Abstract
Squamous cell carcinoma of the rectum is a rare malignancy (0.3% of all rectal cancers), with no known risk factor. These tumours are assessed as rectal cancer using immunohistochemical and radiological tests, and certain criteria (localisation, relationship with neighbouring structures) have to be fulfilled to make the diagnosis. Some clinicians used to stage them with the anal cancer TNM (tumour–node–metastasis), whereas others used the rectal cancer TNM. When localised, the tendency nowadays is to treat those tumours like squamous anal cancers with definitive chemoradiotherapy (5-fluorouracil and mitomycin) and to skip surgery. For metastatic disease there is no clearly validated regimen and treatment should be based on recommendations of squamous anal cancers because of their common histology. Concerning follow-up after a curative approach, techniques should follow those for anal cancer as well, evaluating a delayed response.
Key words: squamous rectal cancer, rare disease, definitive chemoradiotherapy
Highlights
-
•
Rectal squamous cell carcinoma (rSCC) is a rare entity for which we have limited knowledge and no clear recommendations.
-
•
Creation of an international registry and a biological repository could increase our understating of this rare entity.
-
•
Definitive chemoradiotherapy (CRT) should be the gold standard treatment of local/locally-advanced rSCC.
-
•
Clarification of clinical and pathologic response rates with CRT or radiotherapy alone and patterns of failure is important.
-
•
Timing for tumour response assessment is paramount. Data tilt towards waiting until 6 months after definitive treatment.
Introduction
Rectal cancer (RC) represents 35% of colorectal cancers (CRCs) and is considered a distinct entity with different aetiologies, molecular profile and risk factors compared with colon cancer. From a histological point of view, 90% of RCs are adenocarcinomas (ADCs). Pure rectal squamous cell carcinoma (rSCC) is rare (0.3% of RCs). In addition to rSCC, other rare malignant histologies can be found in the rectum such as mixed adenosquamous, neuroendocrine tumours, carcinoids, lymphomas, leiomyosarcomas and gastrointestinal stromal tumours.
Given the rarity of rSCC, its epidemiology, pathogenesis, prognosis and therapeutic management are not well defined. Consequently, neither the European Society of Medical Oncology (ESMO) nor the National Comprehensive Cancer Network (NCCN) societies have published recommendations for rSCC. Nowadays, the trend is to treat rSCC by analogy to anal SCCs (aSCC) with definitive chemoradiotherapy (CRT), with chemotherapy being 5-fluorouracil (5-FU) and mitomycin (MMC), and to skip surgery. Interestingly, treatment is based on histology (squamous) and not on localisation (rectum). The objective of this work is to provide a critical review of this rare pathology from epidemiology to treatment and follow-up.
Epidemiology
CRC is the second most frequent cancer in Europe and represents 13.2% and 12.7% of all cancer cases in men and women, respectively. The incidence of RC in the European Union is ~125 000 new cases per year, representing ~35% of the total CRC cases. RC prevalence is ~20 cases/100 000 population per year1 and it is associated with significant morbidity and mortality.2 Within the rectum, >90% of malignancies are ADC3 with rSCC representing only 0.1% to 0.3% of all RCs. Apart from rSCC, other rare histologies such as neuroendocrine, carcinoids, lymphomas, leiomyosarcomas and gastrointestinal stromal tumours are found in the rectum.4 Given the rarity of rSCC, some authors question its existence.5 SCC histology in the rest of the colic tube is even more rare.6,7 Schmidtmann8 reported the first case of SCC of the colon in 1919, whereas the first case of rSCC was described by Raiford in 1933.9
In RC, the SCC histology can also be found in mixed histologic patterns such as adenosquamous carcinoma. In a series of 750 cases of colorectal adenomas, Williams et al.10 reported three cases with squamous differentiation. Other authors have reported squamous metaplasia in adenomatous polyps,11,12 like in villous adenoma.13 In case of mixed (glandular and epithelial components) the latter ones have more invasive behaviour.14 These findings may represent the squamous differentiation of a basal colonic cell, with changes inciting a metaplastic change of the developing adenoma.6
From 1946 to 2015 and based on the Surveillance, Epidemiology and End Results (SEER) database from the National Cancer Institute, 142 cases of rSCC were identified by Guerra et al.6 with an age range of 39-93 years old and an average age at diagnosis of 63 years. Female sex predominates, accounting for 57.4% of all cases, with no apparent ethnical or geographic predisposition. Patients most frequently presented with early-stage (stage I/II, 52.8%) or locally advanced disease (stage III, 29.3%).
Pathogenesis—aetiology
The aetiology of rSCC remains unclear and multiple theories have been postulated: malignant transformation of persistent ectopic embryonal nests of ectodermal cells, smoking-induced de novo mutations, previous radiation exposure, chronic rectal inflammation (e.g. ulcerative colitis), enteric chronic infections (amebiasis or schistosomiasis), human immunodeficiency virus (HIV), human papillomavirus (HPV).6,14
The theory of chronic inflammation leading to squamous metaplasia and subsequent carcinoma is one of the most prominent. This idea draws upon the fact that irritation and inflammation can lead to a change in the epithelial lining-metaplasia-phenomenon occurring at different gastrointestinal locations in response to exposure to various stressors (such as ulcerative colitis, radiotherapy and infection as mentioned before).15
Drawing further upon this theory is the idea of pluripotent mucosal stem cells capable of multidirectional differentiation, first postulated in the 1950s.16,17 Furthermore, a work by Nahas et al. in 200718 also supports the fact that keratin profiles vary amongst epithelia but remain constant in neoplastic transformation. They demonstrated that rSCC and ADC of the rectum (rADC) stain for cytokeratin CAM5.2, unlike SCC of the anal margin (aSCC), suggesting a common cell of origin for both RC subtypes. This lends support to an idea that the mucosal lining of the rectum contains a common pluripotent endodermal stem cell, which under certain conditions (inflammation and epithelial damage) can undergo squamous differentiation. This is visualised as an area of metaplasia, which can subsequently undergo dysplasia and carcinomatous change if the stressor is not removed.18
HPV is a factor eliciting dysplastic changes of the squamous epithelium. While a strong association between HPV and SCC at multiple sites (anus, head/neck and cervix) is demonstrated, however, its role in rSCC is not established. Only a limited number of studies exist that have examined HPV’s implication in rSCC with discordant results.2 Audeau et al.19 examined 20 squamous lesions (from metaplasia to carcinoma) but did not find any evidence of HPV 6, 11, 16, 18. Similarly Nahas et al.18 and Frizelle et al.,20 using in situ hybridisation, did not find any evidence of HPV DNA for five and six cases, respectively. By contrast, Sotlar et al.21 (one case), Kong and Welton22 (three cases), Matsuda et al.23 (one case) and Jaworski et al.16 (two cases), all identified HPV 16 by using (contrary to the first authors) a PCR method, implying that the method of detection could matter. Due to the small number of cases, however, the role of HPV in rSCC pathogenesis remains unclear.
Patients with HIV infection have a higher incidence of HPV infection than the general population. Additionally, HIV infection increases susceptibility to virally promoted cancers, amongst them anogenital carcinoma (HPV). Although it could be inferred that the cell- mediated immune deficiency associated with HIV could predispose to rSCC,6 there is no proof that HIV incidence is higher in rSCC and therefore plays a role in its pathogenesis.23,24
Prognosis—prognostic factors
The majority of patients diagnosed with rSCC have local or locoregional disease (82.1%). RSCC has a poorer overall survival (OS) when compared stage for stage with rADC. The overall 5-year survival for rSCC is 48.9% compared with 62.1% for rADC. When localised, the 5-year OS for rSCC is 73.7% versus 91.8% for rADC, being 31.3% for rSCC with regional involvement versus 65.8% for rADC. In the metastatic setting, OS is 8.8% for rSCC compared with 20.8% for rADC.4 With regards to aSCC, the 5-year survival is 82%, 65% and 32% in localised, locally advanced and metastatic disease, respectively.25
Chiu et al.,2 in their population-based analysis (SEER, 1998-2011) with rSCC patients, showed that favourable prognostic factors included: receipt of radiotherapy, local stage, younger age, female sex and white race. In a retrospective analysis by Dutta et al.,26 worse OS was associated with age (>65 years old), male sex, African/American race, higher Charlson comorbidity index (CCI) score, and a lower median income. In this study, tumour grade did not influence survival. Whereas there was a difference in survival from stage I to stage II/III disease (log rank P < 0.001 for each), no survival difference was seen between stage II and stage III disease (log rank P = 0.119).
Recently, based again on the SEER database (study cohort from January 2004 to December 2013), Diao et al.27 have developed a nomogram to predict 3 and 5 years OS for rSCC, with a model containing age, marital status, T and M stage, surgery (yes/no), chemotherapy and radiotherapy. Their nomogram offers superior discrimination over the 8th edition American Joint Committee on Cancer tumour–node–metastasis (AJCC TNM) staging classification.
Clinical presentation
Symptoms associated with rSCC are similar to those seen in rADC and encompass abdominal pain, anorexia, weight loss, alteration in bowel habit (constipation, diarrhoea, tenesmus), pain while defaecating and rectal bleeding.28
Diagnosis
Complete colonoscopy with biopsies of any abnormalities is essential for rSCC diagnosis. Demonstration of the discontinuity of a lesion from the anal squamous mucosa is of great importance to differentiate rSCC from aSCC. RSCC lesions have a versatile endoscopic appearance dependent on the stage of disease,6 ranging from a small mucosal polyp, an ulcerative lesion and through to a large obstructing mass.29
Histologically, well- or moderately-differentiated rSCC and rADC are easily distinguished. SCC (Figure 1A and B), as in other localisations, is composed of polygonal cells of rather large size, forming tumour masses or islets with inward maturation. As in squamous epithelium, the tumour cells are connected to each other by intercellular bridges, visible under an optical microscope. Keratinization may occur. ADC is composed of medium-sized cells with an increased nucleo-cytoplasmic ratio, forming glands or cribriform structures. The percentage of glands formation determines differentiation. Several subtypes of ADC exist, such as mucinous ADC or signet-ring cell carcinoma. Adenosquamous carcinoma has features of both ADC and SCC. Poorly or undifferentiated carcinomas may be difficult to subtype on morphological criteria only. Auxiliary techniques may be required.
Figure 1.
Histology of a keratinizing squamous cell carcinoma.
Biopsy of an ulcerated rectal mass in an 83-year old female showing a keratinizing squamous cell carcinoma, with carcinomatous proliferation forming nests and cords centred by keratin pearls (A, haematoxylin–eosin, grossing ×80); residual rectal glands are seen at the periphery of the tumour (B, haematoxylin–eosin, grossing ×100).
Concerning the localization of SCC, it is not possible to determine whether it is of rectal, anal or metastatic origin, based on morphological characteristics. Immunohistochemistry staining can aid to better define the organ of origin and the subtype of the tumour.6 The most used markers are AE1/AE3, 34bE12, p63, CAM5.2 and CK 7/2028 (Table 1).
Table 1.
Immunohistochemical diagnostic tests
| rSCC | rADC | aSCC | |
|---|---|---|---|
| CAM5.2 | + | + | − |
| AE1/AE3 | + | + | + |
| 34bE12 | + | ± | + |
| CK 7 | ± | ± | ± |
| CK 20 | − | + | − |
| p63 | + | − | + |
aSCC, anal squamous cell carcinoma; rADC, adenocarcinoma of the rectum; rSCC, rectal squamous cell carcinoma.
CAM5.2 is an antibody that identifies keratins 8 and 18. It shares a similar expression pattern in rSCC and rADC (but not in aSCC) and favours a common pluripotent endodermal stem cell origin for both those RC subtypes. According to Nahas et al.18 cytokeratin CAM5.2 is a discriminatory test aiding in the differentiation of rSCC from aSCC, characteristically staining for rSCC or rADC but not aSCC.
SCC-associated antigen (SCCAg) is a serum tumour marker expressed by epidermoid tumours, including aSCC. It is weakly correlated with nodal involvement or disease relapse in aSCC. Its clinical utility in diagnosis and follow-up is controversial in aSCC according to ESMO guidelines.30, 31, 32 There are very limited data of the utility of SCCAg in rSCC; it is therefore not recommended.33
To discriminate rSCC from aSCC, Williams et al.10 have proposed four criteria which must be fulfilled: (i) absence of SCC in another primary site; (ii) the tumour must not have a squamous-lined fistula tract to the affected bowel; (iii) the tumour cannot represent proximal extension of SCC of the anus and (iv) histological SCC confirmation.
Diagnosis and initial staging (Table 2) involve evaluation of the primary tumour and assessment for regional and metastatic disease. For locoregional evaluation, as with rADC, magnetic resonance imaging (MRI) and endorectal ultrasound (ERUS) should be carried out.34 ERUS is essential at determining the depth of tumour invasion, particularly with T1/2 lesions,6 as well as excluding an anal origin with rectal extension. For more advanced T3/4 tumours and in order to determine locoregional nodal involvement, pelvic MRI provides improved definition.35,36 Contrary to colon cancer, there is no evidence in literature that serum levels of carcinoembryonic antigen (CEA) should be part of the initial diagnosis (neither for tumour evaluation during treatment nor the follow-up) of rSCC, not to mention the low sensitivity and specificity of this tumour marker.
Table 2.
Diagnostic work-up in rSCC, rADC, aSCC
| rSCC | rADC | aSCC | |
|---|---|---|---|
| Rectoscopy | + | + | − |
| Colonoscopy | + | + | + |
| Pelvic MRI | + | + | + |
| CT scan | + | + | + |
| FDG-PET/CT | + | (+) | + |
| SCCAg | − | − | (+) |
aSCC, anal squamous cell carcinoma; CT, computed tomography; FDG, fluorodeoxyglucose; MRI, magnetic resonance imaging; PET, positron emission tomography; rADC, adenocarcinoma of the rectum; rSCC, rectal squamous cell carcinoma; SCCAg, squamous cell carcinoma-associated antigen.
As for anal cancers, fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT), although optional, is recommended for initial staging of rSCC as well as to assess the tumour response to treatment.37
Staging
Accurate staging of rSCC is of critical importance. It confers prognosis and dictates management as in aSCC and rADC. RSCC follows the same pattern of lymphatic spread as rADC. The most common metastatic organs are liver, lung and bones.20
There is no consensus for rSCC staging as to which TNM classification should be used. Some authors propose to use the rADC TNM classification due to the shared localisation and others prefer to use the aSCC TNM classification due to their common histology.5 As a result, there is controversy as to whether the T stage of this malignancy should be based on size, as in aSCC (AJCC-anus), or on depth of invasion, as in rADC (AJCC-rectum).
Goffredo et al.,38 in their population-based study including 2881 rSCC patients, separated them in to two different groups according to AJCC-anus and AJCC-rectum classification. Stages I and IV were represented by the same subset of patients in both staging systems (anal or rectal) and therefore had the same 5-year disease-specific survival (DSS) (93% and 21%, respectively, for stage I and IV). When patients were staged according to AJCC-rectum, the 5-year DSS for stages I, II and III was 80%, 61% and 62%, respectively. When the same was done using the AJCC-anus classification, 5-year DSS across all three stages (I, I and III) was 87%, 72% and 59% respectively.38 Goffredo et al.38 concluded that the AJCC-anus staging system (based on histology and therefore size) has a better prognostic discrimination and should be used to predict rSCC survival.
Treatment options
Though histologically similar to aSCC and anatomically identical to rADC, rSCC is a rare and unique malignancy for which evidence and clinical consensus surrounding treatment are lacking.39 No treatment guidelines (ESMO or NCCN) are available and treatment options are extrapolated from rADC and aSCC.34 In the past, rSCC treatment has traditionally involved surgery with significant morbidity (13%-46%) and mortality (1%-7%),39,40 in some cases preceded or followed by radiotherapy and/or chemotherapy.6 At the beginning of the 20th century, surgery was progressively abandoned with a shift towards CRT. Nowadays, poor quality data support treating rSCC as aSCC with high-dose definitive CRT. This change of treatment paradigm derives primarily from case reports, case series, one large population-based study2 and one meta-analysis.6 In view of the rarity of the disease and in order to plan the best treatment strategy, each patient’s case should be discussed in a multidisciplinary specialists’ meeting.
Non-metastatic disease
aSCC is treated with CRT consisting of 50.4 Gy of radiotherapy over 28 days, combined with 5-FU and MMC,41 surgery being an option only as rescue treatment in case of local recurrence or incomplete response. Definitive CRT has become the accepted standard treatment of aSCC.38,42, 43, 44
In their literature (487 articles, spanning from 1946 to 2015), Guerra et al.6 show that patients with rSCC undergoing definitive CRT have a superior OS (86%) to those operated on (48%). Likewise, local recurrence (25% versus 10%) and metastasization rates (30% versus 13%) are also improved with CRT compared with surgery upfront.6 These results are corroborated by a SEER registry population-based analysis of 999 rSCC patients treated from 1998 to 2011.2 Patients with rSCC greatly benefited from radiotherapy (improvement in OS from 51 to 135 months). Based on those results these authors recommended a radiotherapy-based treatment of rSCC patients. Finally, in a large retrospective analysis (data from the National Cancer Database) with 2296 rSCC patients, Dutta et al.26 showed that adding surgery after concomitant CRT (trimodality treatment) did not impact OS compared with CRT alone (P = 0.909 on multivariate analysis) supporting the effectiveness of CRT alone, without surgery.26 This is consistent with a prior report by Kulaylat et al.3 who showed that performing salvage surgery (i.e. ≥12 weeks after CRT) had no survival impact in patients with locally advanced rSCC.
In the largest (23 patients) prospective case series of rSCC, Loganadane et al.,7 treated 21 patients with CRT and 2 with preoperative CRT followed by surgery. With a median follow-up of 85 months, the clinical complete response rate was 83% and the 5-year disease-free survival, colostomy-free survival and OS rates were 81%, 65% and 86%, respectively. Based on the high local control rate and prolonged survival, the authors recommended that rSCC should be treated similarly to aSCC.
Given the current knowledge and taking under consideration the most important case series (Table 3), but in the absence of robust evidence and guidelines, it is reasonable to suggest that primary treatment should be concomitant definitive CRT. Proposed radiation doses vary (at least 45 Gy going up to 65 Gy) and are delivered as external beam radiation over 28 days (with an average of 1.8 Gy per fraction), combined with 1000 mg/m2/day of 5-FU given as a continuous infusion over days 1 through 4 and repeated on days 29 through 32 (week 5), with 10 mg/m2 (maximal dose of 20 mg) of MMC injected on days 1 and 29. Radiotherapy should target the tumour, mesorectum, presacral nodes and internal iliac nodal basins.39 Similar to aSCC treatment guidelines, most case reports and series of rSCC have used 5-FU associated with MMC or cisplatin44, 45, 46, 47 as the CRT regimen (Table 3) with encouraging outcomes (most patients alive with no evidence of disease in a long follow-up).
Table 3.
Most important reported case series to date in which chemoradiotherapy was the primary treatment
| Authors | Year of publication | Number of patients included | Initial schema of chemotherapy | Initial regimen of RT (Gy) | Follow-up (months), median (range) | Outcome | Disease-free survival, % |
|---|---|---|---|---|---|---|---|
| Clark et al.45 | 2008 | 7 | 5-FU/cisplatin (4) 5-FU/MMC (3) |
50.4-54 | 18 (5-31) | ANED (7) | 100 |
| Sturgeon et al.46 | 2017 | 14 | 5-FU/cisplatin (10) Capecitabin/cisplatin (4) |
38-58.8 | 54 (21.6-195.6) | ANED (11) LR (3) |
72 (5 years) |
| Loganadane et al.7 | 2016 | 23 | 5-FU/cisplatin (12) 5-FU/MMC (3) Capecitabine/MMC (4) Cisplatin weekly (2) None (1) |
45-65 | 85 (12-161) | ANED (18) LR (2) M (2) LR + M (1) |
81 (5 years) |
| Musio et al.5 | 2015 | 8 | 5-FU/MMC (6) Raltitrexed + oxaliplatin (2) |
45-76.5 | 41.75 (1-164) | ANED (7) LR (1) |
87.5 |
| Nahas et al.18 | 2007 | 9 | 5-FU/MMC (6) 5-FU/cisplatin (3) |
50.4 | 31.2 (6-192) | ANED (9) | 100 |
| Péron et al.40 | 2015 | 10 | 5-FU/MMC (4) 5-FU/cisplatin (5) Capecitabine (1) |
45-62 | 42 (6-133) | ANED (8) LR (2) |
80 |
5-FU, 5-fluorouracil; ANED, alive with no evidence of disease; LR, local recurrence; LR + M, both local recurrence and metastasis; M, metastasis; MMC, mitomycin C; RT, radiotherapy.
Metastatic disease
In the whole literature there is no retrospective cohort evaluating the ideal systemic treatment of metastatic disease. All information available is summarized in some isolated case reports referring to patients with metastatic rSCC, with neither reference to the choice of treatment given (type of chemotherapy), nor the patients’ outcome. In the only case series found, Mayo Clinic reported a total of 52 cases of patients with rSCC detected in its tissue registry during the period 1907-1992. Among them, some (exact number not provided) presented metastatic disease in different sites (by decreasing frequency): lymph nodes, liver, peritoneum, lung and bone.20 With no single evidence of the best systemic treatement in the setting of metastatic rSCC, chemotherapy based on the principles of metastatic aSCC (5-FU + cisplatin or carboplatin + paclitaxel or 5-FU + oxaliplatin) should be considered for those cases.48,49
Treatment evaluation–follow-up–recurrence
As with rADC and aSCC, one of the most pertinent issues with CRT is determining treatment response, which currently can only be confirmed by histopathology.50 A suspicious residual mass or scar should be considered for selective biopsy. A rigorous monitoring of residual masses need to be carried out to avoid useless rectal biopsy, given the substantial risk of radionecrosis.40
Response to CRT for rSCC has been assessed variably, from 6 to 8 weeks up to 6 months after the conclusion of treatment. Contrary to rADC where the evaluation of neoadjuvant radiotherapy should be done in a window of 8-12 weeks, as surgery follows afterwards,1 it has been suggested that for rSCC a more prolonged assessment, with regular endoscopic and radiological controls even up to 6 months, could be required for a better evaluation of tumour response. This is in line with the finding that patients who will end up being in pathological complete response had clinical and radiological findings, suggestive of persistent disease, in the early ‘post’ CRT stage.18 A delayed tumour response may continue for 4-6 months after the completion of CRT,5 as is shown in the ACT II trial by Glynne-Jones et al.,51 suggesting that a ‘wait and see’ approach of 6 months, provided absence of clear tumour progression, is the optimal one to assess response in rSCC.
Evaluation involves a combination of clinical and radiological methods (Table 4) by rectoscopy with biopsy (if needed) and imaging assessment with MRI ± (PET) CT.45,52 Use of FDG-PET/CT has been assessed in rSCC in a recent publication to monitor treatment response and select candidates who request surgery in case of incomplete tumour response.7 Questions such as the efficiency of existing imaging methods and optimal timing of surgery, are raised.
Table 4.
Treatment evaluation—follow-up techniques
| rSCC | rADC | aSCC | |
|---|---|---|---|
| Anoscopy | − | − | + |
| Rectoscopy | + | + | − |
| MRI | + | + | + |
| (FDG-PET)/CTa | + | + | + |
| Endoscopic-US | + | + | + |
aSCC, anal squamous cell carcinoma; CT, computed tomography; FDG, fluorodeoxyglucose; MRI, magnetic resonance imaging; PET, position emission tomography; rADC, adenocarcinoma of the rectum; rSCC, rectal squamous cell carcinoma; US, ultrasound.
FDG-PET/CT can be considered for treatment evaluation and research of post-CRT resistant disease, but not for long-term follow-up.
Patients with complete clinical and radiological response should undergo follow-up at regular intervals (physical examination with palpation of inguinal lymph nodes, blood biochemical control) with reducing frequency out to 5 years, generally every 3-6 months for the first 2 years and 6-12 monthly out to 5 years.6 One could recommend pelvic MRI as well as rectoscopy on a 6-monthly basis for the 3 first years in the research of local recurrence, which, in analogy to aSCC, should be more frequent than a distant one. Bearing in mind that very few relapses occur after 3 years in the setting of aSCC, extended imaging surveillance after this time should not be recommended, nor for rSCC; a thoraco-abdominal CT scan should be carried out once per year up to 3 years of follow-up (going up to 5 years if clinically indicated).
In the case of local relapse after complete response or in the case of partial response, salvage surgery is the only option and should be considered. In the case of distant recurrence, systemic therapy (see ‘Metastatic disease’ paragraph) should be proposed and the situation should be discussed in a multidisciplinary tumour board.
Discussion
Our literature review has one important limitation that lies in the quality of the data collected from mainly retrospective studies. It is possible that patients, included in cited studies, did not meet the criteria proposed by Williams et al.10 and therefore skew observation and conclusion towards anal cancer. To minimise this possible bias, we carefully selected studies using criteria proposed by Williams et al.10 or selected patients with criteria close to those proposed by Williams et al.10
Although some will argue that rSCCs do not exist, biological data show otherwise. rSCC is a rare entity with no known risk factors and biological behaviour. Due to its rectal localisation, rSCC has been traditionally treated with surgery first, with poor survival results. Retrospective series and registry analyses show that diagnostic work-out is similar to that of rADC, but staging should be based on the AJCC-anus staging system.
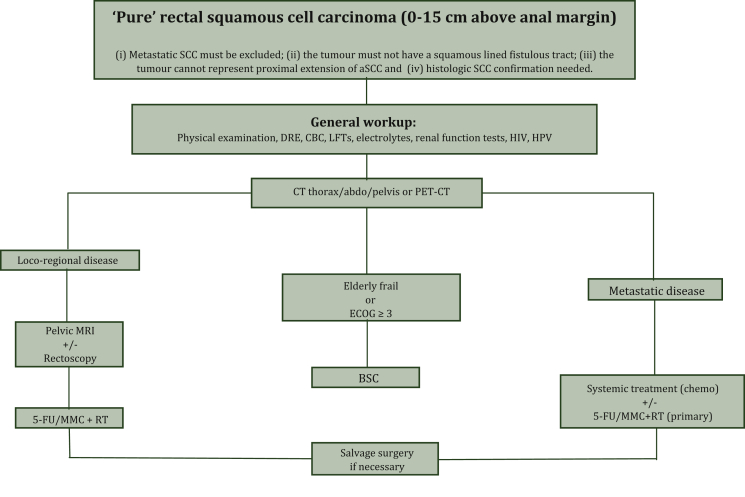
Currently, there are no official guidelines concerning therapeutic strategies. For localised disease, retrospective evidence points towards treating similarly to aSCC, with definitive CRT (based on 5-FU and MMC associated with concomitant external radiotherapy) with no surgery. Response assessment following treatment is again inspired by aSCC with endoscopy, MRI and (PET)/CT. In the case of local relapse, surgery is the treatment of choice (Figure 21,39,53). Every case should benefit from a multidisciplinary tumour board discussion.
Figure 2.
Proposed treatment algorithm for rSCC. Inspired, influenced and adapted from references Glynne-Jones et al.,1 Song et al.39 and Lukovic et al.53aSCC, anal squamous cell carcinoma; BSC, best supportive care; CBC, complete blood count; CT, computed tomography; DRE, digital rigid exam; ECOG, Eastern Cooperative Oncology Group; 5-FU, 5-fluoruracil; HIV, human immunodeficiency virus; HPV, human papillomavirus; LFTs, liver function tests; MMC, mitomycin; MRI, magnetic resonance imaging; PET, positron emission tomography; RT, radiotherapy; SCC, squamous cell carcinoma.
For advanced disease, data in the literature are extremely poor to provide any firm recommendation. Due to the histotype of rSCC and its close localisation to aSCC, we are inclined to recommend systemic treatment which is effective in aSCC, such as paclitaxel with carboplatin.
Much is unknown about this rare entity, such as the association between HPV and rSCC, the molecular profile of this tumour or the most effective treatments. We, as others, think that the creation of an international registry and a biological repository could increase our understanding of this rare entity.
By contrast, it would be important to know the exact clinical and pathologic response rates for rSCC with CRT or radiotherapy alone, as well as patterns of failure. Timing for tumour response is paramount and data are not clear but tilt toward waiting until 6 months after definitive treatment, as in aSCC. Follow-up is similar to that of aSCC.
Acknowledgments
Funding
None declared.
Disclosure
TK discloses consulting or advisory role for Merck Sharp & Dohme (MSD), Bristol-Myers Squibb (BMS), Lilly, Roche, Boehringer Ingelheim and Servier. All other authors have declared no conflicts of interest.
References
- 1.Glynne-Jones R., Wyrwicz L., Tiret E. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22–iv40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 2.Chiu M.S., Verma V., Bennion N.R. Comparison of outcomes between rectal squamous cell carcinoma and adenocarcinoma. Cancer Med. 2016;5(12):3394–3402. doi: 10.1002/cam4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulaylat A.S., Hollenbeak C.S., Stewart D.B. Squamous cancers of the rectum demonstrate poorer survival and increased need for salvage surgery compared with squamous cancers of the anus. Dis Colon Rectum. 2017;60(9):922–927. doi: 10.1097/DCR.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 4.Kang H., O’Connell J.B., Leonardi M.J., Maggard M.A., McGory M.L., Ko C.Y. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis. 2006;22(2):183–189. doi: 10.1007/s00384-006-0145-2. [DOI] [PubMed] [Google Scholar]
- 5.Musio D., De Felice F., Manfrida S. Squamous cell carcinoma of the rectum: the treatment paradigm. Eur J Surg Oncol. 2015;41(8):1054–1058. doi: 10.1016/j.ejso.2015.03.239. [DOI] [PubMed] [Google Scholar]
- 6.Guerra G.R., Kong C.H., Warrier S.K., Lynch A.C., Heriot A.G., Ngan S.Y. Primary squamous cell carcinoma of the rectum: an update and implications for treatment. World J Gastrointest Surg. 2016;8(3):252. doi: 10.4240/wjgs.v8.i3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loganadane G., Servagi-Vernat S., Schernberg A. Chemoradiation in rectal squamous cell carcinoma: bi-institutional case series. Eur J Cancer. 2016;58:83–89. doi: 10.1016/j.ejca.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Schmidtmann M. Zur Kenntnis seltener Krebsformen. Virchows Arch Pathol Anat Physiol Klin Med. 1919;226:100–118. [Google Scholar]
- 9.Raiford T. Epitheliomata of the lower rectum and anus. Surg Gynaecol Obstet. 1933;57:21–35. [Google Scholar]
- 10.Williams G.T., Blackshaw A.J., Morson B.C. Squamous carcinoma of the colorectum and its genesis. J Pathol. 1979;129:139–147. doi: 10.1002/path.1711290306. [DOI] [PubMed] [Google Scholar]
- 11.Chen K.T.K. Colonic adenomatous polyp with focal squamous metaplasia. Hum Pathol. 1981;12(9):848–849. doi: 10.1016/s0046-8177(81)80090-1. [DOI] [PubMed] [Google Scholar]
- 12.Almagro U.A., Pintar K., Zellmer R.B. Squamous metaplasia in colorectal polyps. Cancer. 1984;53:2679–2682. doi: 10.1002/1097-0142(19840615)53:12<2679::aid-cncr2820531219>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Lundquest D.E., Marcus J.N., Thorson A.G., Massop D. Primary squamous cell carcinoma of the colon arising in a villous adenoma. Hum Pathol. 1988;19(3):362–364. doi: 10.1016/s0046-8177(88)80532-x. [DOI] [PubMed] [Google Scholar]
- 14.Ozuner G., Aytac E., Gorgun E., Bennett A. Colorectal squamous cell carcinoma: a rare tumor with poor prognosis. Int J Colorectal Dis. 2015;30(1):127–130. doi: 10.1007/s00384-014-2058-9. [DOI] [PubMed] [Google Scholar]
- 15.LeBlanc L.J., Buie L.A., Dockerty M.B. Squamous-cell epithelioma of the rectum. Ann Surg. 1950;131(3):392–399. doi: 10.1097/00000658-195003000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaworski R.C., Biankin S.A., Baird P.J. Squamous cell carcinoma in situ arising in inflammatory cloacogenic polyps: report of two cases with PCR analysis for HPV DNA. Pathology. 2001;33:312–314. [PubMed] [Google Scholar]
- 17.Ouban A., Nawab R.A., Coppola D. Diagnostic and pathogenetic implications of colorectal carcinomas with multidirectional differentiation: a report of 4 cases. Clin Colorectal Cancer. 2002;1(4):243–248. doi: 10.3816/CCC.2002.n.006. [DOI] [PubMed] [Google Scholar]
- 18.Nahas C.S.R., Shia J., Joseph R. Squamous-cell carcinoma of the rectum: a rare but curable tumor. Dis Colon Rectum. 2007;50(9):1393–1400. doi: 10.1007/s10350-007-0256-z. [DOI] [PubMed] [Google Scholar]
- 19.Audeau A., Han H.W., Johnston M.J., Whitehead M.W., Frizelle F.A. Does human papilloma virus have a role in squamous cell carcinoma of the colon and upper rectum? Eur J Surg Oncol. 2002;28(6):657–660. doi: 10.1053/ejso.2002.1304. [DOI] [PubMed] [Google Scholar]
- 20.Frizelle F.A., Hobday K.S., Batts K.P., Nelson H. Adenosquamous and squamous carcinoma of the colon and upper rectum: a clinical and histopathologic study. Dis Colon Rectum. 2001;44(3):341–346. doi: 10.1007/BF02234730. [DOI] [PubMed] [Google Scholar]
- 21.Sotlar K., Köveker G., Aepinus C., Selinka H., Kandolf R., Bültmann B. Human papillomavirus type 16–associated primary squamous cell carcinoma of the rectum. Gastroenterology. 2001;120(4):988–994. doi: 10.1053/gast.2001.22523. [DOI] [PubMed] [Google Scholar]
- 22.Kong C.S., Welton M.L. Role of human papillomavirus in squamous cell metaplasia-dysplasia-carcinoma of the rectum. Am J Surg Pathol. 2007;31(6):7. doi: 10.1097/01.pas.0000213441.86030.fc. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda A., Takahashi K., Yamaguchi T. HPV infection in an HIV-positive patient with primary squamous cell carcinoma of rectum. Int J Clin Oncol. 2009;14(6):551–554. doi: 10.1007/s10147-009-0890-7. [DOI] [PubMed] [Google Scholar]
- 24.Choi H., Lee H.W., Ann H.W. A case of rectal squamous cell carcinoma with metachronous diffuse large B cell lymphoma in an HIV-infected patient. Infect Chemother. 2014;46(4):257. doi: 10.3947/ic.2014.46.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anal cancer’s survival rates. https://www.cancer.org/cancer/anal-cancer/detection-diagnosis-staging/survival-rates.html Available at. Accessed June 3, 2021.
- 26.Dutta S.W., Alonso C.E., Waddle M.R., Khandelwal S.R., Janowski E.-M., Trifiletti D.M. Squamous cell carcinoma of the rectum: practice trends and patient survival. Cancer Med. 2018;7(12):6093–6103. doi: 10.1002/cam4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diao J.-D., Wu C.-J., Cui H.-X. Nomogram predicting overall survival of rectal squamous cell carcinomas patients based on the SEER database: a population-based STROBE cohort study. Medicine (Baltimore) 2019;98(46):e17916. doi: 10.1097/MD.0000000000017916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyson T., Draganov P.V. Squamous cell cancer of the rectum. World J Gastroenterol. 2009;15(35):4380. doi: 10.3748/wjg.15.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Errasti Alustiza J., Espín Basany E., Reina Duarte Á. Rare tumors of the rectum. Narrative Review. Cir Esp. 2014;92(9):579–588. doi: 10.1016/j.ciresp.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Glynne-Jones R., Nilsson P.J., Aschele C. Anal cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii10–iii20. doi: 10.1093/annonc/mdu159. [DOI] [PubMed] [Google Scholar]
- 31.Petrelli N.J., Palmer M., Herrera L., Bhargava A. The utility of squamous cell carcinoma antigen for the follow-up of patients with squamous cell carcinoma of the anal canal. Cancer. 1992;70(1):35–39. doi: 10.1002/1097-0142(19920701)70:1<35::aid-cncr2820700106>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.Williams M., Swampillai A., Osborne M. Squamous cell carcinoma antigen: a potentially useful prognostic marker in squamous cell carcinoma of the anal canal and margin. Cancer. 2013;119(13):2391–2398. doi: 10.1002/cncr.28055. [DOI] [PubMed] [Google Scholar]
- 33.Rasheed S., Yap T., Zia A., McDonald P.J., Glynne-Jones R. Chemo-radiotherapy: an alternative to surgery for squamous cell carcinoma of the rectum - report of six patients and literature review. Colorectal Dis. 2009;11(2):191–197. doi: 10.1111/j.1463-1318.2008.01560.x. [DOI] [PubMed] [Google Scholar]
- 34.Ballestero Pérez A., Abadía Barnó P., García-Moreno Nisa F., Die Trill J., Galindo Álvarez J. Primary squamous cell carcinoma of the rectum: an atypical histology. Rev Esp Enferm Dig. 2016;108:826–835. doi: 10.17235/reed.2016.3975/2015. [DOI] [PubMed] [Google Scholar]
- 35.Dewhurst C., Rosen M.P., Blake M.A. ACR appropriateness criteria® pretreatment staging of colorectal cancer. J Am Coll Radiol. 2012;9(11):775–781. doi: 10.1016/j.jacr.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 36.Wu J. Rectal cancer staging. Clin Colon Rectal Surg. 2007;20(3):148–157. doi: 10.1055/s-2007-984859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal A., Marcus C., Xiao J., Nene P., Kachnic L.A., Subramaniam R.M. FDG PET/CT in the management of colorectal and anal cancers. Am J Roentgenol. 2014;203(5):1109–1119. doi: 10.2214/AJR.13.12256. [DOI] [PubMed] [Google Scholar]
- 38.Goffredo P., Robinson T.J., Frakes J.M., Utria A.F., Scott A.T., Hassan I. Comparison of anal versus rectal staging in the prognostication of rectal squamous cell carcinoma: a population-based analysis. Dis Colon Rectum. 2019;62(3):302–308. doi: 10.1097/DCR.0000000000001205. [DOI] [PubMed] [Google Scholar]
- 39.Song E.J., Jacobs C.D., Palta M., Willett C.G., Wu Y., Czito B.G. Evaluating treatment protocols for rectal squamous cell carcinomas: the Duke experience and literature. J Gastrointest Oncol. 2020;11(2):242–249. doi: 10.21037/jgo.2018.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Péron J., Bylicki O., Laude C., Martel-Lafay I., Carrie C., Racadot S. Nonoperative management of squamous-cell carcinoma of the Rectum. Dis Colon Rectum. 2015;58(1):60–64. doi: 10.1097/DCR.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 41.Nigro N.D., Vaitkevicius V.K., Considine B.J. Combined therapy for cancer of the anal: a preliminary report. Dis Colon Rectum. 1974;17:354–356. doi: 10.1007/BF02586980. [DOI] [PubMed] [Google Scholar]
- 42.Northover J., Glynne-Jones R., Sebag-Montefiore D. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I) Br J Cancer. 2010;102(7):1123–1128. doi: 10.1038/sj.bjc.6605605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flam M., John M., Pajak T.F. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14(9):2527–2539. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 44.James R.D., Glynne-Jones R., Meadows H.M. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2×2 factorial trial. Lancet Oncol. 2013;14(6):516–524. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 45.Clark J., Cleator S., Goldin R., Lowdell C., Darzi A., Ziprin P. Treatment of primary rectal squamous cell carcinoma by primary chemoradiotherapy: should surgery still be considered a standard of care? Eur J Cancer. 2008;44(16):2340–2343. doi: 10.1016/j.ejca.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Sturgeon J.D., Crane C.H., Krishnan S. Definitive chemoradiation for squamous cell carcinoma of the rectum. Am J Clin Oncol. 2017;40(2):163–166. doi: 10.1097/COC.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 47.Sundriyal D., Shirsi N., Kotwal S., Dawar R. Squamous cell carcinoma of rectum: how to treat? Indian J Surg Oncol. 2015;6(3):300–302. doi: 10.1007/s13193-015-0434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao S., Sclafani F., Eng C. InterAACT: a multicentre open label randomised phase II advanced anal cancer trial of cisplatin (CDDP) plus 5-fluorouracil (5-FU) vs carboplatin (C) plus weekly paclitaxel (P) in patients (pts) with inoperable locally recurrent (ILR) or metastatic treatment naïve disease - An International Rare Cancers Initiative (IRCI) trial. Ann Oncol. 2018;29:viii715–viii716. [Google Scholar]
- 49.Matsunaga M., Miwa K., Oka Y. Successful treatment of metastatic anal canal adenocarcinoma with mFOLFOX6 + Bevacizumab. Case Rep Oncol. 2016;9(1):249–254. doi: 10.1159/000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker A.S., Zwintscher N.P., Johnson E.K. Future directions for monitoring treatment response in colorectal cancer. J Cancer. 2014;5(1):44–57. doi: 10.7150/jca.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glynne-Jones R., Sebag-Montefiore D., Meadows H.M. Best time to assess complete clinical response after chemoradiotherapy in squamous cell carcinoma of the anus (ACT II): a post-hoc analysis of randomised controlled phase 3 trial. Lancet Oncol. 2017;18(3):347–356. doi: 10.1016/S1470-2045(17)30071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M.L.C., Heriot A., Leong T., Ngan S.Y.K. Chemoradiotherapy in the management of primary squamous-cell carcinoma of the rectum: squamous-cell carcinoma of the rectum. Colorectal Dis. 2011;13(3):296–301. doi: 10.1111/j.1463-1318.2009.02154.x. [DOI] [PubMed] [Google Scholar]
- 53.Lukovic J., Kim J.J., Krzyzanowska M., Chadi S.A., Taniguchi C.M., Hosni A. Anal adenocarcinoma: a rare malignancy in need of multidisciplinary management. JCO Oncol Pract. 2020;16(10):635–640. doi: 10.1200/OP.20.00363. [DOI] [PubMed] [Google Scholar]