Abstract
A high degree of regional, temporal and molecular specificity is evident in the regulation of GABAergic signaling in stress-responsive circuitry, hampering the use of systemic GABAergic modulators for the treatment of stress-related psychopathology. Here we investigated the effectiveness of local intervention with the GABA synthetic enzymes GAD65 and GAD67 in the dorsal dentate gyrus (dDG) vs ventral DG (vDG) to alleviate anxiety-like behavior and stress-induced symptoms in the rat. We induced shRNA-mediated knock down of either GAD65 or GAD67 with lentiviral vectors microinjected into the dDG or vDG of young adult male rats and examined anxiety behavior, learning and memory performance. Subsequently we tested whether reducing GAD65 expression in the dDG would also confer resilience against juvenile stress-induced behavioral and physiological symptoms in adulthood. While knock down of either isoform in the vDG increased anxiety levels in the open field and the elevated plus maze tests, the knock down of GAD65, but not GAD67, in the dDG conferred a significant reduction in anxiety levels. Strikingly, this manipulation also attenuated juvenile stress evoked anxiety behavior, cognitive and synaptic plasticity impairments. Local GABAergic circuitry in the DG plays an important and highly region-specific role in control of emotional behavior and stress responding. Reduction of GAD65 expression in the dDG appears to provide resilience to juvenile stress-induced emotional and cognitive deficits, opening a new direction towards addressing a significant risk factor for developing stress and trauma-related psychopathologies later in life.
Keywords: PTSD, GABA, GAD65, GAD67, Dentate gyrus, Stress resilience
Highlights
-
•
GAD67/65 in the dorsal/ventral dentate gyrus differentially modulate anxiety.
-
•
Reduced GAD65 expression in the dorsal dentate gyrus supports stress resilience.
-
•
The dorsal dentate gyrus plays a key role in stress resilience.
1. Introduction
The use of γ-amino butyric acid (GABA) modulators has been tremendously successful in the treatment of anxiety states, but their effectiveness to alleviate symptoms of trauma-related disorders is poorly defined (Guina et al., 2015). This failure may relate to an inability of systemic GABA modulation to selectively modify activity in the relevant stress-responsive brain circuitry. In fact, a high degree of regional, temporal and isoform-specificity has been observed in the regulation of GABAergic interneuron function in the brain in response to different stress experiences (Albrecht et al., 2017).
This specificity is particularly evident in the regulation of the GABA synthetic enzymes glutamic acid decarboxylase (GAD)65 and GAD67 in stress (Bergado-Acosta et al., 2008; Bowers et al., 1998; Gilabert-Juan et al, 2011, 2017; Heldt and Ressler, 2007; Lussier et al., 2013; Makinson et al., 2015). GAD65 and GAD67, which together account for >99% of the GABA synthesis in the mammalian brain, are typically co-expressed in GABAergic cells, are encoded by separate genes and their total and relative expression levels differ between regions and cell populations. They are also differently regulated by phosphorylation and proteolytic cleavage and use different mechanisms of membrane association (summarized in (Lee et al., 2019). In general, while GAD67 is responsible for most of the constitutive GABA production, GAD65 activity is highly regulated and dependent on the cofactor pyridoxal phosphate. However, activity dependent regulation of inhibition through GAD67 has also been reported (Lau and Murthy, 2012). Moreover, both isozymes also can interact directly, forming homo- and heteromultimeric holoenzymes and compensate for each other after genetic disruption (Obata and Otsuka, 2013). Selective alteration in the expression level of these enzymes may thus provide a mechanism of endogenous adaptive modulation in stress circuit activity.
Indeed, a highly specific expression regulation has been observed in the dentate gyrus (DG) and CA3, amygdala, bed nucleus of the stria terminalis (BNST), and various hypothalamic subregions, following conditioned fear (Bergado-Acosta et al., 2008; Heldt and Ressler, 2007), acute stress and chronic stress (Banasr et al., 2017; Bowers et al., 1998; Makinson et al., 2015). Different levels of GAD65 and GAD67 expression have also been reported in the dorsal and ventral dentate gyrus (dDG/vDG) of naïve rodents (Czéh et al., 2013). These two hippocampal sub-regions have been related to different cognitive and emotional aspects of behavior (Fanselow and Dong, 2010), such that the cognitive functions of the hippocampus are considered to be mediated mostly by its dorsal sector, while the ventral hippocampus appears to be more associated with control of behavioral inhibition, stress and emotional memory. The dorsal and ventral hippocampus are differentially recruited upon stress experience, with the vDG assuming a more influential position, re-routing hippocampal output more towards limbic, rather than cortical areas (Segal et al., 2010). Under stress a dynamic routing of hippocampal connectivity occurs favoring the ventral route to the amygdala, while the dorsal route to the neocortex is suppressed. Such a differential modulation of inhibitory functions in the dDG and vDG has also been observed in the juvenile stress (JVS) model of a risk factor to Post-traumatic Stress Disorder (PTSD) (Albrecht et al., 2017). In line with these topographic specifications, we could previously demonstrate region- and layer-specific regulation of GAD65/67 expression in the dDG and vDG, associated with changes in inhibitory control in response to paired-pulse stimulation following JVS (Albrecht et al., 2016). We moreover found that GAD65 expression in the dDG decreases only after controllable but not after uncontrollable stress, whereas its expression in the BLA is reduced regardless of stressor controllability, supporting a distinctive regulation of stress processing by the dDG(Hadad-Ophir et al., 2016).
The role of GAD65 in stress response has previously been addressed with selective genetic intervention. In fact, GAD65(−/−) mice display increased anxiety and avoidance behavior, hyperarousal, generalization of fear memories and deficits in fear extinction, modeling key aspects of PTSD (Müller et al., 2015). These behavioral changes are associated with increased activation of the amygdala and DG (Bergado-Acosta et al., 2014) and disturbances of amygdalo-hippocampal network synchronization (Bergado-Acosta et al., 2008; Sangha et al., 2009). By contrast, GAD65 haplodeficient mice reveal resilience to JVS -induced contextual generalization of fear memory (Müller et al., 2014) and a selective reduction of GAD65 gene expression in the adult DG (Richter-Levin et al., 2019a).
Constitutive deletion of GAD67 in mice is lethal and GAD67 haplodeficiency does not cause apparent change in anxiety levels (Sandhu et al., 2014). However, a selective ablation of GAD67 in somatostatin interneurons has been shown to enhance anxiety like behavior (Miyata et al., 2019). Moreover, a recent study in GAD67 knock out rats has also demonstrated enhancement of contextual fear memory (Fujihara et al., 2021). We propose these gene-dosage -dependent and isozyme-specific effects to be related to a differential role of GAD65 and GAD67 in the dDG and vDG during stressful experiences.
In the current study, we therefore set out to investigate stress-related impact of GAD65/67 deficiency in a regionally specific manner, using a locally restricted lentivirus-mediated knock down of GAD65/67 gene expression in the dDG vs vDG of adult rats. Our findings further led us to examine the effects of GAD65 knock down on behavioral and physiological changes induced by JVS and a potential role of this GAD isozyme controlling stress-induced behavioral changes.
2. Methods
(More detailed description is provided in the Supplementary material).
2.1. Lentivirus production and validation (Raza et al., 2017)
To specifically silence GAD65, the oligonucleotide sequence 5′-GCATGCTTCCTACCTCTTTCA-3’ (corresponding to NM 008078, base pairs 1599–1619 in mouse and NM_012563.1, base pairs 1337–1357 in rat) was selected for generation of shRNA hairpin constructs as described previously (Rehberg et al., 2014). For GAD67, the oligonucleotide sequence 5′-GCTGGAAGTGGTAGACATACT-3’ (corresponding to NM 008077, base pairs 616–636 in mouse and NM017007 base pairs 531–551, in rat) was used in the same manner. A random sequence shRNA ((5′- TCGTCATGACGTGCATAGG -3′ (Thiere et al., 2016), and an anti-luciferase shRNA (shLuc) from pMIR-mU6-Luc (Rehberg et al., 2014) were used as controls. All shRNA constructs under U6 promoter were cloned into pll3.7 vector (Rubinson et al., 2003) using Hpa1- Xho1 restriction sites. Validation of constructs and efficiency is described in the Supplementary material and Tables S1–S7.
Generation of lentiviral particles was done in HEK293T cells essentially as described in the Supplementary material. To determine the viral titer, a test transduction on HEK293T cells was done using serial dilutions of the virus and GFP expression from pll3.7 backbone was quantified using FACS analysis (FACScalibur, BD Biosciences).
2.2. Animals
Male Sprague-Dawley rats (Harlan, Jerusalem, Israel) were purchased at postnatal day (PND) 22, weighing 30–50 g. A separate group was ordered to arrive at PND 50, weighting 200–230 g. Animals were group housed (22 ± 2 °C; light–dark cycle: 12/12 h), with water/food ad libitum. All experiments were carried out during the light phase (8:00 a.m.–5:00 p.m.), in accordance with the NIH guidelines for the care and use of laboratory animals and were approved by the University of Haifa ethical committee.
2.3. Stereotaxic virus injection
Rats (PND 60) received bilateral microinjections of a vector expressing shRNA against GAD65 (shGAD65), GAD67 (shGAD67) or a random sequence control shRNA (shCTR), into dDG or vDG. After suturing the scalp, Antisedan (10 mg/kg s.c.) was injected and animals were allowed 2 weeks recovery before behavioral assessment (Saha et al., 2018).
2.4. Behavioral assessments
2.4.1. Juvenile stress (JVS)
Juvenile rats were exposed to variable psychological stress during PND 27–29 (Albrecht et al., 2017; Horovitz et al., 2012). Rats were exposed to three different stressors for three consecutive days (PND 27–29): Day 1, forced swimming (10 min); Day 2, elevated platform stress for 3 × 30 min (1-h inter trial interval in the home cage); Day 3, 2 h in a restraint apparatus.
2.4.2. Open field test (OF)
Locomotor activity and anxiety-like behavior were tested at PND 67 as previously described (Ardi et al., 2016). Briefly, rats were placed in the corner of a square, dimly-lit plexiglass box (90 × 90 × 50 cm) and allowed to freely explore the arena for 5 min.
2.4.3. Elevated plus maze test (EPM)
Anxiety-like behaviour was assessed at PND 68, 24 h after the OF test as previously described in (Ardi et al., 2016). Entries to open and closed arms were evaluated as measures of anxiety-like behaviour and exploratory activity.
2.4.4. Two way shuttle avoidance (TWSA)
The TWSA task (adapted from (Hadad-Ophir et al., 2016) was used to assess the learning performance of rats over time in an active avoidance paradigm (Supplementary material). Rat location in the TWSA was tracked automatically and collected for offline-analysis via the ShuttAvoid Software (Panlab, Harvard Apparatus, Barcelona, Spain).
2.4.5. Morris water maze (MWM)
The MWM (Hadad-Ophir et al., 2014) was used to assess spatial learning (the latency to reach a platform) and memory (time spent in the correct quadrant). Rat behavior in the OF, EPM and MWM was recorded and analyzed using an EthoVision XT8 tracking system (Noldus, Wageningen, Netherlands).
2.5. Experimental groups and design
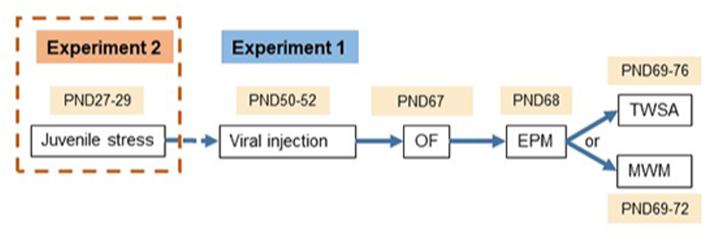
The experimental design is depicted in Fig. 1. Following viral injections on PND 50–52, rats were assigned to two experimental paradigms:
Fig. 1.
Schematic illustration of the experimental design for the analysis of GAD65 knock down effects on anxiety and learning behavior. Following viral injections on PND 50–52, rats were assigned to two experimental paradigms: Experiment (1), to assess the effects of GAD65/67 expression reduction on emotional behavior, learning and memory. Viral gene reduction was conducted in naïve rats at PND 50–52. Separate animal groups were injected in the dDG with shGAD65/shshCTR (N = 25/22), shGAD67/shCTR (N = 13/13), and in the vDG (N = 21/17 and N = 18/15, respectively); then tested in the OF (PND 67), EPM (PND 68), followed by either TWSA test (PND 69–76) or MWM (PND 69–72). For the latter two tests, the groups were split as follows: TWSA, injected to the dDG: shGAD65/shCTR (N = 11/8) and shGAD67/shCTR (N = 5/5); injected to the vDG: shGAD65/shCTR (N = 11/8) and shGAD67/shCTR (N = 10/7); MWM, injected to the dDG: shGAD65/shCTR (N = 10/10) and shGAD67/shCTR (N = 8/8); injected to the vDG: shGAD65/shCTR (N = 10/9) and shGAD67/shCTR (N = 8/8). Experiment (2), viral intervention was performed in rats pre-exposed to juvenile stress (JVS) at puberty (PND 27–29) before viral injection at adulthood with either shCTR (JS + shCTR) or shGAD65 (JS + shGAD65), in the dDG only on PND 50–52. Another group of rats that did not undergo any surgical procedure was included as a naïve control and split into exposed and non-exposed to JVS ((NAIVE group (N = 24), NAÏVE + JS, N = 25)). Behavioral testing was conducted as described for paradigm 1. For TWSA and MWM, groups were split as follows: TWSA (NAIVE = 10, NAIVE + JS = 10, JS + shCTR = 11, JS + shGAD65 = 9) or MWM/physiology tests (NAIVE = 14, NAIVE + JS = 15, JS + shCTR = 11, JS + shGAD65 = 11). In vivo LTP was conducted two weeks after the last behavior testing on PND 90. All behavioral experiments were conducted by experimenters blinded for which viral vector was injected.
2.5.1. Experiment 1
Effects of GAD65/67 expression reduction on emotional behavior, learning and memory. At PND 50–52 rats were injected in the dDG with shGAD65/shCTR (N = 25/22), shGAD67/shCTR (N = 13/13), as well as in the vDG ((N = 21/17 and N = 18/15, respectively, see expression profile in Tables S1–S4)); then tested in the OF (PND 67), EPM (PND 68), followed by either TWSA test (PND 69–76) or MWM (PND 69–72).
2.5.2. Experiment 2
Examining the impact of reducing GAD65 expression in the dDG on JVS- induced symptoms in adulthood. Rats pre-exposed to JVS (PND 27–29), were injected at PND 50–52 in the dDG with either shCTR (JS + shCTR, N = 22) or shGAD65 (JS + shGAD65, N = 21) (see expression profile in Tables S5–S7). A naïve, non-injected control group was split into exposed (NAIVE + JS, N = 25) and non-exposed to JVS (NAIVE, N = 24). Behavioral testing was conducted as described for Experiment 1. In vivo LTP was performed two weeks after the last behavior testing on PND 90. All behavioral tests were conducted by experimenters blind to treatment.
2.6. Behavioral profiling
Profiling animals as “Affected” or “Unaffected” was done according to the Behavioral Profiling approach (Ardi et al., 2016), in which the performance of the animal in each behavioral parameter is compared to the averaged performance of the naïve control group.
2.7. In vivo electrophysiology
At PND 90 rats were deeply anesthetized with 40% urethane and 5% chloral hydrate and were placed on stereotaxic frame (Stoelting, Wood Dale, IL) and body temperature maintained at 36.5–37.5 °C. After fixing the head, small holes were drilled for the stereotaxic insertion of recording and stimulating electrodes, according to coordinates. Baseline recordings of EPSP were assessed during 20 min, at frequency of 0.1 Hz. LTP was induced by theta burst stimulation (TBS) (Ardi et al., 2014): three sets of 10 trains, each with 10 pulses (100 Hz), were administrated with 200 ms inter-train interval, and 1 min inter-set interval. LTP was recorded for 60 min after TBS.
2.8. Brain removal and histology
For details see supplementary material
2.9. Statistics
Each data set was tested for normality distribution and equal variance by Shapiro-Wilk test. Reduced protein levels of GAD65 and GAD67 in vitro and in vivo were analyzed with One-Way ANOVA followed by just Fisher LSD or Student's t-test, respectively. OF and EPM data were analyzed with t-test (in experiment 1) and Kruskal Wallis test (in experiment 2). TWSA learning, and acquisition phase of the MWM, were analyzed with repeated measures ANOVA and OneWay ANOVA post hoc comparison where appropriate. The MWM probe test was analyzed with t-test or One-Way ANOVA followed by Bonferroni. p < 0.05 was considered significant. LTP was analyzed by with repeated measures ANOVA and Bonferroni post hoc comparison.
In experiment 2, following behavioral profiling, we applied Pearson's chi-squared test in order to calculate the distribution of affected vs unaffected populations.
3. Results
3.1. Verification of the viral knockdown in vitro and in vivo
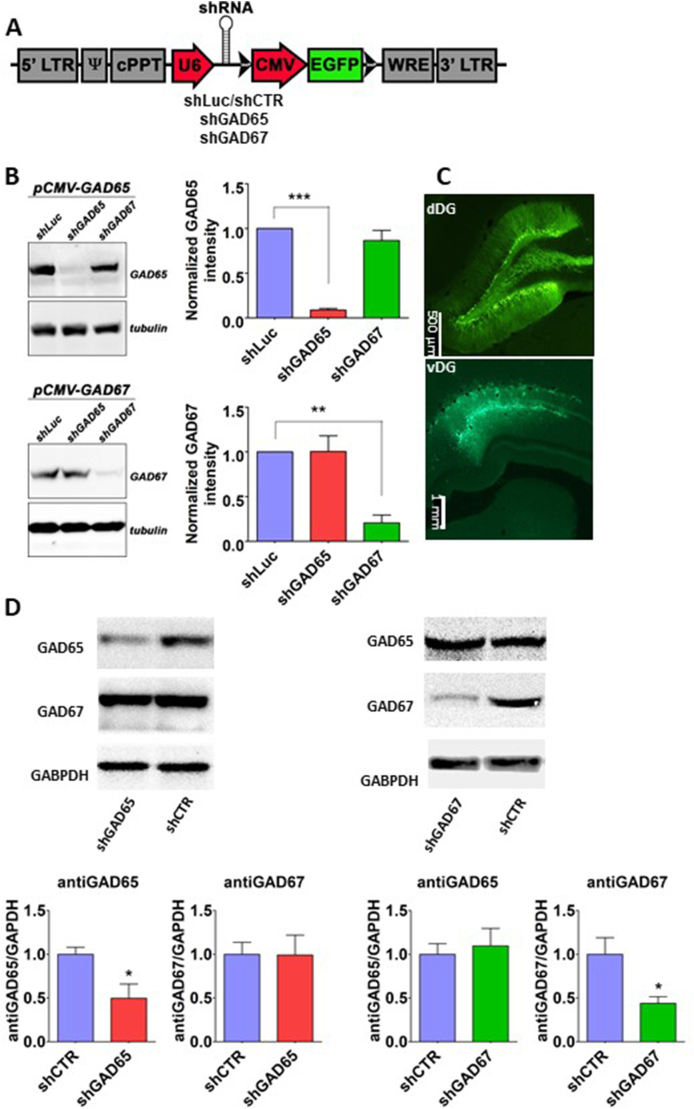
Efficiency and specificity of knock down constructs (Fig. 2A) were first tested using acute transfections of HEK293T cells. With heterologous expression of either GAD65 and GAD67 constructs, we could demonstrate that the transfection of shGAD65, but not shGAD67, efficiently suppressed GAD65 expression compared to that of shLuc control (F2,9 = 53.22, P = 0.001) (92% reduction with shGAD65, Fig. 2B top). On the other hand, in GAD67 expressing cells, shGAD67 reduced GAD67 protein levels significantly (80% reduction) while shGAD65 did not differ from controls (Fig. 2B bottom) (F2,6 = 16.22, P = 0.003). Injection of shRNA lentiviral vectors into the dDG (Fig. 2 C) revealed similar efficiency and specificity against endogenous GAD65 and GAD67 proteins in vivo (51% and 57%, respectively, Fig. 2D) (t8 = 2.76, P = 0.025 and t8 = 2.74, P = 0.025, performed on normalized values, respectively). Furthermore, immunohistochemical analysis following GAD65 knock down in rat dDG, revealed a reduced total immunofluorescence intensity and number of GAD65 cells in the hilus of DG (Figs. S1C and S1D, respectively).
Fig. 2.
Knock down of GAD isozymes. (A) Schematic illustration of the various viral constructs. (B) In vitro validation of viral constructs. Upper panel: expression of shGAD65 in HEK293T showing a specific reduction of GAD65 protein levels. Lower panel: expression of shGAD67 showing a specific reduction of GAD67 protein levels. No cross reactivity is evident. Relative expression levels were normalized to tubulin as housekeeping gene and to control shRNA (N = 4 independent experiments, **p ≤ 0.01, ***p ≤ 0.001). (C) In vivo validation: viral vectors were bilaterally injected to the dorsal and ventral dentate gyrus (dDG and vDG, respectively). Representative photomicrograph showing confirmation of injection site according to the expression of GFP; bar, 500 μm and 1 mm). (D) Western blot shows that shGAD65 injection (N = 5) led to a significant knock down of GAD65 in the dDG. Efficient and selective knock down of GAD67 (N = 5) was achieved with shGAD67 construct (N = 5 in each group). Mean ± SEM are shown for all the graphs, *p ≤ 0.05. Abbreviations: CMV, cytomegaly virus promoter; cPPT, central polypurine traxt; EGFP, enhanced green fluorescence protein; LTR, long terminal repeat; Psi, Psi packaging element; U6, U6 promoter; WRE, woodchuck hepatitis virus posttranscriptional regulatory element. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Experiment 1: effect of selective knock down of GAD65 and GAD67 in dDG and vDG on anxiety and learning
3.2.1. Effect of selective knock down of GAD65 and GAD67 on of exploration
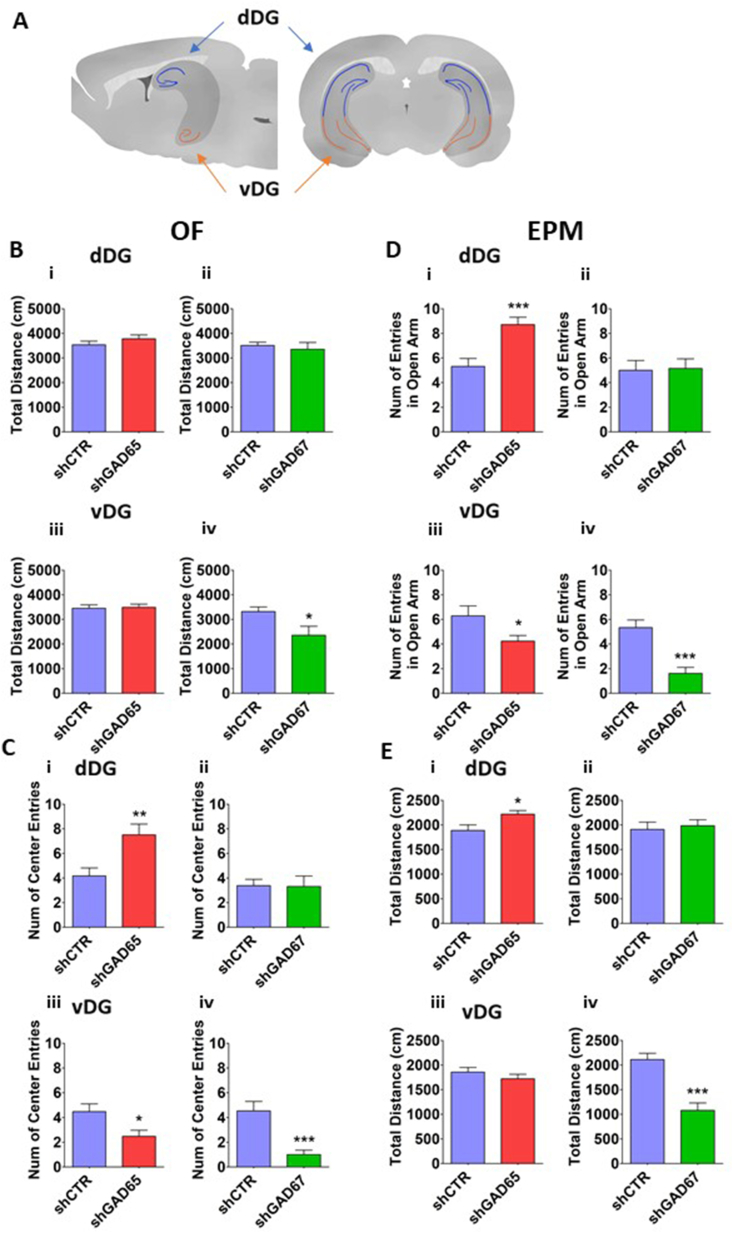
No change in locomotor activity, measured by the total distance covered in the OF arena during the test, was found in animals injected with shGAD65 or shGAD67 in the dDG (Fig. 3B–i, ii), or shGAD65 in the vDG (Fig. 3B–iii). Conversely, a significant reduction in locomotion was evident when GAD67 was knocked down in the vDG (Fig. 3B–iv).
Fig. 3.
Effect of GAD65 and GAD67 knockdown on locomotor activity and anxiety. The schematic lay out of the experimental timeline and group size are illustrated in Fig. 1. Adult rats were injected with shRNA65, shRNA67 and shRNA control at PND 50–52 and later subjected to OF and EPM tests at PND 67 and PND 68 respectively. (A) Virus injections were targeted to the dDG (blue arrows) or vDG (orange arrows). The control group (CTR) consisted of rats injected with the shRNA random sequence-containing virus. (B) Exploratory activity in the OF, measured as the total path length. (C) Number of entries into the central area of the OF. (D) Proportion of entries to open arms of the EPM. (E) Exploratory activity in the EPM measured by total distance traveled. B, C for OF; D, E for EPM; i, ii for dDG; iii, iv for vDG. Mean ± SEM is shown for all graphs. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
By contrast, the reduction of GAD65 in the dDG profoundly increased exploratory behavior in the central area compared with control shRNA (Fig. 3C–i shCTR; 4.18 ± 0.64, and shGAD65; 7.52 ± 0.87). This anxiolytic-like change was isoform-specific as no such effect was observed with shGAD67 compared with rats treated with respective control shRNA (shCTR, Fig. 3C–ii). Moreover, reducing the levels of either GAD65 or GAD67 in the vDG resulted in a lower number of entries into in the central area (Fig. 3C–iii,iv).
3.2.2. Effect of selective knock down of GAD65 and GAD67 on behavior in the EPM
In line with the observations in the OF, knock down of GAD65 in the dDG led to an increased percentage of entries into open arms (Fig. 3D–i shCTR; 5.31 ± 0.64; shGAD65; 8.72 ± 0.59). No such anxiolytic-like change was seen in rats with decreased expression of GAD67 in the same region (Fig. 3D–ii). Moreover, both constructs generated anxiogenic-like effects when injected to the vDG, reducing the proportion of entries into the open arms (Fig. 3D–iii, iv). These changes were accompanied by altered overall activity, which was increased by knock down of GAD65, but not GAD67 in the dDG, as measured by total distance traveled (Fig. 3E–i, ii). Decreasing the levels of GAD67 in vDG had the opposite effect (Fig. 3E–iv).
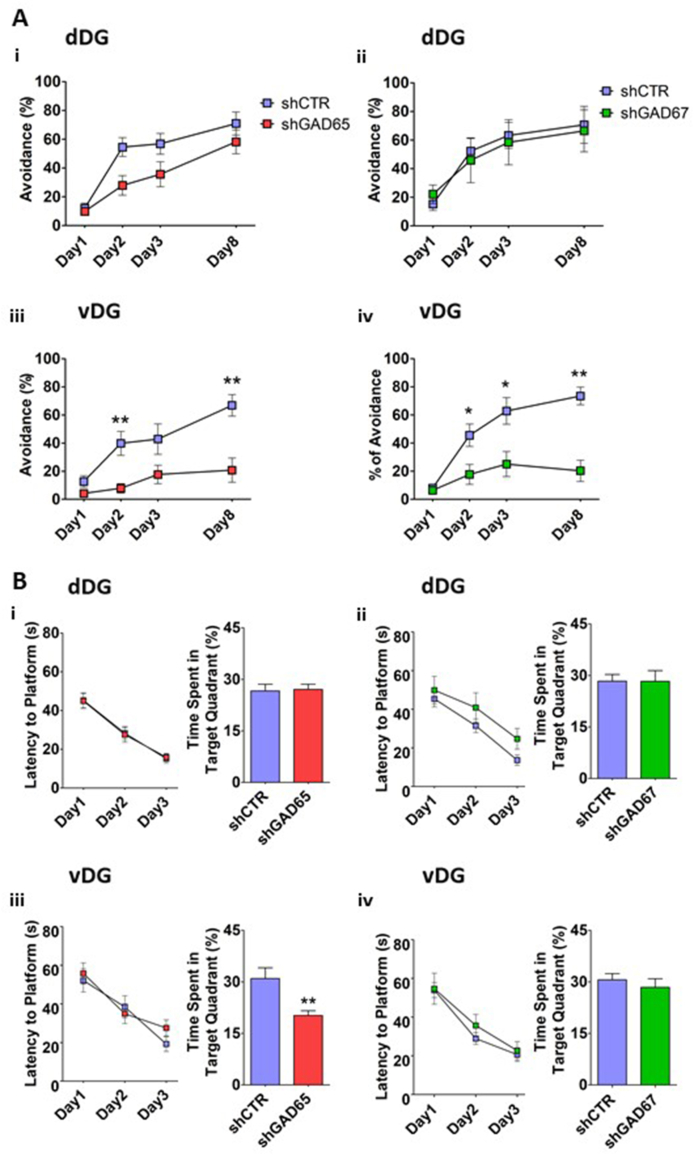
3.2.3. Effect of selective knock down of GAD65 or GAD67 on performance in the TWSA
Despite a non-significant trend for reduced initial performance in shGAD65 injected rats, neither shGAD65 nor shGAD67 significantly affected performance in the TWSA test, when knocked down in the dDG (repeated-measures ANOVA, effect of days; F(2,34) = 16.141, p = 0.000, group effect; F(1,17) = 4.013, p = 0.061, effect of days X groups; F(2,34) = 1.330, p = 0.278) (Fig. 4A i, ii). By contrast, both constructs clearly impaired performance when targeted to the vDG, resulting in reduced avoidance behavior from the second day of training onwards (Fig. 4A–iii, iv; repeated-measures ANOVA for shGAD65: days F(1.443,34) = 7.134, p = 0.007, days X group F(1.443,34) = 1.972, p = 0.169, group; F(1,17) = 14.913, p = 0.001; shGAD67: days F(2,30) = 5.726 p = 0.008 Days X group; F(2,30) = 3.591 p = 0.040 Groups; F(1,15) = 14.991 p = 0.002. Further OneWay ANOVA shows the effect per day: for shGAD65, Day2; F(1,18) = 15.136, p = 0.001; Day3; F(1,18) = 4.417, p = 0.051; for GAD67, Day2; F(1,16) = 6.693, p = 0.02; Day3; F(1,16) = 7.966, p = 0.02. Moreover, consolidation of learning at retest on day 8 was strongly impaired by both constructs upon injection to vDG (shGAD65: t17 = 3.848 p = 0.001; shGAD67: t15 = 5.054, P = 0.001).
Fig. 4.
Effect of GAD65 and GAD67 knock down on learning and memory. The schematic lay out of the experimental timeline and group size are illustrated in Fig. 1 (A) Performance of rats in the TWSA task on 3 consecutive days of learning, and consolidation of learning at retest day 8, by targeting both shGAD65 and shGAD67 to the dDG (i, ii) and vDG (iii, iv). *p < 0.05, **p ≤ 0.001). B) Spatial learning and reference memory (bar graphs) in the MWM, targeting shGAD65 and shGAD67 to the dDG (i, ii) and the vDG (iii, iv). Mean ± SEM are shown for all the graphs. **p ≤ 0.05.
3.2.4. Effect of selective knock down of GAD65 and GAD67 on performance in the MWM
None of the constructs impaired performance during the learning phase irrespective of the injection region (Fig. 4B, i-iv). Further, no effect of either shGAD65 or shGAD67 injection to the dDG was observed in the probe test (Fig. 4B–i, ii). However, injections of shGAD65 to the vDG led to a reduced performance during the probe test (t(17) = 3.287, p = 0.004; Fig. 4B–iii). (repeated-measures ANOVA for the dDG: shGAD65, effect of days; F(2,36) = 39.926, p = 0.001, effect of group; F(1,18) = 0.002, p = 0.962, days X group; F(2,36) = 0.030, p = 0.970; shGAD67, effect of days; F(1.373,19.216) = 16.835, p = 0.001, effect of group; F(1,14) = 2.886, p = 0.111, days X group; F(1.373,19.216) = 0.004, p = 0.981; for the vDG: shGAD65, effect of days; F(1.512,25.709) = 24.709, p = 0.001, group effect; F(1,17) = 0.376, p = 0.548, days X group; F(1.512,25.709) = 0.944, p = 0.378; for the vDG GAD67, effect of days; F(2,28) = 36.938, p = 0.001, group effect; F(1,14) = 0.315, p = 0.583, days X group; F(2,28) = 0.333, p = 0.719).
3.3. Experiment 2: effects of reducing GAD65 in the dDG on JVS–induced alterations in adulthood
Findings of experiment 1, tested both in the OF and the EPM, showed that the selective reduction of GAD65, but not GAD67 in the dDG resulted in a significant decline in the anxiety levels. In experiment 2 we examined whether this anxiolytic effect would protect against the long-term impact of JVS.
3.3.1. Protective effects of GAD65 knock down in the OF
In the OF, significant group differences were observed concerning both the path length in the arena and number of center entries. The JVS exposed rats (NAÏVE + JS) showed a significant reduction of total exploratory activity compared to that of the unexposed, NAÏVE group (Fig. 5B), while this effect was not evident in the virus injected groups, pre-exposed to JVS (Kruskal-Wallis test values, H(3) = 43.895, p = 0.001; p = 0.001 between NAÏVE JS vs NAÏVE, NAÏVE JS vs JS + shGAD65, and JS vs JS + shCTR). We would have expected a reduction in the activity of the shCTR injected animals similar to that seen in naive juvenile stressed rats. In fact, non-linear effects on animal behavior of multiple stress exposure during development and adulthood have been discussed intensively (Daskalakis et al., 2013). As a result, it may happen that exposure of a particular group of animals to a stressor will not result the well-established and expected outcome in a singular parameter, despite other parameters indicating a clear stress effect. Therefore, truly shGAD65 mediated effects on stress induced behavior should be considered in comparison to both the JS group and the shCTR group. For this reason, animals were further examined on several behavioral parameters, and subjected to a Behavioral Profiling analysis of the behavior (see section 3.4).
Fig. 5.
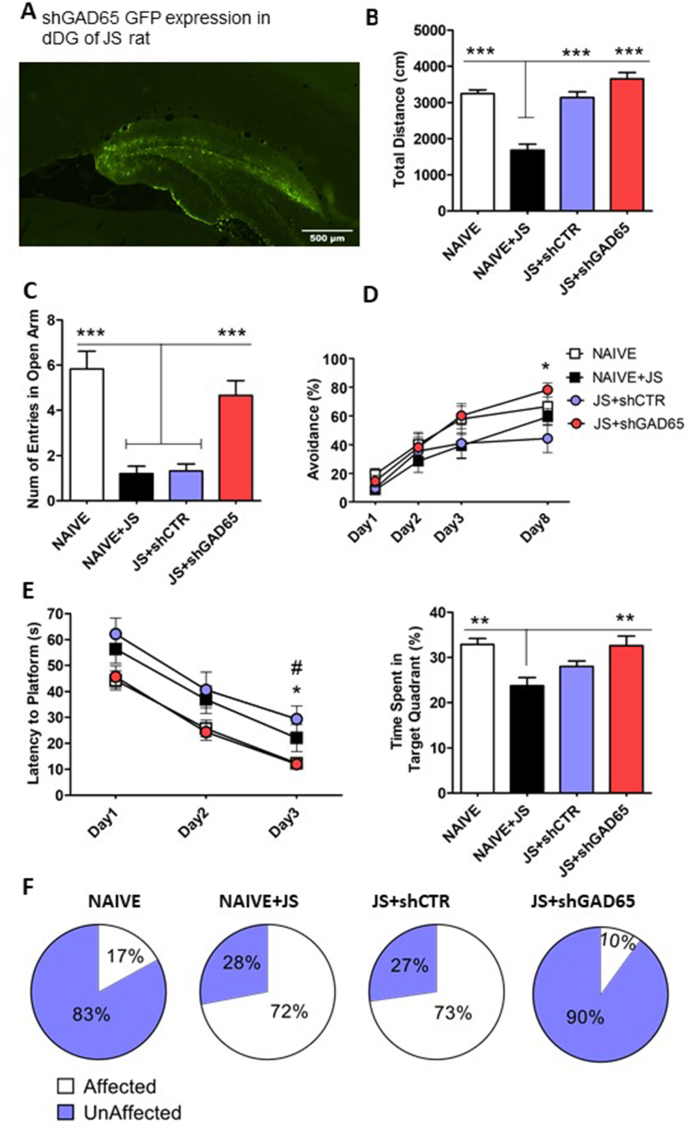
Effect of GAD65 knock down in the dDG on behavioral responses of adult rats after JVS: The schematic lay out of the experimental timeline and group size are illustrated in Fig. 1 (A) Representative photomicrograph depicting injection site of shGAD65 and random sequence control vectors targeted to the dDG and expression of green fluorescence protein (GFP). Scale bar, 500 μm. (B) Juvenile stress-induced effects in the OF. (C) Attenuation of stress-induced anxiety in the EPM, as measured by the proportion of open arm entries. N for all groups is the same as in (B) and as described in Fig. 1. ***p ≤ 0.001. (D) Improved performance of rats in the TWSA. Training was done for 3 days and memory retrieval was on day 8. *p ≤ 0.05. (E) Recovery of memory performance in the MWM; the latency to reach the hidden platform was evaluated over 3 days of training. *p < 0.05; #p < 0.05 for NAÏVE vs JS + shCTR. The probe test was done on day 4 (on the right represented by bars). **p ≤ 0.01. Mean ± SEM are shown for all the graphs (B–E). (F) Behavioral profiling analysis, was conducted to examine the impact of the shGAD65 manipulation on the prevalence of affected versus unaffected individuals (see Supplemental Information). Values are the percentage of unaffected and affected animals in each group. (x2 = 32.807, p < 0.001). NAÏVE + JS, non-vector-injected naïve rats exposed to juvenile stress (JVS); NAÏVE, unexposed rats; JS + shCTR, control shRNA injected groups pre-exposed to JVS; shGAD65, shGAD65 injected groups pre-exposed to JVS. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
A significant reduction of center exploration was evident in JVS exposed rats compared to that of unexposed naïve rats (Fig. S2A), an effect which was fully recovered by the injection of shGAD65, but not by the control virus (Kruskal-Wallis test values; H(3) = 40.202, p = 0.001, NAÏVE + JS vs. NAÏVE, p = 0.001; NAÏVE + JS vs JS + shGAD65, p = 0.001; JS + shCTR vs. NAÏVE, p = 0.010; JS + shCTR vs JS + shGAD65, p = 0.001).
3.3.2. Attenuation of stress-induced anxiety in the EPM upon GAD65 knock down
Results in the EPM were compatible with those in the OF. Group comparison showed a significant decrease in open arm entries upon JVS by naive rats (NAÏVE + JS). This effect was counteracted by shGAD65 injection but not by shRNA-injected rats (JS + shCTR) (Fig. 5C; Kruskal-Wallis test, H(3) = 41.654, p = 0.001; p = 0.001 between NAÏVE + JS-JS + shGAD65; NAÏVE + JS-NAÏVE,; JS + shCTR-NAÏVE; JS + shCTR-JS + shGAD65). Similar results were obtained for total locomotor activity (Fig. S2B; Kruskal-Wallis test; H(3) = 39.598, p = 0.001, JS + shCTR-JS + shGAD65, p = 0.030; JS + shCTR- NAÏVE, p = 0.002; NAÏVE + JS-JS + shGAD65 and NAÏVE + JS-NAÏVE, p = 0.001).
3.3.3. Improved performance of stressed rats in the TWSA task upon GAD65 knock down
During TWSA learning, all four groups steadily improved their performance on days 2 and 3 of training, compared to that of first day and maintained high levels of avoidance on retrieval day 8 ((Fig. 5D; repeated-measures ANOVA, effect of day; F(1.645, 59.207) = 44.132, p = 0.001, days X group effect; F(4.934, 59.207) = 3.052, p = 0.017, group effect; F(3,36) = 1.224, p = 0.315)). Although NAÏVE + JS and JS + shCTR groups showed a tendency for lower avoidance learning during the first three days, the difference from NAÏVE did not reach a significance level at any time point. In contrast, shGAD65 treatment resulted in a significant improvement in TWSA performance on day 8 compared to that of JS + shCTR (One-Way ANOVA F(3,39) = 2.930, p = 0.047, Posthoc Bonferroni JS + shCTR-JS + shGAD65, p = 0.041; Fig. 5D).
3.3.4. Attenuation of stress-induced deficits in the MWM by GAD65 knock down
All groups steadily reduced the latency to reach the hidden platform on days 2 and 3 of training compared to that of first day (Fig. 5E, left). However, clear group differences existed as the NAÏVE + JS and JS + shCTR groups displayed increased latencies throughout the test ((repeated-measures of ANOVA, effect of days; F(2, 94) = 97.142, p = 0.001, days X group; F(6,94) = 0.078, p = 0.998, group effect; F(1, 47) = 4.697, p = 0.006)). Moreover, at day 3, shGAD65 rats pre-exposed to stress exhibited a significant reduction in time to reach the platform (One-Way ANOVA F(3,50) = 4.262, p = 0.010, Posthoc Bonferroni JS + shCTR–Naïve, p = 0.024, JS + shCTR-JS + shGAD65, p = 0.032). In the probe test, the JVS group showed a reduction in time spent in the correct target quadrant, which was fully recovered by injection of shGAD65, but not shCTR ((Fig. 5E, right, One-Way ANOVA F(3,50) = 7.466, p = 0.001, Post hoc Bonferroni NAIVE-NAIVE + JS, p = 0.001, JS + shGAD65-NAÏVE + JS, p = 0.003)).
3.4. Behavioral profiling analysis of the impact of GAD65 knock down in the dDG
Relying on analysis of group averages may mask the impact of individual differences (Richter-Levin et al., 2019b). We thus employed a behavioral profiling analysis (Ritov et al., 2016), which enables examining the impact of the shGAD65 manipulation on the prevalence of affected versus unaffected individuals (detailed in the Supplemental Information). Determination of the classification criteria was based on the lower or upper 25th percentiles of naive control animal distribution. Every rat that demonstrated a behavior profile that falls within a minimum of four out of the five behavioral parameters were considered “affected” (Fig. 5 F). Pearson X2 analysis revealed that compared to the control value (mean±1SD of naïve) 72% of JVS- exposed naïve rats and 73% of shRNA injected rats exposed to JVS developed such a phenotype (x2 = 32.807, p = 0.001). However, upon injection of shGAD65 (reduced GAD65 levels) to the dDG this proportion was reduced to 10%, similar to the level observed in the naïve controls (p = 0.001).
3.5. Effect of selective knock down of GAD65 in the dDG of adult rats on long-term potentiation (LTP)
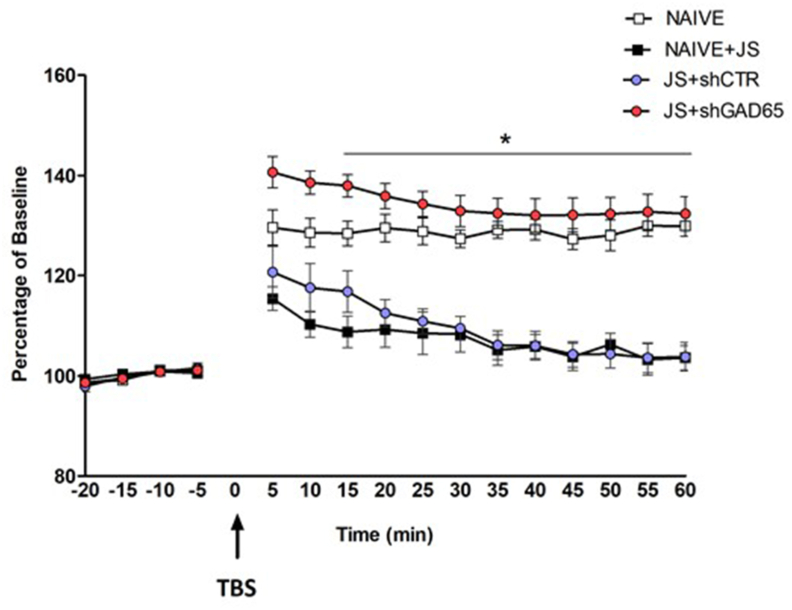
As alterations in GAD65 restricted to the dorsal hippocampus proved to attenuate anxiety levels, we set out to test the effect of GAD65 knock down in this region following JVS on in vivo plasticity. For this, LTP was monitored upon theta-burst stimulation (TBS) to the perforant path-DG pathway, and recording in the dDG. No significant difference in baseline recording was found prior to TBS (repeated-measures ANOVA; Greenhouse-Geisser effect, for Time; F(1.75, 40.24) = 7.11, p = 0.003, Time x group; F(5.25, 40.24) = 0.30, p = 0.917, Group; F(3, 23) = 1.49, p = 0.243) (Fig. 6). Following TBS, both NAÏVE + JS and JS + shCTR rats displayed a reduced LTP, which returned to baseline within 40 min, compared to that of unexposed-naïve rats, ((repeated-measures of ANOVA, time effect; F(2.257, 51.919) = 11.267, p = 0.001, time X group; F(6.772, 51.919) = 1.945, p = 0.083, group effect; F(3,23) = 31.874, p = 0.001. However, targeted reduction of GAD65 in the dDG of JVS rats eliminated the impact of JVS on LTP, such that LTP of the JS + shGAD65 group reached levels similar to those of the naïve control group (One-Way ANOVA F(3,26) = 25.326, p = 0.001, post-hoc (40 min), JS + shGAD65-NAÏVE + JS, p = 0.001, JS + shGAD65-JS + shCTR, p = 0.001).
Fig. 6.
GAD65 knock down in the dDG of JVS rats affects LTP in adult rats. LTP was assessed upon theta-burst stimulation (TBS) to the perforant path-DG pathway, and recording in the dDG. No significant difference in baseline recording was found prior to TBS. The results are the Mean ± SEM. *p ≤ 0.001, different from NAÏVE + JS and JS + shCTR at 40 min.
4. Discussion
Accumulating evidence indicates that childhood stress-induced altered GABAergic functioning may contribute to psychopathology later in life (Albrecht et al., 2017; Brambilla et al., 2003; Pearl and Gibson, 2004; Rudolph and Möhler, 2014). Specifically, the hippocampal DG has been implicated in childhood-trauma induced vulnerability. However, the role of local GABAergic inhibitory circuitry within different hippocampal subfields and their contribution to emotional modulation is yet to be established. Here we provide evidence for a subarea- and isoform specific function of the GABA synthetic enzyme GAD65 in controlling anxiety-like behavior and stress coping via the DG.
We have previously shown that stress at juvenility increases vulnerability later in life and alters limbic GABAergic activity (Ardi et al., 2019; Jacobson-Pick and Richter-Levin, 2012; Ritov et al., 2016; Saha et al., 2017). We further demonstrated a differential long-term regulation of GABAergic functions in dDG and vDG following JVS, as JVS exposure in rats led to a lasting altered expression of the astrocytic GABA transporter GAT-3, causing imbalance of excitation/inhibition ratios in the dDG. Strikingly, no such regulation was observed in the vDG (Albrecht et al., 2016).
In the current study, we investigated the differential role of the GABA synthetic enzymes GAD65 and GAD67 in control of emotional and cognitive functions, and in the ability to cope with JVS experience in rats. We purposely implemented a model of pre-puberty/juvenile stress at a specified time point, which more closely resembles exposure in human childhood (Horovitz et al, 2012, 2014). The time window of exposure is of importance as exemplified in our earlier studies where shifting to stress at ‘adolescence’ or adulthood, yielded different outcomes with respect to its effect on GABAergic mechanisms and behavior, compared to exposure in juvenility (Tsoory and Richter-Levin, 2006; Zitman and Richter-Levin, 2013).
Previous evidence showed that haplodeficiency of GAD65 (GAD65+/−) confers stress-resilience in animals with a history of JVS (Müller et al., 2015), although no conclusion could be made on its region-specific function. Here we report that selective knock down of GAD65, in the dorsal DG, but not the ventral DG at adulthood, conferred naïve animals a significant reduction in their innate anxiety levels, as shown by the increased OF center entries and EPM open arm entries, alluding to a differential role of GAD65 in the dDG vs vDG during stressful life experiences. We cannot completely rule out a possible influence of increased locomotion activity of rats, in the EPM, on anxiolytic measures of GAD65 shRNA injection to the dDG, though the magnitude of the locomotion effect was lower compared with that of the open arm entries number. No such effect was evident following GAD67 isoform knockdown in the same dorsal portion of the DG, indicating an isoform specific effect of GAD65 in this region. This isoform dissociation is further manifested by the anxiety-like behavior of naïve rats that were knocked down in the vDG with shGAD67. The difference may be related to altered relative GAD65 and GAD67 expression levels in dorsal and ventral portions of the DG (Czéh et al., 2013) and/or to differences in the functionality of inhibitory local circuit neurons (Albrecht et al., 2021). In fact, increased inhibitory tone and enhanced activation of local circuit inhibition in the vDG has been described previously (Pofantis et al., 2015; Schreurs et al., 2017). It will be important, in future studies, to investigate the contribution of GAD isozymes to stress-induced changes in tonic and phasic GABA release in different subregions of the DG (Holm et al., 2011; Lee et al., 2016).
The classical view of hippocampal functioning suggests a dichotomy of functions between the dorsal and ventral hippocampus, assigning an emotional role to the ventral hippocampus, while the dorsal part of the hippocampus is typically associated with cognitive and spatial functions (Bannerman et al., 2014; Fanselow and Dong, 2010). Indeed, there is a body of findings demonstrating that the ventral hippocampus is more responsive to emotional challenges and to amygdala activation (e.g. (Ardi et al., 2014; Sood et al., 2014). However, as reviewed in (Albrecht et al., 2017), this preference is not categorical, with the dorsal hippocampus contributing to emotional processing and the ventral to cognitive functions, an interactoion which probably serves as a kind of a cognitive-emotional interface. This integrative view receives further support from the results showing that the viral-targeted reduction of GAD65 in the dDG attenuates anxiety behavior and cognitive impairment resulting from exposure to JVS. In line with that, we have recently demonstrated that activation of NPY-positive interneurons in the dDG, but not vDG provides resilience, whereas activation of CCK-interneurons in the vDG, but not dDG relates to vulnerability of rats following JVS (Regev-Tsur et al., 2020). These findings are further supported by a previous report showing that uncontrollable stress causes elevation of GAD65 mRNA levels only in dDG but not vDG (Hadad-Ophir et al., 2017). Thus, the dDG, which responds differently to various stressors, seems to integrate emotional and cognitive aspects of an experience and share that data with the hippocampus proper, as well as with a network of brain regions such as the amygdala and prefrontal cortex (Fa et al., 2014).
The translational validity of our findings was corroborated by the behavioral profiling method, which improved the classification of JVS exposed rats into affected/non-affected and provided a sharper appraisal of the positive effect of GAD65 reduction on JVS symptoms. This approach tracks individual variations in animals across several behavioral performances in comparison to a non-stress control population, thus approximating the scale-based diagnosis criteria in humans (Richter-Levin et al., 2019b; Ritov et al., 2016). Furthermore, this method helps to address potential effects that might arise from the viral manipulation on behavior. The current results reveal a full reversal of water-maze learning deficits and reduced LTP in the JVS group by the reduction of GAD65 within the dDG, suggesting an involvement of the GABAergic system in the synaptic plasticity-induced recovery. Both behavioral manifestations have been reported in JVS (Avital and Richter-Levin, 2005; Grigoryan et al., 2015). In support of that, we previously found that prolonged uncontrollable stress in rats resulted in altered expression of GABA-related genes, increased inhibitory activity and reduced LTP in the dDG (Hadad-Ophir et al., 2017). We here focus mainly on the differential effects of GAD65 knock down in the dDG and vDG, because of the dDG GAD65 knock down effect of reducing anxiety. However, the potential role of GAD67 in stress responding also needs to be acknowledged. Induction of GAD67 expression has been observed following restraint stress (Bowers et al., 1998) and increased number of GAD67 positive cells was reported in the DG of stress-resistant rats in a model of PTSD (Skórzewska et al., 2020). We find here that a reduction in expression of GAD67 in the vDG, but not in the dDG, is associated with increased anxiety. Clearly, this result is an indication of a dDG-vDG differential role also of GAD67 relating to anxiety. The different phenotype of mice with GAD67 deficiency in somatostatinergic or parvalbuminergic interneurons (Fujihara et al., 2015; Miyata et al., 2019) demonstrates the cell-type dependence of observed GAD effects. It is thus likely, that the differential roles of dDG and vDG in control of emotional and cognitive behavior involve also GAD67-dependent mechanism not identified in the current study.
To conclude, our results suggest that changes in the expression of the GABA synthesizing enzymes can mediate both stress vulnerability and stress resilience in an isozyme- and brain region dependent manner. They further lend support to the dynamic routing hypothesis of stress processing in the hippocampus and the position of the dDG at an “emotional-cognitive” interface, where GAD65 plays a dual role in controlling both emotional behavior in response to stress as well as learning and memory functions. These together culminate in a protective role for dDG GAD65 towards anxiety and learning deficits induced in a JVS paradigm that models an increased probability to develop psychopathologies upon traumatic incidents at adulthood (Richter-Levin et al., 2019b). Our current results suggest that selective modulation the GABAergic pathways in the dDG may be envisioned to develop new therapeutic strategies in animal models of trauma-related psychopathologies.
CRediT authorship contribution statement
Kuldeep Tripathi: Investigation, Formal analysis, Visualization. Yunus Emre Demiray: Investigation, Visualization. Stefanie Kliche: Methodology, Validation. Liang Jing: Investigation. Somoday Hazra: Investigation. Joyeeta Dutta Hazra: Investigation. Gal Richter-Levin: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. Oliver Stork: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
None.
Acknowledgements
We are grateful to S. Stork, R. Anunu for expert technical assistance and to S. Mandel for editing the manuscript. This work was supported by funds of the German Research Foundation (STO488/6 to OS and GRL, CRC779 TPB5 to OS) and by The Israeli Science Foundation (grant no. 1517/16), and by the State of Israel Ministry of Science & Technology award (Grant no. 3-14356).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100350.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Albrecht A., Ivens S., Papageorgiou I.E., Çalişkan G., Saiepour N., Brück W., Richter-Levin G., Heinemann U., Stork O. Shifts in excitatory/inhibitory balance by juvenile stress: a role for neuron-astrocyte interaction in the dentate gyrus. Glia. 2016;64:911–922. doi: 10.1002/glia.22970. [DOI] [PubMed] [Google Scholar]
- Albrecht A., Müller I., Ardi Z., Çalışkan G., Gruber D., Ivens S., Segal M., Behr J., Heinemann U., Stork O., Richter-Levin G. Neurobiological consequences of juvenile stress: a GABAergic perspective on risk and resilience. Neurosci. Biobehav. Rev. 2017;74:21–43. doi: 10.1016/j.neubiorev.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Albrecht A., Redavide E., Regev-Tsur S., Stork O., Richter-Levin G. Hippocampal GABAergic interneurons and their co-localized neuropeptides in stress vulnerability and resilience. Neurosci. Biobehav. Rev. 2021 doi: 10.1016/j.neubiorev.2020.11.002. [DOI] [PubMed] [Google Scholar]
- Ardi Z., Albrecht A., Richter-Levin A., Saha R., Richter-Levin G. Behavioral profiling as a translational approach in an animal model of posttraumatic stress disorder. Neurobiol. Dis. 2016;88:139–147. doi: 10.1016/j.nbd.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Ardi Z., Richter-Levin A., Xu L., Cao X., Volkmer H., Stork O., Richter-Levin G. The role of the GABAA receptor Alpha 1 subunit in the ventral hippocampus in stress resilience. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-49824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardi Z., Ritov G., Lucas M., Richter-Levin G. The effects of a reminder of underwater trauma on behaviour and memory-related mechanisms in the rat dentate gyrus. Int. J. Neuropsychopharmacol. 2014;17:571–580. doi: 10.1017/S1461145713001272. [DOI] [PubMed] [Google Scholar]
- Avital A., Richter-Levin G. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int. J. Neuropsychopharmacol. 2005;8:163–173. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- Banasr M., Lepack A., Fee C., Duric V., Maldonado-Aviles J., Dileone R., Sibille E., Duman R.S., Sanacora G. Vol. 1. 2017. (Characterization of GABAergic marker expression in the chronic unpredictable stress model of depression). journals.sagepub.com. 247054701772045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman D.M., Sprengel R., Sanderson D.J., Mchugh S.B., Rawlins J.N.P., Monyer H., Seeburg P.H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci. 2014;15:181–192. doi: 10.1038/nrn3677. [DOI] [PubMed] [Google Scholar]
- Bergado-Acosta J.R., Müller I., Richter-Levin G., Stork O. The GABA-synthetic enzyme GAD65 controls circadian activation of conditioned fear pathways. Behav. Brain Res. 2014;260:92–100. doi: 10.1016/j.bbr.2013.11.042. [DOI] [PubMed] [Google Scholar]
- Bergado-Acosta J.R., Sangha S., Narayanan R.T., Obata K., Pape H.-C., Stork O. Vol. 15. 2008. pp. 162–171. (Critical role of the 65-kDa isoform of glutamic acid decarboxylase in consolidation and generalization of Pavlovian fear memory). learnmem.cshlp.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers G., Cullinan W.E., Herman J.P. Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. Soc. Neurosci. 1998;18:5938–5947. doi: 10.1523/JNEUROSCI.18-15-05938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P., Perez J., Barale F., Schettini G., Soares J.C. GABAergic dysfunction in mood disorders. Mol. Psychiatr. 2003 doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- Czéh B., Ábrahám H., Tahtakran S., Houser C.R., Seress L. Number and regional distribution of GAD65 mRNA-expressing interneurons in the rat hippocampal formation. Acta Biol. Hung. 2013;64:395–413. doi: 10.1556/ABiol.64.2013.4.1. [DOI] [PubMed] [Google Scholar]
- Daskalakis N.P., Bagot R.C., Parker K.J., Vinkers C.H., de Kloet E.R. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38:1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fa M.X., Xia L., Anunu R., Kehat O., Kriebel M., Volkmer H., Richter-Levin G. Stress modulation of hippocampal activity - spotlight on the dentate gyrus. Neurobiol. Learn. Mem. 2014;112:53–60. doi: 10.1016/j.nlm.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S., Dong H.W. Are the dorsal and ventral Hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara K., Miwa H., Kakizaki T., Kaneko R., Mikuni M., Tanahira C., Tamamaki N., Yanagawa Y. Glutamate decarboxylase 67 deficiency in a subset of GABAergic neurons induces schizophrenia-related phenotypes. Neuropsychopharmacology. 2015;40:2475–2486. doi: 10.1038/npp.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara K., Sato T., Miyasaka Y., Mashimo T., Yanagawa Y. Genetic deletion of the 67-kDa isoform of glutamate decarboxylase alters conditioned fear behavior in rats. FEBS Open Bio. 2021;11:340–353. doi: 10.1002/2211-5463.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert-Juan J., Bueno-Fernandez C., Castillo-Gomez E., Nacher J. Reduced interneuronal dendritic arborization in CA1 but not in CA3 region of mice subjected to chronic mild stress. Brain Behav. 2017;7 doi: 10.1002/brb3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert-Juan J., Castillo-Gomez E., Pérez-Rando M., Moltó M.D., Nacher J. Chronic stress induces changes in the structure of interneurons and in the expression of molecules related to neuronal structural plasticity and inhibitory neurotransmission in the amygdala of adult mice. Exp. Neurol. 2011;232:33–40. doi: 10.1016/j.expneurol.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Grigoryan G., Ardi Z., Albrecht A., Richter-Levin G., Segal M. Juvenile stress alters LTP in ventral hippocampal slices: involvement of noradrenergic mechanisms. Behav. Brain Res. 2015;278:559–562. doi: 10.1016/j.bbr.2014.09.047. [DOI] [PubMed] [Google Scholar]
- Guina J., Rossetter S.R., Derhodes B.J., Nahhas R.W., Welton R.S. Winner of resident paper award 2014: benzodiazepines for PTSD: a systematic review and meta-analysis. J. Psychiatr. Pract. 2015;21:281–303. doi: 10.1097/PRA.0000000000000091. [DOI] [PubMed] [Google Scholar]
- Hadad-Ophir O., Albrecht A., Stork O., Richter-Levin G. Amygdala activation and GABAergic gene expression in hippocampal sub-regions at the interplay of stress and spatial learning. Front. Behav. Neurosci. 2014;8:3. doi: 10.3389/fnbeh.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadad-Ophir O., Ardi Z., Brande-Eilat N., Kehat O., Anunu R., Richter-Levin G. Exposure to prolonged controllable or uncontrollable stress affects GABAergic function in sub-regions of the hippocampus and the amygdala. Neurobiol. Learn. Mem. 2017;138:271–280. doi: 10.1016/j.nlm.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Hadad-Ophir O., Brande-Eilat N., Richter-Levin G. Differential effects of controllable stress exposure on subsequent extinction learning in adult rats. Front. Behav. Neurosci. 2016;9 doi: 10.3389/fnbeh.2015.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt S.A., Ressler K.J. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur. J. Neurosci. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M.M., Nieto-Gonzalez J.L., Vardya I., Henningsen K., Jayatissa M.N., Wiborg O., Jensen K. Hippocampal GABAergic dysfunction in a rat chronic mild stress model of depression. Hippocampus. 2011;21:422–433. doi: 10.1002/hipo.20758. [DOI] [PubMed] [Google Scholar]
- Horovitz O., Tsoory M.M., Hall J., Jacobson-Pick S., Richter-Levin G. Post-weaning to pre-pubertal ('juvenile’) stress: a model of induced predisposition to stress-related disorders. Neuroendocrinology. 2012 doi: 10.1159/000331393. [DOI] [PubMed] [Google Scholar]
- Horovitz O., Tsoory M.M., Yovell Y., Richter-Levin G. A rat model of pre-puberty (Juvenile) stress-induced predisposition to stress-related disorders: sex similarities and sex differences in effects and symptoms. World J. Biol. Psychiatr. 2014;15:36–48. doi: 10.3109/15622975.2012.745604. [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S., Richter-Levin G. Short-and long-term effects of juvenile stressor exposure on the expression of GABA. Stress. 2012;15:416–424. doi: 10.3109/10253890.2011.634036. [DOI] [PubMed] [Google Scholar]
- Lau C.G., Murthy V.N. Activity-dependent regulation of inhibition via GAD67. J. Neurosci. 2012;32:8521–8531. doi: 10.1523/JNEUROSCI.1245-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.E., Lee Y., Lee G.H. The regulation of glutamic acid decarboxylases in GABA neurotransmission in the brain. Arch Pharm. Res. (Seoul) 2019 doi: 10.1007/s12272-019-01196-z. [DOI] [PubMed] [Google Scholar]
- Lee V., MacKenzie G., Hooper A., Maguire J. Reduced tonic inhibition in the dentate gyrus contributes to chronic stress-induced impairments in learning and memory. Hippocampus. 2016;26:1276–1290. doi: 10.1002/hipo.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier A.L., Romay-Tallón R., Caruncho H.J., Kalynchuk L.E. Altered GABAergic and glutamatergic activity within the rat hippocampus and amygdala in rats subjected to repeated corticosterone administration but not restraint stress. Neuroscience. 2013;231:38–48. doi: 10.1016/j.neuroscience.2012.11.037. [DOI] [PubMed] [Google Scholar]
- Makinson R., Lundgren K.H., Seroogy K.B., Herman J.P. Chronic social subordination stress modulates glutamic acid decarboxylase (GAD) 67 mRNA expression in central stress circuits. Physiol. Behav. 2015;146:7–15. doi: 10.1016/j.physbeh.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S., Kumagaya R., Kakizaki T., Fujihara K., Wakamatsu K., Yanagawa Y. Loss of glutamate decarboxylase 67 in somatostatin-expressing neurons leads to anxiety-like behavior and alteration in the akt/gsk3β signaling pathway. Front. Behav. Neurosci. 2019;13 doi: 10.3389/fnbeh.2019.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I., Çalişkan G., Stork O. The GAD65 knock out mouse - a model for GABAergic processes in fear- and stress-induced psychopathology. Gene Brain Behav. 2015 doi: 10.1111/gbb.12188. [DOI] [PubMed] [Google Scholar]
- Müller I., Obata K., Richter-Levin G., Stork O. GAD65 haplodeficiency conveys resilience in animal models of stress-induced psychopathology. Front. Behav. Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K., Otsuka M. Vol. 89. 2013. pp. 139–156. (Review Synaptic inhibition and .-aminobutyric acid in the mammalian central nervous system). jstage.jst.go.jp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl P.L., Gibson K.M. Clinical aspects off the disorders of GABA metabolism in children. Curr. Opin. Neurol. 2004 doi: 10.1097/00019052-200404000-00005. [DOI] [PubMed] [Google Scholar]
- Pofantis H., Georgopoulos P., Petrides T., Papatheodoropoulos C. Differences in paired-pulse inhibition and facilitation in the dentate gyrus and CA3 field between dorsal and ventral rat hippocampus. Brain Res. 2015;1608:21–30. doi: 10.1016/j.brainres.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Raza S.A., Albrecht A., Çallşkan G., Müller B., Demiray Y.E., Ludewig S., Meis S., Faber N., Hartig R., Schraven B., Lessmann V., Schwegler H., Stork O. HIPP neurons in the dentate gyrus mediate the cholinergic modulation of background context memory salience. Nat. Commun. 2017;8 doi: 10.1038/s41467-017-00205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev-Tsur S., Demiray Y.E., Tripathi K., Stork O., Richter-Levin G., Albrecht A. Region-specific involvement of interneuron subpopulations in trauma-related pathology and resilience. Neurobiol. Dis. 2020;143 doi: 10.1016/j.nbd.2020.104974. [DOI] [PubMed] [Google Scholar]
- Rehberg K., Kliche S., Madencioglu D.A., Thiere M., Müller B., Meineke B.M., Freund C., Budinger E., Stork O. The serine/threonine kinase Ndr2 controls integrin trafficking and integrin-dependent neurite growth. Soc. Neurosci. 2014;34:5342–5354. doi: 10.1523/JNEUROSCI.2728-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Levin G., Müller I., Tripathi K., Stork O. Stress Resilience: Molecular and Behavioral Aspects. Elsevier; 2019. Active resilience in response to traumatic stress; pp. 95–106. [DOI] [Google Scholar]
- Richter-Levin G., Stork O., Schmidt M.V. Animal models of PTSD: a challenge to be met. Mol. Psychiatr. 2019;24:1135–1156. doi: 10.1038/s41380-018-0272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritov G., Boltyansky B., Richter-Levin G. A novel approach to PTSD modeling in rats reveals alternating patterns of limbic activity in different types of stress reaction. Mol. Psychiatr. 2016;21:630–641. doi: 10.1038/mp.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinson D.A., Dillon C.P., Kwiatkowski A.V., Sievers C., Yang L., Kopinja J., Zhang M., McManus M.T., Gertler F.B., Scott M.L., Van Parijs L. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat. Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- Rudolph U., Möhler H. GABAA receptor subtypes: therapeutic potential in down syndrome, affective disorders, schizophrenia, and autism. Annu. Rev. Pharmacol. Toxicol. 2014 doi: 10.1146/annurev-pharmtox-011613-135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha R., Knapp S., Chakraborty D., Horovitz O., Albrecht A., Kriebel M., Kaphzan H., Ehrlich I., Volkmer H., Richter-Levin G. GABAergic synapses at the axon initial segment of basolateral amygdala projection neurons modulate fear extinction. Neuropsychopharmacology. 2017;42:473–484. doi: 10.1038/npp.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha R., Kriebel M., Volkmer H., Richter-Levin G., Albrecht A. Neurofascin knock down in the basolateral amygdala mediates resilience of memory and plasticity in the dorsal dentate gyrus under stress. Mol. Neurobiol. 2018;55:7317–7326. doi: 10.1007/s12035-018-0930-2. [DOI] [PubMed] [Google Scholar]
- Sandhu K.V., Lang D., Müller B., Nullmeier S., Yanagawa Y., Schwegler H., Stork O. Glutamic acid decarboxylase 67 haplodeficiency impairs social behavior in mice. Gene Brain Behav. 2014;13:439–450. doi: 10.1111/gbb.12131. [DOI] [PubMed] [Google Scholar]
- Sangha S., Narayanan R.T., Bergado-Acosta J.R., Stork O., Seidenbecher T., Pape H.-C. Behavioral/systems/cognitive deficiency of the 65 kDa isoform of glutamic acid decarboxylase impairs extinction of cued but not contextual fear memory. Soc. Neurosci. 2009;29:15713–15720. doi: 10.1523/JNEUROSCI.2620-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs A., Sabanov V., Balschun D. Distinct properties of long-term potentiation in the dentate gyrus along the dorsoventral Axis: influence of age and inhibition. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-05358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M., Richter-Levin G., Maggio N. Stress-induced dynamic routing of hippocampal connectivity: a hypothesis. Hippocampus. 2010;20:1332–1338. doi: 10.1002/hipo.20751. [DOI] [PubMed] [Google Scholar]
- Skórzewska A., Lehner M., Wisłowska-Stanek A., Turzyńska D., Sobolewska A., Krząścik P., Szyndler J., Maciejak P., Chmielewska N., Kołosowska K., Płaźnik A. Individual susceptibility or resistance to posttraumatic stress disorder-like behaviours. Behav. Brain Res. 2020;386 doi: 10.1016/j.bbr.2020.112591. [DOI] [PubMed] [Google Scholar]
- Sood R., Ritov G., Boltyansky B., Spector-Chotiner A., Richter-Levin G., Barki-Harrington L. Underwater trauma causes a long-term specific increase in the expression of cyclooxygenase-2 in the ventral CA1 of the hippocampus. Psychoneuroendocrinology. 2014;49:62–68. doi: 10.1016/j.psyneuen.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Thiere M., Kliche S., Müiler B., Teuber J., Nold I., Stork O. Integrin activation through the hematopoietic adapter molecule ADAP regulates dendritic development of hippocampal neurons. Front. Mol. Neurosci. 2016;9 doi: 10.3389/fnmol.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoory M., Richter-Levin G. Learning under stress in the adult rat is differentially affected by “juvenile” or “adolescent” stress. Int. J. Neuropsychopharmacol. 2006;9:713–728. doi: 10.1017/S1461145705006255. [DOI] [PubMed] [Google Scholar]
- Zitman F.M.P., Richter-Levin G. Age and sex-dependent differences in activity, plasticity and response to stress in the dentate gyrus. Neuroscience. 2013;249:21–30. doi: 10.1016/j.neuroscience.2013.05.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.