Highlights
-
•
First Case of hematogenously metastasized sclerosing epithelioid fibrosarcoma arising primarily in the cervix uteri.
-
•
Tumor cells were strongly and diffusely positive for MUC4.
-
•
Tumor showed a rare EWSR1-CREB3L2 gene fusion.
Keywords: Sclerosing, Epithelioid, Fibrosarcoma, Uterine, Cervix, SEF
1. Introduction
Sarcoma of the uterine cervix is a rare entity and accounts only for about 1% of cervical neoplasms. Carcinosarcomas are the most common subtype representing ~40% of cases, followed by adenosarcomas and leiomyosarcomas (each representing 21% of cases) (Bansal et al., 2010).
Sclerosing epithelioid fibrosarcoma is a rare variant of fibrosarcomas first described in 1995 (Meis-Kindblom, Kindblom, and Enzinger, 1995). Immunohistochemically, most cases show cytoplasmic staining for glycoprotein mucin 4 (MUC4) (Doyle et al., 2012). On a molecular level, they commonly display a EWSR1-CREB3L1 fusion. However, rare cases of EWSR1-CREB3L2 and FUS-CREB3L2 (among others) rearrangements have been described (Prieto-Granada et al., 2015, Tsuda et al., 2020).
We report the first case of a hematogenously metastasized sclerosing epithelioid fibrosarcoma arising primarily in the cervix uteri with a rare EWSR1-CREB3L2 gene fusion.
2. Case presentation
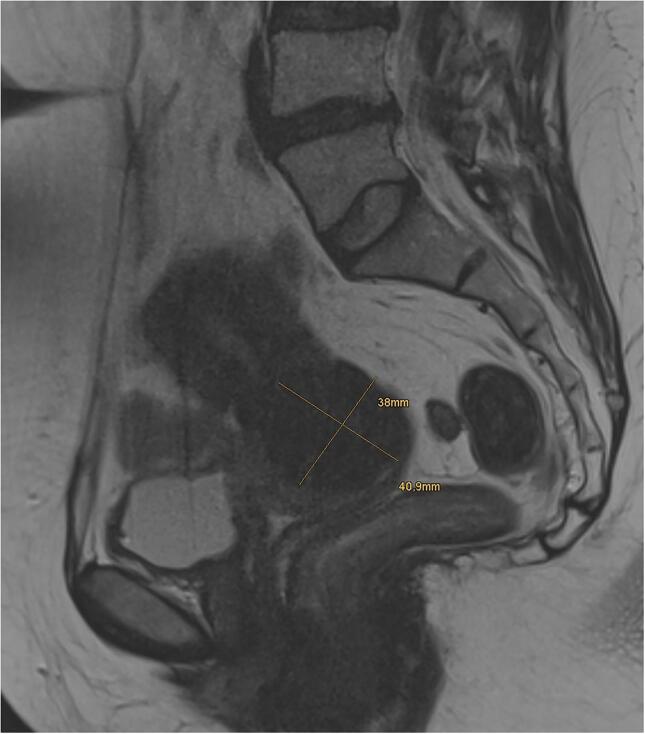
The patient is a 44 year-old woman referred to our tertiary referral center from a secondary care hospital with a lesion of the uterine cervix. Initial biopsy performed at the secondary care hospital was sent for reference pathology. Diagnosis was spindle cell lesion, not otherwise specified. MRI showed a 3.8 × 4.0 cm T2 hypointense lesion (Fig. 1). Subsequently, she underwent a radical hysterectomy with adnexectomy.
Fig. 1.
Sagittal T2 weighted MRI of the pelvis showing a 3.8 × 4.0 cm mass.
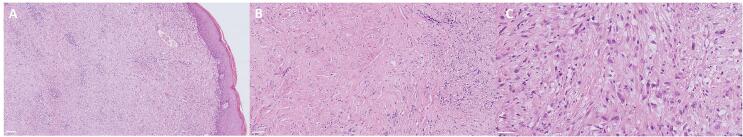
Macroscopically, the specimen showed a 4.5 cm measuring ill-defined nodule in the uterine cervix with a white whorled surface extending to the over lining dorsal serosa. Microscopically, the tumor was composed round-to-plump spindle cells intermixed with less cellular, abundantly hyalinized stroma. The tumor cells mostly grew in cords and nests and had mild cytologic atypia, clear cytoplasm, and inconspicuous nucleoli. Just focal areas showed slightly higher-grade cytologic atypia. No mitotic activity or necrosis was seen (Fig. 2).
Fig. 2.
Low (A), medium (B) and high (C) magnification H&E stains demonstrating tumor cells growing in cords and nests with mild cytologic atypia, inconspicuous nucleoli, no mitotic activity, and no necrosis with overlaying regular cervical squamous epithelium. Scale bars: (A) 100 µm; (B) 50 µm; (C) 50 µm.
Immunohistochemically, the tumor cells stained negative for S-100, β-Catenin, HMB45, Melan A, CD10, caldesmon, actin, desmin, STAT6, CD34 and pancytokeratin AE1/AE3. Epithelial membrane antigen (EMA) was focally positive. The proliferative activity measured by Ki67 was 1%.
To test for the differential diagnosis of low-grade fibromyxoid sarcoma, FISH analysis for FUS break apart was performed showing no FUS rearrangement.
Diagnosis was mesenchymal tumor of the uterine cervix, locally completely excised, no further subclassification possible.
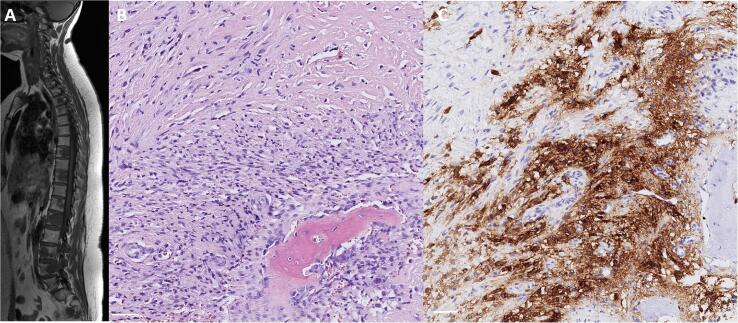
Three years later the patient presented with lower back pain. MRI showed multiple T2 hyperintense, T1 hypointense bone, pulmonary, and lymph node lesions. A diagnostic bone biopsy revealed a spindle cell tumor with only mild atypia and epithelioid tumor cells similar to the one in the uterine cervix. The tumor cells stained strongly and diffusely for MUC4 (Fig. 3). EMA was focally weakly positive. The tumor stained negative for CD34, S-100, desmin, and pancytokeratin AE1/AE3. Proliferation activity measured by Ki67 was focally up to 10%.
Fig. 3.
Sagittal T1 weighted MRI showing multiple vertebral lesions (A). Bone biopsy revealed a spindle cell tumor with only mild atypia and epithelioid tumor cells (B) and strong and diffuse positivity for MUC4 (C). Scale bars: 50 µm.
Taking the multiple lesions into account, fusion analysis of the primary cervix tumor was performed using the ArcherDX FusionPlex sarcoma kit (30 target genes) and Illumina NextSeq 550. A EWSR1 (exon 19) - CREB3L2 (intron 6) fusion was detected with genomic location of breakpoint being chr22:29683123 and chr7:137590578.
Knowing the clinical history, the strong immunoreaction for MUC4, and the EWSR1-CREB3L2 fusion, the cervical tumor as well as the bone lesion were classified as sclerosing epithelioid fibrosarcoma. Subsequently the patient received 6 cycles of chemotherapy with doxorubicin for her metastatic disease as well as 20 Gy radiation therapy for her bone lesions. Last follow-up CT scan showed stable metastatic disease. The patient is follow-upped regularly every three months.
3. Discussion
We report a case of hematogenously metastasized sclerosing epithelioid fibrosarcoma arising primarily in the uterine cervix. Immunohistochemically, the tumor showed an overexpression of MUC4. On a molecular level, the tumor displayed a EWSR1-CREB3L2 rearrangement.
In general, sarcomas of the uterine cervix are rare with carcinosarcomas, adenosarcomas and leiomyosarcomas being the most common subtypes (Bansal et al., 2010). Our case showed a bland looking spindle cell tumor in the uterine cervix. Differential diagnosis included low-grade fibromyxoid sarcoma, solitary fibrous tumor, malignant peripheral nerve sheet tumor, and leiomyosarcoma, as well as a benign lesion. Sclerosing epithelioid fibrosarcomas have been described mainly in the bone, and deep soft tissue of the lower extremities, limb girdles or trunk. However, rare cases of sclerosing epithelioid fibrosarcoma involving coecum (Frattini et al., 2007), pancreas (Kramer et al., 2020), liver (Tomimaru et al., 2009), kidney (Argani et al., 2015) and uterine corpus (Braun et al., 2019), but not the uterine cervix, have been reported. The clinical presentation (primary tumor manifestation in the uterine cervix with surgical excision and bone lesions three years later) argues against the assumption of a primary osseous and secondary uterine tumor. Although usually distinguishable on standard histology from low-grade fibromyxoid sarcomas, they show in some cases morphological overlap. In this setting hybrid low-grade fibromyxoid sarcoma/sclerosing epithelioid fibrosarcoma have been identified and authors have proposed that these entities are a spectrum of the same disease (Prieto-Granada et al., 2015). On standard histology, sclerosing epithelioid fibrosarcoma and low-grade fibromyxoid sarcoma show a relatively low-grade histology. Both entities typically exhibit an upregulation of MUC4. A cytoplasmic positivity for MUC4 on immunohistochemistry can help to distinguish sclerosing epithelioid fibrosarcoma from other neoplasms with epithelioid morphology and low-grade fibromyxoid sarcoma from other bland spindle cell neoplasms. However, a small proportion of tumors are negative for MUC4. In appropriate cases, and to objectify a MUC4-positive tumor, molecular analyses should be considered. The broad RNA-based fusion analyses are superior to the classical FISH analyses, because rare fusions and unknown fusion partners can also be detected. On a molecular level, low-grade fibromyxoid sarcomas most commonly show a FUS-CREB3L1 or FUS-CREB3L2 gene fusion, (Matsuyama et al., 2006, Mertens et al., 2005) whereas sclerosing epithelioid fibrosarcomas mainly show a EWSR1 (or PAX5)-CREB3L1 fusion (Warmke and Meis 2020). Rare cases of sclerosing epithelioid fibrosarcoma with a EWSR1-CREB3L2 fusion have also been reported (Prieto-Granada et al., 2015).
Sclerosing epithelioid fibrosarcoma is a rare malignant soft tissue tumor with a relatively bland cytologic appearance, but an aggressive clinical behavior. These tumors show a high rate of local recurrence and metastasis to the lungs and bones (Antonescu et al., 2001). Only a limited responsiveness to conventional chemotherapy is described (Chew et al., 2018).
In summary, we present the first case of a hematogenously metastasized sclerosing epithelioid fibrosarcoma arising primarily in the cervix uteri with a rare EWSR1-CREB3L2 gene fusion.
4. Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request
CRediT authorship contribution statement
Marie-Lisa Eich: Data curation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Bernd Morgenstern: Resources, Writing - review & editing. Michael Puesken: Writing - review & editing. Roberto Pappesch: Formal analysis, Writing - review & editing. Alexander Quaas: Conceptualization, Investigation, Writing - review & editing. Birgid Schoemig-Markiefka: Supervision, Investigation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antonescu C.R., Rosenblum M.K., Pereira P., Nascimento A.G., Woodruff J.M. Sclerosing epithelioid fibrosarcoma: a study of 16 cases and confirmation of a clinicopathologically distinct tumor. Am. J. Surg. Pathol. 2001;25(6):699–709. doi: 10.1097/00000478-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Argani P., Lewin J.R., Edmonds P., Netto G.J., Prieto-Granada C., Zhang L., Jungbluth A.A., Antonescu C.R. Primary renal sclerosing epithelioid fibrosarcoma: report of 2 cases with EWSR1-CREB3L1 gene fusion. Am. J. Surg. Pathol. 2015;39(3):365–373. doi: 10.1097/PAS.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal S., Lewin S.N., Burke W.M., Deutsch I., Sun X., Herzog T.J., Wright J.D. Sarcoma of the cervix: natural history and outcomes. Gynecol. Oncol. 2010;118(2):134–138. doi: 10.1016/j.ygyno.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Braun M., Kuncman W., Szumera-Cieckiewicz A., Kosela-Paterczyk H., Musial J., Jesionek-Kupnicka D., Rys J., Kordek R. Benign-looking primary fibrosarcoma of the uterus. Pol. J. Pathol. 2019;70(2):148–152. doi: 10.5114/pjp.2019.87108. [DOI] [PubMed] [Google Scholar]
- Chew W., Benson C., Thway K., Hayes A., Miah A., Zaidi S., Lee A.T.J., Messiou C., Fisher C., van der Graaf W.T., Jones R.L. Clinical characteristics and efficacy of chemotherapy in sclerosing epithelioid fibrosarcoma. Med. Oncol. 2018;35(11):138. doi: 10.1007/s12032-018-1192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle L.A., Wang W.L., Dal Cin P., Lopez-Terrada D., Mertens F., Lazar A.J., Fletcher C.D., Hornick J.L. MUC4 is a sensitive and extremely useful marker for sclerosing epithelioid fibrosarcoma: association with FUS gene rearrangement. Am. J. Surg. Pathol. 2012;36(10):1444–1451. doi: 10.1097/PAS.0b013e3182562bf8. [DOI] [PubMed] [Google Scholar]
- Frattini J.C., Sosa J.A., Carmack S., Robert M.E. Sclerosing epithelioid fibrosarcoma of the cecum: a radiation-associated tumor in a previously unreported site. Arch. Pathol. Lab. Med. 2007;131(12):1825–1828. doi: 10.1043/1543-2165(2007)131[1825:SEFOTC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kramer S.P., Bowman C.J., Wang Z.J., Sheahon K.M., Nakakura E.K., Cho S.J., Umetsu S.E., Behr S.C. Hybrid low-grade fibromyxoid sarcoma and sclerosing epithelioid fibrosarcoma of the pancreas. J Gastrointest Cancer. 2020;51(3):1025–1029. doi: 10.1007/s12029-020-00369-0. [DOI] [PubMed] [Google Scholar]
- Matsuyama A., Hisaoka M., Shimajiri S., Hayashi T., Imamura T., Ishida T., Fukunaga M., Fukuhara T., Minato H., Nakajima T., Yonezawa S., Kuroda M., Yamasaki F., Toyoshima S., Hashimoto H. Molecular detection of FUS-CREB3L2 fusion transcripts in low-grade fibromyxoid sarcoma using formalin-fixed, paraffin-embedded tissue specimens. Am. J. Surg. Pathol. 2006;30(9):1077–1084. doi: 10.1097/01.pas.0000209830.24230.1f. [DOI] [PubMed] [Google Scholar]
- Meis-Kindblom J.M., Kindblom L.G., Enzinger F.M. Sclerosing epithelioid fibrosarcoma. A variant of fibrosarcoma simulating carcinoma. Am. J. Surg. Pathol. 1995;19(9):979–993. doi: 10.1097/00000478-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Mertens F., Fletcher C.D., Antonescu C.R., Coindre J.M., Colecchia M., Domanski H.A., Downs-Kelly E., Fisher C., Goldblum J.R., Guillou L., Reid R., Rosai J., Sciot R., Mandahl N., Panagopoulos I. Clinicopathologic and molecular genetic characterization of low-grade fibromyxoid sarcoma, and cloning of a novel FUS/CREB3L1 fusion gene. Lab. Invest. 2005;85(3):408–415. doi: 10.1038/labinvest.3700230. [DOI] [PubMed] [Google Scholar]
- Prieto-Granada C., Zhang L., Chen H.W., Sung Y.S., Agaram N.P., Jungbluth A.A., Antonescu C.R. A genetic dichotomy between pure sclerosing epithelioid fibrosarcoma (SEF) and hybrid SEF/low-grade fibromyxoid sarcoma: a pathologic and molecular study of 18 cases. Genes Chromosom. Cancer. 2015;54(1):28–38. doi: 10.1002/gcc.22215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomimaru Y., Nagano H., Marubashi S., Kobayashi S., Eguchi H., Takeda Y., Tanemura M., Kitagawa T., Umeshita K., Hashimoto N., Yoshikawa H., Wakasa K., Doki Y., Mori M. Sclerosing epithelioid fibrosarcoma of the liver infiltrating the inferior vena cava. World J. Gastroenterol. 2009;15(33):4204–4208. doi: 10.3748/wjg.15.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda Y., Dickson B.C., Dry S.M., Federman N., Suurmeijer A.J.H., Swanson D., Sung Y.S., Zhang L., Healey J.H., Antonescu C.R. Clinical and molecular characterization of primary sclerosing epithelioid fibrosarcoma of bone and review of the literature. Genes Chromosom. Cancer. 2020;59(4):217–224. doi: 10.1002/gcc.22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke L.M., Meis J.M. Sclerosing epithelioid fibrosarcoma: a distinct sarcoma with aggressive features. Am. J. Surg. Pathol. 2020 doi: 10.1097/PAS.0000000000001559. [DOI] [PubMed] [Google Scholar]