Abstract
Background/Aim: This study aimed to access the effectiveness of serum neutrophil-to-lymphocyte ratio (NLR) in patients undergoing prostate needle biopsy with a prostate specific antigen (PSA) between 4.0 and 10.0 ng/ml. Patients and Methods: A total of 633 cases were eligible. We evaluated several factors including age, PSA, PSA-density (PSAD), platelet-to-lymphocyte ratio (PLR) and NLR in the presence or absence of prostate cancer (PCa), retrospectively. We evaluated statistically the associations between each factor and pathological findings or Gleason score. Results: A total of 201 were evaluated in this study. Regarding the presence or absence of prostate cancer, there were statistically significant differences in age, PSA levels, PSAD, the PLR and NLR. The mean NLR value of the patients with PCa was significantly lower compared to the entire cohort. Multivariate analysis showed that age, PSAD, and NLR were independent risk factors predicting PCa. Conclusion: For patients having a PSA between 4.0 and 10.0 ng/ml, NLR was a predicting factor of PCa prior to prostate needle biopsy and an effective biomarker and useful tool for avoiding unnecessary biopsies.
Keywords: Neutrophil-to-lymphocyte ratio, prostate cancer, gray zone, prostate biopsy
In various carcinomas, the development and progression of cancers has been associated with chronic inflammation within the tissue microenvironment (1). In the last few years, increasing evidence has indicated that cancer development and progression depend on complex interactions between tumor and host factors, including the systemic inflammatory response (2,3).
Prostate specific antigen (PSA) is the only biomarker used for the early detection of prostate cancer (PCa). PSA is highly specific for prostate but not for PCa. Approximately 70% of men with an increased serum PSA between 4 and 10 ng/ml do not have PCa and hence, this PSA range is referred to as the “grey area” (4). Some PSA-related testing parameters [e.g., PSA-density (PSAD), free/total PSA ratio (F/T), PSA-doubling time (PSA-DT), and prostate health index test] have been used to improve the accuracy of PCa prediction (5). Regarding the indication for prostate biopsy (PBx), not only the PSA value but also digital rectal examination, magnetic resonance imaging (MRI) findings, PSAD, F/T ratio and PSA-DT should be used. It is important to avoid unnecessary PBx by taking an informed decision. Moreover, PBx has been associated with various complications such as pain, macrohematuria, dysuria and severe infections. Therefore, identification of new biomarkers of PCa that are easy to assess and could be used to determine the optimal indications for PBx is needed.
The neutrophil-to-leukocyte ratio (NLR) is the proportion of systemic neutrophils and lymphocytes, and it is a recognized circulating biomarker in multiple cancers. Although NLR is a parameter of inflammation, it has been reported as a prognostic factor for solid cancers including PCa (6,7). Also, NLR has been reported to be a new biomarker to predict PCa in men undergoing PBx (8). Another report, however, has suggested that low serum neutrophil count may be a positive predictor of PCa detection by transrectal ultrasound (TRUS)-guided biopsy (9). Although some histopathological, clinical, and epidemiological evidence suggest that chronic inflammation plays a role in prostate carcinogenesis, an association between inflammatory markers and the overall detection rate of PCa remains controversial (10,11).
In the present study, we investigated whether NLR could be used to predict PCa in patients with PSA levels between 4.0 and 10.0 ng/ml.
Patients and Methods
Study subjects. This is a retrospective study that used data extracted from electronic records. Between April 2009 and August 2018, 633 cases with PSA 4.0-10.0 ng/ml out of 1,435 cases, which underwent PBx at the Department of Urology, Teikyo University Chiba Medical Center (Ichihara, Japan), were included. PBx was performed transrectally under ultrasound sonography with local anesthesia and routinely performed by using 14-region template in all patients. The biopsy specimens were examined by a dedicated histopathologist at our hospital. Complete blood counts (CBCs) including absolute neutrophil and lymphocyte counts were performed for all patients. Peripheral blood was collected at our hospital within one month of the PBx. The institutional review board of Teikyo University approved this study [20-114].
Clinical and laboratory assessments. The NLR was calculated using neutrophil and lymphocyte count via CBCS obtained simultaneously with PSA. We determined the cut-off point of NLR according to the sensitivity and specificity levels derived from the area under receiver operator characteristics (AUROC) curve plotted using the presence or absence of PCa. We evaluated the factors including age, PSA, PSAD, platelet-to-lymphocyte ratio (PLR) and NLR in the presence or absence of PCa, retrospectively. The NLR was calculated by dividing the neutrophil count by the lymphocyte count, while the PLR was calculated by dividing the platelet count by the lymphocyte count. The patients were pathologically classified into benign prostate hypertrophy (BPH), prostatitis, and PCa groups. In addition, they were divided according to Gleason score (GS), as GS=6, GS=7 and ≥GS 8. Then, we statistically evaluated the association of each factor with pathological finding and GS.
Statistical analysis. Results are shown as the mean±SD. Patient characteristics and preoperative factors were analyzed using Mann-Whitney U-test and Kruskal-Wallis test. Analyses were performed with JMP version 10 (SAS Institute Inc., Cary, NC, USA). A probability of <0.05 was considered statistically significant.
Results
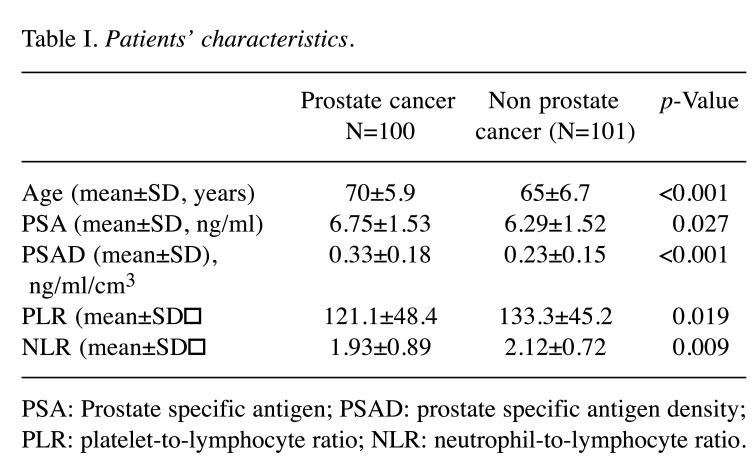
Of the 633 eligible patients, 201 were evaluated in this study. The mean age, PSA levels, PSAD, PLR, NLR were 68 years, 6.34 ng/ml, 0.23, 117.7 and 1.84, respectively. Of these, 100 were positive for PCa. None of these patients were found to have metastatic disease at the time of the diagnosis of PCa. The mean age of the PCa group (70±5.9) was significantly higher than that of the non-PCa group (65±6.7; p<0.001). The mean PSA levels of the PCa group (6.75±1.53) were significantly higher than that of the non-PCa group (6.29±1.52; p=0.027). The mean PSAD value of the PCa group (0.33±0.18) was significantly higher than that of the non-PCa group (0.23±0.15; p<0.001). The mean PLR value of the PCa group (121.1±48.4) was significantly lower than that of the non-PCa group (133.3±45.2; p=0.019). The mean NLR value of the PCa group (1.93±0.89) was significantly lower than that of the non-PCa group (2.12±0.72; p=0.009) (Table I).
Table I. Patients’ characteristics.
PSA: Prostate specific antigen; PSAD: prostate specific antigen density; PLR: platelet-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio.
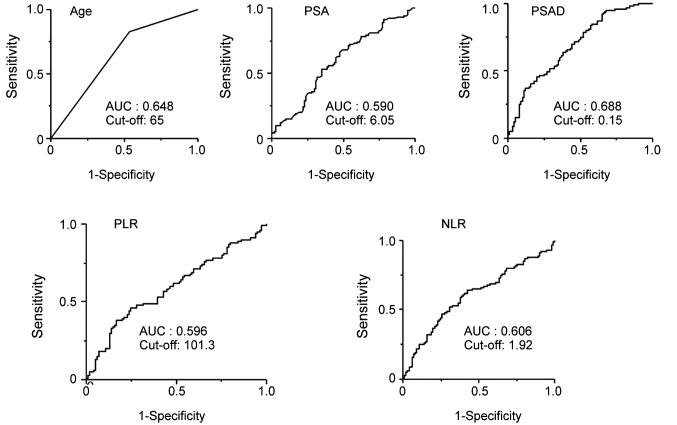
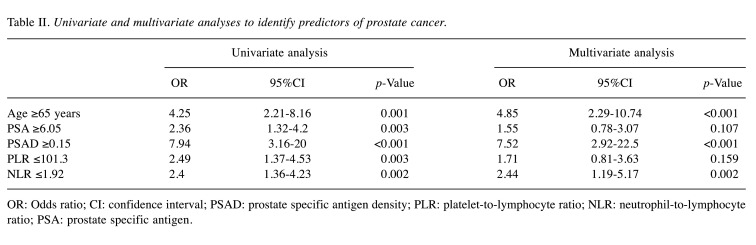
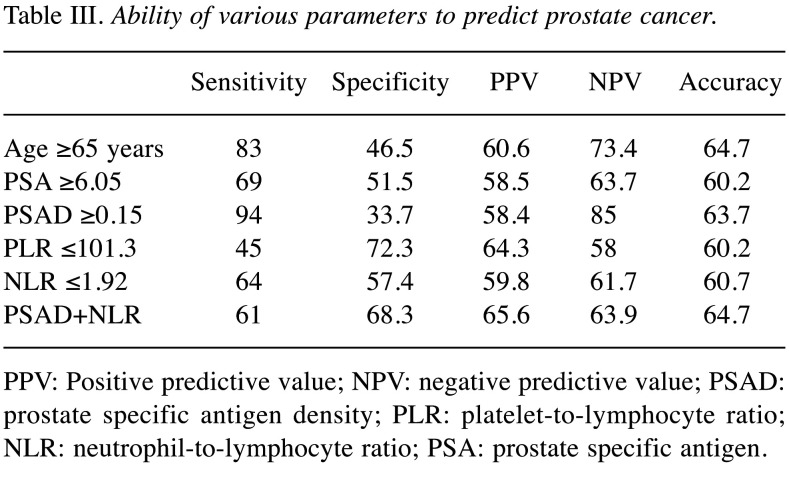
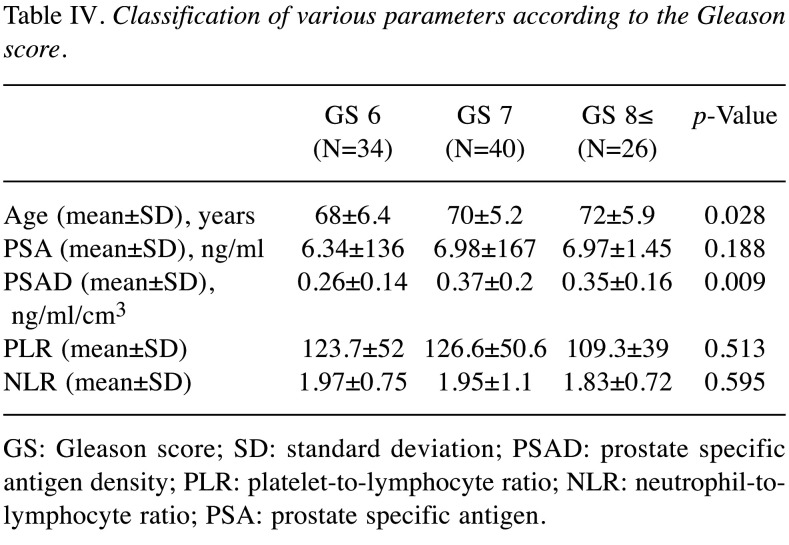
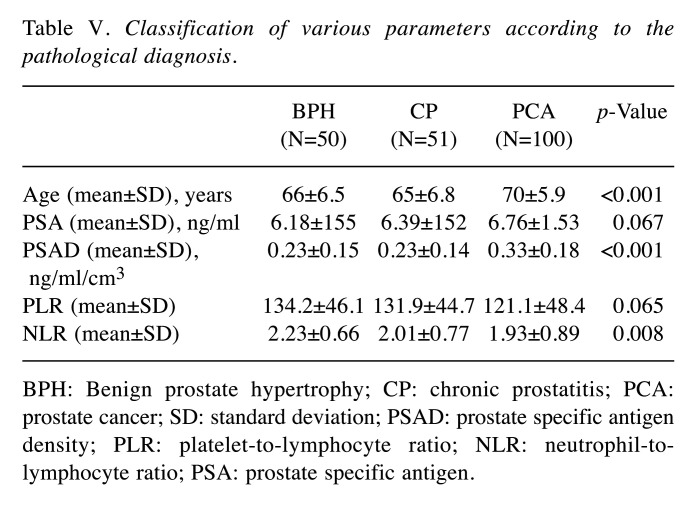
We performed receiver operative characteristic (ROC) curve analysis to assess the sensitivity and specificity of each factor for predicting PCa. Subsequently, the cut-off values for each factor were set (Figure 1). Based on the AUROC curve, the cut-off levels for age, PSA, PSAD, PLR and NLR were set at 65, 6.05, 0.15, 101.3 and 1.92 for predicting PCa, respectively. Multivariate analysis showed that age [odds ratio (OR)=4.85, 95% confidence interval (CI)=2.29-10.74, p<0.001], PSAD (OR=7.52, 95%CI=2.92-22.5, p<0.001), and NLR (OR=2.44, 95%CI=1.19-5.17, p=0.002) were independent risk factors predicting PCa (Table II). The sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV), and accuracy of these factors are shown in Table III. The PPV and accuracy of the combination of PSAD and NLR were higher (65.6% and 64.7%, respectively) compared to the PSAD cut-off group (58.4% and 63.7%, respectively) and NLR cut-off group (59.8% and 60.7%, respectively) (Table III). The patients in the PCa group were divided into three groups according to Gleason score (GS) (Table IV). GS was available for all PCa patients: 34 (34%) GS6, 40 (40%) GS7, 26 (26%) GS8 over (Table IV). Significant differences in age and PSAD were observed between each group. Regarding the pathological findings, there were 50, 51 and 100 patients with BPH, prostatitis and PCa, respectively. When we compared the three groups, we recognized that the mean age and PSAD value of the PCa group were significantly higher than those of the other two groups. Moreover, the NLR value of the PCa group was significantly lower than those of the other two groups (Table V).
Figure 1. AUROC curve values of variables for predicting prostate cancer. Based on AUROC curve values, the optimal age, PSA, PSAD, PLR, and NLR cut-off levels for predicting PCa were set at 65, 6.05, 0.15, 101.3, and 1.92, respectively.
Table II. Univariate and multivariate analyses to identify predictors of prostate cancer.
OR: Odds ratio; CI: confidence interval; PSAD: prostate specific antigen density; PLR: platelet-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; PSA: prostate specific antigen.
Table III. Ability of various parameters to predict prostate cancer.
PPV: Positive predictive value; NPV: negative predictive value; PSAD: prostate specific antigen density; PLR: platelet-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; PSA: prostate specific antigen.
Table IV. Classification of various parameters according to the Gleason score.
GS: Gleason score; SD: standard deviation; PSAD: prostate specific antigen density; PLR: platelet-to-lymphocyte ratio; NLR: neutrophil-tolymphocyte ratio; PSA: prostate specific antigen.
Table V. Classification of various parameters according to the pathological diagnosis.
BPH: Benign prostate hypertrophy; CP: chronic prostatitis; PCA: prostate cancer; SD: standard deviation; PSAD: prostate specific antigen density; PLR: platelet-to-lymphocyte ratio; NLR: neutrophil-tolymphocyte ratio; PSA: prostate specific antigen.
Discussion
In the present study, it was revealed that NLR, which was used for the diagnosis of PCa with PSA between 4.0 and 10.0 ng/ml, showed a high accuracy rate for PCa. Moreover, when NLR was combined with PSAD, it was validated that the accuracy rate was further increased. Therefore, we suggest that NLR would be an effective and useful tool in the determining the indication for PBx in patients having a PSA between 4.0 and 10.0 ng/ml.
It has been reported that NLR is an important prognostic factor for advanced PCa (12). Gu et al. (13) have reported that increased pretreatment NLR was associated with poor overall survival (OS) (HR=1.38) and progression free survival/recurrence free survival (PFS/RFS) (HR=1.24). They suggested that an increased NLR could predict poor diagnosis in patients with PCa. Nuhn et al. (14) have shown that that the NLR was significantly associated with clinical outcomes in men receiving first-line chemotherapy with docetaxel for metastatic castration-resistant PCa. Langsenlehner et al. (15) have reported that the NLR seems to represent an independent prognostic marker and should be considered for future individual risk assessment in patients with prostate cancer. However, Naito et al. (16) have reported that there was no significant difference in the NLR value between localized PCa and metastatic PCa.
In our study, NLR was statistically significantly higher in the non-PCa group than in the PCa group. Fujita et al. (9) have demonstrated that a low serum neutrophil count is a predictor of positive prostate biopsy in 323 Japanese men (OR=0.408, p<0.002). The value of NLR in patients with PSA below 10 ng/ml was 2.09 in the negative biopsy group and 1.90 in the positive biopsy group. It was thought that this result was sufficient similar to our data. Therefore, this finding indicated that NLR could be a predicting factor of PCa in the gray zone. Furthermore, Naito et al. (16) have reported that the median NLR of PCa patients with a PSA below 10 ng/ml was 1.834, which was lower than the overall median of 1.945, which included that of the non-PCa patients. In the case of PSA below 10 ng/ml, the NLR value was high in the non-prostate cancer. This finding supports our results.
Also, NLR exhibited higher specificity, PPV, and accuracy for predicting PCa than the PSA levels. So, NLR may be a biomarker comparable to PSA. In the multivariate analysis, the OR of NLR was 2.44, which suggested that NLR could be a useful predictor of PCa. In addition, it was found that the combination of NLR with PSAD resulted in even more accurate predictions of PCa. Regarding the indications for PBx, it is suggested that NLR could facilitate auxiliary diagnosis, as has been found for other factors. Therefore, the use of the combination of NLR with PSAD may prevent unnecessary PBx. Kamali et al. (17) have evaluated 500 patients who underwent PBx but no statistically significant difference was not obtained between the NLR of the patients with positive biopsy and those with negative biopsy (p=0.112). NLR was not described as a predictive factor for positive PCa biopsy. Although it was demonstrated that the AUC value under ROC was 0.58 (8), in the present study was 0.60, so, it was almost consistent with other reports. Moreover, based on the AUROC in the present study, the NLR cut-off value was determined to be 1.92 for patients with PSA between 4.0-10.0 ng/ml. In another report, the NLR cut-off value was determined to be 2.40 in the gray zone (8). In several studies analyzing advanced pancreatic cancer, the NLR cut-off values were approximately 5 (18). In intrahepatic cholangiocarcinoma and liver metastasis from colorectal carcinoma, the NLR cut-off value was also set as 5 (19). In renal cell carcinoma varied in different studies from 2 to 5 (19). Thus, the optimal NLR cut-off point for predicting PCa varies depending on the target disease and organ. Furthermore, the NLR value changes depending on the progression of the disease even in the same organ. Therefore, it seems difficult to set a universal NLR cut-off point for predicting PCa.
It has been previously demonstrated that chronic inflammation is involved in prostate carcinogenesis through disrupting of the immune response and regulating of the tumor microenvironment (20). However, regarding the present study, it was considered that the relationship is not well established during the early stages of carcinogenesis where PSA levels are still low. Kawahara et al. (8) have reported that the median NLR value was 2.05 in patients with PSA 4 ng/ml below and 2.00 in patients with PSA 4.0-10 ng/ml. In the gray zone, the NLR value was rather low. Regarding the PCa in the gray zone, the reason the NLR value was lower than that in the non-PCa group was unknown. We hypothesized that significant inflammation rather than carcinogenesis may characterize the prostate tissue in the gray zone. Molecular pathological evaluation is necessary in the future. Although it has been reported that there is a positive correlation between NLR and PSA (16), we could not confirm the association of NLR and PSA in the present study. Hashimoto et al. (21) have reported that ΔNLR was the most accurate marker to improve the total predictive value in repeat PBx for diagnosing PCa. In the case of gray zone, the NLR value was not constant, therefore the ΔNLR may be a marker of a change from non- cancer status to cancer-positive status. Hence, we suggest that the assessment of NLR, regardless of its value, is meaningful. Regarding the association between NLR and GS, Mehmet et al. (22) have demonstrated that higher GS is associated with higher NLR in patients with PCa. In the present study of PCa in the gray zone, NLR showed an insignificant negative correlation with high GS. The various cancer growth patterns and histopathological findings in the gray zone might have affected our results. No evaluations of NLR were performed in cases in which digital rectal examinations produced positive results. If the NLR were evaluated in such cases, it would be possible to clarify the relationship between the degree of malignancy and the NLR. Regarding the evaluation of the histopathology, it has been reported that the value of NLR in the gray zone is higher in prostatitis compared with prostate cancer (22). In the present study, the NLR value was the highest in the BPH group, and gradually decreased in the prostatitis and PCa groups.
As for the other biomarker, Volkan Caglayan et al. (23) have reported that lymphocyte-to-monocyte (LMR) is a useful tool for detecting PCa especially in patients with PSA value between 4 and 10 ng/dl. The combination of free/total PSA ratio and LMR improved the diagnostic accuracy more than the use of free/total PSA ratio alone. They demonstrated that in the 4-10 ng/dl PSA range, LMR value was extremely lower in patients with positive biopsy than in those with a negative biopsy (p=0.012). However, unfortunately, there has not been any detailed discussion about the reasons or the pathophysiology underlying this relationship in the literature. Also, Ozgur Haki Yuksel et al. (24) have revealed that PLR values in the PCa group were significantly higher compared with the BPH group. They concluded that PLR could be an additional predictor of PCa and can prevent unnecessary PBx in the diagnosis of PCa. However, in their report, the reason why PLR value was high in the PCa was not mentioned. Conversely, in our study of patients with PSA between 4.0-10.0 ng/ml, PLR was higher in the BPH group compared with the PCa group.
There are several limitations in our study. First, it was a retrospective cohort study, which involved the extraction of electronically stored clinical data. Second, it had a small sample size compared with previous studies. Third, we could not perform a comparison of NLR between the different stages of PCa. It is possible that the NLR might differ between advanced PCa and non-advanced PCa in the gray zone. However, because of its small sample size, it was difficult to perform such comparisons and evaluations. It is necessary to validate the findings of this retrospective analysis in prospective studies, including a randomized study, with larger populations in the future. The NLR, which can be measured easily, could help to determine the optimal indications for PBx. Moreover, the importance of routinely obtaining a CBC was demonstrated in this study.
Conclusion
The present study suggests that the NLR could be a useful pre-PBx predictor of PCa, and hence, could help to avoid unnecessary PBx, in patients with PSA levels of 4.0-10.0 ng/ml. Although the NLR value for PCa in the gray zone was statistically significantly lower than for non-PCa, we suggested that the measurement of NLR might be very meaningful regardless of its value. Moreover, the combination of NLR with PSAD likely improves both PPV and accuracy of PBx in patients with PSA value of 4.0-10.0 ng/ml. These findings suggest that it is necessary to perform a CBC as a routine test in such patients.
Conflicts of Interest
The Authors have stated that they have no conflicts of interest in relation to this study.
Authors’ Contributions
HM designed the study and acquired the data. HM prepared and edited the manuscript. KM, KO, KH, TS, KA, SK and YN were involved in a patient care and reviewed the electronic records. All Authors read and approved the manuscript.
References
- 1.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 3.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel T, Zattoni F, European Association of Urology EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59(1):61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 6.Dalpiaz O, Pichler M, Mannweiler S, Martín Hernández JM, Stojakovic T, Pummer K, Zigeuner R, Hutterer GC. Validation of the pretreatment derived neutrophil-lymphocyte ratio as a prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. Br J Cancer. 2014;110(10):2531–2536. doi: 10.1038/bjc.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohno Y, Nakashima J, Ohori M, Hatano T, Tachibana M. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol. 2010;184(3):873–878. doi: 10.1016/j.juro.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Kawahara T, Fukui S, Sakamaki K, Ito Y, Ito H, Kobayashi N, Izumi K, Yokomizo Y, Miyoshi Y, Makiyama K, Nakaigawa N, Yamanaka T, Yao M, Miyamoto H, Uemura H. Neutrophil-to-lymphocyte ratio predicts prostatic carcinoma in men undergoing needle biopsy. Oncotarget. 2015;6(31):32169–32176. doi: 10.18632/oncotarget.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita K, Imamura R, Tanigawa G, Nakagawa M, Hayashi T, Kishimoto N, Hosomi M, Yamaguchi S. Low serum neutrophil count predicts a positive prostate biopsy. Prostate Cancer Prostatic Dis. 2012;15(4):386–390. doi: 10.1038/pcan.2012.27. [DOI] [PubMed] [Google Scholar]
- 10.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutcliffe S, Platz EA. Inflammation in the etiology of prostate cancer: an epidemiologic perspective. Urol Oncol. 2007;25(3):242–249. doi: 10.1016/j.urolonc.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Templeton AJ, Pezaro C, Omlin A, McNamara MG, Leibowitz-Amit R, Vera-Badillo FE, Attard G, de Bono JS, Tannock IF, Amir E. Simple prognostic score for metastatic castration-resistant prostate cancer with incorporation of neutrophil-to-lymphocyte ratio. Cancer. 2014;120(21):3346–3352. doi: 10.1002/cncr.28890. [DOI] [PubMed] [Google Scholar]
- 13.Gu X, Gao X, Li X, Qi X, Ma M, Qin S, Yu H, Sun S, Zhou D, Wang W. Prognostic significance of neutrophil-to-lymphocyte ratio in prostate cancer: Evidence from 16,266 patients. Sci Rep. 2016;6:22089. doi: 10.1038/srep22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuhn P, Vaghasia AM, Goyal J, Zhou XC, Carducci MA, Eisenberger MA, Antonarakis ES. Association of pretreatment neutrophil-to-lymphocyte ratio (NLR) and overall survival (OS) in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with first-line docetaxel. BJU Int. 2014;114(6b):E11–E17. doi: 10.1111/bju.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langsenlehner T, Thurner EM, Krenn-Pilko S, Langsenlehner U, Stojakovic T, Gerger A, Pichler M. Validation of the neutrophil-to-lymphocyte ratio as a prognostic factor in a cohort of European prostate cancer patients. World J Urol. 2015;33(11):1661–1667. doi: 10.1007/s00345-015-1494-7. [DOI] [PubMed] [Google Scholar]
- 16.Naito H, Sugimoto M, Taketa S, Kakehi Y. [Clinical significance of neutrophil-to-lymphocyte ratio in patients with prostate cancer] Hinyokika Kiyo. 2017;63(2):63–67. doi: 10.14989/ActaUrolJap_63_2_63. [DOI] [PubMed] [Google Scholar]
- 17.Kamali K, Ashrafi M, Shadpour P, Ameli M, Khayyamfar A, Abolhasani M, Azizpoor A. The role of blood neutrophil count and the neutrophil-to-lymphocyte ratio as a predictive factor for prostate biopsy results. Urologia. 2018;85(4):158–162. doi: 10.1177/0391560318766822. [DOI] [PubMed] [Google Scholar]
- 18.Kou T, Kanai M, Yamamoto M, Xue P, Mori Y, Kudo Y, Kurita A, Uza N, Kodama Y, Asada M, Kawaguchi M, Masui T, Mizumoto M, Yazumi S, Matsumoto S, Takaori K, Morita S, Muto M, Uemoto S, Chiba T. Prognostic model for survival based on readily available pretreatment factors in patients with advanced pancreatic cancer receiving palliative chemotherapy. Int J Clin Oncol. 2016;21(1):118–125. doi: 10.1007/s10147-015-0864-x. [DOI] [PubMed] [Google Scholar]
- 19.McDonald AC, Vira MA, Vidal AC, Gan W, Freedland SJ, Taioli E. Association between systemic inflammatory markers and serum prostate-specific antigen in men without prostatic disease - the 2001-2008 National Health and Nutrition Examination Survey. Prostate. 2014;74(5):561–567. doi: 10.1002/pros.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taverna G, Pedretti E, Di Caro G, Borroni EM, Marchesi F, Grizzi F. Inflammation and prostate cancer: friends or foe. Inflamm Res. 2015;64(5):275–286. doi: 10.1007/s00011-015-0812-2. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto M, Matsumura N, Ohzeki T, Hongo S, Sugimoto K, Shimizu N, Mori Y, Minami T, Nozawa M, Nose K, Tahara H, Yoshimura K, Uemura H. The change in neutrophil lymphocyte ratio from the first to the last repeat prostate biopsy proposed as a marker of carcinogenesis. Urol Int. 2018;101(1):74–79. doi: 10.1159/000489400. [DOI] [PubMed] [Google Scholar]
- 22.Gokce MI, Hamidi N, Suer E, Tangal S, Huseynov A, Ibiş A. Evaluation of neutrophil-to-lymphocyte ratio prior to prostate biopsy to predict biopsy histology: Results of 1836 patients. Can Urol Assoc J. 2015;9(11-12):E761–E765. doi: 10.5489/cuaj.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caglayan V, Onen E, Avci S, Sambel M, Kilic M, Oner S, Aydos MM, Yıldız HE. Lymphocyte-to-monocyte ratio is a valuable marker to predict prostate cancer in patients with prostate specific antigen between 4 and 10 ng/dl. Arch Ital Urol Androl. 2019;90(4):270–275. doi: 10.4081/aiua.2018.4.270. [DOI] [PubMed] [Google Scholar]
- 24.Yuksel OH, Urkmez A, Akan S, Yldirim C, Verit A. Predictive value of the platelet-to-lymphocyte ratio in diagnosis of prostate cancer. Asian Pac J Cancer Prev. 2015;16(15):6407–6412. doi: 10.7314/apjcp.2015.16.15.6407. [DOI] [PubMed] [Google Scholar]