Abstract
Human schistosomiasis is a debilitating, life-threatening disease affecting more than 229 million people in as many as 78 countries. There is only one drug of choice effective against all three major species of Schistosoma, praziquantel (PZQ). However, as with many monotherapies, evidence for resistance is emerging in the field and can be selected for in the laboratory. Previously used therapies include oxamniquine (OXA), but shortcomings such as drug resistance and affordability resulted in discontinuation. Employing a genetic, biochemical and molecular approach, a sulfotransferase (SULT-OR) was identified as responsible for OXA drug resistance. By crystallizing SmSULT- OR with OXA, the mode of action of OXA was determined. This information allowed a rational approach to novel drug design. Our team approach with schistosome biologists, medicinal chemists, structural biologists and geneticists has enabled us to develop and test novel drug derivatives of OXA to treat this disease. Using an iterative process for drug development, we have successfully identified derivatives that are effective against all three species of the parasite. One derivative CIDD-0149830 kills 100% of all three human schistosome species within 5 days. The goal is to generate a second therapeutic with a different mode of action that can be used in conjunction with praziquantel to overcome the ever-growing threat of resistance and improve efficacy. The ability and need to design, screen, and develop future, affordable therapeutics to treat human schistosomiasis is critical for successful control program outcomes.
Keywords: Schistosomiasis, Drug discovery, Drug resistance, Control programs, Sulfotransferase, Oxamniquine
Graphical abstract
Highlights
-
•
Identification of gene for oxamniquine drug resistance.
-
•
Identify the mode of action of oxamniquine.
-
•
Develop an iterative approach to drug discovery.
-
•
Identify oxamniquine derivatives that will kill the three major species of Schistosoma.
1. Introduction
Schistosomiasis is a major human parasitic disease caused by three species of Schistosoma: S. mansoni, S. haematobium, and S. japonicum. Current estimates indicate that globally schistosomiasis affects over 229 million people in 78 countries (Gryseels et al., 2006; Steinmann et al., 2006; WHO fact sheet, 2016). Of those infected, over 100 million are estimated to be symptomatic, 20 million experience long term complications due to infection, and anywhere from 20,000–200,000 people are estimated to die from the disease annually (WHO Tech Rep Ser, 2002; van der Werf et al., 2003; Chitsulo et al., 2004). Furthermore, these three major species account for the majority of global burden (King, 2007, 2008, 2010; DALYs, GBD and H Collaborators, 2016). Currently, there is no effective vaccine against human schistosomiasis; however, there is a drug that is effective against all three human schistosome species: praziquantel (PZQ). The mainstay of schistosome control programs has used repeated mass chemotherapy with PZQ to treat the at-risk and infected populations of human hosts (Fenwick et al., 2009; Vos et al., 2012; Vale et al., 2017). In addition to development of drug resistance as an ever-present concern, the cure rate for PZQ is usually 60–90% but never 100% (Doenhoff 1998). According to Vos et al. (2012) an estimated 240 million people had schistosomiasis in 2010 and over 66.5 million people received chemotherapy in 2015 in 52 endemic countries (WHO, 2016). Zwang and Olliaro (2014) recent meta-analysis for praziquantel efficacy estimated cure rates of 77.1% and 76.7% for S. haematobium and S. mansoni infections, respectively. Another study reported 60–90% cure rates in sub-Saharan Africa where 90% of infections occur (Doenhoff et al., 2009). Mass Drug Administration (MDA) with praziquantel has brought about reductions in morbidity in both urogenital and intestinal schistosomiasis. However, some locations have maintained high levels of infection prevalence and intensity despite MDA. In western Côte d’Ivoire, an overall reduction in S. mansoni infection prevalence and intensity was achieved one year after a single school-based MDA, yet 10% of schools saw an increase in S. mansoni infection prevalence by 25% or more (Assare et al., 2016). In fact, persistent hot spots in Kenya have been described after MDA strategies were less effective at reducing prevalence and intensity in the hotspot after 5 years of treatment compared to other villages (Wiegand et al., 2017). Treatment of schistosomiasis relies precariously on just one drug, praziquantel (PZQ). This mono chemotherapeutic strategy of schistosomiasis control presents challenges as PZQ is not active against juvenile but only adult stage schistosomes, does not prevent reinfection and the development of PZQ resistant parasites remains a continuous threat. Evidence for drug resistance in the field and laboratory has been reported (Fallon and Doenhoff, 1994; Gryseels et al., 1994; Ismail et al., 1999; Couto et al., 2011; Wang et al., 2012; Alonso et al., 2006). Therefore, new drugs with a different mode of action to be used in combination with PZQ are urgently needed. Our focus has been on oxamniquine (OXA), a prodrug drug effective against S. mansoni but not S. haematobium or S. japonicum.
The goal of this minireview is to present a rational approach to identifying novel drugs that can be used in combination with PZQ. Previous treatments for S. mansoni include oxamniquine (OXA) and hycanthone (HYC). OXA was used extensively in Brazil (Cioli et al., 1995; Katz et al., 2008) until the patent on PZQ expired and PZQ usage became more prevalent (Hagan et al., 2004; Vale et al., 2017). OXA was used to treat millions of people with results comparable to those of PZQ, with respect to safety and efficacy. However, OXA is only effective against the adult worm stage of S. mansoni, HYC is effective against the adult worm stage of S. mansoni and S. haematobium but has been shown to be a carcinogen (Archer and Yarinsky, 1972; Haese and Bueding, 1976; Hartman and Hulbert, 1975) and thus has fallen out of use. Drug resistance against OXA has been demonstrated in the laboratory and in the field (Rogers and Bueding, 1971; Katz et al., 1973). Genetic complementation studies indicated that mutations in the same gene are responsible for both HYC and OXA resistance (Pica-Mattoccia et al., 1993). Further genetic studies demonstrated that OXA resistance was a double recessive trait (Cioli et al., 1992). To initiate these studies, we first had to identify the gene responsible for OXA drug resistance. To do this, we took advantage of the fact that schistosomes are dioecious and that a monomiracidial infection will result in thousands of progeny (cercariae) of the same genotype (sex, Fig. 1). Sex (male/female) of cercariae can be determined by PCR (Webster et al., 1989; Chevalier et al., 2016b).
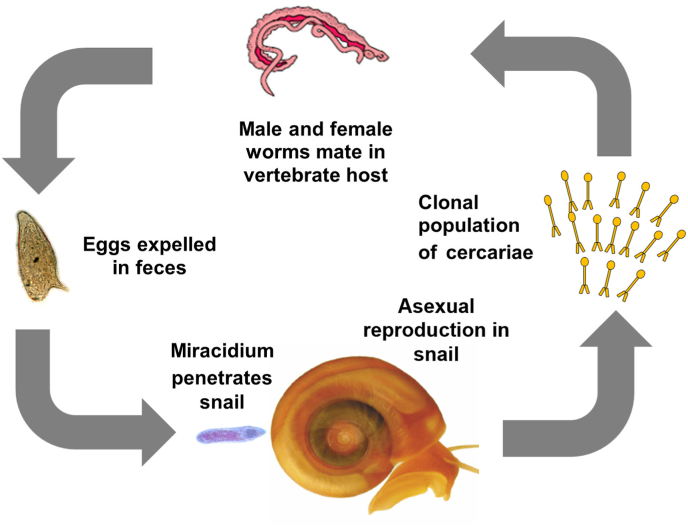
Fig. 1.
Schistosome life cycle is amenable to genetic studies.
Male and female worms inhabit the vasculature where they mate and produce progeny (eggs) by sexual reproduction. Eggs are expelled in the feces where they reach fresh water to continue the life cycle. A miracidium of a single genotype is released and must find a suitable snail host where it penetrates and undergoes several rounds of asexual reproduction producing cercariae, the infective stage for a vertebrate host. The asexual proliferation in the snail host results in a clonal population of cercariae all of the same genotype. Adapted from Anderson et al. (2018).
To identify the gene responsible for drug resistance, an OXA-sensitive S. mansoni (SmLE) from Brazil and OXA-resistant S. mansoni (SmHR) strain from Puerto Rico were crossed (Valentim et al., 2013). Hamsters were infected with male HR cercariae from a single miracidia snail infection and female OXA-susceptible cercariae from a second single miracidia infection. The F1 progeny were mated to isolate multiple F2 progeny. Resistance phenotypes were measured in parents, F1s and F2 progeny, by placing groups of adult worms of a single genotype in wells of a 24-well plate, exposing them to 500 μg/ml OXA for 45 min and plotting survival curves. All of the F1 individuals (heterozygotes) and 136 out of 182 (74.7%) F2 progeny were OXA-sensitive, whereas 36 out of 182 (25.3%) F2 progeny were OXA-resistant, consistent with recessive trait inheritance (Cioli et al., 1992). Parental parasites, F1 individuals, and 144 F2 progeny were genotyped using 62 microsatellite markers (Valentim et al., 2013) distributed at 20 cM intervals across the genome. This was amenable to study as the S. mansoni genome had been sequenced and a 5 cM genetic map had been constructed (Berriman et al., 2009; Criscione et al., 2009). A gene of interest (Smp089320) was localized to the p end of chromosome 6. Using a biochemical and molecular approach, Smp089320, a sulfotransferase, was demonstrated to be responsible for OXA resistance and named SmSULT- OR (Valentim et al., 2013). A biochemical assay took advantage of a previous study that demonstrated homogenates of resistant worms do not bind labelled OXA (Pica-Mattoccia et al., 1992, 2006). OXA binding was rescued by complementing the reaction with the addition of recombinant sulfotransferase protein demonstrating that this sulfotransferase enzyme (SmSULT- OR) is the active principle for OXA activity (Valentim et al., 2013). OXA-sensitive parasites were shown to be OXA-resistant when SmSULT- OR was knocked down using RNAi (Valentim et al., 2013). The sulfotransferase in sensitive worm extracts activate OXA by transferring sulfate groups from the universal sulfate donor 3′-phosphoadenosine-5′-phosphosulfate (PAPS) to the drug (Valentim et al., 2013). To validate that Smp089320 is a sulfotransferase, a sulfation assay using quercetin as substrate was performed (Valentim et al., 2013). Cioli and colleagues (Pica-Mattoccia et al., 2006) had correctly predicted that the active principle for the prodrug OXA was a sulfotransferase. To facilitate linkage mapping to identify any gene of interest, an exome capture and extreme QTL method was employed using identification of the OXA resistance gene as a proof of principle (Chevalier et al., 2014).
2. Mode of action
The mechanism of OXA activity and the mechanism for OXA resistance were identified by further genetic and crystallographic studies (Valentim et al., 2013; Taylor et al., 2017). OXA binds to a specific S. mansoni sulfotransferase, (SmSULT- OR) where it is transiently sulfated (Fig. 2). Oxamniquine was proposed to exert its schistosomicidal activity upon the decay of the sulfated product of the SmSULT- OR reaction to a reactive ethylene with toxic alkylating activity within the parasite (Pica-Mattoccia et al., 2006; Valentim et al., 2013). Continuous-flow mass spectral analyses established directly that the SULT- OR of all three major schistosome species catalyze a sulfotransferase reaction with oxamniquine as substrate, but the proposed ethylene product was not detected (Taylor et al., 2017). Rather, the sulfur group on the released sulfate ester undergoes a nucleophilic attack by various buffer components in the mass spectral analyses (Taylor et al., 2017). Thus, in an SN2-like reaction, activated OXA forms adducts with DNA and other macromolecules, resulting in killing of the worms (Valentim et al., 2013; Taylor et al., 2017). This affects both adult sexes but mainly the males, causing the parasites to detach from hepatoportal circulation and move into the liver where they are eliminated, in part, by the formation of an adduct on DNA thus blocking DNA replication and transcription (Pica-Mattoccia et al., 1989, 2006; Valentim et al., 2013; Taylor et al., 2017). Males express 5X more SmSULT- OR than females which may account for the sex difference in killing (Guzman et al., 2020). If the female worms are not killed, the lack of male worms causes the female worms to revert to an immature state and cease producing eggs (LoVerde et al., 2004). However, male and female worms are both killed by the OXA derivatives (Guzman et al., 2020).
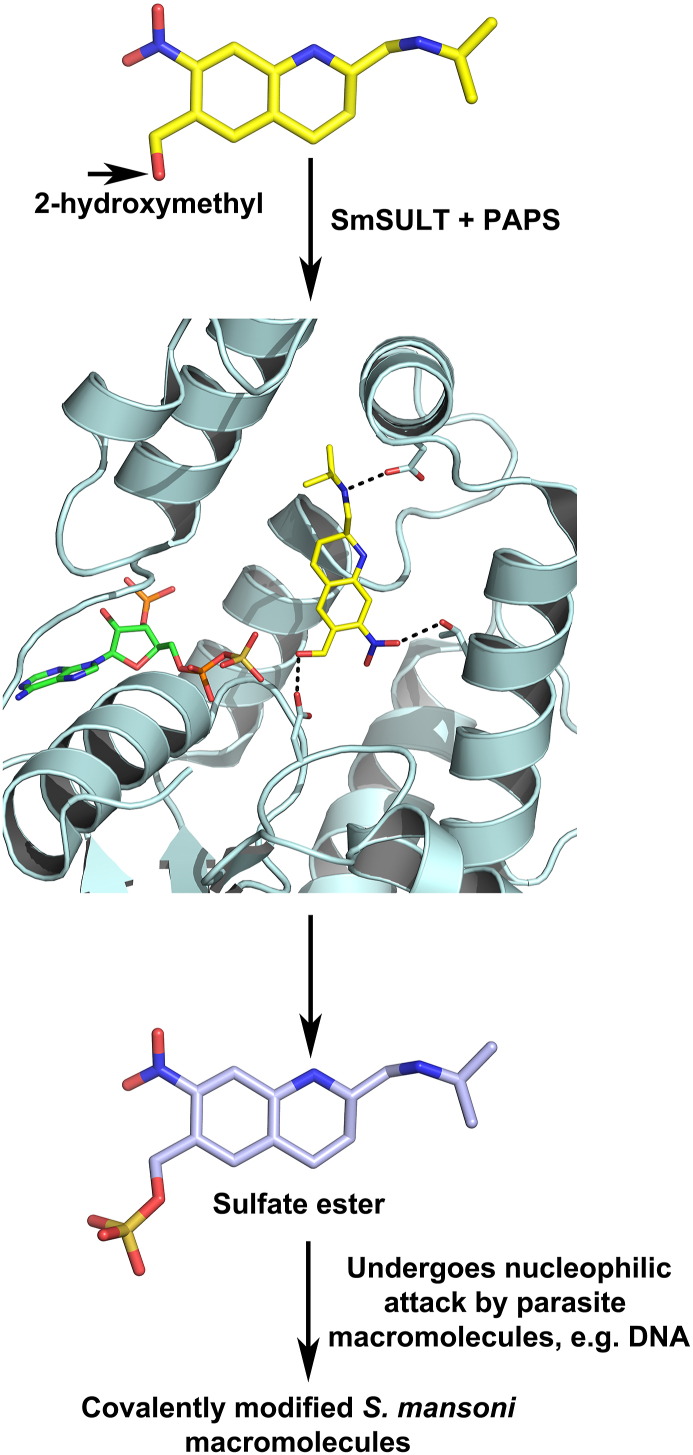
Fig. 2.
OXA mode of action. OXA is a prodrug that is enzymatically activated in the parasite. A sulfotransferase (SULT) transfers a sulfuryl group from the co-factor 3′-phosphoadenine 5′ phosphosulfate (PAPS) to the 2-hydoxymethyl moiety. The sulfate ester reacts with nucleophiles in an SN2-like reaction with the sulfate as the leaving group. This leads to covalent modification of DNA, proteins and other macromolecules and eventually to the death of the parasite. Adapted from Valentim et al. (2013), Taylor et al. (2017).
Because the focus of the research is to develop OXA derivatives that improve the efficiency of S. mansoni killing and will kill S. haematobium and S. japonicum, SULT- ORs from the three main species responsible for almost all human blood fluke infections were crystallized and the structures were determined (Taylor et al., 2017). The SULT- ORs have the hallmark characteristics of PAPS-dependent sulfotransferases, including the β strand-loop-α helix PAPS-binding motif (Taylor et al., 2017). Sequence variations between the three SULT- OR orthologs compared among the molecular structures were proposed to limit OXA binding in the active sites. For example, S. mansoni SULT- OR has a phenylalanine near the active site while S. haematobium and S. japonicum have a tyrosine and therefore change in polarity that was thought to negatively impact OXA binding (Valentim et al., 2013). Later, it was found that OXA could be soaked into S. haematobium crystals and was observed bound in the active site. A phosphatase-coupled assay further showed that SULT- ORs from S. haematobium and S. japonicum each have activity against OXA in vitro, although at lower catalytic efficiencies compared to S. mansoni (Taylor et al., 2017).
Since OXA is activated by a SULT- OR and is lethal to S. mansoni, the question of why oxamniquine fails to kill S. haematobium and S. japonicum adult worms becomes relevant. A study was conducted to ascertain if one or any combination of differences in the OXA contact residues were responsible for S. haematobium or S. japonicum inability to activate OXA. SmSULT- OR, ShSULT- OR and SjSULT- OR each have sulfotransferase activity. Kinetic analyses allowed direct comparison of SmSULT- OR, ShSULT - OR and SjSULT- OR enzymatic activities and showed SmSULT- OR has the highest catalytic efficiency with OXA as substrate based on the kcat/KM values. ShSULT- OR is less active than SmSULT- OR by a factor of about one-half and SjSULT- OR is less active by about an order of magnitude. These lower levels of catalytic efficiency may in part explain why OXA fails to kill S. haematobium and S. japonicum infection (Valentim et al., 2013; Taylor et al., 2017). An amino acid alignment between SmSULT- OR and ShSULT- OR showed 3 differences in OXA contact residues. Adding SjSULT- OR to the analysis showed that SjSULT- OR was 52% identical and 69% similar with 6 differences in OXA contact residues. The PAPS contact residues were conserved for the three schistosome species (Valentim et al., 2013; Rugel et al., 2020) (Fig. 3).
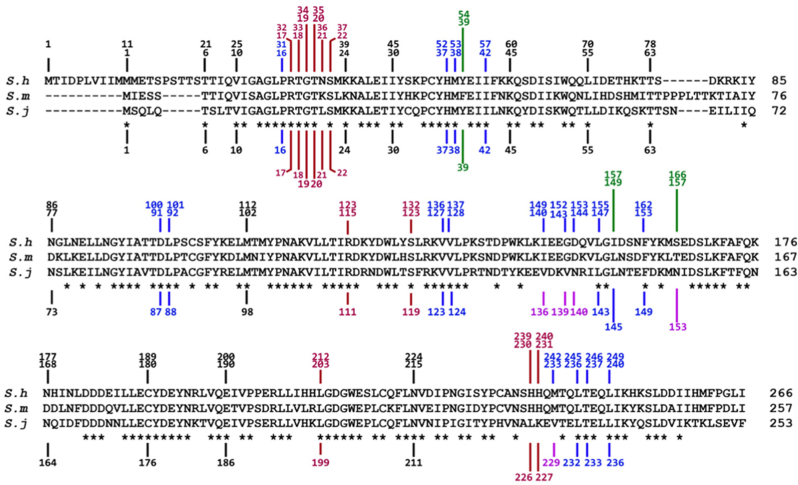
Fig. 3.
Amino acid alignment of S. mansoni SULT-OR, S. haematobium SULT-OR and S. japonicum SULT-OR. Red lines indicate residues that contact co-factor (PAPS), blue lines indicate residues that contact OXA. The green lines indicate amino acids of special interest that differ between SmSULT-OR and ShSULT-OR. The purple lines indicate amino acids that differ between SmSULT-OR and SjSULT-OR. All contacts are ≤4.5 Å. S. haematobium SULT-OR vs S. mansoni SULT-OR: 70.6% Identity; 80.9% Similarity; S. japonicum SULT-OR vs S. mansoni SULT-OR: 52% Identity; 69% Similarity. Adapted from Rugel et al. (2020). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To perform follow up studies, an in vitro activation assay was developed in which the SULT- OR, tritiated OXA, co-factors and sheared schistosome DNA was used as a target (Chevalier et al., 2016a). These studies demonstrated using single, double and triple mutant proteins to interconvert rSmSULT- OR and rShSULT- OR showed no effect on the ability of the recombinant sulfotransferase to activate OXA (Rugel et al., 2020) (Fig. 4A). Therefore, the hypothesis predicting that the differences in contact residues between SmSULT- OR and ShSULT- OR are responsible for the OXA resistance exhibited by S. haematobium does not explain the natural resistance of the S. haematobium worms to OXA.
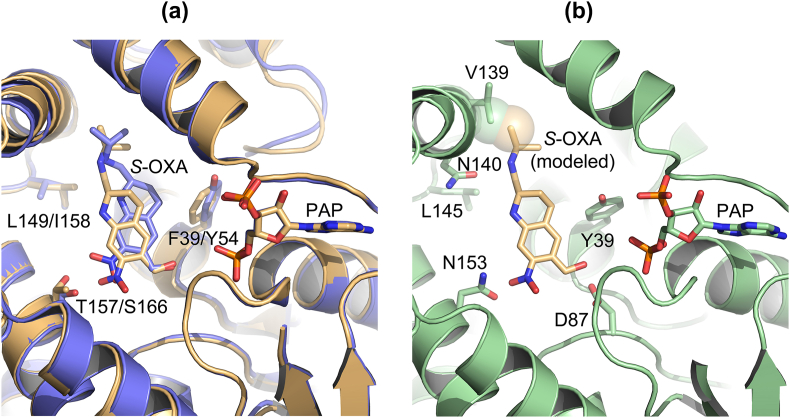
Fig. 4.
Recombinant mutant SULTs were constructed. A. Crystal structure of OXA-bound SmSULT (light orange): F39Y, L148I, T157S, F39Y/L149I, F39Y/T157S, F39Y/L149I/T157S; OXA-bound ShSULT (light blue): Y45F, I158L, S166T, Y54F/I158L. B. Crystal structure of substrate-free SjSULT with OXA (light orange) modeled in the binding pocket using coordinates from the OXA-bound SmSULT crystal structure. The V139 side chain of SjSULT creates a steric clash with OXA (light orange sphere). SjSULT is shown in the same orientation with equivalent amino acid side chains in panel A and with additional contact residues D87 (catalytic aspartate) and N140 (predicted OXA contact). Adapted from Rugel et al. (2020). Some secondary structure has been removed for clarity. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In the case of S. japonicum, rSjSULT- OR failed to activate OXA. Structural comparison suggested that a glycine to valine change at residue 139 might account for the inability of SjSULT- OR to activate OXA (Taylor et al., 2017; Rugel et al., 2020). The V139G mutation was made to activate OXA in a recombinant protein activation assay. The mutation results in an approximate 10-fold increase in activation of OXA, indicating that SjSULT- OR V139 can account in part for the lack of SjSULT- OR's activation of OXA (Rugel et al., 2020) (Fig. 4B). These results are consistent with the hypothesis that amino acid changes are, in part, responsible for the lack of OXA activation in S. japonicum. Data presented argue that the ability of SULT- ORs to sulfate and thus activate OXA and its derivatives is linked to the ability of OXA and OXA-derivative to fit in the binding pocket to allow the transfer of a sulfur group. Additional insight into OXA resistance was derived from the structures when mapping resistance mutations E142del and C35R which appear to disrupt the substrate binding region and PAPS binding region, respectively (Valentim et al., 2013). Their mechanisms of resistance could be described as sulfotransferase knock-downs in the parasites, since they seem to disrupt the sulfotransferase fold (Fig. 5). There is a selective toxicity of oxamniquine toward S. mansoni and not humans, which is likely attributable to the structural variation between SULT homologs of human and schistosome, and the possible availability of an alternative detoxification pathway in humans (Taylor et al., 2017).
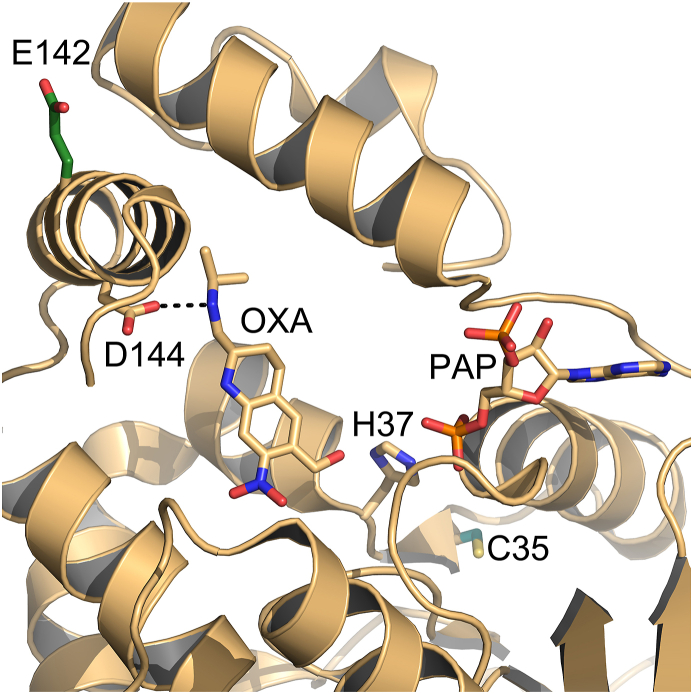
Fig. 5.
Crystal structure of SmSULT with highlighted resistance mutations. E142del, shown in dark green, is predicted to disrupt an α helix that contains D144, which makes a specific interaction with oxamniquine. The C35R mutation (teal) likely disrupts co-substrate PAPS binding as it is predicted to displace secondary structures and nearby H37 (proposed to stabilize the transition state). Some secondary structure has been removed for clarity. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Modeling OXA as oriented in schistosome SULT- ORs into known mammalian sulfotransferase structures suggest steric hindrance, so expanding on the OXA scaffold for derivatives has potential to maintain low toxicity to humans. Interestingly, OXA is the first human anthelminthic drug for which the basis for resistance and the mode of action of the drug has been determined (Valentim et al., 2013).
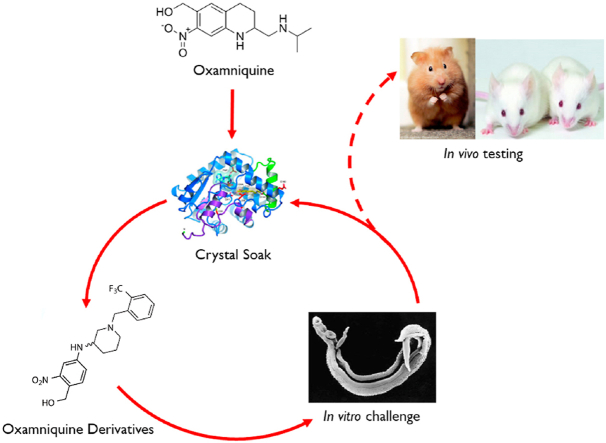
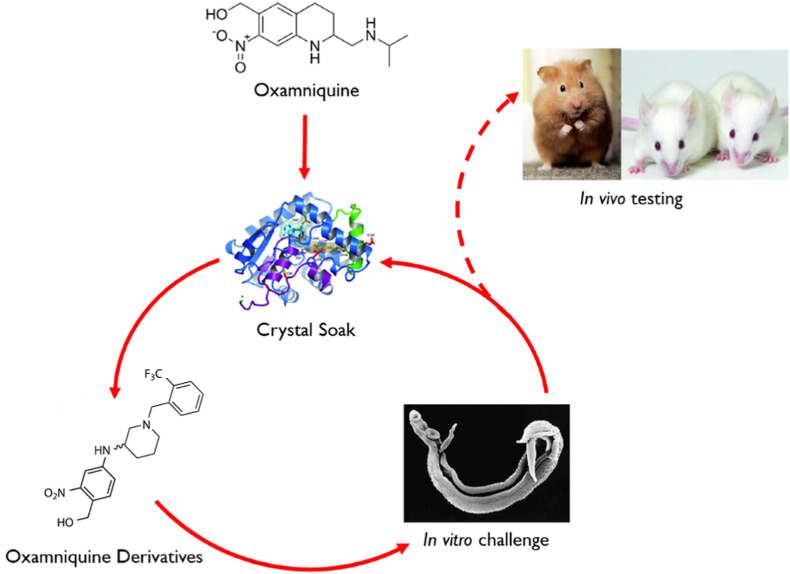
3. Iterative process. We used an iterative approach to identify derivatives of OXA that kill human schistosomes
OXA is soaked into S. mansoni sulfotransferase (SmSULT- OR) and the structural relationships are determined. This information is used to design and synthesize new derivatives based on the structural information. The OXA derivatives are tested for killing activity using worm motility assays in cultured parasites (Marcellino et al., 2012). Those derivatives that show the most potent activity are soaked into new crystals and the process repeated (Fig. 6) (Rugel et al., 2018; Guzman et al., 2020).
Fig. 6.
Iterative process of identifying novel drugs. We soaked OXA into S. mansoni sulfotransferase (SmSULT) and determined the structural relationships. We then designed and synthesized new derivatives based on the structural information. The OXA derivatives are tested for killing activity using worm motility assays or direct observation in cultured parasites. Those derivatives that showed the best cidal activity were soaked into new crystals and the process repeated. This innovative approach has produced highly efficacious OXA derivatives that will kill the three major species of Schistosoma where OXA would only kill S. mansoni. The very best cidal derivatives are tested in in vivo Schistosoma models.
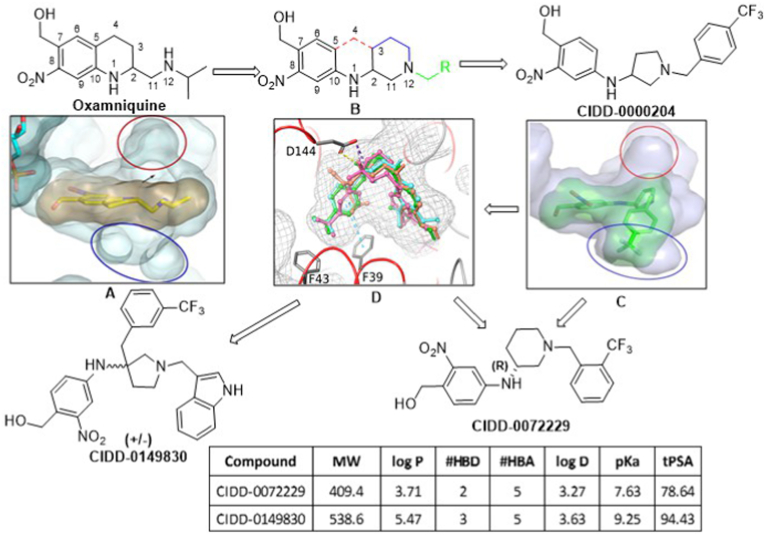
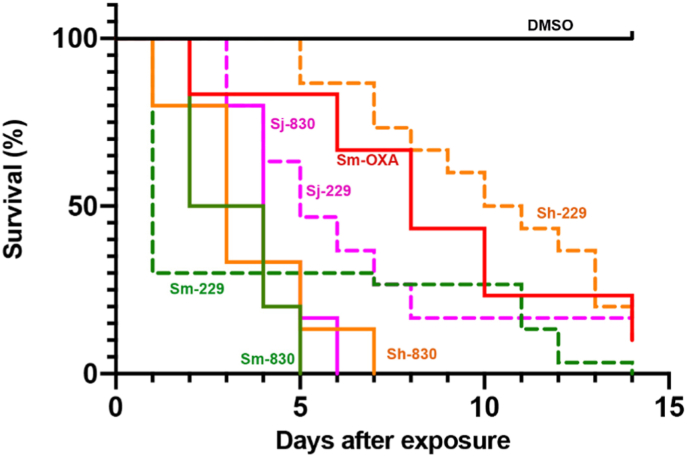
Our goal is to design broad-acting anti-schistosomal agents based on the hypothesis that accessing the binding cavities from the structural information on S. mansoni, S. haematobium and S. japonicum identified in sulfotransferase crystal structures would offer the opportunity to access new binding modes for derivatives capable of being active against all three schistosome species. To design broad-acting anti-schistosome agents, we tested the hypothesis that accessing two cavities identified in sulfotransferase crystal structures (structure A, blue and red circles) would offer the opportunity to access new binding modes for derivatives capable of being active against all three schistosome species (Fig. 7). The design strategy, represented by compound B, removed the rigid tetrahydroquinoline ring of OXA (structure B, red bonds), created new heterocyclic rings between C2 to N12 (structure B, blue bonds), created two new rotatable bonds between C10–N1 and N1–C2, allowing lipophilic groups (structure B, green) to access the targeted cavities. Lead compound CIDD-0000204 exemplifies this approach and the observed binding in the crystal structure SmSULT-OR·CIDD204 supported the design hypothesis (structure C, blue circle). Using this iterative process, structure-activity relationship (SAR) and in silico docking models were used to guide new chemotype design (structure D). Using these models, we hypothesized that 3,3′-disubstituted pyrrolidines (CIDD-0149830) might afford new scaffolds that could access both binding cavities (red and blue circles) simultaneously. The project team designed, synthesized and screened >300 novel compounds in two, structurally distinct chemical series. From these, several derivatives stand out, such as CIDD–0072229 and CIDD-149830 (Guzman et al., 2020). All of the drugs synthesized are racemic mixtures. As CIDD-0066790 demonstrated broad species activity killing S. mansoni (75%), S. haematobium (40%) and S. japonicum (83%) in in vitro killing assays (Rugel et al., 2018), we analyzed the enantiomers of CIDD-0066790. The (R)-enantiomer killed 93% S. mansoni, 95% S. haematobium and 80% of S. japonicum worms in an in vitro killing assay. However, the derivative CIDD-0149830, kills 100% of the S. mansoni, S. haematobium and S. japonicum worms in 5, 6 and 7 days, respectively compared to 14 days for OXA (Fig. 8).
Fig. 7.
New compound design. The design strategy involved modifying oxamniquine by removing the element of rigidity by breaking the tetrahydroquinoline ring system shown in structure B (red bonds), introduce two rotatable bonds between C10–N1 and N1–C2, new substituted cyclic systems between C2 to N12 (structure B, blue bonds) and various R groups (Structure B, green) to capitalize on SmSULT, ShSULT and SjSULT residue interactions. We retained the critical ortho-nitro benzyl alcohol moiety necessary for activation by SULT. The new design allows for more simplistic chemistry as compared to OXA structure. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 8.
OXA Derivative Screen against Schistosoma sp. in vitro. OXA derivatives CIDD-00149830, a racemic derivative of OXA and CIDD-0072229, R-enantiomer of CIDD- 0066790 were tested against adult male Schistosoma worms in vitro. All derivatives were solubilized in 100% DMSO and administered at a final concentration of 143 μM per well. All screens were performed in experimental and biological triplicate. Survival was plotted as a percentage over time using the Kaplan-Meier curves. Pair wise comparison was performed using a log-rank test with Bonferroni correction for multiple testing. The p-value threshold for each derivative compared to DMSO was <0.001. Green was S. mansoni, purple S. japonicum, gold S. haematobium, red oxamniquine, a positive control against S. mansoni and black a negative control DMSO. Solid lines are CIDD-00149830, dashed lines are CIDD-0072229. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
These efforts culminated in the discovery of CIDD-0072229 and CIDD-0149830, which show varying degrees of pan-anti-schistosome activity across all three species (Fig. 8, Rugel et al., 2018; Guzman et al., 2020). To support these efforts, efficient syntheses were developed, allowing manipulation of multiple functional groups to synergize schistosome SAR with optimization of “drug-like” physicochemical property calculations and in silico molecular modeling and docking studies to aid in compound design cycles. Thus, favorable “drug-like” physiochemical properties (LogP, tPSA, MW and number of hydrogen bond donors/acceptors) were manipulated across all analogs to further enhance aqueous solubility properties, and improve in vitro ADME properties such as microsomal clearance, permeability and Cyp450 inhibition (Lipinski et al., 2001; Lu et al., 2004, Fig. 7).
The initial screen was against S. mansoni adult worms, performed in triplicate (10 worms per well), compared to DMSO (no drug control) and OXA (parent drug) (Rugel et al., 2018; Guzman et al., 2020). Each derivative was tested at 143 μM which allowed direct comparison of each derivative against OXA and the other derivatives. Those derivatives that demonstrated killing activity as good as or better than OXA were then tested on S. haematobium and S. japonicum adult worms. The derivative that demonstrated the best killing was soaked into new SmSULT- OR crystals to determine structural interactions and continue the process of synthesizing new derivatives that are tested in an in vitro killing assay. The most promising compounds will be screened using in vivo worm killing assays within infected animals to evaluate efficacy before moving to safety and toxicity studies. The design of the new analogs also avoided structural features or functional groups associated with known toxicities or drug development-associated hurdles. Future studies will include determining the physical chemical properties of the best derivatives, an improvement of desirable drug properties and improvement of the formulation of the derivative. Studies will include a test of derivatives against PZQ resistant parasites, the impact of combination therapy with PZQ and determination of a biomarker to inform control programs of drug resistance to therapy. Since cost-effectiveness of a new therapy is of high importance due to the impact of the schistosomiasis endemic in developing and poor rural communities, these new analogs will be prepared via a short and efficient 6-step, high-yielding synthesis starting from inexpensive and readily available reagents and materials (Rugel et al., 2018; Guzman et al., 2020). Infected patients are treated with 15–50 mg/kg depending on the geographical location of parasite. OXA has a half -life of 1.5–2 h. If a therapeutic dose of 15 mg/kg is given, then the maximum plasma concentration is 1–4 mg/L which is achieved 1–4 h after administration (Foster, 1987; Kokwaro and Taylor, 1991; Ridi and Tallima, 2013).
Other derivatives of OXA have been synthesized and tested for efficacy (Filho et al., 2002; da Rocha Pitta et al., 2013; da Silva et al., 2017). For example, OXA was subjected to the Mannich reaction which gave three unexpected products which when tested in a mouse model of S. mansoni (List, 2000) showed promise but had higher toxicities than OXA (Filho et al., 2007). Three new biopolymers derived from oxamniquine were designed and synthesized to act as prodrugs. Efficacy trials did not demonstrate better effects on reducing worm burden than OXA. A series of studies (Hess et al., 2017; Buchter et al., 2018, 2020) has demonstrated excellent efficacy of OXA derivatives against S. mansoni both in vitro (100% killing) and in vivo (100% killing with a 200 mg/kg dose) and S. haematobium in vitro (75% killing activity). Hess et al. (2017) synthesized ruthenocenyl- and ferrocenyl- and benzyl-derivatives-based organometallic OXA conjugates to improve ADME and physicochemical properties. Current studies focus on increasing bioavailability to improve in vivo activity (Buchter et al., 2020).
One issue with these other studies is that OXA, even though it demonstrates exceptional safety, and efficacy in humans, is in short supply. This in part is a result of high production costs partially due to a biotransformation hydroxylation process and lower cost of praziquantel (Straathof and Adlercreutz, 2003; Beck et al., 2001). This fact discouraged its use outside of South America, where only S. mansoni exists. The restricted market of OXA prevented its competitive production and the expected price reduction, so that today PZQ is cheaper than OXA and has replaced it even in countries, like Brazil, where OXA has been for many years the successful cornerstone of control programs (Richter, 2003). Thus, studies which are dependent on starting material from the parent OXA drug will be compromised.
4. Conclusions
The innovative approach guided by data from X-ray crystallographic studies and Schistosoma worm killing assays has produced a robust SAR program that identified several new lead compounds with effective worm killing (Rugel et al., 2018; Guzman et al., 2020) and has produced highly efficacious OXA derivatives that will kill the three major species of Schistosoma where OXA would only kill S. mansoni. What is important is that novel drugs with a different mode of action from PZQ can be used in combination with PZQ to improve efficacy and mitigate the development of drug resistance. This minireview demonstrates how research can move from a genetic cross to drug redesign through the power of team science to identify novel anti-schistosome compounds.
Declaration of competing interest
None.
Acknowledgements
This work is dedicated to Dr. Livia Pica-Mattoccia whose contributions and insights into drugs and drug development will be sorely missed. The research was supported by a grant to PTL from the NIH, NIAID R01 AI115691. Schistosome infected snails were provided by BRI via the NIAID schistosomiasis resource center under NIH-NIAID Contract No. HHSN272201000005I. The Structural Biology Core Facilities - X-ray Crystallography Core Laboratory is a part of the Institutional Research Cores at the University of Texas Health Science Center at San Antonio supported by the Office of the Vice President for Research and the Mays Cancer Center Drug Discovery and Structural Biology Shared Resource (NIH P30 CA054174). This work is based upon research conducted at the Northeastern Collaborative Access Team beamlines, which are funded by the National Institute of General Medical Sciences from the National Institutes of Health (P30 GM124165). The Eiger 16M detector on 24-ID-E is funded by a NIH-ORIP HEI grant (S10OD021527). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
References
- Alonso D., Muñoz J., Gascón J., Valls M.E., Corachan M. Failure of standard treatment with praziquantel in two returned travelers with Schistosoma haematobium infection. Am. J. Trop. Med. Hyg. 2006;74:342–344. [PubMed] [Google Scholar]
- Anderson T.J.C., LoVerde P.T., Le Clec'h W., Chevalier F.D. Genetic crosses and linkage mapping in schistosome parasites. Trends Parasitol. 2018;34:982–996. doi: 10.1016/j.pt.2018.08.001. Epub 2018 Aug 24. PMID: 30150002. PMCID: PMC6382074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer S., Yarinsky A. Progress in drug research Fortschritte der Arzneimittelforschung Progres des recherches pharmaceutiques. Vol. 16. 1972. Recent developments in the chemotherapy of schistosomiasis; pp. 11–66. Epub 1972/01/01. [DOI] [PubMed] [Google Scholar]
- Assaré R.K., Tian-Bi Y.N., Yao P.K., N'Guessan N.A., Ouattara M., Yapi A., Coulibaly J.T., Meïté A., Hürlimann E., Knopp S., Utzinger J., N'Goran E.K. Sustaining control of schistosomiasis mansoni in western cote d'Ivoire: results from a SCORE study, one year after initial praziquantel administration. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck L., Favre T.C., Pieri O.S., Zani L.C., Domas G.G., Barbosa C.S. Replacing oxamniquine by praziquantel against Schistosoma mansoni infection in a rural community from the sugar-cane zone of Northeast Brazil: an epidemiological follow-up. Mem. Inst. Oswaldo Cruz. 2001;96:165–167. doi: 10.1590/s0074-02762001000900025. PMID: 11586444. [DOI] [PubMed] [Google Scholar]
- Berriman M., Haas B.J., LoVerde P.T. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchter V., Hess J., Gasser G., Keiser J. Assessment of tegumental damage to Schistosoma mansoni and S. haematobium after in vitro exposure to ferrocenyl, ruthenocenyl and benzyl derivatives of oxamniquine using scanning electron microscopy. Parasites Vectors. 2018;11:580. doi: 10.1186/s13071-018-3132-x. PMID: 30400935; PMCID: PMC6219169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchter V., Ong Y.C., Mouvet F., Ladaycia A., Lepeltier E., Rothlisberger U., Keiser J., Gasser G. Multidisciplinary preclinical investigations on three oxamniquine analogues as novel drug candidates for schistosomiasis. Chem. Eur J. 2020;26:15232–15241. doi: 10.1002/chem.202002856. [DOI] [PubMed] [Google Scholar]
- Chevalier F.D., Le Clec'h W., Eng N., Rugel A.R., Assis R.R., Oliveira G., Holloway S.P., Cao X., Hart P.J., LoVerde P.T., Anderson T.J. Independent origins of loss-of-function mutations conferring oxamniquine resistance in a Brazilian schistosome population. Int. J. Parasitol. 2016;46:417–424. doi: 10.1016/j.ijpara.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier F.D., Valentim C.L.L., LoVerde P.T., Anderson T.J.C. Efficient linkage mapping using exome capture and extreme QTL in schistosome parasites. BMC Genom. 2014;15:617. doi: 10.1186/1471-2164-15-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier F.D., Le Clec'h W., Alves de Mattos A.C., LoVerde P.T., Anderson T.J. Real-time PCR for sexing Schistosoma mansoni cercariae. Mol. Biochem. Parasitol. 2016;205:35–38. doi: 10.1016/j.molbiopara.2016.03.010. Epub 2016 Mar 26. PubMed PMID: 27021570. PubMed Central PMCID: PMC4841722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitsulo L., LoVerde P., Engels D. Focus: schistosomiasis. Nat. Rev. Microbiol. 2004;2:12. doi: 10.1038/nrmicro801. [DOI] [PubMed] [Google Scholar]
- Cioli D., Pica-Mattoccia L., Moroni R. Schistosoma mansoni: hycanthone/oxamniquine resistance is controlled by a single autosomal recessive gene. Exp. Parasitol. 1992;75:425–432. doi: 10.1016/0014-4894(92)90255-9. [PubMed: 1493874] [DOI] [PubMed] [Google Scholar]
- Cioli D., Pica-Mattoccia L., Archer S. Antischistosomal drugs: past, present … and future? Pharmacol. Ther. 1995;68:35–85. doi: 10.1016/0163-7258(95)00026-7. Epub 1995/01/01. [DOI] [PubMed] [Google Scholar]
- Couto F.F., Coelho P.M., Araújo N., Kusel J.R., Katz N., Jannotti-Passos L.K., Mattos A.C. Schistosoma mansoni: a method for inducing resistance to praziquantel using infected Biomphalaria glabrata snails. Mem. Inst. Oswaldo Cruz. 2011;106:153–157. doi: 10.1590/s0074-02762011000200006. [DOI] [PubMed] [Google Scholar]
- Criscione C.D., Valentim C.L., Hirai H., LoVerde P.T., Anderson T.J. Genomic linkage map of the human blood fluke Schistosoma mansoni. Genome Biol. 2009;10:R71. doi: 10.1186/gb-2009-10-6-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALYs G.B.D., Collaborators H. Global, regional, and national disability adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha Pitta M.G., da Rocha Pitta M.G., de Melo Rêgo M.J., Galdino S.L. The evolution of drugs on schistosoma treatment: looking to the past to improve the future. Mini Rev. Med. Chem. 2013;13:493–508. doi: 10.2174/1389557511313040003. PMID: 23373654. [DOI] [PubMed] [Google Scholar]
- da Silva V.B.R., Campos B.R.K.L., de Oliveira J.F., Decout J.L., do Carmo Alves de Lima M. Medicinal chemistry of antischistosomal drugs: praziquantel and oxamniquine. Bioorg. Med. Chem. 2017;1:3259–3277. doi: 10.1016/j.bmc.2017.04.031. Epub 2017 Apr 27. PMID: 28495384. [DOI] [PubMed] [Google Scholar]
- Doenhoff M.J. Is schistosomicidal chemotherapy sub-curative? Implications for drug resistance. Parasitol. Today. 1998;14:434–435. doi: 10.1016/s0169-4758(98)01315-5. [DOI] [PubMed] [Google Scholar]
- Doenhoff M.J., Hagan P., Cioli D., Southgate V., Pica-Mattoccia L., Botros S., Coles G., Tchuem Tchuenté L.A., Mbaye A., Engels D. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology. 2009;136:1825–1835. doi: 10.1017/S0031182009000493. [DOI] [PubMed] [Google Scholar]
- Fallon P.G., Doenhoff M.J. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am. J. Trop. Med. Hyg. 1994;51:83–88. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- Fenwick A., Webster J.P., Bosque-Oliva E., Blair L., Fleming F.M., Zhang Y., Garba A., Stothard J.R., Gabrielli A.F., Clements A.C., Kabatereine N.B., Toure S., Dembele R., Nyandindi U., Mwansa J., Koukounari A. The Schistosomiasis Control Initiative (SCI): rationale,development and implementation from 2002-2008. Parasitology. 2009;136:1719–1730. doi: 10.1017/S0031182009990400. [DOI] [PubMed] [Google Scholar]
- Filho S.B., Gargioni C., Silva Pinto P.L., Chiodelli S.G., Gurgel Vellosa S.A., da Silva R.M., da Silveira M.A. Synthesis and evaluation of new oxamniquine derivatives. Int. J. Pharm. 2002;21:35–41. doi: 10.1016/s0378-5173(01)00917-6. PMID: 11897408. [DOI] [PubMed] [Google Scholar]
- Filho R.P., de Souza Menezes C.M., Pinto P.L., Paula G.A., Brandt C.A., da Silveira M.A. Design, synthesis, and in vivo evaluation of oxamniquine methacrylate and acrylamide prodrugs. Bioorg. Med. Chem. 2007;15:1229–1236. doi: 10.1016/j.bmc.2006.11.027. Epub 2006 Nov 16. PMID: 17134907. [DOI] [PubMed] [Google Scholar]
- Foster R. A review of clinical experience with oxamniquine. Trans. R. Soc. Trop. Med. Hyg. 1987;81:55–59. doi: 10.1016/0035-9203(87)90282-3. [DOI] [PubMed] [Google Scholar]
- Gryseels B., Stelma F.F., Talla I., van Dam G.J., Polman K., Sow S., Diaw M., Sturrock R.F., Doehring-Schwerdtfeger E., Kardorff R. Epidemiology, immunology and chemotherapy of Schistosoma mansoni infections in a recently exposed community in Senegal. Trop. Geogr. Med. 1994;46:209–219. [PubMed] [Google Scholar]
- Gryseels B., Polman K., Clerinx J., Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- Guzman M.A., Rugel A.R., Tarpley R.S., Alwan S.N., Chevalier F.D., Kovalskyy D.P., Cao X., Holloway S.P., Anderson T.J.C., Taylor A.B., McHardy S.F., LoVerde P.T. An iterative process produces oxamniquine derivatives that kill the major species of schistosomes infecting humans. PLoS Neglected Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haese W.H., Bueding E. Long-term hepatocellular effects of hycanthone and of two other anti-Schistosomal drugs in mice infected with Schistosoma mansoni. J. Pharmacol. Exp. Therapeut. 1976;197:703–713. Epub 1976/06/01. [PubMed] [Google Scholar]
- Hagan P., Appleton C.C., Coles G.C., Kusel J.R., Tchuem-Tchuente L.A. Schistosomiasis control: keep taking the tablets. Trends Parasitol. 2004;20:92–97. doi: 10.1016/j.pt.2003.11.010. Epub 2004/01/30. [DOI] [PubMed] [Google Scholar]
- Hartman P.E., Hulbert P.B. Genetic activity spectra of some antischistosomal compounds, with particular emphasis on thioxanthenones and benzothiopyranoindazoles. J. Toxicol. Environ. Health. 1975;1:243–270. doi: 10.1080/15287397509529325. Epub 1975/11/01. [DOI] [PubMed] [Google Scholar]
- Hess J., Panic G., Patra M., Mastrobuoni L., Spingler B., Roy S., Keiser J., Gasser G. Ferrocenyl, ruthenocenyl, and benzyl oxamniquine derivatives with cross-species activity against Schistosoma mansoni and Schistosoma haematobium. ACS Infect. Dis. 2017;3:645–652. doi: 10.1021/acsinfecdis.7b00054. [DOI] [PubMed] [Google Scholar]
- Ismail M., Botros S., Metwally A., William S., Farghally A., Tao L.F., Day T.A., Bennett J.L. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am. J. Trop. Med. Hyg. 1999;60:932–935. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- Katz N., Dias E.P., Araujo N., Souza C.P. Estudo de uma cepa humana de Schistosoma mansoni resistente a agentes esquistossomicidas. Rev. Soc. Bras. Med. Trop. 1973;7:381–387. [Google Scholar]
- Katz N., Coelho P.M. Clinical therapy of schistosomiasis mansoni: the Brazilian contribution. Acta Trop. 2008;108:72–78. doi: 10.1016/j.actatropica.2008.05.006. Epub 2008/07/12. [DOI] [PubMed] [Google Scholar]
- King C.H. Lifting the burden of schistosomiasis--defining elements of infection associated disease and the benefits of antiparasite treatment. J. Infect. Dis. 2007;196:653–655. doi: 10.1086/520522. [DOI] [PubMed] [Google Scholar]
- King C.H. Schistosomiasis japonica: the DALYs recaptured. PLoS Neglected Trop. Dis. 2008;2 doi: 10.1371/journal.pntd.0000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.H. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokwaro G.O., Taylor G. Oxamniquine pharmacokinetics in healthy Kenyan African volunteers. East Afr. Med. J. 1991;68:359–364. [PubMed] [Google Scholar]
- Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- List B. The direct catalytic asymmetric three-component Mannich reaction. J. Am. Chem. Soc. 2000;122:9336–9337. [Google Scholar]
- LoVerde P.T., Niles E.G., Osman A., Wu W. Schistosoma mansoni male–female interactions. Can. J. Zool. 2004;82:357–374. [Google Scholar]
- Lu J.J., Crimin K., Goodwin J.T., Crivori P., Orrenius C., Xing L., Tandler P.J., Vidmar T.J., Amore B.M., Wilson A.G.E., Stouten P.F.W., Burton P.S. Influence of molecular flexibility and polar surface area metrics on oral bioavailability in the rat. J. Med. Chem. 2004;47:6104–6107. doi: 10.1021/jm0306529. [DOI] [PubMed] [Google Scholar]
- Marcellino C., Gut J., Lim K.C., Singh R., McKerrow J., Sakanari J. WormAssay: a novel computer application for whole-plate motion-based screening of macroscopic parasites. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001494. PMCID: PMC3269415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Cioli D., Archer S. Binding of oxamniquine to the DNA of schistosomes. Trans. R. Soc. Trop. Med. Hyg. 1989;83:373–376. doi: 10.1016/0035-9203(89)90508-7. Epub 1989/05/01. [DOI] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Archer S., Cioli D. Hycanthone resistance in schistosomes correlates with the lack of an enzymatic activity which produces the covalent binding of hycanthone to parasite macromolecules. Mol. Biochem. Parasitol. 1992;55:167–175. doi: 10.1016/0166-6851(92)90137-9. [PubMed: 1435868] [DOI] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Dias L.C., Moroni R., Cioli D. Schistosoma mansoni: genetic complementation analysis shows that two independent hycanthone/oxamniquine-resistant strains are mutated in the same gene. Exp. Parasitol. 1993;77:445–449. doi: 10.1006/expr.1993.1104. Epub 1993/12/01. [DOI] [PubMed] [Google Scholar]
- Pica-Mattoccia L., Carlini D., Guidi A., Cimica V., Vigorosi F., Cioli D. The schistosome enzyme that activates oxamniquine has the characteristics of a sulfotransferase. Mem. Inst. Oswaldo Cruz. 2006;101:307–312. doi: 10.1590/s0074-02762006000900048. [DOI] [PubMed] [Google Scholar]
- Richter J. The impact of chemotherapy on morbidity due to schistosomiasis. Acta Trop. 2003;86:161–183. doi: 10.1016/S0001-706X(03)00032-9. [PMID: 12745135] [DOI] [PubMed] [Google Scholar]
- Ridi R.A.F., Tallima H.A.M. Novel therapeutic and prevention approaches for schistosomiasis: review. J. Adv. Res. 2013;4:467–478. doi: 10.1016/j.jare.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S.H., Bueding E. Hycanthone resistance: development in Schistosoma mansoni. Science. 1971;172:1057–1058. doi: 10.1126/science.172.3987.1057. Epub 1971/06/04. [DOI] [PubMed] [Google Scholar]
- Rugel A., Tarpley R.S., Lopez A., Menard T., Guzman M.A., Taylor A.B., Cao X., Kovalskyy D., Chevalier F.D., Anderson T.J.C., Hart P.J., LoVerde P.T., McHardy S.F. Design, synthesis, and characterization of novel small molecules as broad range antischistosomal agents. ACS Med. Chem. Lett. 2018;9:967–973. doi: 10.1021/acsmedchemlett.8b00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugel A., Guzman M.A., Taylor A.B., Chevalier F.D., Tarpley R.S., McHardy S.F., Cao X., Holloway S.P., Anderson T.J.C., Hart P.J., LoVerde P.T. Why does oxamniquine kill Schistosoma mansoni and not S. haematobium and S. japonicum? Int. J. Parasitol. Drugs Drug Resist. 2020;13:8–15. doi: 10.1016/j.ijpddr.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann P., Keiser J., Bos R., Tanner M., Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- Straathof A.J.J., Adlercreutz P. CRC Press; 2003. Applied Biocatalysis. [Google Scholar]
- Taylor A.B., Roberts K.M., Cao X., Clark N.E., Holloway S.P., Donati E., Polcaro C.M., Pica-Mattoccia L., Tarpley R.S., McHardy S.F., Cioli D., LoVerde P.T., Fitzpatrick P.F., Hart P.J. Structural and enzymatic insights into species-specific resistance to schistosome parasite drug therapy. J. Biol. Chem. 2017;292:11154–11164. doi: 10.1074/jbc.M116.766527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale N., Gouveia M.J., Rinaldi G., Brindley P.J., Gartner F., Correia da Costa J.M. Praziquantel for schistosomiasis: single-drug metabolism revisited, mode of action, and resistance. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.02582-16. e02582-16. Epub 2017/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentim C.L., Cioli D., Chevalier F.D., Cao X., Taylor A.B., Holloway S.P., Pica-Mattoccia L., Guidi A., Basso A., Tsai I.J., Berriman M., Carvalho-Queiroz C., Almeida M., Aguilar H., Frantz D.E., Hart P.J., LoVerde P.T., Anderson T.J. Genetic and molecular basis of drug resistance and species specific drug action in schistosome parasites. Science. 2013;342:1385–1389. doi: 10.1126/science.1243106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf M.J., de Vlas S.J., Brooker S., Looman C.W., Nagelkerke N.J., Habbema J.D., Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Vos T., Flaxman A.D., Naghavi M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang L., Liang Y.S. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol. Res. 2012;111:1871–1877. doi: 10.1007/s00436-012-3151-z. [DOI] [PubMed] [Google Scholar]
- Webster P., Mansour T.E., Bieber D. Isolation of a female-specific, highly repeated Schistosoma mansoni DNA probe and its use in an assay of cercarial sex. Mol. Biochem. Parasitol. 1989;36:217–222. doi: 10.1016/0166-6851(89)90169-2. [DOI] [PubMed] [Google Scholar]
- Wiegand R.E., Mwinzi P.N.M., Montgomery S.P., Chan Y.L., Andiego K., Omedo M., Muchiri G., Ogutu M.O., Rawago F., Odiere M.R., Karanja D.M.S., Secor W.E. A persistent hotspot of schistosoma mansoni infection in a five-year randomized trial of praziquantel preventative chemotherapy strategies. J. Infect. Dis. 2017;216:1425–1433. doi: 10.1093/infdis/jix496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Expert Committee . 2002. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis. World Health Organ Tech Rep Ser. 912:i-vi, 1-57, back cover. PMID: 12592987. [PubMed] [Google Scholar]
- WHO Fact sheet: schistosomiasis. 2016. http://www.who.int/mediacentre/factsheets/fs115/en Available from.
- WHO Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2015. Wkly. Epidemiol. Rec. 2016;91(49–50):585–595. [PubMed] [Google Scholar]
- Zwang J., Olliaro P.L. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis-a meta-analysis of comparative and noncomparative clinical trials. PLoS Neglected Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003286. [DOI] [PMC free article] [PubMed] [Google Scholar]