cells | Not reported | Not reported | [64] |
| 16 | Ladanein (BJ486K) | 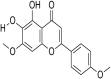 |
HCV strains: 1a&1b, 2b as well 3a, 4a, 5a, & 6a | In-vitro | IC50 = 2.5μmol/L | CC50 = 98.04 μmol/L Against Huh7-Lunet/CD81 cells | Entry | Inhibition of post attachment entry into the host cells | [33] |
| 17 | Kaempferol | 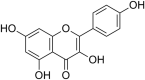 |
Enterovirus EV-A71 | In-vitro | At a concentration of 35 μM for 24 h, 80% virus activity decreased | Greater than 50 μM against RD cells | Replication | Interference with the Replication process of the Virus | [42] |
| Its IC50 is not reported. | |||||||||
| SARS-CoV-1 | In-vitro | At 20 μM Kaempferol decreased the 3a channel activity by factor of 3–5 times. | Not reported | Secretion of virus from host cells | Inhibition of the 3a-channel protein, which is used for virus release from host cells | [47] | |||
| 18 | Juglanin | 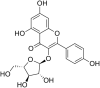 |
SARS‐CoV-1 | In-vitro | IC50 = 2.3 μM | Not reported | Secretion of virus from host cells | Blocking of the 3a-channel protein, which is used for virus release from host cells | [47] |
| 19 | Herbacetin | 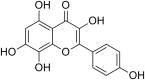 |
SARS‐CoV-1 Coronavirus | In-vitro | IC50 = 33.17 μM | Not reported | Poly-protein processing | Inhibition of SARS-CoV-1 3CL-protease enzyme required for viral poly-protein processing | [62] |
| 20 | Gossypetin | 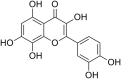 |
Ebola Virus | In-silico | Glide (binding) energy of −34.633 Kcal/mol with VP24 Glide (binding) energy of −31.628 Kcal/mol Kcal/mol with VP35 | – | VP24 & VP35 Viral Protein inhibition | Blocking the active site of VP24&35 which suppresses host interferon production. | [70] |
| 21 | Genistein | 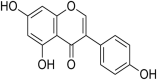 |
HIV-1 | In-vitro | 80 μM caused 50% inhibition. IC50 not reported | Not reported | Virus release from infected host cells | Blocking of Vpu-Ion-channel (viral-protein-U), which is necessary for virus release from infected host cells. | [48] |
| HCMV Cytomegalovirus | In-vitro | IC50 = 38 μM | CC50 = 100 μM for HEL 299 | Replication | DNA synthesis by interfering with DNA-polymerase | [61] | |||
| HSV-1 | In-vitro | EC50 = 1.35 μg/ml | CC50 = 250 μM (Vero cells) | Not reported | Reduction in CPE | [65] | |||
| 22 | Galangin | 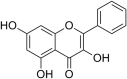 |
HSV-1 | In-vitro | EC50 = 0.64 μg/ml | CC50 = 1000 μM (Vero cells0 | Not reported | Reduction in CPE | [65] |
| 23 | Fisetin | 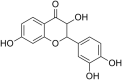 |
CHIKV Chikungunya virus | In-vitro | IC50 = 8.44 µg/ml | CC50 were 194.45 μg/ml | Intracellular stage | Targeted the Replication process of Virus | [27] |
| 24 | Epigallocatechin Gallate (EGCG) | 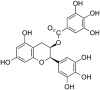 |
HBV | In-vitro | IC50 = 39.4 μ mol/L | No cytotoxicity at 100 μmol/L to HepG2.117 cells | Envelop proteins & Replication | Reduction in Precore-mRNA & HBeAg. | [35] |
| ZIKV Zika virus | In-vitro | EC50 = 21.4 μM | > 100 μM for C6/36 cells | Entry | Blocking of Envelop proteins | [55] | |||
| HSV-1 | In-vitro | IC50 = 12–25 μM For different HSV-1 Strains | > 100 μM (Vero & CV-1 cells) | Entry | Inhibition of gB and gD viral Envelop proteins of HSV-1,2 | [66] | |||
| HSV-2 | IC50 = 6–12.5 μM For different HSV-2 Strains | ||||||||
| 25 | Epicatechin gallate | 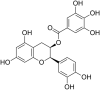 |
HSV-1 | In-vitro | EC50 = 0.72 μg/ml | CC50 = 500 μM (Vero cells) | Not reported | Reduction in CPE | [64] |
| 26 | Dihydroquercetin | 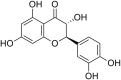 |
Coxsackievirus - B4 strain | In-vivo | 75–150 mg/kg/day for 5-days (p.i) | Not reported | Replication | Suppression of Viral Replication process. | [73] |
| 27 | ( ± ) Dihydromyricetin | 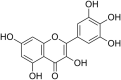 |
Influenza Virus H1N1 | In-vitro | IC50 = 2.8 μg/ml | Not reported | Viral attachment | Blocking of Viral surface protein attachment to host cells | [16] |
| 28 | cyanidin-3-(p-coumaroyl)-rutinoside-5-glucoside |  |
Sudan Ebolavirus SEBOV | In-silico | ∆G-binding value −9,6941 kcal/mol | – | Entry and attachment | In-silico simulation showed strong interaction and attachment of this Flavonoid with the envelop glyco-proteins (GP) of Ebola virus. | [69] |
| 29 | Baicalin |  |
HIV-1 | In-vitro | 4 μM causes 50% inhibition. (IC50 not reported) | Not reported | Entry | Inhibition of HIV-1 Env-Protein | [37] |
| DENV-2 | In-vitro | IC50 = 14.9 μg/ml | CC50 = 290.9 μg/ml (Vero cells) | Intracellular | Inhibition of Nsps of Ebola Virus | [25] | |||
| IC50 = 13.50 μg/ml | replication | Not reported | |||||||
| Enterovirus EV-A71 | In-vitro | IC50 = 4.96 μg/ml | CC50 = 823.53 μg/ml (RD Cells) | Replication | Inhibition of Polymerase | [41] | |||
| 30 | Amentoflavone |  |
Coxsackie virus B3 | In-vitro | IC50 = 19 ± 1.6–41 ± 1.2 μg/ml | CC50 = 53 ± 0.9 μg/ml (HEp-2 cells) | – | Direct virucidal effect. | [75] |
| SARS-CoV-1 | In-vitro | IC50 = 8.3 μM | Not reported | Poly-Protein processing | Inhibition of SARS-CoV 3CLprotease enzyme. | [63] | |||
| 31 | Apigenin | 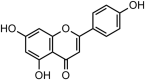 |
HCV | In-vitro | At 5 μM significant inhibition was noted(IC50 not reported) | Not reported | Replication | Inhibition of gene expression of miRNA122. | [31] |
| Entero virus EV-71 | In-vitro | EC50 of 10.3 μM | CC50 = 79.0 μM RD Cells | Host factor modulation | Viral RNA association with hnRNP A1 and A2 proteins | [39] | |||
| Entero virus EV-71 | In-vivo | 50 mg/Kg Mice model (88.89% survival rate) | Not reported | Replication & Protein synthesis | Viral genomic RNA inhibition | [40] | |||
| 32 | Baicalein | 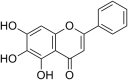 |
HCMV | In-vitro | IC50 = 0.4 ± 0.04 μM | CC50 = 100 μM | Replication | Inhibition of immediate early proteins | [61] |
| CHIKV | In-vitro | IC50 = 3.243 µg/ml | CC50 356.65 μg/ml (BHK-21 cells) | Replication | CHIKV replicon | [27] | |||
| IC50 = 9.934 µg/ml | Extracellular | Adsorption | |||||||
| IC50 = 1.891 µg/ml | Intracellular | Replication of CHIKV | |||||||
| JEV | In-vitro | IC50 = 7.27 ± 1.08 μg/ml | CC50 = 115.2 ± 0.2 μg/ml | Replication | Inhibition of Viral RNA synthesis | [22] | |||
| IC50 = 5.8 ± 1.09 μg/ml | Entry | Blocking of internalization | |||||||
| 33 | 7-O-(6-feruoylglucosyl)isoorientin | 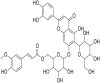 |
Sudan Ebola virus SEBOV | In-silico | ∆G-binding values of −9.0058 kcal/mol | – | Entry and attachment | In-silico simulation showed strong interaction and attachment of this Flavonoid with the envelop glyco-proteins (GP) of Ebola virus | [69] |
| 34 | 6-chloro-4′-oxazolinyl flavanone | 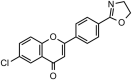 |
Polio-virus type-2 | In-vitro | IC50 = 2.791 μM | > 12.5 μM (Hela Cells) | Plaque reduction | Inhibition of viral replication | [45] |
| Human Rhino Virus (HRV-1B) | In-vitro | IC50 = 5.161 μM | > 12.5 μM (Hela Cells) | Plaque reduction | Inhibition of viral replication | ||||
| 35 | 6-chloro-3-Methoxyflavone-4′-carboxylic acid | 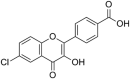 |
Human Rhino Virus (HRV-1B) | In-vitro | IC50 = 3.82 μM | > 25 μM (Hela Cells) | Plaque reduction | Inhibition of viral replication | [45] |
| 36 | 6-Chloro-3-methoxy-4′-oxazolinylflavone | 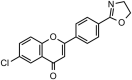 |
Human Rhino Virus (HRV-1B) | In-vitro | IC50 = 4.47 μM | > 25 μM (Hela Cells) | Plaque reduction | Inhibition of viral replication | [45] |
| 37 | 6-chloro-3-hydroxyflavone-4′-carboxylic acid | 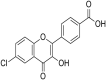 |
Polio virus PV-2 | In-vitro | IC50 = 1.91 μM | > 12.5 μM (Hela Cells) | Plaque reduction | Inhibition of viral replication | [45] |
| 38 | 5,7,3′,4′-tetra-O-methylquercetin | 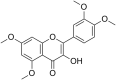 |
Influenza virus H1N1 | In-vitro | IC50 = 0.13 μg/ml | Not reported | Viral attachment | Blocking of Viral surface Glyco-proteins and its attachment to host cells | [16] |
| 39 | 3-methyl Quercetin | 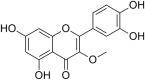 |
Polio Virus PV-1 Mahoney strain | In-vitro | 1.5 μM Prevented appearance of viral proteins (IC50 not reported) | Not reported | Viral RNA Replication | strong blockade of poliovirus plus-stranded RNA synthesis | [44] |
| 40 | (-)Epigallocatechin (EGC) | 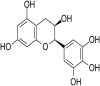 |
Influenza A&B | In-vitro | 1–3 log reduction (IC50 not reported) | CC50 = 400 μg /ml on MDCK cells | Intracellular | prevention of acid-catalyzed fusion reaction of Virus | [17] |
| HSV-1 | In-vitro | EC50 = 0.725 μg/ml | No cytotoxicity At 100 μM to Vero cells | Not reported | Reduction in CPE | [65] | |||
| 41 | (-)Epicatechin | 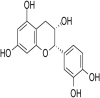 |
HSV-1 | In-vitro | EC50 = 0.725 μg/ml | No cytotoxicity At 100 μM to Vero cells | Not reported | Reduction in CPE | [65] |
| 42 | Quercetin | 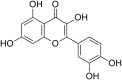 |
Influennza-A | ||||||
| 1)A/Puerto Rico/8/34 strain | In-vitro | IC50 = 7.756 μg/ml | Higher than 250 μg/ml For MDCK Cells | Entry | Blocking of Hemagglutinin (HA) Glycoprotein (GP) | [19] | |||
| 2) A/FM-1/47/1 strain | IC50 = 6.225 μg/ml | ||||||||
| 3) A/Aichi/2/68 (H3N2) | IC50 = 2.738 μg/ml | ||||||||
| DENV-2 | In-vitro | IC50 = 35.7 μg /ml | 252.6 ± 0.17 μg/ml for Vero cells | Replication | Reduction of Viral RNA levels | [24] | |||
| HCV | In-vitro | 10 μg/ml | No cytotoxicity at a concentration of 10 μg/ml | Replication and NS3 protease | Blocking of both the RNA synthesis and NS3 protease enzyme | [32] | |||
| Cardio Virus different strains | In-vivo | 80–240 mg/kg | No toxicity at 240 mg/kg in mice model | Immune-mediated infection control | Macrophage activation | [51] | |||
| Rhino Virus | In-vitro | 10 μg /ml | Not reported | Transcription | Inhibition of endocytosis and PI-3 Kinase | [53] | |||
| HSV-1&2 | In-vitro | EC50 = 1.69 μg/ml | No cytotoxicity on Vero cells up to of 100 μM | Not reported | Reduction in CPE caused by HSV-1&2 | [65] | |||
| Ebola Virus EBOV | In-vitro | IC50 = 7.4 μM | CC50 was greater than 100 μM for HEK293T cells | Inhibition of VP24 protein | Restoration of immune system by InhibitingVP24 viral protein | [71] | |||
| Zika virus | In-vitro | IC50 = 2.30 μM | CC50 = >500 μM Vero cells | Replication | Targeting ZIKV RNA production | [57] | |||
| 43 | Myricetin | 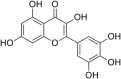 |
Zika virus | In-vitro | IC50 = 0.58 μM | CC50 = >500 μM Vero cells | Replication | Targeting ZIKV RNA production | [57] |
| 45 | Isoquercitrin | 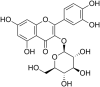 |
Zika virus | In-vitro | IC50 = 9.7 ± 1.2 μM | CC50 = 582.2 ± 41.4 μM for SH-SY5Y cells | Entry | Blocked virus internalization into host cells | [56] |
| 46 | Silymarin | 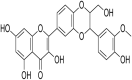 |
HCV | In-vivo | 5–20 mg/kg infusions | Not reported | Replication | By inhibition of HCV RNA replicon | [30] |
| Influenza-A,B | In-vitro | At 100 μg/ml concentration 98% inhibition was noted | No cytotoxicity at 100 μg/ml to MDCK cells | Replication | By targeting the Viral RNA synthesis process | [59] | |||
| 47 | Sorbifolin | 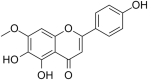 |
HCV | In-vitro | 45.0% efficient at inhibiting viral entry | No cytotoxicity towards the tested cells. | Entry | Virucidal effect | [34] |
| 48 | Pedalitin | 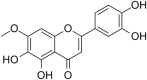 |
HCV | In-vitro | 78.7% efficient at inhibiting viral entry | No cytotoxicity towards the tested cells | Entry | inhibit virus entry by interfering with virus and host cell. | [34] |
(IC50) The half-maximal inhibitory concentration is a measure of a substance's ability to inhibit biological activity.
(EC50) Half-maximal effective concentration is the concentration required to obtain a 50% effect.
(CC50) is the compound's concentration required for reducing cell viability by 50%.
26.8. Naringin and other flavonoids
Jain et al. [87] performed in silico docking studies of different flavonoids to see which one bind best to the S-protein of SARS-CoV-2. The naringin & s-protein complex showed the least binding energy of −9.8 kcal/mol, and additional molecular simulations were performed to observe the stability of the naringin-spike protein complex. In contrast to the RMSD value of apo-protein, the RMSD value of the naringin-spike protein complex tended to be more stable relative to that of the protein in the 0.2–0.4 nm range [87]. These results serve as proof of the potential of using flavonoid against SARS-CoV-2. Data about the flavonoids, which are active against SARS-CoV-2, are given in Table 2.
Table 2.
Active Flavonoids against Coronavirus SARS-CoV-2.
| S.No | Virus | Flavonoid | Experiment model | IC50/Binding Energies | Inhibition target |
|---|---|---|---|---|---|
| 1 | Coronavirus. SARS-CoV-2 | Epigallocatechin Gallate (EGCG) | In-vitro | IC50 = 7.58 μg/ml | Main protease-MPro |
| 2 | Theaflavin | In-vitro | IC50 = 8.44 μg/ml | Main protease-MPro | |
| 3 | Myricitrin | In-silico | Binding energy = −8.9 kcal/mol | Main protease-MPro | |
| 4 | Taiwanhomoflavone A | In-silico | Binding energy = −9.6 kcal/mol | RNA-dependent RNA-Polymerase (RdRp) and Human Angiotensin Converting Enzyme-2 (hACE-2) | |
| 5 | Rutin | In-silico | Binding energy = −8.2072 kcal/mol | Main protease-MPro | |
| 6 | Fisetin | In-silico | binding affinity score= −8.5 kcal/mol | spike protein | |
| 7 | Biochanin A | In-silico | Docking energy score= −78.41 | Protease domain-ACE-2 & RBD-Spike protein | |
| 8 | Silymarin | In-silico | Docking energy score= −121.28 kcal/mol | Protease domain-ACE-2 & RBD-Spike protein | |
| 9 | Quercetin | In-silico | binding affinity score= −8.5 kcal/mol | spike protein | |
| binding affinity score= −9.2 kcal/mol | Main protease-MPro | ||||
| 10 | Narcissin | In-silico | binding affinity score= −8.2530 and | Main protease-MPro | |
| 11 | kaempferol-3-O-rutinoside | In-silico | binding affinity score= −8.1203 kcal/mol | Main protease-MPro | |
| 12 | Naringin | In-silico | binding energy = −9.8 kcal/mol | Spike protein |
(IC50) The half-maximal inhibitory concentration is a measure of a substance's ability to inhibit biological activity. Binding affinity, energy scores are the energy values when a ligand bind to a protein in Molecular Docking studies, Or The binding free energy is defined as the sum of the ligand's inter-molecular interactions with the protein and the ligand's internal steric energy.
27. Structure activity relationship of flavonoids against different viruses
Flavonoids phytochemicals has different classes and they are divided into these classes based on the position of ring-B (Benzene) and the attached Hydroxyl (OH) groups of the Flavonoid nucleus. Flavonoids has two fused rings, ring-A and ring-C. A B-ring is attaced to ring C ( Fig. 1 below), and by changing the position of ring-B around the ring-C, different classes such as iso-flavonoids and neoflavonoids are formed [60]. Groups of Hydroxyl (OH) at different flavonoid positions Rings-A, C and B play a major role in these flavonoids' antiviral activity against viruses. The molecular docking studies of the gossepetin flavonoid were performed with envelope protein of EBOV. The gossepetin flavonoid, for example, showed that hydroxyl (OH) groups at position 5, 7 and 8 on ring-A, hydroxyl (OH) on position 3 at ring-C, and hydroxyl (OH) groups at position 3′ and 4′ on ring-B showed strong binding interaction with different amino acid of the viral envelop protein of the Ebola Virus [70].
Fig. 1.
General structure of a flavonoid.
It is also worth noting that prenylation of flavonoids at position 8 of ring-A showed strong antiviral action. Leachianone-G prenylated flavonoid, for example, inhibited HSV-1 at a very low concentration and resulted in a very low IC50 = 1.6 μg/ml of leachianone-G [64]. Substitution of a glycoside on Ring-C at position of 3–OH resulted in high solubility in water, and potent antiviral activity For instance, kaempferol had a lower antiviral activity as compared to its glycosylated derivative juglanin against SARS-CoV-1 [47]. Moreover, the addition of gallate moiety to catechin flavonoid Ring-C at position-3 had increased antiviral potency against the HSV virus in comparison to the catechins with no gallate moiety on HSV virus [66].
27.1. Synthesis of flavonoids
27.1.1. Microwave-assisted flavonoids synthesis
Ghani et al. [88] utilized the Baker–Venkataraman rearrangement synthesis process for the preparation of multiple synthetic flavonoids and their derivatives ( Fig. 2). Multiple synthetic flavones were synthesized by using 2-hydroxyacetophenones; a microwave-assisted condensation process was used to close the resulting diones. First, pyridine solutions of the properly substituted O-hydroxyacetophenones were prepared and then treated with acid chlorides of choice in slight excess and two equivalents of 1, 8-Diazabicyclo [5.4.0]undec-7-ene (DBU) were also added to this mixture; then this mixture was heated for 16 h at 80 °C. It resulted in the synthesis of 1,3-diketones, and then purified by the use of column chromatography. The crude 1,3-diketones reaction product was used for convenience. Then, it was followed by a cyclization reaction in the presence of ethyl alcohol and a small quantity of conc. H2SO4 (100:1 by volume); this process of cyclization was performed using microwave-assisted cyclization [88].
Fig. 2.
Synthesis of Flavonoids Using Baker–Venkataraman rearrangement.
27.1.2. Synthesis of fluorine substituted flavonoids
Conti et al. synthesized synthetic flavonoids substituted with fluorine, and they characterized their structures using multiple analytical techniques (i.e. IR and NMR) [90]. After the synthesis of these flavonoids, they were then tested for their antiviral activity on Human rhinoviruses (HRV). They utilized the method previously described by Chang et al. [89] where several halogeno-flavonoids were synthesized [89]; they used the same method for the preparation of Fluorine substituted 6-Fluoroflavone and 6-Fluoroflavanone synthetic flavonoids [90].
27.1.2.1. Synthesis of 6-Fluoroflavone and 6-Fluoroflavanone flavonoids
First, 1-(5-Fluoro-2-hydroxyphenyl)−3-phenylpropen-1-one) was prepared, which is the starting material for the synthesis of these flavonoids. In order to prepare 1-(5-Fluoro-2-hydroxyphenyl)−3-phenylpropen-1-one ( Fig. 3a), 10 mmol Barium hydroxide octahydrate was taken and was then added to benzaldehyde (10 mmol) and 5′-fluoro2′-hydroxyacetophenone (10 mmol). This mixture is finally added to a methanol solution of (200 ml). The mixture was agitated for 6 h at 50 °C. The solvent was evaporated under reduced pressure after cooling. After addition of water to the residue, dilute hydrochloric acid (6 N) was used to acidify the resulting suspension. A filtration process was used to gather the 1-(5-Fluoro-2-hydroxyphenyl)− 3-phenylpropen-1-one precipitate, which was then cleaned with water and crystalized. Its synthesis pathway diagram is given below.
Fig. 3.
(a) Synthesis of fluorinated ketone intermediates. (b) Synthesis of 6-fluoroflavone. (c) Synthesis of 6-Fluoroflavanone through acetic acid.
Then, into a solution of 10 mmol 1-(5-fluoro-2-hydroxyphenyl)3-phenylpropen-1-one in dimethyl sulphoxide, (70 ml), selenium (IV) oxide (17.5 mmol) was added. Then, this mixture was heated at 130 °C for 2.5 h with constant stirring. The mixture was then filtered after cooling, and the filtrate was added into ice-water. After that, filtration was used to extract the precipitate of 6-Fluoroflavone, which was then washed and crystallized from AcOEt (Fig. 3b). The yield of 6-Fluoroflavone was 81%, and its m.p. was 130–131 °C. 6-Fluoroflavone also acted on the tested HRV-14 virus with an IC50 = >12.50 µM. its synthesis pathway diagram is given in Fig. 3.
For the synthesis of 6-Fluoroflavanone, the same starting material of 1-(5-fluoro-2-hydroxyphenyl)3-phenylpropen-1-one was used, but in a different reaction medium. (10 mmol) of 1-(5-fluoro-2hydroxyphenyl)− 3-phenylpropen-1-one was added to a concentrated hydrochloric acid (15 ml) and concentrated acetic acid (130 ml) solution and was heated for 17 h at 120 °C with stirring. The mixture was cooled, then poured into ice water, where the precipitate of 6-Fluoroflavanone was filtered, washed with water, and crystallized with the help of petroleum ether. The yield of 6-Fluoroflavanone was 87%, and its m.p. noted was 73–76 °C (Fig. 3c). 6 -Fluoroflavanone showed anti-viral activity against the HRV-B1 virus and HRV-14 virus strain with an IC50 = 9.21 and an IC50 = 12.50 µM, respectively [90]. The synthesis pathway of 6-Fluoroflavanone is diagrammed in Fig. 3c.
27.2. Synthesis flavonoids via Heck & Friedel–Crafts reaction
Bianco et al. [91] utilized the Heck & Friedel–Crafts reaction for the synthesis of multiple synthetic flavonoids. First for the synthesis of the α, β-Unsaturated ketone called compound (1), they used a modified method as described by Stetter et al. (1973) [97], [98]. In the first Friedel–Crafts reaction, a compound known as resorcinol dimethyl ether was acylated by β -Bromopropionyl chloride with TiCl3 acting as a Lewis acid. In the second step, the resultant Compound (10) was then dehydro-halogenated into the required α, β-Unsaturated ketone compound (1); the yield of this reaction was 78%. The synthesis pathway of the reaction is given below in the diagram ( Fig. 4a).
Fig. 4.
(a) Initial Steps of synthesis of starting material for flavonoids. (b) Synthesis of chalcone for making flavonoids derivatives.
Afterwards, the α, β-Unsaturated ketone compound (1) was taken in equimolar amounts to compound 2. 0.34 mmol of α, β-Unsaturated ketone compound (1) (65 mg) & compound (2) aryl iodide (88 mg) are then dissolved in acetonitrile 3.5 ml and Et3N (1 ml). PPh3 (5.24 mg) & Pd(OAc)2 (0.75 mg) were also added to this mixture and refluxed at 85 °C under argon, and stirred. The reaction completed in four hours, and then quenched by adding ice, and then acidified with hydrochloric acid (1 N). The crude material was purified using silica-gel chromatography after extraction with diethyl ether and the standard procedure yielded about 120 mg crude-material. CHCl3 was used as eluent and this reaction resulted in the synthesis of compound (3) called chalcone. The chalcone compound (3) has a yield of 94% (103 mg, 0.32 mmol). The synthesis pathway of the reaction is given below in in Fig. 4b.
The acetyl group in chalcone compound (3) was hydrolyzed, and then demethylated by sodium ethanethiolate, which resulted in a racemic mixture of multiple flavanones and chalcones ( Fig. 5) [91]. The synthesis pathway of the reaction is given below in Fig. 5.
Fig. 5.
Synthesis of flavanone and flavonoids.
27.3. Synthesis of myricetin flavonoid derivatives
Chen et al. [92] synthesized derivatives of myricetin that have 1,2,4-triazole Schiff bases with high potency against the tobacco mosaic virus. The initial hydrazine hydrate was heated at 50 °C in water for thirty minutes, and then carbon disulfide was added and heated at 90 °C for an hour. The solution was cooled and crystalized to get the first intermediate product. This initial intermediate was refluxed in glacial acetic acid for four hours and then cooled. The unreacted acetic acid was removed and the precipitate of the second intermediate was washed with water. This intermediate was fully dissolved in ethanol and then it was refluxed for six hours with aldehyde to get the final third intermediate product.
In the second phase, myricetin was treated with methyl iodide for forty-eight hours at room temperature and highly concentrated hydrochloric acid deglucoside was added to the flask to get a first intermediate of myricetin. In the next step, this intermediate was stirred for an hour at room temperature and then dibromoalkane was added to it, and the reaction proceed for twelve hours to get the next intermediate of myricetin. The third intermediate of hydrazine reaction was taken in an amount of 1.2 mmol and to it 3.0 mmol of potassium carbonate and 20 ml of dimethyl formamide were added taken in a 50 ml 3-neck round bottom flask equipped with a magnetic stirrer. These reactants were stirred at 24 °C for half an hour and then, 1.0 mmol of the myricetin intermediate was added, stirred at 100 °C for six hours, and continuously checked through thin layer chromatography. The product was cooled and 100 ml of deionized water was added to it. 30 ml dichloromethane were added to the product flask for extraction and the procedure was repeated thrice. The organic material was washed twice with 5% sodium hydroxide, H2O, and saturated aqueous sodium chloride. The solvent was dried through evaporation and the yield was in the range 40–75%. The products were characterized through different spectroscopic techniques. These derivatives were checked for their antiviral activity against the tobacco mosaic virus. The percent inhibition was more than 52%, and was better than the standard drug ribavirin (39%), while equal to ningnanmycin (52.7%) [92]. Fig. 6.
Fig. 6.
Synthesis of myricetin derivatives with antiviral activities against tobacco mosaic virus.
28. Comparison of anti-viral activity of flavonoids with standard anti-viral therapeutic drugs and other antiviral phytochemicals
In comparison of anti-viral activity of certain flavonoids discussed here in this review, some of the flavonoids showed increased anti-viral activity compared to the standard drugs. Ferenci et al. described the in vivo anti-viral activity of Silibinin, which is a flavo-Lignan complex. The results showed that chronic HCV Patients whose treatment was in progress did not responded to the therapy of standard antiviral drugs like Ribavirin & pegylated Interferon, but when doses of Silibinin were given to these Patients intravenously (IV), the HCV RNA decreased by almost 1.32 0.55 log. When Silibinin was given in combination with Ribavirin & pegylated Interferon in 7 patients the HCV RNA in these patients were undetectable. Choi et al. [19] described and compared the anti-viral activities of standard drug Ribavirin, Q7R flavonoid, and other phytochemicals. It was found that Q7R flavonoid has IC50 = 0.014 μg/ml compared to the standard drug Ribavirin’s IC50 which was 4.1 μg/ml against the PEDV Virus. Furthermore, it was also compared to the coumarin phytochemical whose IC50 was 9 μg/ml on the PEDV virus. It can be inferred from these results that flavonoid Q7R had more potent antiviral activity than the standard drug and other tested phytochemicals. In another study of antiviral activities, it was noted that quercetin had 0.2 μg/ml maximum inhibitory concentration compared to Acyclovir, which had maximum inhibitory concentration of 1.6 μg/ml against the Herpes Simplex Virus-1 [93]. Other than flavonoids, other phytochemicals like polysaccharides were also shown to be active against a multitude of viruses [94]. However, the major hurdle to develop phytochemical such as flavonoids, polysaccharides, and alkaloids as anti-viral agents are because of very few in vivo studies of these phytochemicals performed in live organisms, which if pursued could result in new and effective antivirals from these plant derived compounds [95]. A comparison of the anti-viral inhibitory concentrations of some flavonoids and standard anti-viral drugs are given below in the Table 3.
Table 3.
Comparison of the inhibitory concentration of flavonoids & standard antiviral compounds.
| S/N | Flavonoid | Inhibitory Concentration | Standard Drug | Inhibitory Concentration | Virus | Ref. |
|---|---|---|---|---|---|---|
| 1 | Silymarin | 10 mg/kg/day | Ribavarin+IFN | 10–20 mg/kg/day | HCV | [30] |
| 2 | Genistein | 0.4 μg/ml | Acyclovir | 1.6 μg/ml | PI-3 | [93] |
| 3 | Quercetin | 0.2 μg/ml | Acyclovir | 1.6 μg/ml | HSV-1 | [93] |
| 4 | 5, 7, 30, 40-tetra-O-methylquercetin | 0.36 μM | Oseltamivir | 0.32 μM | H1N1 | [16] |
| 5 | Apigenin | 1.6 μg/ml | Acyclovir | 1.6 μg/ml | HSV-1 | [93] |
| 6 | Fisetin | 8.444 µg/ml | Ribavirin | 11.07 µg/ml | CHIKV | [27] |
| 7 | Silymarin | 100 μg/ml 98% Inhibition | Oseltamivir | 100 μg/ml 52%- inhibition | H1N1 | [59] |
| 8 | Leachianone-G | 1.6 μg/ml | Acyclovir | 1.6 μg/ml | HSV-1 | [64], [93] |
| 9 | Galangin | 0.64 μg/ml | Acyclovir | 1.6 μg/ml | HSV-1 | [65], [93] |
| 10 | Baicalein | 1.891 µg/ml | Ribavirin | 11.07 µg/ml | CHIKV | [27] |
| 11 | Q7R | 0.014 μg/ml | Ribavirin | 4.1 μg/ml | PEDV | [77] |
| 12 | Silibinin | 1.6 μg/ml | Oseltamivir | 1.6 μg/ml | PI-3 | [93] |
| 13 | Narinen | 0.2 μg/ml | Oseltamivir | 1.6 μg/ml | PI-3 | [93] |
| 14 | Quercetin | 1.69 μg/ml | Acyclovir | 1.6 μg/ml | HSV-1 | [65], [93] |
| 15 | Genistein | 1.35 μg/ml | Acyclovir | 1.6 μg/ml | HSV-1 | [65], [93] |
| 16 | DHQ | 75–150 mg/kg/day | Ribavirin | 75–150 mg/kg/day | CV-B4 | [73] |
29. Conclusions
The flavonoids, we reviewed here showed strong antiviral activity against a multitude of viruses. Several of these viruses we discussed still have no viable treatment available, and the available drugs which are in use for the prevention of viral diseases also have negative side effects on patients. The tested flavonoids showed more than 90% strong antiviral activities without affecting the cell viability at higher concentrations. Based on their CC50 values and other tests, it was observed that these flavonoids are safe and non-toxic. The in vivo (mice-model) studies also confirmed that flavonoids can protect mice from lethal doses of viruses and can even provide them with prophylactic protection. Some of the flavonoids reviewed here showed potent antiviral activities similar to those of commercially available drugs. Further in vivo studies on flavonoids can provide more insights into the effectiveness of these phytochemicals against viruses. Future studies may include clinical trials so that flavonoids and their conjugated compounds can be used safely. From these studies, it can be assumed that flavonoid phytochemicals can be produced as therapeutic candidates and as an antiviral agents with great potential.
Conflict of interest statement
All the authors declare that they have no conflict of interest.
References
- 1.Samanta A., Das G., Das S.K.J.C. Roles of flavonoids in plants. Int. J. Pharm. Sci.Technol. 2011;100(6):12–35. [Google Scholar]
- 2.Feng W., Hao Z., Li MJF, from biosynthesis to human health/Ed. by Justino GC Intech Open. Isolation and structure identification of flavonoids. 2017:17–43.
- 3.M. Burak Y.J.T.K.T.B.D. Imen Flavonoids Antioxid. Prop. 19 1999 296 304.
- 4.Dewick PJMNPABA, Second Edition, John Wiley, Sons L, Chichester, UK. Front Matter and Index. 2001.
- 5.Castañeda-Ovando A., de Lourdes Pacheco-Hernández M., Páez-Hernández M.E., Rodríguez J.A., CAJFc Galán-Vidal. Chemical studies of anthocyanins: a review. Food Chem. 2009;113(4):859–871. [Google Scholar]
- 6.Das S., J.P.N. Rosazza. Microbial and enzymatic transformations of flavonoids. J. Nat. Prod. 2006;69(3):499–508. doi: 10.1021/np0504659. [DOI] [PubMed] [Google Scholar]
- 7.Peluso M.R.J.E.B. Flavonoids attenuate cardiovascular disease, inhibit phosphodiesterase, and modulate lipid homeostasis in adipose tissue and liver. Medicine. 2006;231(8):1287–1299. doi: 10.1177/153537020623100802. [DOI] [PubMed] [Google Scholar]
- 8.Cushnie T.T., AJJIjoaa Lamb. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26(5):343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panthong A., Kanjanapothi D., Tuntiwachwuttikul P., Pancharoen O., Reutrakul V.J.P. Antiinflammatory activity of flavonoids. Phytomedicine. 1994;1(2):141–144. doi: 10.1016/S0944-7113(11)80032-2. [DOI] [PubMed] [Google Scholar]
- 10.Friedman MJMn, research f. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. 2007;51(1):116–134. [DOI] [PMC free article] [PubMed]
- 11.Nardini M., Natella F., Scaccini C.J.P. Role of dietary polyphenols in platelet aggregation. Rev. Suppl. Stud. 2007;18(3):224–243. doi: 10.1080/09537100601078083. [DOI] [PubMed] [Google Scholar]
- 12.Lalani S., Poh C.L.J.V. Flavonoids as antiviral agents for Enterovirus A71 (EV-A71) Viruses. 2020;12(2):184. doi: 10.3390/v12020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin K.W., EJAt Ernst. Antiviral agents from plants and herbs: a systematic review. Antivir. Ther. 2003;8(2):77. [PubMed] [Google Scholar]
- 14.Helenius A.J.Jomb. Virus entry: looking back and moving forward. J. Mol. Biol. 2018;430(13):1853–1862. doi: 10.1016/j.jmb.2018.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad A., Kaleem M., Ahmed Z., Shafiq H.J.F.R.I. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—a review. Food Res. Int. 2015;77:221–235. [Google Scholar]
- 16.Roschek B., Jr., Fink R.C., McMichael M.D., Li D., Alberte R.S.J.P. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry. 2009;70(10):1255–1261. doi: 10.1016/j.phytochem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 17.A. Bahuguna, I. Khan, V.K. Bajpai, Kang SCJBJoP . MTT assay to evaluate the cytotoxic potential of a drug. 2017;12(2):Online: Apr 8–2017.
- 18.Imanishi N., Tuji Y., Katada Y., Maruhashi M., Konosu S., Mantani N., Terasawa K., Ochiai H.J.M. Additional inhibitory effect of tea extract on the growth of influenza A and B viruses in MDCK cells. Microbiol. Immunol. 2002;46(7):491–494. doi: 10.1111/j.1348-0421.2002.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 19.Choi H.J., Song J.H., Park K.S., Kwon D.H.J.E.Jo.P.S. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur. J. Pharm. Sci . 2009;37(3–4):329–333. doi: 10.1016/j.ejps.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Wu W., Li R., Li X., He J., Jiang S., Liu S., Yang J.J.V. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses. 2016;8(1):6. doi: 10.3390/v8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumiyoshi H., Mori C., Fuke I., Morita K., Kuhara S., Kondou J., Kikuchi Y., Nagamatu H., Igarashi A.J.V. Complete nucleotide sequence of the Japanese encephalitis virus genome RNA. Virology. 1987;161(2):497–510. doi: 10.1016/0042-6822(87)90144-9. [DOI] [PubMed] [Google Scholar]
- 22.Johari J., Kianmehr A., Mustafa M.R., Abubakar S., Zandi K.J.Ijoms. Antiviral activity of baicalein and quercetin against the Japanese encephalitis virus. Int. J. Mol. Sci. 2012;13(12):16785–16795. doi: 10.3390/ijms131216785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phadungsombat J., Lin M.Y.-C., Srimark N., Yamanaka A., Nakayama E.E., Moolasart V., Suttha P., Shioda T., Uttayamakul S.J.P.O. Emergence of genotype Cosmopolitan of dengue virus type 2 and genotype III of dengue virus type 3 in Thailand. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0207220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zandi K., Teoh B.-T., Sam S.-S., Wong P.-F., Mustafa M.R., AbuBakar S.J.Vj. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011;8(1):1–11. doi: 10.1186/1743-422X-8-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moghaddam E., Teoh B.-T., Sam S.-S., Lani R., Hassandarvish P., Chik Z., Yueh A., Abubakar S., Zandi K.J.Sr. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014;4:5452. doi: 10.1038/srep05452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsetsarkin K.A., Chen R., Sherman M.B., Weaver S.C.J.Coiv. Chikungunya virus: evolution and genetic determinants of emergence. Curr. Opin. Virol. 2011;1(4):310–317. doi: 10.1016/j.coviro.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lani R., Hassandarvish P., Shu M.-H., Phoon W.H., Chu J.J.H., Higgs S., Vanlandingham D., Bakar S.A., Zandi K.J.Ar. Antiviral activity of selected flavonoids against Chikungunya virus. Antiviral Res. 2016;133:50–61. doi: 10.1016/j.antiviral.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Chen S.L., Morgan T.R.J.Ijoms. The natural history of hepatitis C virus (HCV) infection. Int. J. Med. Sci. 2006;3(2):47. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nahmias Y., Goldwasser J., Casali M., Van Poll D., Wakita T., Chung R.T., Yarmush M.L.J.H. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology. 2008;47(5):1437–1445. doi: 10.1002/hep.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferenci P., Scherzer T.M., Kerschner H., Rutter K., Beinhardt S., Hofer H., Schöniger–Hekele M., Holzmann H., Steindl–Munda P.J.G. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008;135(5):1561–1567. doi: 10.1053/j.gastro.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 31.Shibata C., Ohno M., Otsuka M., Kishikawa T., Goto K., Muroyama R., Kato N., Yoshikawa T., Takata A., Koike K.J.V. The flavonoid apigenin inhibits hepatitis C virus replication by decreasing mature microRNA122 levels. Virology. 2014;462:42–48. doi: 10.1016/j.virol.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Bachmetov L., Gal‐Tanamy M., Shapira A., Vorobeychik M., Giterman‐Galam T., Sathiyamoorthy P., Golan‐Goldhirsh A., Benhar I., Tur‐Kaspa R., Zemel R.J.Jovh. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral Hepat. 2012;19(2):e81–e88. doi: 10.1111/j.1365-2893.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- 33.Haid S., Novodomská A., Gentzsch J., Grethe C., Geuenich S., Bankwitz D., Chhatwal P., Jannack B., Hennebelle T., Bailleul F.J.G. A plant-derived flavonoid inhibits entry of all HCV genotypes into human hepatocytes. Gastroenterology. 2012;143(1):213–222. e215. doi: 10.1053/j.gastro.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu J.F., Lima C.S., Pereira C.M., Bittar C., Batista M.N., Nazaré A.C., Polaquini C.R., Zothner C., Harris M., Rahal P.J.Sr. Flavonoids from Pterogyne nitens Inhibit Hepatitis C Virus Entry. Sci. Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-16336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He W., Li L.-X., Liao Q.-J., Liu C.-L., Chen X.-L.J.WjogW. Epigallocatechin gallate inhibits HBV DNA synthesis in a viral replication - inducible cell line. World J. Gastroenterol. 2011;17(11):1507. doi: 10.3748/wjg.v17.i11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson M.J.Nm. HIV-1 pathogenesis. Nat. Med. 2003;9(7):853–860. doi: 10.1038/nm0703-853. [DOI] [PubMed] [Google Scholar]
- 37.Li B.Q., Fu T., Dongyan Y., Mikovits J.A., Ruscetti F.W., Wang J.M.J.B. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem. Biophys. Res. Commun. 2000;276(2):534–538. doi: 10.1006/bbrc.2000.3485. [DOI] [PubMed] [Google Scholar]
- 38.Solomon T., Lewthwaite P., Perera D., Cardosa M.J., McMinn P., Ooi M.H.J.T.Lid. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010;10(11):778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W., Qiao H., Lv Y., Wang J., Chen X., Hou Y., Tan R., Li E.J.Po. Apigenin inhibits enterovirus-71 Infection by disrupting viral RNA association with trans-acting factors. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0110429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai W., Bi J., Li F., Wang S., Huang X., Meng X., Sun B., Wang D., Kong W., Jiang C.J.V. Antiviral efficacy of flavonoids against enterovirus 71 infection in vitro and in newborn mice. Viruses. 2019;11(7):625. doi: 10.3390/v11070625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X., Liu Y., Wu T., Jin Y., Cheng J., Wan C., Qian W., Xing F., Shi W.J.V. The antiviral effect of baicalin on enterovirus 71 in vitro. Viruses. 2015;7(8):4756–4771. doi: 10.3390/v7082841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai F.-J., Lin C.-W., Lai C.-C., Lan Y.-C., Lai C.-H., Hung C.-H., Hsueh K.-C., Lin T.-H., Chang H.C., Wan L.J.Fc. Kaempferol inhibits enterovirus 71 replication and internal ribosome entry site (IRES) activity through FUBP and HNRP proteins. Food Chem. 2011;128(2):312–322. doi: 10.1016/j.foodchem.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Racaniello V.R.J.V. One hundred years of poliovirus pathogenesis. Virology. 2006;344(1):9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez M., Martínez-Abarca F., Carrasco L.J.A.C. Flavonoids: potent inhibitors of poliovirus RNA synthesis. Chemotherapy. 1990;1(3):203–209. [Google Scholar]
- 45.Desideri N., Conti C., Sestili I., Tomao P., Stein M., Orsi N.J.A.C. In vitro evaluation of the anti-picornavirus activities of new synthetic flavonoids. Chemotherapy. 1995;6(5):298–306. [Google Scholar]
- 46.De Wit E., Van Doremalen N., Falzarano D., Munster V.J.J.N.R.M. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(8):523. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarz S., Sauter D., Wang K., Zhang R., Sun B., Karioti A., Bilia A.R., Efferth T., Schwarz W.J.Pm. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014;80(02–03):177. doi: 10.1055/s-0033-1360277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauter D., Schwarz S., Wang K., Zhang R., Sun B., Schwarz W.J.Pm. Genistein as antiviral drug against HIV ion channel. Planta Med. 2014;80(08/09):682–687. doi: 10.1055/s-0034-1368583. [DOI] [PubMed] [Google Scholar]
- 49.Tao J., Hu Q., Yang J., Li R., Li X., Lu C., Chen C., Wang L., Shattock R., Ben K.J.Ar. In vitro anti-HIV and -HSV activity and safety of sodium rutin sulfate as a microbicide candidate. Antivir. Res. 2007;75(3):227–233. doi: 10.1016/j.antiviral.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y., Zhao J., Zheng M., Liu Z., Li W., Fu X., Lin Y., Yuan J., Zhao J., Shen Q.J.Vj. A novel cardiovirus in wild rats. Virol. J. 2018;15(1):58. doi: 10.1186/s12985-018-0968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veckenstedt A., RJAr Pusztai. Mechanism of antiviral action of quercetin against cardiovirus infection in mice. Antivir. Res. 1981;1(4):249–261. doi: 10.1016/0166-3542(81)90015-2. [DOI] [PubMed] [Google Scholar]
- 52.Vandini S., Biagi C., Fischer M., Lanari M.J.V. Impact of rhinovirus infections in children. Viruses. 2019;11(6):521. doi: 10.3390/v11060521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ganesan S., Faris A.N., Comstock A.T., Wang Q., Nanua S., Hershenson M.B., Sajjan U.S.J.Ar. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012;94(3):258–271. doi: 10.1016/j.antiviral.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manet C., Simon-Lorière E., Jouvion G., Hardy D., Prot M., Conquet L., Flamand M., Panthier J.-J., Sakuntabhai A., Montagutelli X.J.Jo.V. Genetic diversity of collaborative cross mice controls viral replication, clinical severity, and brain pathology induced by Zika virus infection, Independently of Oas1b. J. Virol. 2020;94(3) doi: 10.1128/JVI.01034-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carneiro B.M., Batista M.N., Braga A.C.S., Nogueira M.L., Rahal P.J.V. The green tea molecule EGCG inhibits Zika virus entry. Virology. 2016;496:215–218. doi: 10.1016/j.virol.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 56.Gaudry A., Bos S., Viranaicken W., Roche M., Krejbich-Trotot P., Gadea G., Desprès P., El-Kalamouni C.J.Ijoms. The flavonoid isoquercitrin precludes initiation of zika virus infection in human cells. Int. J. Mol. Sci. 2018;19(4):1093. doi: 10.3390/ijms19041093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou M., Liu H., Li J., Yao X., Chen Y., Ke C., Liu S.J.B.P. Structure-activity relationship of flavonoid bifunctional inhibitors against Zika virus infection. Biochem. Pharmacol. 2020 doi: 10.1016/j.bcp.2020.113962. [DOI] [PubMed] [Google Scholar]
- 58.Cataneo A.H.D., Kuczera D., Koishi A.C., Zanluca C., Silveira G.F., de Arruda T.B., Suzukawa A.A., Bortot L.O., Dias-Baruffi M., Verri W.A.J.Sr. The citrus flavonoid naringenin impairs the in vitro infection of human cells by Zika virus. Sci. Rep. 2019;9(1):1–15. doi: 10.1038/s41598-019-52626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song J., Choi H.J.P. Silymarin efficacy against influenza A virus replication. Phytomedicine. 2011;18(10):832–835. doi: 10.1016/j.phymed.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 60.Jean Beltran P.M., Cristea I.M.J.Erop. The life cycle and pathogenesis of human cytomegalovirus infection: lessons from proteomics. Expert Rev. Proteom. 2014;11(6):697–711. doi: 10.1586/14789450.2014.971116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evers D.L., Chao C.-F., Wang X., Zhang Z., Huong S.-M., Huang E.-S.J.A.R. Human cytomegalovirus-inhibitory flavonoids: studies on antiviral activity and mechanism of action. Antiviral Res. 2005;68(3):124–134. doi: 10.1016/j.antiviral.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jo S., Kim S., Shin D.H., Kim M.-S.J.Joei. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020;35(1):145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryu Y.B., Jeong H.J., Kim J.H., Kim Y.M., Park J.-Y., Kim D., Naguyen T.T.H., Park S.-J., Chang J.S., Park K.H.J.B. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CL(pro) inhibition. Bioorg. Med. Chem. 2010;18(22):7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du J., He Z.-D., Jiang R.-W., Ye W.-C., Xu H.-X., But P.P.-H.J.P. Antiviral flavonoids from the root bark of Morus alba L. Phytochemistry. 2003;62(8):1235–1238. doi: 10.1016/s0031-9422(02)00753-7. [DOI] [PubMed] [Google Scholar]
- 65.Lyu S.-Y., Rhim J.-Y., Park W.-B.J.Aopr. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch. Pharm. Res. 2005;28(11):1293–1301. doi: 10.1007/BF02978215. [DOI] [PubMed] [Google Scholar]
- 66.Isaacs C.E., Wen G.Y., Xu W., Jia J.H., Rohan L., Corbo C., Di Maggio V., Jenkins E.C., Hillier S.J.Aa. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. chemotherapy. 2008;52(3):962–970. doi: 10.1128/AAC.00825-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Lee J., Loe M.W.C., Lee R.C.H., Chu J.J.H.J.A.R. Antiviral activity of pinocembrin against Zika virus replication. Antivir. Res. 2019;167:13–24. doi: 10.1016/j.antiviral.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Qiu X., Kroeker A., He S., Kozak R., Audet J., Mbikay M., Chrétien M.J.Aa. Prophylactic efficacy of quercetin 3-β-Od-glucoside against Ebola virus infection. Chemotherapy. 2016;60(9):5182–5188. doi: 10.1128/AAC.00307-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Putra R.P., Alkaff A.H., Nasution M.A.F., Corona A., Kantale B., Tambunan U.S.F.J.I.J. Searching of flavonoid compounds as a new antiviral for Sudan Ebolavirus glycoprotein using in silico methods. Int. J. GEOMATE. 2018;15(49):78–84. [Google Scholar]
- 70.Raj U., Varadwaj P.K.J.I.S.C.L.S. Identification of inhibitors against metastasis protein “ survivin:” in silico discovery using virtual screening and molecular docking studies. Pharmacogn. Mag. 2016;8(2):132–141. doi: 10.4103/pm.pm_178_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fanunza E., Iampietro M., Distinto S., Corona A., Quartu M., Maccioni E., Horvat B., Tramontano E.J.A.A. Quercetin blocks Ebola Virus infection by counteracting the VP24 Interferon inhibitory function. Chemotherapy. 2020 doi: 10.1128/AAC.00530-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sin J., Mangale V., Thienphrapa W., Gottlieb R.A., Feuer R.J.V. Recent progress in understanding coxsackievirus replication, dissemination, and pathogenesis. Virology. 2015;484:288–304. doi: 10.1016/j.virol.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Galochkina A.V., Anikin V.B., Babkin V.A., Ostrouhova L.A., Zarubaev V.V.J.Aov. Virus-inhibiting activity of dihydroquercetin, a flavonoid from Larix sibirica, against coxsackievirus B4 in a model of viral pancreatitis. Arch. Virol. 2016;161(4):929–938. doi: 10.1007/s00705-016-2749-3. [DOI] [PubMed] [Google Scholar]
- 74.Xu L., Su W., Jin J., Chen J., Li X., Zhang X., Sun M., Sun S., Fan P., An D.J.V. Identification of luteolin as enterovirus 71 and coxsackievirus A16 inhibitors through reporter viruses and cell viability-based screening. Viruses. 2014;6(7):2778–2795. doi: 10.3390/v6072778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yin D., Li J., Lei X., Liu Y., Yang Z., Chen K.J.E.-B.C. Antiviral activity of total flavonoid extracts from Selaginella moellendorffii Hieron against coxsackie virus B3 in vitro and in vivo. Med. A. 2014:2014. doi: 10.1155/2014/950817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jung K., Saif L.J.J.T.V.J. Porcine epidemic diarrhea virus infection: etiology. Epidemiol. Pathog. Immunoprophyl. 2015;204(2):134–143. doi: 10.1016/j.tvjl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi H.-J., Kim J.-H., Lee C.-H., Ahn Y.-J., Song J.-H., Baek S.-H., Kwon D.-H.J.A.R. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antivir. Res. 2009;81(1):77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R.J.N.E.Jo.M. A Novel Coronavirus from Patients with Pneumonia in China, 2019. New Engl. J. Med. 2019:2020. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X.J.Tl. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jang M., Park Y.-I., Cha Y.-E., Park R., Namkoong S., Lee J.I., Park J.J.E.-B.C. Tea polyphenols EGCG and theaflavin inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Evid. Based Complement. Altern. Med. 2020;2020:1–7. doi: 10.1155/2020/5630838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Joshi R.S., Jagdale S.S., Bansode S.B., Shankar S.S., Tellis M.B., Pandya V.K., Chugh A., Giri A.P., Kulkarni M.J.J.Jo.B.S. Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main protease. Dynamics. 2020:1–16. doi: 10.1080/07391102.2020.1760137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abd El-Mordy F.M., El-Hamouly M.M., Ibrahim M.T., Abd El-Rheem G., Aly O.M., Abd El-kader A.M., Youssif K.A., Abdelmohsen U.R.J.R.A. Inhibition of SARS-CoV-2 main protease by phenolic compounds from Manilkara hexandra (Roxb.) Dubard assisted by metabolite profiling and in silico virtual screening†. RSC Adv. 2020;10(53):32148–32155. doi: 10.1039/d0ra05679k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pandey P., Rane J.S., Chatterjee A., Kumar A., Khan R., Prakash A., Ray S.J.Jo.B.S. Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurringphytochemicals: an in silico study for drugdevelopment. J. Biomol. Struct. Dyn. 2020:1–11. doi: 10.1080/07391102.2020.1796811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gorla U.S., Rao G.K., Kulandaivelu U.S., Alavala R.R., Panda S.P.J.Cc. Lead finding from selected flavonoids with antiviral (SARS-CoV-2) potentials against COVID-19: an in-silico evaluation. Comb. Chem. High Throughput Screen. 2020 doi: 10.2174/1386207323999200818162706. [DOI] [PubMed] [Google Scholar]
- 85.Vijayakumar B.G., Ramesh D., Joji A., Kannan T.J.Ejop. In silico pharmacokinetic and molecular docking studies of natural flavonoids and synthetic indole chalcones against essential proteins of SARS-CoV-2. Eur. J. Pharmacol. 2020;886 doi: 10.1016/j.ejphar.2020.173448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Owis A.I., El-Hawary M.S., El Amir D., Aly O.M., Abdelmohsen U.R., Kamel M.S.J.R.A. Molecular docking reveals the potential of Salvadora persica flavonoids to inhibit COVID-19 virus main protease. RSC Adv. 2020;10(33):19570–19575. doi: 10.1039/d0ra03582c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jain A.S., Sushma P., Dharmashekar C., Beelagi M.S., Prasad S.K., Shivamallu C., Prasad A., Syed A., Marraiki N., Prasad K.S.J.Sjobs. In silico evaluation of flavonoids as effective antiviral agents on the spike glycoprotein of SARS-CoV-2. Saudi J. Biol. Sci. 2021;28(1):1040–1051. doi: 10.1016/j.sjbs.2020.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ghani S.B.A., Weaver L., Zidan Z.H., Ali H.M., Keevil C.W., Brown R.C. Microwave-assisted synthesis and antimicrobial activities of flavonoid derivatives. Bioorg. Med. Chem. Lett. 2008;18(2):518–522. doi: 10.1016/j.bmcl.2007.11.081. [DOI] [PubMed] [Google Scholar]
- 89.Chang C., Chen F., Chen T., Hsu K., Ueng T., Hung M. 664. Synthesis of 6-halogenoflavones and related compounds. J. Chem. Soc. Resumed. 1961:3414–3417. [Google Scholar]
- 90.Conti C., Mastromarino P., Goldoni P., Portalone G., Desideri N. Synthesis and anti-rhinovirus properties of fluoro-substituted flavonoids. Antivir. Chem. Chemother. 2005;16(4):267–276. doi: 10.1177/095632020501600406. [DOI] [PubMed] [Google Scholar]
- 91.Bianco A., Cavarischia C., Farina A., Guiso M., Marra C. A new synthesis of flavonoids via Heck reaction. Tetrahedron Lett. 2003;44(51):9107–9109. [Google Scholar]
- 92.Chen Y., Li P., Su S., Chen M., He J., Liu L., He M., Wang H., Xue W. Synthesis and antibacterial and antiviral activities of myricetin derivatives containing a 1, 2, 4-triazole Schiff base. RSC Adv. 2019;9(40):23045–23052. doi: 10.1039/c9ra05139b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Özçelik B., Kartal M., Orhan I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011;49(4):396–402. doi: 10.3109/13880209.2010.519390. [DOI] [PubMed] [Google Scholar]
- 94.Chen L., Huang G. The antiviral activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018;115:77–82. doi: 10.1016/j.ijbiomac.2018.04.056. [DOI] [PubMed] [Google Scholar]
- 95.Asad U., Sidra M., Syed L.B., Noreen K., Lubna G., Benjamin G.P., Abdul-Hamid E., Mariusz J. Important flavonoids and their role as a therapeutic agent. Molecules. 2021;25(22):5243. doi: 10.3390/molecules25225243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Noreen, Ali R., Badshah S.L., Faheem M., Abbasi S.W., Ullah R., Bari A., Jamal S.B., Mahmood H.M., Haider A., Haider S. Identification of potential inhibitors of Zika virus NS5 RNA-dependent RNA polymerase through virtual screening and molecular dynamic simulations. Saudi Pharm. J. 2021;28(12):1580–1591. doi: 10.1016/j.jsps.2020.10.005. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nasir A., Farman A., Badshah S.L., Rahman A.U., Rashid H.U., Khan K. Molecular modeling, simulation and docking study of ebola virus glycoprotein. J. Mol. Graph. Model. 2017;72:266–271. doi: 10.1016/j.jmgm.2016.12.010. In this issue. [DOI] [PubMed] [Google Scholar]
- 98.Stetter H., Manfred S. A new method for addition of aldehydes to activated double bonds. Angew. Chem. Int. Ed. Engl. 1973;12(1):81. doi: 10.1002/anie.197300811. [DOI] [Google Scholar]
- 99.Fareena S., Noreen, Ali R., Badshah S.L., Jamal S.B., Ullah R., Bari A., Mahmood H.M., Sohaib M., Ansari S.A. Identification of Potential HCV Inhibitors Based on the interaction of epigallocatechin-3-gallate with viral envelope proteins. Molecules. 2021;26(5) doi: 10.3390/molecules26051257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lal B.S., Naeem A., Mabkhot Y. The new high resolution crystal structure of NS2B-NS3 protease of Zika virus. Viruses. 2017;9(1) doi: 10.3390/v9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nasir A., Rehman A.U., Badshah S.L., Ullah A., Mohammad A., Khan K. Molecular dynamics simulation of zika virus NS5 RNA dependent RNA polymerase with selected novel non-nucleoside inhibitors. J. Mol. Struct. 2020;1203 doi: 10.1016/j.molstruc.2019.127428. [DOI] [Google Scholar]
- 102.Lal B.S., Ahmad N., Rehman A.U., Khan K., Ullah A., Alsayari A., Muhsinah A., Mabkhot Y. Molecular docking and simulation of Zika virus NS3 helicase. BMC Chemistry. 2019;13 doi: 10.1186/s13065-019-0582-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lal B.S., Ullah A., Badshah S.H., Ahmad I. Spread of Novel coronavirus by returning pilgrims from Iran to Pakistan. J. Travel Med. 2020;27(3) doi: 10.1093/jtm/taaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lal B.S., Ullah A. Spread of coronavirus disease-19 among devotees during religious congregations. Ann. Thorac. Med. 2020;15(3):105–106. doi: 10.4103/atm.ATM_162_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nasir A., Badshah S.L., Junaid M., Rehman A.U., Muhammad A., Khan K. Structural insights into the Zika virus NS1 protein inhibition using a computational approach. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1759453. [DOI] [PubMed] [Google Scholar]