Abstract
Twin to twin transfusion syndrome (TTTS) is a common complication that typically presents in the second trimester of pregnancy in 10–15% of monochorionic twins due to net transfer of volume and hormonal substances from one twin to the other across vascular anastomoses on the placenta. Without recognition and treatment, TTTS is the greatest contributor to fetal loss prior to viability in 90–100% of advanced cases. Ultrasound diagnosis of monochorionicity is most reliable in the first trimester and sets the monitoring strategy for this type of twins. The diagnosis of TTTS is made by ultrasound with the findings of polyhydramnios due to volume overload and polyuria in one twin and oligohydramnios due to oliguria of the co-twin. Assessment of bladder filling as well as arterial and venous Doppler patterns are required for staging disease severity. Assessment of fetal cardiac function also provides additional insight into the fetal cardiovascular impacts of the disease as well as help identify fetuses that may require postnatal follow up. Fetoscopic laser ablation of the communicating vascular anastomoses between the twins is the standard treatment for TTTS. It aims to cure the condition by interrupting the link between their circulations and making them independent of one another. Contemporary outcome data after laser surgery suggests survival for both fetuses can be anticipated in up to 65% of cases and survival of a single fetus in up to 88% of cases. However, preterm birth remains a significant contributor to postnatal morbidity and mortality. Long term outcomes of TTTS survivors indicate that up to 11% of children may show signs of neurologic impairment. Strategies to minimize preterm birth after treatment and standardized reporting by laser centers are important considerations to improve overall outcomes and understand the long-term impacts of TTTS.
Keywords: Fetoscopy; placental diseases, pregnancy, twin; twin to twin transfusion syndrome (TTTS)
Introduction
Twin to twin transfusion syndrome (TTTS) is a disease that occurs in 10−15% of monochorionic twins as a result of volume imbalance across the vascular anastomoses between the twins and is the largest contributor to previable pregnancy loss for this type of twins. Diagnosis of monochorionicity in the first trimester and adherence to international guidelines for close surveillance of these pregnancies at least every 2 weeks after 16 weeks provides the best opportunity and early diagnosis and definitive treatment with fetoscopic laser surgery. The current technique allows >70% survival of at least one twin but preterm birth is a common consequence of the intervention. This unique features of the monochorionic placenta that contribute to the TTTS, as well as diagnosis, treatment and anticipated outcomes are reviewed.
Features of the monochorionic placenta
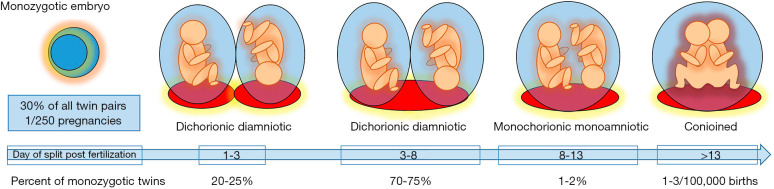
Monozygotic twins are classically considered the result of division of a single embryo and account for approximately 30% of all twin pairs worldwide (1,2). The timing of the split is related to the observed number of placentas and amniotic sacs, with earlier division leading to more complete separation (Figure 1) (1,3). Chorionicity refers to the number of placentas in the pregnancy. This can be determined in the first trimester by ultrasound identification of a single placental mass with a thin dividing membrane that inserts directly into the placental surface (T-sign) and absence of placental tissue extending in between the intertwin membrane (λ-sign) with a sensitivity and specificity of up to 98–100% (Figure 2) (4-7). Correct identification of monochorionicity is critical because it defines the risk profile and the range of complications that can occur. The mortality for monochorionic twins is twice that of dichorionic twins and four times that of singleton pregnancies with a highest rate of previable pregnancy loss prior to viability most commonly attributable to the unique features of the monochorionic placenta (8,9).
Figure 1.
Timeline for division of the monozygotic embryo and proportion of all monozygotic twin pairs. Earlier division of the monozygotic embryo results in more complete separation of the twin pair beginning from two separate placentas and amniotic sacs in dichorionic diamniotic twins when the division occurs in the first three days to conjoined twins when the division of the embryo occurs after 13 days.
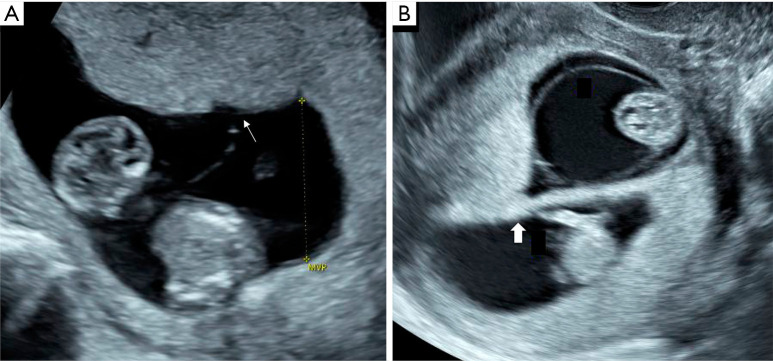
Figure 2.
First trimester ultrasound appearance of a monochorionic and dichorionic twin pregnancy. A monochorionic twin pregnancy is diagnosed when the membrane is thin and inserts directly on the placental surface (T-sign) as indicated by a thin arrow (A). A dichorionic twin pregnancy is diagnosed when the intertwin membrane is thick with intervening placental tissue at its base (λ-sign) as indicated by a thick arrow (B).
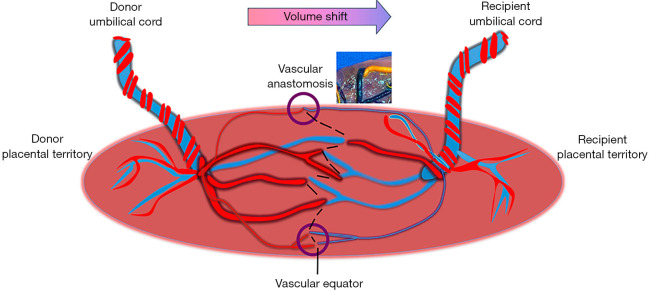
In a monochorionic twin placenta, the umbilical cord for each fetus can insert either centrally, at the placental edge (marginal) or into the membranes (velamentous). The fetal vessels originate from the base of the umbilical cord, branch and extend over the surface of the placenta essentially claiming their respective portion of the placenta that provides the predominant nutrient supply for fetal growth. Near universally, fetal vessels from each twin meet along the border between the placental territories for each twin (10). They may connect directly creating superficial vascular anastomoses or perfuse a shared placental cotyledon with deep anastomoses of arterioles and venules. The imaginary line along the surface of the placenta that connects the anastomosis is referred to as the vascular equator. This portion of the shared placenta may account for 5–10% of the shared vascular volume for each twin and is referred to as the third circulation (Figure 3) (11).
Figure 3.
Features of the monochorionic placenta include separate placental cord insertions with presence vascular anastomoses (circles and placental dye injection image) that link the fetal circulation. The natural line along the placenta where the vessels from each twin meet is the vascular equator (dotted line). Volume and substance shift from the donor to the recipient twin is responsible for development of twin to twin transfusion syndrome. Fetoscopic laser surgery is performed by coagulating the individual anastomoses and the intervening chorionic plate to dichorionize the placenta (dotted line).
Vascular anastomoses may be found in any number, size and arrangement between arteries and veins. Arteriovenous anastomoses occur when a shared placental cotyledon is perfused by the artery from one twin and drained by a vein from the co-twin (12). This results in a unidirectional transfer of volume, hemoglobin and substances from one fetus to the other. Artery-to-artery or vein-to-vein anastomoses directly connect to one another along the chorionic surface and allow bidirectional flow between the twins based on pressure gradients (10). In the majority of monochorionic twin pairs, the net exchange between the twins remains balanced in their shared circulation.
Pathophysiology of twin to twin transfusion syndrome
In 10–15% of monochorionic twins the balance becomes skewed due to volume shunting across arteriovenous vascular anastomoses resulting in TTTS (13). Placental observations in uncomplicated monochorionic twins compared to those with TTTS demonstrate that unbalanced arteriovenous anastomoses are the prerequisite to develop the condition (14,15). Surface artery to artery or vein to vein anastomoses are considered protective from TTTS by allowing redistribution of volume more efficiently across a range of vessel diameters compared to reciprocal artery to vein anastomoses (16).
This chronic net transfer of volume and vasoactive substances from one twin to the other leads to an abnormal intravascular volume status and compensatory response of both twins. Observable findings on ultrasound include discordance in amniotic fluid, bladder filling, and cardiovascular manifestations. The recipient twin experiences increased preload demonstrated by higher umbilical venous flow (17). Increased stretch on the cardiac chambers triggers release of atrial natriuretic peptide and brain natriuretic peptide, which stimulates diuresis leading to polyhydramnios (18,19). Additionally, the potent vasoconstrictor endothelin is increased contributing to recipient hypertension and consequently cardiac hypertrophy and valvular regurgitation (19,20). In contrast, the donor twin experiences hypovolemia and subsequently decreased urine production resulting in oligohydramnios and minimal or no visible bladder filling. In response there is upregulation of the renin-angiotensin system, which passes to the hypervolemic recipient via their shared circulation amplifying recipient hypertension and cardiomyopathy that cannot be explained solely by changes in volume status (21,22).
The hemodynamic impacts of TTTS can contribute to both functional and ultimately structural cardiac disease for each fetus. Changes in recipient cardiac function may be observed prior to development of overt TTTS and may include cardiac enlargement, biventricular hypertrophy, valvular regurgitation, impaired contractility (23,24). Right ventricular hypertrophy and hypertension coupled with tricuspid regurgitation may lead to decreased flow through the pulmonary valve and right outflow tract, essentially creating a functional subvalvular right ventricular outflow obstruction in up to 9% of recipients (25-27). These findings may resolve after treatment but persistent pulmonary stenosis or functional atresia may result and require postnatal treatment (28). Structural heart disease and cardiac dysfunction are less likely to be diagnosed in donor fetuses, however decreased flow through the aortic isthmus from hypovolemia, decreased venous return and higher placental resistance may evolve into coarctation (29,30).
Staging criteria
The initial diagnosis of TTTS is made by ultrasound identification of both polyhydramnios for the recipient twin by a maximum vertical pocket (MVP) of amniotic fluid of >8 cm and oligohydramnios for the donor twin with a MVP <2 cm, which is well above the 95th percentile and below the 5th percentile across gestational age (31). Measurement of the MVP should be performed with the patient in the dorsal supine position and in an area free of fetal parts or the umbilical cord in order to avoid underestimation of the amniotic fluid volume. Due to oligohydramnios, the donor twin may appear “stuck” to the placenta or uterine wall. On occasion the fetus may appear suspended from the uterine wall, termed the “chandelier sign” from being wrapped in the membrane and the folded membrane may appear thicker from being folded on itself. Use of the Quintero staging criteria is widely accepted as the standard to communicate the severity of disease (32). It includes assessment of bladder filling, Doppler assessment of the umbilical artery, ductus venosus and umbilical vein, presence of hydrops or fetal demise (Table 1). Importantly, each component of the classification system is a categorical assessment. For instance, to meet criteria for critically abnormal Dopplers absent or reversed end diastolic velocity in the umbilical artery or ductus venosus a-wave is required. Increased resistance or abnormal indices are not sufficient to satisfy criteria for TTTS Stage 3 or higher but may provide important insight into the overall clinical assessment.
Table 1. Staging criteria for twin to twin transfusion syndrome.
| Stage | Recipient | Donor |
|---|---|---|
| 1 | MVP >8 cm | MVP <2 cm |
| 2 | Visible bladder | No bladder filling |
| 3 | UA A/REDV, DV absent/reversed a-wave, UV pulsations in either twin | |
| 4 | Hydrops of either twin | |
| 5 | Single or double fetal demise | |
A/REDV, absent/reversed end diastolic velocity; DV, ductus venosus; MVP, maximum vertical pocket; UA, umbilical artery; UV, umbilical vein.
Cardiovascular manifestations of TTTS may be evident in early stage disease (33), so additional scoring systems that incorporate fetal cardiac function can be used to complement the assessment (34,35). Recipient twins are more likely to demonstrate signs of myocardial hypertrophy, impaired diastolic function, and valvular regurgitation that ultimately leads to abnormal venous Dopplers. Fetal hydrops resulting from fetal cardiac failure is a late manifestation of the disease. Donor twins are more likely to display signs of elevated placental resistance reflected in the umbilical artery Doppler waveform and much less likely to exhibit cardiac dysfunction (20). Since the Quintero staging criteria only accounts for late reflections of cardiovascular dysfunction, this explains the discrepancy commonly observed between scoring systems (34). Although prospective evaluation of the cardiovascular score may not be predictive of ultimate pregnancy outcome (36), it allows recognition of significant cardiac dysfunction across the spectrum of disease. Therefore, fetal echocardiogram is recommended before and after laser surgery to monitor for disease resolution and identify fetuses with persistent cardiac disease that require neonatal echocardiogram and follow-up.
Twin anemia polycythemia sequence (TAPS)
Another condition along the spectrum of monochorionic transfusion syndromes is TAPS. This condition occurs spontaneously in about 5% of monochorionic twins due to chronic transfusion predominantly of red blood cells via small diameter vascular anastomoses (<1 mm) from one twin to the other resulting in anemia of one and polycythemia of the co-twin (37-39). The features of TAPS are more subtle since it may occur in the absence of fluid discordance (40). Its incidence is likely underestimated since international screening protocols do not always include assessment of fetal anemia as a part routine surveillance of monochorionic twins. Fetal anemia is suspected when the velocity in the middle cerebral artery is elevated >1.5 multiples of the median (MoM) for gestational age (41). However, performance of the middle cerebral artery peak systolic velocity (MCA-PSV) to detect fetal polycythemia is unvalidated (42). Accordingly, a small prospective study demonstrated that discordance of the MCA-PSV was more strongly correlated with postnatal intertwin hemoglobin difference than the MCA-PSV MoM of the polycythemic twin (43). Another retrospective study by Tollenaar et al., demonstrated that discordance of MCA-PSV of >0.5 MoM was a better predictor of hemoglobin differences at birth than absolute cut-offs for the MCA-PSV even if both twins had normal measurements (44). Although the diagnostic and staging criteria are not standardized, a recent Delphi consensus of international experts supported use of either absolute MCA PSV cutoffs of >1.5 MoM for the donor and <1 MoM for the recipient or a discordance of >1 MoM to make the diagnosis (45).
Evaluation for TTTS
TTTS most commonly occurs between 16–26 weeks of pregnancy (13). The combination of serial ultrasound assessment beginning in the first trimester and every two weeks after 16 weeks combined with maternal education about symptoms of polyhydramnios allows for early risk stratification and timely identification of the condition (46-48). A recent meta-analysis identified first trimester intertwin discordance in the nuchal translucency or crown rump length, nuchal translucency >95th percentile, or reversed ductus venosus a-wave identify monochorionic pregnancies that are increased risk for developing TTTS (49). In the second trimester, discordance in amniotic fluid, placental cord insertions, and the abdominal circumference can be helpful to identify >70% of monochorionic pregnancies at risk for adverse pregnancy outcome but has a positive predictive value for TTTS of only 22%. This is likely due to the fact that these factors contribute to the entire range of complications that are specific to monochorionic pregnancies (46). For this reason, there it is international agreement that ultrasound surveillance of monochorionic twins should include determination of chorionicity followed by an assessment at least every two weeks after 16 weeks to evaluate for complications including TTTS (48,50,51).
Natural history of TTTS
Untreated TTTS has a very poor prognosis. Although stage I disease may remain stable or regress in up to 30% of expectantly managed cases, progression, fetal demise, or previable birth may occur (52,53). Advanced TTTS results in 90–100% mortality from either single or double twin demise or pregnancy loss from preterm labor due to overdistention of the uterus from polyhydramnios especially when it occurs at <28 weeks gestation (54-56). When TTTS presents in the third trimester, outcomes are more favorable since delivery may be considered. Acute TTTS occurs rarely in labor but may contribute to abnormal fetal heart rate patterns before birth or significant discrepancies in fetal hemoglobin or even hypovolemic shock in the donor (57).
Management of TTTS/TAPS
Fetoscopic laser ablation of placental vessels is the only intervention that aims to cure TTTS by closing the interconnecting vascular communications between the twins giving each fetus a chance for survival (58-60). The procedure is typically performed between 16–26 weeks gestation using local anesthesia with intravenous sedation as needed, epidural or occasionally with general anesthesia (61,62). Preoperative ultrasound mapping of the placental cord insertions, intertwin membrane and consideration of fetal size discordance is used to estimate the location and orientation of the vascular equator (63). Using ultrasound guidance, the sac of the recipient fetus is entered with a fetoscope that has an outer trocar diameter of <4 mm. The intertwin membrane and vascular equator are identified under direct visualization. A 400–600 µm laser fiber is advanced through the operative channel of the fetoscope and the vessels are coagulated at the site of the anastomosis. The chorionic plate is also coagulated between each vascular anastomosis along the vascular equator (Figure 2). This is termed the “Solomon technique” and leads to the highest reported rates of double twin survival of up to 65% (64), while also minimizing the risk for residual anastomoses (65), recurrence or development of post-laser TAPS to <5% (66,67). Prior to removal of the fetoscope, amnioreduction is performed to achieve a normal fluid pocket around the recipient.
Postoperative complications after laser are largely related to issues of membrane integrity and contribute to preterm birth (68). Visualization of fluid in between the chorion and amnion after laser surgery is a risk factor for preterm premature rupture of membranes (PPROM) and shorter interval to delivery (69). The rate of PPROM increases over time from surgery to delivery and is reported in up to 39% of cases by 34 weeks gestation, with the highest risk for early PPROM occurring when surgery is performed at <17 weeks (68,70). Preoperative cervical shortening is also a major contributor to spontaneous preterm birth (71,72). In a multi-center cohort of 449 patients prospectively followed with TTTS, a cervical length <28 mm increased the risk for preterm birth and was associated with a shorter interval to delivery after laser (73). Although several interventions for cervical shortening have been utilized perioperatively including cerclage, progesterone, and pessary, optimal management of a short cervix remains elusive (74-76).
Maternal complications are less common but are estimated to occur in >5% of cases and include intraperitoneal amniotic fluid or bleeding, placental abruption, mirror syndrome, pulmonary edema and occasionally need for intensive care. Underreporting of maternal complications is assumed since many minor complications are self-limited and not universally reported outcomes (77,78).
TTTS presenting after 26 weeks requires additional considerations. The procedure may be technically more challenging due to increased amniotic fluid turbidity and larger vessel diameter. However, outcomes for TTTS cases treated before and after 26 weeks appear to be comparable with no differences in duration of surgery, complication rate or delivery timing observed (79,80). Definitive treatment with laser affords the potential to avoid a very preterm delivery with decreased neurologic morbidity compared to amnioreduction (81), but thresholds for intervention for fetal status after viability require shared decision making between parents and physicians after counseling.
Alternative management options for TTTS include amnioreduction, selective fetal reduction, or pregnancy termination. Amnioreduction is a consideration for situations where referral to a laser center is not feasible or as a temporizing measure particularly if late in gestation. Selective fetal reduction is typically performed for cases with associated fetal anomalies of one twin or where survival is unlikely after treatment for TTTS (82). Some parents may also choose previable pregnancy termination if the perioperative risks and potential outcomes are not acceptable to the family (51).
For cases of TTTS Stage V with a single fetal demise, there is about a 15% risk of death to the surviving co-twin and up to 30% risk for neurologic impairment due to sudden reversed perfusion from the surviving fetus to the demised fetus at the time of death (83,84). No interventions have been shown to alleviate these risks including rescue transfusion of the surviving twin (85). When a single fetal demise is identified in a monochorionic pair, evidence of cerebral injury may not be evident for several weeks by ultrasound or magnetic resonance imaging. The findings are typically consistent with hypoxic-ischemic injuries resulting in periventricular leukomalacia, encephalomalacia, ventriculomegaly, intraventricular hemorrhage, and infarction (86,87).
Management options for TAPS similarly include observation, fetoscopic laser ablation, selective fetal reduction, intrauterine transfusion with or without partial exchange transfusion, termination of pregnancy or early delivery. There is an ongoing international multi-center randomized trial to investigate whether treatment with fetoscopic laser surgery improves outcomes compared to the alternative treatments (ClinicalTrials.gov Identifier: NCT04432168).
Surveillance after treatment for TTTS
After the immediate postoperative period, close ultrasound surveillance is required to monitor for resolution of the condition. In our observations, resolution of TTTS generally occurs over the first two weeks after laser surgery. Initially, weekly ultrasound surveillance is recommended with the option to extend monitoring to two-week intervals if the clinical picture is stable. Since residual anastomoses on the surface or deep to the chorionic plate may be present after laser (65,88-90), the potential for recurrent TTTS or TAPS exists until delivery (91).
The optimal strategy for antenatal surveillance in the third trimester with non-stress testing or biophysical profile is unknown, however a similar strategy for other pregnancies at high risk for fetal compromise or deterioration can be considered. Preterm delivery after laser surgery is common with a median gestational age at birth <34 weeks across several large series from experienced laser centers. A course of betamethasone is recommended since the risk for preterm birth is elevated once TTTS is diagnosed and treated (51). The timing may be determined based on the clinical circumstances. When the postoperative course is uncomplicated and surveillance is reassuring, the pregnancy can be continued until 34–36 weeks. Mode of delivery can also be planned based on obstetric indications if fetal status is reassuring. In cases of a single survivor, delivery timing and mode can also follow recommendations for a singleton pregnancy.
Perinatal and long-term outcomes
Overall fetal survival has improved with experience and modification of the laser technique since its initial description. A systematic review of 34 studies including 3,868 pregnancies evaluated the outcomes treated with laser surgery over 25 years. Mean gestational age at delivery was 32.4±1.3 weeks across the time period but mean survival increased for a both fetuses from 35% to 65% and for a single fetus from 70–88% (64). After laser surgery, hemorrhagic or ischemic cerebral lesions are observed in about 2–10% of both recipient and donor fetuses on ultrasound or MRI (92,93). This risk is not affected by single fetal demise after surgery but it was higher in cases of recurrent TTTS or post-laser TAPS (93). This is important because prenatally detected severe cerebral lesions are related to neurodevelopmental outcome at age 2 years of age with an odds ratio of 34.86; (95% confidence interval, 11.83–102.75; P<0.01). A systematic review of TTTS survivors showed that 11% of children showed signs of neurologic impairment beginning in infancy (94). This may manifest as delays in cognitive, motor, or verbal skills as well as cerebral palsy but the rates are lower than those managed conservatively or with amnioreduction (95). Furthermore, prematurity has an independent impact on neurodevelopment with each gestational age week so some of these observations may be attributable to the effects of prematurity rather than TTTS (96). Longer term follow up to 6 years of age is consistent with rates of severe neurodevelopmental impairment ranging from 4–13% of children evaluated (97).
Cardiac dysfunction resulting from volume loading as well as hormonal aberrations in fetal and placental tissues have both short and potentially longer term impacts into childhood (20-22,97). Although cardiac function improves in many fetuses shortly after laser surgery (98), identifying former recipients with residual pulmonary valve stenosis or atresia may be important for delivery location and management planning in the newborn period (28,99). In the neonatal period, about 4% of former recipient twins may have persistent pulmonary hypertension of the newborn (100). Hypertension is also more common in the first year of life but normalizes through childhood with normal values observed in both recipient and donors by 10 years of age (101,102).
Conclusion
Although curative treatment for TTTS with laser surgery is more widely available with acceptable fetal and maternal outcomes, this condition remains a significant contributor to morbidity and mortality in monochorionic twins. Strategies to minimize ruptured membranes and preterm birth after treatment are elusive and the equipment used contemporarily has changed minimally over time. In order to further improve perinatal outcomes, accurate diagnosis (103) and referral of TTTS cases to a laser center (51) combined with effective treatments to reduce preterm birth particularly in the setting of short cervix are necessary advancements. Standardization for reporting of core outcomes measures are important steps for treatment centers to adopt in order to improve the framework for comparison studies and long-term outcome monitoring (104).
Supplementary
The article’s supplementary files as
Acknowledgments
Dr. Ahmet Baschat for ongoing mentorship as well as providing content for figures.
Funding: None.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Eric B. Jelin and George B. Mychaliska) for the series “Fetal Surgery” published in Translational Pediatrics. The article has undergone external peer review.
Peer Review File: Available at http://dx.doi.org/10.21037/tp-20-264
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-264). The series “Fetal Surgery” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
References
- 1.Hall JG. Twinning. Lancet 2003;362:735-43. 10.1016/S0140-6736(03)14237-7 [DOI] [PubMed] [Google Scholar]
- 2.Hall JG. Twinning: mechanisms and genetic implications. Curr Opin Genet Dev 1996;6:343-7. 10.1016/S0959-437X(96)80012-8 [DOI] [PubMed] [Google Scholar]
- 3.Mutchinick OM, Luna-Muñoz L, Amar E, Bakker MK, et al. Conjoined twins: a worldwide collaborative epidemiological study of the International Clearinghouse for Birth Defects Surveillance and Research. Am J Med Genet C Semin Med Genet 2011;157C:274-87. 10.1002/ajmg.c.30321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenhouse E, Hardwick C, Maharaj S, et al. Chorionicity determination in twin pregnancies: how accurate are we? Ultrasound Obstet Gynecol 2002;19:350-2. 10.1046/j.1469-0705.2002.00679.x [DOI] [PubMed] [Google Scholar]
- 5.Carroll SGM, Soothill PW, Abdel-Fattah SA, et al. Prediction of chorionicity in twin pregnancies at 10-14 weeks of gestation. BJOG 2002;109:182-6. [DOI] [PubMed] [Google Scholar]
- 6.Menon DK. A retrospective study of the accuracy of sonographic chorionicity determination in twin pregnancies. Twin Res Hum Genet 2005;8:259-61. 10.1375/twin.8.3.259 [DOI] [PubMed] [Google Scholar]
- 7.Dias T, Arcangeli T, Bhide A, et al. First-trimester ultrasound determination of chorionicity in twin pregnancy. Ultrasound Obstet Gynecol 2011;38:530-2. 10.1002/uog.8956 [DOI] [PubMed] [Google Scholar]
- 8.Sebire NJ, Snijders RJ, Hughes K, et al. The hidden mortality of monochorionic twin pregnancies. Br J Obstet Gynaecol 1997;104:1203-7. 10.1111/j.1471-0528.1997.tb10948.x [DOI] [PubMed] [Google Scholar]
- 9.Lewi L, Van Schoubroeck D, Gratacós E, et al. Monochorionic diamniotic twins: complications and management options. Curr Opin Obstet Gynecol 2003;15:177-94. 10.1097/00001703-200304000-00013 [DOI] [PubMed] [Google Scholar]
- 10.Denbow ML, Cox P, Taylor M, et al. Placental angioarchitecture in monochorionic twin pregnancies: relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am J Obstet Gynecol 2000;182:417-26. 10.1016/S0002-9378(00)70233-X [DOI] [PubMed] [Google Scholar]
- 11.Glennon CL, Shemer SA, Palma-Dias R, et al. The History of Treatment of Twin-to-Twin Transfusion Syndrome. Twin Res Hum Genet 2016;19:168-74. 10.1017/thg.2016.27 [DOI] [PubMed] [Google Scholar]
- 12.Paepe MED. Examination of the twin placenta. Semin Perinatol 2015;39:27-35. 10.1053/j.semperi.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 13.Lewi L, Jani J, Blickstein I, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol 2008;199:514.e1-8. 10.1016/j.ajog.2008.03.050 [DOI] [PubMed] [Google Scholar]
- 14.Diehl W, Hecher K, Zikulnig L, et al. Placental vascular anastomoses visualized during fetoscopic laser surgery in severe mid-trimester twin-twin transfusion syndrome. Placenta 2001;22:876-81. 10.1053/plac.2001.0710 [DOI] [PubMed] [Google Scholar]
- 15.Bermúdez C, Becerra CH, Bornick PW, et al. Placental types and twin-twin transfusion syndrome. Am J Obstet Gynecol 2002;187:489-94. 10.1067/mob.2002.124280 [DOI] [PubMed] [Google Scholar]
- 16.Umur A, van Gemert MJC, Nikkels PGJ, et al. Monochorionic twins and twin-twin transfusion syndrome: the protective role of arterio-arterial anastomoses. Placenta 2002;23:201-9. 10.1053/plac.2001.0758 [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto M, Nasr B, Ortqvist L, et al. Intertwin discordance in umbilical venous volume flow: a reflection of blood volume imbalance in twin-to-twin transfusion syndrome. Ultrasound Obstet Gynecol 2007;29:317-20. 10.1002/uog.3959 [DOI] [PubMed] [Google Scholar]
- 18.Van Mieghem T, Doné E, Gucciardo L, et al. Amniotic fluid markers of fetal cardiac dysfunction in twin-to-twin transfusion syndrome. Am J Obstet Gynecol 2010;202:48.e1-7. 10.1016/j.ajog.2009.08.013 [DOI] [PubMed] [Google Scholar]
- 19.Habli M, Cnota J, Michelfelder E, et al. The relationship between amniotic fluid levels of brain-type natriuretic peptide and recipient cardiomyopathy in twin-twin transfusion syndrome. Am J Obstet Gynecol 2010;203:404.e1-7. 10.1016/j.ajog.2010.06.070 [DOI] [PubMed] [Google Scholar]
- 20.Bajoria R, Sullivan M, Fisk NM. Endothelin concentrations in monochorionic twins with severe twin-twin transfusion syndrome. Hum Reprod 1999;14:1614-8. 10.1093/humrep/14.6.1614 [DOI] [PubMed] [Google Scholar]
- 21.Mahieu-Caputo D, Meulemans A, Martinovic J, et al. Paradoxic activation of the renin-angiotensin system in twin-twin transfusion syndrome: an explanation for cardiovascular disturbances in the recipient. Pediatr Res 2005;58:685-8. 10.1203/01.PDR.0000180558.03164.E8 [DOI] [PubMed] [Google Scholar]
- 22.Galea P, Barigye O, Wee L, et al. The placenta contributes to activation of the renin angiotensin system in twin-twin transfusion syndrome. Placenta 2008;29:734-42. 10.1016/j.placenta.2008.04.010 [DOI] [PubMed] [Google Scholar]
- 23.Michelfelder E, Gottliebson W, Border W, et al. Early manifestations and spectrum of recipient twin cardiomyopathy in twin-twin transfusion syndrome: relation to Quintero stage. Ultrasound Obstet Gynecol 2007;30:965-71. 10.1002/uog.5211 [DOI] [PubMed] [Google Scholar]
- 24.Stirnemann JJ, Mougeot M, Proulx F, et al. Profiling fetal cardiac function in twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2010;35:19-27. 10.1002/uog.7488 [DOI] [PubMed] [Google Scholar]
- 25.Lougheed J, Sinclair BG, Fung Kee Fung K, et al. Acquired right ventricular outflow tract obstruction in the recipient twin in twin-twin transfusion syndrome. J Am Coll Cardiol 2001;38:1533-8. 10.1016/S0735-1097(01)01549-2 [DOI] [PubMed] [Google Scholar]
- 26.Eschbach SJ, Ten Harkel ADJ, Middeldorp JM, et al. Acquired right ventricular outflow tract obstruction in twin-to-twin transfusion syndrome; a prospective longitudinal study. Prenat Diagn 2018;38:1013-9. 10.1002/pd.5378 [DOI] [PubMed] [Google Scholar]
- 27.Michelfelder E, Tan X, Cnota J, et al. Prevalence, Spectrum, and Outcome of Right Ventricular Outflow Tract Abnormalities in Twin-twin Transfusion Syndrome: A Large, Single-center Experience. Congenit Heart Dis 2015;10:209-18. 10.1111/chd.12215 [DOI] [PubMed] [Google Scholar]
- 28.Herberg U, Gross W, Bartmann P, et al. Long term cardiac follow up of severe twin to twin transfusion syndrome after intrauterine laser coagulation. Heart 2006;92:95-100. 10.1136/hrt.2004.057497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Boom J, Battin M, Hornung T. Twin-twin transfusion syndrome, coarctation of the aorta and hypoplastic aortic arch: a case series report. J Paediatr Child Health 2010;46:76-9. 10.1111/j.1440-1754.2009.01641.x [DOI] [PubMed] [Google Scholar]
- 30.Manning N, Archer N. Cardiac Manifestations of Twin-to-Twin Transfusion Syndrome. Twin Res Hum Genet 2016;19:246-54. 10.1017/thg.2016.20 [DOI] [PubMed] [Google Scholar]
- 31.Magann EF, Doherty DA, Ennen CS, et al. The ultrasound estimation of amniotic fluid volume in diamniotic twin pregnancies and prediction of peripartum outcomes. Am J Obstet Gynecol 2007;196:570.e1-6; discussion 570.e6-8. 10.1016/j.ajog.2007.01.030 [DOI] [PubMed] [Google Scholar]
- 32.Quintero RA, Morales WJ, Allen MH, et al. Staging of twin-twin transfusion syndrome. J Perinatol 1999;19:550-5. 10.1038/sj.jp.7200292 [DOI] [PubMed] [Google Scholar]
- 33.Barrea C, Alkazaleh F, Ryan G, et al. Prenatal cardiovascular manifestations in the twin-to-twin transfusion syndrome recipients and the impact of therapeutic amnioreduction. Am J Obstet Gynecol 2005;192:892-902. 10.1016/j.ajog.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 34.Rychik J, Tian Z, Bebbington M, et al. The twin-twin transfusion syndrome: spectrum of cardiovascular abnormality and development of a cardiovascular score to assess severity of disease. Am J Obstet Gynecol 2007;197:392.e1-8. 10.1016/j.ajog.2007.06.055 [DOI] [PubMed] [Google Scholar]
- 35.Shah AD, Border WL, Crombleholme TM, et al. Initial fetal cardiovascular profile score predicts recipient twin outcome in twin-twin transfusion syndrome. J Am Soc Echocardiogr 2008;21:1105-8. 10.1016/j.echo.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stirnemann JJ, Nasr B, Proulx F, et al. Evaluation of the CHOP cardiovascular score as a prognostic predictor of outcome in twin-twin transfusion syndrome after laser coagulation of placental vessels in a prospective cohort. Ultrasound Obstet Gynecol 2010;36:52-7. 10.1002/uog.7713 [DOI] [PubMed] [Google Scholar]
- 37.Lopriore E, Deprest J, Slaghekke F, et al. Placental characteristics in monochorionic twins with and without twin anemia-polycythemia sequence. Obstet Gynecol 2008;112:753-8. 10.1097/AOG.0b013e318187e1ff [DOI] [PubMed] [Google Scholar]
- 38.Gucciardo L, Lewi L, Vaast P, et al. Twin anemia polycythemia sequence from a prenatal perspective. Prenat Diagn 2010;30:438-42. 10.1002/pd.2491 [DOI] [PubMed] [Google Scholar]
- 39.de Villiers SF, Slaghekke F, Middeldorp JM, et al. Placental characteristics in monochorionic twins with spontaneous versus post-laser twin anemia-polycythemia sequence. Placenta 2013;34:456-9. 10.1016/j.placenta.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 40.Lopriore E, Middeldorp JM, Oepkes D, et al. Twin Anemia-Polycythemia Sequence in Two Monochorionic Twin Pairs Without Oligo-Polyhydramnios Sequence. Placenta 2007;28:47-51. 10.1016/j.placenta.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 41.Mari G, Deter RL, Carpenter RL, et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N Engl J Med 2000;342:9-14. 10.1056/NEJM200001063420102 [DOI] [PubMed] [Google Scholar]
- 42.Lucewicz A, Fisher K, Henry A, et al. Review of the correlation between blood flow velocity and polycythemia in the fetus, neonate and adult: appropriate diagnostic levels need to be determined for twin anemia-polycythemia sequence. Ultrasound Obstet Gynecol 2016;47:152-7. 10.1002/uog.14782 [DOI] [PubMed] [Google Scholar]
- 43.Fishel-Bartal M, Weisz B, Mazaki-Tovi S, et al. Can middle cerebral artery peak systolic velocity predict polycythemia in monochorionic-diamniotic twins? Evidence from a prospective cohort study. Ultrasound Obstet Gynecol 2016;48:470-5. 10.1002/uog.15838 [DOI] [PubMed] [Google Scholar]
- 44.Tollenaar LSA, Lopriore E, Middeldorp JM, et al. Improved prediction of twin anemia--polycythemia sequence by delta middle cerebral artery peak systolic velocity: New antenatal classification system. Ultrasound Obstet Gynecol 2019;53:788-93. 10.1002/uog.20096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khalil A, Gordijn S, Ganzevoort W, et al. Consensus diagnostic criteria and monitoring of twin anemia polycythemia sequence: a Delphi procedure. Ultrasound Obstet Gynecol 2020;56:388-94. 10.1002/uog.21882 [DOI] [PubMed] [Google Scholar]
- 46.Lewi L, Lewi P, Diemert A, et al. The role of ultrasound examination in the first trimester and at 16 weeks’ gestation to predict fetal complications in monochorionic diamniotic twin pregnancies. Am J Obstet Gynecol 2008;199:493.e1-7. 10.1016/j.ajog.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 47.Sueters M, Middeldorp JM, Lopriore E, et al. Timely diagnosis of twin-to-twin transfusion syndrome in monochorionic twin pregnancies by biweekly sonography combined with patient instruction to report onset of symptoms. Ultrasound Obstet Gynecol 2006;28:659-64. 10.1002/uog.3819 [DOI] [PubMed] [Google Scholar]
- 48.Khalil A, Rodgers M, Baschat A, et al. ISUOG Practice Guidelines: role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol 2016;47:247-63. 10.1002/uog.15821 [DOI] [PubMed] [Google Scholar]
- 49.Stagnati V, Zanardini C, Fichera A, et al. Early prediction of twin-to-twin transfusion syndrome: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017;49:573-82. 10.1002/uog.15989 [DOI] [PubMed] [Google Scholar]
- 50.Emery SP, Bahtiyar MO, Dashe JS, et al. The North American Fetal Therapy Network Consensus Statement: prenatal management of uncomplicated monochorionic gestations. Obstet Gynecol 2015;125:1236-43. 10.1097/AOG.0000000000000723 [DOI] [PubMed] [Google Scholar]
- 51.Society for Maternal-Fetal Medicine , Simpson LL. Twin-twin transfusion syndrome. Am J Obstet Gynecol 2013;208:3-18. 10.1016/j.ajog.2012.10.880 [DOI] [PubMed] [Google Scholar]
- 52.Emery SP, Hasley SK, Catov JM, et al. North American Fetal Therapy Network: intervention vs expectant management for stage I twin-twin transfusion syndrome. Am J Obstet Gynecol 2016;215:346.e1-7. 10.1016/j.ajog.2016.04.024 [DOI] [PubMed] [Google Scholar]
- 53.O’Donoghue K, Cartwright E, Galea P, et al. Stage I twin-twin transfusion syndrome: rates of progression and regression in relation to outcome. Ultrasound Obstet Gynecol 2007;30:958-64. 10.1002/uog.5189 [DOI] [PubMed] [Google Scholar]
- 54.Lewi L, Gucciardo L, Van Mieghem T, de Koninck P, et al. Monochorionic diamniotic twin pregnancies: natural history and risk stratification. Fetal Diagn Ther 2010;27:121-33. 10.1159/000313300 [DOI] [PubMed] [Google Scholar]
- 55.Gul A, Aslan H, Polat I, et al. Natural history of 11 cases of twin-twin transfusion syndrome without intervention. Twin Res 2003;6:263-6. [DOI] [PubMed] [Google Scholar]
- 56.Berghella V, Kaufmann M. Natural history of twin-twin transfusion syndrome. J Reprod Med 2001;46:480-4. [PubMed] [Google Scholar]
- 57.Skupski DW, Sylvestre G, Di Renzo GC, et al. Acute twin-twin transfusion syndrome in labor: pathophysiology and associated factors. J Matern Fetal Neonatal Med 2012;25:456-60. 10.3109/14767058.2011.637146 [DOI] [PubMed] [Google Scholar]
- 58.Senat M-V, Deprest J, Boulvain M, et al. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med 2004;351:136-44. 10.1056/NEJMoa032597 [DOI] [PubMed] [Google Scholar]
- 59.De Lia JE, Cruikshank DP, Keye WR, Jr. Fetoscopic neodymium:YAG laser occlusion of placental vessels in severe twin-twin transfusion syndrome. Obstet Gynecol 1990;75:1046-53. [PubMed] [Google Scholar]
- 60.Roberts D, Neilson JP, Kilby MD, et al. Interventions for the treatment of twin-twin transfusion syndrome. Cochrane Database Syst Rev 2014;1:CD002073. 10.1002/14651858.CD002073.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferschl MB, Feiner J, Vu L, et al. A comparison of spinal anesthesia versus monitored anesthesia care with local anesthesia in minimally invasive fetal surgery. Anesth Analg 2020;130:409-15. 10.1213/ANE.0000000000003947 [DOI] [PubMed] [Google Scholar]
- 62.Duron VD, Watson-Smith D, Benzuly SE, et al. Maternal and fetal safety of fluid-restrictive general anesthesia for endoscopic fetal surgery in monochorionic twin gestations. J Clin Anesth 2014;26:184-90. 10.1016/j.jclinane.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 63.Miller JL, Block-Abraham DM, Blakemore KJ, et al. Preoperative ultrasound prediction of essential landmarks for successful fetoscopic laser treatment of Twin-Twin Transfusion Syndrome. Fetal Diagn Ther 2019;45:295-301. 10.1159/000489119 [DOI] [PubMed] [Google Scholar]
- 64.Akkermans J, Peeters SHP, Klumper FJ, et al. Twenty-five years of fetoscopic laser coagulation in Twin-Twin Transfusion Syndrome: a systematic review. Fetal Diagn Ther 2015;38:241-53. 10.1159/000437053 [DOI] [PubMed] [Google Scholar]
- 65.Slaghekke F, Lewi L, Middeldorp JM, et al. Residual anastomoses in twin-twin transfusion syndrome after laser: the Solomon randomized trial. Am J Obstet Gynecol 2014;211:285.e1-7. 10.1016/j.ajog.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 66.Baschat AA, Barber J, Pedersen N, et al. Outcome after fetoscopic selective laser ablation of placental anastomoses vs equatorial laser dichorionization for the treatment of twin-to-twin transfusion syndrome. Am J Obstet Gynecol 2013;209:234.e1-8. 10.1016/j.ajog.2013.05.034 [DOI] [PubMed] [Google Scholar]
- 67.Ruano R, Rodo C, Peiro JL, et al. Fetoscopic laser ablation of placental anastomoses in twin-twin transfusion syndrome using “Solomon technique.” Ultrasound Obstet Gynecol 2013;42:434-9. 10.1002/uog.12492 [DOI] [PubMed] [Google Scholar]
- 68.Snowise S, Mann LK, Moise K, et al. Preterm prelabor rupture of membranes after fetoscopic laser surgery for twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2017;49:607-11. 10.1002/uog.15958 [DOI] [PubMed] [Google Scholar]
- 69.Papanna R, Mann LK, Johnson A, et al. Chorioamnion separation as a risk for preterm premature rupture of membranes after laser therapy for twin-twin transfusion syndrome. Obstet Gynecol 2010;115:771-6. 10.1097/AOG.0b013e3181d57335 [DOI] [PubMed] [Google Scholar]
- 70.Stirnemann J, Djaafri F, Kim A, et al. Preterm premature rupture of membranes is a collateral effect of improvement in perinatal outcomes following fetoscopic coagulation of chorionic vessels for twin-twin transfusion syndrome: a retrospective observational study of 1092 cases. BJOG 2018;125:1154-62. 10.1111/1471-0528.15147 [DOI] [PubMed] [Google Scholar]
- 71.Malshe A, Snowise S, Mann LK, et al. Preterm delivery after fetoscopic laser surgery for twin-twin transfusion syndrome: etiology and risk factors. Ultrasound Obstet Gynecol 2017;49:612-6. 10.1002/uog.15972 [DOI] [PubMed] [Google Scholar]
- 72.Chmait RH, Korst LM, Llanes A, et al. Perioperative characteristics associated with preterm birth in twin-twin transfusion syndrome treated by laser surgery. Am J Obstet Gynecol 2013;209:264.e1-8. 10.1016/j.ajog.2013.05.025 [DOI] [PubMed] [Google Scholar]
- 73.Papanna R, Mann LK, Baschat AA, et al. Cervical length in prediction of preterm birth after laser surgery for twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2015;45:175-82. 10.1002/uog.14696 [DOI] [PubMed] [Google Scholar]
- 74.Papanna R, Habli M, Baschat AA, et al. Cerclage for cervical shortening at fetoscopic laser photocoagulation in twin-twin transfusion syndrome. Am J Obstet Gynecol 2012;206:425.e1-7. 10.1016/j.ajog.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 75.Carreras E, Arévalo S, Bello-Muñoz JC, et al. Arabin cervical pessary to prevent preterm birth in severe twin-to-twin transfusion syndrome treated by laser surgery. Prenat Diagn 2012;32:1181-5. 10.1002/pd.3982 [DOI] [PubMed] [Google Scholar]
- 76.Aboudiab MS, Chon AH, Korst LM, et al. Management of twin-twin transfusion syndrome with an extremely short cervix. J Obstet Gynaecol 2018;38:359-62. 10.1080/01443615.2017.1330324 [DOI] [PubMed] [Google Scholar]
- 77.Rustico MA, Lanna MM, Faiola S, et al. Fetal and maternal complications after selective fetoscopic laser surgery for twin-to-twin transfusion syndrome: a single-center experience. Fetal Diagn Ther 2012;31:170-8. 10.1159/000336227 [DOI] [PubMed] [Google Scholar]
- 78.Merz W, Tchatcheva K, Gembruch U, et al. Maternal complications of fetoscopic laser photocoagulation (FLP) for treatment of twin-twin transfusion syndrome (TTTS). J Perinat Med 2010;38:439-43. 10.1515/jpm.2010.061 [DOI] [PubMed] [Google Scholar]
- 79.Valsky DV, Eixarch E, Martinez-Crespo JM, et al. Fetoscopic laser surgery for twin-to-twin transfusion syndrome after 26 weeks of gestation. Fetal Diagn Ther 2012;31:30-4. 10.1159/000330369 [DOI] [PubMed] [Google Scholar]
- 80.Baud D, Windrim R, Keunen J, et al. Fetoscopic laser therapy for twin-twin transfusion syndrome before 17 and after 26 weeks’ gestation. Am J Obstet Gynecol 2013;208:197.e1-7. 10.1016/j.ajog.2012.11.027 [DOI] [PubMed] [Google Scholar]
- 81.Middeldorp JM, Lopriore E, Sueters M, et al. Twin-to-twin transfusion syndrome after 26 weeks of gestation: is there a role for fetoscopic laser surgery? BJOG 2007;114:694-8. 10.1111/j.1471-0528.2007.01337.x [DOI] [PubMed] [Google Scholar]
- 82.Rossi AC, D’Addario V. Umbilical cord occlusion for selective feticide in complicated monochorionic twins: a systematic review of literature. Am J Obstet Gynecol 2009;200:123-9. 10.1016/j.ajog.2008.08.039 [DOI] [PubMed] [Google Scholar]
- 83.Ong SS, Zamora J, Khan KS, Kilby MD. Prognosis for the co-twin following single-twin death: a systematic review. BJOG 2006;113:992-8. 10.1111/j.1471-0528.2006.01027.x [DOI] [PubMed] [Google Scholar]
- 84.Hillman SC, Morris RK, Kilby MD. Co-twin prognosis after single fetal death: a systematic review and meta-analysis. Obstet Gynecol 2011;118:928-40. 10.1097/AOG.0b013e31822f129d [DOI] [PubMed] [Google Scholar]
- 85.Quarello E, Stirnemann J, Nassar M, et al. Outcome of anaemic monochorionic single survivors following early intrauterine rescue transfusion in cases of feto-fetal transfusion syndrome. BJOG 2008;115:595-601. 10.1111/j.1471-0528.2007.01659.x [DOI] [PubMed] [Google Scholar]
- 86.van Klink JMM, van Steenis A, Steggerda SJ, et al. Single fetal demise in monochorionic pregnancies: incidence and patterns of cerebral injury. Ultrasound Obstet Gynecol 2015;45:294-300. 10.1002/uog.14722 [DOI] [PubMed] [Google Scholar]
- 87.Conte G, Righini A, Griffiths PD, et al. Brain-injured survivors of monochorionic twin pregnancies complicated by single intrauterine death: MR findings in a multicenter study. Radiology 2018;288:582-90. 10.1148/radiol.2018171267 [DOI] [PubMed] [Google Scholar]
- 88.Knijnenburg PJC, Slaghekke F, Tollenaar LSA, et al. Incidence of and risk factors for residual anastomoses in Twin-Twin Transfusion Syndrome treated with laser surgery: A 15-year single-center experience. Fetal Diagn Ther 2019;45:13-20. 10.1159/000485932 [DOI] [PubMed] [Google Scholar]
- 89.Lewi L, Jani J, Cannie M, et al. Intertwin anastomoses in monochorionic placentas after fetoscopic laser coagulation for twin-to-twin transfusion syndrome: is there more than meets the eye? Am J Obstet Gynecol 2006;194:790-5. 10.1016/j.ajog.2005.08.062 [DOI] [PubMed] [Google Scholar]
- 90.Wee LY, Taylor M, Watkins N, et al. Characterisation of deep arterio-venous anastomoses within monochorionic placentae by vascular casting. Placenta 2005;26:19-24. 10.1016/j.placenta.2004.04.007 [DOI] [PubMed] [Google Scholar]
- 91.Lopriore E, Slaghekke F, Middeldorp JM, et al. Residual anastomoses in twin-to-twin transfusion syndrome treated with selective fetoscopic laser surgery: localization, size, and consequences. Am J Obstet Gynecol 2009;201:66.e1-4. 10.1016/j.ajog.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 92.Lopriore E, van Wezel-Meijler G, Middeldorp JM, et al. Incidence, origin, and character of cerebral injury in twin-to-twin transfusion syndrome treated with fetoscopic laser surgery. Am J Obstet Gynecol 2006;194:1215-20. 10.1016/j.ajog.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 93.Stirnemann J, Chalouhi G, Essaoui M, et al. Fetal brain imaging following laser surgery in twin-to-twin surgery. BJOG 2018;125:1186-91. 10.1111/1471-0528.14162 [DOI] [PubMed] [Google Scholar]
- 94.Rossi AC, Vanderbilt D, Chmait RH. Neurodevelopmental outcomes after laser therapy for twin-twin transfusion syndrome: a systematic review and meta-analysis. Obstet Gynecol 2011;118:1145-50. 10.1097/AOG.0b013e318231827f [DOI] [PubMed] [Google Scholar]
- 95.Miralles-Gutiérrez A, Narbona-Arias I, González-Mesa E. Neurological complications after therapy for fetal-fetal transfusion syndrome: a systematic review of the outcomes at 24 months. J Perinat Med 2018;46:991-7. 10.1515/jpm-2017-0217 [DOI] [PubMed] [Google Scholar]
- 96.Wolke D, Strauss VY, Johnson S, et al. Universal gestational age effects on cognitive and basic mathematic processing: 2 cohorts in 2 countries. J Pediatr 2015;166:1410-6.e62. 10.1016/j.jpeds.2015.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hecher K, Gardiner HM, Diemert A, et al. Long-term outcomes for monochorionic twins after laser therapy in twin-to-twin transfusion syndrome. Lancet Child Adolesc Health 2018;2:525-35. 10.1016/S2352-4642(18)30127-5 [DOI] [PubMed] [Google Scholar]
- 98.Wohlmuth C, Boudreaux D, Moise KJ, et al. Cardiac pathophysiology in twin-twin transfusion syndrome: new insights into its evolution. Ultrasound Obstet Gynecol 2018;51:341-8. 10.1002/uog.17480 [DOI] [PubMed] [Google Scholar]
- 99.Ortiz JU, Masoller N, Gómez O, et al. Rate and outcomes of pulmonary stenosis and functional pulmonary atresia in recipient twins with twin-twin transfusion syndrome. Fetal Diagn Ther 2017;41:191-6. 10.1159/000448075 [DOI] [PubMed] [Google Scholar]
- 100.Gijtenbeek M, Haak MC, Ten Harkel DJ, et al. Persistent pulmonary hypertension of the newborn in twin-twin transfusion syndrome: a case-control study. Neonatology 2017;112:402-8. 10.1159/000478844 [DOI] [PubMed] [Google Scholar]
- 101.Gardiner HM, Taylor MJO, Karatza A, et al. Twin-twin transfusion syndrome: the influence of intrauterine laser photocoagulation on arterial distensibility in childhood. Circulation 2003;107:1906-11. 10.1161/01.CIR.0000060543.64250.80 [DOI] [PubMed] [Google Scholar]
- 102.Gardiner HM, Barlas A, Matsui H, et al. Vascular programming in twins: the effects of chorionicity and fetal therapy for twin-to-twin transfusion syndrome. J Dev Orig Health Dis 2012;3:182-9. 10.1017/S204017441200013X [DOI] [PubMed] [Google Scholar]
- 103.Baud D, Windrim R, Van Mieghem T, et al. Twin--twin transfusion syndrome: a frequently missed diagnosis with important consequences. Ultrasound Obstet Gynecol 2014;44:205-9. 10.1002/uog.13328 [DOI] [PubMed] [Google Scholar]
- 104.Perry H, Duffy JMN, Umadia O, et al. International Collaboration to Harmonise Outcomes for Twin-Twin Transfusion Syndrome (CHOOSE). Outcome reporting across randomized trials and observational studies evaluating treatments for twin-twin transfusion syndrome: systematic review. Ultrasound Obstet Gynecol 2018;52:577-85. 10.1002/uog.19068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as