Abstract
Background/Aim: Intestinal mucositis with diarrhea is a dose-limiting toxicity of 5-fluorouracil (5-FU). M40403, a superoxide dismutase mimetic, was evaluated on whether it improves the mucositis with diarrhea. Materials and Methods: BALB/c mice were treated with daily intraperitoneal injections of 5-FU±M40403 for five consecutive days. Following treatment, light microscopy (apoptosis), electron microscopy (autophagy), and analyses for the expression of apoptosis/autophagy-related proteins were performed in analysing small intestinal samples. Body weight, diarrhea score, blood cytokine levels, complete blood count, and blood chemistries were measured. The in vivo anti-tumor activity of 5-FU±M40403 was also evaluated. Results: M40403 improved 5-FU-induced intestinal mucositis (apoptosis and autophagy) and attenuated 5-FU-induced changes in the expression of apoptosis/autophagy-related proteins, weight loss, diarrhea score, and serum TNF-α levels. M40403 neither added further adverse effects nor compromised the anti-tumor activity during 5-FU treatment. Conclusion: M40403 can be useful in improving 5-FU-induced intestinal mucositis with diarrhea.
Keywords: Diarrhea, 5-fluorouracil, M40403, mucositis, superoxide dismutase
Cancer is still a leading cause of death worldwide and systemic chemotherapy plays a crucial role in the treatment of cancer (1). 5-Fluorouracil (5-FU) is one of the most popular cytotoxic agents that is used widely in solid tumors such as gastrointestinal adenocarcinoma, breast adenocarcinoma, and squamous cell carcinoma of the head and neck (2). Alimentary tract mucositis is one of the most troublesome side-effects of 5-FU with symptoms such as severe pain, nausea, vomiting, and diarrhea (3,4). Diarrhea induced by 5-FU is one of the main dose-limiting side effects that can occur in up to 50% of patients receiving the drug and can lead to hospitalization and even treatment-related death (5-7). 5-FU-induced diarrhea can also worsen the quality of life, delay further chemotherapies, and consequently lead to poorer treatment outcomes (8,9). However, until today, despite the fact that many agents such as amifostine, loperamide, octreotide, sucralfate, and anti-inflammatory drugs have been used to ameliorate 5-FU-induced diarrhea, no specific or definite preventive or therapeutic agents exist (8).
5-FU and other chemotherapeutic agents have been well known to induce the production of reactive oxygen species (ROS) in addition to inducing DNA damage (10-13). Although the mechanism of development of chemotherapy-induced intestinal mucositis has not been completely understood, an overlapping five-phase model in which ROS play important roles is widely accepted. Dynamic biochemical interactions occur between chemotherapeutic agents, constituents of the intestinal mucosa, and indirect biological signals including ROS in the model (8,14): i) Initiation, where DNA and non-DNA damages of the basal epithelium and submucosa occur along with the generation of ROS that further damage the mucosa. ii) Up-regulation, where in response to chemotherapy and ROS, multiple inflammatory cytokines such as TNF-α, IL-1β, and IL-6, and apoptosis are up-regulated. iii) Signal amplification, where up-regulated inflammatory cytokines further damage the epithelium and subepithelium by signal amplification. iv) Ulceration, where the loss of mucosal integrity occurs. v) Healing, where the restoration of mucosa ultimately occurs (14). Thus, it can be suggested that ROS, inflammatory cytokines, and apoptosis play important roles in the development of 5-FU-induced intestinal mucositis.
Apoptosis and autophagy are important cellular responses responsible for the determination of cell death or survival (15). It is also well known that ROS play important roles in both apoptosis and autophagic cell death (15). Therefore, it was hypothesized that restricting ROS may improve the 5-FU-induced intestinal mucosal damage and its associated diarrhea in the present study.
Superoxide dismutases (SOD’s) are metalloproteins that act as the first-line defense against ROS by dismutating superoxide radicals to hydrogen peroxide and oxygen (16,17). There are two forms of SOD’s in eukaryotes. One is a manganese-containing enzyme (SOD2) in the mitochondria and the other is copper/zink-containing enzyme in the cytosol (SOD1) or extracellular surfaces (SOD3) (18). However, the therapeutic use of native SOD’s has many limitations because of their large molecular size, instability, immunogenicity, and high susceptibility to proteolytic digestion (19).
M40403 (imisopasem manganese) is a synthetic non-peptidyl manganese-containing SOD mimetic of human SOD2, which has a much smaller molecular size (MW 483 vs. 30000; M40403 vs. native enzyme) and higher stability in vivo (18). M40403 can also catalytically remove superoxide anions at a higher rate and selectivity, without interacting with other ROS (18,19). It has shown protective effects on ROS-related tissue damages such as chemical pleurisy, intestinal ischemia and reperfusion injury, and colitis (19-21).
In the present study, it was tested whether M40403 ameliorates the 5-FU-induced intestinal mucositis and diarrhea in a mouse model. Changes in serum inflammatory cytokines and small intestinal apoptosis and autophagy were evaluated. We also evaluated the possibility that M40403 modulates 5-FU-induced changes in complete blood count (CBC) and blood chemistries and the possibility that M40403 interferes with the anti-tumor activity of 5-FU.
Materials and Methods
Animals and experimental design. Specific pathogen-free BALB/c mice (6-8 weeks of age) were purchased from Damul Experimental Animal Center (Daejeon, Republic of Korea) and housed at the Jeonbuk National University Hospital Animal Care Facility. Mice were maintained under a 12h dark-light cycle at a temperature of 20-22˚C (45-70% humidity) and were provided with food and water ad libitum. Mice were age- and sex-matched at the onset of each experiment.
Mice were randomly assigned to four groups (untreated control group, 5-FU alone-treated group, 5-FU/5 mg/kg M40403-treated group, and 5-FU/10 mg/kg M40403-treated group). Mice of treatment groups had daily intraperitoneal injections of 30 mg/kg 5-FU (Ildong Pharmaceutical Co., Republic of Korea) with or without 5 mg/kg or 10 mg/kg M40403 (MedKoo Biosciences, Inc., NC, USA) for five consecutive days (from day 1 to day 5). M40403 was dissolved in a vehicle, 26 mM sodium bicarbonate buffer (pH 8.3). To exclude the therapeutic effect of the vehicle (26 mM sodium bicarbonate buffer, pH 8.3), groups receiving intraperitoneal injections of the vehicle alone or 5-FU/vehicle were added in some experiments. On day 8, mice were euthanized by CO2 inhalation for the collection of blood and small intestinal samples. A simplified flow chart of the experiment is presented in Figure 1. In some experiments evaluating diarrhea, body weight change, and survival, experimental days were extended when necessary. All experiments were approved by the Jeonbuk National University Animal Care and Use Committee (JBNU 2020-047) and performed according to its guidelines.
Figure 1. Simplified flow chart of the experiment.
Body weight and diarrhea. Mice were assessed for body weight daily and diarrhea score on days 4, 5, 9, and 10. To evaluate the diarrhea score correctly, defecated stools should be collected as intact as possible. A new stool collecting method was therefore devised by placing the mouse-dwelling portion and its steel wire net floor of the metabolic cage (Nalgene, Rochester, NY, USA) on paper towels. Mice (4 mice/group) were kept in the dwelling portion of the metabolic cage for 1h to collect defecated stools on the paper towel. After the collection, each stool was scored as follows. 1=normal pellet (neither wet nor unshaped stool); 2=wet pellet (when the stool has gross wetness on its contacting area of the paper towel), unshaped stool, or complete diarrhea. After the scoring, the mean±SD of the diarrhea score per group was compared between experimental groups.
Small intestinal histology with hematoxylin and eosin stain. The small intestine was collected for histologic analyses. After the euthanasia of mice, proximal small bowel segments positioned between 2 and 5 cm distal from the distal end of the stomach were harvested (3 cm in length). They were washed with phosphate-buffered saline, fixed with 10% formalin, and then dehydrated to be embedded in paraffin. They were then sectioned in 5 μm thickness and stained with hematoxylin and eosin (HE). The villus length was measured from the top of the villus to the villus-crypt junction under light microscopy using a calibrated micrometer (×200). Ten random well-oriented villi of each sample originated from five mice per group were measured, and the mean±SD of the villi lengths was calculated for the comparison between experimental groups.
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) stain. Unstained intestinal tissue slides originated from 5 mice per group were prepared as above described in “Small intestinal histology with hematoxylin and eosin stain”. TUNEL staining was performed using a commercially available in situ apoptosis detection kit (ApopTag, S7100, Chemicon, Temecula, CA, USA), according to the manufacturer’s instructions. The number of TUNEL-positive cells (brown-colored cells) was counted per field (10 random fields) under magnification (×400). Mean±SD of the number of TUNEL-positive cells/sample was calculated for the comparison between experimental groups.
Transmission electron microscopy (TEM). The intestinal tissues were cut into 1 μL cubes and fixed with 2% glutaraldehyde and 2% paraformaldehyde in 0.05 sodium cacodylate buffer (pH 7.2) (Electron Microscopy Sciences, EMS, Hatfield, PA, USA). Afterward, they were post-fixed in 1% buffered osmium tetroxide (EMS) and dehydrated in graded ethanol and propylene oxide (EMS). After dehydration, samples were embedded in epoxy resin (Embed 812, nadic methyl anhydride, dodecenylsuccinic anhydride, and DMP-30) (EMS). Using an ultramicrotome (Leica, Vienna, Austria), ultra-thin sections were cut and stained with uranyl acetate and lead citrate (EMS). Specimens were examined with Hitachi H-7650 transmission electron microscope (Hitachi High-Technologies, Tokyo, Japan) installed at the Center for University-Wide Research Facilities, Jeonbuk National University (Jeonju, Republic of Korea).
Western blot analyses. Intestinal tissues were lysed with a protein extraction solution (PRO-PREP™, iNtRON Biotechnology, Gyeonggi, Republic of Korea). After centrifugation at 16,000 ×g for 10 min, the extracted proteins were quantified using the Bradford method. Fifty μg of protein samples were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride (PVDF) membranes, and then washed in a Tris-buffered saline-Tween 20 (TBST) (Fisher Biotech, Fair Lawn, NJ, USA). After blocking with TBST containing 5% non-fat dry milk, the membranes were incubated with anti-Bcl-xL (BD Biosciences, San Diego, CA, USA), anti-Bcl2 (BD Biosciences), anti-Beclin-1 (Cell Signaling Technology, Beverly, MA, USA), anti-ATG5 (Novus, Littleton, CO, USA), anti-LC3 (Cell Signaling Technology), anti-p62 (Cell Signaling Technology), and anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies. The membranes were then washed and incubated with alkaline phosphatase-conjugated secondary antibodies to rabbit immunoglobulin G (Santa Cruz Biotechnology). A chemiluminescence reagent (SUPEX, Pohang University of Science and Technology, Pohang, Republic of Korea) was used to detect bound antibodies. Blots for β-actin were used as indicators for the equal amount of protein loading. Densitometric band quantification was performed by employing Fusion Solo S (Vilber Lourmat, France). Mean±SD of relative density ratios of target protein/β-actin of three similar experiments was presented.
Measurement of serum cytokines (TNF-α, IL-1β, IL-10, IL-12, IL-6, and IFN-γ). A mouse premixed multi-analyte kit (Magnetic LuminexR Screening Assay, R&D Systems, MN, USA) capable of detecting TNF-α, IL-1β, IL-10, IL-12, IL-6, and IFN-γ was used and the cytokines were assayed on mouse sera harvested on day 8 according to the manufacturer’s instructions. Mouse sera were prepared by leaving the whole blood-containing tube in a standing position for 30 min followed by centrifuging it at 1,500 ×g for 10 min and then harvesting the supernatant. The sera were stored at -80℃ and melted right before the cytokine assay. Each serum sample was assayed in duplicate and the mean of the duplicate measurements was employed as the cytokine level of the sample. Equivalent amounts of samples were incubated with antibody-coated capture beads for 2 h at room temperature. After washing, beads were further incubated with biotin-labeled anti-mouse cytokine antibodies for 1 h followed by incubation with streptavidin-phycoerythrin for 30 min. Samples were analyzed using Luminex 200™ (Luminex, Austin, TX, USA) and MasterPlex QT 2010 software (MiraiBio, Hitachi, CA, USA). Standard curves of known concentrations of recombinant mouse cytokines were used to convert median fluorescence intensity to cytokine concentration in pg/ml.
Complete blood counts (CBC) and blood chemistries. Blood samples were obtained by a cardiac puncture method. Complete blood counts (CBC) were performed on 150 μL samples of EDTA anti-coagulated blood using Technicon H1 analyzer (Technicon, Tarrytown, NY, USA). Multiphasic serum chemistry panels were performed on 250 μL samples of serum using Hitachi 911 analyzer (Boehringer Mannheim, Indianapolis, IN, USA). Levels of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine were measured.
Effect of M40403 administration on 5-FU-induced anti-tumor activity. To evaluate the effect of M40403 administration on 5-FU-induced anti-tumor responses, the survival duration was measured employing the Meth-A tumor ascites model (22). Four animal groups (untreated control group, 5-FU alone-treated group, 5-FU/5 mg/kg M40403-treated group, and 5-FU/10 mg/kg M40403-treated group) were employed. All mice (5 mice/group) were intraperitoneally injected with 5×106 Meth-A tumor cells on day 1. On day 2, 5-FU with or without M40403 were intraperitoneally injected daily for four consecutive days (from day 2 to day 5). Untreated Meth-A ascites tumor-bearing mice served as a control. Survival was measured by observing the mouse cages daily. Survival duration was defined from day 2 to the day the mouse died. Three similar experiments were performed. Because the results were similar in each experiment, they were pooled for analysis.
Statistical analysis. Data were expressed as mean±standard deviation (SD). Differences between two groups were analyzed by Student’s t-test and those between multiple groups by one-way ANOVA with post hoc analysis for inter-group differences. Data were analyzed with SPSS statistics 23 (IBM, USA) and graphed using Microsoft Excel 2010 (Microsoft, USA). p<0.05 (two-sided tests) was considered statistically significant.
Results
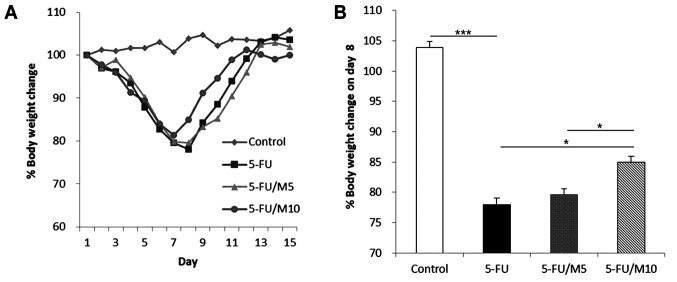
M40403 attenuated 5-FU-induced body weight loss and diarrhea. Percent body weight changes of mice were measured daily (Figure 2A). The control group showed no significant weight loss during the experimental period. The mice receiving 5-FU±M40403 showed body weight reductions without significant differences among experimental groups until day 7. However, the nadir of body weight and the time taken to reach the nadir were different among groups receiving 5-FU±M40403. The nadir was reached on day 8 in mice receiving 5-FU alone and 5-FU/5 mg/kg M40403 (79.78±2.50% and 80.52±1.33%, respectively, p=0.827, 5-FU alone vs. 5-FU/5 mg/kg M40403), but was reached on day 7 (84.06±2.62%) in mice receiving 5-FU/10 mg/kg M40403 (higher dose of M40403). 5-FU/10 mg/kg M40403-treated group also showed a significantly less weight loss on day 8 (85.41±0.66%, p=0.049 and p=0.042 vs. 5-FU alone and 5-FU/5 mg/kg M40403 groups, respectively) (Figure 2B). All 5-FU±M40403-treated groups started to show weight recovery after the nadir. Mice receiving 5-FU/10 mg/kg M40403 showed a faster weight gain than those receiving 5-FU alone or 5-FU/5 mg/kg M40403 (Figure 2A).
Figure 2. Effects of M40403 on the 5-FU-induced change in body weight. Mice (4 mice/group) were started on a 5-day course of daily intraperitoneal injection of 30 mg/kg 5-FU±5 mg/kg or 10 mg/kg M40403 on day 1. Untreated mice served as a control. Body weight was measured daily and percent body weight was calculated in comparison with the weight on day 1. A representative result of three similar experiments was presented (A). Percent body weight changes on day 8 were compared between experimental groups. Data are expressed as mean±SD of three similar experiments (B). ***p<0.001, *p<0.05; 5-FU: 5-Fluorouracil; M5: 5 mg/kg M40403; M10: 10 mg/kg M40403.
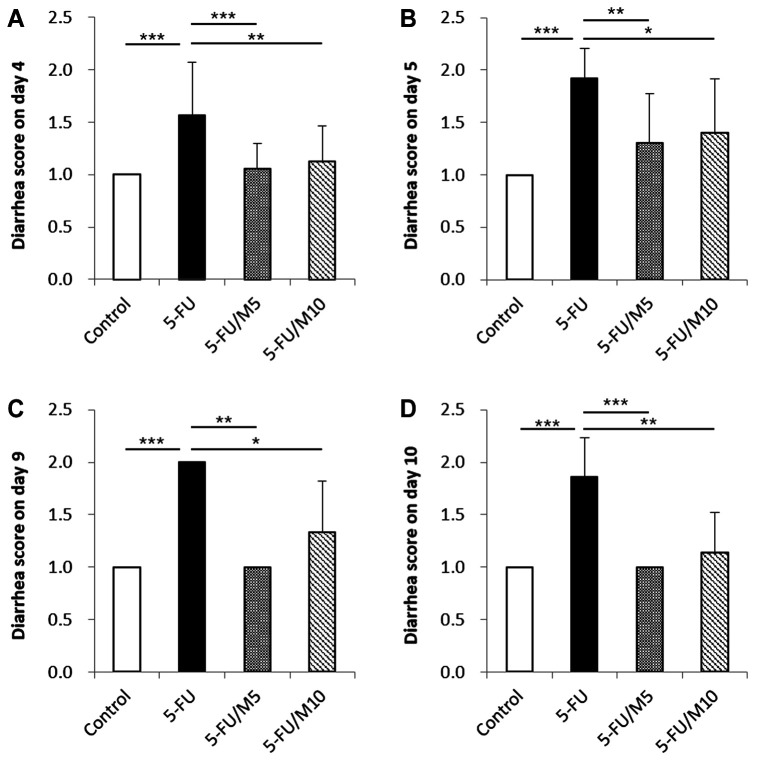
Diarrhea scores were measured on days 4, 5, 9, and 10. Control mice showed no diarrhea during the experimental period. All groups treated with 5-FU, regardless of M40403 co-administration, began to show diarrhea from day 4. Compared to 5-FU alone group, mice receiving 5-FU/M40403 had significantly lower diarrhea scores, regardless of the injection doses of M40403 on days 4, 5, 9, and 10 (Figure 3).
Figure 3. Effects of M40403 on the 5-FU-induced change in diarrhea score. Mice (4 mice/group) were started on a 5-day course of daily intraperitoneal injection of 30 mg/kg 5-FU±5 mg/kg or 10 mg/kg M40403 on day 1. On days 4, 5, 9, and 10 (A, B, C, and D, respectively), defecated stools were collected on paper towels for 1 h. The diarrhea score was determined for each stool. Untreated mice served as a control. A representative result of three similar experiments was presented. Data were expressed as mean±SD. *p<0.05, **p<0.01, ***p<0.001; 5-FU: 5-Fluorouracil; M5: 5 mg/kg M40403; M10: 10 mg/kg M40403.
None of the mice in each group died in the experiments, even though the daily survival observation was extended until day 22 (data not shown).
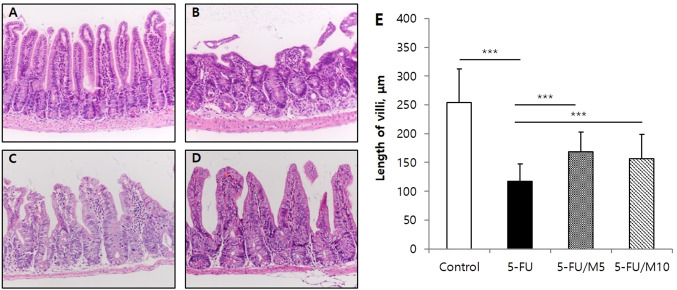
M40403 attenuated 5-FU-induced small intestinal mucosal injury. Intestinal mucosal damage can be reflected by measuring villi lengths (23). Under a light microscope, intestinal villi structures and lengths were assessed. The control group showed evenly tall villi structures with regularly arranged mucosal epithelium. The group receiving 5-FU alone showed severe histologic damages of villi structures and decreases in villi lengths compared to the control group (253.8±58.6 μm in control group vs. 117.4±29.7 μm in 5-FU alone group, p<0.0001). However, the co-administration of M40403 (5 mg/kg or 10 mg/kg) attenuated the 5-FU-induced decrease in villi lengths (168.8±34.3 μm in 5 mg/kg M40403 group, and 156.3±42.8 μm in 10 mg/kg M40403 group, p<0.0001 and p=0.0008, respectively vs. 5-FU alone group) and appeared to partially restore the 5-FU-induced damages of villi structures (Figure 4). Additional groups of mice treated with the vehicle (26 mM sodium bicarbonate buffer, pH 8.3) alone and 5-FU/vehicle revealed similar histologic findings of the intestine to those of control and 5-FU alone-treated mice, respectively (data not shown).
Figure 4. Effects of M40403 on the 5-FU-induced change in the length of small intestinal villi. Mice (5 mice/group) were started on a 5-day course of daily intraperitoneal injection of 30 mg/kg 5-FU±5 mg/kg or 10 mg/kg M40403 on day 1. On day 8, small intestinal samples were subjected to microscopic examinations employing hematoxylin and eosin stain. The length of 10 random well-oriented villi of each sample was measured using a calibrated micrometer (×200). Untreated mice served as a control. Representative microscopic pictures (A, B, C, and D) and mean±SD of the length of villi (E) of each group were presented. Representative data obtained from one of three similar experiments were shown. ***p<0.001, (A) Control: untreated control group, (B) 5-FU: 5-FU alone-treated group, (C) 5-FU/M5: 5-FU/5 mg/kg M40403-treated group, (D) 5-FU/M10: 5- FU/10 mg/kg M40403-treated group.
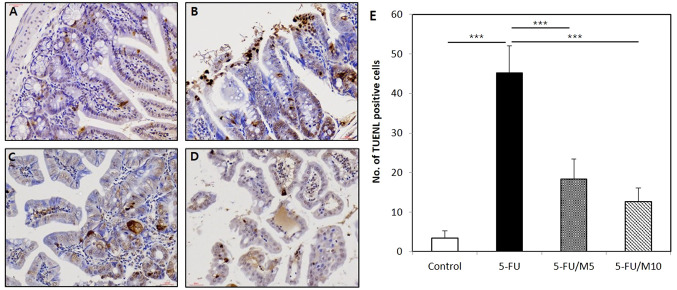
M40403 attenuated the 5-FU-induced increase in the number of apoptotic cells in the small intestine (TUNEL stain). The number of TUNEL-positive cells was significantly increased in 5-FU alone group (45.2±6.9) compared to the control group (3.4±1.8, p<0.0001). Groups treated with 5-FU/M40403 had significantly less TUNEL-positive cells compared to 5-FU alone group (18.4±5.1 in 5-FU/5 mg/kg M40403 group and 12.6±3.6 in 5-FU/10 mg/kg M40403 group vs. 5-FU alone group, p=0.0001 and p<0.0001, respectively) (Figure 5).
Figure 5. Effects of M40403 on the 5-FU-induced apoptosis of the small intestinal epithelium. Mice (5 mice/group) were started on a 5-day course of daily intraperitoneal injection of 30 mg/kg 5-FU±5 mg/kg or 10 mg/kg M40403 on day 1. On day 8, small intestinal samples were subjected to microscopic examinations employing TUNEL stain. The number of TUNEL-positive cells was counted per 10 random fields under magnification (×400). Untreated mice served as a control. Representative microscopic pictures (A, B, C, and D) and mean±SD of the number of TUNEL-positive cells (E) of each group were presented. ***p<0.001, (A) Control: untreated control group, (B) 5-FU: 5-FU alone-treated group, (C) 5-FU/M5: 5- FU/5 mg/kg M40403-treated group, (D) 5-FU/M10: 5-FU/10 mg/kg M40403-treated group. TUNEL: Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling.
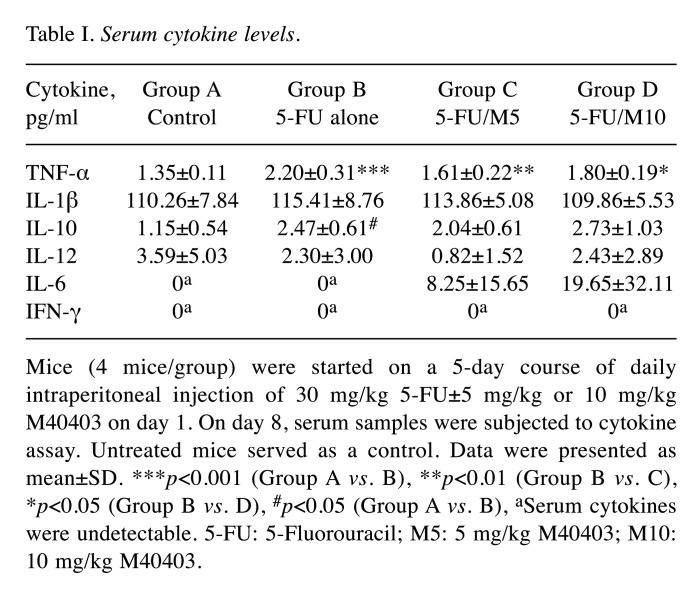
Cytokine levels in serum. Serum levels of TNF-α, IL-1β, IL-10, IL-12, IL-6, and IFN-γ were measured (Table I). Mice treated with 5-FU alone showed an increase in serum TNF-α levels (2.20±0.31 pg/ml, p<0.0001) compared to control group (1.35±0.11 pg/ml). The co-administration of M40403 significantly attenuated the 5-FU-induced increase in serum TNF-α levels, regardless of the doses of M40403 (1.61±0.22 pg/ml in 5-FU/5 mg/kg M40403 group and 1.80±0.19 pg/ml in 5-FU/10 mg/kg M40403 group, p=0.004 and p=0.021, respectively vs. 5-FU alone group). IL-1β tended to increase in 5-FU alone group (115.41±8.76 pg/ml) compared to the control group (110.26±7.84 pg/ml), and tended to decrease in 5-FU/M40403 groups (113.86±5.08 pg/ml in 5-FU/5 mg/kg M40403 group and 109.86±5.53 pg/ml in 5-FU/10 mg/kg M40403 group) compared to 5-FU alone group, but statistical significance was not reached. Serum IL-10 levels showed an increase in 5-FU alone group compared to the control group (2.47±0.61 pg/ml in 5-FU alone group vs. 1.15±0.54 pg/ml in the control group, p=0.023), but co-administration of M40403 failed to show any significant changes in the 5-FU-induced increase in IL-10 levels. Serum IL-12 levels showed no significant intergroup differences. Serum IL-6 was undetectable in control and 5-FU alone groups. However, 5-FU/M40403-treated groups showed increased serum IL-6 levels (8.25±15.65 pg/ml in 5-FU/5 mg/kg M40403 group and 19.65±32.11 pg/ml in 5-FU/10 mg/kg M40403 group) without statistical significances compared to untreated control and 5-FU alone-treated groups. Serum IFN-γ was undetectable in all groups.
Table I. Serum cytokine levels.
Mice (4 mice/group) were started on a 5-day course of daily intraperitoneal injection of 30 mg/kg 5-FU±5 mg/kg or 10 mg/kg M40403 on day 1. On day 8, serum samples were subjected to cytokine assay. Untreated mice served as a control. Data were presented as mean±SD. ***p<0.001 (Group A vs. B), **p<0.01 (Group B vs. C), *p<0.05 (Group B vs. D), #p<0.05 (Group A vs. B), aSerum cytokines were undetectable. 5-FU: 5-Fluorouracil; M5: 5 mg/kg M40403; M10: 10 mg/kg M40403.
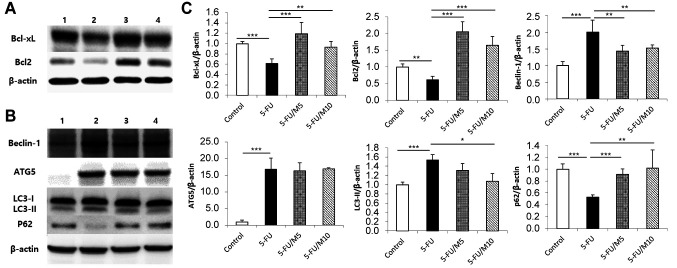
Western blot analyses for apoptosis- and autophagy-related signals in the small intestine. Bcl-xL and Bcl2 are representative molecules having anti-apoptotic functions (15,24). 5-FU alone group showed decreased Bcl-xL and Bcl2 expressions compared to the control group. In contrast, 5-FU/M40403 groups had increased Bcl-xL and Bcl2 expressions compared to 5-FU alone group (Figure 6A and C). Beclin-1, ATG5, and LC3-II are signaling molecules whose expressions are increased during autophagy (25). p62 is also one of the autophagy-related molecules whose expression is decreased during autophagy (25). Compared to the control group, 5-FU alone group showed increased expression of Beclin-1, ATG5, and LC3-II, and decreased expression of p62. On the other hand, 5-FU/M40403 groups had decreased Beclin-1 expression and increased p62 expression compared to 5-FU alone group. ATG5 expression appeared to have no differences between 5-FU alone group and 5-FU/M40403 groups. The 5-FU-induced increase in LC3-II expression was inhibited in the 5-FU/10 mg/kg M40403 (higher dose) group, but not in the 5-FU/5 mg/kg M40403 (lower dose) group (Figure 6B and C).
Figure 6. Western blot analyses for apoptosis- and autophagy-related proteins. Mice were started on a 5-day course of daily intraperitoneal injection of 30 mg/kg 5-FU±5 mg/kg or 10 mg/kg M40403 on day 1. On day 8, small intestinal samples were subjected to immunoblot analyses. Untreated mice served as a control. Representative immunoblots (A and B) and mean±SD of relative density ratios of target protein/β-actin (C) of three similar experiments were presented. *p<0.05, **p<0.01, ***p<0.001, 1: Control, untreated control group; 2: 5-FU, 5-FU alone-treated group; 3: 5-FU/M5, 5-FU/5 mg/kg M40403-treated group; 4: 5-FU/M10, 5-FU/10 mg/kg M40403-treated group.
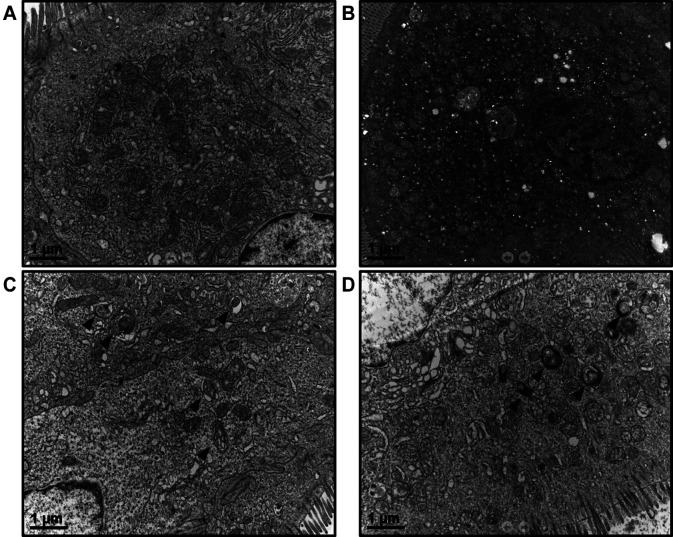
Effects of M40403 on the 5-FU-induced change in the ultrastructure of enterocytes. Under a transmission electron microscope, control enterocytes showed few signs of rupture of mitochondrial cristae, dilatation of endoplasmic reticulum cisternae, or autophagy. Enterocytes of 5-FU-treated mice showed signs of cellular injuries such as nuclear distortion and condensation, and numerous cytoplasmic vacuoles indicating misshapen and dilated organelles and autophagy. M40403 treatment appeared to partially restore the 5-FU-induced ultrastructural changes, showing more preserved cytoplasmic organelles and nucleus, and fewer vacuoles in a trend of a concentration-dependent manner (Figure 7).
Figure 7. Effects of M40403 on the 5-FU-induced changes in transmission electron microscopic findings of enterocytes. Mice were started on a 5-day course of daily intraperitoneal injection of 30 mg/kg 5-FU±5 mg/kg or 10 mg/kg M40403 on day 1. On day 8, small intestinal samples were subjected to transmission electron microscopy. Representative results of three similar experiments are presented. An untreated control mouse showed a well-preserved ultrastructure of enterocytes (A). A 5-FU alone-treated mouse showed numerous vacuoles indicating increased autophagy and cellular injuries (B). 5-FU/5 mg/kg M40403- and 5-FU/10 mg/kg M40403-treated mice showed improvements of 5-FU-induced ultrastructural injuries including autophagy (C and D, respectively). Arrowheads indicate autophagy.
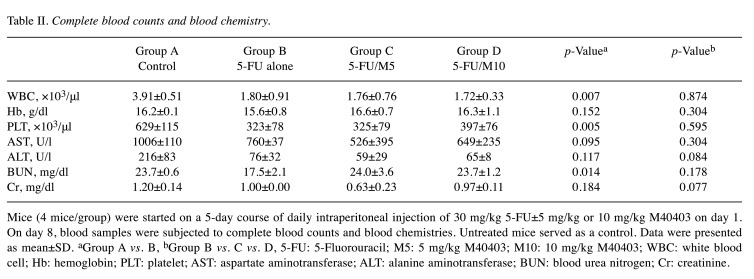
Effects of M40403 on the 5-FU-induced change in CBC and blood chemistries. Compared to the control group, 5-FU alone-treated group showed decreased levels of WBC (3.91±0.51 vs. 1.80±0.91 x103/μl, p=0.007), hemoglobin (16.2±0.1 vs. 15.6±0.8 g/dl, p=0.152), platelets (629±115 vs. 323±78 x103/μl, p=0.005), AST (1006±110 vs. 760±37 U/l, p=0.095), ALT (216±83 vs. 76±32 U/l, p=0.117), BUN (23.7±0.6 vs. 17.5±2.1 mg/dl, p=0.014), and creatinine (1.20±0.14 vs. 1.00±0.00 mg/dl, p=0.184). The co-administration of M40403 with 5-FU induced no significant differences in CBC, and serum levels of AST, ALT, BUN, and creatinine between 5-FU alone and 5-FU/M40403 groups, regardless of the co-administered doses of M40403 (Table II).
Table II. Complete blood counts and blood chemistry.
Mice (4 mice/group) were started on a 5-day course of daily intraperitoneal injection of 30 mg/kg 5-FU±5 mg/kg or 10 mg/kg M40403 on day 1. On day 8, blood samples were subjected to complete blood counts and blood chemistries. Untreated mice served as a control. Data were presented as mean±SD. aGroup A vs. B, bGroup B vs. C vs. D, 5-FU: 5-Fluorouracil; M5: 5 mg/kg M40403; M10: 10 mg/kg M40403; WBC: white blood cell; Hb: hemoglobin; PLT: platelet; AST: aspartate aminotransferase; ALT: alanine aminotransferase; BUN: blood urea nitrogen; Cr: creatinine.
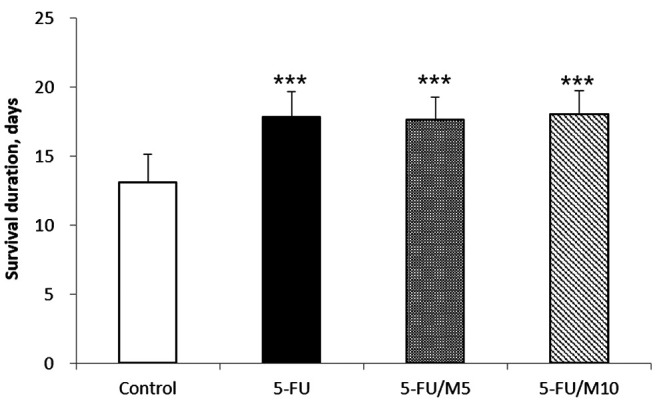
Effects of M40403 on the 5-FU-induced anti-tumor responses. Compared to the control group, all the 5-FU-treated groups, regardless of the co-administration of M40403, showed increased mean survival durations [13.1±2.0 days in the control group vs. 17.9±1.8 days (p<0.001), 17.7±1.6 days (p<0.001), and 18.1±1.7 days (p<0.001) in 5-FU alone-, 5-FU/5 mg/kg M40403-, and 5-FU/10 mg/kg M40403-treated groups, respectively] (Figure 8). However, no differences were shown in the mean survival durations among the 5-FU-treated groups, regardless of the co-administration of M40403 (Figure 8).
Figure 8. Effects of M40403 on the anti-tumor activity of 5-FU. Meth-A ascites tumor-bearing mice (5 mice/group) were started on a 4-day course of daily intraperitoneal injection of 30 mg/kg 5-FU±5 mg/kg or 10 mg/kg M40403. Untreated Meth-A ascites tumor-bearing mice served as a control. Survival duration was measured from the first day of the treatment to the day the mouse died. The same experiments were performed three times. The results were similar among the experiments and were pooled for analysis. Survival durations were expressed as mean±SD. ***p<0.001 vs. Control; 5-FU: 5-Fluorouracil; M5: 5 mg/kg M40403; M10: 10 mg/kg M40403.
Discussion
5-FU and other chemotherapeutic agents have been well known to induce the production of ROS in addition to inducing DNA damage (10-13). Chemotherapy-induced intestinal mucositis and diarrhea were suggested to develop during the interplay between chemotherapy-induced ROS, inflammatory cytokines, and apoptosis (14). ROS are thought to be involved from the initiation phase in the development of mucositis. Therefore, removing ROS by SOD mimetics would reduce the following inflammatory cytokine productions and apoptosis. Consequently, it would inhibit the development of intestinal mucositis and diarrhea. In the present study, M40403, a synthetic manganese-containing SOD mimetic, was demonstrated to attenuate 5-FU-induced intestinal mucositis and diarrhea in a mouse model.
The present study showed that the intraperitoneal injection of 30 mg/kg 5-FU for 5 consecutive days induced intestinal mucosal injuries along with diarrhea. The finding is consistent with other studies (26,27). Histological examinations showed that 5-FU treatment caused a shortening of villi lengths and destruction of mucosal structures. By adding M40403, the 5-FU-induced microscopic damage of the intestine was attenuated, showing more preserved villi lengths and structures. This was also true for the 5-FU-induced increase in diarrhea score which was improved by co-administration of M40403. The decrease in body weight was not significantly different between 5-FU alone and 5-FU/M40403 groups until day 7. However, the co-administration of 10 mg/kg M40403 (higher dose) with 5-FU improved the nadir of body weight loss and enhanced the rate of body weight recovery, compared to 5-FU alone treatment. On the other hand, there were no significant differences in the nadir of body weight loss and the rate of body weight recovery between 5-FU alone- and 5-FU/5 mg/kg M40403-treated groups. These results suggest that M40403 may have a dose-dependent effect against the 5-FU-induced intestinal injury which is consistent with a previous study that showed a trend of dose-dependency in an ischemia-reperfusion intestinal injury model (20).
In the present study, the proximal small intestine rather than the distal one was sampled as the site for histological and western blot analyses, since the proximal intestine had been known to be more susceptible to acute stress-induced damages (28).
Many previous studies have employed different diarrhea-scoring methods to assess the severity of diarrhea in mouse models (29-34). A frequently used one is the four-step diarrhea-scoring method as follows: 0 (normal stool), 1 (slightly wet and soft stool), 2 (wet and unformed stool with moderate perianal staining of the fur coat), and 3 (watery stool with severe perianal staining of the fur coat) (35). However, it was often difficult to determine the exact score because of the overlapping ambiguous borders between scores, intermingled, crushed, and contaminated stools with mouse beds in normal cages, and fur coats stained with other causes than diarrhea. It did not either consider the drying of stools in the cage during inter-observation periods. In the present study, a 2-step-scoring method was, therefore, developed and employed by placing the mouse-dwelling portion of metabolic cages on paper towels for 1 h to collect stools without time differences of stool sampling, intermingling with mouse beds, crushing, or drying.
Serum inflammatory cytokines other than TNF-α failed to show intergroup differences. Inflammatory cytokines such as TNF-α, IL-1β, and IL-6 were suggested to play major roles in the induction of intestinal mucositis (14). In the present study, serum TNF-α was significantly increased in 5-FU alone group compared to the control group, and M40403 treatment significantly inhibited the 5-FU-induced increase in serum TNF-α levels. IL-1β tended to increase in 5-FU alone group compared to the control group and tended to decrease in 5-FU/M40403 groups compared to 5-FU alone group, but statistical significance was not reached. IL-10 was increased in 5-FU alone group compared to the control group, but co-administration of M40403 had no effects on the 5-FU-induced increase of IL-10. Many serum samples showed undetectable levels of IL-6, IL-12, and IFN-γ. In other studies, employing M40403 against other inflammatory conditions, M40403 showed significant decreases in inflammatory cytokine expression levels (19-21,36). In those studies, samples obtained from the primarily affected organs (such as pleural exudates or colon tissues) were evaluated. However, in the present study, we employed serum samples of mice. It can be assumed that cytokine levels in the systemic circulation could be low compared to those in the primarily affected organs. This sampling difference could affect the results of the present study in which only TNF-α levels were significantly different between groups. The timing of serum sampling on day 8 might also affected the results. These assumptions may also explain the reason why IL-6, IL-12, and IFN-γ were undetectable in many serum samples in the present study. Nevertheless, if it is assumed that 5-FU induced only TNF-α elevation, but not other cytokines assayed, in the primarily affected site, therefore TNF-α might be one of the crucial cytokines that causes the 5-FU-induced intestinal mucositis and diarrhea.
TUNEL staining is a method of identifying apoptotic cells by labeling apoptotic DNA fragments (37). The results of TUNEL staining revealed that 5-FU treatment increased the small intestinal apoptosis. This 5-FU-induced apoptosis was significantly suppressed by co-administration of M40403. These findings were also supported by the results of western blot analyses for Bcl-xL and Bcl2, which are known as anti-apoptotic molecules (15,23). In other words, 5-FU treatment inhibited the expression of Bcl-xL and Bcl2 in the intestine, whereas administration of M40403 partially restored 5-FU-induced inhibition of the expression of Bcl-xL and Bcl2. It is known that 5-FU-induced ROS is an important mechanism for causing intestinal mucositis (14). Therefore, the results of TUNEL staining and western blot analyses for Bcl-xL and Bcl2 suggest that M40403 inhibited 5-FU-induced ROS production, thereby inhibiting the intestinal apoptosis and its associated mucositis.
Autophagy is primarily responsible for preventing cellular damage and promoting the survival of cells during various cellular stresses, such as starvation or pathogen infections (15,24). In contrast, it can also induce autophagic cell death (15,24). ROS are well known to induce autophagy (38). The close relationship between apoptosis and autophagy in many cell death processes suggested that autophagic cell death may play a role in the development of 5-FU-induced intestinal mucositis (15,24). In transmission electron microscopy, the enterocytes of 5-FU-treated mice showed a markedly increased number of cytoplasmic vacuoles or autophagy, and severely damaged cytoplasmic and nuclear structures compared to those of control mice. On the other hand, co-administration of M40403 significantly restored the 5-FU-induced ultrastructural cellular injuries, demonstrating its improving activity against the 5-FU-induced autophagy.
Among autophagy-related molecules, Beclin-1, ATG5, and LC3-II are known to increase their expression during autophagy, whereas expression p62 is decreased (24). In this study, the expression of Beclin-1, ATG5, and LC3-II was increased in the 5-FU-treated group, and the expression of p62 was decreased, compared to the control group. This result indicates that 5-FU treatment increased autophagy in enterocytes, and also supports the electron microscopic findings in which autophagy was increased by the 5-FU treatment. Administration of M40403 suppressed the changes in the expression of the autophagy-related molecules caused by 5-FU except for the changes in the expression of ATG5. This result is also consistent with the electron microscopic finding in which M40403 inhibited the 5-FU-induced intestinal autophagy. Combining the results of TUNEL staining, electron microscopy, and western blot analysis suggests that 5-FU-induced ROS cause intestinal mucositis by increasing apoptosis and autophagy, and M40403 suppresses it and thus its associated mucositis.
M40403 catalyzes the dismutation of the superoxide radical (O−2) into the oxygen (O2) and hydrogen peroxide (H2O2) at a high rate and specificity without interacting with other ROS including nitric oxide, peroxynitrite, hydrogen peroxide, oxygen, or hydroxyl radicals. M40403 is not deactivated by peroxynitrite, while native SOD’s are nitrated and deactivated by peroxynitrite (19). Thus, compared to native SOD’s, M40403 has merits of better in vivo catalytic activity and stability with low molecular weight, 483. Superoxide is a crucial mediator involved in the crosstalk between autophagy and apoptosis by regulating the expression of apoptosis/autophagy-related proteins such as Beclin-1 and Bcl2 (39). Superoxide modulates the Beclin-1-Bcl2 complex formation by phosphorylating these proteins (39). Superoxide also induces the expression of various inflammatory mediators such as cytokines, chemokines, inflammatory enzymes, and adhesion molecules (19). The mechanisms by which superoxide modulates these events remain to be clarified. An attractive possibility would be the activation of transcription factors such as NF-kB or AP-1 which regulate the expression of a variety of genes (40). M40403 might modulate these superoxide-mediated events by removing superoxide at a high rate and specificity in the absence of peroxynitrite-induced deactivation.
In addition to the findings of apoptosis and autophagy, transmission electron microscopic examinations of enterocytes of 5-FU-treated mice revealed the signs of necrosis such as the distorted nucleus and cytoplasmic organelles (41). Those necrotic findings were also attenuated by co-administration of M40403 with 5-FU. The results suggest that necrosis is another potential mechanism of the 5-FU-induced death of enterocytes. It has been known that severe forms of apoptosis might be destined for necrosis (15). Autophagy can be instinctively induced before apoptosis when cells are stimulated by stresses, and apoptosis can be induced if autophagy is ineffective (15). Thus, three types of cell death mechanisms (apoptosis, autophagy, and necrosis) may be induced successively or simultaneously during 5-FU treatment.
A potential mechanism that mediates the M40403-induced attenuation of 5-FU-induced intestinal apoptosis, autophagy, and necrosis is the removal of the 5-FU-induced ROS by the SOD mimetic activity of M40403. The attenuation of apoptosis and autophagy caused by M40403 can also be induced in cancer cells during 5-FU chemotherapy and have a potential for modulating the anti-tumor activity of 5-FU. We, therefore, tested whether M40403 interferes with the anti-tumor activity of 5-FU, employing Meth-A ascites tumor-bearing mice. No differences were shown in the survival duration between 5-FU alone- and 5-FU/M40403-treated groups. This finding suggests that M40403 can be safely administered to improve the 5-FU-induced intestinal mucositis without compromising the anti-tumor activity of 5-FU.
The toxic effect of M40403 was also evaluated by employing complete blood counts and blood chemistries for liver and renal functions. The 5-FU alone and 5-FU/M40403 groups did not show any significant differences in those measurements, suggesting that M40403 did not add any further toxicities in the hematopoietic, hepatic, and renal functions during 5-FU treatment, at the doses of 5 and 10 mg/kg. There were phase I and II clinical trials employing GC4419, a mirror-image isomer of M40403, to test its effect on the chemoradiation-induced oral mucositis in oropharyngeal cancer (42). In that study, toxicities related to GC4419 were nausea/vomiting and paresthesia, which were manageable.
Further studies are needed to elucidate more detailed action mechanisms of M40403 and to evaluate its clinical usefulness in improving intestinal mucositis and its associated diarrhea caused by inadvertent 5-FU intoxication as well as usual doses of 5-FU. It is also needed to evaluate whether M40403 has protective effects against intestinal mucositis and diarrhea caused by other chemotherapeutic agents than 5-FU.
Conclusion
M40403, a SOD mimetic, has an attenuating effect against 5-FU-induced intestinal mucositis and its associated diarrhea. The experimental findings suggested that the inhibition of the 5-FU-induced increase in TNF-α, and the attenuation of the 5-FU-induced intestinal apoptosis and autophagy were potential mechanisms for the M40403-induced improvement of the 5-FU-induced intestinal mucositis and diarrhea.
Conflicts of Interest
The Authors have no conflicts of interest to declare.
Authors’ Contributions
S.K.Y., S.O.L., S.W.K., S.Y.S., and S.T.L. conceived and designed the experiments. S.K.Y., K.M.K., C.H.L., E.K.S., and S.H.K. performed the experiments. S.K.Y., S.Y.S., I.H.K., S.W.K., S.O.L., and S.T.L. analyzed the data and wrote the article.
Acknowledgements
This paper was supported by the fund of Biomedical Research Institute, Jeonbuk National University Hospital which had no role in the design, execution, interpretation, or writing of the study.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Breda M, Barattè S. A review of analytical methods for the determination of 5-fluorouracil in biological matrices. Anal Bioanal Chem. 2010;397(3):1191–1201. doi: 10.1007/s00216-010-3633-8. [DOI] [PubMed] [Google Scholar]
- 3.Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O, Peterson DE, Raber-Durlacher JE, Sonis ST, Elad S, Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120(10):1453–1461. doi: 10.1002/cncr.28592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi I, Kawamata R, Makino K. A rat model of oral mucositis induced by cancer chemotherapy for quantitative experiments. Anticancer Res. 2020;40(5):2701–2706. doi: 10.21873/anticanres.14241. [DOI] [PubMed] [Google Scholar]
- 5.Petrelli N, Herrera L, Rustum Y, Burke P, Creaven P, Stulc J, Emrich LJ, Mittelman A. A prospective randomized trial of 5-fluorouracil versus 5-fluorouracil and high-dose leucovorin versus 5-fluorouracil and methotrexate in previously untreated patients with advanced colorectal carcinoma. J Clin Oncol. 1987;5(10):1559–1565. doi: 10.1200/JCO.1987.5.10.1559. [DOI] [PubMed] [Google Scholar]
- 6.Petrelli N, Douglass HO Jr, Herrera L, Russell D, Stablein DM, Bruckner HW, Mayer RJ, Schinella R, Green MD, Muggia FM. The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: A prospective randomized phase III trial. Gastrointestinal Tumor Study Group. J Clin Oncol. 1989;7(10):1419–1426. doi: 10.1200/JCO.1989.7.10.1419. [DOI] [PubMed] [Google Scholar]
- 7.Benson AB 3rd, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA Jr, McCallum R, Mitchell EP, O’Dorisio TM, Vokes EE, Wadler S. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol. 2004;22(14):2918–2926. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- 8.Van Sebille YZ, Stansborough R, Wardill HR, Bateman E, Gibson RJ, Keefe DM. Management of mucositis during chemotherapy: From pathophysiology to pragmatic therapeutics. Curr Oncol Rep. 2015;17(11):50. doi: 10.1007/s11912-015-0474-9. [DOI] [PubMed] [Google Scholar]
- 9.Davila M, Bresalier RS. Gastrointestinal complications of oncologic therapy. Nat Clin Pract Gastroenterol Hepatol. 2008;5(12):682–696. doi: 10.1038/ncpgasthep1277. [DOI] [PubMed] [Google Scholar]
- 10.Focaccetti C, Bruno A, Magnani E, Bartolini D, Principi E, Dallaglio K, Bucci EO, Finzi G, Sessa F, Noonan DM, Albini A. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS One. 2015;10(2):e0115686. doi: 10.1371/journal.pone.0115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang IT, Chung YM, Kim JJ, Chung JS, Kim BS, Kim HJ, Kim JS, Yoo YD. Drug resistance to 5-FU linked to reactive oxygen species modulator 1. Biochem Biophys Res Commun. 2007;359(2):304–310. doi: 10.1016/j.bbrc.2007.05.088. [DOI] [PubMed] [Google Scholar]
- 12.Hwang PM, Bunz F, Yu J, Rago C, Chan TA, Murphy MP, Kelso GF, Smith RA, Kinzler KW, Vogelstein B. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat Med. 2001;7(10):1111–1117. doi: 10.1038/nm1001-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Y, Yang G, Zhu F, Peng C, Li W, Li H, Kim HG, Bode AM, Dong Z, Dong Z. Antioxidants decrease the apoptotic effect of 5-Fu in colon cancer by regulating Src-dependent caspase-7 phosphorylation. Cell Death Dis. 2014;5:e983. doi: 10.1038/cddis.2013.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4(4):277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Kang J, Fu C. The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduct Target Ther. 2018;3:18. doi: 10.1038/s41392-018-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 17.Hunter GJ, Trinh CH, Bonetta R, Stewart EE, Cabelli DE, Hunter T. The structure of the Caenorhabditis elegans manganese superoxide dismutase MnSOD-3-azide complex. Protein Sci. 2015;24(11):1777–1788. doi: 10.1002/pro.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvemini D, Muscoli C, Riley DP, Cuzzocrea S. Superoxide dismutase mimetics. Pulm Pharmacol Ther. 2002;15(5):439–447. doi: 10.1006/pupt.2002.0374. [DOI] [PubMed] [Google Scholar]
- 19.Salvemini D, Mazzon E, Dugo L, Riley DP, Serraino I, Caputi AP, Cuzzocrea S. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. Br J Pharmacol. 2001;132(4):815–827. doi: 10.1038/sj.bjp.0703841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286(5438):304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 21.Cuzzocrea S, Mazzon E, Dugo L, Caputi AP, Riley DP, Salvemini D. Protective effects of M40403, a superoxide dismutase mimetic, in a rodent model of colitis. Eur J Pharmacol. 2001;432(1):79–89. doi: 10.1016/s0014-2999(01)01427-3. [DOI] [PubMed] [Google Scholar]
- 22.Yim CY, McGregor JR, Kwon OD, Bastian NR, Rees M, Mori M, Hibbs JB Jr, Samlowski WE. Nitric oxide synthesis contributes to IL-2-induced antitumor responses against intraperitoneal Meth A tumor. J Immunol. 1995;155(9):4382–4390. [PubMed] [Google Scholar]
- 23.Wright TH, Yazbeck R, Lymn KA, Whitford EJ, Cheah KY, Butler RN, Feinle-Bisset C, Pilichiewicz AN, Mashtoub S, Howarth GS. The herbal extract, Iberogast, improves jejunal integrity in rats with 5-fluorouracil (5-FU)-induced mucositis. Cancer Biol Ther. 2009;8(10):923–929. doi: 10.4161/cbt.8.10.8146. [DOI] [PubMed] [Google Scholar]
- 24.Warren CFA, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10(3):177. doi: 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Kim JH, Moon W, Park J, Park SJ, Song GA, Han SH, Lee JH. Rebamipide attenuates 5-fluorouracil-induced small intestinal mucositis in a mouse model. Biol Pharm Bull. 2015;38(2):179–183. doi: 10.1248/bpb.b14-00400. [DOI] [PubMed] [Google Scholar]
- 27.Huang TY, Chu HC, Lin YL, Ho WH, Hou HS, Chao YC, Liao CL. Minocycline attenuates 5-fluorouracil-induced small intestinal mucositis in mouse model. Biochem Biophys Res Commun. 2009;389(4):634–639. doi: 10.1016/j.bbrc.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 28.Novosad VL, Richards JL, Phillips NA, King MA, Clanton TL. Regional susceptibility to stress-induced intestinal injury in the mouse. Am J Physiol Gastrointest Liver Physiol. 2013;305(6):G418–G426. doi: 10.1152/ajpgi.00166.2013. [DOI] [PubMed] [Google Scholar]
- 29.Pereira VB, Melo AT, Assis-Júnior EM, Wong DV, Brito GA, Almeida PR, Ribeiro RA, Lima-Júnior RC. A new animal model of intestinal mucositis induced by the combination of irinotecan and 5-fluorouracil in mice. Cancer Chemother Pharmacol. 2016;77(2):323–332. doi: 10.1007/s00280-015-2938-x. [DOI] [PubMed] [Google Scholar]
- 30.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112(11):1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sezaki T, Hirata Y, Hagiwara T, Kawamura YI, Okamura T, Takanashi R, Nakano K, Tamura-Nakano M, Burkly LC, Dohi T. Disruption of the TWEAK/Fn14 pathway prevents 5-fluorouracil-induced diarrhea in mice. World J Gastroenterol. 2017;23(13):2294–2307. doi: 10.3748/wjg.v23.i13.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taghipour N, Molaei M, Mosaffa N, Rostami-Nejad M, Asadzadeh Aghdaei H, Anissian A, Azimzadeh P, Zali MR. An experimental model of colitis induced by dextran sulfate sodium from acute progresses to chronicity in C57BL/6: Correlation between conditions of mice and the environment. Gastroenterol Hepatol Bed Bench. 2016;9(1):45–52. [PMC free article] [PubMed] [Google Scholar]
- 33.Sakai H, Sagara A, Matsumoto K, Hasegawa S, Sato K, Nishizaki M, Shoji T, Horie S, Nakagawa T, Tokuyama S, Narita M. 5-fluorouracil induces diarrhea with changes in the expression of inflammatory cytokines and aquaporins in mouse intestines. PLoS One. 2013;8(1):e54788. doi: 10.1371/journal.pone.0054788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocha-Pereira J, Kolawole AO, Verbeken E, Wobus CE, Neyts J. Post-exposure antiviral treatment of norovirus infections effectively protects against diarrhea and reduces virus shedding in the stool in a mortality mouse model. Antiviral Res. 2016;132:76–84. doi: 10.1016/j.antiviral.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurita A, Kado S, Kaneda N, Onoue M, Hashimoto S, Yokokura T. Modified irinotecan hydrochloride (CPT-11) administration schedule improves induction of delayed-onset diarrhea in rats. Cancer Chemother Pharmacol. 2000;46(3):211–220. doi: 10.1007/s002800000151. [DOI] [PubMed] [Google Scholar]
- 36.Salvemini D, Mazzon E, Dugo L, Serraino I, De Sarro A, Caputi AP, Cuzzocrea S. Amelioration of joint disease in a rat model of collagen-induced arthritis by M40403, a superoxide dismutase mimetic. Arthritis Rheum. 2001;44(12):2909–2921. doi: 10.1002/1529-0131(200112)44:12<2909::aid-art479>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Kyrylkova K, Kyryachenko S, Leid M, Kioussi C. Detection of apoptosis by TUNEL assay. Methods Mol Biol. 2012;887:41–47. doi: 10.1007/978-1-61779-860-3_5. [DOI] [PubMed] [Google Scholar]
- 38.Yun HR, Jo YH, Kim J, Shin Y, Kim SS, Choi TG. Roles of autophagy in oxidative stress. Int J Mol Sci. 2020;21(9):3289. doi: 10.3390/ijms21093289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Zhang W, Guo X, Ren J, Gao A. The crosstalk between autophagy and apoptosis was mediated by phosphorylation of Bcl-2 and beclin1 in benzene-induced hematotoxicity. Cell Death Dis. 2019;10(10):772. doi: 10.1038/s41419-019-2004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85(8):753–766. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- 41.Kamiński M, Masaoka M, Karbowski M, Kedzior J, Nishizawa Y, Usukura J, Wakabayashi T. Ultrastructural basis for the transition of cell death mode from apoptosis to necrosis in menadione-treated osteosarcoma 143B cells. J Electron Microsc (Tokyo) 2003;52(3):313–325. doi: 10.1093/jmicro/52.3.313. [DOI] [PubMed] [Google Scholar]
- 42.Anderson CM, Sonis ST, Lee CM, Adkins D, Allen BG, Sun W, Agarwala SS, Venigalla ML, Chen Y, Zhen W, Mould DR, Holmlund JT, Brill JM, Buatti JM. Phase 1b/2a trial of the superoxide dismutase mimetic GC4419 to reduce chemoradiotherapy-induced oral mucositis in patients with oral cavity or oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2018;100(2):427–435. doi: 10.1016/j.ijrobp.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]