Highlights
-
•
US-assisted photocatalysis by ZnO leads to efficient mineralization of diclofenac.
-
•
Synergistic effects are observed only when ZnO nanorods are used.
-
•
Tests in simulated water matrix show enhanced performance with respect to TiO2.
-
•
Reaction intermediates of photo- and sonophotocatalysis are identified.
Keywords: Advanced oxidation processes, ZnO, Diclofenac, Piezo-enhanced photodegradation, Mineralization, Transformation products
Abstract
The degradation of diclofenac has been realized for the first time by a piezo-enhanced sonophotocatalytic approach based on ZnO. The sonophotocatalytic degradation showed a slight enhancement in the degradation of the parent compound, whereas strong synergistic effects were observed for the mineralization process when suitable ZnO morphologies are used, reaching 70% of complete degradation of 25 ppm diclofenac using 0.1 g/L ZnO in 360 min. Tests in a complex water matrix show enhanced diclofenac removal, outperforming a TiO2 benchmark photocatalyst. These promising experimental results promote this process as a good alternative to traditional degradation approaches for remediation of real water matrices.
Owing to the growth and aging of world population, in the last years a high consumption of drugs has been recorded. As a result, concentrations of pharmaceuticals in surface waters are on the rise, also due to the low removal efficiency of wastewater treatment plants. Diclofenac (DFC) is a non-steroidal and anti-inflammatory drug, widely used in the form of a sodium salt as analgesic, antiarthritic and antirheumatic [1]. As many other pharmaceuticals, DCF is a recalcitrant contaminant hard to remove by conventional techniques, such as activated sludge [2]. The global increase in water consumption and the water scarcity issues faced by numerous regions urge us to develop innovative approaches for water decontamination and reuse. Advanced oxidation processes (AOP) represent a class of treatment methods that not only remove hazardous pollutants but also degrade them. Ozonation, heterogeneous photocatalysis, Fenton oxidation, and sonolysis are among the most investigated technologies [3], [4], [5]. A crucial disadvantage of these methods is represented by the possible formation of undesired by-products, which can be more dangerous than the parent compound. The use of combined techniques can positively affect the efficiency of the process, speeding up the degradation and reducing noxious by-product formation. In this respect, the combination of ultrasound (US) with photocatalysis has been reported to enhance the formation of highly active radical species [6], [7], promote mass transfer and contribute to continuously regenerating the surface of the photocatalyst [8]. In this respect, TiO2 has been, by far, the most investigated photocatalyst for sonophotocatalytic water remediation. However, TiO2 presents several disadvantages. For instance, our recent investigation on sonophotocatalytic degradation of DCF by micro-sized TiO2 revealed the crucial role of the water medium, showing a decrease in performance of the photocatalyst in terms of DCF abatement, number of by-products formed and their degradation, when drinking water is used instead of ultrapure water [9].
Piezoelectric-assisted photocatalysis (piezophotocatalysis) is a new concept in the realm of innovative pollutant degradation methods [10]. By this approach a mechanical energy (e.g., ultrasonic vibration) enhances the separation of photoinduced charge carriers by piezoelectric effect [11], [12], [13], [14]. The piezophotocatalytic ability of ZnO has been very recently applied for water decontamination [15], [16]. However, to the best of our knowledge, the DCF degradation by ultrasound-assisted photocatalysis by ZnO has scarcely been investigated [17].
Two ZnO samples (ZnO_1 and ZnO_2), which have been previously tested for different applications showing very similar activity [18], are here investigated for the piezo-assisted photocatalytic DCF degradation in different water matrices (ultrapure and simulated drinking water). The two ZnO samples are characterized by similar surface area, phase composition (Fig. S2) and optical properties (Table S2), but different morphology. In particular, the ZnO_1 morphology is characterized by nanorods with hexagonal base (Figs. S3a and S4a,b), indicative of a peculiar directional growth; conversely, ZnO_2 exhibits star-shaped clusters made up of large aggregates of crystals, as observed by HR-TEM images, indicative of a growth by random orientation (Figs. S3b and S4c,d). As reported in the literature [16], morphology can play a key role in the piezoelectric effect of the materials, as we here demonstrate for DCF degradation.
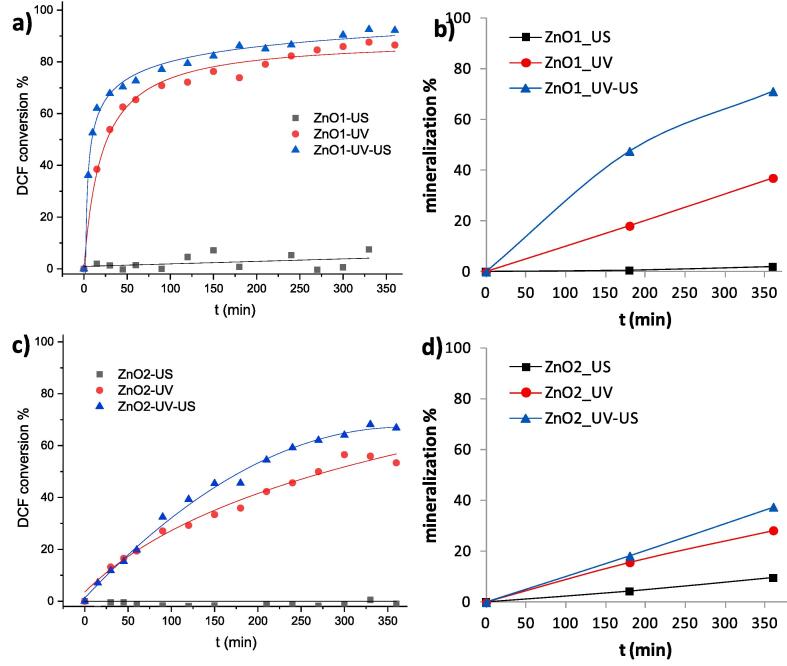
Samples were tested towards the degradation of DCF using sonocatalysis (tests labeled with the suffix _US), photocatalysis (_UV) and sonophotocatalysis (_UV-US), as detailed in Section S1. Fig. 1 reports degradation test results in terms of DCF removal and mineralization degree. It is clear that ZnO_1 presents a much higher photocatalytic and sonophotocatalytic activity than ZnO_2, both in terms of DCF disappearance and mineralization. It should be noted that both samples presented comparable dark adsorption.
Fig. 1.
DCF conversion (a,c) and mineralization degree (b,d) during tests using ZnO_1 (a,b) and ZnO_2 (c,d) in ultrapure water. Lines are reported only as guide for the eye.
When comparing the combined and single degradation tests, it is apparent that no clear sonocatalytic degradation can be observed and that DCF removal during photocatalytic tests closely mirrors that of sonophotocatalytic tests for both samples. However, mineralization data of ZnO_1 clearly show synergistic effects when ultrasound and photocatalysis are combined. To the authors’ best knowledge, this is the first evidence of synergistic effects in ultrasound-assisted photocatalysis of diclofenac by ZnO. A direct comparison between the present results and those reported in the scientific literature is not easy, because reaction conditions differ, in particular in terms of catalyst concentration, light irradiation, and pollutant concentration [17], [19], [20]. Moreover, mineralization data are seldom reported. However, the present sonophotocatalytic tests with ZnO_1 led to a quantitative DCF degradation with a rather low photocatalyst loading (0.1 g/L) and working with a relatively high initial DCF concentration (25 ppm) and, more important, to a high degree of mineralization in reasonable time. The different performances of ZnO_1 and ZnO_2 are notable considering their similar surface area and structural features (Table S2). It is noteworthy that the two materials exhibit very different morphologies, with ZnO_1 showing nanorod structures. Piezoelectric effects are well known to be dependent on the sample morphology, with elongated morphologies enhancing charge separation phenomena [21], [22]. The presence of a mechanical energy can induce positively and negatively charged dipoles on the surface of elongated ZnO rods [23]. The electric polarization of dielectric materials is known to act as driving force for transporting free charge carriers to the surface. These phenomena can support radical formation, such as hydroxyl radicals, and promote DCF degradation.
Moreover, ZnO_1 notably outperforms the TiO2 benchmark during sonophotocatalytic tests in terms of mineralization degree (Fig. S5), reaching over 70% total mineralization after 180 min with respect to 52% of TiO2_ref (Fig. S5). It should be noted that TiO2_ref gives rise to a complete DCF disappearance in less than 120 min, whereas DCF traces are still appreciatble after 180 min in tests with ZnO_1. This suggests for the first time a difference in the reaction mechanism for TiO2 and ZnO, as also supported by data on ZnO_2: the latter shows ca. 37% mineralization after 360 min, when DCF disappearance is merely 66%, whereas TiO2_ref shows a comparable mineralization after 180 min when the disappearance of the parent compound is complete.
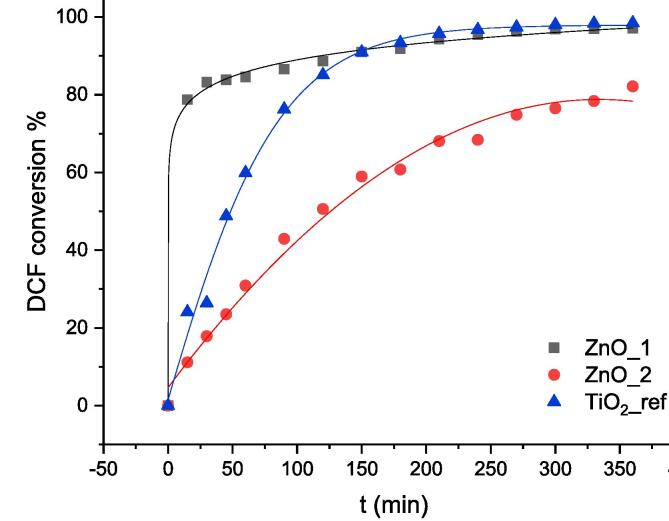
Even more interestingly, ZnO samples not only retained their degradation performance when using a simulated drinking water matrix, but the DCF conversion showed a slight improvement (Fig. 2): for ZnO_1 an almost complete disappearance of DCF (>95%) is observed in 240 min in drinking water matrix, while ZnO_2 displayed an improvement in DCF conversion at 180 min from 45% to over 60% when changing the water matrix. It is noteworthy that TiO2 worsens its photocatalytic and sonophotocatalytic performance in simulated drinking water (Fig. 3), in agreement with previous reports regarding to complex water matrices [9], due the presence of common electrolytes like chlorides and carbonates [24]. The enhanced performance of ZnO could possibly be attributed to the slightly alkaline pH of drinking water, which promotes the stability of ZnO [25].
Fig. 2.
DCF conversion by sonophotocatalysis in simulated drinking water by ZnO_1 and ZnO_2. Lines are reported only as a guide to better identify the trend.
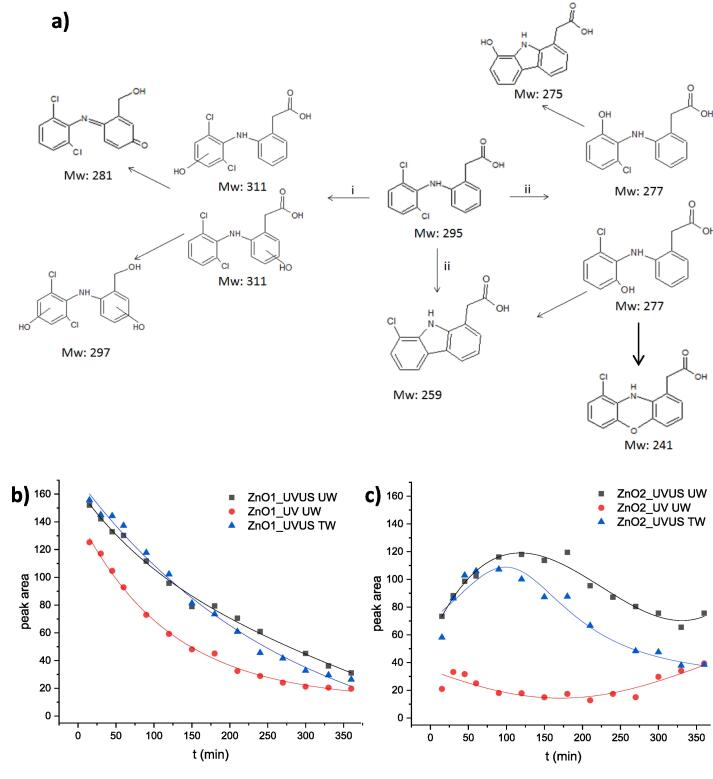
Fig. 3.
Proposed photodegradation pathway of DCF (A), Time profile of TP with Mw 311 in tests using ZnO_1 (B) and ZnO_2 (C) in either ultrapure water (UW) or tap water (TW). Lines are reported only as a guide to better identify the trend.
The photocatalytic and sonophotocatalytic reaction pathways of DCF degradation in ultrapure water by ZnO were investigated via UPLC-MS. The analysis of the time-profiles of the transformation products (TPs) arising from the incomplete DCF mineralization reveals two main possible reactions: i) oxidation and ii) dechlorination (Fig. 3a).
The type of TPs and their time-profiles are the same for both the degradation tests carried out by sonophoto- and photocatalysis and exhibit a bell-shaped time-profile both in ultrapure and simulated drinking water (Figs. 3b,c and S6) by the use of both photocatalysts. Unlike what was previously observed for TiO2 [9], none of the TPs tend to accumulate over time, promoting the piezo-enhanced photodegradation process as a good alternative to traditional degradation approaches, eliminating by-products accumulation in water matrix. More in detail, the time profile plots reveal that the TP at 311 m/z is the prominent peak, whereas the other co-products are present in trace amount. Moreover, owing to its higher activity at the beginning of the ultrasound-assisted photodegradation process, ZnO_1 generates this TP with higher intensity with respect to ZnO_2 but with a subsequently faster abatement. Moreover, while the TPs from sonophotocatalytic degradation by ZnO_2 seem to reach an equilibrium concentration after about 300 min treatment, the more effective ZnO_1 continues their degradation, strongly reducing their peak area, in good agreement with the TOC results. However, both ZnO photocatalysts lead to the production of a number of TPs far lower than that previously observed with TiO2 [9], corroborating the hypothesis of different reaction mechanisms that are under investigation. Furthermore, comparing the time profiles of TPs produced by TiO2 [9] and ZnO_1 (Fig. S6) in ultrapure water under sonophotocatalysis, it is evident that the peak areas of TPs from •OH addition pathway (isomers with Mw 311) exhibit similar bell-shaped trends. On the contrary, the peak area of TP from the dechlorination route (Mw 277) is very low for ZnO_1, whereas a growing trend was appreciable for TiO2. These results are in agreement with the different mineralization degrees observed for the two oxides (Figs. 1a and S5).
In summary, an innovative piezo-enhanced sonophotocatalytic process for diclofenac degradation is here reported. In the presence of ZnO_1 photocatalyst an important synergistic effect was observed particularly for the mineralization process. It has been demonstrated that, by this approach, the presence of electrolytes does not hinder the performances of ZnO, paving the way to applications in real matrices.
CRediT authorship contribution statement
Daniela Meroni: Conceptualization, Methodology, Validation, Formal analysis, Supervision, Writing - original draft, Writing - review & editing. Claudia L. Bianchi: Conceptualization, Supervision, Funding acquisition. Daria C. Boffito: Investigation, Data curation. Giuseppina Cerrato: Investigation, Data curation. Anna Bruni: Investigation, Data curation, Writing - original draft. Marta Sartirana: Investigation, Data curation, Writing - original draft. Ermelinda Falletta: Conceptualization, Methodology, Validation, Formal analysis, Supervision, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Velux Stiftung Foundation for the financial support through the project 1381 “SUNFLOAT – Water decontamination by sunlight-driven floating photocatalytic systems” and Dr Alessandro Di Michele of the Department of Physics and Geology, Università degli Studi di Perugia, for the SEM images.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105615.
Contributor Information
Daniela Meroni, Email: daniela.meroni@unimi.it.
Ermelinda Falletta, Email: ermelinda.falletta@unimi.it.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhang N., Liu G., Liu H., Wang Y., He Z., Wang G. Diclofenac photodegradation under simulated sunlight: effect of different forms of nitrogen and kinetics. J. Hazard. Mater. 2011;192:411–418. doi: 10.1016/j.jhazmat.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 2.S. Bae, D. Kim, W. Lee, Degradation of diclofenac by pyrite catalyzed Fenton oxidation, Appl. Catal., B 134–135 (2013) 93–102. https://doi.org/10.1016/j.apcatb.2012.12.031.
- 3.Guan R., Yuan X., Wu Z., Jiang L., Li Y., Zeng G. Principle and application of hydrogen peroxide based advanced oxidation processes in activated sludge treatment: a review. Chem. Eng. J. 2018;339:519–530. [Google Scholar]
- 4.Salimi M., Esrafili A., Gholami M., Jonidi Jafari A., Rezaei Kalantary R., Farzadkia M., Kermani M., Sobhi H.R. Contaminants of emerging concern: a review of new approach in AOP technologies. Environ. Monit. Assess. 2017;189:414. doi: 10.1007/s10661-017-6097-x. [DOI] [PubMed] [Google Scholar]
- 5.Miklos D.B., Remy C., Jekel M., Linden K.G., Drewes J.E., Hübner U. Evaluation of advanced oxidation processes for water and wastewater treatment – a critical review. Water Res. 2018;139:118–131. doi: 10.1016/j.watres.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 6.Madhavan J., Grieser F., Ashokkumar M. Combined advanced oxidation processes for the synergistic degradation of ibuprofen in aqueous environments. J. Hazard. Mater. 2010;178:202–208. doi: 10.1016/j.jhazmat.2010.01.064. [DOI] [PubMed] [Google Scholar]
- 7.Joseph C.G., Li Puma G., Bono A., Krishnaiah D. Sonophotocatalysis in advanced oxidation process: a short review. Ultrason. Sonochem. 2009;16:583–589. doi: 10.1016/j.ultsonch.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 8.P. Wang, W. Ji, M. Li, G. Zhang, J. Wang, Bi25VO40 microcube with step surface for visible light photocatalytic reduction of Cr(VI): enhanced activity and ultrasound assisted regeneration, Ultrason. Sonochem. 38 (2017) 289–297. https://doi.org/101016/j.ultsonch.2017.03.016. [DOI] [PubMed]
- 9.Meroni D., Jiménez-Salcedo M., Falletta E., Bresolin B.M., Fai Kait C., Boffito D.C., Bianchi C.L., Pirola C. Sonophotocatalytic degradation of sodium diclofenac using low power ultrasound and micro sized TiO2. Ultrason. Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105123. [DOI] [PubMed] [Google Scholar]
- 10.S. Tu, Y. Guo, Y. Zhang, C. Hu, T. Zhang, T. Ma, H. Huang, Piezocatalysis and Piezo‐Photocatalysis: Catalysts Classification and Modification Strategy, Reaction Mechanism, and Practical Application, Adv. Funct. Mater. 2020, 30. 2005158(1-31). https://doi.org/10.1002/adfm.202005158.
- 11.Marino A., Becker R. Piezoelectric effect and growth control in bone. Nature. 1970;228:473–474. doi: 10.1038/228473a0. [DOI] [PubMed] [Google Scholar]
- 12.Katsouras I., Asadi K., Li M., van Driel T.B., Kjær K.S., Zhao D., Lenz T., Gu Y., Blom P.W.M., Damjanovic D., Nielsen M.M., de Leeuw D.M. The negative piezoelectric effect of the ferroelectric polymer poly(vinylidene fluoride) Nat Mater. 2016;15:78–84. doi: 10.1038/nmat4423. [DOI] [PubMed] [Google Scholar]
- 13.Jia Y.M., Luo H.S., Zhao X.Y., Wang F.F. Giant magnetoelectric response from a piezoelectric/magnetostrictive laminated composite combined with a piezoelectric transformer. Adv. Mat. 2008;20:4776–4779. doi: 10.1002/adma.200800565. [DOI] [Google Scholar]
- 14.Starr M.B., Wang X. Fundamental analysis of piezocatalysis process on the surfaces of strained piezoelectric materials. Sci. Rep. 2013;3:2160. doi: 10.1038/srep02160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun C., Fu Y., Wang Q., Xing L., Liu B. Xinyu Xue, Ultrafast piezo-photocatalytic degradation of organic pollutions by Ag2O/tetrapod-ZnO nanostructures under ultrasonic/UV exposure. RSC Adv. 2016;6:87446. doi: 10.1039/c6ra13464e. [DOI] [Google Scholar]
- 16.Chimupala Y., Phromma C., Yimklan S., Semakul N., Ruankham P. Dye wastewater treatment enabled by piezoenhanced photocatalysis of single-component ZnO nanoparticles. RSC Adv. 2020;10:28567. doi: 10.1039/d0ra04746e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madhavan J., Kumar P.S.S., Anandan S., Zhou M., Grieser F., Ashokkumar M. Ultrasound assisted photocatalytic degradation of diclofenac in an aqueous environment. Chemosphere. 2010;80:747–752. doi: 10.1016/j.chemosphere.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Meroni D., Gasparini C., Di Michele A., Ardizzone S., Bianchi C.L. Ultrasound-assisted synthesis of ZnO photocatalysts for gas phase pollutant remediation: Role of the synthetic parameters and of promotion with WO3. Ultrason. Sonochem. 2020;66 doi: 10.1016/j.ultsonch.2020.105119. [DOI] [PubMed] [Google Scholar]
- 19.Sarro M., Gule N.P., Laurenti E., Gamberini R., Paganini M.C., Mallon P.E., Calza P. ZnO-based materials and enzymes hybrid systems as highly efficient catalysts for recalcitrant pollutants abatement. Chem. Eng. J. 2018;334:2530. doi: 10.1016/j.cej.2017.11.146. [DOI] [Google Scholar]
- 20.Silvestri S., Dias Ferreira C., Oliveira V., Varejão J.M.T.B., Labrincha J.A., Tobaldi D.M. Synthesis of PPy-ZnO composite used as photocatalyst for the degradation of diclofenac under simulated solar irradiation. J. Photochem. Photobiol. A. 2019;375:261. doi: 10.1016/j.jphotochem.2019.02.034. [DOI] [Google Scholar]
- 21.Pagano R., Ingrosso C., Giancane G., Valli L., Bettini S. Wet synthesis of elongated hexagonal ZnO microstructures for applications as photo-piezoelectric catalysts. Materials. 2020;13:2938. doi: 10.3390/ma13132938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song J., Zhou J., Wang Z.L. Piezoelectric and semiconducting coupled power generating process of a single ZnO belt/wire. A technology for harvesting electricity from the environment. Nano Lett. 2006;6:1656. doi: 10.1021/nl060820v. [DOI] [PubMed] [Google Scholar]
- 23.Lops C., Ancona A., Di Cesare K., Dumontel B., Garino N., Canavese G., Hérnandez S., Cauda V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B. 2019;243:629. doi: 10.1016/j.apcatb.2018.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.L. Rimoldi, D. Meroni, E. Falletta, V. Pifferi, L. Falciola, G. Cappelletti, S. Ardizzone, Emerging pollutant mixture mineralization by TiO2 photocatalysts. The role of the water medium, Photochem. Photobiol. Sci., 16 (2017) 60. https://doi.org/10.1039/c6pp00214e. [DOI] [PubMed]
- 25.Fatehah M.O., Aziz H.A., Stoll S. Stability of ZnO nanoparticles in solution. influence of ph, dissolution, aggregation and disaggregation effects. J. Colloid Sci Biotechnol. 2014;3:75. doi: 10.1166/jcsb.2014.1072. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.