Abstract
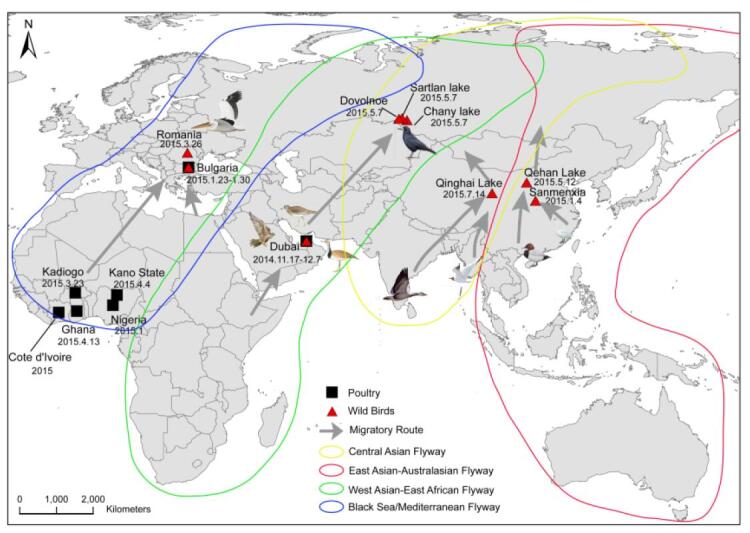
A novel Clade 2.3.2.1c H5N1 reassortant virus caused several outbreaks in wild birds in some regions of China from late 2014 to 2015. Based on the genetic and phylogenetic analyses, the viruses possess a stable gene constellation with a Clade 2.3.2.1c HA, a H9N2-derived PB2 gene and the other six genes of Asian H5N1-origin. The Clade 2.3.2.1c H5N1 reassortants displayed a high genetic relationship to a human H5N1 strain (A/Alberta/01/2014). Further analysis showed that similar viruses have been circulating in wild birds in China, Russia, Dubai (Western Asia), Bulgaria and Romania (Europe), as well as domestic poultry in some regions of Africa. The affected areas include the Central Asian, East Asian-Australasian, West Asian-East African, and Black Sea/Mediterranean flyways. These results show that the novel Clade 2.3.2.1c reassortant viruses are circulating worldwide and may have gained a selective advantage in migratory birds, thus posing a serious threat to wild birds and potentially humans.
Electronic Supplementary Material
Supplementary material is available for this article at 10.1007/s12250-016-3750-4 and is accessible for authorized users.
Keywords: H5N1, highly pathogenic avian influenza virus, Clade 2.3.2.1c, outbreak, migratory birds
Electronic supplementary material
Supplementary material, approximately 3.96 MB.
Supplementary material, approximately 32 KB.
Footnotes
These authors contributed equally to this article.
ORCID: 0000-0002-5595-363X
ORCID: 0000-0002-8717-2942
Contributor Information
Yuhai Bi, Phone: +86-10-64806013, Email: Beeyh@im.ac.cn.
Weifeng Shi, Phone: +86-538-622502, Email: shiwf@ioz.ac.cn.
References
- Bi Y, Zhang Z, Liu W, Yin Y, Hong J, Li X, Wang H, Wong G, Chen J, Li Y, Ru W, Gao R, Liu D, Liu Y, Zhou B, Gao GF, Shi W, Lei F. Highly Pathogenic Avian Influenza A(H5N1) Virus Struck Migratory Birds in China in 2015. Sci Rep. 2015;5:12986. doi: 10.1038/srep12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi YH, Mei K, Shi WF, Liu D, Yu XL, Gao ZM, Zhao LH, Gao GF, Chen JJ, Chen QJ. Two novel reassortants of avian influenza A (H5N6) virus in China. J Gen Virol. 2015;96:975–981. doi: 10.1099/vir.0.000056. [DOI] [PubMed] [Google Scholar]
- Cauldwell AV, Long JS, Moncorgé O, Barclay WS. Viral determinants of influenza A virus host range. J Gen Virol. 2014;95:1193–1210. doi: 10.1099/vir.0.062836-0. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO. 2015). H5N1 HPAI spread in Nigeria and increased risk for neighbouring countries in West Africa. http://www.fao.org/3/ai4561e.pdf.
- Hatta M, Gao P, Halfmann P, Kawaoka Y. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science. 2001;293:1840–1842. doi: 10.1126/science.1062882. [DOI] [PubMed] [Google Scholar]
- Hay AJ, Wolstenholme AJ, Skehel JJ, Smith MH. The molecular basis of the specific anti-influenza action of amantadine. The EMBO J. 1985;4:3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Liu D, Wang M, Yang L, Zhu Q, Li L, Gao GF. Clade 2.3.2 avian influenza virus (H5N1), Qinghai Lake region, China, 2009–2010. Emerg Infect Dis. 2011;17:560–562. doi: 10.3201/eid1703.100948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhung MA, Nelson DI. Outbreaks of avian influenza A (H5N2), (H5N8), and (H5N1) among birds—United States, December 2014-January 2015. MMWR (Morb Mortal Wkly Rep) 2015;64:111. [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xiao H, Lei F, Zhu Q, Qin K, Zhang XW, Zhang XL, Zhao D, Wang G, Feng Y, Ma J, Liu W, Wang J, Gao GF. Highly pathogenic H5N1 influenza virus infection in migratory birds. Science. 2005;309:1206. doi: 10.1126/science.1115273. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Swayne DE, Thomas C, Rameix-Welti M-A, Naffakh N, Warnes C, Altholtz M, Donis R, Subbarao K. Neuraminidase stalk length and additional glycosylation of the hemagglutinin influence the virulence of influenza H5N1 viruses for mice. J Virol. 2009;83:4704–4708. doi: 10.1128/JVI.01987-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok CKP, Yen HL, Yu MYM, Yuen KM, Sia SF, Chan MCW, Qin G, Tu WW, Peiris JSM. Amino acid residues 253 and 591 of the PB2 protein of avian influenza virus A H9N2 contribute to mammalian pathogenesis. J Virol. 2011;85:9641–9645. doi: 10.1128/JVI.00702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monne I, Meseko C, Joannis T, Shittu I, Ahmed M, Tassoni L, Fusaro A, Cattoli G. Highly pathogenic avian influenza A (H5N1) Virus in poultry, Nigeria, 2015. Emerg Infect Dis. 2015;21:1275–1277. doi: 10.3201/eid2107.150421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib MM, Kinne J, Chen H, Chan KH, Joseph S, Wong PC, Woo PC, Wernery R, Beer M, Wernery U, Harder TC. Outbreaks of highly pathogenic avian influenza H5N1 Clade 2.3.2.1c in hunting falcons and kept wild birds in Dubai implicate intercontinental virus spread. J Gen Virol. 2015;96:3212–3212. doi: 10.1099/jgv.0.000274. [DOI] [PubMed] [Google Scholar]
- Pabbaraju K, Tellier R, Wong S, Li Y, Bastien N, Tang JW, Drews SJ, Jang Y, Davis CT, Fonseca K, Tipples GA. Full-genome analysis of avian influenza A(H5N1) virus from a human, North America, 2013. Emerg Infect Dis. 2014;20:887–891. doi: 10.3201/eid2005.140164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–528. doi: 10.1016/0092-8674(92)90452-I. [DOI] [PubMed] [Google Scholar]
- Sakoda Y, Ito H, Uchida Y, Okamatsu M, Yamamoto N, Soda K, Nomura N, Kuribayashi S, Shichinohe S, Sunden Y, Umemura T, Usui T, Ozaki H, Yamaguchi T, Murase T, Ito T, Saito T, Takada A, Kida H. Reintroduction of H5N1 highly pathogenic avian influenza virus by migratory water birds, causing poultry outbreaks in the 2010–2011 winter season in Japan. J Gen Virol. 2012;93:541–550. doi: 10.1099/vir.0.037572-0. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J, Blixt O, Tumpey TM, Taubenberger JK, Paulson JC, Wilson IA. Structure and receptor specificity of the hemagglutinin from an H5N1 influenza virus. Science. 2006;312:404–410. doi: 10.1126/science.1124513. [DOI] [PubMed] [Google Scholar]
- Subbarao EK, London W, Murphy BR. A single amino-acid in the Pb2-Gene of influenza a virus is a determinant of host range. J Virol. 1993;67:1761–1764. doi: 10.1128/jvi.67.4.1761-1764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JH, Herfst S, Fouchier RAM. How a virus travels the world. Science. 2015;347:616–617. doi: 10.1126/science.aaa6724. [DOI] [PubMed] [Google Scholar]
- Wang G, Zhan D, Li L, Lei F, Liu B, Liu D, Xiao H, Feng Y, Li J, Yang B, Yin Z, Song X, Zhu X, Cong Y, Pu J, Wang J, Liu J, Gao GF, Zhu Q. H5N1 avian influenza re-emergence of Lake Qinghai: phylogenetic and antigenic analyses of the newly isolated viruses and roles of migratory birds in virus circulation. J Gen Virol. 2008;89:697–702. doi: 10.1099/vir.0.83419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Peiris M, Chen H, Guan Y. H5N1 outbreaks and enzootic influenza. Emerg Infect Dis. 2006;12:3–8. doi: 10.3201/eid1201.051024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization WHO. Revised and updated nomenclature for highly pathogenic avian influenza A (H5N1) viruses. Influenza Other Respir Viruses. 2014;8:384–388. doi: 10.1111/irv.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Shi Y, Lu XS, Shu YL, Qi JX, Gao GF. An airborne transmissible avian influenza H5 hemagglutinin seen at the atomic level. Science. 2013;340:1463–1467. doi: 10.1126/science.1236787. [DOI] [PubMed] [Google Scholar]
- Zhou B, Pearce MB, Li Y, Wang JR, Mason RJ, Tumpey TM, Wentworth DE. 2013. Asparagine substitution at PB2 residue 701 enhances the replication, pathogenicity, and transmission of the 2009 pandemic H1N1 influenza A virus. PloS One, 8. [DOI] [PMC free article] [PubMed]
- Zhou H, Yu Z, Hu Y, Tu J, Zou W, Peng Y, Zhu J, Li Y, Zhang A, Yu Z. The special neuraminidase stalk-motif responsible for increased virulence and pathogenesis of H5N1 influenza A virus. PLoS One. 2009;4:6277. doi: 10.1371/journal.pone.0006277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material, approximately 3.96 MB.
Supplementary material, approximately 32 KB.


