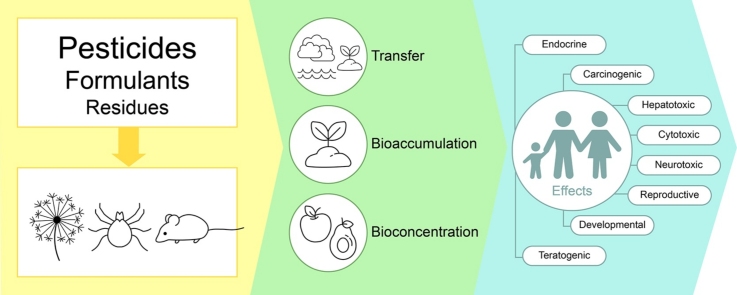
Graphical abstract
Keywords: Pesticides, Agricultural crops, Health consequences, Formulants, Risk assessment
Highlights
-
•
Pesticide residues cause problems in non-target species and unbalance ecosystems.
-
•
Initial toxicity of pesticides may change after entering the organism.
-
•
Inert components of pesticide products pose implicit threat.
Abstract
Pesticides are commonly used in agriculture to enhance crop production and control pests. Therefore, pesticide residues can persist in the environment and agricultural crops. Although modern formulations are relatively safe to non-target species, numerous theoretical and experimental data demonstrate that pesticide residues can produce long-term negative effects on the health of humans and animals and stability of ecosystems. Of particular interest are molecular mechanisms that mediate the start of a cascade of adverse effects. This is a review of the latest literature data on the effects and consequences of contamination of agricultural crops by pesticide residues. In addition, we address the issue of implicit risks associated with pesticide formulations. The effects of pesticides are considered in the context of the Adverse Outcome Pathway concept.
1. Introduction
Pesticides include a wide variety of chemicals, which are increasingly being used all over the world. The EU Pesticides Database [1] lists more than 1378 active ingredients, 466 of which have been approved and 858 have not been approved for use in the EU. The great variety of molecular mechanisms responsible for the effects of pesticides on their target organisms are shown in Table 1S (Supplementary materials). Each of them has its merits and demerits. For instance, carbamate-based insecticides are commonly used because of their low bioaccumulation, relatively low toxicity to mammals, and effectiveness in controlling a wide range of pests [2]. Organochlorine pesticides, which were commonly used in agriculture in the 1950s [3], at the present time are listed as persistent organic pollutants (POPs) and banned in most countries, in accordance with the Stockholm Convention [4]. Nevertheless, high concentrations of these pesticides and their transformation products (TPs) and degradation products, such as dichlorodiphenyltrichloroethane (DDT), which are very persistent and have long lifetimes, are still detected in agricultural soils [[5], [6], [7]]. Therefore, in recent years, obsolete organochlorine pesticides have been increasingly replaced with more effective and safer alternatives with faster biodegradation rates such as organophosphorus pesticides and neonicotinoids [8,9]. However, some of them still can affect non-target species via water, soil, and contaminated plant tissues [10,11].
Modern formulations, also called current-use pesticides (CUPs), are invented to avoid PBT (persistent, bioaccumulative, and toxic) properties. Nevertheless, they are sufficiently persistent to migrate over long distances. Thus, a number of studies report the discovery of CUPs in air and surface water samples in the Arctic [12,13]. CUPs often appear as representatives of emerging pollutants (EPs) – chemicals without regulatory status that can affect the environment, but with no sufficient toxicologically evaluated data on their properties, metabolism and ecological fate [14,15]. Guida et al. conclude that CUPs seemed to behave like pseudo-persistent pollutants because of their broad and permanent use [16]. The problem is that, despite the advantages of modern pesticide formulations, they also have disadvantages. As the modes of action of pesticides are not always specific, they may be hazardous to non-target species, including humans [17,18] or wild animals by accidental exposure [[19], [20], [21]].
One more problem is that the toxic effects of commercial pesticide formulations are equated to the effects of their active ingredients, which may result in incorrect assessment of their safety. Considerable research effort has been devoted recently to separation of the effects and detection of hidden hazards [[22], [23], [24], [25], [26], [27], [28], [29], [30]].
A less obvious problem is that pesticides, as other chemical substances, can affect each other according to the additivity and interaction concepts, decreasing (antagonism) or increasing (synergism) each other’s effect [31]. Another important effect is potentiation, when a non-toxic substance enhances the effect of another substance. Thus, to ensure that the effects of pesticide mixtures are not unpredictable, the data should be based on cumulative risk assessment rather than summing of the effects of all compounds assessed separately [32].
In the framework of modern approaches to risk assessment, it is necessary to consider not a single episode of exposure and effect of pesticides on non-target organisms, but a sequence of related events. This is in good agreement with the concept of the adverse outcome pathway (AOP). The AOP connects key events occurring at different levels of the biological organization that can initiate negative consequences at the level of the whole organism and even several generations of organisms in a population [33,34]. Thus, the concept provides a holistic view of the many key events involved in the toxicity of pesticides and allows estimating the scale of the effect of chemical compounds on biological processes – this plays an important role in predicting adverse effects in the framework of risk assessment.
This review mainly focuses on the distribution pathways and transfer of pesticides into living organisms and negative effects of large-scale use of pesticides on non-target species. A particular emphasis is on inert ingredients of pesticide formulations and their effects based on the currently available data.
2. Commercial formulations of pesticides
The main side effects of pesticides on the environment are associated with their toxicity to non-target species [17,18] and persistence in the environment [5,6,35] and, thus, long-term effects [[36], [37], [38]]. Another issue, however, is the lack of systematic science-based approach to developing formulations [39] and incorporation of adjuvants whose toxicity has not been sufficiently investigated [22,23]. The general terms formulants or co-formulants refer to the inert ingredients that are usually added to enhance the effect and stability of pesticide formulations [40]. For instance, the herbicide mobility and intake by leaves of weeds are enhanced by adding inert surfactants [39], penetrant enhancers, and co-solvents [41]. Another insufficiently studied aspect is the safety of safeners – inert agrochemicals used to protect crops from herbicide toxicity by enhancing their biochemical response [42].
Surfactants are often used as formulants. The most commonly used auxiliary ingredients are non-ionic surfactants such as alkylphenol ethoxylates (APEOs), alcohol ethoxylates (AEOs), and alkylamine ethoxylates (ANEOs), organosilicone polyethoxylates, polyethoxylated tallow amines, and cosolvents such as N-methyl-2-pyrrolidone (NMP). They improve the solubility of the active ingredient and protect it against rapid degradation. It has been shown that seed oil additives and surfactants increase the half-life of metazachlor and reduce its rate of destruction in soil [43]. Formulants also act as spreaders, (anti)foaming agents, dyes, and drift retardants, thus altering the physicochemical properties of the active ingredients of pesticide products.
Due to the inert ingredients present in the formulation, pesticide products with the same active ingredient are marketed in the form of granules, dusts, solutions, wettable powder, flowable suspension concentrate, emulsifiable concentrate, sprayable oils, aerosols [24]. For example, there are approximately 750 different glyphosate-based pesticide formulations around the world [44]. The rate and the way of active substance release, the application method, and stability depend on the form of the pesticide preparation. For example, imidacloprid in granular formulation was more persistent than in wettable powder formulation [45]. Forms with the same active ingredient may differ in toxicity to both pests and non-target species. For instance, emulsifiable formulation is more toxic than aqueous capsule suspension [25]. Pesticide products from different manufacturers have different proportions of active ingredient and formulants. This explains the variability of formulations and, as a consequence, the complex effect of pesticides on non-target organisms.
According to a report from the European Chemicals Agency, there is strong evidence that some formulants are potentially capable of independently exhibiting toxic properties, resulting in higher toxicity in the final pesticide product [46]. For example, polyethoxylated alkylamines, POEA, (surfactants) used as constituents of glyphosate-based herbicides are potentially toxic [23,26,39,47]. Data on the increased toxicity of commercial formulations compared with active ingredients alone were also obtained for pesticides of other classes and mechanisms of action: bifenthrin and fipronil [27], carbaryl, malathion, imidacloprid [48]; azoxystrobin, tebuconazole [28]. Biotesting methods have shown that an insecticide based on neonicotinoid clothianidin, containing the sulfonic acid formulant, was 46.5 times more toxic for Daphnia magna than clothianidin alone [29]. Moreover, in a similar study, the combination of another neonicotinoid, acetamiprid, with linear alkylbenzenesulfonates acting as auxiliary components produced a pronounced synergistic toxic effect on Daphnia magna [49]. Results demonstrating that the toxicity of commercial pesticide formulations is higher than that of pure active ingredients have been obtained in other in vitro bioassays [50]. The same can be said for the potential harm to non-target organisms, for instance, the N-methyl-2-pyrrolidone (NMP) co-solvent was toxic to honey bees [41]. The effect of inert ingredients on non-target species has been shown for pesticide formulations based on pyraclostrobin [51], clomazone [25], acetamiprid [30]. Toxic effects have also been shown for herbicide safeners [52]. Some inert substances have such a pronounced toxic effect that they are capable of separately acting as pesticides: for example, surfactants [53,54].
Since formulants are defined as inert diluents, they are usually not required to be tested for chronic toxicity [47,55]. Usually pesticide formulations are only selectively tested for acute toxicity; the main package of toxicological tests is applied only to the active ingredient [56], and it includes comprehensive information about the structure, expected impact on target species, and ecotoxicological studies of the active ingredient [57,58].
There is evidence of the effects of commercial formulations on human tissues. A number of in vitro studies have shown that negative impacts on nerve cell cultures, placenta cell cultures, DNA [56], male gametes [59], endocrine system [60], mitochondrial and enzyme activities [61] are more pronounced after the action of commercial formulations than pure active ingredients.
In addition, the auxiliary ingredients increase the penetration of the active ingredient not only into the target organism, but also into the skin of those who use them – that is, agricultural workers and farmers are vulnerable groups. This conclusion is based on the results of a comparative study of the transdermal penetration of active ingredients and commercial formulations of pesticides [62,63]. Combination of ingredients in commercial formulations may change the percutaneous penetration of pesticides: significant differences in the penetration rate, lag-time, and amount of the penetrating substance between the pesticide product and the active ingredient are reported for methiocarb and pirimicarb [64]. This is an important factor for the correct assessment of the toxic effects of pesticides on workers and farmers.
Formulants are assumed to differ by the degree of toxicity as well: in a comparative retrospective study, exposure of the patients to POEA-containing herbicides had more serious consequences than ingestion of herbicides with other co-formulants [65]. The main difficulty in testing the safety of commercial pesticide preparations is the impossibility of testing all the constituent components, since manufacturers usually do not disclose exact compositions of the formulants and the data on the environmental toxicity of the formulation to consumers and users [22,41,66]. In accordance with FIFRA (Federal Insecticide, Fungicide and Rodenticide Act), only active ingredients must generally be indicated on pesticide product labels. All formulants can be combined into the category of inert ingredients and are listed as a percentage of product’s total weight to protect confidential commercial information [66,67]. It is obvious that testing of the pesticide formulations intended for sale is a mandatory step in the authorization process; however, product’s marketing documentation seldom reveals any information about the ecotoxicity of the product as a whole [22].
Thus, confidentiality of commercial information, the definition of auxiliary ingredients of pesticide formulations as "inert", and, as a consequence, disregard for their toxicity result in incorrect calculation of threshold values for pesticide consumption [68]. However, as the formulants are conditionally inert, their toxicity is often neglected, and the illusion of safety is created [69]. The absence of the publicly known data on pesticide formulation ingredients hampers risk assessment of the product. It is necessary, though, to know how the formulants can alter metabolism and toxicity of the pesticide formulation to be able to carry out a comprehensive assessment of risks for non-target species including humans [47].
3. Transport pathways of pesticides in the environment
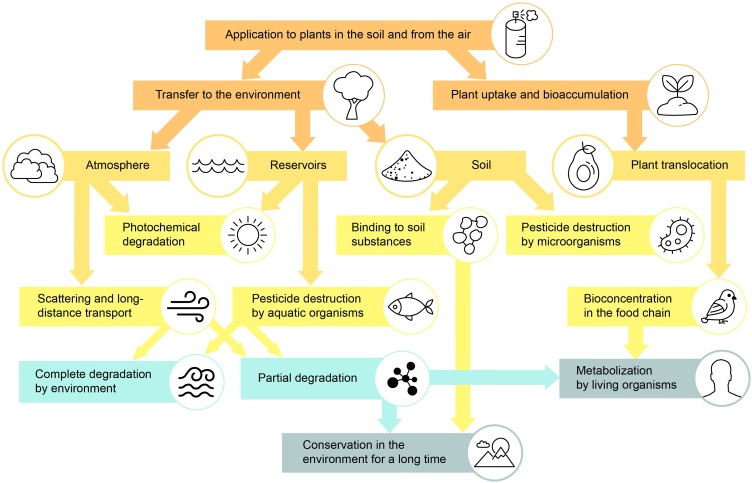
Pesticides not only accumulate in the crops but they can be transported through air, soil, and water over long distances, constituting a major pollution source in ecosystems [70,71]. (Fig. 1).
Fig. 1.
A schematic representation of pesticide transport routes after application. Having been applied to soil or used to treat crops, pesticides are capable of migrating within various environments and, ultimately, accumulate in food chains or persist as degradation products.
The main sources of air pollution by agrochemicals are ground or aerial spraying procedures. Semivolatile pesticides, which are mainly adsorbed on atmospheric aerosol particles, have half-lives in particulate phase from several days to a month and are able to remain stable towards gas-phase reactions toward hydroxyl radicals in the atmosphere [72]. Airborne pesticides can migrate over considerable distances: gaseous stable chemicals can be transported all over the world [38]. CUPs are able to persist in the gas phase of air samples irrespective of land use intensity or sampling year. In studies at various points on the planet, the atrazine and terbuthylazine herbicides and the carbaryl and chlorpyrifos insecticides were detected with high frequency [73,74]. POPs are transported in the environment over long distances as well [70,75,76]. Oxidation and photochemical reactions can transform the airborne pesticide residues into products that are more toxic than the original ones [38].
The movement of pesticides to remote regions is likely facilitated by a combination of properties: both the properties of pesticides (low solubility in water) and climatic factors (dry weather and relatively high temperatures in spring and summer) [77]. An important source of CUPs in the air is volatilization from surface water [74]. The elevated ambient temperatures may accelerate the volatilization of pesticides in warm seasons.
Atmospheric precipitation in turn can transport pesticides to water bodies. In addition to that, water flow gradually removes agrochemicals from the field soil to various water environments, where they can affect aquatic organisms [35]. Chemical processes and microorganisms transform pesticides into products that can be transported via water pathways to surface waters [78].
Dissolved CUPs are widely present in water bodies. For example, comparative studies of China aquatic systems report high detection frequencies of napropamide, atrazine [79], chlorpyrifos, dicofol [74,80]. In a similar study in Argentina, less than 30 % of all detected pesticides were quantified, and glyphosate and AMPA showed the highest concentrations in surface water [81].
Dissolved pesticides and pesticides bound to soil particles can be transported in the river, accumulating in river sediments. Among the pesticides transported with soil particles are pyrethroids [81], which have high affinity for solid particles [82]. The persistence of hydrophobic and cationic compounds is increased due to their ability to form long-lasting bound residues with the soil, while their mobility and availability for biotic and abiotic degradation are reduced [83].
Soil contamination chiefly occurs when pesticides are applied directly to soil to protect crops. For example, farmers in Southeast Asia lacking special knowledge often follow the advice of a sales clerk in a pesticide store and, thus, use far too large amounts of pesticides to control pests in their fields [83]. When applied onto crops, pesticides can be transported downward with the water flow and adsorb, desorb, and break down during their passage through soil. The velocity of passage through soil is determined by the properties of the pesticide, soil properties, and the prevailing environmental conditions [84]. The degree of pesticide leaching to groundwater may be increased considerably because of soil fumigation [85]. Soils, in turn, can be a secondary source of pollutants because of air-soil exchange [86].
Since pesticides differ significantly in their physicochemical properties, the processes of their degradation or accumulation in the environment will also be different. Thus, in comparative studies of soil samples, triazine herbicides and conazole fungicides were more common than other pesticides [87,88]. Large-scale analysis of soil samples collected in the European Union countries for the presence of chemical pollution showed that more than 80 % of the samples contained at least one pesticide residue, the most frequently occurring of which were glyphosate, DDT, and broad-spectrum fungicides (boscalide, epoxiconazole, and tebuconazole) [89]. However, due to the complex interactions between particular pesticides and soils and other confounding factors, there are no significant correlations between pesticide prevalence and soil properties [87].
The problem is aggravated by the formation and accumulation in the soil of not only CUPs, but also their TPs: for example, when atrazine was not detected, its TP, hydroxyatrazine, was often present in the tested soils, which may be explained by previous intensive use of the parent herbicide [88]. Concentrations of hydroxyatrazine usually exceed concentrations of atrazine and its other TPs in soils and sediments [90]. Hydroxyatrazine may be present in soils in significant concentrations for more than 20 years after cessation of use [91].
In natural media, some bacteria, fungi, and microalgae are able to degrade pesticides completely, without causing secondary environmental pollution, which often results from chemical and physical degradation, but microbial degradation may be ineffective [92]. Metribuzin is microbially degraded, and, thus, climatic conditions affect its decomposition: under cold conditions, it is degraded at a slower rate [93]. The persistence of metribuzin is determined by its rate of adsorption to soil particles, increasing in soils with a high organic matter content [94]. Pendimethalin is strongly adsorbed to soil organic matter and is not subjected to microbial degradation, but it is degraded by light in aquatic systems [95]. Metolachlor is subjected to the slow microbial and anaerobic degradation rates; due to its ability to leach through soil, metolachlor has the potential to contaminate groundwater [96,97].
Unintentional spills or accidents at chemical industries also release pollutants hazardous to humans that persist in the environment. A spill of pesticides from the pesticide factory at Wheathampstead, U.K., in the 1960s caused long-term contamination of the River Lee (Lea) with DDT and TPs [35]; improper storage of chemicals at the former pesticide factory in Loma del Gallo, El Salvador, Central America, caused the elevated levels of toxic compounds in samples of surface and ground waters [98]. Obsolete pesticides are still stored in developing regions of Asia, Latin America, and Africa [99].
4. Transfer and bioaccumulation, bioavailability of pesticides
Collateral effects of pesticides are associated with their ability to take part in biological processes. As pesticide residues are sequentially transferred from soil to plants and then to humans, they can be transformed and accumulated. Whether the pesticides will be removed from the human body without causing any significant damage to it or whether they will accumulate, producing long-term subclinical and clinical effects depends on the type of the pesticide and its interactions with the body at different levels – from molecular to organismic ones.
The uptake and translocation of toxic compounds from soil to plants is determined by the physicochemical properties of the pesticides [100] – such as pesticide mobility in soil, solubility, and dissipation of pesticides; abiotic factors – soil composition, pH, temperature, and moisture content; physiological properties of plants – plant transpiration rate, plant growth, cultivation techniques, varieties of fruits and vegetables [101]. It is necessary to take into account the solubility of pesticides, since this parameter can be of great importance for the transfer of pollutants. The presence of dissolved and particulate organic matter and the lipophilicity of compounds can significantly alter the bioavailability and overall toxicity of the CUPs. Therefore, widely known predictors of bioavailable fraction – the values of log KOC, log KOW, log KOA – are used to describe the behavior of pesticides.
Pesticides with a log KOW less than 3 (for example, triazines and carbamates) have low lipophilicity, and their bioavailability is weakly influenced by the impact of particles [102]. For example, neonicotinoids are readily soluble in water, which ensures their systemic effect (with an increase in solubility, the bioavailability of the pesticide increases to protect plants from pests) and removal from the environment. However, in dry soils with a high content of organic matter, the persistence of neonicotinoids in the soil increases significantly [103]. The most lipophilic CUPs (log KOW 5–7, log KOC more than 3.7), for example, pyrethroids, during interaction with organic matter, demonstrate a decrease in toxicity [102]. Pesticides with intermediate lipid solubility (log KOW values ranging from 3 to 5) and high octanol-air partitioning (log KOA values ranging from 7 to 11) are likely to have optimal parameters for bioaccumulation in marine and terrestrial food chains [13].
Also an important factor is soil composition: humic acids and colloidal clay can serve as adsorbents for certain chemicals [100,104]. Strong adsorption of pesticides onto soil particles may result in lower uptake of the pesticides by plants [101]. However, due to the complex interactions of pesticides with particles, as well as the different physicochemical properties of pesticides, predicting of bioavailability and bioaccumulation only based on KOW and KOC is insufficient for an accurate assessment.
Cultivation techniques contribute to an increase in bioconcentration of pesticides by plants. For instance, insecticides and fungicides are detected more frequently in tomatoes grown in greenhouses than in field-grown tomatoes. The reasons for this are that, on the one hand, the rates of application of agrochemicals in the greenhouse are higher because of different pesticide-use regimes or slower rates of pesticide removal [105] and, on the other, pesticides are removed from the open soil by large amounts of precipitation, especially in wet regions [106].
Some plant species accumulate greater amounts of pesticides in some of their organs, i.e. are prone to bioaccumulation, as supported by research evidence [107,108]. Leaf vegetables (spinach, lettuce, cabbage, etc.) accumulate greater concentrations of pesticides than root vegetables [75,109], exceeding maximum residue limits (MRLs) in comparative studies [110]. This may be caused by intensive photosynthesis and transpiration processes in plant edible parts and by their growing conditions: located close to the soil surface, they are attacked by insects more frequently, and, hence, larger amounts of pesticides are applied to protect them.
As pesticides move up the food chain, biomagnification occurs. Insignificant concentrations of pesticides enter into the food chain at a low trophic level, but they increase cumulatively at higher trophic levels [111].
A realistic scenario of the adverse effects of pollutants on organisms should be based on the accurate data on their bioavailability, toxicokinetics, uptake, distribution, metabolism, and excretion by the organism.
Bioaccumulation by the organism is the result of accumulation of pesticides and their TPs that the organism has received from different sources and that have accumulated in different organs. Pesticides belonging to the POPs and CUPs probably differ fundamentally in bioaccumulation: the highest concentrations of POPs are usually found in organisms of the highest trophic level (mammals) – thus, biomagnification of persistent pesticides is observed. The situation is different with CUPs, whose concentrations were the highest in invertebrates and decreased with increasing trophic level [13]. Such specific biomagnification with trophic dilution in food webs probably indicates the metabolization of these pesticides by non-target organisms [112].
Pesticides are usually distributed in the organism by binding with plasma proteins, blood cells, and lipids in different organs and peripheral tissues [113]. The strength of binding is determined by the lipophilicity of molecules. Thus, the data on the water solubility of toxicants provide the basis for estimating their bioaccumulation, biodegradation, hydrolysis, and adsorption [114]. The lipophilicity of pesticides increases both their effectiveness in pest control and their subsequent bioaccumulation [115]. The hydrophobicity and chemical structure of both the starting product and pesticide TPs play an important role [78].
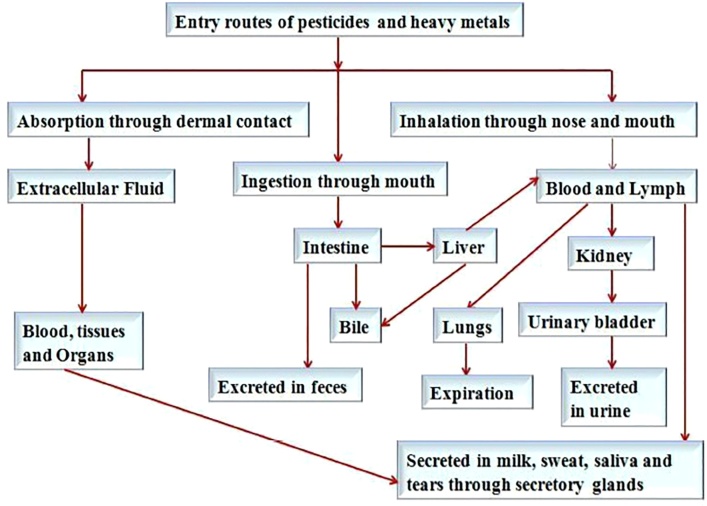
Pesticide (and heavy metal) transfer affects all systems in the human body, often resulting in bioaccumulation of toxic compounds in different organs (Fig. 2). Pesticides can be removed from the body via several routes, including urinary, biliary, and respiratory ones. CUPs are rapidly metabolized after entering the body and are mainly excreted in the urine as polar metabolites. This allows the body to get rid of dangerous compounds, but in some cases, the biotransformation products themselves can be bioaccumulative [13]. Chemical substances are effectively eliminated by the body through secretory glands. Residues of organochlorinated pesticides were more often detected in samples of sweat than in blood serum [116]. However, the most serious cause for concern is that pesticides can be transferred to the breast milk and then to the baby (Fig. 2).
Fig. 2.
Routes of uptake, distribution, and excretion associated with the exposure to heavy metals and pesticides in the humans. Reproduced from [117] with permission from Frontiers Media S.A. publisher.
Gestational exposure to pesticides, even at low concentrations, also causes concern. CUP bitertanol is rapidly metabolized after adsorption from the gastrointestinal tract, but it was detected in the amniotic fluid of orally exposed Wistar rats, which suggested potential risk to the fetus [118].
Pesticides undergo transformations in the body. Pesticide TPs may be more toxic and persistent than the initial pesticide [119]. For instance, more pronounced negative effects in comparison with the parent compound are shown for the main bioactive metabolites of glyphosate – aminomethylphosphonic acid (AMPA) and N-(phosphonomethyl)iminodiacetic acid (PMIDA) [40,120]; carbosulfan – carbofuran and 3-hydroxycarbofuran [121] and imidacloprid [18]. Moreover, the impact can be implicitly expressed. Despite the fact that the degradation products of glyphosate and atrazine – AMPA and hydroxyatrazine, respectively, – did not cause morphological and physiological changes in the model plant Arabidopsis thaliana, they induced clear metabolic and genetic effects, possibly through novel mechanisms of toxicity [122]. Although biotransformation is not the major process in pesticide toxicokinetics [123], it should be taken into account.
Bioaccumulation of pollutants during digestion is largely determined by the bioavailability of the substances [124], i.e. their ability to penetrate into the body and be distributed among the tissues [125]. Several studies reported that bioavailability of pollutants can vary depending on pH and the type of the food being digested [[126], [127], [128]]. Pesticide sedimentation rate is significantly affected by the fat content of food [126,127,129,130]. The diet rich in fish and other foods of animal origin increases the effect of POPs compared to the diet rich in foods of vegetable origin [131]. Digestion time influences the effectiveness of absorption as well [132].
A number of studies suggest a contribution of human membrane drug transporters to pesticide toxicokinetics in the body, as they can interact with different types of pesticides [133], but further studies are needed to understand their interaction mechanisms.
Pesticide bioavailability and, hence, hazard, may be increased by administration of antibiotics. The interaction between antibiotics and pesticides is mediated by the intestinal microbial community: the antibiotic-altered gut microbiota affects intestinal absorption of toxicants, decreasing metabolic enzyme gene expression [134]. Other factors such as diseases of the gastrointestinal tract, eating behavior, and unhealthy habits also alter microbiota, making it more susceptible to the effects of pesticides. For instance, the effect of organophosphates on gut microbiome was found to bring about neurotoxic damage [135]. Joint effects of several pesticides may alter toxicokinetics of individual compounds, thus changing the predicted toxicity [32]. In addition, the presence of one pesticide may affect the absorption rate and metabolism of another pesticide [136].
5. Health effects after pesticide exposure
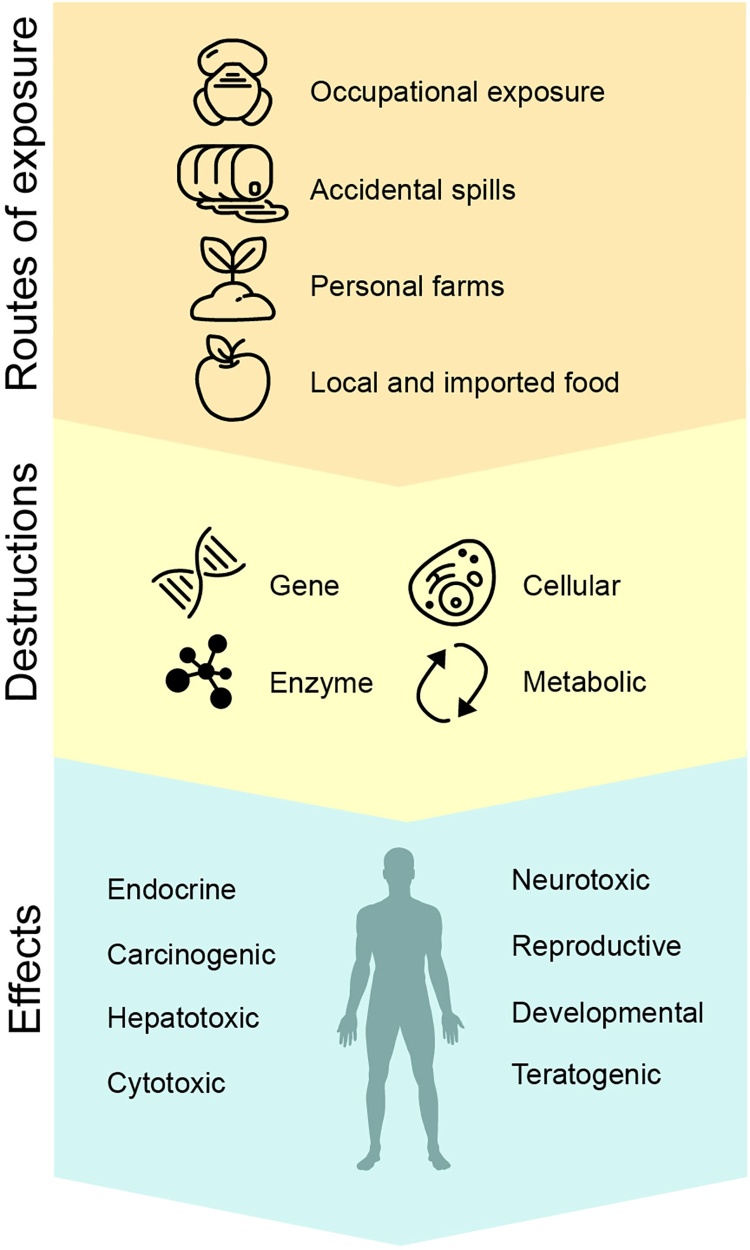
Pesticides can affect humans both directly and indirectly, via various routes (Fig. 3). Food, however, is the primary source of direct consumption of toxic substances by humans. Vegetables and fruits grown on contaminated agricultural soils accumulate pesticides in their edible and inedible parts in concentrations that are high enough to cause clinical problems in animals and humans [[137], [138], [139], [140]].
Fig. 3.
Routes of exposure to pesticides and potential effects on humans.
Pesticides penetrate the human body through skin, mouth, eyes, and respiratory system [17], and, thus, scientifically confirmed acute diseases associated with pesticides include headaches, stomachaches, vomiting, skin rash, respiratory disorders, eye irritation, sneezing, convulsions, and coma [141]. Direct exposure to pesticides may even cause death [142,143].
Oral exposure to pesticides is the key factor determining their toxicity [37,133]. Frequent consumption of food based on agricultural crops grown on soil with pesticides results in short-duration (acute) and long-duration (chronic) diseases and disorders [144,145]. Acute pesticide poisoning has now become a rare event, but long-term subclinical effects remain an issue. Chronic toxicity caused by long-duration exposure to low doses of pesticides can become evident much later [8]. Chronic diseases include cancer, asthma, dermatitis, endocrine disorders, reproductive dysfunctions, immunotoxicity, neurobehavioral disorders, and congenital defects [17,146]. Chronic disorders may result from disturbance of cellular homeostasis caused by the primary action of pesticides (disorders of enzymes, ionic channels, and receptors; morphological changes of mitochondria) [147] and accumulation of DNA damage [36].
For example, the relationship of pesticides and carcinogenesis is widely discussed in the scientific literature. A positive association between the risk of developing tumors and pesticide exposure has been found for glyphosate [148] and several other pesticides [149,150]. Environmental concentrations of neonicotinoids and pyrethroids could contribute to the genetic and molecular changes and potentially induce carcinogenic processes [151,152]. However, for some pesticides previously considered carcinogenic, a relationship with the risk of developing cancer in more recent studies is rarely established [153] or marked as contradictory [154].
Sometimes, under long-duration pesticide exposure, acute effects of toxic exposure can concur with subclinical symptoms. Both acute and chronic effects were identified in persons receiving occupational pesticide exposure, such as farm workers [40,155]. Long-term health effects of occupational exposure include reproductive disorders and congenital defects, which can be mediated via DNA damage. These data are supported by recent epidemiological studies involving farmers and rural workers [[156], [157], [158]].
In Fig. 3, we summed up the major groups of pesticide-related effects on humans. The literature data suggest that chronic effects include hepatotoxic, carcinogenic, cytotoxic, teratogenic, neurotoxic, reproductive, and endocrine disorders [3,17,18,32,36,131,135,146,147,[159], [160], [161]]. These effects, however, are underpinned by numerous disorders in biochemical reactions of the body.
A number of studies suggested that certain pesticides as well as other synthetic chemicals can be endocrine disruptors and function as pseudo-hormones [32,[159], [160], [161], [162]]. The endocrine-disruptive effect was shown for more than one hundred pesticides of different classes and with different modes of action [99], e.g., fipronil, ziram, zineb, pyrimethanil, thiazopyr [160], vinclozolin, dicofol, atrazine [111] – active ingredients of pesticides in different functional groups.
The endocrine-disruptive properties of pesticides are often determined by their ability to disrupt hormonal signaling mediated by nuclear hormone receptors due to changes in their transcriptional activity [149]. The study by [163] showed that a large number of pesticides with various structures and mechanisms of action can act as agonists of human pregnane X receptor (hPXR). Moreover, in a recent review [162] other mechanisms that mediate the negative effects of endocrine disrupting pesticides are also considered; among the main ones are interactions with membrane-associated receptors and ion-channels, suppression of key signaling pathway in cells, DNA methylation and histone modifications. Thus, pesticides are potentially capable of disrupting the complex hormonal regulation, causing a cascade of disturbances: for example, atrazine [164], cypermethrin [165] and ziram [166] could alter the levels of steroidogenic enzymes associated with reproductive functions.
In addition, pesticide TPs are able to exhibit stronger endocrine disrupting effects than their corresponding initial pesticides; this is also based on changes in gene expression and hormonal secretion [167]. Since endocrine disrupting chemicals affect epigenetic marks such as DNA methylation and histone modifications, they have the potential to cause chronic effects associated with alterations in epigenome and pass them on to future generations [168].
Positive correlation was revealed between pesticide effects and thyroid disorders: a case–control study established that in regions with increased long-duration pesticide use, the incidence and the risk of thyroid diseases were considerably higher [169]. Systemic toxicity for thyroid and liver was reported in other studies [158,170]. In some cases, formulants rather than active ingredients of pesticides exhibit endocrine disrupting properties [26,69]. However, it is important to keep in mind that the effects of pesticides on human thyroid function are still limited. The mechanisms underlying thyroid toxicity caused by realistic environmental levels of pesticides are complicated, and the sensitivity to thyroid hormone disturbances differs between species [171]. Therefore, thorough research is needed to correctly assess the risk.
One of the less obvious effects of pesticides is their effect on the gut microbiome of non-target organisms. There are comprehensive reviews [172,173] that discuss a possible antimicrobial capability of various types of pesticides against select groups of beneficial bacteria in the microbiome. Mesnage et al. [174] showed that glyphosate could potentially block the shikimate pathway of gastrointestinal microorganisms in rodents. Studies have been conducted on representatives of various taxonomic groups. Direct or indirect effects of administration of pesticide formulations on the gut microbiome were mainly detected in rats and mice, but there are data on the effects of pesticides on the gut microbiome of worms [175], pigs [176], fish [177], and honey bees. For example, glyphosate affects susceptibility of the gut microbiome of honey bees to pathogens, making them more vulnerable to infections [178].
We cannot leave unmentioned the well-known adverse effects of highly toxic persistent pesticides. For instance, chlordecone mostly accumulates in liver, disrupting its function [179]; lindane and endosulfan are associated with the damage to the reproductive system [180]; carbofuran can disrupt the function of acetylcholinesterase, penetrating through the blood-brain barrier [181].
5.1. Pesticide effects at the cellular and molecular levels
Toxic effects of pesticides are based on their ability to trigger the processes that result in damage at the cellular and molecular levels. Pesticides change enzymatic reaction rate, affecting the activities of various enzymes such as superoxide dismutase, catalase and glutathione peroxidase [182], alanine transaminase and aspartate transaminase, alkaline phosphatase [18,183,184], lactate dehydrogenase [111], whose elevated levels in cells are indicative of the toxic effects on the organism. Carbamate pesticides inhibit the function of acetylcholinesterase, and this can serve as a biomarker of neurotoxicity [181]. Pesticides are capable of inhibiting activity of carboxylesterases – enzymes responsible for detoxification [32]. As enzymes are highly sensitive to the side effects of pesticides, they are widely used in assays for selective and integrated detection of residual commercial formulations in various natural environments [185,186].
Some pesticides can significantly decrease activity of NADH-dehydrogenase – the main enzyme of the mitochondrial electron transport chain. Impairment of NADH-dehydrogenase activity caused by chlorpyrifos may mediate oxidative stress and neurotoxicity [147]. Moreover, pesticides are able to induce generation of reactive oxygen species (ROS) [182] and reactive nitrogen species (RNS) in cells, which ultimately leads to oxidative stress and damage to cell structures. Enhanced production of ROS/RNS in mammals during metabolism and biotransformation of toxic substances is the cause of hepatotoxicity [18]. The ability of ROS to interact with macromolecules of cells mediates inactivation of enzymes and DNA damage, which can finally result in cell necrosis or apoptosis [111].
A study of the effect of glyphosate and its main metabolite AMPA on the DNA molecule revealed damage to single- and double-stranded DNA, which most likely occurred through ROS-mediated effects [187]. A similar experiment, carried out to estimate the effects of low concentrations of pesticide mixtures, showed that DNA damage was mediated by mitochondrial dysfunction, causing ROS production. Accumulation of DNA damage finally resulted in inhibition of repair activity of enzymes [36]. Frequently used pesticides such as diazinon and malathion exhibited an ability to change gene promoter DNA methylation levels in the in vitro experiments, inducing carcinogenesis [188]. DNA methylation in patients with Parkinson’s disease is also associated with the chronic exposure to low concentrations of organophosphates [189]. The molecular mechanisms that determine the ability of pesticides to influence DNA and potentially explain the long-term effects are intricate and still under investigation. A study of atrazine showed that they could be associated with the ability of that pesticide to alter DNA methylation by affecting epigenetic enzyme expression levels [190].
Toxic effects of pesticides on RNA should be also evaluated. The article [191] reports a study of the effects of various herbicides on the transcriptome in the HepaRG human liver cell line. Genes associated with fatty acid metabolic pathways were found to be affected. In addition, the authors of that study noted a possible non-linear dose response in pesticide effects on non-target organisms, which indicated the complexity of metabolic processes underlying their toxicity.
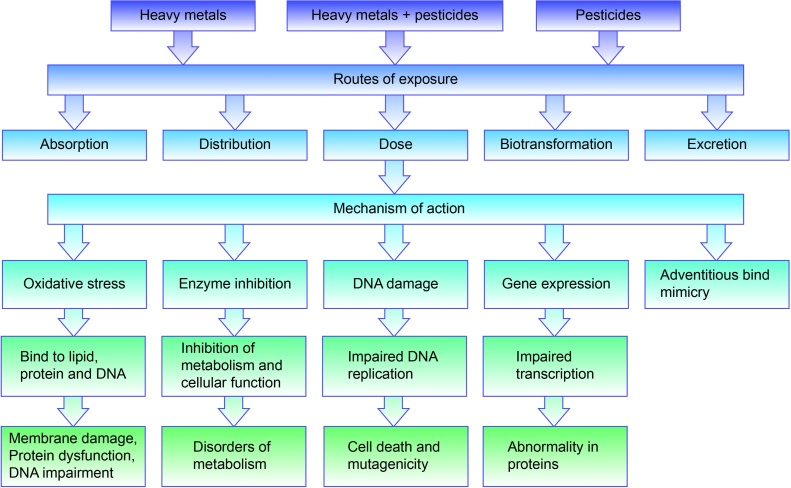
Thus, pesticides alone and together with heavy metals are able to damage the organism severely, through mediated processes and mechanisms. Damages at the molecular and cellular levels tend to accumulate, exhibiting subclinical effects for long stretches of time. Impaired enzyme functions, DNA expression, damaged membranes taken together (Fig. 4) will finally cause inevitable disturbance in metabolism and functions of systems of organs. Eventually, this may cause diseases and disorders described above.
Fig. 4.
Routes of exposure and mechanisms of action of heavy metals and pesticides. Reproduced from [117] with permission from Frontiers Media S.A. publisher.
5.2. High-risk groups
Although toxic pollutants of food may be hazardous to the health of people of all ages, they present the greatest hazard to children – the most sensitive population [17,138]. Children are disproportionally impacted because they are still developing and consume greater amounts of food and fluids relative to their bodyweight [131]. Long-term effects of pesticides on this group of population include cancer, asthma, neurobehavioral disorders, learning and developmental disabilities, and congenital defects.
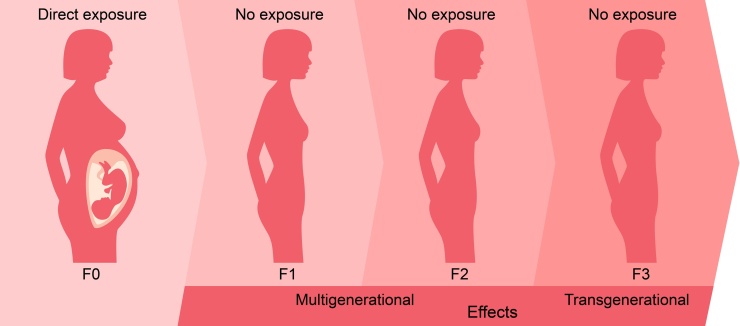
Experiment carried out by a team of U.S. scientists showed that school and preschool children could receive considerable amounts of pesticides with their daily food [192]. The data obtained in experiments with animals also demonstrated that pesticides could cause transgenerational epigenetic changes (Fig. 5). Altered epigenetic markers were even detected in the third-generation rats [137,193]. Data supporting the possibility of epigenetic transgenerational inheritance were also reported in other studies: in the case of pesticide mixture containing permethrin and N,N-Diethyl-meta-toluamide [194] and atrazine [195], effects of pesticides on gestating rats resulted in an increase in anomalies and diseases in F3 generation. Of course, more in-depth research, primarily epidemiological, is needed to establish mechanisms and cause-effect relationships. However, the already available data suggest the conclusion that epigenetic alterations can potentially mediate pesticide toxicity to human health [196].
Fig. 5.
Multi- and transgenerational effects of pesticides.
Similar damage can be done to organisms other than human. Although pesticides are impressively diverse and have selective modes of action, they may sometimes cause disturbance of metabolic functions and immunotoxicity in non-target organisms [99]. One of the implicit consequences of pesticide contamination in individuals is a long-term negative effect on populations and even ecosystems. A study by Köhler and Triebskorn [99] showed that even insignificant endocrine and metabolic disruptions in individuals could result in tremendous changes of the entire ecosystem.
Negative effects of commonly used pesticides are detected in non-target species at all levels of the food chain: plants [197], relict plants [198], non-target insects, including honey bees [11], and aquatic organisms [199].
In recent years, numerous data have been collected on harmful effects of modern pesticides such as pyrethroids and neonicotinoid insecticides, which were previously considered as safe to non-target organisms. Although these substances are more selective, they can be toxic to other plants and animals [200]. Negative effects of neonicotinoid insecticides on honey bees have been reported in many studies. For instance, imidacloprid produced a harmful effect on young bees, thus affecting the entire colony [11]. Another example: in summer 2019, in several regions of Central Russia, about 40 000 bee colonies (tens of millions of bees) were killed by neonicotinoids such as the widely used imidacloprid and clothianidin.
The indirect effects and biomagnification of pesticides in food chains result in unpredictable negative consequences. As neonicotinoid insecticides, e.g. imidacloprid, damage non-target invertebrates, the staple food for birds, bird population decreases year by year [201]. In recent years, imidacloprid has been associated with direct negative effects on large vertebrates such as white-tailed deer [10] and a number of damaging effects on reproductive function of mammals [202].
The death of useful organisms often causes severe and almost irreparable disruptions in interactions in ecosystems, involving the loss of biodiversity. As pesticides are used everywhere, their effects are detected even in the non-agricultural species such as seals, Polar bears [203], and sea snails [199]. Authors of [204] propose non-chemical methods for pest management as an alternative to pesticides, which threaten non-target species, but further research is needed to develop and use these methods.
6. Conclusion and future outlook
Development, use, disposal, and storage of pesticides still remain a concern. Although modern agrochemical companies are committed to developing safe formulations to avoid persistent and bioaccumulative properties of substances, it cannot be denied that it is impossible to anticipate and prevent all potential negative effects of pesticides on human health and on whole ecosystems. CUPs show long-range transport potential and are found in air, water, and sediments in remote areas. Even modern pesticides with an allegedly short half-life under certain conditions can persist in soil for up to several years, becoming a source of potential risk for soil invertebrates [103]. In pursuit of safety, alternative strategies (for example, the development of biopesticides) can lead to weakening of toxic properties and faster development of resistance to pesticides and pesticide mixtures in target species [205], which will likely result in more intensive use of those pesticides to achieve efficient crop production.
Current trends in agriculture and monitoring tend to reduce the harmful impacts of pesticides on humans and biota. Pesticide formulations become less dangerous and persistent, and innovative strategies are proposed for their accurate delivery to target species [206].
The ecosystem-based strategy of Integrated Pest Management (IPM) in the framework of the Concept of Sustainable Agriculture [207] focuses on long-term prevention of pests through a combination of techniques among which are, for example, biological control and use of resistant varieties [208]. The strategy promotes an environmental approach to pest control allowing reduction in the amounts of pesticides used.
Monitoring the use of pesticides is becoming more conscious and rigorous. A new model based on Mathematical Programming-based Multi-Agent System (MPMAS), simulation software that allows assessing ex-ante the impact of alternative pesticide use reduction strategies, has shown reducing average use of pesticides by 34 % over current levels without adverse effects on the average farm income [209].
To maintain a predetermined concentration of pesticide for a sufficient time, controlled release systems (CRS) of pesticides are proposed. They enable relocation of an active ingredient from entrapped compartments to a targeted surface, thus reducing unwanted losses of pesticides due to various factors and achieving the desired impact on the target pests [210].
As the concern about the hidden hazards posed by formulants as components of pesticide formulations is growing, interactions between ingredients should be studied in a more systematic way. Many manufacturers of pesticides do not disclose their composition, thus, making it impossible to assess accurately the effects of potentially toxic chemicals [39,69]. Authorization to commercialize the pesticide does not usually imply assessment of potential risks of additives, impurities, and formulants, which are included in the pesticide product [114]. For example, the fact that formulants may contribute to transdermal penetration of the active ingredient into non-target organisms is the reason for taking greater precautions when handling pesticides. Inert ingredients increase persistence of the product in the environment, which may be underestimated.
To reduce the proportion of hazardous formulants in pesticide products, new technologies are being actively developed. For example, nanocarriers also reduce pesticide losses, preventing premature release, and increase the accuracy of delivery of active ingredients due to enhanced affinity to the target pest species [211,212]. Moreover, pesticide nanocarriers are biodegradable.
An important aspect of ensuring safety of agricultural products to consumers is government regulations. Although developed countries have established complex systems for pesticide approval and controlling pesticide sale and use, other countries do not always follow the same strict rules [138,139,213]. Preventive measures include testing of the safety of formulants for non-target species, based on complete and reliable data on their effects, performing mandatory assessment of chronic effects of adjuvants, and enhancing predictive power of in silico methods in toxicology. The main preventive measure, however, is correct and justified application of chemicals in cultivation of crops: in the context of the increasing environmental awareness and decreasing impact on ecosystems and the Earth, judicious use of pesticides is the imperative of our time.
Funding
The research was funding by the Government of Krasnoyarsk Territory, Krasnoyarsk Regional Fund of Science and Russian Foundation for Basic Research (project No 20-44-242001).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Dr. Aristides M. Tsatsakis
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2021.06.004.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.European Commission; 2016. EU Pesticides Database.https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN (accessed November 16, 2019) [Google Scholar]
- 2.Wei J.C., Wei B., Yang W., He C.W., Su H.X., Wan J.B., Li P., Wang Y.T. Trace determination of carbamate pesticides in medicinal plants by a fluorescent technique. Food Chem. Toxicol. 2018;119:430–437. doi: 10.1016/j.fct.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Thompson L.A., Darwish W.S., Ikenaka Y., Nakayama S.M.M., Mizukawa H., Ishizuka M. Organochlorine pesticide contamination of foods in Africa: incidence and public health significance. J. Vet. Med. Sci. 2017;79:751–764. doi: 10.1292/jvms.16-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.2019. Stockholm Convention.http://pops.int/ (accessed November 16, 2019) [Google Scholar]
- 5.Zhang A., Luo W., Sun J., Xiao H., Liu W. Distribution and uptake pathways of organochlorine pesticides in greenhouse and conventional vegetables. Sci. Total Environ. 2015;505:1142–1147. doi: 10.1016/j.scitotenv.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 6.Fosu P.O., Donkor A., Ziwu C., Dubey B., Kingsford-Adaboh R., Asante I., Nyarko S., Tawiah R., Nazzah N. Surveillance of pesticide residues in fruits and vegetables from Accra Metropolis markets, Ghana, 2010–2012: a case study in Sub-Saharan Africa. Environ. Sci. Pollut. Res. 2017;24:17187–17205. doi: 10.1007/s11356-017-9287-8. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez-Vargas G., Sosa-Hernández J.E., Saldarriaga-Hernandez S., Villalba-Rodríguez A.M., Parra-Saldivar R., Iqbal H.M.N. Electrochemical biosensors: a solution to pollution detection with reference to environmental contaminants. Biosensors. 2018;8 doi: 10.3390/bios8020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capoferri D., Della Pelle F., Del Carlo M., Compagnone D. Affinity sensing strategies for the detection of pesticides in food. Foods. 2018;7:1–48. doi: 10.3390/foods7090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cimino A.M., Boyles A.L., Thayer K.A., Perry M.J. Effects of neonicotinoid pesticide exposure on human health: a systematic review. Environ. Health Perspect. 2017;125:155–162. doi: 10.1289/EHP515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berheim E.H., Jenks J.A., Lundgren J.G., Michel E.S., Grove D., Jensen W.F. Effects of neonicotinoid insecticides on physiology and reproductive characteristics of captive female and fawn white-tailed deer. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-40994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonalons C.M., Farina W.M. Impaired associative learning after chronic exposure to pesticides in young adult honey bees. J. Exp. Biol. 2018;221 doi: 10.1242/jeb.176644. [DOI] [PubMed] [Google Scholar]
- 12.Zhong G., Xie Z., Cai M., Möller A., Sturm R., Tang J., Zhang G., He J., Ebinghaus R. Distribution and air-sea exchange of current-use pesticides (CUPs) from East Asia to the high Arctic Ocean. Environ. Sci. Technol. 2012;46:259–267. doi: 10.1021/es202655k. [DOI] [PubMed] [Google Scholar]
- 13.Balmer J.E., Morris A.D., Hung H., Jantunen L., Vorkamp K., Rigét F., Evans M., Houde M., Muir D.C.G. Levels and trends of current-use pesticides (CUPs) in the arctic: an updated review, 2010–2018. Emerg. Contam. 2019;5:70–88. doi: 10.1016/j.emcon.2019.02.002. [DOI] [Google Scholar]
- 14.Geissen V., Mol H., Klumpp E., Umlauf G., Nadal M., van der Ploeg M., van de Zee S.E.A.T.M., Ritsema C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015;3:57–65. doi: 10.1016/j.iswcr.2015.03.002. [DOI] [Google Scholar]
- 15.Thomaidis N.S., Asimakopoulos A.G., Bletsou A.A. Emerging contaminants: a tutorial mini-review. Glob. Nest J. 2012;14:72–79. doi: 10.30955/gnj.000823. [DOI] [Google Scholar]
- 16.Guida YdeS., Meire R.O., Torres J.P.M., Malm O. Air contamination by legacy and current-use pesticides in Brazilian mountains: an overview of national regulations by monitoring pollutant presence in pristine areas. Environ. Pollut. 2018;242:19–30. doi: 10.1016/j.envpol.2018.06.061. [DOI] [PubMed] [Google Scholar]
- 17.Kim K.H., Kabir E., Jahan S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017;575:525–535. doi: 10.1016/j.scitotenv.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Mahajan L., Verma P.K., Raina R., Sood S. Toxic effects of imidacloprid combined with arsenic: oxidative stress in rat liver. Toxicol. Ind. Health. 2018;34:726–735. doi: 10.1177/0748233718778993. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-Suárez N., Boada L.D., Henríquez-Hernández L.A., González-Moreo F., Suárez-Pérez A., Camacho M., Zumbado M., Almeida-González M., del Mar Travieso-Aja M., Luzardo O.P. Continued implication of the banned pesticides carbofuran and aldicarb in the poisoning of domestic and wild animals of the Canary Islands (Spain) Sci. Total Environ. 2015;505:1093–1099. doi: 10.1016/j.scitotenv.2014.10.093. [DOI] [PubMed] [Google Scholar]
- 20.Wagner N., Reichenbecher W., Teichmann H., Tappeser B., Lötters S. Questions concerning the potential impact of glyphosate-based herbicides on amphibians. Environ. Toxicol. Chem. 2013;32:1688–1700. doi: 10.1002/etc.2268. [DOI] [PubMed] [Google Scholar]
- 21.Plaza P.I., Martínez-López E., Lambertucci S.A. The perfect threat: pesticides and vultures. Sci. Total Environ. 2019;687:1207–1218. doi: 10.1016/j.scitotenv.2019.06.160. [DOI] [PubMed] [Google Scholar]
- 22.Queirós L., Vidal T., Nogueira A.J.A., Gonçalves F.J.M., Pereira J.L. Ecotoxicological assessment of the herbicide Winner Top and its active substances—are the other formulants truly inert? Ecotoxicology. 2018;27:945–955. doi: 10.1007/s10646-018-1939-z. [DOI] [PubMed] [Google Scholar]
- 23.Vanlaeys A., Dubuisson F., Seralini G.E., Travert C. Formulants of glyphosate-based herbicides have more deleterious impact than glyphosate on TM4 Sertoli cells. Toxicol. In Vitro. 2018;52:14–22. doi: 10.1016/j.tiv.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Muntz H., Miller R., Alston D. 2016. Understanding Pesticide Risks: Toxicity and Formulation.http://npic.orst.edu/factsheets/formulations.pdf (accessed March 24, 2021) [Google Scholar]
- 25.Stevanovic M., Gasic S., Pipal M., Blahova L., Brkic D., Neskovic N., Hilscherova K. Toxicity of clomazone and its formulations to zebrafish embryos (Danio rerio) Aquat. Toxicol. 2017;188:54–63. doi: 10.1016/j.aquatox.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Defarge N., Spiroux de Vendômois J., Séralini G.E. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol. Reports. 2018;5:156–163. doi: 10.1016/j.toxrep.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beggel S., Werner I., Connon R.E., Geist J.P. Sublethal toxicity of commercial insecticide formulations and their active ingredients to larval fathead minnow (Pimephales promelas) Sci. Total Environ. 2010;408:3169–3175. doi: 10.1016/j.scitotenv.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Gomes S.I.L., Ammendola A., Casini S., Amorim M.J.B. Toxicity of fungicides to terrestrial non-target fauna – formulated products versus active ingredients (azoxystrobin, cyproconazole, prothioconazole, tebuconazole) – a case study with Enchytraeus crypticus (Oligochaeta) Sci. Total Environ. 2021;754:142098. doi: 10.1016/j.scitotenv.2020.142098. [DOI] [PubMed] [Google Scholar]
- 29.Takács E., Klátyik S., Mörtl M., Rácz G., Kovács K., Darvas B., Székács A. Effects of neonicotinoid insecticide formulations and their components on Daphnia magna–the role of active ingredients and co-formulants. Int. J. Environ. Anal. Chem. 2017;97:885–900. doi: 10.1080/03067319.2017.1363196. [DOI] [Google Scholar]
- 30.Cossi P.F., Herbert L.T., Yusseppone M.S., Pérez A.F., Kristoff G. Toxicity evaluation of the active ingredient acetamiprid and a commercial formulation (Assail® 70) on the non-target gastropod Biomphalaria straminea (Mollusca: planorbidae) Ecotoxicol. Environ. Saf. 2020;192:110248. doi: 10.1016/j.ecoenv.2020.110248. [DOI] [PubMed] [Google Scholar]
- 31.Silins I., Högberg J. Combined toxic exposures and human health: biomarkers of exposure and effect. Int. J. Environ. Res. Public Health. 2011;8:629–647. doi: 10.3390/ijerph8030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernández A.F., Parrón T., Tsatsakis A.M., Requena M., Alarcón R., López-Guarnido O. Toxic effects of pesticide mixtures at a molecular level: their relevance to human health. Toxicology. 2013;307:136–145. doi: 10.1016/j.tox.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Leist M., Ghallab A., Graepel R., Marchan R., Hassan R., Bennekou S.H., Limonciel A., Vinken M., Schildknecht S., Waldmann T., Danen E. Adverse outcome pathways: opportunities, limitations and open questions. Arch. Toxicol. 2017;91:3477–3505. doi: 10.1007/s00204-017-2045-3. [DOI] [PubMed] [Google Scholar]
- 34.Ankley G.T., Bennett R.S., Erickson R.J., Hoff D.J., Hornung M.W., Johnson R.D., Mount D.R., Nichols J.W., Russom C.L., Schmieder P.K., Serrrano J.A., Tietge J.E., Villeneuve D.L. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- 35.Jürgens M.D., Crosse J., Hamilton P.B., Johnson A.C., Jones K.C. The long shadow of our chemical past – high DDT concentrations in fish near a former agrochemicals factory in England. Chemosphere. 2016;162:333–344. doi: 10.1016/j.chemosphere.2016.07.078. [DOI] [PubMed] [Google Scholar]
- 36.Alleva R., Manzella N., Gaetani S., Bacchetti T., Bracci M., Ciarapica V., Monaco F., Borghi B., Amati M., Ferretti G., Tomasetti M. Mechanism underlying the effect of long-term exposure to low dose of pesticides on DNA integrity. Environ. Toxicol. 2018;33:476–487. doi: 10.1002/tox.22534. [DOI] [PubMed] [Google Scholar]
- 37.Quijano L., Yusà V., Font G., Pardo O. Chronic cumulative risk assessment of the exposure to organophosphorus, carbamate and pyrethroid and pyrethrin pesticides through fruit and vegetables consumption in the region of Valencia (Spain) Food Chem. Toxicol. 2016;89:39–46. doi: 10.1016/j.fct.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Woodrow J.E., Gibson K.A., Seiber J.N. Rev. Environ. Contam. Toxicol. Springer; New York LLC: 2019. Pesticides and related toxicants in the atmosphere; pp. 147–196. [DOI] [PubMed] [Google Scholar]
- 39.Castro M.J.L., Ojeda C., Cirelli A.F. Advances in surfactants for agrochemicals. Environ. Chem. Lett. 2014;12:85–95. doi: 10.1007/s10311-013-0432-4. [DOI] [Google Scholar]
- 40.Vandenberg L.N., Blumberg B., Antoniou M.N., Benbrook C.M., Carroll L., Colborn T., Everett L.G., Hansen M., Landrigan P.J., Lanphear B.P., Mesnage R., vom Saal F.S., Welshons W.V., Myers J.P. Is it time to reassess current safety standards for glyphosate-based herbicides? J. Epidemiol. Community Health. 2017;71:613–618. doi: 10.1136/jech-2016-208463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mullin C.A. Effects of “inactive” ingredients on bees. Curr. Opin. Insect Sci. 2015;10:194–200. doi: 10.1016/j.cois.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Kral A.E., Pflug N.C., McFadden M.E., LeFevre G.H., Sivey J.D., Cwiertny D.M. Photochemical transformations of dichloroacetamide safeners. Environ. Sci. Technol. 2019;53:6738–6746. doi: 10.1021/acs.est.9b00861. [DOI] [PubMed] [Google Scholar]
- 43.Kucharski M., Sadowski J. Behaviour of metazachlor applied with additives in soil: laboratory and field studies. J. Food, Agric. Environ. 2011;9:723–726. https://www.cabdirect.org/cabdirect/abstract/20113407274 (accessed February 15, 2021) [Google Scholar]
- 44.Guyton K.Z., Loomis D., Grosse Y., El Ghissassi F., Benbrahim-Tallaa L., Guha N., Scoccianti C., Mattock H., Straif K., Blair A., Fritschi L., McLaughlin J., Sergi C.M., Calaf G.M., Le Curieux F., Baldi I., Forastiere F., Kromhout H., ’t Mannetje A., Rodriguez T., Egeghy P., Jahnke G.D., Jameson C.W., Martin M.T., Ross M.K., Rusyn I., Zeise L. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015;16:490–491. doi: 10.1016/S1470-2045(15)70134-8. [DOI] [PubMed] [Google Scholar]
- 45.Armbrust K.L., Peeler H.B. Effects of formulation on the run-off of imidacloprid from turf. Pest Manag. Sci. 2002;58:702–706. doi: 10.1002/ps.518. [DOI] [PubMed] [Google Scholar]
- 46.BAuA . 2016. CLH Report Proposal for Harmonised Classification and Labelling Based on Regulation (EC) No 1272/2008 (CLP Regulation), Annex VI, Part 2 CLH REPORT FOR GLYPHOSATE 2 CONTENTS. [Google Scholar]
- 47.Mesnage R., Benbrook C., Antoniou M.N. Insight into the confusion over surfactant co-formulants in glyphosate-based herbicides. Food Chem. Toxicol. 2019;128:137–145. doi: 10.1016/j.fct.2019.03.053. [DOI] [PubMed] [Google Scholar]
- 48.Puglis H.J., Boone M.D. Effects of technical-grade active ingredient vs. Commercial formulation of seven pesticides in the presence or absence of UV radiation on survival of green frog tadpoles. Arch. Environ. Contam. Toxicol. 2011;60:145–155. doi: 10.1007/s00244-010-9528-z. [DOI] [PubMed] [Google Scholar]
- 49.Mörtl M., Takács E., Klátyik S., Székács A. Aquatic toxicity and loss of linear alkylbenzenesulfonates alone and in a neonicotinoid insecticide formulation in surface water. Sci. Total Environ. 2019;652:780–787. doi: 10.1016/j.scitotenv.2018.10.211. [DOI] [PubMed] [Google Scholar]
- 50.van de Merwe J.P., Neale P.A., Melvin S.D., Leusch F.D.L. In vitro bioassays reveal that additives are significant contributors to the toxicity of commercial household pesticides. Aquat. Toxicol. 2018;199:263–268. doi: 10.1016/j.aquatox.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 51.Tadei R., Menezes-Oliveira V.B., Silva-Zacarin E.C.M. Silent effect of the fungicide pyraclostrobin on the larval exposure of the non-target organism Africanized Apis mellifera and its interaction with the pathogen Nosema ceranae in adulthood. Environ. Pollut. 2020;267:115622. doi: 10.1016/j.envpol.2020.115622. [DOI] [PubMed] [Google Scholar]
- 52.Liu S., Deng X., Zhou X., Bai L. Assessing the toxicity of three “inert” herbicide safeners toward Danio rerio: effects on embryos development. Ecotoxicol. Environ. Saf. 2021;207:111576. doi: 10.1016/j.ecoenv.2020.111576. [DOI] [PubMed] [Google Scholar]
- 53.Mullin C.A., Fine J.D., Reynolds R.D., Frazier M.T. Toxicological risks of agrochemical spray adjuvants: organosilicone surfactants may not Be safe. Front. Public Health Serv. Syst. Res. 2016;4:92. doi: 10.3389/fpubh.2016.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu T.-X., Stansly P.A. Insecticidal activity of surfactants and oils against silverleaf whitefly (Bemisia argentifolii) nymphs (Homoptera: aleyrodidae) on collards and tomato. Pest Manag. Sci. 2000;56:861–866. [Google Scholar]
- 55.Mesnage R., Bernay B., Séralini G.E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology. 2013;313:122–128. doi: 10.1016/j.tox.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Cox C., Surgan M. Unidentified inert ingredients in pesticides: implications for human and environmental health. Environ. Health Perspect. 2006;114:1803–1806. doi: 10.1289/ehp.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.European Commission . 2020. Guidelines on Active Substances and Plant Protection Products.https://ec.europa.eu/food/plant/pesticides/approval_active_substances/guidance_documents_en (accessed October 18, 2020) [Google Scholar]
- 58.US EPA . 2020. Data Requirements for Pesticide Registration.https://www.epa.gov/pesticide-registration/data-requirements-pesticide-registration (accessed October 18, 2020) [Google Scholar]
- 59.Nerozzi C., Recuero S., Galeati G., Bucci D., Spinaci M., Yeste M. Effects of Roundup and its main component, glyphosate, upon mammalian sperm function and survival. Sci. Rep. 2020;10:11026. doi: 10.1038/s41598-020-67538-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Defarge N., Takács E., Lozano V.L., Mesnage R., de Vendômois J.S., Séralini G.E., Székács A. Co-formulants in glyphosate-based herbicides disrupt aromatase activity in human cells below toxic levels. Int. J. Environ. Res. Public Health. 2016;13:264. doi: 10.3390/ijerph13030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mesnage R., Defarge N., Spiroux De Vendômois J., Séralini G.E. Major pesticides are more toxic to human cells than their declared active principles. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/179691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brand R.M. Transdermal penetration of atrazine, Alachlor, and trifluralin: effect of formulation. Toxicol. Sci. 2002;68:18–23. doi: 10.1093/toxsci/68.1.18. [DOI] [PubMed] [Google Scholar]
- 63.Nagy K., Duca R.C., Lovas S., Creta M., Scheepers P.T.J., Godderis L., Ádám B. Systematic review of comparative studies assessing the toxicity of pesticide active ingredients and their product formulations. Environ. Res. 2020;181:108926. doi: 10.1016/j.envres.2019.108926. [DOI] [PubMed] [Google Scholar]
- 64.Holmgaard R., Nielsen J.B. Dermal absorption of pesticides – evaluation of variability and prevention. Danish Environ. Prot. Agency. 2009;116 https://www2.mst.dk/udgiv/publications/2009/978-87-7052-980-8/pdf/978-87-7052-981-5.pdf (accessed February 17, 2021) [Google Scholar]
- 65.Langrand J., Blanc-Brisset I., Boucaud-Maitre D., Puskarczyk E., Nisse P., Garnier R., Pulce C. Increased severity associated with tallowamine in acute glyphosate poisoning. Clin. Toxicol. 2020;58:201–203. doi: 10.1080/15563650.2019.1623406. [DOI] [PubMed] [Google Scholar]
- 66.Weinhold B. Mystery in a bottle: will the EPA require public disclosure of inert pesticide ingredients? Environ. Health Perspect. 2010;118:A168. doi: 10.1289/ehp.118-a168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.US EPA . 2019. Basic Information About Pesticide Ingredients.https://www.epa.gov/ingredients-used-pesticide-products/basic-information-about-pesticide-ingredients (accessed April 2, 2020) [Google Scholar]
- 68.Mesnage R., Antoniou M.N. Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Front. Public Health Serv. Syst. Res. 2018;5:1. doi: 10.3389/fpubh.2017.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mesnage R., Defarge N., Spiroux de Vendômois J., Séralini G.E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015;84:133–153. doi: 10.1016/j.fct.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 70.Qu C., Albanese S., Lima A., Hope D., Pond P., Fortelli A., Romano N., Cerino P., Pizzolante A., De Vivo B. The occurrence of OCPs, PCBs, and PAHs in the soil, air, and bulk deposition of the Naples metropolitan area, southern Italy: implications for sources and environmental processes. Environ. Int. 2019:89–97. doi: 10.1016/j.envint.2018.12.031. [DOI] [PubMed] [Google Scholar]
- 71.Toumi H., Burga-Perez K.F., Ferard J.F. Acute and chronic ecotoxicity of carbaryl with a battery of aquatic bioassays. J. Environ. Sci. Heal. - Part B Pestic. Food Contam. Agric. Wastes. 2016;51:57–62. doi: 10.1080/03601234.2015.1080500. [DOI] [PubMed] [Google Scholar]
- 72.Socorro J., Durand A., Temime-Roussel B., Gligorovski S., Wortham H., Quivet E. The persistence of pesticides in atmospheric particulate phase: an emerging air quality issue. Sci. Rep. 2016;6:1–7. doi: 10.1038/srep33456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuhrimann S., Klánová J., Přibylová P., Kohoutek J., Dalvie M.A., Röösli M., Degrendele C. Qualitative assessment of 27 current-use pesticides in air at 20 sampling sites across Africa. Chemosphere. 2020;258:127333. doi: 10.1016/j.chemosphere.2020.127333. [DOI] [PubMed] [Google Scholar]
- 74.Liu L., Tang J., Zhong G., Zhen X., Pan X., Tian C. Spatial distribution and seasonal variation of four current-use pesticides (CUPs) in air and surface water of the Bohai Sea, China. Sci. Total Environ. 2018;621:516–523. doi: 10.1016/j.scitotenv.2017.11.282. [DOI] [PubMed] [Google Scholar]
- 75.Olatunji O.S. Evaluation of selected polychlorinated biphenyls (PCBs) congeners and dichlorodiphenyltrichloroethane (DDT) in fresh root and leafy vegetables using GC-MS. Sci. Rep. 2019;9 doi: 10.1038/s41598-018-36996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meire R.O., Khairy M., Targino A.C., Galvão P.M.A., Torres J.P.M., Malm O., Lohmann R. Use of passive samplers to detect organochlorine pesticides in air and water at wetland mountain region sites (S-SE Brazil) Chemosphere. 2016;144:2175–2182. doi: 10.1016/j.chemosphere.2015.10.133. [DOI] [PubMed] [Google Scholar]
- 77.Muir D.C.G., Teixeira C., Wania F. Empirical and modeling evidence of regional atmospheric transport of current-use pesticides. Environ. Toxicol. Chem. 2004;23:2421. doi: 10.1897/03-457. [DOI] [PubMed] [Google Scholar]
- 78.Iwafune T. Studies on the behavior and ecotoxicity of pesticides and their transformation products in a river. J. Pestic. Sci. 2018;43:297–304. doi: 10.1584/jpestics.J18-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen C., Zou W., Cui G., Tian J., Wang Y., Ma L. Ecological risk assessment of current-use pesticides in an aquatic system of Shanghai, China. Chemosphere. 2020;257:127222. doi: 10.1016/j.chemosphere.2020.127222. [DOI] [PubMed] [Google Scholar]
- 80.Zhen X., Liu L., Wang X., Zhong G., Tang J. Fates and ecological effects of current-use pesticides (CUPs) in a typical river-estuarine system of Laizhou Bay, North China. Environ. Pollut. 2019;252:573–579. doi: 10.1016/j.envpol.2019.05.141. [DOI] [PubMed] [Google Scholar]
- 81.Carpenter K.D., Kuivila K.M., Hladik M.L., Haluska T., Cole M.B. Storm-event-transport of urban-use pesticides to streams likely impairs invertebrate assemblages. Environ. Monit. Assess. 2016;188:1–18. doi: 10.1007/s10661-016-5215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J., Lin K., Taylor A., Gan J. In vitro assessment of pyrethroid bioaccessibility via particle ingestion. Environ. Int. 2018;119:125–132. doi: 10.1016/j.envint.2018.05.043. [DOI] [PubMed] [Google Scholar]
- 83.Schreinemachers P., pu Chen H., Nguyen T.T.L., Buntong B., Bouapao L., Gautam S., Le N.T., Pinn T., Vilaysone P., Srinivasan R. Too much to handle? Pesticide dependence of smallholder vegetable farmers in Southeast Asia. Sci. Total Environ. 2017;593–594:470–477. doi: 10.1016/j.scitotenv.2017.03.181. [DOI] [PubMed] [Google Scholar]
- 84.Katagi T. Springer; New York: 2015. Soil Column Leaching of Pesticides Article in Reviews of Environmental Contamination and Toxicology. [DOI] [PubMed] [Google Scholar]
- 85.Huang B., Yan D., Wang X., Wang X., Fang W., Zhang D., Ouyang C., Wang Q., Cao A. Soil fumigation alters adsorption and degradation behavior of pesticides in soil. Environ. Pollut. 2019:264–273. doi: 10.1016/j.envpol.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 86.Pokhrel B., Gong P., Wang X., Chen M., Wang C., Gao S. Distribution, sources, and air–soil exchange of OCPs, PCBs and PAHs in urban soils of Nepal. Chemosphere. 2018;200:532–541. doi: 10.1016/j.chemosphere.2018.01.119. [DOI] [PubMed] [Google Scholar]
- 87.Hvězdová M., Kosubová P., Košíková M., Scherr K.E., Šimek Z., Brodský L., Šudoma M., Škulcová L., Sáňka M., Svobodová M., Krkošková L., Vašíčková J., Neuwirthová N., Bielská L., Hofman J. Currently and recently used pesticides in Central European arable soils. Sci. Total Environ. 2018;613–614:361–370. doi: 10.1016/j.scitotenv.2017.09.049. [DOI] [PubMed] [Google Scholar]
- 88.Kosubová P., Škulcová L., Poláková, Hofman J., Bielská L. Spatial and temporal distribution of the currently-used and recently-banned pesticides in arable soils of the Czech Republic. Chemosphere. 2020;254:126902. doi: 10.1016/j.chemosphere.2020.126902. [DOI] [PubMed] [Google Scholar]
- 89.Silva V., Mol H.G.J., Zomer P., Tienstra M., Ritsema C.J., Geissen V. Pesticide residues in European agricultural soils – A hidden reality unfolded. Sci. Total Environ. 2019;653:1532–1545. doi: 10.1016/j.scitotenv.2018.10.441. [DOI] [PubMed] [Google Scholar]
- 90.Lerch R.N., Thurman E.M., Blanchard P.E. Hydroxyatrazine in soils and sediments. Environ. Toxicol. Chem. 1999;18:2161–2168. doi: 10.1002/etc.5620181007. [DOI] [PubMed] [Google Scholar]
- 91.Jablonowski N.D., Köppchen S., Hofmann D., Schäffer A., Burauel P. Persistence of 14C-labeled atrazine and its residues in a field lysimeter soil after 22 years. Environ. Pollut. 2009;157:2126–2131. doi: 10.1016/j.envpol.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 92.Huang Y., Xiao L., Li F., Xiao M., Lin D., Long X., Wu Z. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: a review. Molecules. 2018;23 doi: 10.3390/molecules23092313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stenrød M., Perceval J., Benoit P., Almvik M., Bolli R.I., Eklo O.M., Sveistrup T.E., Kværner J. Cold climatic conditions: effects on bioavailability and leaching of the mobile pesticide metribuzin in a silt loam soil in Norway. Cold Reg. Sci. Technol. 2008;53:4–15. doi: 10.1016/j.coldregions.2007.06.007. [DOI] [Google Scholar]
- 94.López-Piñeiro A., Peña D., Albarrán A., Becerra D., Sánchez-Llerena J. Sorption, leaching and persistence of metribuzin in Mediterranean soils amended with olive mill waste of different degrees of organic matter maturity. J. Environ. Manage. 2013;122:76–84. doi: 10.1016/j.jenvman.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 95.Willkommen S., Pfannerstill M., Ulrich U., Guse B., Fohrer N. How weather conditions and physico-chemical properties control the leaching of flufenacet, diflufenican, and pendimethalin in a tile-drained landscape. Agric. Ecosyst. Environ. 2019;278:107–116. doi: 10.1016/j.agee.2019.03.017. [DOI] [Google Scholar]
- 96.Rice P.J., Anderson T.A., Coats J.R. Degradation and persistence of metolachlor in soil: effects of concentration, soil moisture, soil depth, and sterilization. Environ. Toxicol. Chem. 2002;21:2640–2648. doi: 10.1002/etc.5620211216. [DOI] [PubMed] [Google Scholar]
- 97.Sun Y., Zhao L., Li X., Hao Y., Xu H., Weng L., Li Y. Stimulation of earthworms (Eisenia fetida) on soil microbial communities to promote metolachlor degradation. Environ. Pollut. 2019;248:219–228. doi: 10.1016/j.envpol.2019.01.058. [DOI] [PubMed] [Google Scholar]
- 98.Quinteros E., Ribó A., Mejía R., López A., Belteton W., Comandari A., Orantes C.M., Pleites E.B., Hernández C.E., López D.L. Heavy metals and pesticide exposure from agricultural activities and former agrochemical factory in a Salvadoran rural community. Environ. Sci. Pollut. Res. 2017;24:1662–1676. doi: 10.1007/s11356-016-7899-z. [DOI] [PubMed] [Google Scholar]
- 99.Köhler H.R., Triebskorn R. Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science (80-.) 2013;341:759–765. doi: 10.1126/science.1237591. [DOI] [PubMed] [Google Scholar]
- 100.Pullagurala V.L.R., Rawat S., Adisa I.O., Hernandez-Viezcas J.A., Peralta-Videa J.R., Gardea-Torresdey J.L. Plant uptake and translocation of contaminants of emerging concern in soil. Sci. Total Environ. 2018;636:1585–1596. doi: 10.1016/j.scitotenv.2018.04.375. [DOI] [PubMed] [Google Scholar]
- 101.Hwang J.-I., Lee S.-E., Kim J.-E. Comparison of theoretical and experimental values for plant uptake of pesticide from soil. PLoS One. 2017;12:e0172254. doi: 10.1371/journal.pone.0172254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Knauer K., Homazava N., Junghans M., Werner I. The influence of particles on bioavailability and toxicity of pesticides in surface water. Integr. Environ. Assess. Manag. 2017;13:585–600. doi: 10.1002/ieam.1867. [DOI] [PubMed] [Google Scholar]
- 103.Bonmatin J.M., Giorio C., Girolami V., Goulson D., Kreutzweiser D.P., Krupke C., Liess M., Long E., Marzaro M., Mitchell E.A., Noome D.A., Simon-Delso N., Tapparo A. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015;22:35–67. doi: 10.1007/s11356-014-3332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Besse-Hoggan P., Alekseeva T., Sancelme M., Delort A.M., Forano C. Atrazine biodegradation modulated by clays and clay/humic acid complexes. Environ. Pollut. 2009;157:2837–2844. doi: 10.1016/j.envpol.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 105.Allen G., Halsall C.J., Ukpebor J., Paul N.D., Ridall G., Wargent J.J. Increased occurrence of pesticide residues on crops grown in protected environments compared to crops grown in open field conditions. Chemosphere. 2015;119:1428–1435. doi: 10.1016/j.chemosphere.2014.10.066. [DOI] [PubMed] [Google Scholar]
- 106.Bojacá C.R., Arias L.A., Ahumada D.A., Casilimas H.A., Schrevens E. Evaluation of pesticide residues in open field and greenhouse tomatoes from Colombia. Food Control. 2013;30:400–403. doi: 10.1016/j.foodcont.2012.08.015. [DOI] [Google Scholar]
- 107.White J.C. Plant-facilitated mobilization and translocation of weathered 2,2-bis(p-chlorophenyl)-1,1-dichloroethylene (p,p’-DDE) from an agricultural soil. Environ. Toxicol. Chem. 2001;20:2047. doi: 10.1002/etc.5620200925. [DOI] [PubMed] [Google Scholar]
- 108.Wang W., Xu W., Zhou K., Xiong Z. Research progressing of present contamination of Cd in soil and restoration method. Wuhan Univ. J. Nat. Sci. 2015;20:430–444. doi: 10.1007/s11859-015-1116-7. [DOI] [Google Scholar]
- 109.EWG’s . 2019. Shopper’s Guide to Pesticides in Produce | Dirty Dozen, (2019)https://www.ewg.org/foodnews/dirty-dozen.php (accessed November 16, 2019) [Google Scholar]
- 110.Park D.W., Kim K.G., Choi E.A., Kang G.R., Kim T.S., Yang Y.S., Moon S.J., Ha D.R., Kim E.S., Cho B.S. Pesticide residues in leafy vegetables, stalk and stem vegetables from south korea: a long-term study on safety and health risk assessment. Food Addit. Contam. - Part A Chem. Anal. Control. Expo. Risk Assess. 2015;33:105–118. doi: 10.1080/19440049.2015.1108524. [DOI] [PubMed] [Google Scholar]
- 111.Lushchak V.I., Matviishyn T.M., Husak V.V., Storey J.M., Storey K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018;17:1101–1136. doi: 10.17179/excli2018-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morris A.D., Muir D.C.G., Solomon K.R., Letcher R.J., McKinney M.A., Fisk A.T., McMeans B.C., Tomy G.T., Teixeira C., Wang X., Duric M. Current-use pesticides in seawater and their bioaccumulation in polar bear-ringed seal food chains of the Canadian Arctic. Environ. Toxicol. Chem. 2016;35:1695–1707. doi: 10.1002/etc.3427. [DOI] [PubMed] [Google Scholar]
- 113.Clark R.D. Predicting mammalian metabolism and toxicity of pesticides in silico. Pest Manag. Sci. 2018;74:1992–2003. doi: 10.1002/ps.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Winkler D.A. Overview of quantitative structure-activity relationships (QSAR) Mol. Anal. Genome Discov. 2005:347–367. doi: 10.1002/0470020202.ch16. [DOI] [Google Scholar]
- 115.Ali N., Rajeh N., Wang W., Abualnaja K.O., Kumosani T.A., Albar H.M.S., Eqani S.A.M.A.S., Ismail I.M.I. Organohalogenated contaminants in type 2 diabetic serum from Jeddah, Saudi Arabia. Environ. Pollut. 2016;213:206–212. doi: 10.1016/j.envpol.2016.01.087. [DOI] [PubMed] [Google Scholar]
- 116.Genuis S.J., Lane K., Birkholz D. Human elimination of organochlorine pesticides: blood, urine, and sweat study. Biomed Res. Int. 2016;2016 doi: 10.1155/2016/1624643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh N., Gupta V.K., Kumar A., Sharma B. Synergistic effects of heavy metals and pesticides in living systems. Front. Chem. 2017;5:1–9. doi: 10.3389/fchem.2017.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bossi R., Vinggaard A.M., Taxvig C., Boberg J., Bonefeld-Jørgensen E.C. Levels of pesticides and their metabolites in wistar rat amniotic fluids and maternal urine upon gestational exposure. Int. J. Environ. Res. Public Health. 2013;10:2271–2281. doi: 10.3390/ijerph10062271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kotthoff L., Keller J., Lörchner D., Mekonnen T.F., Koch M. Transformation products of organic contaminants and residues—overview of current simulation methods. Molecules. 2019;24 doi: 10.3390/molecules24040753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kwiatkowska M., Jarosiewicz P., Michałowicz J., Koter-Michalak M., Huras B., Bukowska B. The impact of glyphosate, its metabolites and impurities on viability, ATP level and morphological changes in human peripheral blood mononuclear cells. PLoS One. 2016;11:e0156946. doi: 10.1371/journal.pone.0156946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cui J., Wang F., Gao J., Zhai W., Zhou Z., Liu D., Wang P. Bioaccumulation and metabolism of Carbosulfan in zebrafish (Danio rerio) and the toxic effects of its metabolites. J. Agric. Food Chem. 2019 doi: 10.1021/acs.jafc.9b03674. [DOI] [PubMed] [Google Scholar]
- 122.Serra A.A., Nuttens A., Larvor V., Renault D., Couée I., Sulmon C., Gouesbet G. Low environmentally relevant levels of bioactive xenobiotics and associated degradation products cause cryptic perturbations of metabolism and molecular stress responses in Arabidopsis thaliana. J. Exp. Bot. 2013;64:2753–2766. doi: 10.1093/jxb/ert119. [DOI] [PubMed] [Google Scholar]
- 123.Rösch A., Anliker S., Hollender J. How biotransformation influences toxicokinetics of azole fungicides in the aquatic invertebrate Gammarus pulex. Environ. Sci. Technol. 2016;50:7175–7188. doi: 10.1021/acs.est.6b01301. [DOI] [PubMed] [Google Scholar]
- 124.Shen H., Starr J., Han J., Zhang L., Lu D., Guan R., Xu X., Wang X., Li J., Li W., Zhang Y., Wu Y. The bioaccessibility of polychlorinated biphenyls (PCBs) and polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs) in cooked plant and animal origin foods. Environ. Int. 2016;94:33–42. doi: 10.1016/j.envint.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 125.McLaughlin M.J., Lanno R. Use of “Bioavailability” as a term in ecotoxicology. Integr. Environ. Assess. Manag. 2014;10:138–140. doi: 10.1002/ieam.1497. [DOI] [Google Scholar]
- 126.Chitindingu K., Benhura M.A.N., Muchuweti M. Invitro bioaccessibility assessment of phenolic compounds from selected cereal grains: a prediction tool of nutritional efficiency. LWT - Food Sci. Technol. 2015;63:575–581. doi: 10.1016/j.lwt.2015.02.026. [DOI] [Google Scholar]
- 127.Liu Y.Y., Xiao J.J., Fu Y.Y., Liao M., Cao H.Q., Shi Y.H. Study of factors influencing the bioaccessibility of triazolone in cherry tomatoes using a static SHIME model. Int. J. Environ. Res. Public Health. 2018;15 doi: 10.3390/ijerph15050993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shi Y.H., Xiao J.J., Feng R.P., Liu Y.Y., Liao M., Wu X.W., Hua R.M., Cao H.Q. Factors affecting the bioaccessibility and intestinal transport of difenoconazole, hexaconazole, and Spirodiclofen in human Caco-2 cells following in vitro digestion. J. Agric. Food Chem. 2017;65:9139–9146. doi: 10.1021/acs.jafc.7b02781. [DOI] [PubMed] [Google Scholar]
- 129.Peixoto R.R.A., Devesa V., Vélez D., Cervera M.L., Cadore S. Study of the factors influencing the bioaccessibility of 10 elements from chocolate drink powder. J. Food Anal. 2016;48:41–47. doi: 10.1016/j.jfca.2016.02.002. [DOI] [Google Scholar]
- 130.Krishnan R., Meera M.S. Assessment of inhibitory factors on bioaccessibility of iron and zinc in pearl millet (Pennisetum glaucum (L.) R. Br.) cultivars. J. Food Sci. Technol. 2017;54:4378–4386. doi: 10.1007/s13197-017-2911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vogt R., Bennett D., Cassady D., Frost J., Ritz B., Hertz-Picciotto I. Cancer and non-cancer health effects from food contaminant exposures for children and adults in California: A risk assessment. Environ. Heal. A Glob. Access Sci. Source. 2012;11 doi: 10.1186/1476-069X-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shi Y.H., Xiao J.J., Feng R.P., Liu Y.Y., Liao M., Wu X.W., Hua R.M., Cao H.Q. In-vitro bioaccessibility of five pyrethroids after human ingestion and the corresponding gastrointestinal digestion parameters: a contribution for human exposure assessments. Chemosphere. 2017;182:517–524. doi: 10.1016/j.chemosphere.2017.05.081. [DOI] [PubMed] [Google Scholar]
- 133.Chedik L., Mias-Lucquin D., Bruyere A., Fardel O. In silico prediction for intestinal absorption and brain penetration of chemical pesticides in humans. Int. J. Environ. Res. Public Health. 2017;14 doi: 10.3390/ijerph14070708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhan J., Liang Y., Liu D., Ma X., Li P., Liu C., Liu X., Wang P., Zhou Z. Antibiotics may increase triazine herbicide exposure risk via disturbing gut microbiota. Microbiome. 2018;6 doi: 10.1186/s40168-018-0602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roman P., Cardona D., Sempere L., Carvajal F. Microbiota and organophosphates. Neurotoxicology. 2019;75:200–208. doi: 10.1016/j.neuro.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 136.Cedergreen N. Quantifying synergy: a systematic review of mixture toxicity studies within environmental toxicology. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mostafalou S., Abdollahi M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013;268:157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 138.WHO . WHO; 2008. Diet, Nutrition and the Prevention of Chronic Diseases.https://www.who.int/dietphysicalactivity/publications/trs916/en/ (accessed November 16, 2019) [Google Scholar]
- 139.WHO . WHO; 2019. Highly Hazardous Pesticides.https://www.who.int/ipcs/assessment/public_health/pesticides/en/ (accessed November 16, 2019) [Google Scholar]
- 140.Yousaf B., Liu G., Wang R., Imtiaz M., Zia-ur-Rehman M., Munir M.A.M., Niu Z. Bioavailability evaluation, uptake of heavy metals and potential health risks via dietary exposure in urban-industrial areas. Environ. Sci. Pollut. Res. 2016;23:22443–22453. doi: 10.1007/s11356-016-7449-8. [DOI] [PubMed] [Google Scholar]
- 141.Raschke A.M., Burger A.E.C. Risk assessment as a management tool used to assess the effect of pesticide use in an irrigation system, situated in a semi-desert region. Arch. Environ. Contam. Toxicol. 1997;32:42–49. doi: 10.1007/s002449900153. [DOI] [PubMed] [Google Scholar]
- 142.Page A., Liu S., Gunnell D., Astell-Burt T., Feng X., Wang L., Zhou M. Suicide by pesticide poisoning remains a priority for suicide prevention in China: analysis of national mortality trends 2006–2013. J. Affect. Disord. 2017;208:418–423. doi: 10.1016/j.jad.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 143.Dasgupta S., Meisner C., Huq M. The World Bank; 2005. Health Effects And Pesticide Perception As Determinants Of Pesticide Use : Evidence From Bangladesh. [DOI] [Google Scholar]
- 144.Macharia I. Pesticides and health in vegetable production in Kenya. Biomed Res. Int. 2015;2015 doi: 10.1155/2015/241516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chiu Y.H., Afeiche M.C., Gaskins A.J., Williams P.L., Petrozza J.C., Tanrikut C., Hauser R., Chavarro J.E. Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Hum. Reprod. 2015;30:1342–1351. doi: 10.1093/humrep/dev064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Horrigan L., Lawrence R.S., Walker P. How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ. Health Perspect. 2002;110:445–456. doi: 10.1289/ehp.02110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen T., Tan J., Wan Z., Zou Y., Afewerky H.K., Zhang Z., Zhang T. Effects of commonly used pesticides in China on the mitochondria and ubiquitin-proteasome system in Parkinson’s disease. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18122507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thongprakaisang S., Thiantanawat A., Rangkadilok N., Suriyo T., Satayavivad J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. 2013;59:129–136. doi: 10.1016/j.fct.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 149.Sabarwal A., Kumar K., Singh R.P. Hazardous effects of chemical pesticides on human health–Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018;63:103–114. doi: 10.1016/j.etap.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 150.Schinasi L., Leon M.E. Non-hodgkin lymphoma and occupational exposure to agricultural pesticide chemical groups and active ingredients: a systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2014;11:4449–4527. doi: 10.3390/ijerph110404449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Caron-Beaudoin É., Viau R., Sanderson J.T. Effects of neonicotinoid pesticides on promoter-specific aromatase (CYP19) expression in Hs578t breast cancer cells and the role of the VEGF pathway. Environ. Health Perspect. 2018;126 doi: 10.1289/EHP2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Navarrete-Meneses M.D.P., Pérez-Vera P. Pyrethroid pesticide exposure and hematological cancer: Epidemiological, biological and molecular evidence. Rev. Environ. Health. 2019;34:197–210. doi: 10.1515/reveh-2018-0070. [DOI] [PubMed] [Google Scholar]
- 153.Hooker E.P., Fulcher K.G., Gibb H.J. Aldrin and dieldrin: A reevaluation of the cancer and noncancer dose-response assessments. Risk Anal. 2014;34:865–878. doi: 10.1111/risa.12145. [DOI] [PubMed] [Google Scholar]
- 154.Boffetta P., Desai V. Exposure to permethrin and cancer risk: a systematic review. Crit. Rev. Toxicol. 2018;48:433–442. doi: 10.1080/10408444.2018.1439449. [DOI] [PubMed] [Google Scholar]
- 155.Gangemi S., Gofita E., Costa C., Teodoro M., Briguglio G., Nikitovic D., Tzanakakis G., Tsatsakis A.M., Wilks M.F., Spandidos D.A., Fenga C. Occupational and environmental exposure to pesticides and cytokine pathways in chronic diseases (Review) Int. J. Mol. Med. 2016;38:1012–1020. doi: 10.3892/ijmm.2016.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dhananjayan V., Ravichandran B. Occupational health risk of farmers exposed to pesticides in agricultural activities. Curr. Opin. Environ. Sci. Heal. 2018;4:31–37. doi: 10.1016/j.coesh.2018.07.005. [DOI] [Google Scholar]
- 157.Sapbamrer R., Hongsibsong S., Sittitoon N., Amput P. DNA damage and adverse neurological outcomes among garlic farmers exposed to organophosphate pesticides. Environ. Toxicol. Pharmacol. 2019;72:103241. doi: 10.1016/j.etap.2019.103241. [DOI] [PubMed] [Google Scholar]
- 158.Bernieri T., Rodrigues D., Barbosa I.R., Ardenghi P.G., Basso da Silva L. Occupational exposure to pesticides and thyroid function in Brazilian soybean farmers. Chemosphere. 2019;218:425–429. doi: 10.1016/j.chemosphere.2018.11.124. [DOI] [PubMed] [Google Scholar]
- 159.Colborn T., Vom Saal F.S., Soto A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Matisová E., Hrouzková S. Analysis of endocrine disrupting pesticides by capillary GC with mass spectrometric detection. Int. J. Environ. Res. Public Health. 2012;9:3166–3196. doi: 10.3390/ijerph9093166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li C., Sun H., Juhasz A.L., Cui X., Ma L.Q. Predicting the relative bioavailability of DDT and its metabolites in historically contaminated soils using a tenax-improved physiologically based extraction test (TI-PBET) Environ. Sci. Technol. 2016;50:1118–1125. doi: 10.1021/acs.est.5b03891. [DOI] [PubMed] [Google Scholar]
- 162.Warner G.R., Mourikes V.E., Neff A.M., Brehm E., Flaws J.A. Mechanisms of action of agrochemicals acting as endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2020;502:110680. doi: 10.1016/j.mce.2019.110680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kojima H., Sata F., Takeuchi S., Sueyoshi T., Nagai T. Comparative study of human and mouse pregnane X receptor agonistic activity in 200 pesticides using in vitro reporter gene assays. Toxicology. 2011;280:77–87. doi: 10.1016/j.tox.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 164.Pogrmic-Majkic K., Samardzija D., Stojkov-Mimic N., Vukosavljevic J., Trninic-Pjevic A., Kopitovic V., Andric N. Atrazine suppresses FSH-induced steroidogenesis and LH-dependent expression of ovulatory genes through PDE-cAMP signaling pathway in human cumulus granulosa cells. Mol. Cell. Endocrinol. 2018;461:79–88. doi: 10.1016/j.mce.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 165.Ji C., Yu C., Yue S., Zhang Q., Yan Y., Fan J., Zhao M. Enantioselectivity in endocrine disrupting effects of four cypermethrin enantiomers based on in vitro models. Chemosphere. 2019;220:766–773. doi: 10.1016/j.chemosphere.2018.12.158. [DOI] [PubMed] [Google Scholar]
- 166.Guo X., Zhou S., Chen Y., Chen X., Liu J., Ge F., Lian Q., Chen X., Ge R.-S. Ziram Delays Pubertal Development of Rat Leydig Cells. Toxicol. Sci. 2017;160:329–340. doi: 10.1093/toxsci/kfx181. [DOI] [PubMed] [Google Scholar]
- 167.Ji C., Song Q., Chen Y., Zhou Z., Wang P., Liu J., Sun Z., Zhao M. The potential endocrine disruption of pesticide transformation products (TPs): the blind spot of pesticide risk assessment. Environ. Int. 2020;137:105490. doi: 10.1016/j.envint.2020.105490. [DOI] [PubMed] [Google Scholar]
- 168.Alavian-Ghavanini A., Rüegg J. Understanding epigenetic effects of endocrine disrupting chemicals: from mechanisms to novel test methods. Basic Clin. Pharmacol. Toxicol. 2018;122:38–45. doi: 10.1111/bcpt.12878. [DOI] [PubMed] [Google Scholar]
- 169.Requena M., López-Villén A., Hernández A.F., Parrón T., Navarro Á., Alarcón R. Environmental exposure to pesticides and risk of thyroid diseases. Toxicol. Lett. 2019;315:55–63. doi: 10.1016/j.toxlet.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 170.Van Ravenzwaay B., Herold M., Kamp H., Kapp M.D., Fabian E., Looser R., Krennrich G., Mellert W., Prokoudine A., Strauss V., Walk T., Wiemer J. Metabolomics: A tool for early detection of toxicological effects and an opportunity for biology based grouping of chemicals-From QSAR to QBAR. Mutat. Res. - Genet. Toxicol. Environ. Mutagen. 2012;746:144–150. doi: 10.1016/j.mrgentox.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 171.Hernández A.F., Bennekou S.H., Hart A., Mohimont L., Wolterink G. Mechanisms underlying disruptive effects of pesticides on the thyroid function. Curr. Opin. Toxicol. 2020;19:34–41. doi: 10.1016/j.cotox.2019.10.003. [DOI] [Google Scholar]
- 172.Tsiaoussis J., Antoniou M.N., Koliarakis I., Mesnage R., Vardavas C.I., Izotov B.N., Psaroulaki A., Tsatsakis A. Effects of single and combined toxic exposures on the gut microbiome: current knowledge and future directions. Toxicol. Lett. 2019;312:72–97. doi: 10.1016/j.toxlet.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 173.Utembe W., Kamng’ona A.W. Gut microbiota-mediated pesticide toxicity in humans: methodological issues and challenges in risk assessment of pesticides. Chemosphere. 2021;271:129817. doi: 10.1016/j.chemosphere.2021.129817. [DOI] [PubMed] [Google Scholar]
- 174.Mesnage R., Teixeira M., Mandrioli D., Falcioni L., Ducarmon Q.R., Zwittink R.D., Mazzacuva F., Caldwell A., Halket J., Amiel C., Panoff J.M., Belpoggi F., Antoniou M.N. Use of shotgun metagenomics and metabolomics to evaluate the impact of glyphosate or roundup mon 52276 on the gut microbiota and serum metabolome of sprague-dawley rats. Environ. Health Perspect. 2021;129:1–15. doi: 10.1289/EHP6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Owagboriaye F., Mesnage R., Dedeke G., Adegboyega T., Aladesida A., Adeleke M., Owa S., Antoniou M.N. Impacts of a glyphosate-based herbicide on the gut microbiome of three earthworm species (Alma millsoni, Eudrilus eugeniae and Libyodrilus violaceus): a pilot study. Toxicol. Reports. 2021;8:753–758. doi: 10.1016/j.toxrep.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Krause J.L., Haange S.B., Schäpe S.S., Engelmann B., Rolle-Kampczyk U., Fritz-Wallace K., Wang Z., Jehmlich N., Türkowsky D., Schubert K., Pöppe J., Bote K., Rösler U., Herberth G., von Bergen M. The glyphosate formulation Roundup® LB plus influences the global metabolome of pig gut microbiota in vitro. Sci. Total Environ. 2020;745:140932. doi: 10.1016/j.scitotenv.2020.140932. [DOI] [PubMed] [Google Scholar]
- 177.Wang X., Shen M., Zhou J., Jin Y. Chlorpyrifos disturbs hepatic metabolism associated with oxidative stress and gut microbiota dysbiosis in adult zebrafish. Comp. Biochem. Physiol. Part - C Toxicol. Pharmacol. 2019;216:19–28. doi: 10.1016/j.cbpc.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 178.Motta E.V.S., Raymann K., Moran N.A. Glyphosate perturbs the gut microbiota of honey bees. Proc. Natl. Acad. Sci. U. S. A. 2018;115:10305–10310. doi: 10.1073/pnas.1803880115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Multigner L., Kadhel P., Rouget F., Blanchet P., Cordier S. Chlordecone exposure and adverse effects in French West Indies populations. Environ. Sci. Pollut. Res. 2016;23:3–8. doi: 10.1007/s11356-015-4621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Du H., Wang M., Wang L., Dai H., Wang M., Hong W., Nie X., Wu L., Xu A. Reproductive toxicity of endosulfan: implication from germ cell apoptosis modulated by mitochondrial dysfunction and genotoxic response genes in Caenorhabditis elegans. Toxicol. Sci. 2015;145:118–127. doi: 10.1093/toxsci/kfv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gupta V.K., Pathak A., Siddiqi N.J., Sharma B. Carbofuran modulating functions of acetylcholinesterase from rat brain in vitro. Adv. Biol. 2016;2016:1–7. doi: 10.1155/2016/3760967. [DOI] [Google Scholar]
- 182.Sharma D., Sangha G.K., Khera K.S. Triazophos-induced oxidative stress and histomorphological changes in ovary of female Wistar rats. Pestic. Biochem. Physiol. 2015;117:9–18. doi: 10.1016/j.pestbp.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 183.Amjad S., Sharma A.K., Serajuddin M. Toxicity assessment of cypermethrin nanoparticles in Channa punctatus: behavioural response, micronuclei induction and enzyme alteration. Regul. Toxicol. Pharmacol. 2018;100:127–133. doi: 10.1016/J.YRTPH.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 184.Rehman H., Aziz A.T., Saggu S., Vanwert A.L., Zidan N., Saggu S. Additive toxic effect of deltamethrin and cadmium on hepatic, hematological, and immunological parameters in mice. Toxicol. Ind. Health. 2017;33:495–502. doi: 10.1177/0748233716684710. [DOI] [PubMed] [Google Scholar]
- 185.Esimbekova E.N., Kalyabina V.P., Kopylova K.V., Torgashina I.G., Kratasyuk V.A. Design of bioluminescent biosensors for assessing contamination of complex matrices. Talanta. 2021;233:122509. doi: 10.1016/j.talanta.2021.122509. [DOI] [PubMed] [Google Scholar]
- 186.Esimbekova E.N., Torgashina I.G., Kalyabina V.P., Kratasyuk V.A. Enzymatic Biotesting : Scientific Basis and Application. 2021;14:290–304. doi: 10.1134/S1995425521030069. [DOI] [Google Scholar]
- 187.Woźniak E., Sicińska P., Michałowicz J., Woźniak K., Reszka E., Huras B., Zakrzewski J., Bukowska B. The mechanism of DNA damage induced by Roundup 360 PLUS, glyphosate and AMPA in human peripheral blood mononuclear cells - genotoxic risk assessement. Food Chem. Toxicol. 2018;120:510–522. doi: 10.1016/j.fct.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 188.Zhang X., Wallace A.D., Du P., Kibbe W.A., Jafari N., Xie H., Lin S., Baccarelli A., Soares M.B., Hou L. DNA methylation alterations in response to pesticide exposure in vitro. Environ. Mol. Mutagen. 2012;53:542–549. doi: 10.1002/em.21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Paul K.C., Chuang Y.H., Cockburn M., Bronstein J.M., Horvath S., Ritz B. Organophosphate pesticide exposure and differential genome-wide DNA methylation. Sci. Total Environ. 2018;645:1135–1143. doi: 10.1016/j.scitotenv.2018.07.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sánchez O.F., Lin L., Bryan C.J., Xie J., Freeman J.L., Yuan C. Profiling epigenetic changes in human cell line induced by atrazine exposure. Environ. Pollut. 2020;258:113712. doi: 10.1016/j.envpol.2019.113712. [DOI] [PubMed] [Google Scholar]
- 191.Mesnage R., Biserni M., Wozniak E., Xenakis T., Mein C.A., Antoniou M.N. Comparison of transcriptome responses to glyphosate, isoxaflutole, quizalofop-p-ethyl and mesotrione in the HepaRG cell line. Toxicol. Reports. 2018;5:819–826. doi: 10.1016/j.toxrep.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Luis G., Hernández C., Rubio C., González-Weller D., Gutiérrez Á., Revert C., Hardisson A. Metales traza y metales tóxicos en tomates producidos intensivamente (lycopersicon esculentum) Nutr. Hosp. 2012;27:1605–1609. doi: 10.3305/nh.2012.27.5.5944. [DOI] [PubMed] [Google Scholar]
- 193.How Toxic Pollutants Can Harm Future, Unexposed Generations. EWG; 2017. The evidence of transgenerational toxicity.https://www.ewg.org/research/how-toxic-pollutants-can-harm-future-unexposed-generations/2-evidence-transgenerational (accessed November 16, 2019) [Google Scholar]
- 194.Manikkam M., Tracey R., Guerrero-Bosagna C., Skinner M.K. Pesticide and insect repellent mixture (permethrin and DEET) induces epigenetic transgenerational inheritance of disease and sperm epimutations. Reprod. Toxicol. 2012;34:708–719. doi: 10.1016/j.reprotox.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.McBirney M., King S.E., Pappalardo M., Houser E., Unkefer M., Nilsson E., Sadler-Riggleman I., Beck D., Winchester P., Skinner M.K. Atrazine induced epigenetic transgenerational inheritance of disease, lean phenotype and sperm epimutation pathology biomarkers. PLoS One. 2017;12:e0184306. doi: 10.1371/journal.pone.0184306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Collotta M., Bertazzi P.A., Bollati V. Epigenetics and pesticides. Toxicology. 2013;307:35–41. doi: 10.1016/j.tox.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 197.Soares C., Pereira R., Spormann S., Fidalgo F. Is soil contamination by a glyphosate commercial formulation truly harmless to non-target plants? – Evaluation of oxidative damage and antioxidant responses in tomato. Environ. Pollut. 2019:256–265. doi: 10.1016/j.envpol.2019.01.063. [DOI] [PubMed] [Google Scholar]
- 198.Ferreira M.F., Carolina T., Bracamonte E., Leonardo G. Effects of the herbicide glyphosate on non-target plant native species from Chaco forest (Argentina) Ecotoxicol. Environ. Saf. 2017;144:360–368. doi: 10.1016/j.ecoenv.2017.06.049. [DOI] [PubMed] [Google Scholar]
- 199.Milan M., Dalla Rovere G., Smits M., Ferraresso S., Pastore P., Marin M.G., Bogialli S., Patarnello T., Bargelloni L., Matozzo V. Ecotoxicological effects of the herbicide glyphosate in non-target aquatic species: transcriptional responses in the mussel Mytilus galloprovincialis. Environ. Pollut. 2018;237:442–451. doi: 10.1016/j.envpol.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 200.Mužinić V., Želježić D. Non-target toxicity of novel insecticides. Arh. Hig. Rada Toksikol. 2018;69:86–102. doi: 10.2478/aiht-2018-69-3111. [DOI] [PubMed] [Google Scholar]
- 201.Hallmann C.A., Foppen R.P.B., Van Turnhout C.A.M., De Kroon H., Jongejans E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature. 2014;511:341–343. doi: 10.1038/nature13531. [DOI] [PubMed] [Google Scholar]
- 202.hua Gu Y., Li Y., feng Huang X., fen Zheng J., Yang J., Diao H., Yuan Y., Xu Y., Liu M., juan Shi H., ping Xu W. Reproductive effects of two neonicotinoid insecticides on mouse sperm function and early embryonic development in vitro. PLoS One. 2013;8:e70112. doi: 10.1371/journal.pone.0070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Brown T.M., Macdonald R.W., Muir D.C.G., Letcher R.J. The distribution and trends of persistent organic pollutants and mercury in marine mammals from Canada’s Eastern Arctic. Sci. Total Environ. 2018;618:500–517. doi: 10.1016/j.scitotenv.2017.11.052. [DOI] [PubMed] [Google Scholar]
- 204.Jactel H., Verheggen F., Thiéry D., Escobar-Gutiérrez A.J., Gachet E., Desneux N. Alternatives to neonicotinoids. Environ. Int. 2019;129:423–429. doi: 10.1016/j.envint.2019.04.045. [DOI] [PubMed] [Google Scholar]
- 205.Siegwart M., Graillot B., Lopez C.B., Besse S., Bardin M., Nicot P.C., Lopez-Ferber M. Resistance to bio-insecticides or how to enhance their sustainability: a review. Front. Plant Sci. 2015;6:1–19. doi: 10.3389/fpls.2015.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Neuwirthová N., Trojan M., Svobodová M., Vašíčková J., Šimek Z., Hofman J., Bielská L. Pesticide residues remaining in soils from previous growing season(s) - Can they accumulate in non-target organisms and contaminate the food web? Sci. Total Environ. 2019;646:1056–1062. doi: 10.1016/j.scitotenv.2018.07.357. [DOI] [PubMed] [Google Scholar]
- 207.FAO, Sustainable Food and Agriculture, (2020). http://www.fao.org/sustainability/en/ (accessed May 31, 2020).
- 208.Integrated Pest Management (IPM) | Agricultural Sustainability Institute, (2020). https://asi.ucdavis.edu/programs/ucsarep/about/what-is-sustainable-agriculture/practices/ipm (accessed May 26, 2020).
- 209.Grovermann C., Schreinemachers P., Riwthong S., Berger T. Smart’ policies to reduce pesticide use and avoid income trade-offs: an agent-based model applied to Thai agriculture. Ecol. Econ. 2017;132:91–103. doi: 10.1016/j.ecolecon.2016.09.031. [DOI] [Google Scholar]
- 210.Singh A., Dhiman N., Kar A.K., Singh D., Purohit M.P., Ghosh D., Patnaik S. Advances in controlled release pesticide formulations: prospects to safer integrated pest management and sustainable agriculture. J. Hazard. Mater. 2020;385:121525. doi: 10.1016/j.jhazmat.2019.121525. [DOI] [PubMed] [Google Scholar]
- 211.Liang Y., Gao Y., Wang W., Dong H., Tang R., Yang J., Niu J., Zhou Z., Jiang N., Cao Y. Fabrication of smart stimuli-responsive mesoporous organosilica nano-vehicles for targeted pesticide delivery. J. Hazard. Mater. 2020;389:122075. doi: 10.1016/j.jhazmat.2020.122075. [DOI] [PubMed] [Google Scholar]
- 212.Kumar S., Nehra M., Dilbaghi N., Marrazza G., Hassan A.A., Kim K.H. Nano-based smart pesticide formulations: emerging opportunities for agriculture. J. Control. Release. 2019;294:131–153. doi: 10.1016/j.jconrel.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 213.WHO . WHO; 2009. The WHO Recommended Classification of Pesticides by Hazard.https://www.who.int/ipcs/publications/pesticides_hazard/en/ (accessed November 16, 2019) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.