Abstract
Background: Wire-guided localization is the gold-standard for the detection of non-palpable breast lesions, although with acknowledged limitations. The aim of this study was to evaluate the combined use of LOCalizerr™ (Hologic, Santa Carla, CA, USA), and intraoperative ultrasound (IOUS) for localization and surgery of non-palpable breast cancer. Patients and Methods: Patients with non-palpable breast lesions underwent localization procedure with LOCalizer™ and IOUS. After the placement of the marker, eight measures were made to guide the excision. LOCalizerr™ Pencil and IOUS were performed to obtain the distance between the dissection plane and the margins of lesions. Results: The procedure was feasible in the five enrolled patients and associated with clear oncological margins in all cases. Moreover, a high satisfaction according to Likert scale for surgeons, radiologists and patients, performing limited and tailored resections, was reported. Conclusion: Combining LOCalizerr™ and IOUS is an effective method for locating non-palpable breast cancer, guarantying excellent oncological and cosmetic results.
Keywords: Non-palpable breast cancer, LOCalizer™, radiofrequency localization, intraoperative ultrasound
Worldwide screening mammography campaigns and the improvement of 3D-tomosynthesis detection has led to an increase in the diagnosis of non-palpable breast lesions (1). It is acknowledged that one-third of breast cancers are occult at first clinical evaluation. The number of patients with non-palpable breast cancer (NPBC) who are candidates for surgery might rise substantially considering the increasing use of neo-adjuvant treatments and downstaging of palpable lesions (2). Therefore, accurate preoperative localization of non-palpable lesions is mandatory for correct surgical excision. In patients with early-stage NPBC, wire-guided localization (WGL) is widely adopted as the method of localization (3), despite being associated with several disadvantages, such as migration, trauma, breakage, patient discomfort, and risk of pneumothorax. Moreover, the placement of wires should be carried out during hospitalization, on the same day of surgery (4).
Since the turn of the century, different innovative localizing wireless techniques have been tested and adopted in the search for the ideal system. Radioactive seed localization (RSL) consists of a system using an intra-operative gamma probe capable of identifying a 5-mm I125 pellet with a titanium shell, the radioactive seed. Similar surgical outcomes after its application are reported, including oncological safety, reoperation rates, minimal invasiveness and cosmesis. The main limit of this technique is patient exposure to radioactivity (5-8).
The SCOUT Radar Device (Merit Medical, San Josè, CA, USA) is a non-radioactive, non-wire localization device using infrared light and radar technology, and was approved by the U.S. Food and Drug Administration (FDA) in 2014 for localization of breast lesions. Despite positive results, its main limit is related to the significant cost of the intraoperative probe compared to WGL and RSL (9).
The most recently introduced system is the MAGSEED device (Endomagnetics, Inc, Austin, TX, USA). It was approved by the FDA in March 2016 and consists of a metal marker containing iron particles. A dedicated Sentimag probe uses the MAGSEED to generate an alternating magnetic field. The main limits to its use are the absence of a detectable signal through the skin if placed deeper than 4.0 cm, artifacts at magnetic resonance imaging (MRI), and the necessity for use of non-magnetic tools during surgery (10).
Intraoperative ultrasound (IOUS) is a fast and simple method for the detection of NPBC (11). It is affected by operator experience but is cheap and reproducible.
Recently, a new device was approved by the FDA, the Faxitron LOCalizer™. It allows the identification of NPBC through the positioning of a radiographic, radiofrequency identification (RFID) tag which is recognized by a dedicated probe (LOCalizer™ Pencil) but in literature, although the preliminary results compared to conventional WGL were positive, only scarce data on its use have been reported (12).
The current study aimed to evaluate the accuracy and feasibility of the combined use of Faxitron LOCalizer™ and IOUS in the detection of NPBC.
Patients and Methods
Study design. A prospective, randomized pilot study was conducted to evaluate the feasibility of the combined use of Faxitron LOCalizer™ and IOUS as a method for localization of NPBC. This study was reviewed and approved by the local institutional Ethics Committee (203/20).
Study setting and study population. From 15 June to 15 July 2020, a series of patients with NPBC and referred to the General Mini-invasive, Oncological, and Bariatric Surgery Unit of L. Vanvitelli Campania University in Naples were included. Inclusion criteria were: A diagnosis of NPBC confirmed by mammography, ultrasound and core biopsy; a preoperative TNM classification (eighth edition) (13) up to T1c with maximum diameter <2 cm; female gender; age between 18 and 70 years.
The patient’s surgical and oncological management were discussed during a multidisciplinary committee. Patients were provided with detailed informed regarding the study protocol. We proposed two different procedural options: Wireless tumor localization using the LOCalizer™ system associated with IOUS, or the current gold standard, WGL. All risks and benefits for both techniques were explained and the patients were able to choose their procedure. All patients gave their informed written consent to the procedure.
Faxitron LOCalizer™. Faxitron LOCalizer™ is a composite device composed an RFID chip named a Tag, a Tag applicator, a Reader, and a surgical probe named a Pencil, which guides the surgeon looking for the marked lesion. Each inert Tag measures about 1 cm and has a unique identification number, which is very useful when there are two or more lesions to excise. A Tag transmits its digital data only when triggered by an electromagnetic interrogation pulse from the LOCalizer™ Pencil, releasing an acoustic signal, indicating its distance in millimeters. The pitch and the volume of the sound increase when the Pencil approaches the Tag.
The placement of the Tag was performed at the Division of Diagnostic Imaging of L. Vanvitelli Campania University (Naples), 10 days before the planned surgery. Two radiologists participated in the current pilot trial. Tags were placed by introducing a percutaneous 12-gauge applicator under US, with local anesthesia (14).
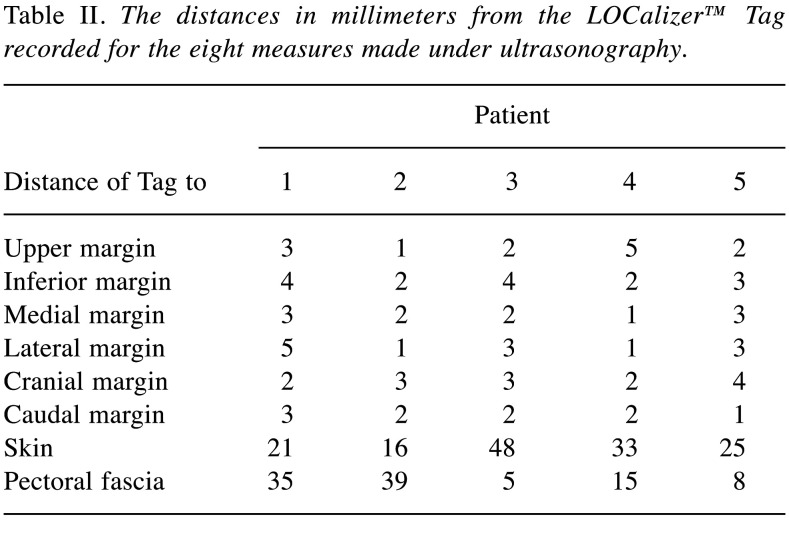
Since the Tag is clearly identifiable by US, immediately after placement, the radiologist checked the position of the Tag and measured its distance from the margins of the lesion. Eight measures were made and recorded from the Tag to: i) Upper margin, ii) inferior margin, iii) medial margin, iv) lateral margin, v) cranial margin, vi) caudal margin, vii) skin, viii) pectoral fascia. Following the procedure, radiologists completed a questionnaire and were asked about the placement duration, and their satisfaction with Tag centering, and Applicator and Tag visibility at US, according to a Likert scale (range=0-10) (15). Moreover, they gave their evaluation of the overall procedure 24 h and 1 month after the procedure.
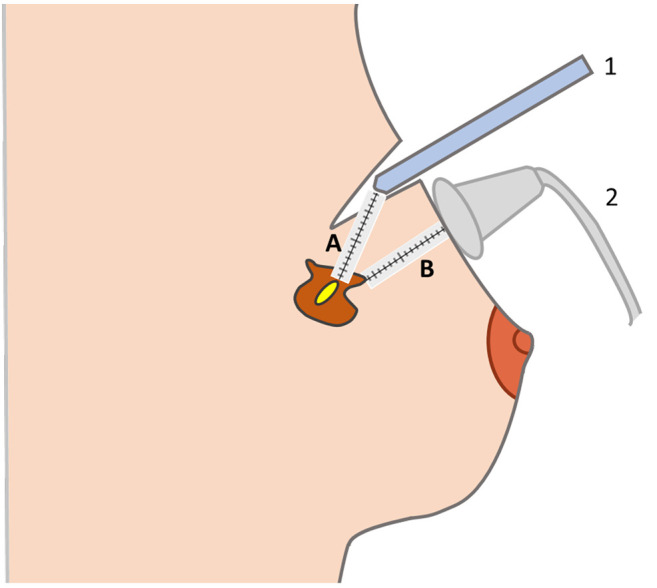
Surgical procedure and intraoperative combined localization. All surgical procedures were performed by three experienced oncology breast surgeons, with extensive US experience (almost 3,000 intraoperative US examinations). Lumpectomies were performed under general anesthesia. Before the incision, LOCalizer™ Pencil was used to identify the Tag’s site. A dermographic pen was used to mark the supposed incisional cut. US examination was adopted to confirm the Tag’s location. Before incision, the surgeons repeated the eight measurements reported by the radiologists in order to verify their correspondence, and assess migration. Therefore, the surgeons started the lumpectomy using the LOCalizer™ Pencil. The surgeons were guided by the sound and the indication of the Tag Pencil distance on the display of the LOCalizer™ Reader. Whilst the LOCalizer™ allows the distance between the Pencil and the Tag placed into or near the lesion to be verified, lesion margins remained occult because the LOCalizer™ system provides information about the Tag, not about the perimeter of the NPBC. To improve selection of the correct section line and to visualize the lump margins, intraoperative US was performed when the reported distance on the display between the Pencil and the Tag was <2.5 cm. (Figure 1). Subsequently, the patients underwent conventional lumpectomy with sentinel lymph node biopsy (16).
Figure 1. The image shows the intraoperative combined use of LOCalizer™ and intraoperative ultrasound. The Pencil (labeled 1) approaches the lesion, following the indication of the distance in millimeters (A) from the Tag but the surgeon cannot recognize the margins of the lesion. Intraoperative ultrasound (labeled 2) enables identification of the perimeter of the lesion and the distance to the margin (B), improving oncological safety and enabling minimally invasive resection.
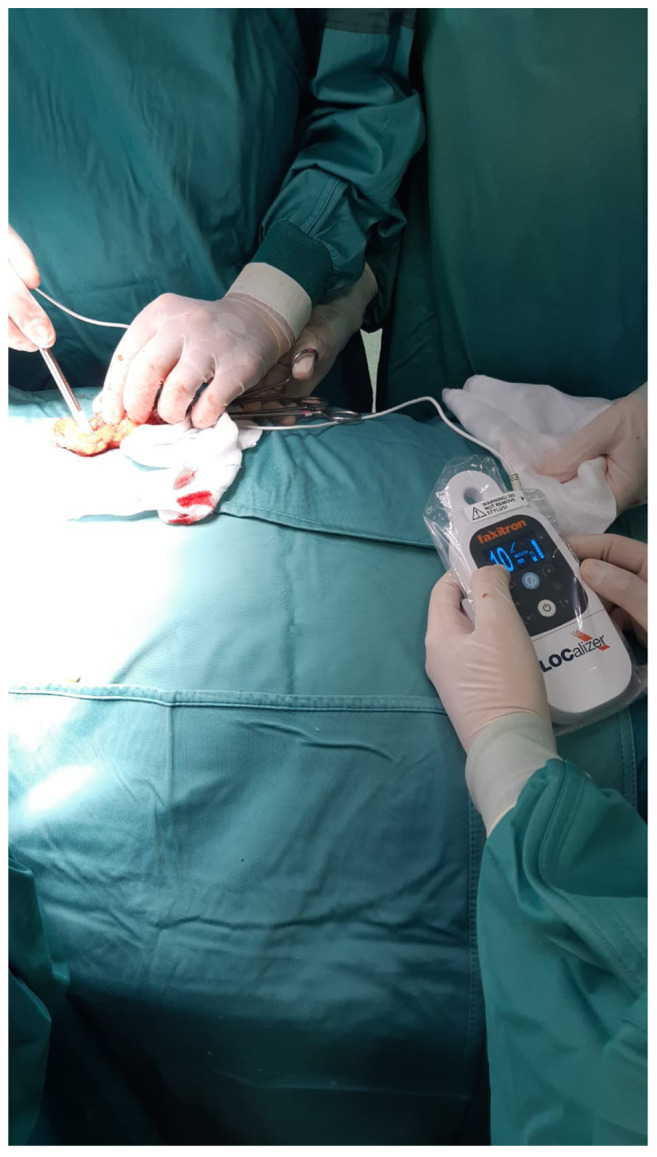
After lump removal, the surgeons confirmed the removal of Tags using the Pencil on the resected specimen (Figure 2). The Probe was also positioned into the breast to exclude the existence of a residual Tag. A further check was performed using US on the resected lump containing the Tag, which was clearly visible. Finally, the pathologist carried out an extemporaneous examination of each specimen.
Figure 2. Intraoperative image of the LOCalizer™ system in use, showing the surgeon verifying the presence of the LOCalizer™ Tag in the resected specimen.
After the procedure, the surgeons completed a questionnaire evaluating six main domains: i) Tag migration, ii) duration of excision (minutes), iii) Tag visibility at US, iv) margin visibility, v) US and localizer correspondence, vi) oncological radicality according to US of specimens. The qualitative parameters were evaluated according to a Likert scale. Like radiologists, surgeons expressed their overall impression 24 h and 1 month after the procedure, collecting their immediate reactions after the procedure and their global evaluation knowing the histological results.
All the patients were clinically followed-up regularly at 1, 3 and 6 months after surgery with outpatient visits. Twenty-four hours after surgery and at 1 month follow-up, patients received a survey asking about: i) Pain during the placement, ii) the existence of painful episodes in the period between the Tag placement and surgery, iii) cosmetic results. The Visual Analog Scale (VAS) (17) was adopted to evaluate pain and the Likert scale was used for evaluating the cosmetic results. The patients were asked to give their overall evaluation of the procedure 24 h and 1 month after it.
Study outcomes. The primary outcome of the study was the assessment of the feasibility of performing a minimally invasive lumpectomy with oncological safety margins, using the double technique of LOCalizer™ combined with IOUS.
Secondary outcomes were: i) Evaluation of successful Tag placement under US, ii) identification of the incisional site, iii) Tag identification, and iv) identification of NPBC margins by US, v) evaluation of weight and volume of resected lesions, vii) evaluation of patients, radiologists, and surgeons’ opinions of the procedure.
Data were described according to each variable type. Continuous variables are given as the mean with its standard deviation. Non-continuous variables were evaluated by rating scale such as VAS for pain and the Likert scale for physician and patient agreement.
Results
Of the nine patients affected by NPBC referred to our center between 15 June and 15 July 2020, seven met the inclusion criteria. Two patients preferred conventional WGL. Five patients agreed to wireless localization and were enrolled in the current pilot study and underwent NPBC localization using LOCalizer™ combined with IOUS.
All the patients were women, and the mean age was 51±9.56 years. All patients underwent lumpectomy with sentinel lymph node biopsy. According to TNM classification, definitive pathology showed a T1b N0 M0 tumor in three patients, a case of T1a N0 M0, and a T1c N0 M0 lesion for the other patient (all the lesions measured less than 2 cm and were clinical impalpable) (Table I). Tag placement was performed under US 10 days before surgery. The mean placement took 3.8±1.3 min. The details of margin distances for all the patients are reported in Table II.
Table I. Patient characteristics.
Table II. The distances in millimeters from the LOCalizer™ Tag recorded for the eight measures made under ultrasonography.
Regarding the primary outcome, combined localization using LOCalizer™ with IOUS was feasible in all patients, allowing surgical radicality in all patients, as confirmed by the US evaluation and the examination of the resected specimen (clear margins in all cases). The definitive pathology confirmed oncological radicality and diagnosis of breast carcinoma of no special type in all cases. The mean specimen volume was 23.4±5.0 cm3 and the mean weight was 15.4±6.0 g. During the immediate post-surgical period and follow-up, no complications were reported. No patient underwent reoperation.
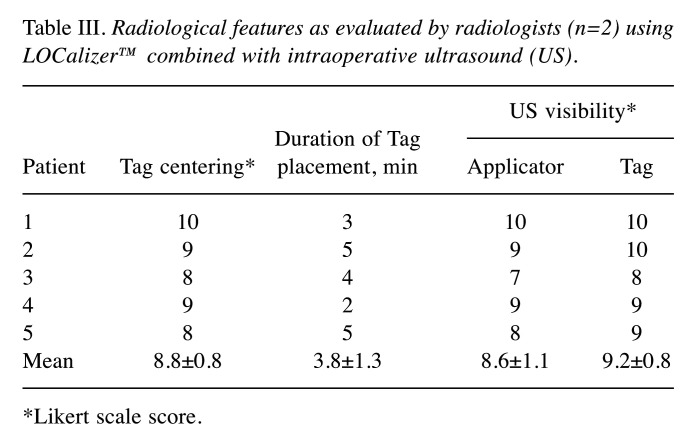
Radiologists were asked about their satisfaction with Tag centering according to the Likert scale, providing a score of 8.8±0.8. The score for LOCalizer™-Applicator visibility under US imaging was 8.6±1.1, while LOCalizer™-Tag visibility scored 9.2±0.8 (Table III).
Table III. Radiological features as evaluated by radiologists (n=2) using LOCalizer™ combined with intraoperative ultrasound (US).
*Likert scale score.
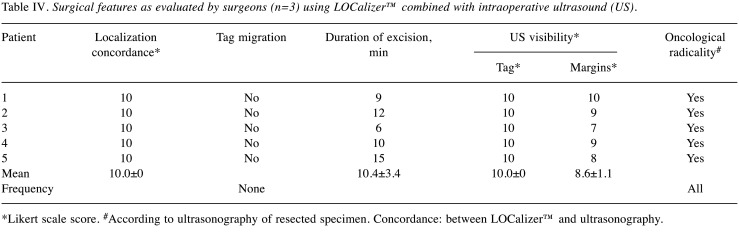
In all five cases, no migration was detected, pre-operative US measures were totally concordant with the previous data. The mean duration of lumpectomy was 10.4±3.4 min. Surgeons assigned the maximum Likert scale score of 10 to Tag visibility at US. Margin visibility on IOUS had a mean score of 8.6±1.1, showing complete IOUS and Localizer concordance (all scored as 10.0) (Table IV).
Table IV. Surgical features as evaluated by surgeons (n=3) using LOCalizer™ combined with intraoperative ultrasound (US).
*Likert scale score. #According to ultrasonography of resected specimen. Concordance: between LOCalizer™ and ultrasonography.
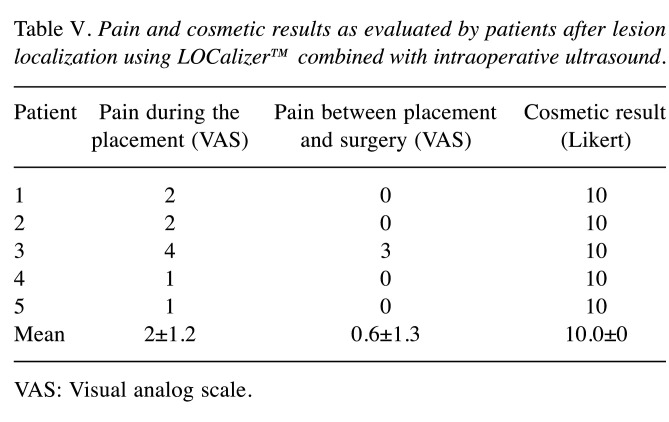
Regarding patients, pain during Tag placement was almost absent, thanks to local anesthesia (VAS score for pain: 0.8±0.8) and no particular discomfort in the period between placement and surgery (VAS score: 0.4±0.5) was reported. Cosmetic results were also excellent (10.0) (Table V).
Table V. Pain and cosmetic results as evaluated by patients after lesion localization using LOCalizer™ combined with intraoperative ultrasound.
VAS: Visual analog scale.
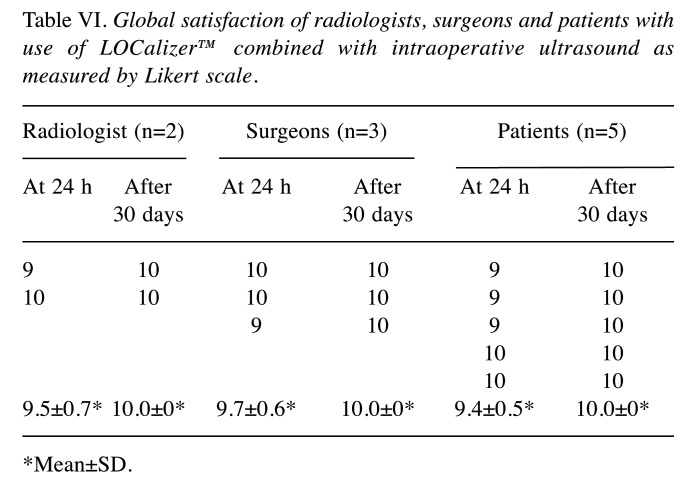
Radiologists’ overall satisfaction was 9.5±0.7 and 10.0 at 24 h and 1 month after the procedure, respectively, whilst for surgeons, scores were 9.7±0.6 and 10.0, respectively. Overall evaluation by patients was 9.4±0.5 and 10.0, respectively. The global agreement at 24 h and 1 month after the surgery by radiologist, surgeons and patients, was >9.4 for each category (Table VI).
Table VI. Global satisfaction of radiologists, surgeons and patients with use of LOCalizer™ combined with intraoperative ultrasound as measured by Likert scale.
*Mean±SD.
Discussion
The current study aimed to evaluate the feasibility of combining Faxitron LOCalizer™ with IOUS as an effective method for localization of NPBC. The localization of clinically occult breast lesions represents an arduous challenge. This has prompted imaging improvement, the spread of neoadjuvant chemotherapy, and neoplasm downstaging. It has been estimated that one-third of breast cancer lesions are not palpable at diagnosis and accurate localizing systems are advocated for oncological and tailored surgery (18). Currently the most widely adopted approach (up to 80% reported in literature) in guided breast-conserving surgery for excising non-palpable breast lesions is WGL [reviewed in (19)]. Nevertheless, many limitations are known: Patient anxiety, infection, migration and wire breakage. When a hook wire is used, the placement should be performed immediately before surgery to reduce the abovementioned inconveniences. Moreover, the risk of pneumothorax is not negligible. Wire dislocations can also result in the resection of an incorrect area of breast tissue, with the consequent possible need for re-operation (14). Other localization techniques have been performed but none have replaced the wire guide. The use of RSL has been proposed for its advantage of being able to be placed immediately before surgery. Conversely, the surgical procedure should be performed within 5 days so as not to nullify its efficacy. Moreover, the seeds are associated with radioactive exposure for the patient and the operators, and disposal management might be difficult (7,20). Further localizing systems were developed to improve intraoperative detection of NPBC. For example, in 1996 in Italy, clinicians referring to the European Oncological Institute proposed radio-guided localization of occult lesions (21). This is a modern alternative method with the advantage of being able to combine lesion localization with the investigation of the sentinel lymph node, performing a mixed procedure named sentinel node and occult lesion localization (SNOLL). Nevertheless, its association with increased weight and volume of resected specimens compared to WGL is reported. Moreover, the results are correlated to the variability of the insertion site (22-24). MAGSEED is a metal marker generating a magnetic field which is detected by a dedicated probe. Therefore, ferrous-steel surgical tools produce undesirable interference with detection, requiring the adoption of non-magnetic instruments. Moreover, MAGSEED is not indicated for lesions deeper than 4 cm because the signal might not be detectable (9) and the marker has a 5-mm diameter more suitable for mammographic detection rather than US-vision or surgical exploration.
It is worth noting that none of the abovementioned systems are able to give quantitative information about the distance to the dissection site: surgeons are guided only by a sound increasing when the lesion has been approached. Moreover, all the markers can be placed into or very near the lesion, but none of the conventional systems are able to indicate a lesion’s margins. However, an accurate and dimensional evaluation can be achieved by US, during lumpectomy. IOUS is, in fact, a readily available and cheap method for NPBC detection and has been evaluated as localization method. Two IOUS techniques have been described: Krekel et al. used US as an absolute system, highlighting the ability to determine the exact location of the lesion, identifying its margins (11). Ivanovic et al. signaled the lesion with a marker pointing out the lump during IOUS (25). Both of these procedures had promising oncological and time-saving results, but the efficacy of the localization is primarily related to the skill and experience of the sonographer surgeon, especially in the evaluation of artifacts linked to the penetration of fluids or air during the surgery (26-32).
The current observational study is the first Italian study about the use of RFID technology for localization of NPBC. Moreover, it is also the first evaluating the combined adoption of Faxitron LOCalizer™ and IOUS to improve the identification, oncological safety and minimal invasiveness of a wireless localizing system. Faxitron LOCalizer™ is based on a 9-mm Tag associated with an RFID chip, placed into or near a lesion. It has been approved for long-term placement over 30 days prior to surgery (33) and its main peculiarity is the ability to indicate the actual distance in millimeters between the surgical Pencil and the Tag. To the best of our knowledge, several non-European studies demonstrated its effectiveness without the use of IOUS. In a study performed by DiNome et al., in Los Angeles, the LOCalizer™ was utilized for 50 breast lumpectomies (33 cancers/50 cases). For 93.9% of patients, the specimen margins were clean, and the mean volume of breast cancer was 36.3 cm3 (4). Using the double localization method, in the current study, oncological radicality was achieved in all five patients and the mean volume of specimens was 23.4±5.0 cm3. McGugin et al., in Boston, compared LOCalizer™ to WGL, performing 356 lumpectomies after WGL and 147 with LOCalizer (12). The mean duration was shorter with WGL (49 vs. 57 min), while the mean volume was similar (8.2 cm3 with LOCalizer vs. 8.0 cm3 with WGL). For 19.1% of the patients treated with LOCalizer™, re-operation was required (16.8% of reoperations in the group which underwent WGL).
In Europe, only three studies testing LOCalizer™ were performed. In Cologne, in 2019, Malter et al. evaluated four cases using LOCalizer™ localization, showing oncological radicality without significant surgical complications. Moreover, surgeons agreed the feasibility of use of the LOCalizer™ system and reported a satisfaction rating of 90% (33). In the United Kingdom, Wazir et al. demonstrated that wireless localization using RFID is an effective and time-efficient alternative to WGL, with low margin positivity and reoperation rates. In their study, 10 patients underwent surgical localization with LOCalizer™. The mean time for deployment of the RFID tag was 5.4 min (range=2-20 min), slightly slower than that for our series (3.8±1.3 min). This difference is probably due to the enrollment in their study of patients with heterogeneous breast lesions, such as ductal hyperplasia, which are sometimes more difficult to detect ultrasonographically. The mean duration for surgical excision was 10.2 min (range=6-20 min), similar to our observation (10.4±3.4 min). Mean specimen weight was 19.6 g (range=4.5-42 g) for malignant lesions (34), while our result was 8 g (range=4-15 g), p<0.001. It is possible to argue that combined localization might allow a more tailored resection, avoiding removal of healthy tissue. However, the heterogeneity of their population should be considered, as they also included T2 lesions (diameter>2 cm) and benign lesions such as fibroadenoma and papilloma. The most recent study about LOCalizer™ was published by Tayeh et al. in 2020 and compared the wireless techniques LOCalizer™ and Magseed vs. the gold standard WGL. Twenty-two patients underwent Tag or Magseed localization. The mean time was 3.4 min for the placement and 8.8 min for the excision. In one case, re-operation was necessary to obtain clean margins (2). Wireless systems produced better results than WGL procedures, suggesting that WGL can be replaced as the gold standard.
Radiologists were asked about their satisfaction according to the Likert scale, with Tag centering resulting in a score of 8.8±0.8, LOCalizer™-Applicator visibility under US imaging scoring 8.6±1.1, while LOCalizer™-Tag visibility scored 9.2±0.8. Ours is the first reported evaluation by radiologists of radiological performance, although. Malter et al., in fact, evaluated the satisfaction of surgeons about aspects of Tag placement (33).
The current study, even if on a limited population, obtained excellent results regarding margin safety and a minimally invasive resection, mainly thanks to the support of IOUS in the identification of the lesion margins.
Many device features could be improved: The caliber of the Tag Applicator is large, and can produce discomfort for the patients or unaesthetic scars; the Tag is not suitable for evaluations under MRI because the materials produce artefacts (34); the latter also prevents its use in patients undergoing neoadjuvant chemotherapy who are candidates for MRI evaluation before and after their medical therapies; the Tag may also be too large for lesions under 1 cm in size.
The study has some limitations to address. Firstly, the small sample size and number of surgeons is not negligible. Secondly, the limited follow-up period (6 months) did not allow investigation of long-term recurrences.
Conclusion
Combined use of LOCalizer™ and IOUS system is a feasible localizing technique. LOCalizer™ allows a quantitative measure of the distance from the lesion to be obtained, while IOUS is able to improve the detection of lesion margins. Performing this double technique, surgical duration is minimally prolonged but the oncological and cosmetic results appear better than with other localizing systems. Large prospective cohort studies comparing LOCalizer™–IOUS with other localization systems are required to address this issue.
Conflicts of Interest
The Authors declare no conflicts of interest.
Authors’ Contributions
Simona Parisi: Initiated and designed the study, and proofread, wrote and finalized the article. Roberto Ruggiero: Designed and supervised the study. Giorgia Gualtieri: Data collection. Mariachiara Lanza Volpe: Editing. Serena Rinaldi: Data collection. Giusiana Nesta: Data collection. Lidija Bogdanovich: Wrote and edited language. Francesco Saverio Lucido: Proofread the study. Salvatore Tolone: proofread the study. Domenico Parmeggiani: Proofread the study. Claudio Gambardella: Editing and supervised the study. Ludovico Docimo: Supervised the study.
References
- 1.Shapiro S, Strax P, Venet L. Evaluation of periodic breast cancer screening with mammography. Methodology and early observations. JAMA. 1966;195(9):731–738. [PubMed] [Google Scholar]
- 2.Tayeh S, Gera R, Perry N, Michell M, Malhotra A, Mokbel K. The use of magnetic seeds and radiofrequency identifier tags in breast surgery for non-palpable lesions. Anticancer Res. 2020;40(1):315–321. doi: 10.21873/anticanres.13955. [DOI] [PubMed] [Google Scholar]
- 3.Frank HA, Hall FM, Steer ML. Preoperative localization of nonpalpable breast lesions demonstrated by mammography. N Engl J Med. 1976;295(5):259–260. doi: 10.1056/NEJM197607292950506. [DOI] [PubMed] [Google Scholar]
- 4.DiNome ML, Kusske AM, Attai DJ, Fischer CP, Hoyt AC. Microchipping the breast: An effective new technology for localizing non-palpable breast lesions for surgery. Breast Cancer Res Treat. 2019;175(1):165–170. doi: 10.1007/s10549-019-05143-w. [DOI] [PubMed] [Google Scholar]
- 5.Jakub JW, Gray RJ, Degnim AC, Boughey JC, Gardner M, Cox CE. Current status of radioactive seed for localization of non palpable breast lesions. Am J Surg. 2010;199(4):522–528. doi: 10.1016/j.amjsurg.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 6.McGhan LJ, McKeever SC, Pockaj BA, Wasif N, Giurescu ME, Walton HA, Gray RJ. Radioactive seed localization for nonpalpable breast lesions:Review of 1,000 consecutive procedures at a single institution. Ann Surg Oncol. 2011;18(11):3096–3101. doi: 10.1245/s10434-011-1910-1. [DOI] [PubMed] [Google Scholar]
- 7.Hughes JH, Mason MC, Gray RJ, McLaughlin SA, Degnim AC, Fulmer JT, Pockaj BA, Karstaedt PJ, Roarke MC. A multi-site validation trial of radioactive seed localization as an alternative to wire localization. Breast J. 2008;14(2):153–157. doi: 10.1111/j.1524-4741.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 8.Sharek D, Zuley ML, Zhang JY, Soran A, Ahrendt GM, Ganott MA. Radioactive seed localization versus wire localization for lumpectomies: A comparison of outcomes. AJR Am J Roentgenol. 2015;204(4):872–877. doi: 10.2214/AJR.14.12743. [DOI] [PubMed] [Google Scholar]
- 9.Hayes MK. Update on preoperative breast localization. Radiol Clin North Am. 2017;55(3):591–603. doi: 10.1016/j.rcl.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Zacharioudakis K, Down S, Bholah Z, Lee S, Khan T, Maxwell AJ, Howe M, Harvey J. Is the future magnetic? Magseed localisation for non palpable breast cancer. A multi-centre non randomised control study. Eur J Surg Oncol. 2019;45(11):2016–2021. doi: 10.1016/j.ejso.2019.06.035. [DOI] [PubMed] [Google Scholar]
- 11.Krekel NM, Haloua MH, Lopes Cardozo AM, de Wit RH, Bosch AM, de Widt-Levert LM, Muller S, van der Veen H, Bergers E, de Lange de Klerk ES, Meijer S, van den Tol MP. Intraoperative ultrasound guidance for palpable breast cancer excision (COBALT trial): A multicentre, randomised controlled trial. Lancet Oncol. 2013;14(1):48–54. doi: 10.1016/S1470-2045(12)70527-2. [DOI] [PubMed] [Google Scholar]
- 12.McGugin C, Spivey T, Coopey S, Smith B, Kelly B, Gadd M, Hughes K, Dontchos B, Specht M. Radiofrequency identification tag localization is comparable to wire localization for non-palpable breast lesions. Breast Cancer Res Treat. 2019;177(3):735–739. doi: 10.1007/s10549-019-05355-0. [DOI] [PubMed] [Google Scholar]
- 13.Sawaki M, Shien T, Iwata H. TNM classification of malignant tumors (Breast Cancer Study Group) Jpn J Clin Oncol. 2019;49(3):228–231. doi: 10.1093/jjco/hyy182. [DOI] [PubMed] [Google Scholar]
- 14.Dauphine C, Reicher JJ, Reicher MA, Gondusky C, Khalkhali I, Kim M. A prospective clinical study to evaluate the safety and performance of wireless localization of nonpalpable breast lesions using radiofrequency identification technology. AJR Am J Roentgenol. 2015;204(6):W720–W723. doi: 10.2214/AJR.14.13201. [DOI] [PubMed] [Google Scholar]
- 15.Norman G. Likert scales, levels of measurement and the “laws” of statistics. Adv Health Sci Educ Theory Pract. 2010;15(5):625–632. doi: 10.1007/s10459-010-9222-y. [DOI] [PubMed] [Google Scholar]
- 16.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, Harbeck N, Aguilar Lopez B, Barrios CH, Bergh J, Biganzoli L, Boers-Doets CB, Cardoso MJ, Carey LA, Cortés J, Curigliano G, Diéras V, El Saghir NS, Eniu A, Fallowfield L, Francis PA, Gelmon K, Johnston SRD, Kaufman B, Koppikar S, Krop IE, Mayer M, Nakigudde G, Offersen BV, Ohno S, Pagani O, Paluch-Shimon S, Penault-Llorca F, Prat A, Rugo HS, Sledge GW, Spence D, Thomssen C, Vorobiof DA, Xu B, Norton L, Winer EP. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)†. Ann Oncol. 2018;29(8):1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: A critical review. Psychol Med. 1988;18(4):1007–1019. doi: 10.1017/s0033291700009934. [DOI] [PubMed] [Google Scholar]
- 18.Lovrics PJ, Cornacchi SD, Farrokhyar F, Garnett A, Chen V, Franic S, Simunovic M. The relationship between surgical factors and margin status after breast-conservation surgery for early stage breast cancer. Am J Surg. 2009;197(6):740–746. doi: 10.1016/j.amjsurg.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Chan BK, Wiseberg-Firtell JA, Jois RH, Jensen K, Audisio RA. Localization techniques for guided surgical excision of non-palpable breast lesions. Cochrane Database Syst Rev. 2015;(12):CD009206. doi: 10.1002/14651858.CD009206.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy JO, Moo TA, King TA, Van Zee KJ, Villegas KA, Stempel M, Eaton A, St Germain JM, Morris E, Morrow M. Radioactive seed localization compared to wire localization in breast-conserving surgery: Initial 6-month experience. Ann Surg Oncol. 2013;20(13):4121–4127. doi: 10.1245/s10434-013-3166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Cicco C, Pizzamiglio M, Trifirò G, Luini A, Ferrari M, Prisco G, Galimberti V, Cassano E, Viale G, Intra M, Veronesi P, Paganelli G. Radioguided occult lesion localisation (ROLL) and surgical biopsy in breast cancer. Technical aspects. Q J Nucl Med. 2002;46(2):145–151. [PubMed] [Google Scholar]
- 22.Postma EL, Verkooijen HM, van Esser S, Hobbelink MG, van der Schelling GP, Koelemij R, Witkamp AJ, Contant C, van Diest PJ, Willems SM, Borel Rinkes IH, van den Bosch MA, Mali WP, van Hillegersberg R, ROLL Study Group Efficacy of ‘radioguided occult lesion localisation’ (ROLL) versus ‘wire-guided localisation’ (WGL) in breast conserving surgery for non-palpable breast cancer: A randomised controlled multicentre trial. Breast Cancer Res Treat. 2012;136(2):469–478. doi: 10.1007/s10549-012-2225-z. [DOI] [PubMed] [Google Scholar]
- 23.van der Ploeg IM, Hobbelink M, van den Bosch MA, Mali WP, Borel Rinkes IH, van Hillegersberg R. ‘Radioguided occult lesion localisation’ (ROLL) for non-palpable breast lesions: A review of the relevant literature. Eur J Surg Oncol. 2008;34(1):1–5. doi: 10.1016/j.ejso.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Nadeem R, Chagla LS, Harris O, Desmond S, Thind R, Titterrell C, Audisio RA. Occult breast lesions: A comparison between radioguided occult lesion localisation (ROLL) vs. wire-guided lumpectomy (WGL) Breast. 2005;14(4):283–289. doi: 10.1016/j.breast.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Ivanovic NS, Zdravkovic DD, Skuric Z, Kostic J, Colakovic N, Stojiljkovic M, Opric S, Stefanovic Radovic M, Soldatovic I, Sredic B, Granic M. Optimization of breast cancer excision by intraoperative ultrasound and marking needle - technique description and feasibility. World J Surg Oncol. 2015;13:153. doi: 10.1186/s12957-015-0568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Rijk MC, Tanis PJ, Nieweg OE, Loo CE, Olmos RA, Oldenburg HS, Rutgers EJ, Hoefnagel CA, Kroon BB. Sentinel node biopsy and concomitant probe-guided tumor excision of nonpalpable breast cancer. Ann Surg Oncol. 2007;14(2):627–632. doi: 10.1245/s10434-006-9070-4. [DOI] [PubMed] [Google Scholar]
- 27.Bennett IC, Greenslade J, Chiam H. Intraoperative ultrasound-guided excision of nonpalpable breast lesions. World J Surg. 2005;29(3):369–374. doi: 10.1007/s00268-004-7554-6. [DOI] [PubMed] [Google Scholar]
- 28.Ngô C, Pollet AG, Laperrelle J, Ackerman G, Gomme S, Thibault F, Fourchotte V, Salmon RJ. Intraoperative ultrasound localization of nonpalpable breast cancers. Ann Surg Oncol. 2007;14(9):2485–2489. doi: 10.1245/s10434-007-9420-x. [DOI] [PubMed] [Google Scholar]
- 29.Haid A, Knauer M, Dunzinger S, Jasarevic Z, Köberle-Wührer R, Schuster A, Toeppker M, Haid B, Wenzl E, Offner F. Intra-operative sonography: A valuable aid during breast-conserving surgery for occult breast cancer. Ann Surg Oncol. 2007;14(11):3090–3101. doi: 10.1245/s10434-007-9490-9. [DOI] [PubMed] [Google Scholar]
- 30.Fortunato L, Penteriani R, Farina M, Vitelli CE, Piro FR. Intraoperative ultrasound is an effective and preferable technique to localize non-palpable breast tumors. Eur J Surg Oncol. 2008;34(12):1289–1292. doi: 10.1016/j.ejso.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Barentsz MW, van Dalen T, Gobardhan PD, Bongers V, Perre CI, Pijnappel RM, van den Bosch MA, Verkooijen HM. Intraoperative ultrasound guidance for excision of non-palpable invasive breast cancer: A hospital-based series and an overview of the literature. Breast Cancer Res Treat. 2012;135(1):209–219. doi: 10.1007/s10549-012-2165-7. [DOI] [PubMed] [Google Scholar]
- 32.Pan H, Wu N, Ding H, Ding Q, Dai J, Ling L, Chen L, Zha X, Liu X, Zhou W, Wang S. Intraoperative ultrasound guidance is associated with clear lumpectomy margins for breast cancer: a systematic review and meta-analysis. PLoS One. 2013;8(9):e74028. doi: 10.1371/journal.pone.0074028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malter W, Holtschmidt J, Thangarajah F, Mallmann P, Krug B, Warm M, Eichler C. First reported use of the Faxitron LOCalizer™ radiofrequency identification (RFID) system in Europe - A feasibility trial, surgical guide and review for non-palpable breast lesions. In Vivo. 2019;33(5):1559–1564. doi: 10.21873/invivo.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wazir U, Tayeh S, Perry N, Michell M, Malhotra A, Mokbel K. wireless breast localization using radio-frequency identification tags: The first reported European experience in breast cancer. In Vivo. 2020;34(1):233–238. doi: 10.21873/invivo.11765. [DOI] [PMC free article] [PubMed] [Google Scholar]