Abstract
Background/Aim: The aim of this study is to identify and describe randomized controlled studies evaluating the therapeutic effect of EPA and DHA supplementation in companion animal diseases. Materials and Methods: A systematic search was conducted in PubMed database and the information collected was summarized and evaluated according to the risk of bias, using the revised Cochrane tool (RoB2). Results: Twenty-three studies were eligible for inclusion: twenty performed in dogs and three in cats. A therapeutic benefit was found in canine allergic dermatitis, haircoat disorder, keratoconjunctivitis sicca, valvular disease, and canine and feline osteoarthritis. Dogs diagnosed with chronic heart failure and lymphoma and cats with allergic dermatitis also seem to benefit from supplementation with omega-3 fatty acids, but studies with improved methodological quality are needed to strengthen this evidence. Conclusion: EPA and DHA supplementation has proven benefits in the adjuvant treatment of various neoplastic and non-neoplastic diseases in dogs and cats.
Keywords: Eicosapentaenoic acid, EPA, docosahexaenoic acid, DHA, dogs, cats, omega-3 fatty acids, supplementation, review
The use of nutraceuticals in the prevention and treatment of canine and feline diseases has been increasing as a result of a greater awareness of their effects by veterinarians. Omega-3 fatty acids are one of the most frequently prescribed products in small animal practices, mainly in the form of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (1).
EPA and DHA are two types of omega-3 polyunsaturated fatty acids (PUFAs) that are derived from alpha-linolenic acid (ALA) through a metabolic pathway that includes chain elongation and desaturation. Since their bioconversion is very limited in dogs and cats, these omega-3 fatty acids need to be supplied to the animal through its diet (2,3). The main source of EPA and DHA are fish and fish-oil products, such as menhaden, cod liver and salmon oils, even though crustaceans, bivalves, cephalopods and marine algae are also rich sources (4).
Supplementation with EPA and DHA has shown benefits in the treatment of several human disorders, such as diabetes, cardiovascular problems, psychiatric and psychological disturbances, rheumatoid arthritis and even cancer (4). Regarding veterinary patients, inflammatory skin disorders, cardiovascular conditions, renal disease and osteoarthritis are the main non-neoplastic diseases where the EPA and DHA seem to have the most impact. Omega-3 fatty acid supplementation has also been used in adjuvant treatment of neoplastic diseases, however, the studies reported in these patients are more scarce (5). It is currently assumed that the benefit of these fatty acids is due to their immunomodulatory and lower-inflammatory effects because of its competitive inhibition of the arachidonic acid cascade, whose final metabolites (prostaglandins, thromboxanes and leukotrienes) have a higher pro-inflammatory activity (4). Additionally, they are also precursors of potent anti-inflammatory and pro-resolving mediators (6). The increased circulating levels of EPA and DHA also result in the decreased expression of matrix metalloproteinases (MMP’s) (7,8), interleukins (IL-1 and IL-2) (8), cyclooxygenase-2 (COX-2) (8) and tumour necrosis factor- α (TNF- α) (8), enhancing their effect.
There are several published clinical and experimental studies evaluating the effect of nutraceuticals in either clinically healthy or sick dogs and cats, although a systematic evaluation is lacking in veterinary medicine. Only one systematic review evaluates the effect of various nutraceuticals on a particular disease (9), not the effect of a single nutraceutical on different disorders.
Our purpose is to identify in which canine and feline diseases the therapeutic benefit of EPA and DHA supplementation has been shown to have a proven effect. We believe that with this type of information and level of evidence, we can contribute to a more conscious and safe use of EPA and DHA fatty acids by veterinary doctors and also increase the interest for this scope of research.
Materials and Methods
In order to find studies evaluating the therapeutic effect of EPA and DHA in dogs and cats, the PubMed database was searched on July 13th, 2020, for terms: i) “EPA”, ii) “eicosapentaenoic”, iii) “DHA”, iv) “docosahexaenoic”, v) “fatty acids”, vi) “omega-3”, vii) “dog”, viii) “cat”, ix) “canine” and x) “feline”. Thus, the following search strategy was used: (epa OR eicosapentaenoic OR dha OR docosahexaenoic) AND [(Fatty Acids, Omega-3(MeSH Terms)) OR (Fatty Acids, Omega-3)] AND [(cats(MeSH Terms)) OR (dogs(MeSH Terms)) OR (dog OR canine OR feline OR cat)].
According to the inclusion criteria, studies were eligible if: i) were published in English, French, Portuguese or Spanish; ii) were performed in canine and feline patients diagnosed with a disease or a health problem; iii) evaluated the therapeutic effect of supplementation with the omega-3 fatty acids EPA and DHA; iv) were performed as randomized clinical trials. Regarding the exclusion criteria, studies were rejected if they were: i) performed in humans, rats or other animal species; ii) performed in healthy dogs and cats; iii) evaluated non-spontaneous or experimentally induced diseases; iv) carried out in vitro; v) performed as non-randomized clinical trials. Finally, to ensure that no relevant articles were unidentified, the reference lists of the previous selected articles were scrutinized.
Title and abstract screening were performed by two authors working independently (TRM and ALL) and after the individual selection, disagreements were solved by consensus or through the final decision of a third author (FLQ). Then, data was collected from each study, regarding i) the study population, ii) protocol, iii) outcome measured, iv) supplements or diet composition, v) EPA and DHA content and vi) therapeutic results. For bias assessment, two authors (TRM and HG) used the revised Cochrane risk-of-bias tool for randomized trials (RoB2) (10). Once again, in case of disagreement, a third author had the final decision (FLQ).
The design and writing of this review were based on the guidelines recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (11).
Results
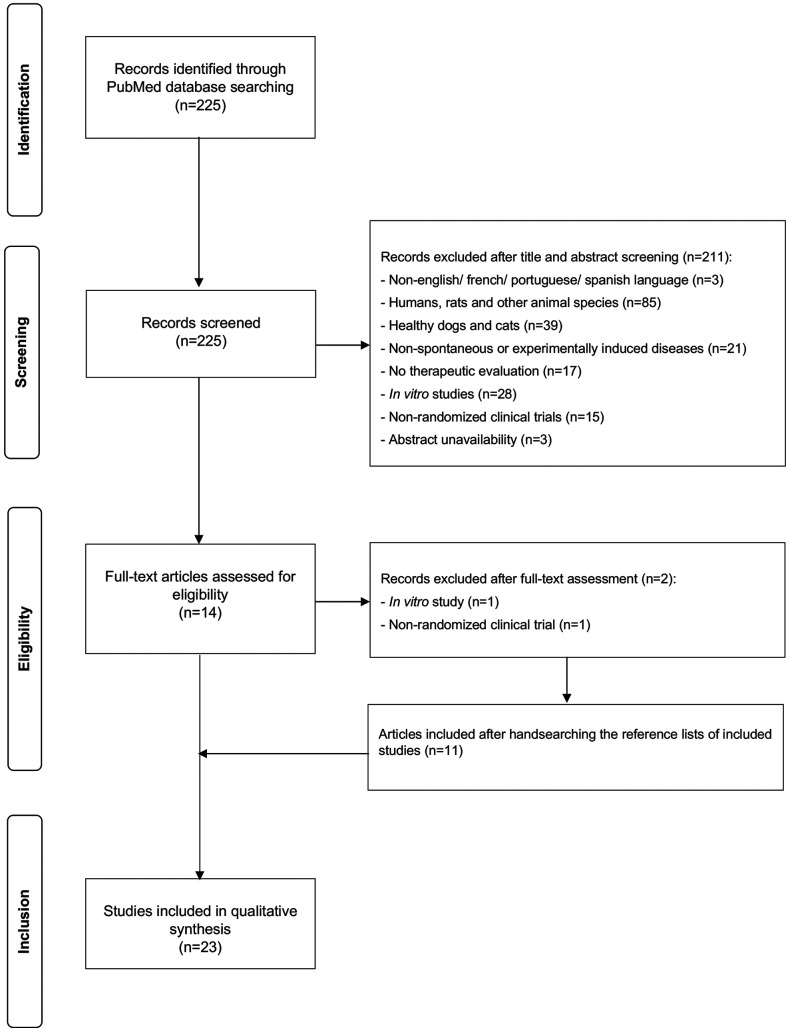
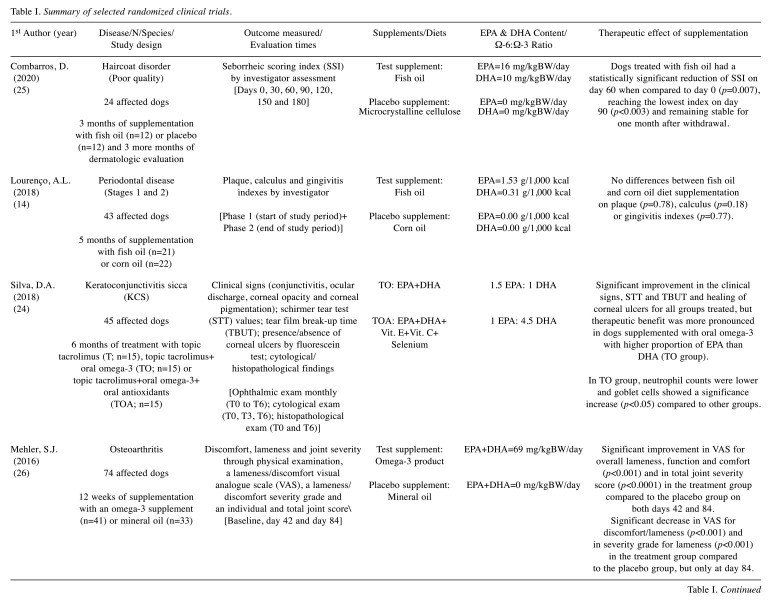
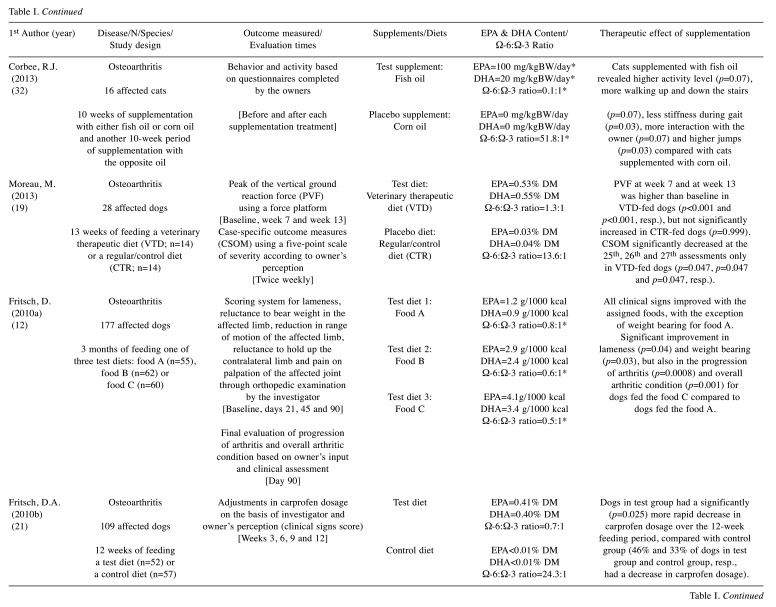
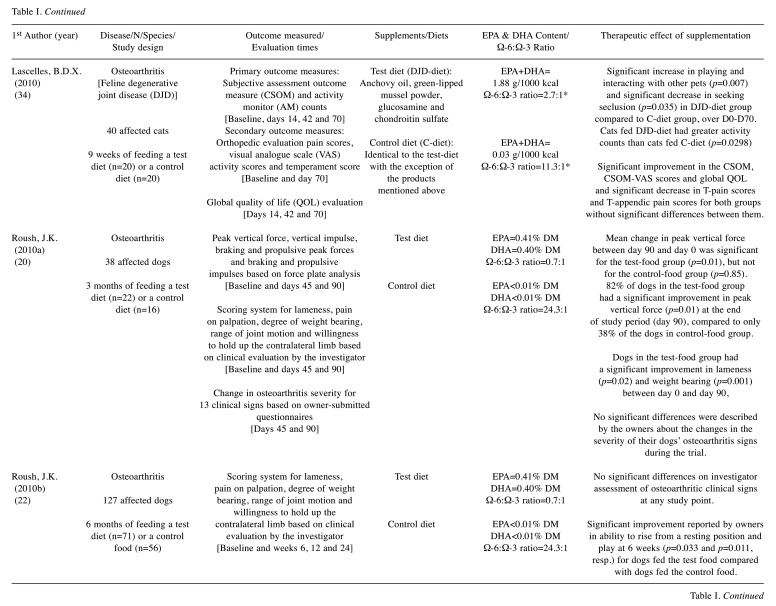
A total of 225 results were identified during the PubMed database search and their respective titles and abstracts were analysed. Based on the inclusion and exclusion criteria, 211 studies were excluded and 14 remained for full-text assessment. Two articles were excluded for not meeting the inclusion criteria. After consulting the reference lists of the originally selected articles, 11 additional manuscripts were considered relevant. Thus, 23 articles were included for final reviewing (Figure 1) from which data were collected and summarized (Table I). Twenty studies were performed in canine patients (12-31) and three studies in feline patients (32-34), with a total of 978 dogs and 68 cats evaluated. In dogs, five types of non-neoplastic diseases were studied: i) allergic dermatitis (13,16,17,27-31), ii) osteoarthritis (12,19-22,26), iii) periodontal disease (14), iv) keratoconjunctivitis sicca (24) and v) cardiovascular disorders (18,23). According to the eligibility criteria, an additional study was reviewed for assessing a health issue, such as a poor quality haircoat, rather than a particular disease (25). Regarding neoplastic diseases, a single study was identified in dogs diagnosed with lymphoma (15). In cats, none of the neoplastic diseases has been evaluated so far; however, two types of non-neoplastic diseases were assessed: i) allergic dermatitis (33) and ii) osteoarthritis (32,34). Overall, cutaneous (13,16,17,25,27-31,33) and osteoarticular disorders (12,19-22,26,32,34) accounted for the largest number of studies included in this review.
Figure 1. PRISMA flowchart of selected studies.
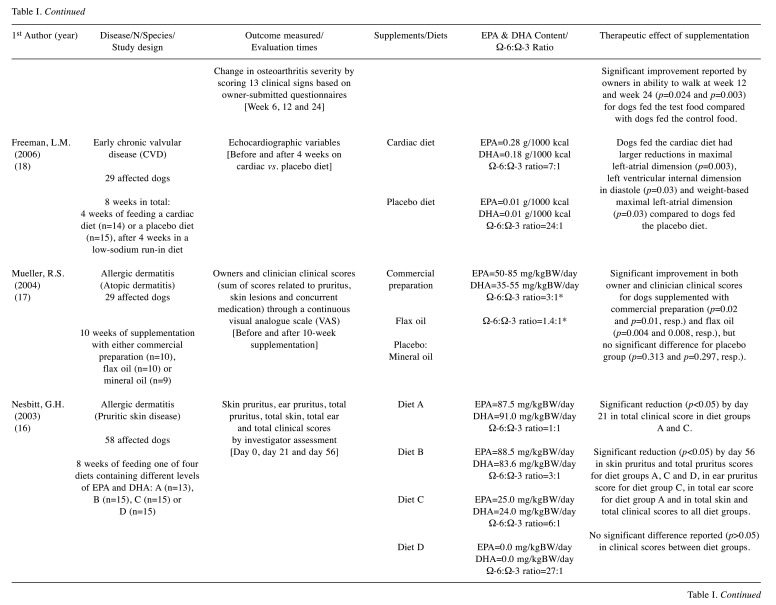
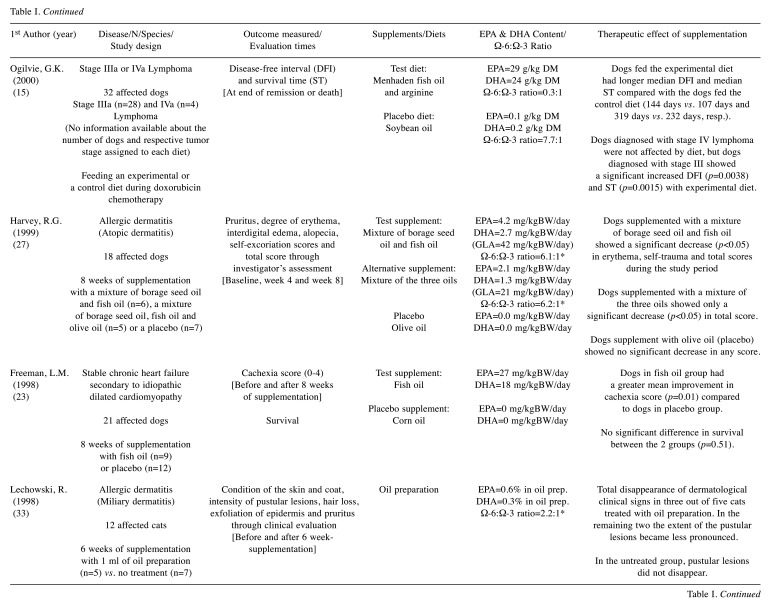
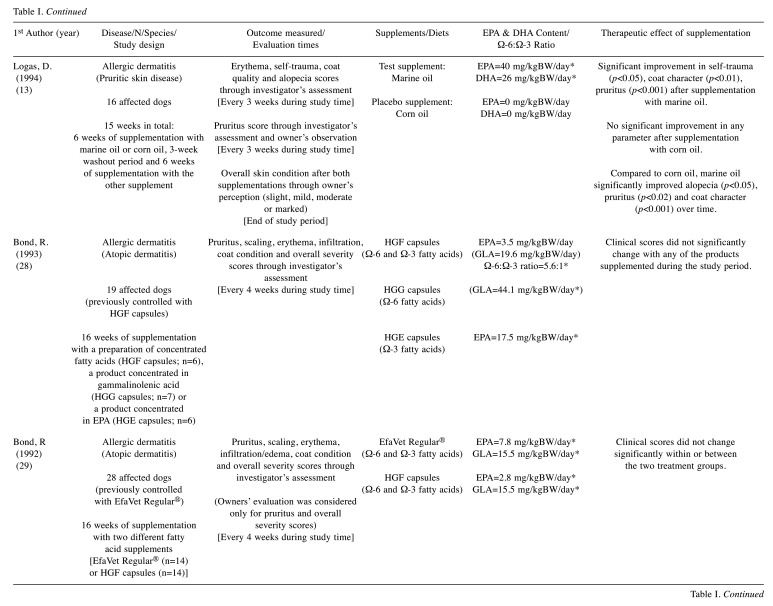
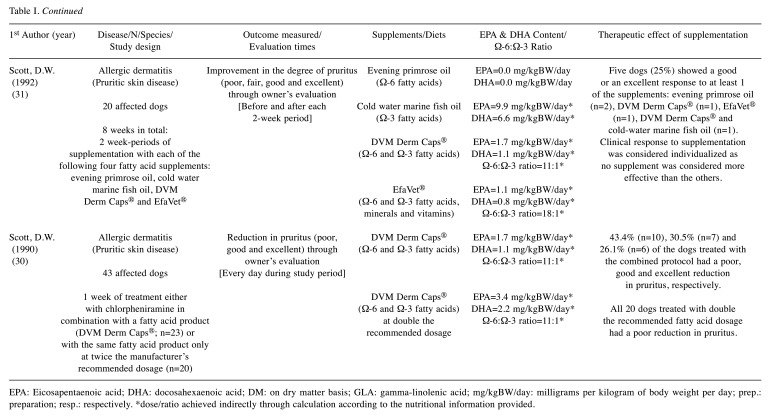
Table I. Summary of selected randomized clinical trials.
EPA: Eicosapentaenoic acid; DHA: docosahexaenoic acid; DM: on dry matter basis; GLA: gamma-linolenic acid; mg/kgBW/day: milligrams per kilogram of body weight per day; prep.: preparation; resp.: respectively. *dose/ratio achieved indirectly through calculation according to the nutritional information provided.
The therapeutic effect of EPA and DHA was addressed according to the information collected and reported by the investigator/clinician (14-16,18,23-26,28,33), the owner (30-32) or both (12,13,17,19-22,27,29,34). The methods most often used in the investigator’s assessment were: i) scoring systems and ii) scales (12-14,16,17,20-23,25-29,34). In addition, patients’ status was assessed through i) subjective clinical evaluation (12,33), ii) objective clinical tests and measurements (18,24), iii) medication adjustment needs (21), iv) visual analogue scale (VAS) (17,26,34), v) force plate analysis (19,20), vi) activity monitor (AM) counts (34) and vii) time periods, such as disease-free interval (DFI) (15) and survival time (ST) (15,23). Regarding the owners’ evaluation, questionnaires were carried out to obtain information on the severity of clinical signs (13,17,19-22,27,34), their overall opinion on the treatment response (12,13,30,31) and quality of life (34), as well as their perception of the clinical condition (29), behavior (32) and activity of their pets (32).
For standardization purposes and to allow a more direct comparison between studies, the daily dosage of EPA and DHA was reported as milligrams per kilogram of body weight (mg/kgBW/day) using i) the information collected directly from the studies (16,17,23,25-28), ii) the calculations performed based on the nutritional data provided (13,29,31,32), and iii) the registered composition of the commercial supplements (30). Unfortunately, this type of information was not provided in all studies, so alternative forms were used, such as grams per 1000 kilocalories (12,14,18,34), grams per kilogram (g/kg) on dry matter basis (15), EPA:DHA ratio (24) and relative percentage on dry matter basis (19-22) or in the oil composition (33).
Only 11 studies provide information on the presence/ absence of adverse effects potentially related to supplementation: 9 trials describe side effects in a small percentage of patients, such as vomiting (12,13,20,32,34), loose stools (30,31), diarrhea (12,26), halitosis (13) and inappetence (20,22), and 2 trials report that animals did not experience any adverse effect at all (25,27).
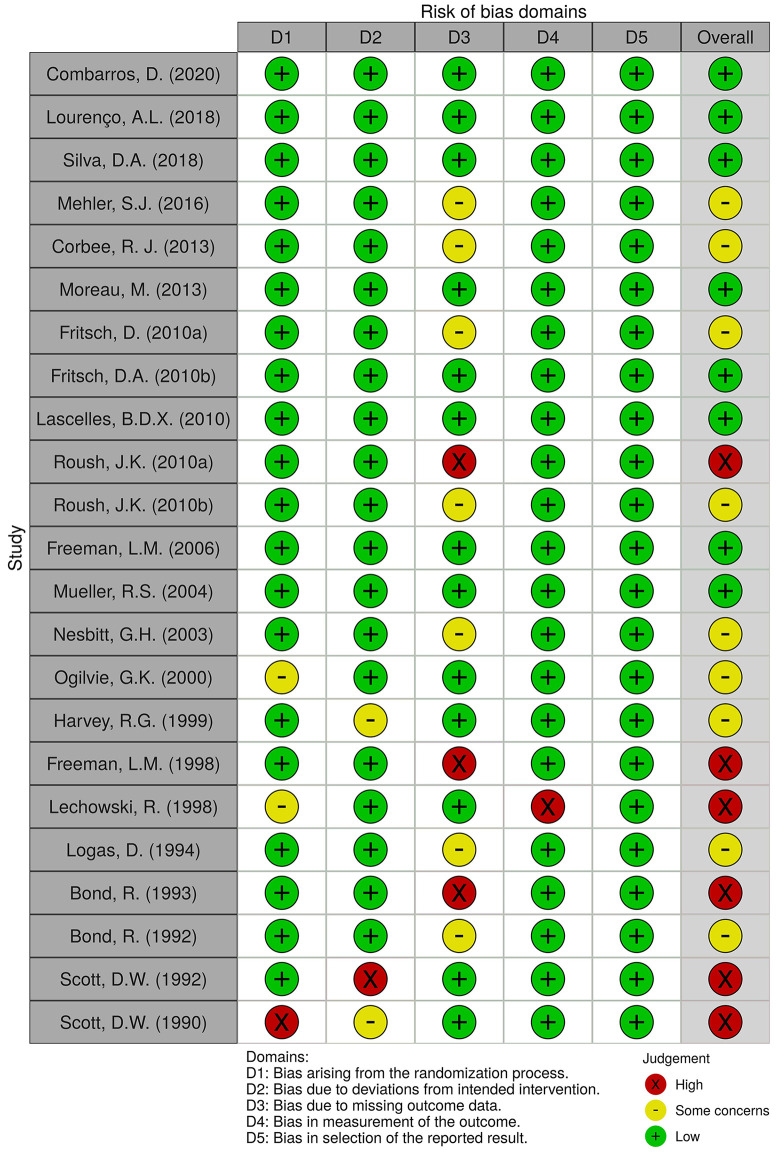
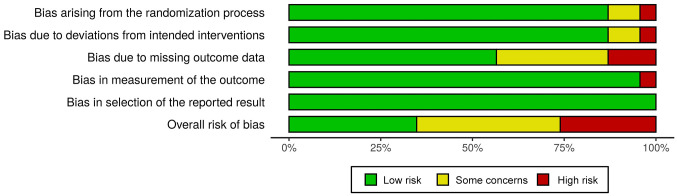
Selected studies were evaluated using the revised Cochrane risk-of-bias tool for randomized trials (RoB2) (10), as represented in Figure 2 and Figure 3, considering the differences between parallel group (12,14-30,33,34) and cross-over trials. Eight studies were associated with a low risk of bias (14,17-19,21,24,25,34), other 9 with having some concerns (12,13,15,16,22,26,27,29,32) and 6 with a high risk of bias (20,23,28,30,31,33).
Figure 2. Risk of bias in selected randomized clinical trials: summary plot.
Figure 3. Risk of bias in selected randomized clinical trials: traffic-light plot.
Discussion
As explored in detail in Table I, several randomized clinical trials have shown the therapeutic impact of a supplement or a diet rich in EPA and DHA fatty acids, based on clinical signs, quality of life and disease progression of affected dogs and cats. Considering these results, the nutraceutical effect of EPA and DHA supplementation on the management and treatment of different diseases or health problems are discussed below.
Neoplastic diseases
Canine lymphoma. Supplementation with EPA and DHA fatty acids has shown benefits in humans diagnosed with cancer, through their metabolic influence on tumor progression and by improving the effectiveness of conventional treatments, such as chemotherapy (35). The same type of effect was expected by Ogilvie et al., (15) who have studied the impact of a diet enriched with EPA and DHA content on the remission and survival time of dogs diagnosed with lymphoma. These dogs were already receiving chemotherapy as the main therapy and the addition of the experimental diet allowed for longer periods of disease-free interval (DFI) and survival time (ST) compared to those obtained with the placebo diet, especially in stage III dogs. Although this study was associated with some concerns, due to the lack of clear information about the selection of patients and the randomization process, the identified relationship between high serum levels of EPA and DHA and this improvement reinforces the idea that dogs diagnosed with lymphoma may benefit from supplementation with these omega-3 fatty acids.
Non-neoplastic diseases
Canine allergic dermatitis. We included in this group all the diseases that promote skin pruritus, such as atopic dermatitis, flea allergy, food allergy and idiopathic pruritic disorder, excluding endocrine or infectious conditions (bacterial, fungal or parasitic). Canine atopy is one of the most frequently diagnosed dermatological diseases in dogs at veterinary centers with a complex and multifactorial pathogenesis that results from the development of hypersensitivity reactions to environmental allergens (36). Although, immunotherapy and treatment with anti-inflammatory and immunomodulatory drugs, such as glucocorticoids, ciclosporin and oclacitinib, are the mainstays of treatment, omega-3 fatty acid supplementation has been described as a viable adjuvant therapy option (36). Regarding the studies included in this systematic review, the main clinical sign assessed was: i) pruritus (13,16,17,27-31), and other dermatological signs, such as ii) erythema (13,16,17,27), iii) alopecia (13,27), iv) self-trauma (13,27), v) edema (16,27), vi) papules (16,17), vii) pustules (16) and viii) crusts (16,17). The majority of the studies (six out of eight) described a significant improvement in the clinical condition of affected dogs after a period of supplementation (13,17,27,30,31) or feeding (16) with omega-3 fatty acids. In the remaining two studies (28,29), dogs were already treated with a commercial product before the trial, so the fact that no clinical difference was noticed with the new supplementation does not mean that the fatty acids had no benefit in alleviating the signs, but only that they did not prove to be better compared to the initial supplement. In fact, we can hypothesize that fatty acids also showed a therapeutic benefit in these individuals, since they were initially treated with a commercial product rich in omega 6 and omega-3 fatty acids and showed either mild clinical signs or none at all, without the need for additional therapy. With respect to bias assessment, only one study was associated with a low risk of bias (17), while four trials were judged as of some concern, due to i) the high percentage of dogs that dropped out during the trial (19-24%), paired with the reasoning for their removal (13,16,29) (different reasons related to health status among the two groups or absence of clear information) and ii) the weakness of the blinding process (27). Moreover, three trials were judged to contain a high risk of bias, due to i) the strong suspicion that the missing outcome data were associated with the effect of supplementation (28), ii) the insufficient “wash-out period” provided (31) and iii) the predictability of the randomization, allocation and blinding processes (30). Despite these limitations, the overall results suggest that supplementation with EPA and DHA fatty acids should be considered as therapeutic agents in skin diseases leading to the clinical manifestation of pruritus, cutaneous inflammation and secondary dermatological lesions.
Canine haircoat disorder. The beneficial effect of EPA and DHA is noted not only on the skin surface, but also on the haircoat, as shown by Combarros et al. (25), through the evaluation of the seborrheic scoring index, in a recent study conducted on dogs with poor coat condition. We judged this trial as a low risk of bias due to its design, detailed information provided and consistency of results. Logas et al. (13) had already reached a similar conclusion at the end of the 20th century, when the haircoat quality of a group of dogs significantly improved after 6 weeks of supplementation with marine oil. Thus, omega-3 fatty acids should be considered for the improvement and maintenance of hair quality. It should also be noted that the therapy must be done for at least 2 months and the clinical effect is expected to remain for one additional month following withdrawal.
Feline allergic dermatitis. Feline allergic dermatitis is a common term used to designate a set of skin diseases caused by hypersensitivity reactions to environmental allergens, flea bites and/or food items. This skin disorder is characterized by pruritus and secondary skin lesions, such as alopecia, erythema, erosions, ulcerations and crusts (37). One of the most common lesion patterns found in cats is miliary dermatitis, which is addressed by Lechowski et al. (33). In this study, the clinical condition of cats diagnosed with this disorder improved after 6 weeks of supplementation with an oil preparation enriched in EPA and DHA omega-3 fatty acids, in comparison with the control group not submitted to any treatment. Although this study has shown clinical improvement with supplementation, the outcome results must be interpreted carefully as we attributed a high risk of bias to this trial due to the small number of cats enrolled (n=12), the unclear allocation process and the subjective evaluation used to measure the outcome. For more robust evidence, further studies are needed, particularly with a larger group of cats and more objective and comparable assessment methods.
Canine and feline osteoarthritis. Osteoarthritis is a common disease in dogs and cats that appears with aging as a result of continuous stress and chronic inflammation in the joints, which limits the animal’s mobility and consequently its quality of life. Although nonsteroidal anti-inflammatory drugs are the most frequent medical approach to control the clinical signs associated with osteoarthritis, other therapeutic options, such as nutraceuticals, have been used for the safe long-term management of these patients (38). Vandeweerd et al. (9) have demonstrated in a systematic review that EPA and DHA fatty acids are the only nutraceuticals with an evident therapeutic benefit in this animal disorder. Supplements (26,32) and diets (12,19-22,34) enriched with these elements are responsible for the improvement of clinical signs, behavior, activity and orthopedic measurements in six trials performed in dogs and two trials in cats diagnosed with osteoarticular conditions. This type of diets is usually also enriched with glucosamine and chondroitin, which raise the doubt if the observed clinical effects are the result of EPA and DHA fatty acids content only. However, as there is no current evidence of the effectiveness of glucosamine and chondroitin in the management of osteoarticular disease (39), we could attribute this therapeutic benefit to the omega-3 fatty acids included in the diet. Regarding the methodological quality of the included studies, three trials are associated with a low risk of bias (19,21,34), while the others are at moderate to high risk of bias. Four studies are associated with some concerns due to the number of animals that were dismissed during the trial and that could have influenced the overall outcome (12,22,26,32). In one study, the reported reasons for withdrawal during the trial differed between the two groups (test diet vs. control diet), so we classified this particular trial with a high risk of bias (20). Nevertheless, in general terms, there is sufficient evidence to consider the benefit of long-chain omega-3 fatty acids, such as EPA and DHA, as adjunctive therapy in canine and feline patients diagnosed with osteoarthritis.
Canine periodontal disease. At two years of age, about eighty percent of dogs already have some degree of periodontal disease (40). Lourenço et al. (14) have failed to prove the hypothesis that supplementation with high levels of EPA and DHA, supplied through fish oil, could delay the progression of this oral disorder. As the authors mentioned, several factors may have influenced the lack of effect on periodontal disease scoring, such as insufficient dosage of EPA and DHA or the fact that there may have been an increase in the gingivitis index due to easier bleeding as a result of reduced platelet aggregation. This phenomenon has been associated with supplementation with omega-3 fatty acids, because particularly EPA promotes an inhibitory effect on the thromboxane A2 synthesis (41). Although there are some promising studies, strong evidence of its effect is lacking in human trials as well (42). Further studies with higher doses of EPA and DHA (similar to those used in the treatment of osteoarticular disease) and with evaluation methods that eliminate the bleeding factor should be performed to confirm the presence or lack of positive effect of these long-chain omega-3 fatty acids supplementation in treating dogs with this disease.
Canine keratoconjunctivitis sicca (KCS). Keratoconjunctivitis sicca (KCS) is an ophthalmic disorder characterized by decreased tear production or increased tear evaporation. KCS has a multifactorial nature, even though an immune-mediated mechanism seems to be the main etiology. Medical treatment is based in the use of artificial tear drops, immunomodulatory drugs, such as topical cyclosporine or tacrolimus, and mucolytic agents and antibiotics, when needed (43). There seems to be a benefit in the use of EPA and DHA fatty acids in the treatment of dry eye disease in humans, however, the evidence is still considered weak (44). Its effect has also been studied in animal species and the therapeutic advantage of topical application of EPA and DHA fatty acids has already been shown through an experimental study conducted in mice (45). Regarding the scope of this review, Silva et al. (24) have described a clinical improvement in canine patients diagnosed with KCS when oral supplementation with long-chain omega-3 fatty acids was added to conventional treatment with topical tacrolimus. This clinical result was superior when a higher proportion of EPA than DHA was provided.
Considering the methodological quality of this study, we judged this trial as a low risk of bias. Therefore, daily administration of these long-chain omega-3 fatty acids should be considered as adjuvant therapy, as it seems to increase the desired anti-inflammatory/immunomodulator effect in these patients.
Canine cardiovascular disorders. The benefit of EPA and DHA supplementation has also been shown in the prevention and treatment of cardiovascular problems in humans (46). The therapeutic effect has also been studied in dogs with stable chronic heart failure and showed an improvement in the management of cardiac cachexia (23). This study has some important limitations, particularly in relation to the number of patients lost to follow-up and their respective imbalance between groups (test supplement vs. placebo supplement), which may have biased the outcome result. Despite the high risk of bias attributed to this study, the effect of EPA and DHA supplementation obtained in this condition is in line with what has been described in human patients (47), which can increase the reliability of these particular results. A more recent trial assessed the effect of a diet enriched with EPA and DHA in canine patients with valvular disease (18) and the results showed an improvement on several echocardiographic variables following 4 weeks of feeding. This second study was evaluated with a low risk of bias, which increases the strength of the evidence. Even so, we cannot rule out the possibility that other diet nutrients, such as arginine, L-carnitine, and taurine, may have influenced the outcome. Thus, more randomized controlled studies focused on these nutraceuticals should be performed and assessing not only echocardiographic findings, but also clinical signs associated with the course and progression of this type of chronic diseases.
In this review we chose to include only randomized controlled trials since this study design is considered the “gold standard” to evaluate the effect of interventions (48). Also, as there are different types of non-randomized studies (NRS), such as non-randomized controlled trials, cohort studies and case series, we considered that adding NRS would increase the heterogeneity of the study selection, making the quality assessment and the respective comparison among studies more difficult. Despite this, we admit the possibility that some relevant information may have been lost due to our methodological decision, since NRS can also contribute valuable clinical evidence. For example, during the study selection process, we found several NRS that evaluated the therapeutic effect of EPA and DHA fatty acids in some of the canine and feline diseases mentioned above, such as canine atopic dermatitis (49,50), but also in other diseases for which there are no randomized controlled studies published yet (51-53). On this issue, we would like to highlight three NRS in particular: i) a pilot study that suggested the benefit of a combination of EPA, DHA, magnesium and zinc in dogs with behavioral disorders (51); ii) a retrospective cohort study that described cats diagnosed with chronic renal failure that showed significantly longer survival times after being fed a kidney diet (particularly with higher EPA content) compared to those that were fed a normal/conventional diet (52); and iii) a blinded, cross-over and placebo-controlled trial that reported no significant difference on seizure frequency and severity between dogs diagnosed with idiopathic epilepsy supplemented with an essential fatty acid product or a placebo (53).
The main limitations of this systematic review are: i) the heterogeneity of prescribed doses, treatment durations and patients’ characteristics among studies; ii) the variability in dosage reporting; and iii) the inherent limitations of the included studies.
In this review, the heterogeneity of the studies included contributed to a more difficult comparison between them, since different animals were treated with a wide range of doses during distinct time periods. Additionally, only canine allergic dermatitis and canine and feline osteoarthritis were represented by more than one study, limiting the available information on the remaining diseases. We also found an imbalance in the literature concerning the number of studies in the two domestic species, which demonstrated the lack of randomized clinical trials in feline patients.
Another problem detected was the variability in reporting the dosage of EPA and DHA fatty acids. We strongly recommend the adoption of a common format to describe the dose of fatty acids used in a given diet or supplement, such as mg/kgBW/day, which could provide uniformity among studies, adequate comparisons between trials and an accurate determination of optimal doses for each disease or health problem diagnosed in dogs and cats. Furthermore, in the supplementation trials, the EPA and DHA content in the supplements should be added to the amount in the diet to reveal the true dosage efficiency. If this information is not disclosed, it may raise the suspicion that there is a great variability in doses, depending on the diet that each owner chooses for their pet. A strategy to improve uniformity is to standardize the food provided during the trial, defining the same diet for all animals studied, as done in some of the included studies (14,23,25,33). Another option is to at least inform about the diets used during the supplementation protocol so as to consider the relative amounts of omega-3 fatty acids (17,26).
Finally, some limitations are inherent to the studies included, such as i) the small number of animals (13,15,17-19,23,25,27-29,31-33), ii) subjective outcome measurements (13,19-22,30-34), iii) confounding influence of other fatty acids (27-31) or other nutraceutical substances (15,18,20-22), iv) lack of data from all enrolled animals (12,22,23,26,28,29) and v) concerns in the randomization, allocation and/or blinding processes (15,27,30,33).
In addition to the above limitations, a major concern is the fact that negative results are not always reported, which contributes to a positive bias in systematic reviews of this nature. For example, in this review we only found three articles (14,28,29) that reported supplementation with none associated beneficial effect on the disease studied. We could maybe hypothesize that different results may have been obtained, but as they were not positive, the research work ended up not being published, leading to the loss of valid and relevant information. Therefore, we would strongly recommend the publication of results of any kind for further scientific evidence about the real effect of these omega-3 fatty acids supplementation in the adjuvant treatment of dogs and cats.
Only 14 trials included in this review have performed fatty acid analysis in blood samples during the study protocol (12,14-16,18,22,23,25,26,28,29,32-34). In our view, this laboratory analysis should be more encouraged in future studies, since it allows investigators to: i) confirm compliance, ii) evaluate serum levels of omega-3 fatty acids reached with supplementation and their respective duration after withdrawal, and iii) establish an association between plasma concentrations of EPA and DHA, different clinical improvements and potential adverse effects.
Regarding the adverse effects of EPA and DHA fatty acids, their supplementation has proved to be a safe therapeutic option, with only a small number of undesirable effects reported. Still, approximately half of the studies included (n=12) did not provide any information regarding this aspect, which could be interpreted as lack of report by the owner, but it may also reflect a non-available information or even a biased way to positively favor the results. Information on adverse effects should be correctly detailed in the results of each trial, as expected in all studies evaluating the supply of a particular product (pharmaco-logical, nutraceutical or other).
Finally, this review allowed to highlight the lack of studies performed in canine and feline patients diagnosed with neoplastic diseases, especially when compared with non-neoplastic diseases, such as cutaneous or osteoarticular disorders, on which more research has been carried out. In view of this knowledge gap, we believe that randomized controlled trials on the effects of long-chain omega-3 fatty acids in cancer treatment of veterinary patients should be encouraged.
To our knowledge, this is the first systematic review that addresses simultaneously the effect of EPA and DHA fatty acids in multiple diseases (neoplastic and non-neoplastic) diagnosed in dogs and cats. We believe this work allows a more clear and objective understanding of the impact of EPA and DHA in small animal practice, encouraging their use by veterinarians and their continued investigation by researchers.
In conclusion, in this systematic review, we assessed the therapeutic effect of EPA and DHA supplementation in dogs and cats diagnosed with a spontaneous disease or health problem, through the information provided by the randomized clinical trials published to date and its respective qualitative analysis.
Taken together, adjuvant prescription of omega-3 fatty acids (EPA and DHA) shows a therapeutic benefit in the management of canine allergic dermatitis, canine haircoat disorder, canine and feline osteoarthritis, canine keratoconjunctivitis sicca and canine valvular disease. Additionally, dogs diagnosed with chronic heart failure and lymphoma and cats affected with allergic dermatitis seem to benefit from this supplementation as well, however, more studies are needed to ascertain its clinical evidence, since the available ones have revealed a moderate to high risk of bias.
Finally, additional randomized controlled trials are needed to corroborate the findings of this review and address other common diseases affecting dogs and cats.
Conflicts of Interest
The Authors declare no conflicts of interest with regards to this study.
Authors’ Contributions
TRM, ALL and FLQ conceived and designed the study; HG defined and conducted the search strategy; TRM, ALL and FLQ screened and analyzed the search results; TRM, HG and FLQ performed the risk of bias assessment; TRM wrote the paper; TRM, ALL, HG and FLQ revised the manuscript.
Acknowledgements
This work was financed by National Funds (FCT/MCTES, Fundação para a Ciência e a Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) under the project UIDB/00211/2020. Authors also want to thank the support received by projects UIDB/04033/2020 and UIDB/CVT/00772/2020, from FCT/MCTES.
References
- 1.Elrod SM, Hofmeister EH. Veterinarians’ attitudes towards use of nutraceuticals. Can J Vet Res. 2019;83(4):291–297. [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer JE. The essential nature of dietary omega-3 fatty acids in dogs. J Am Vet Med Assoc. 2016;249(11):1267–1272. doi: 10.2460/javma.249.11.1267. [DOI] [PubMed] [Google Scholar]
- 3.Bauer JE. Metabolic basis for the essential nature of fatty acids and the unique dietary fatty acid requirements of cats. J Am Vet Med Assoc. 2006;229(11):1729–1732. doi: 10.2460/javma.229.11.1729. [DOI] [PubMed] [Google Scholar]
- 4.Shahidi F, Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol. 2018;9:345–381. doi: 10.1146/annurev-food-111317-095850. [DOI] [PubMed] [Google Scholar]
- 5.Bauer JE. Therapeutic use of fish oils in companion animals. J Am Vet Med Assoc. 2011;239(11):1441–1451. doi: 10.2460/javma.239.11.1441. [DOI] [PubMed] [Google Scholar]
- 6.Weylandt KH, Chiu CY, Gomolka B, Waechter SF, Wiedenmann B. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012;97(3-4):73–82. doi: 10.1016/j.prostaglandins.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Hansen RA, Ogilvie GK, Davenport DJ, Gross KL, Walton JA, Richardson KL, Mallinckrodt CH, Hand MS, Fettman MJ. Duration of effects of dietary fish oil supplementation on serum eicosapentaenoic acid and docosahexaenoic acid concentrations in dogs. Am J Vet Res. 1998;59(7):864–868. [PubMed] [Google Scholar]
- 8.Zainal Z, Longman AJ, Hurst S, Duggan K, Caterson B, Hughes CE, Harwood JL. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):896–905. doi: 10.1016/j.joca.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Vandeweerd JM, Coisnon C, Clegg P, Cambier C, Pierson A, Hontoir F, Saegerman C, Gustin P, Buczinski S. Systematic review of efficacy of nutraceuticals to alleviate clinical signs of osteoarthritis. J Vet Intern Med. 2012;26(3):448–456. doi: 10.1111/j.1939-1676.2012.00901.x. [DOI] [PubMed] [Google Scholar]
- 10.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritsch D, Allen TA, Dodd CE, Jewell DE, Sixby KA, Leventhal PS, Hahn KA. Dose-titration effects of fish oil in osteoarthritic dogs. J Vet Intern Med. 2010;24(5):1020–1026. doi: 10.1111/j.1939-1676.2010.0572.x. [DOI] [PubMed] [Google Scholar]
- 13.Logas D, Kunkle G. Double-blinded crossover study with marine oil supplementation containing high-dose icosapentaenoic acid for the treatment of canine pruritic skin disease. Veterinary Dermatology. 2021;5(3):99–104. doi: 10.1111/j.1365-3164.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 14.Lourenço AL, Booij-Vrieling HE, Vossebeld CB, Neves A, Viegas C, Corbee RJ. The effect of dietary corn oil and fish oil supplementation in dogs with naturally occurring gingivitis. J Anim Physiol Anim Nutr (Berl) 2018;102(5):1382–1389. doi: 10.1111/jpn.12932. [DOI] [PubMed] [Google Scholar]
- 15.Ogilvie GK, Fettman MJ, Mallinckrodt CH, Walton JA, Hansen RA, Davenport DJ, Gross KL, Richardson KL, Rogers Q, Hand MS. Effect of fish oil, arginine, and doxorubicin chemotherapy on remission and survival time for dogs with lymphoma: A double-blind, randomized placebo-controlled study. Cancer. 2000;88(8):1916–1928. [PubMed] [Google Scholar]
- 16.Nesbitt GH, Freeman LM, Hannah SS. Effect of n-3 fatty acid ratio and dose on clinical manifestations, plasma fatty acids and inflammatory mediators in dogs with pruritus. Vet Dermatol. 2003;14(2):67–74. doi: 10.1046/j.1365-3164.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 17.Mueller RS, Fieseler KV, Fettman MJ, Zabel S, Rosychuk RA, Ogilvie GK, Greenwalt TL. Effect of omega-3 fatty acids on canine atopic dermatitis. J Small Anim Pract. 2004;45(6):293–297. doi: 10.1111/j.1748-5827.2004.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 18.Freeman LM, Rush JE, Markwell PJ. Effects of dietary modification in dogs with early chronic valvular disease. J Vet Intern Med. 2006;20(5):1116–1126. doi: 10.1892/0891-6640(2006)20[1116:eodmid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Moreau M, Troncy E, Del Castillo JR, Bédard C, Gauvin D, Lussier B. Effects of feeding a high omega-3 fatty acids diet in dogs with naturally occurring osteoarthritis. J Anim Physiol Anim Nutr (Berl) 2013;97(5):830–837. doi: 10.1111/j.1439-0396.2012.01325.x. [DOI] [PubMed] [Google Scholar]
- 20.Roush JK, Cross AR, Renberg WC, Dodd CE, Sixby KA, Fritsch DA, Allen TA, Jewell DE, Richardson DC, Leventhal PS, Hahn KA. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(1):67–73. doi: 10.2460/javma.236.1.67. [DOI] [PubMed] [Google Scholar]
- 21.Fritsch DA, Allen TA, Dodd CE, Jewell DE, Sixby KA, Leventhal PS, Brejda J, Hahn KA. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(5):535–539. doi: 10.2460/javma.236.5.535. [DOI] [PubMed] [Google Scholar]
- 22.Roush JK, Dodd CE, Fritsch DA, Allen TA, Jewell DE, Schoenherr WD, Richardson DC, Leventhal PS, Hahn KA. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc. 2010;236(1):59–66. doi: 10.2460/javma.236.1.59. [DOI] [PubMed] [Google Scholar]
- 23.Freeman LM, Rush JE, Kehayias JJ, Ross JN Jr, Meydani SN, Brown DJ, Dolnikowski GG, Marmor BN, White ME, Dinarello CA, Roubenoff R. Nutritional alterations and the effect of fish oil supplementation in dogs with heart failure. J Vet Intern Med. 1998;12(6):440–448. doi: 10.1111/j.1939-1676.1998.tb02148.x. [DOI] [PubMed] [Google Scholar]
- 24.Silva DA, Nai GA, Giuffrida R, Sgrignoli MR, Santos DRD, Donadão IV, Nascimento FF, Dinallo HR, Andrade SF. Oral omega 3 in different proportions of EPA, DHA, and antioxidants as adjuvant in treatment of keratoconjunctivitis sicca in dogs. Arq Bras Oftalmol. 2018;81(5):421–428. doi: 10.5935/0004-2749.20180081. [DOI] [PubMed] [Google Scholar]
- 25.Combarros D, Castilla-Castaño E, Lecru LA, Pressanti C, Amalric N, Cadiergues MC. A prospective, randomized, double blind, placebo-controlled evaluation of the effects of an n-3 essential fatty acids supplement (Agepi® ω3) on clinical signs, and fatty acid concentrations in the erythrocyte membrane, hair shafts and skin surface of dogs with poor quality coats. Prostaglandins Leukot Essent Fatty Acids. 2020;159:102140. doi: 10.1016/j.plefa.2020.102140. [DOI] [PubMed] [Google Scholar]
- 26.Mehler SJ, May LR, King C, Harris WS, Shah Z. A prospective, randomized, double blind, placebo-controlled evaluation of the effects of eicosapentaenoic acid and docosahexaenoic acid on the clinical signs and erythrocyte membrane polyunsaturated fatty acid concentrations in dogs with osteoarthritis. Prostaglandins Leukot Essent Fatty Acids. 2016;109:1–7. doi: 10.1016/j.plefa.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Harvey RG. A blinded, placebo-controlled study of the efficacy of borage seed oil and fish oil in the management of canine atopy. Vet Rec. 1999;144(15):405–407. doi: 10.1136/vr.144.15.405. [DOI] [PubMed] [Google Scholar]
- 28.Bond R, Lloyd D. Double-blind comparison of three concentrated essential fatty acid supplements in the management of canine atopy. Veterinary Dermatology. 2021;4(4):185–189. doi: 10.1111/j.1365-3164.1993.tb00216.x. [DOI] [Google Scholar]
- 29.Bond R, Lloyd D. Randomized single-blind comparison of an evening primrose oil and fish oil combination and concentrates of these oils in the management of canine atopy. Veterinary Dermatology. 2021;3(6):215–219. doi: 10.1111/j.1365-3164.1992.tb00176.x. [DOI] [Google Scholar]
- 30.Scott DW, Miller WH Jr. Nonsteroidal management of canine pruritus: Chlorpheniramine and a fatty acid supplement (DVM Derm Caps) in combination, and the fatty acid supplement at twice the manufacturer’s recommended dosage. Cornell Vet. 1990;80(4):381–387. [PubMed] [Google Scholar]
- 31.Scott DW, Miller WH Jr, Decker GA, Wellington JR. Comparison of the clinical efficacy of two commercial fatty acid supplements (EfaVet and DVM Derm Caps), evening primrose oil, and cold water marine fish oil in the management of allergic pruritus in dogs: A double-blinded study. Cornell Vet. 1992;82(3):319–329. [PubMed] [Google Scholar]
- 32.Corbee RJ, Barnier MM, van de Lest CH, Hazewinkel HA. The effect of dietary long-chain omega-3 fatty acid supplementation on owner’s perception of behaviour and locomotion in cats with naturally occurring osteoarthritis. J Anim Physiol Anim Nutr (Berl) 2013;97(5):846–853. doi: 10.1111/j.1439-0396.2012.01329.x. [DOI] [PubMed] [Google Scholar]
- 33.Lechowski R, Sawosz E, Kluciński W. The effect of the addition of oil preparation with increased content of n-3 fatty acids on serum lipid profile and clinical condition of cats with miliary dermatitis. Zentralbl Veterinarmed A. 1998;45(6-7):417–424. doi: 10.1111/j.1439-0442.1998.tb00844.x. [DOI] [PubMed] [Google Scholar]
- 34.Lascelles BD, DePuy V, Thomson A, Hansen B, Marcellin-Little DJ, Biourge V, Bauer JE. Evaluation of a therapeutic diet for feline degenerative joint disease. J Vet Intern Med. 2010;24(3):487–495. doi: 10.1111/j.1939-1676.2010.0495.x. [DOI] [PubMed] [Google Scholar]
- 35.Nabavi SF, Bilotto S, Russo GL, Orhan IE, Habtemariam S, Daglia M, Devi KP, Loizzo MR, Tundis R, Nabavi SM. Omega-3 polyunsaturated fatty acids and cancer: Lessons learned from clinical trials. Cancer Metastasis Rev. 2015;34(3):359–380. doi: 10.1007/s10555-015-9572-2. [DOI] [PubMed] [Google Scholar]
- 36.Saridomichelakis MN, Olivry T. An update on the treatment of canine atopic dermatitis. Vet J. 2016;207:29–37. doi: 10.1016/j.tvjl.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Hobi S, Linek M, Marignac G, Olivry T, Beco L, Nett C, Fontaine J, Roosje P, Bergvall K, Belova S, Koebrich S, Pin D, Kovalik M, Meury S, Wilhelm S, Favrot C. Clinical characteristics and causes of pruritus in cats: A multicentre study on feline hypersensitivity-associated dermatoses. Vet Dermatol. 2011;22(5):406–413. doi: 10.1111/j.1365-3164.2011.00962.x. [DOI] [PubMed] [Google Scholar]
- 38.Rychel JK. Diagnosis and treatment of osteoarthritis. Top Companion Anim Med. 2010;25(1):20–25. doi: 10.1053/j.tcam.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Aragon CL, Hofmeister EH, Budsberg SC. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J Am Vet Med Assoc. 2007;230(4):514–521. doi: 10.2460/javma.230.4.514. [DOI] [PubMed] [Google Scholar]
- 40.Niemiec BA. Periodontal disease. Top Companion Anim Med. 2008;23(2):72–80. doi: 10.1053/j.tcam.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 41.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71(1 Suppl):343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 42.Azzi D, Viafara J, Zangeronimo M, Ribeiro lima R, Marques L, Pereira L. N-3 ingestion may modulate the severity of periodontal disease? Systematic review. Critical Reviews in Food Science and Nutrition. 2020;58(11):1937–1942. doi: 10.1080/10408398.2017.1278677. [DOI] [PubMed] [Google Scholar]
- 43.Dodi PL. Immune-mediated keratoconjunctivitis sicca in dogs: Current perspectives on management. Vet Med (Auckl) 2015;6:341–347. doi: 10.2147/VMRR.S66705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Downie LE, Ng SM, Lindsley KB, Akpek EK. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst Rev. 2019;12:CD011016. doi: 10.1002/14651858.CD011016.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126(2):219–225. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- 46.Jain AP, Aggarwal KK, Zhang PY. Omega-3 fatty acids and cardiovascular disease. Eur Rev Med Pharmacol Sci. 2015;19(3):441–445. [PubMed] [Google Scholar]
- 47.von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: Mechanisms and therapies. Nat Rev Cardiol. 2017;14(6):323–341. doi: 10.1038/nrcardio.2017.51. [DOI] [PubMed] [Google Scholar]
- 48.Hariton E, Locascio JJ. Randomised controlled trials - the gold standard for effectiveness research: Study design: randomised controlled trials. BJOG. 2018;125(13):1716. doi: 10.1111/1471-0528.15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bond R, Lloyd DH. Combined treatment with concentrated essential fatty acids and prednisolone in the management of canine atopy. Vet Rec. 1994;134(2):30–32. doi: 10.1136/vr.134.2.30. [DOI] [PubMed] [Google Scholar]
- 50.Abba C, Mussa PP, Vercelli A, Raviri G. Essential fatty acids supplementation in different-stage atopic dogs fed on a controlled diet. J Anim Physiol Anim Nutr (Berl) 2005;89(3-6):203–207. doi: 10.1111/j.1439-0396.2005.00541.x. [DOI] [PubMed] [Google Scholar]
- 51.Rahimi Niyyat M, Azizzadeh M, Khoshnegah J. Effect of supplementation with omega-3 fatty acids, magnesium, and zinc on canine behavioral disorders: Results of a pilot study. Top Companion Anim Med. 2018;33(4):150–155. doi: 10.1053/j.tcam.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Plantinga EA, Everts H, Kastelein AM, Beynen AC. Retrospective study of the survival of cats with acquired chronic renal insufficiency offered different commercial diets. Vet Rec. 2005;157(7):185–187. doi: 10.1136/vr.157.7.185. [DOI] [PubMed] [Google Scholar]
- 53.Matthews H, Granger N, Wood J, Skelly B. Effects of essential fatty acid supplementation in dogs with idiopathic epilepsy: A clinical trial. Vet J. 2012;191(3):396–398. doi: 10.1016/j.tvjl.2011.04.018. [DOI] [PubMed] [Google Scholar]