Abstract
Background/Aim: Hepatocellular carcinoma (HCC) mainly develops in the damaged liver from hepatitis C virus (HCV) or hepatitis B virus (HBV) infection in Japan. On the other hand, the occurrence of HCCs derived from the liver without viral infection has recently been increasing. Our aim was to identify characteristics specific to HCCs with virus-infected liver (HCC-BC) or those with non-B- and non-C-infected liver (HCC-NBNC), Patients and Methods: We collected preoperative serum α-fetoprotein (AFP) and Des-Gamma-Carboxy Prothrombin (DCP), also known as PIVKA-II values from surgically resected HCC cases during 1994-2017 in our department. Results: Preoperative serum AFP values of HCC-BC cases (n=284) were higher compared to HCC-NBNC cases (n=88) (p=0.016), whereas serum DCP values of HCC-NBNC cases were higher compared to HCC-BC cases (p<0.001). Multivariable analyses indicated that abnormal serum AFP [hazard ratio (HR)=1.46, 95% conficdence interval (CI)=1.03-2.07, p=0.035) was one of the significant recurrence-free survival predictors of HCC-BC cases, while abnormal serum DCP (HR=4.99, 95%CI=1.91-13.01, p=0.001) was one of the significant recurrence-free survival predictors of HCC-NBNC cases. Conclusion: HCC-NBNC cases have a different tumor marker profile from HCC-BC cases. Elevated DCP could be both a diagnostic and prognostic marker of HCC-NBNC patients.
Keywords: AFP, DCP, hepatocellular carcinoma, survival predictor
Hepatocellular carcinoma (HCC) is the 6th most frequently occuring cancer globally and still has a high likelihood of recurrence and a poor prognosis (1). HCCs are mainly derived from the damaged liver caused by various etiological factors, including hepatitis C virus (HCV) or hepatitis B virus (HBV) infection, as well as chronic alcohol abuse (2,3). Among them, HCV (65%) and HBV (15%) are the two major pathogenic factors in Japan (4). Recently, the occurrence of HCCs derived from non-B non-C livers (HCC-NBNC) have been relatively increasing because HBV or HCV treatments have dramatically improved. HCC-NBNC lesions typically arise from non-alcoholic steatohepatitis (NASH) or alcoholic liver disease.
To characterize the background liver status, whole-genome analyses have been widely performed (5,6). Some mutational signatures and altered pathways have been associated with certain histological characteristics of background livers or tumor stages (7,8). For instance, the mutation of catenin beta 1 (CTNNB1), one of the critical cluster of Wnt-signaling, has been related to alcohol-damaged liver. Telomerase reverse transcriptase (TERT), cyclin dependent kinase inhibitor 2A (CDKN2A), SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 2 (SMARCA2) and hepatocyte growth factor (HGF) alterations are also enriched in alcohol-related HCC patients. Tumor protein p53 (TP53) mutations are frequently associated with HBV infection. The integration of HBV into the host genome (9,10) induces upregulation of cancer-related genes, such as TERT, lysine methyltransferase 2B (MLL4), and cyclin E1 (CCNE1) genes. This leads to alterations in the genes functioning downstream of all these genes or cause whole genome chromosomal instability (10,11). Concerning the HCC-NBNC and background liver, Kutlu et al. have reported severral molecular characteristics (12), including a patatin-like phospholipase domain containing 3 (PNPLA3) gene mutation, epigenetic changes of phosphodiedterase 1B (PDE1B) and chromodomain helicase DNA-binding protein 1 (CHD1) , micro RNA deregulation including miR-122, metabolic pathway activating insulin receptor signaling and mitochondrial dysfunction caused by reactive oxygen species and endoplasmic reticulum stress.
We hypothesized that some molecular characteristics distinguishing HCC-NBNC from HCC with virus-infected liver (HCC-BC) may affect the positivity of well-known tumor markers of HCC, such as alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) (13). In this study, we used the HCC resection cohort in our institution and retrospectively compared HCC-NBNC cases with HCC-BC cases from the viewpoint of these well-known HCC serum tumor markers.
Patients and Methods
Patient cohort. Among surgically resected HCC cases from 1994 to 2017 in the Department of Gastroenterological Surgery, at Nagoya University (Aichi, Japan), 372 cases with available preoperative AFP and DCP markers were included (Institute Review Board approvealnumber: 2013-0295). Of these, 284 patients were categorized as HCC-BC and 88 patients as HCC-NBNC. The average follow-up period was 51.4 months. Clinical factors including age, gender, liver damage scores, tumor size and numbers, and pathological factors of tumor differentiation, growth pattern, capsule formation, serosal and vascular invasion were categorically compared between the two groups.
Serum marker collection. Each serum marker was checked by peripheral blood examination preoperatively. The standard institutional cut-off values were 10 ng/ml for AFP and 40 mAU/ml for DCP.
Statistical analysis. Patient clinicopathological characteristics were compared using Fisher’s exact test for categorical variables and Mann-Whitney U-test for continuous variables. Overall survival (OS) was defined as the time from surgery to the date of HCC disease-related death. Recurrence-free survival (RFS) was defined as the time from surgery to the date of recurrence diagnosis. Those who remained alive were censored at the last date they were known to be alive. A log-rank test was applied to compare the survival outcomes of the two groups. The Cox proportional hazards model was used for univariate and multivariable analysis for survival outcomes. All tests were considered statistically significant and clinically promising at p<0.05. Statistical analyses were carried out using the JMP 15 software (SAS Institute Japan, Tokyo, Japan).
Results
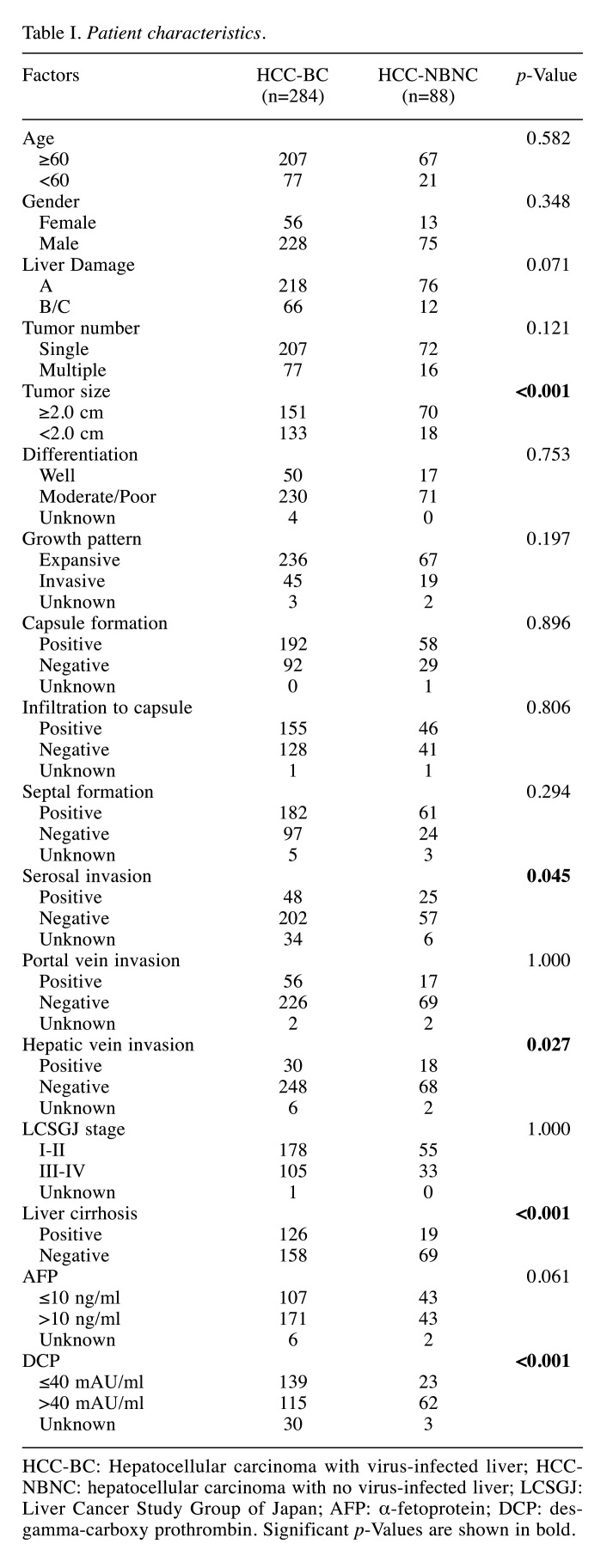
Patients characteristics. Clinicohistological characteristics of both HCC-BC cases (n=284) and HCC-NBNC cases (n=88) are shown in Table I. Due to the viral hepatic damage, liver damage score B/C cases were more frequently found in HCC-BC rather than in HCC-NBNC cases (p=0.071). Histologically advanced cases with large diameter (p<0.001), serosal invasion (p=0.045) and hepatic vein invasion (p=0.027) were frequently found in HCC-NBNC cases, while the cancer stage distributions of Liver Cancer Study Group of Japan (LCSGJ) between the two groups were comparable (p=1.000). The distribution of actual serum values for AFP and DCP were compared between HCC-BC and HCC-NBNC cases, as depicted in Figure 1. AFP values were inclined to exceed the cut-off value in HCC-BC cases (p=0.061), whereas DCP values were significantly higher in HCC-NBNC cases compared to HCC-BC cases (p<0.001).
Table I. Patient characteristics.
HCC-BC: Hepatocellular carcinoma with virus-infected liver; HCCNBNC: hepatocellular carcinoma with no virus-infected liver; LCSGJ: Liver Cancer Study Group of Japan; AFP: α-fetoprotein; DCP: desgamma-carboxy prothrombin. Significant p-Values are shown in bold.
Figure 1. Preoperative AFP and DCP values of HCC-BC and HCC-NBNC cases. HCC-BC cases (n=284) had significantly higher AFP values compared to the NBNC cohort, while HCC-NBNC cases (n=88) had significantly higher DCP values compared to the BC cohort. AFP: Alphafetoprotein; DCP: des-gamma-carboxy prothrombin; HCC-BC: hepatocellular carcinoma with virus-infected liver; HCC-NBNC: hepatocellular carcinoma with no virus-infected liver.
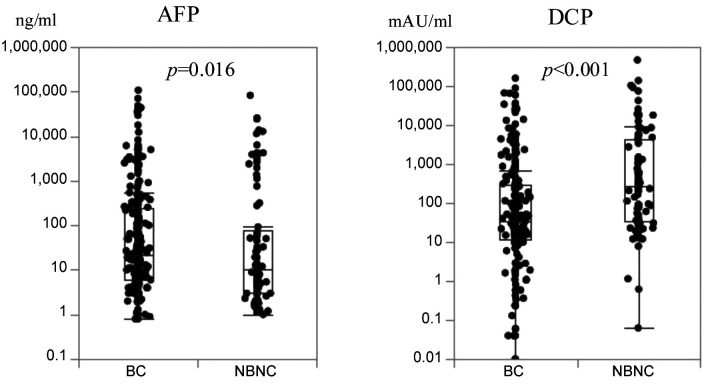
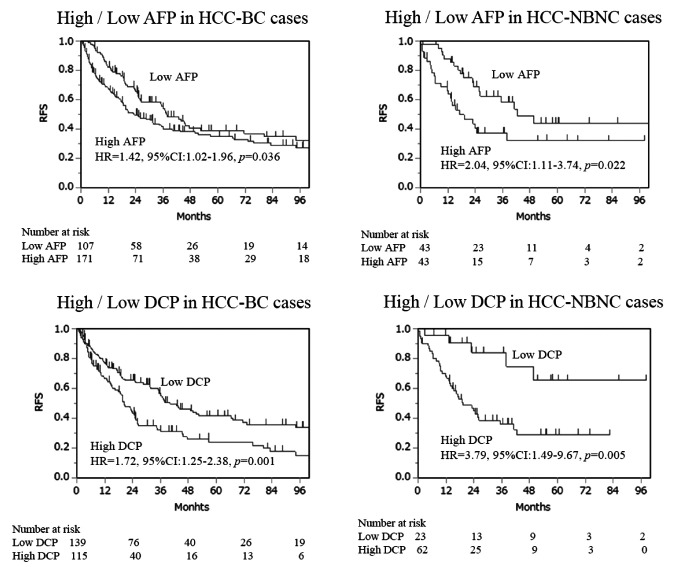
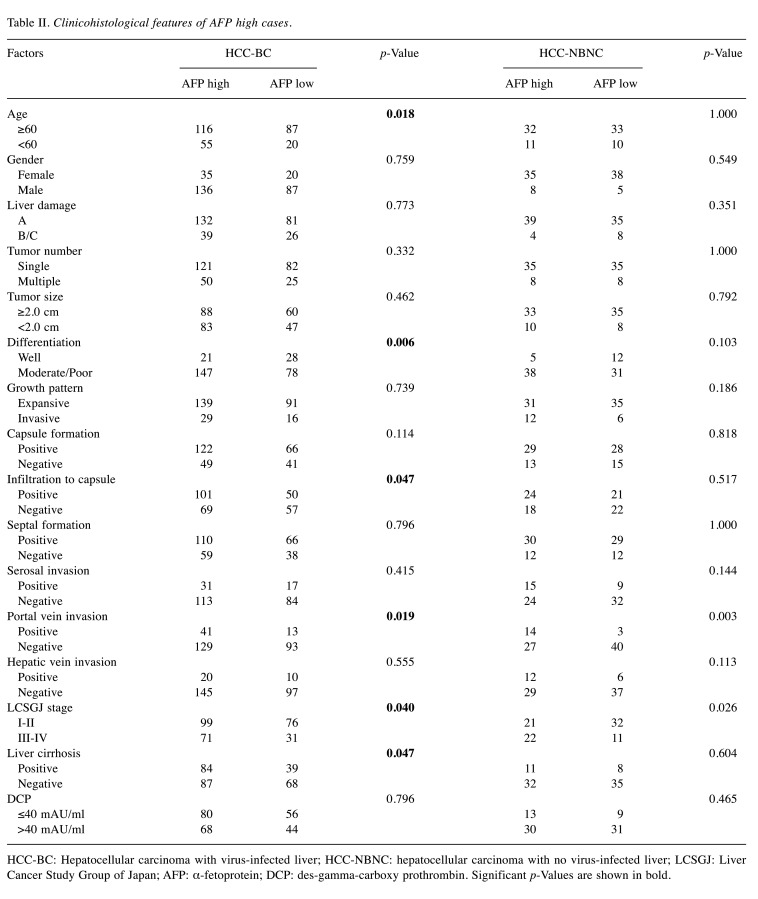
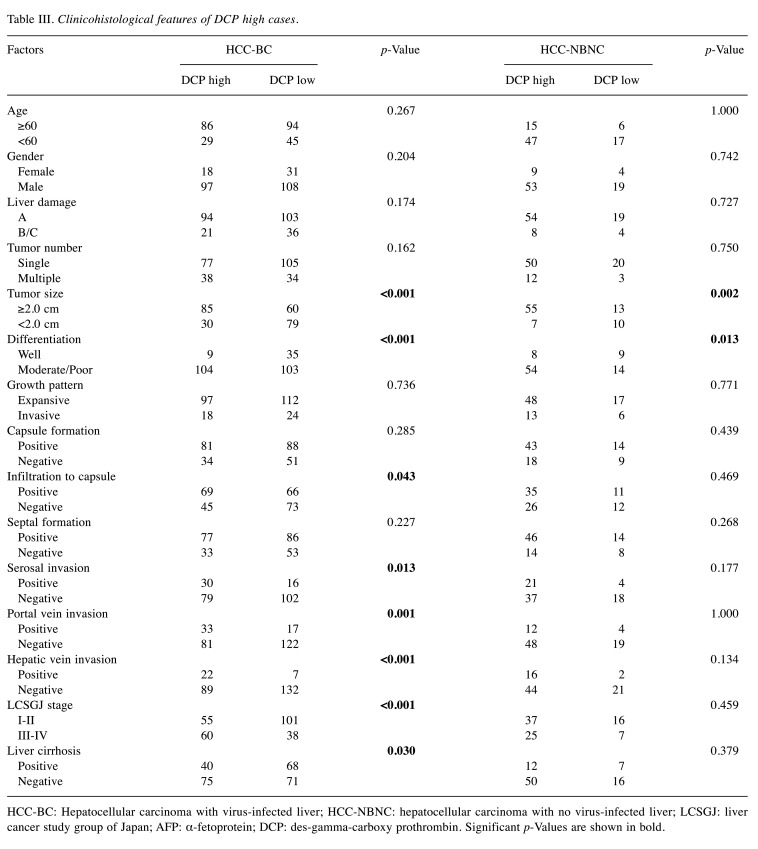
Serum tumor marker and survival outcomes. We compared high and low tumor marker cases based on the cut-off values in each HCC-BC and HCC-NBNC cohort to ascertain the markes’ impact on postoperative RFS and OS. With regards to RFS (Figure 2), cases with aberrantly high values of tumor markers showed significantly poor survival outcomes in both cohorts. Concerning OS (Figure 3), high AFP was associated with a significantly poor prognosis in the HCC-BC cohort. In contrast, patients with high DCP had significantly lower OS in both cohorts, with a vast difference in OS between high and low values in the HCC-HBNC cohort. Then, we compared AFP high with AFP low (Table II) as well as DCP high with DCP low (Table III) in the HCC-BC amd HCC-NBNC cohorts to examine the charactiristics associates with these values in detail. High AFP cases were related to aged people, with i) moderate or poor differentiation, ii) portal vein invasion, iii) advanced tumor stage and iv) positive liver cirrhosis, while high AFP cases were also specific to the HCC-NBNC cohort with both i) portal vein invasion and ii) advanced tumor stage. On the contrary, high DCP cases were significantly correlated with HCC-BC cases with i) a large tumor size, ii) moderate or poor differentiation, iii) infiltration to a capsule, iv) serosal invasion, v) vascular invasion, vi) advanced tumor stage and vii) liver cirrhosis. Also, they were associated with HCC-NBNC with i) large tumor size and ii) moderate or poor differentiation.
Figure 2. Recurrence-free survival curves (RFS). RFS was compared between high AFP cases (AFP>10ng/ml) and low AFP cases, as well as high DCP cases (DCP>40 mAU/ml) and low DCP cases in both HCC-BC and HCC-NBNC cohorts. Both serum markers indicated significantly poor survival outcomes in both cohorts. AFP: Alpha-fetoprotein; DCP: des-gamma-carboxy prothrombin; HCC-BC: hepatocellular carcinoma with virusinfected liver; HCC-NBNC: hepatocellular carcinoma with no virus-infected liver.
Figure 3. Overall survival (OS). OS curves were compared between high AFP cases (AFP>10ng/ml) and low AFP cases, and high DCP cases (DCP>40 mAU/ml) and low DCP cases in the HCC-BC cohort and HCC-NBNC cohort, respectively. High AFP indicated significantly poor survival outcomes in the HCC-BC cohort, while high DCP displayed significantly poor survival outcomes in both cohorts. AFP: Alpha-fetoprotein; DCP: des-gamma-carboxy prothrombin; HCC-BC: hepatocellular carcinoma with virus-infected liver; HCC-NBNC: hepatocellular carcinoma with no virus-infected liver.
Table II. Clinicohistological features of AFP high cases.
HCC-BC: Hepatocellular carcinoma with virus-infected liver; HCC-NBNC: hepatocellular carcinoma with no virus-infected liver; LCSGJ: Liver Cancer Study Group of Japan; AFP: α-fetoprotein; DCP: des-gamma-carboxy prothrombin. Significant p-Values are shown in bold.
Table III. Clinicohistological features of DCP high cases.
HCC-BC: Hepatocellular carcinoma with virus-infected liver; HCC-NBNC: hepatocellular carcinoma with no virus-infected liver; LCSGJ: liver cancer study group of Japan; AFP: α-fetoprotein; DCP: des-gamma-carboxy prothrombin. Significant p-Values are shown in bold.
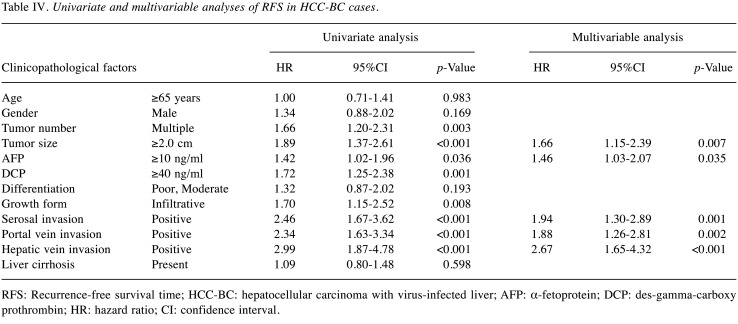
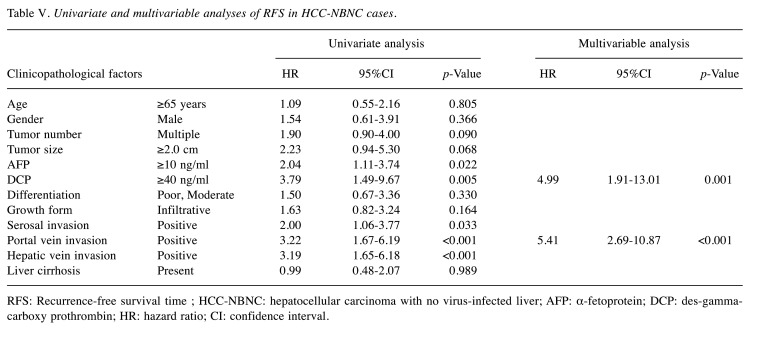
Univariate and multivariable analyses of survival outcomes. Univariate and multivariable analyses of survival outcomes were performed. All significant factors in the univariate analysis were put into the multivariable analysis. The backward stepwise method was performed until the p-Values of all remaining factors became significant. Table IV and Table V summarize the results of RFS in the HCC-BC and HCC-NBNC cohorts. In HCC-BC cases, i) tumor size, ii) AFP elevation, iii) serosal invasion, iv) portal vein invasion and v) hepatic vein invasion were detected as significant prognostic factors of RFS in multivariable analysis. On the other hand, i) DCP elevation and ii) portal vein invasion were significant factors in HCC-NBNC cases.
Table IV. Univariate and multivariable analyses of RFS in HCC-BC cases.
RFS: Recurrence-free survival time; HCC-BC: hepatocellular carcinoma with virus-infected liver; AFP: α-fetoprotein; DCP: des-gamma-carboxy prothrombin; HR: hazard ratio; CI: confidence interval.
Table V. Univariate and multivariable analyses of RFS in HCC-NBNC cases.
RFS: Recurrence-free survival time ; HCC-NBNC: hepatocellular carcinoma with no virus-infected liver; AFP: α-fetoprotein; DCP: des-gammacarboxy prothrombin; HR: hazard ratio; CI: confidence interval.
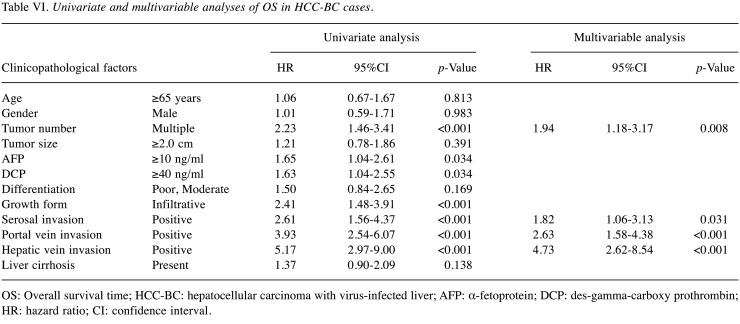
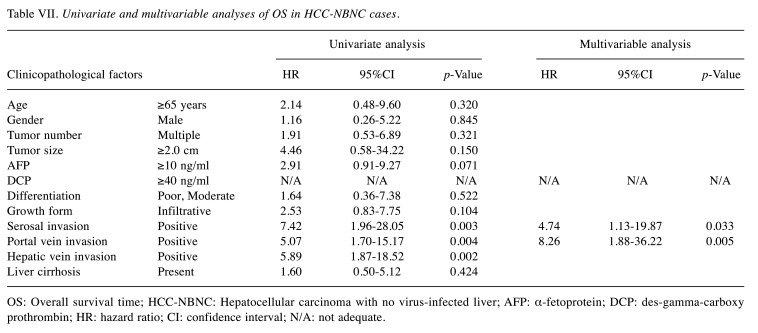
Table VI and Table VII demonstrate the results of OS in each cohort. In the multivariable analysis of HCC-BC cases i) tumor number, ii) serosal invasion, iii) portal vein invasion and iv) hepatic vein invasion were significant predictors. In contrast, i) DCP elevation was an extremely significant predictor of HCC-NBNC cases in addition to ii) serosal invasion and iii) portal vein invasion. None of the low DCP cases died from the disease in our cohort.
Table VI. Univariate and multivariable analyses of OS in HCC-BC cases.
OS: Overall survival time; HCC-BC: hepatocellular carcinoma with virus-infected liver; AFP: α-fetoprotein; DCP: des-gamma-carboxy prothrombin; HR: hazard ratio; CI: confidence interval.
Table VII. Univariate and multivariable analyses of OS in HCC-NBNC cases.
OS: Overall survival time; HCC-NBNC: Hepatocellular carcinoma with no virus-infected liver; AFP: α-fetoprotein; DCP: des-gamma-carboxy prothrombin; HR: hazard ratio; CI: confidence interval; N/A: not adequate.
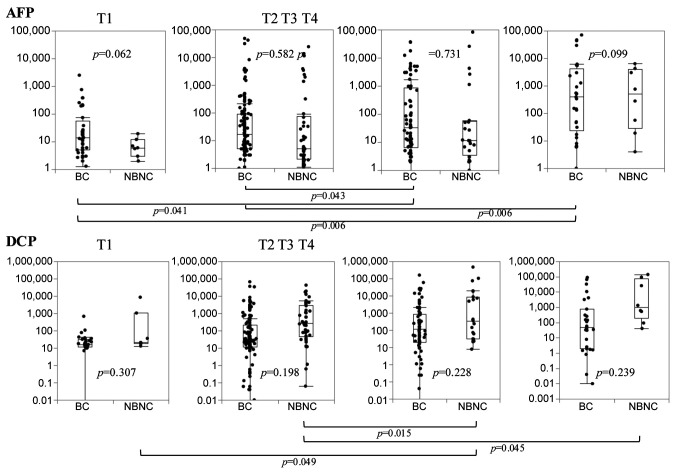
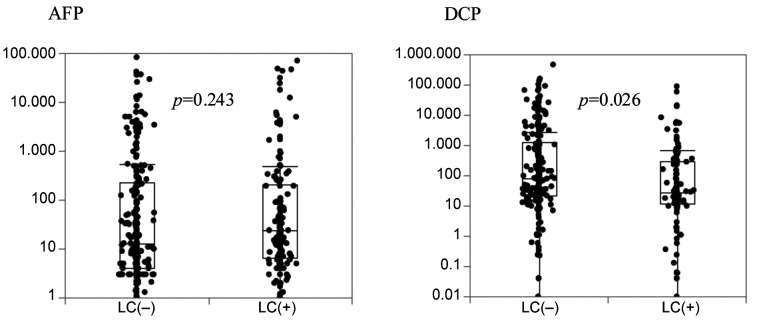
Clinical characteristics of AFP and DCP elevation. AFP values of HCC-BC cases increased depending on tumor T stage, while DCP values of HCC-NBNC cases increased depending on the T stage (Figure 4). Besides, the association of both markers with background liver are shown in Figure 5. AFP does not decrease in the cirrhotic liver, while DCP decreases in them.
Figure 4. Distribution of preoperative AFP and DCP values according to histological T grades. AFP values of HCC-BC cases are gradually increased in parallel with T grades, whereas of HCC-NBNC cases did not. On the contrary, HCC-NBNC cases showed a steady increase in DCP values with T stage, while HCC-BC cases showed no increase. AFP: Alpha-fetoprotein; DCP: des-gamma-carboxy prothrombin; HCC-BC: hepatocellular carcinoma with virus-infected liver; HCC-NBNC: hepatocellular carcinoma with no virus-infected liver.
Figure 5. Association between each tumor marker and liver cirrhosis (LC). AFP values showed no decrease in LC cases, whereas DCP values decreased in LC cases. AFP: Alpha-fetoprotein, DCP: des-gamma-carboxy prothrombin.
Discussion
Clinically, the measurement of both AFP and DCP has been strongly recommended in the Clinical Practice Guidelines for Hepatocellular Carcinoma (14); however, the mechanism of each tumor marker elevation is unknown and may differ bweteen tumor types. HCCs derived from NBNC are reported to have relatively low serum AFP levels compared to hepatitis B-derived HCCs (15). Also, hepatitis C-infected livers usually have high serum AFP levels (16). These findings suggest that AFP elevation is commonly influenced by a viral infection of the background liver. AFP is a glycoprotein derived from the embryonic endoderm. It is closely related to the growth of malignant tumors (17). During embryonic development, AFP is initially produced in the fetal liver and yolk sac. The serum AFP concentration increases during the period between 12-16 weeks of gestation and then it gradually reduces to normal range till adulthood (18). AFP increases again during early stages of hepatocytes’ malignant transformation, and it is activated in the malignant cells. Zheng Y et al., have summarized the AFP production mechanism in HBV-derived hepatitis-based HCCs (17), where the HBV X protein promotes the acceleration of AFP’s accretion, which induces growth signal activation, metastases and bears an immunosuppressive role.
Instead, DCP is abnormal prothrombin and produced due to the defect of the post-translational carboxylation of prothrombin’s precursor (19); however, the detailed mechanism of its production is unclear. Taniguchi T et al. have used mass spectrometry analysis of hepatoma cell lines to reveal that PARP-1 activates prothrombin gene transcription and that this excessive transcription induces DCP production (20). PARP-1 inhibition is also reported as a candidate therapeutic strategy for hepatic triglyceride accumulation, metabolic dysregulation, inflammation and fibrosis in mouse NASH models (21). DCP elevation reflects vascular invasion and tumor recurrences following hepatectomy (22). It has also been reported to increase during epithelial to mesenchymal transition in tumors (23). In other words, DCP goes up by tumor factors.
Interestingly, Suzuki H et al., have reported that mild hypoxia induces HCC to produce DCP, while long-lasting hypoxia impaires DCP production in HCC cells (23), which could partly explain why DCP is elevated in HCC-NBNCs rather than in HCC-BCs. In our study tumor sizes of HCC-NBNCs were significantly larger than HCC-BCs because no intensive follow-up examination was usually performed for NBNC patients. The relatively large HCC-NBNCs sometimes induce intratumoral hypoxia, which is easy to produce DCP (24). Our clinical data clearly indicate that DCP values increased depending on the T stage of HCC-NBNCs. Besides HCC-BCs are derived from the damaged background liver, which is chronically exposed to long-lasting hypoxia (25). Actually, DCP values of the cirrhotic liver tumors were significantly decreased.
Exome sequences of hepatocellular carcinomas have identified new mutational signatures and potential therapeutic targets (7). Depending on the risk factors of hepatocarcinogenesis, responsible gene signatures vary. For instance, CTNNB1, TERT, CDKN2A, SMRCA2 and HGF gene alterations ican be frequently found in alcohol-based hepatitis. TP53 mutation was dominant in hepatitis B cases. In contrast, no distinct signature was identified in hepatitis C or NASH-based HCCs. Totoki et al., have revealed 30 candidate driver genes and 11 core pathway modules from 503 liver cancer genomes (8). TERT or ATRX chromation remodeler (ATRX) genes are widely mutated in all virus-induced HCCs. For NBNC HCCs, AT-rich interaction domain 1A (ARID1A) mutation is frequently found. Moore et al., have demonstrated that ARID1A-deficient livers are more susceptible to high-fat diet-induced liver steatosis and fibrosis in mice models (26). As a detailed mechanism, Qu YL et al., have revealed that ARID1A deficiency impairs fatty acid oxidation by epigenetically downregulating Peroxisome proliferator-activated receptor alpha (PPARα) and other metabolism-related genes, such as carnitine palmitoyltransferase 1A (CPT1A) and acyl-CoA oxidase 1 (ACOX1) (27).
This study has some limitations. First, this is a retrospective study from a single-institution with a modest sample size. Further confirmation with large multicenter data is required. Second, the mechanism of DCP elevation in HCC-NBNC should be explained by specific molecular characteristics, including PNPLA3 mutation, ARID1A deficiency or lipid metabolism-related genes in non-hepatitis livers in future studies.
In conclusion, AFP elevation and DCP elevation were differentially observed depending on the background liver status. Hepatocarcinogenesis in NASH liver was specific to DCP elevation, rather than AFP. DCP seems to be a significant predictive serum marker of survival outcomes, especially for HCC-NBNC cases.
Conflicts of Interest
The Authors declare no conflicts of interest.
Authors’ Contributions
MH, SY and YO designed the project. MH, NT,YO, HT, YI, FS, NT and MK collected the clinical data. MH, NT and YO analyzed the data. MH and SY checked and approved all the statistical analyses. MH prepared the manuscript and DS, NH, CT, GN, MK and YK revised it.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.Utsunomiya T, Shimada M, Kudo M, Ichida T, Matsui O, Izumi N, Matsuyama Y, Sakamoto M, Nakashima O, Ku Y, Takayama T, Kokudo N, Liver Cancer Study Group of Japan A comparison of the surgical outcomes among patients with HBV-positive, HCV-positive, and non-B non-C hepatocellular carcinoma: a nationwide study of 11,950 patients. Ann Surg. 2015;261(3):513–520. doi: 10.1097/SLA.0000000000000821. [DOI] [PubMed] [Google Scholar]
- 5.Brunner SF, Roberts ND, Wylie LA, Moore L, Aitken SJ, Davies SE, Sanders MA, Ellis P, Alder C, Hooks Y, Abascal F, Stratton MR, Martincorena I, Hoare M, Campbell PJ. Somatic mutations and clonal dynamics in healthy and cirrhotic human liver. Nature. 2019;574(7779):538–542. doi: 10.1038/s41586-019-1670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Letouzé E, Shinde J, Renault V, Couchy G, Blanc JF, Tubacher E, Bayard Q, Bacq D, Meyer V, Semhoun J, Bioulac-Sage P, Prévôt S, Azoulay D, Paradis V, Imbeaud S, Deleuze JF, Zucman-Rossi J. Mutational signatures reveal the dynamic interplay of risk factors and cellular processes during liver tumorigenesis. Nat Commun. 2017;8(1):1315. doi: 10.1038/s41467-017-01358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, Calatayud AL, Pinyol R, Pelletier L, Balabaud C, Laurent A, Blanc JF, Mazzaferro V, Calvo F, Villanueva A, Nault JC, Bioulac-Sage P, Stratton MR, Llovet JM, Zucman-Rossi J. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47(5):505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Totoki Y, Tatsuno K, Covington KR, Ueda H, Creighton CJ, Kato M, Tsuji S, Donehower LA, Slagle BL, Nakamura H, Yamamoto S, Shinbrot E, Hama N, Lehmkuhl M, Hosoda F, Arai Y, Walker K, Dahdouli M, Gotoh K, Nagae G, Gingras MC, Muzny DM, Ojima H, Shimada K, Midorikawa Y, Goss JA, Cotton R, Hayashi A, Shibahara J, Ishikawa S, Guiteau J, Tanaka M, Urushidate T, Ohashi S, Okada N, Doddapaneni H, Wang M, Zhu Y, Dinh H, Okusaka T, Kokudo N, Kosuge T, Takayama T, Fukayama M, Gibbs RA, Wheeler DA, Aburatani H, Shibata T. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46(12):1267–1273. doi: 10.1038/ng.3126. [DOI] [PubMed] [Google Scholar]
- 9.Edman JC, Gray P, Valenzuela P, Rall LB, Rutter WJ. Integration of hepatitis B virus sequences and their expression in a human hepatoma cell. Nature. 1980;286(5772):535–538. doi: 10.1038/286535a0. [DOI] [PubMed] [Google Scholar]
- 10.Jhunjhunwala S, Jiang Z, Stawiski EW, Gnad F, Liu J, Mayba O, Du P, Diao J, Johnson S, Wong KF, Gao Z, Li Y, Wu TD, Kapadia SB, Modrusan Z, French DM, Luk JM, Seshagiri S, Zhang Z. Diverse modes of genomic alteration in hepatocellular carcinoma. Genome Biol. 2014;15(8):436. doi: 10.1186/s13059-014-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sung WK, Zheng H, Li S, Chen R, Liu X, Li Y, Lee NP, Lee WH, Ariyaratne PN, Tennakoon C, Mulawadi FH, Wong KF, Liu AM, Poon RT, Fan ST, Chan KL, Gong Z, Hu Y, Lin Z, Wang G, Zhang Q, Barber TD, Chou WC, Aggarwal A, Hao K, Zhou W, Zhang C, Hardwick J, Buser C, Xu J, Kan Z, Dai H, Mao M, Reinhard C, Wang J, Luk JM. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genet. 2012;44(7):765–769. doi: 10.1038/ng.2295. [DOI] [PubMed] [Google Scholar]
- 12.Kutlu O, Kaleli HN, Ozer E. Molecular Pathogenesis of Nonalcoholic Steatohepatitis- (NASH-) Related Hepatocellular Carcinoma. Can J Gastroenterol Hepatol. 2018;2018:8543763. doi: 10.1155/2018/8543763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song T, Wang L, Xin R, Zhang L, Tian Y. Evaluation of serum AFP and DCP levels in the diagnosis of early-stage HBV-related HCC under different backgrounds. J Int Med Res. 2020;48(10):300060520969087. doi: 10.1177/0300060520969087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makuuchi M, Kokudo N. Clinical practice guidelines for hepatocellular carcinoma: the first evidence based guidelines from Japan. World J Gastroenterol. 2006;12(5):828–829. doi: 10.3748/wjg.v12.i5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakiyama S, Matsumoto M, Haruki K, Gocho T, Sakamoto T, Shiba H, Futagawa Y, Ishida Y, Yanaga K. Clinical features and outcome of surgical patients with non-b non-c hepatocellular carcinoma. Anticancer Res. 2017;37(6):3207–3213. doi: 10.21873/anticanres.11682. [DOI] [PubMed] [Google Scholar]
- 16.El Raziky M, Attia D, El Akel W, Shaker O, Khatab H, Abdo S, Elsharkawy A, Esmat G. Hepatic fibrosis and serum alpha-fetoprotein (AFP) as predictors of response to HCV treatment and factors associated with serum AFP normalisation after treatment. Arab J Gastroenterol. 2013;14(3):94–98. doi: 10.1016/j.ajg.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Y, Zhu M, Li M. Effects of alpha-fetoprotein on the occurrence and progression of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(10):2439–2446. doi: 10.1007/s00432-020-03331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Cao D, Zhou L, Zhang Y, Guo X, Li H, Chen Y, Spear BT, Wu JW, Xie Z, Zhang WJ. ZBTB20 is a sequence-specific transcriptional repressor of alpha-fetoprotein gene. Sci Rep. 2015;5:11979. doi: 10.1038/srep11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue P, Gao ZH, Xue X, Cui SX, Zhao CR, Yuan Y, Yin Z, Inagaki Y, Kokudo N, Tang W, Qu XJ. Des-γ-carboxyl prothrombin induces matrix metalloproteinase activity in hepatocellular carcinoma cells by involving the ERK1/2 MAPK signalling pathway. Eur J Cancer. 2011;47(7):1115–1124. doi: 10.1016/j.ejca.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi T, Kishi K, Nakagawa T, Tanaka H, Tanaka T, Tomonari T, Okamoto K, Sogabe M, Miyamoto H, Okahisa T, Muguruma N, Kajimoto M, Sagawa I, Takayama T. Poly-(ADP-Ribose) Polymerase-1 promotes prothrombin gene transcription and produces des-gamma-carboxy prothrombin in hepatocellular carcinoma. Digestion. 2017;95(3):242–251. doi: 10.1159/000470837. [DOI] [PubMed] [Google Scholar]
- 21.Mukhopadhyay P, Horváth B, Rajesh M, Varga ZV, Gariani K, Ryu D, Cao Z, Holovac E, Park O, Zhou Z, Xu MJ, Wang W, Godlewski G, Paloczi J, Nemeth BT, Persidsky Y, Liaudet L, Haskó G, Bai P, Boulares AH, Auwerx J, Gao B, Pacher P. PARP inhibition protects against alcoholic and non-alcoholic steatohepatitis. J Hepatol. 2017;66(3):589–600. doi: 10.1016/j.jhep.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki S, Takayama T, Kurokawa T, Shimamoto N, Mitsuka Y, Yoshida N, Higaki T, Sugitani M. Next-generation des-r-carboxy prothrombin for immunohistochemical assessment of vascular invasion by hepatocellular carcinoma. BMC Surg. 2020;20(1):201. doi: 10.1186/s12893-020-00862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki H, Murata K, Gotoh T, Kusano M, Okano H, Oyamada T, Yasuda Y, Imamura M, Kudo M, Mizokami M, Sakamoto A. Phenotype-dependent production of des-γ-carboxy prothrombin in hepatocellular carcinoma. J Gastroenterol. 2011;46(10):1219–1229. doi: 10.1007/s00535-011-0432-8. [DOI] [PubMed] [Google Scholar]
- 24.Höckel M, Vaupel P. Biological consequences of tumor hypoxia. Semin Oncol. 2001;28(2 Suppl 8):36–41. [PubMed] [Google Scholar]
- 25.Zhu C, Liu X, Wang S, Yan X, Tang Z, Wu K, Li Y, Liu F. Hepatitis C virus core protein induces hypoxia-inducible factor 1α-mediated vascular endothelial growth factor expression in Huh7.5.1 cells. Mol Med Rep. 2014;9(5):2010–2014. doi: 10.3892/mmr.2014.2039. [DOI] [PubMed] [Google Scholar]
- 26.Moore A, Wu L, Chuang JC, Sun X, Luo X, Gopal P, Li L, Celen C, Zimmer M, Zhu H. Arid1a loss drives nonalcoholic steatohepatitis in mice through epigenetic dysregulation of hepatic lipogenesis and fatty acid oxidation. Hepatology. 2019;69(5):1931–1945. doi: 10.1002/hep.30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu YL, Deng CH, Luo Q, Shang XY, Wu JX, Shi Y, Wang L, Han ZG. Arid1a regulates insulin sensitivity and lipid metabolism. EBioMedicine. 2019;42:481–493. doi: 10.1016/j.ebiom.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]