Abstract
Neuroimaging studies using a variety of techniques have demonstrated abnormal patterns of spontaneous brain activity in patients with essential tremor (ET). However, the findings are variable and inconsistent, hindering understanding of underlying neuropathology. We conducted a meta‐analysis of whole‐brain resting‐state functional neuroimaging studies in ET compared to healthy controls (HC), using anisotropic effect‐size seed‐based d mapping, to identify the most consistent brain activity alterations and their relation to clinical features. After systematic literature search, we included 13 studies reporting 14 comparisons, describing 286 ET patients and 254 HC. Subgroup analyses were conducted considering medication status, head tremor status, and methodological factors. Brain activity in ET is altered not only in the cerebellum and cerebral motor cortex, but also in nonmotor cortical regions including prefrontal cortex and insula. Most of the results remained unchanged in subgroup analyses of patients with head tremor, medication‐naive patients, studies with statistical threshold correction, and the large subgroup of studies using functional magnetic resonance imaging. These findings not only show consistent and robust abnormalities in specific brain regions but also provide new information on the biology of patient heterogeneity, and thus help to elucidate the pathophysiology of ET.
Keywords: essential tremor, functional magnetic resonance imaging, meta‐analysis, psychoradiology, resting‐state
Neuroimaging studies using a variety of techniques have demonstrated abnormal patterns of spontaneous brain activity in patients with essential tremor (ET). However, the findings are variable and inconsistent, hindering understanding of underlying neuropathology. We conducted a meta‐analysis of whole‐brain resting‐state functional neuroimaging studies in ET compared to healthy controls, brain activity in ET is altered not only in the cerebellum and cerebral motor cortex, but also in non‐motor cortical regions including prefrontal cortex and insula.
1. INTRODUCTION
Essential tremor (ET), one of the commonest neurological disorders, is an isolated syndrome of bilateral upper limb postural or kinetic tremor, with or without head tremor or tremor in other locations, in the absence of other neurological symptoms such as dystonia, ataxia, or Parkinsonism. Its prevalence increases with age, especially advanced age (Louis & Ferreira, 2010), and it shows marked clinical, pathological, and etiological heterogeneity (Bhatia et al., 2018; Louis, 2018). The pathogenesis of ET involves genetic and environmental factors (Hopfner & Helmich, 2018), and neuropathological investigation has focused on the cerebellum and cerebellar relays (Louis, 2018). Given the high prevalence of phenocopies, the lack of reliable biomarkers in ET hampers diagnosis (Espay et al., 2017; Hopfner & Deuschl, 2018). It is hoped that a better understanding of its neuropathology will help differentiate discrete causes of the disorder, with clinical biomarkers available to separate the subgroups.
Neuroimaging approaches have the potential to define brain structure and function abnormalities in ET (Bhalsing, Saini, & Pal, 2013). In particular, resting‐state functional magnetic resonance imaging (rs‐fMRI), a well‐established and practical tool for investigating intrinsic brain activity (Raichle & Mintun, 2006), has provided valuable insights into the pathogenesis of neuropsychiatric disorders (Gusnard & Raichle, 2001). In fMRI methods, the blood oxygen level dependent (BOLD) signal indirectly reflects neuronal activity. Compared to task‐related fMRI, rs‐fMRI has the practical advantage of minimizing the influences of compliance and task performance. There are several approaches to analyze spontaneous BOLD signals in rs‐fMRI: amplitude of low frequency oscillations (ALFF) assesses the regional intensity of signal fluctuations; regional homogeneity (ReHo) examines similarities between the signals from nearby voxels; other approaches include independent component analysis (ICA) and four‐dimensional (spatiotemporal) consistency of local neural activity (FOCA). A different imaging approach uses the radiotracer techniques of positron emission tomography (PET) or single‐photon emission computed tomography (SPECT) to measure regional cerebral blood flow (rCBF) or glucose metabolism (rCMglu), which also reflect neuronal activity (Cerasa & Quattrone, 2015; Sharifi, Nederveen, Booij, & Rootselaar, 2014).
Although resting‐state brain studies in ET have revealed abnormal patterns of spontaneous activity in cerebello‐thalamo‐cortical circuitry (Fang et al., 2016; Gallea et al., 2015; Pelzer et al., 2017), the results have been variable and inconsistent. For example, one study in ET patients reported decreased cerebellar activity (Fang et al., 2013), another reported increased cerebellar activity (Li et al., 2020), and another found no cerebellar changes (P. Wang et al., 2018). These differences may be ascribed to study differences in sample sizes, demographic and clinical characteristics of the patients, and image acquisition and analysis protocols. Even though early systematic reviews in ET usefully summarized functional neuroimaging findings (Bhalsing et al., 2013; Sharifi et al., 2014), there has not yet been a quantitative meta‐analysis of whole‐brain resting‐state neuroimaging studies in ET. This is what we set out to do. The emphasis on whole‐brain studies is methodologically important: seed‐based and region‐of‐interest (ROI) studies entail a selection bias in the seed or ROI definition, lack ability to test equally for effects in other brain regions, and use a much lower significance threshold. Therefore, as recommended, we limited meta‐analysis to the results of whole‐brain analysis (Müller et al., 2017). Because there are few functional connectivity studies, and methods vary widely between them, this dimension of brain activity was not examined.
The primary aim of the current meta‐analysis was to identify consistent and reliable functional brain alterations in ET by integrating all eligible studies reporting resting‐state brain activity. We used anisotropic effect size‐signed differential mapping (AES‐SDM), a coordinate‐based meta‐analytic tool (Radua, Mataix‐Cols, et al., 2012) which has been widely applied in neuroimaging studies of neurological disorders including Parkinson's disease (Pan et al., 2017; J. Wang, Zhang, Zang, & Wu, 2018; Suo et al. 2021) and Alzheimer's disease (Jacobs, Radua, Luckmann, & Sack, 2013). The second aim was to perform subgroup meta‐analyses addressing the effects of two important clinical factors, namely medication status and the presence of head tremor, and two methodological factors, the statistical correction threshold employed in studies and the imaging technique used. The third aim was to perform meta‐regression analyses to examine the effects of specific clinical/methodological characteristics, namely age of patients, age at onset, illness duration, and severity and statistical correction for multiple comparisons.
2. METHODS
2.1. Literature search
A comprehensive computerized search was performed in the databases PubMed, Web of Science and Embase using the following key words ALFF <or> ReHo <or> rCBF <or> rCMRglu <or> ASL <or> amplitude of low frequency fluctuations <or> low frequency fluctuations <or> regional homogeneity <or> regional cerebral blood flow <or> regional cerebral metabolic <or> arterial spin labeling <or> PET <or> positron emission tomography <or> SPECT <or> single photon emission computed tomography <or> neuroimaging; ET <or> essential tremor; resting‐state <or> rest <or> resting, covering the period from July 1993 to February 2021. Manual searches were also conducted within the reference lists of identified and review articles. Studies were included according to the following inclusion criteria: (a) employing at least one of fMRI, ASL, PET, or SPECT in the resting state; (b) reporting comparisons of ET patients with healthy controls (HC); (c) including coordinates of the activation areas in stereotactic space (Talairach or Montreal Neurological Institute space); (d) using significance thresholds that were either corrected for multiple comparisons or uncorrected with spatial extent thresholds. Where articles reported multiple independent patient samples, the appropriate coordinates were included as separate studies. Where multiple articles were identified as using overlapping patient datasets, the one with the largest sample and the most comprehensive information was selected. Studies were excluded if (a) stereotactic coordinates of the reported changes in the whole brain were not obtainable; (b) analysis was limited to specific ROI or used seed‐voxel‐based analysis procedures; or (c) studies were case reports, letters, meta‐analysis, or reviews. The study selection procedures are summarized in Figure 1.
FIGURE 1.
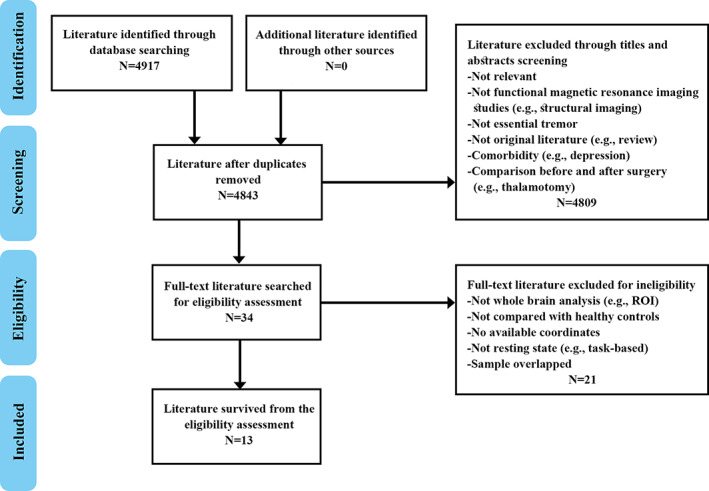
Flowchart of literature search and selection criteria
2.2. Quality assessment
The quality of each selected study was independently assessed by two authors using a 10‐point checklist adapted from previous meta‐analyses (T. Wang et al., 2016). The assessment included the quality of the diagnostic procedures, demographic and clinical characterization, sample size, analysis method and quality of reported results. Each item received a score of 1, 0.5, or 0 according to whether criteria were fully, partially, or not met, respectively. This checklist was used to evaluate the completeness of published studies and provide some objective indication of the rigor of individual studies (see Tables S1 and S2).
2.3. Meta‐analysis
A voxel‐based meta‐analytic approach was conducted using the AES‐SDM software package (http://www.sdmproject.com/software) to analyze regional group differences in brain activity during the resting state. Data extracted from studies included peak coordinates of regions where there were statistically significant group differences at the whole‐brain level, and t‐values or their equivalents (Z‐ or p‐values, which were converted to t‐statistics using the SDM online converter [http://www.sdmproject.com/utilities/?show=Statistics]). If no effect size (t‐, Z‐, or p‐value) was reported, then a “p” was recorded for positive peaks, and “n” for negative peaks. By combining the reported peak coordinates and statistical parameters, AES‐SDM recreates maps of the effect size of group differences in brain activity, calculating both positive and negative differences between groups. Findings of studies that reported no group difference were recreated with anisotropic effect size and variance maps as in any other study, but all voxels were deemed to have a null anisotropic effect size; these were included in the meta‐analysis as usual, thus contributing to the overall meta‐analytic anisotropic effect size. Thresholds were applied (voxel threshold: p ≤ .005, and peak height threshold: peak Z ≥ 1.000) after calculating the meta‐analytic means, with a cluster extent of k > 100. Additional analyses offered by SDM, jackknife, subgroup, and meta‐regression analyses, were used to evaluate the robustness and heterogeneity of results as described below.
2.4. Jackknife sensitivity analysis
A systematic whole‐brain voxel‐based jackknife sensitivity analysis was performed to assess the robustness of the results. The approach is to repeat the analysis over and over, discarding a different study each time. A result is considered replicable if identified alterations in a brain area remain significant in all or most combinations of studies (Radua, Borgwardt, et al., 2012).
2.5. Subgroup analysis
Subgroup analyses were performed to both establish consistency of findings and to ascertain clinical and methodological factors associated with divergent findings. Clinical features of primary interest were studies reporting medication‐naïve and patients with head tremor; methodological features of interest were the use of correction of statistical thresholds for multiple hypothesis testing and studies using fMRI.
2.6. Heterogeneity analysis and publication bias
An inter‐study heterogeneity map was created to identify brain regions in which study findings were more heterogeneous. We examined the statistical (between‐studies) heterogeneity of individual clusters using a random‐effects model with Q statistics, and tested for significance with a permutation approach (uncorrected p < .005). For each cluster with significant ET versus HC differences, we also assessed the asymmetry of funnel plots to examine the possibility of publication bias using the Egger test (Radua et al., 2014).
2.7. Meta‐regression analysis
Finally, meta‐regression analysis was carried out to investigate the potential effects of relevant demographic, clinical, and methodological variables on group differences: mean age, percent of male patients, age at onset, illness duration, Mini‐Mental State Examination (MMSE) score, the illness severity reflected by Fahn–Tolosa–Marin Tremor Rating Scale (FTM‐TRS) score, and statistical correction for multiple comparisons. Data on these variables were extracted from each included study and a more conservative threshold of p < .0005 was adopted to minimize the detection of spurious relationships, and to discard findings in regions other than those detected in the main analyses (Radua, Borgwardt, et al., 2012; Radua & Mataix‐Cols, 2009). Finally, regression plots were visually inspected to discard effects driven by too few studies.
3. RESULTS
3.1. Studies included and sample characteristics
Figure 1 provides a flow diagram showing the screening and selection of studies. A total of 14 datasets from 13 studies (Benito‐León et al., 2015; Czarnecki, Jones, Burnett, Mullan, & Matsumoto, 2011; Fang et al., 2015; Fang et al., 2013; Ha et al., 2015; Jenkins et al., 1993; Li et al., 2020; Song, Park, Chung, & Chung, 2013; L. Wang et al., 2018; P. Wang et al., 2015; P. Wang, Luo, et al., 2018; Wills, Jenkins, Thompson, Findley, & Brooks, 1994; Yin, Lin, Li, Qian, & Mou, 2016) reported 286 patients with ET (mean age 45.0–67.4 years) and 254 HC (mean age 44.4–66.9 years). One of these studies reported multiple independent patient samples (L. Wang, Lei, et al., 2018), comparing ALFF abnormalities in ET patients with and without head tremor with that of HC. One contributed no coordinates as no significant between‐group differences in ALFF were found (P. Wang et al., 2015). Two separate studies used overlapping samples, so we included the studies with the largest sample (Li et al., 2020; Song et al., 2013).
Table 1 summarizes the demographic and clinical characteristics of participants in each study, as well as the neuroimaging methodology used. Of the 13 included studies, eight used fMRI methodology (one with ReHo analysis, four ALFF, two ICA, and one FOCA), four studies used PET or SPECT measurements of rCBF, and one study (Ha et al., 2015) used PET/rCMRglu. The quality scores, ranging from 7.5 to 10 (mean 9.4), show that the included studies were of high quality.
TABLE 1.
Demographic and clinical characteristics of participants in the 13 included studies (14 datasets)
| Study | Modality/analysis | Number (female) | Mean age (y) | Age at onset (y) | Duration (y) | TRS | Medication | Head tremor | Resting tremor | MMSE | Threshold | Quality score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ET | HC | ET | HC | |||||||||||
| Jenkins et al., 1993 | PET/rCBF | 11 (5) | 8 (4) | 63.8 | 57.1 | NA | 25.8 | NA | F | NA | 0 | NA | Corrected | 8.5 |
| Wills et al., 1994 | PET/rCBF | 7 (3) | 6 (NA) | 49.4 | 51.1 | NA | 20.4 | NA | F | 0 | 0 | NA | Corrected | 7.5 |
| Czarnecki et al., 2011 | SPECT/rCBF | 5 (3) | 5 (3) | 67.4 | 61.0 | NA | 12.6 | NA | M | 0 | 0 | NA | Uncorrected | 9 |
| Fang et al., 2013 | rs‐fMRI/ReHo | 20 (8) | 20 (8) | 50.3 | 50.3 | 35.3 | 14.6 | 21.1 | N | 5 | NA | 26.0 | Corrected | 9.5 |
| P. Wang et al., 2015 | rs‐fMRI/ALFF | 7 (3) | 10 (0) | 48.1 | 62.8 | 39.0 | NA | NA | M | NA | NA | NA | Uncorrected | 9.5 |
| Ha et al., 2015 | PET/rCMRglu | 17 (0) | 23 (0) | 67.3 | 65.4 | 57.6 | 9.8 | 15.1 | M | NA | NA | NA | Uncorrected | 9.5 |
| Benito‐León et al., 2015 | rs‐fMRI/ICA | 23 (12) | 22 (12) | 63.3 | 60.6 | NA | 22.9 | 29.3 | NA | NA | NA | NA | Uncorrected | 10 |
| Fang et al., 2015 | rs‐fMRI/ICA | 35 (13) | 35 (13) | 46.8 | 44.4 | 34.0 | 12.8 | 12.7 | N | 5 | 5 | 27.1 | Corrected | 10 |
| Yin et al., 2016 | rs‐fMRI/ALFF | 24 (12) | 23 (11) | 46.4 | 47.2 | 36.8 | 9.6 | NA | N | 7 | NA | NA | Corrected | 10 |
| L. Wang, Lei, et al., 2018 a | rs‐fMRI/ALFF | 20 (13) | 27 (12) | 51.0 | 45.8 | 36.3 | 14.7 | 18.1 | N | 20 | NA | 28.5 | Corrected | 10 |
| 27 (11) | 27 (12) | 45.0 | 45.8 | 32.8 | 12.2 | 17.6 | N | 0 | NA | 28.6 | Corrected | 10 | ||
| P. Wang, Luo, et al., 2018 | rs‐fMRI/FOCA | 17 (7) | 17 (9) | 46.9 | 46.8 | 35.7 | 11.2 | NA | M | 5 | NA | 26.6 | Corrected | 9 |
| Song et al., 2013 | SPECT/rCBF | 23 (14) | 33 (23) | 64.4 | 66.9 | 56.0 | 8.5 | 10.2 | NA | 10 | NA | 27.6 | Uncorrected | 9.5 |
| Li et al., 2020 | rs‐fMRI/ALFF | 50 (34) | 25 (17) | 46.4 | 49.9 | 33.0 | 13.8 | 17.8 | N | 5 | 19 | 23.2 | Corrected | 10 |
Abbreviations: ALFF, amplitude of low‐frequency fluctuation; ET, essential tremor; F, medication‐free; FOCA, four‐dimensional (spatiotemporal) consistency of local neural activities; HC, healthy control; ICA, independent component analysis; MMSE, Mini‐Mental State Exam; Medication status: M, on medication; N, medication‐naive; NA, not available; PET, positron emission tomography; rs‐fMRI, resting‐state functional magnetic resonance imaging; SPECT, single photon emission computed tomography; rCBF, regional cerebral blood flow; ReHo, regional homogeneity; TRS, Fahn‐Tolosa‐Marin Tremor Rating Scale; y, years.
Two data sets included.
3.2. Pooled meta‐analysis
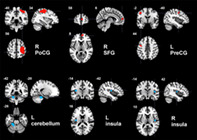
In the pooled meta‐analysis of all included studies, ET patients showed increased resting‐state activity compared to HC in right postcentral gyrus (PoCG) extending anteriorly to include motor cortex and right precentral gyrus (PreCG), and posteriorly to inferior parietal gyri, right superior frontal gyrus (SFG, medial part), and left precentral gyrus (PreCG). ET patients showed decreased activity compared to HC in left cerebellum (including hemispheric lobule IV/V) and bilateral insula (Table 2, Figure 2). Because only one study measured cerebral glucose metabolism (Ha et al., 2015), we repeated the meta‐analysis without it: the results were unchanged, except that the SFG cluster shifted inferiorly to the inferior frontal gyrus in the ET > HC comparison (Table S3).
TABLE 2.
Meta‐analysis results of differences in resting state brain activity between ET and HC
| Brain region | MNI coordinates | SDM Z score | p | No. of voxels | Cluster breakdown (no. of voxels) | Egger's test (p) | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Patients with essential tremor > healthy controls | ||||||||
| R postcentral gyrus | 34 | –40 | 56 | 3.135 | ~0 | 2,209 |
R precentral gyrus, BA 6 (496) R postcentral gyrus, BA 3 (352) R precentral gyrus, BA 4 (250) R inferior parietal (excl. Supramarginal & angular) gyri, BA 40 (242) R postcentral gyrus, BA 4 (172) R inferior parietal (excl. Supramarginal & angular) gyri, BA 2 (141) R postcentral gyrus, BA 2 (132) R superior frontal gyrus, dorsolateral, BA 6 (87) R postcentral gyrus, BA 6 (80) R superior parietal gyrus, BA 2 (56) R superior parietal gyrus, BA 40 (41) R precentral gyrus, BA 3 (31) R postcentral gyrus, BA 40 (20) |
.500 |
| R superior frontal gyrus, medial | 6 | 58 | 8 | 2.027 | .001696348 | 182 |
R superior frontal gyrus, medial, BA 10 (138) R superior frontal gyrus, medial (31) |
.292 |
| L precentral gyrus | −40 | −2 | 44 | 2.343 | .000291586 | 150 |
L precentral gyrus, BA 6 (135) |
.322 |
| Patients with essential tremor < healthy controls | ||||||||
| L cerebellum, hemispheric lobule IV/V | −20 | −42 | −26 | 1.638 | .000543952 | 386 |
L cerebellum, hemispheric lobule IV/V, BA 37 (138) L cerebellum, hemispheric lobule IV/V, BA 30 (92) Middle cerebellar peduncles (60) L fusiform gyrus, BA 37 (42) L cerebellum, hemispheric lobule VI, BA 37 (11) |
.405 |
| L insula | –42 | –14 | 16 | 1.454 | .001908481 | 128 |
L insula, BA 48 (60) L rolandic operculum, BA 48 (46) |
.700 |
| R insula | 42 | –14 | 10 | 1.455 | .001908481 | 102 |
R insula, BA 48 (55) R rolandic operculum, BA 48 (20) R heschl gyrus, BA 48 (14) |
.710 |
Note: Cluster extent threshold: 100 voxels. Regions with fewer than 10 voxels are not reported in the cluster breakdown.
Abbreviations: BA, Brodmann area; ET, essential tremor; HC, healthy controls; L, left; MNI, Montreal Neurological Institute; R, right; SDM, signed differential mapping; SDM‐Z, Seed‐based d Mapping Z score.
FIGURE 2.
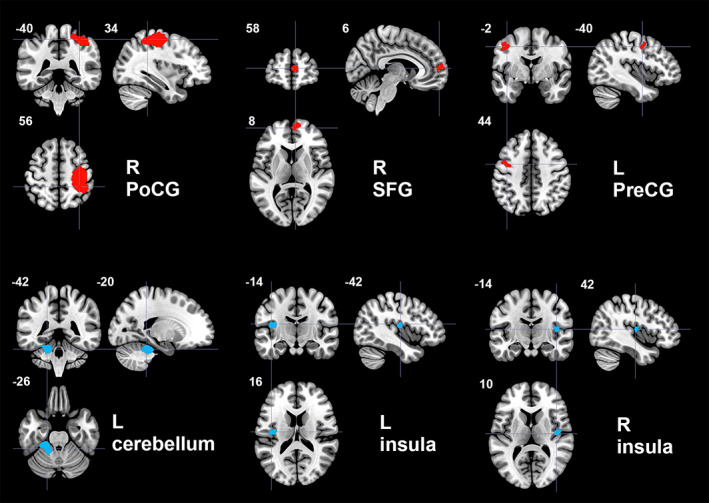
Regions of increased (red color) and decreased (blue color) resting‐state brain activity in patients with essential tremor compared to healthy controls in the pooled meta‐analysis. L, left; PoCG, postcentral gyrus; PreCG, precentral gyrus; R, right; SFG, superior frontal gyrus
3.3. Subgroup meta‐analyses
The results of the subgroup meta‐analyses are presented in the Supporting Information.
The subgroup analysis of patients with head tremor included seven datasets comprising 189 ET patients and 180 HC (Table S4). The results shared four clusters with the results of the pooled meta‐analysis, including increased activity in right PoCG and left PreCG, and decreased activity in the cerebellum and insula. These results are broadly consistent with the main findings. So too were the results of the subgroup meta‐analyses in studies of medication‐naïve patients, studies using threshold correction and fMRI studies (Tables S5, S6, and S7). In addition, we noted decreased activity in cerebellar lobule VI in the subgroup analyses of patients with head tremor and medication‐naïve, respectively.
3.4. Jackknife sensitivity, heterogeneity, and publication bias analysis
In a whole‐brain jack‐knife sensitivity analysis of ET versus HC (Table 3), the findings of increased functional activity in right PoCG of ET patients were highly replicable, being preserved in all combinations of the datasets. Similarly, decreased functional activity in left cerebellum was significant in all but one combination (Fang et al., 2013). Increased functional activity in left PreCG was significant in all but two combinations (Fang et al., 2015; Song et al., 2013). Increased functional activity in right SFG and decreased activity in left insula remained significant in all but three combinations. The results of the pooled meta‐analysis thus showed high replicability and reliability in these regions. The same pattern was seen in all subgroup meta‐analyses (Tables S8–S11).
TABLE 3.
Results of the jackknife sensitivity analysis (No. of datasets: 14)
| Discarded studies | Hyperactivation regions | Hypoactivation regions | ||||
|---|---|---|---|---|---|---|
| R PoCG | R SFG | L PreCG | L cerebellum | L insula | R insula | |
| Jenkins et al., 1993 | Y | Y | Y | Y | Y | Y |
| Wills et al., 1994 | Y | Y | Y | Y | Y | Y |
| Czarnecki et al., 2011 | Y | Y | Y | Y | Y | Y |
| Fang et al., 2013 | Y | Y | Y | N | N | N |
| P. Wang et al., 2015 | Y | Y | Y | Y | Y | Y |
| Ha et al., 2015 | Y | N | Y | Y | Y | Y |
| Benito‐león et al., 2015 | Y | Y | Y | Y | Y | N |
| Fang et al., 2015 | Y | Y | N | Y | Y | Y |
| Yin et al., 2016 | Y | Y | Y | Y | Y | Y |
| L. Wang, Lei, et al., 2018 a | Y | Y | Y | Y | Y | N |
| Y | Y | Y | Y | N | Y | |
| P. Wang, Luo, et al., 2018 | Y | N | Y | Y | Y | Y |
| Song et al., 2013 | Y | N | N | Y | Y | Y |
| Li et al., 2020 | Y | Y | Y | Y | N | N |
| Total Y | 14/14 | 11/14 | 12/14 | 13/14 | 11/14 | 10/14 |
Note: Y, Yes; N, No; “Yes” indicates that the brain regions were significant in the jackknife analysis; “No” indicates that the brain regions were not significant in the jackknife analysis. L, left; R, right; PoCG, postcentral gyrus; SFG, superior frontal gyrus; PreCG, precentral gyrus.
Two data sets included.
In the pooled meta‐analysis, no regions with altered resting‐state functional activity showed significant between‐study heterogeneity. Egger's test was nonsignificant (p > .05 for all comparisons, Table 2), suggesting that there was no publication bias in any cluster.
3.5. Meta‐regression analysis
In regression analyses we examined mean age (available in all studies), percent of male patients (available in all studies), age at onset (available in nine studies), illness duration (available in all but one study), MMSE (available in six studies), and illness severity (available in seven studies): none of these were significantly associated with brain activity measures. We note that linear not nonlinear models were used due to the small sample sizes. In addition, effects in studies using statistical threshold correction did not differ significantly from studies reporting findings with an uncorrected threshold.
4. DISCUSSION
We used AES‐SDM software to perform a comprehensive coordinate‐based meta‐analysis in order to identify the most consistent and reliable alterations in resting‐state brain activity in ET compared with HC. We found increased activity in the right PoCG extending anteriorly to right PreCG and posteriorly to inferior parietal gyri, left PreCG, right SFG, and decreased activity in the left cerebellar hemisphere (lobule IV/V) and bilateral insula. In the subgroup meta‐analyses, decreased activity in cerebellar lobule VI was confirmed in ET patients with head tremor and medication‐naïve subjects. We found no significant correlations with clinical variables.
4.1. The main abnormalities and their potential significance
Our finding of decreased left cerebellar hemisphere activity accords with other evidence of cerebellar pathology in ET: these include postmortem findings of cell loss and axon swelling in cerebellar Purkinje cells (Lin et al., 2014), 1H magnetic resonance spectroscopy findings of decreased cerebellar N‐acetylaspartate/creatine, taken as an index of neuronal degeneration (Louis et al., 2002), and other changes including histopathology (Louis, Faust, & Vonsattel, 2011), electrophysiology (Hellwig et al., 2001), clinical (Louis, Frucht, & Rios, 2009), neuroimaging (Cerasa & Quattrone, 2015; Raethjen & Deuschl, 2012) and therapeutic effects (Popa et al., 2013). A recent study on the structural correlates of the sensorimotor cerebellum in PD and ET points to sensorimotor lobules IV and V as particularly relevant to tremor symptoms (Lopez et al., 2020), and here we also found decreased functional activity in that region. Moreover, the subgroup meta‐analysis of patients with head tremor and medication‐naïve patients revealed decreased activity in the cerebellar hemisphere lobule VI. Lobule VI has been linked to upper extremity somatomotor function, as well as affective and cognitive function (Stoodley & Schmahmann, 2010). As head tremor is often accompanied by upper limb tremor (Louis, 2005), we speculate that abnormal activity of cerebellar lobule VI may contribute to head tremor and perhaps also upper extremity tremor in untreated ET. Future investigations using an ROI approach are needed to explore the specific functions of the different cerebellar regions in ET.
We found significantly increased activity in sensorimotor cortex, including PreCG and PoCG. Electrophysiological studies have identified the motor cortex as an important source of both voluntary physiological movements and involuntary pathological movements, including tremor in ET patients (Raethjen & Deuschl, 2012). Furthermore, the tremor of ET patients can be reduced by subdural stimulation of the motor cortex (Moro et al., 2011). Previous neuroimaging findings of motor cortex in ET include increased functional connectivity between thalamus and motor cortex (Fang et al., 2016), abnormal functional activity in sensorimotor cortex and inferior parietal lobule (Archer et al., 2018), and a variety of structural MRI abnormalities related to motor symptoms (Caligiuri et al., 2017; Louis, 2010; Raethjen, Govindan, Kopper, Muthuraman, & Deuschl, 2007).
A popular theory of ET pathophysiology involves the cerebello‐thalamo‐cortical network, known as the tremor network (Sharifi et al., 2014). Our finding of abnormalities in the cerebellum and sensorimotor cortex are consistent with this theory, and with a recent network‐level connectivity study in ET patients that found abnormal functional connectivity between sensorimotor cortex and cerebellum (DeSimone, Archer, Vaillancourt, & Wagle Shukla, 2019). Notably, we observed decreased activity in cerebellar hemispheres and increased activity in cerebral cortex. A similar pattern has been seen in task‐based fMRI in ET (Neely et al., 2015). It has been suggested that increased cerebral motor cortex activity may represent a compensation for cerebellar degenerative changes in ET (Yin et al., 2016).
Interestingly, our meta‐analysis did not reveal abnormal activity in the thalamus. This relay station for reciprocal projections from cerebellum to cerebral cortex plays an important role in the motor symptoms of ET (Bhalsing et al., 2013; Bucher, Seelos, Dodel, Reiser, & Oertel, 1997; Jenkins et al., 1993; Louis & Ferreira, 2010). Also, stereotactic surgery of the ventral intermediate nucleus of the thalamus is an effective treatment for ET (Flora, Perera, Cameron, & Maddern, 2010). Our overall negative finding has several possible explanations. First, only 4 of the 13 studies included in our meta‐analysis reported changes in this region (Fang et al., 2013; L. Wang, Lei, et al., 2018; P. Wang, Luo, et al., 2018; Wills et al., 1994). Second, there is heterogeneity in the exact location and alterations in different studies: one study reported decreased activity in the mediodorsal and ventral intermediate nuclei (Fang et al., 2013), while another reported increased activity in this region (L. Wang, Lei, et al., 2018). The thalamus is heterogeneous in structure and function, and the resolution of typical resting‐state fMRI studies especially when considered in a meta‐analytic framework may not be sufficient to parse effects that may differ across nuclei. Third, it is noteworthy that intraoperative microelectrode recording does not reveal thalamic activity related to tremor at rest (Hua & Lenz, 2005), suggesting that resting alterations of thalamic neural activity may be restricted to specific nuclei, or more modest than in neocortex and cerebellum.
We found widespread alterations of activity in ET relative to HC in the nonmotor cortices including right SFG (medial) and bilateral insula. These belong to the DMN and limbic system (Roxo, Franceschini, Zubaran, Kleber, & Sander, 2011; Smith et al., 2009), whose dysfunction may lead to various nonmotor symptoms such as cognitive impairment, anxiety and depression that have been associated with ET (Coste, Sadaghiani, Friston, & Kleinschmidt, 2011; Smith et al., 2009). In particular, prefrontal cortex plays a vital role in controlling cognitive processes (Rossi, Pessoa, Desimone, & Ungerleider, 2009), and the insula is also important for a variety of aspects of emotion and cognition (Nagai, Kishi, & Kato, 2007). In line with our findings, task‐based fMRI studies using the Stroop test and working memory paradigms have revealed increased activation of the prefrontal cortex in ET (Cerasa et al., 2010; Passamonti et al., 2011). A diffusion tensor imaging study in ET found that abnormal projections of the insula were significantly related to memory and executive function (Sengul et al., 2020). Thus, regional activity changes in medial frontal cortex and insula may be related to emotional and cognitive dysfunction in patients with ET. It is important to note that patients in our study did not have frank dementia (MMSE score >24), so these findings outside the motor systems are not dementia‐related, but still be sufficient to contribute to neuropsychiatric symptoms in ET patients. The relations of alterations in motor cortex and heteromodal association cortex remain to be understood in mechanistic terms, and developing understanding of how ET induces clinically relevant functional alterations in higher brain functions remains an important research direction.
4.2. Implications of negative findings in meta‐regression analysis
Although no significant correlations were found between clinical variables and brain findings, several factors may potentially impact brain activity, among which illness duration and severity were of particular interest. In the three studies reporting brain activity changes in relation to illness duration and severity, the results vary widely and were too inconsistent to yield a significant effect. Due to the small sample size and heterogeneity of patients with respect to medication, family history and tremor features, our meta‐regression may have lacked sufficient power to detect such effects.
4.3. Limitations and future directions
This meta‐analysis has certain limitations. First, like previous meta‐analyses (Amad, Radua, Vaiva, Williams, & Fovet, 2019; Koch et al., 2016; Kuhn & Gallinat, 2013), we included studies using different neuroimaging methods as long as they were focused on brain activity, in order to provide the most comprehensive overview of resting‐state abnormalities in ET. All included methods reflect intrinsic neural activity, but their different physiological bases and underpinning assumptions may affect the meta‐analysis (Amad et al., 2019). To address this issue, we performed a meta‐analysis including only the fMRI methodological subgroup (which was large enough for separate analysis). The results were much the same as the main analysis. Second, the number of studies (N = 13) and their sample size were relatively small. Although we employed jackknife sensitivity analysis to evaluate the robustness and reliability of the results, caution is necessary in interpretation. Third, despite providing an optimal balance between sensitivity and false positive rate (Pico‐Perez et al., 2020), the default AES‐SDM statistical thresholds based on uncorrected P‐values and the inclusion of studies with uncorrected statistical thresholds may bias the results. However, in the present study, meta‐regression analyses showed that uncorrected thresholds did not significantly affect identified abnormalities. Fourth, coordinate‐based meta‐analysis summarizes reported coordinates instead of working with the original data, which may affect the precision and accuracy of identified spatial location of group differences (Salimi‐Khorshidi, Smith, Keltner, Wager, & Nichols, 2009). Finally, excluding studies that did not report stereotactic coordinates may bias findings in unclear ways.
As is always the case for meta‐analyses, it is possible that as more case–control studies are published in the future, more spatially refined findings and perhaps new findings in additional brain areas may be identified. Also, longitudinal studies that investigate the development of brain activity changes and their relationship to cognitive function will help advance understanding of ET neuropathology as it evolves over time and impacts heteromodal neocortical regions. High resolution functional studies of thalamus, focused on both specific nuclei and relations with cerebellar and neocortical function, would be helpful for understanding circuit level pathology. Future research is needed to determine whether medication therapies alter brain function in a clinically relevant way. Studies might also examine relations to tremor severity (using kinesiological measures of tremor amplitude and frequency), more extensively evaluate neuropsychological functions, and implement multimodal imaging methods to more fully define the pathology of ET.
5. CONCLUSION
This was the first comprehensive meta‐analysis in ET that defined whole‐brain activity alterations, considering 13 published resting‐state functional brain imaging studies. We confirmed that alterations are not only present in the cerebellum and cerebral motor cortex, but also in certain nonmotor cortices including prefrontal cortex and insula. These effects beyond motor systems may contribute to nonmotor neuropsychiatric alterations in ET patients. Clarifying the causes and functional effects of alterations outside motor systems remains an important direction for future studies. These findings have potentially important implications for the systems‐level pathophysiology of ET and their broader neurobehavioral significance. Of note, this study adds to the field of psychoradiology (Sun et al., 2018; Huang et al., 2019; Gong, 2020), an evolving subspecialty of radiology, which is primed to be of major clinical importance in guiding diagnostic and therapeutic decision making in patients with neuropsychiatric disorders.
CONFLICT OF INTERESTS
Dr. Sweeney is a consultant for VersSci. None of the remaining authors have financial conflicts of interest.
Supporting information
Table S1 Quality assessment checklist.
Table S2. Quality assessment details.
Table S3. Meta‐analysis results of differences in resting state brain activity between ET and HC after excluding Ha et al., 2015.
Table S4. Subgroup meta‐analysis results in studies in ET patients with head tremor showing hyper‐ and hypoactivity of brain regions.
Table S5. Subgroup meta‐analysis results in medication‐naïve ET patients showing hyper‐ and hypoactivity of brain regions.
Table S6. Subgroup meta‐analysis results in studies with threshold correction showing hyper‐ and hypoactivity of brain regions.
Table S7. Subgroup meta‐analysis results in studies using fMRI methods showing hyper‐ and hypoactivity of brain regions.
Table S8. Jackknife sensitivity analysis of subgroup meta‐analysis in ET patients with head tremor.
Table S9. Jackknife sensitivity analysis of subgroup meta‐analysis in medication‐naïve ET patients.
Table S10. Jackknife sensitivity analysis of subgroup meta‐analysis in studies with correction threshold.
Table S11. Jackknife sensitivity analysis of subgroup meta‐analysis in studies using fMRI methods.
Table S12. Jackknife sensitivity analysis of meta‐analysis results after excluding Ha et al., 2015.
ACKNOWLEDGMENTS
This research has received funding from the National Natural Science Foundation (Grant Nos. 81621003, 81820108018 and 82001800), the China Postdoctoral Science Foundation (Grant No. 2020M683317), and the Post‐Doctor Research Project, West China Hospital, Sichuan University (Grant No. 2019HXBH104).
Lan H, Suo X, Li W, et al. Abnormalities of intrinsic brain activity in essential tremor: A meta‐analysis of resting‐state functional imaging. Hum Brain Mapp. 2021;42:3156–3167. 10.1002/hbm.25425
Huan Lan and Xueling Suo contributed equally to this work.
Funding information China Postdoctoral Science Foundation, Grant/Award Number: 2020M683317; National Natural Science Foundation of China, Grant/Award Numbers: 81621003, 81820108018, 82001800; Post‐Doctor Research Project, West China Hospital, Sichuan University, Grant/Award Number: 2019HXBH104
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- Amad, A. , Radua, J. , Vaiva, G. , Williams, S. C. , & Fovet, T. (2019). Similarities between borderline personality disorder and post traumatic stress disorder: Evidence from resting‐state meta‐analysis. Neuroscience and Biobehavioral Reviews, 105, 52–59. 10.1016/j.neubiorev.2019.07.018 [DOI] [PubMed] [Google Scholar]
- Archer, D. B. , Coombes, S. A. , Chu, W. T. , Chung, J. W. , Burciu, R. G. , Okun, M. S. , … Vaillancourt, D. E. (2018). A widespread visually‐sensitive functional network relates to symptoms in essential tremor. Brain, 141(2), 472–485. 10.1093/brain/awx338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito‐León, J. , Louis, E. D. , Romero, J. P. , Hernández‐Tamames, J. A. , Manzanedo, E. , Álvarez‐Linera, J. , … Rocon, E. (2015). Altered functional connectivity in essential tremor: A resting‐state fMRI study. Medicine (Baltimore), 94(49), e1936. 10.1097/md.0000000000001936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalsing, K. S. , Saini, J. , & Pal, P. K. (2013). Understanding the pathophysiology of essential tremor through advanced neuroimaging: A review. Journal of the Neurological Sciences, 335(1–2), 9–13. 10.1016/j.jns.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Bhatia, K. P. , Bain, P. , Bajaj, N. , Elble, R. J. , Hallett, M. , Louis, E. D. , … Movement Disorder, S. (2018). Consensus Statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Movement Disorders, 33(1), 75–87. 10.1002/mds.27121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher, S. F. , Seelos, K. C. , Dodel, R. C. , Reiser, M. , & Oertel, W. H. (1997). Activation mapping in essential tremor with functional magnetic resonance imaging. Annals of Neurology, 41(1), 32–40. 10.1002/ana.410410108 [DOI] [PubMed] [Google Scholar]
- Caligiuri, M. E. , Arabia, G. , Barbagallo, G. , Lupo, A. , Morelli, M. , Nistico, R. , … Quattrone, A. (2017). Structural connectivity differences in essential tremor with and without resting tremor. Journal of Neurology, 264(9), 1865–1874. 10.1007/s00415-017-8553-5 [DOI] [PubMed] [Google Scholar]
- Cerasa, A. , Passamonti, L. , Novellino, F. , Salsone, M. , Gioia, M. C. , Morelli, M. , … Quattrone, A. (2010). Fronto‐parietal overactivation in patients with essential tremor during Stroop task. Neuroreport, 21(2), 148–151. 10.1097/WNR.0b013e328335b42c [DOI] [PubMed] [Google Scholar]
- Cerasa, A. , & Quattrone, A. (2015). Linking essential tremor to the cerebellum—Neuroimaging evidence. The Cerebellum, 15(3), 263–275. 10.1007/s12311-015-0739-8 [DOI] [PubMed] [Google Scholar]
- Coste, C. P. , Sadaghiani, S. , Friston, K. J. , & Kleinschmidt, A. (2011). Ongoing brain activity fluctuations directly account for intertrial and indirectly for intersubject variability in Stroop task performance. Cerebral Cortex, 21(11), 2612–2619. 10.1093/cercor/bhr050 [DOI] [PubMed] [Google Scholar]
- Czarnecki, K. , Jones, D. T. , Burnett, M. S. , Mullan, B. , & Matsumoto, J. Y. (2011). SPECT perfusion patterns distinguish psychogenic from essential tremor. Parkinsonism & Related Disorders, 17(5), 328–332. 10.1016/j.parkreldis.2011.01.012 [DOI] [PubMed] [Google Scholar]
- DeSimone, J. C. , Archer, D. B. , Vaillancourt, D. E. , & Wagle Shukla, A. (2019). Network‐level connectivity is a critical feature distinguishing dystonic tremor and essential tremor. Brain, 142(6), 1644–1659. 10.1093/brain/awz085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay, A. J. , Lang, A. E. , Erro, R. , Merola, A. , Fasano, A. , Berardelli, A. , & Bhatia, K. P. (2017). Essential pitfalls in "essential" tremor. Movement Disorders, 32(3), 325–331. 10.1002/mds.26919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, W. , Chen, H. , Wang, H. , Zhang, H. , Liu, M. , Puneet, M. , … Luo, T. (2015). Multiple resting‐state networks are associated with tremors and cognitive features in essential tremor. Movement Disorders, 30(14), 1926–1936. 10.1002/mds.26375 [DOI] [PubMed] [Google Scholar]
- Fang, W. , Chen, H. , Wang, H. , Zhang, H. , Puneet, M. , Liu, M. , … Lu, X. (2016). Essential tremor is associated with disruption of functional connectivity in the ventral intermediate nucleus—Motor cortex—Cerebellum circuit. Human Brain Mapping, 37(1), 165–178. 10.1002/hbm.23024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, W. , Lv, F. , Luo, T. , Cheng, O. , Liao, W. , Sheng, K. , … Zhang, H. (2013). Abnormal regional homogeneity in patients with essential tremor revealed by resting‐state functional MRI. PLoS One, 8(7), e69199. 10.1371/journal.pone.0069199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora, E. D. , Perera, C. L. , Cameron, A. L. , & Maddern, G. J. (2010). Deep brain stimulation for essential tremor: A systematic review. Movement Disorders, 25(11), 1550–1559. 10.1002/mds.23195 [DOI] [PubMed] [Google Scholar]
- Gallea, C. , Popa, T. , Garcia‐Lorenzo, D. , Valabregue, R. , Legrand, A. P. , Marais, L. , … Meunier, S. (2015). Intrinsic signature of essential tremor in the cerebello‐frontal network. Brain, 138 (Pt 10), 2920–2933. 10.1093/brain/awv171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, Q. (2020). Psychoradiology, Neuroimaging Clinics of North America (vol. 30, pp. 1–123). New York: Elsevier Inc.
- Gusnard, D. A. , & Raichle, M. E. (2001). Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews. Neuroscience, 2(10), 685–694. [DOI] [PubMed] [Google Scholar]
- Ha, S. W. , Yang, Y. S. , Song, I. U. , Chung, Y. A. , Oh, J. K. , & Chung, S. W. (2015). Changes in regional brain glucose metabolism measured with F‐18‐FDG‐PET in essential tremor. Acta Radiologica, 56(4), 482–486. 10.1177/0284185114531414 [DOI] [PubMed] [Google Scholar]
- Hellwig, B. , Häußler, S. , Schelter, B. , Lauk, M. , Guschlbauer, B. , Timmer, J. , & Lücking, C. H. (2001). Tremor‐correlated cortical activity in essential tremor. The Lancet, 357(9255), 519–523. 10.1016/s0140-6736(00)04044-7 [DOI] [PubMed] [Google Scholar]
- Hopfner, F. , & Deuschl, G. (2018). Is essential tremor a single entity? European Journal of Neurology, 25(1), 71–82. 10.1111/ene.13454 [DOI] [PubMed] [Google Scholar]
- Hopfner, F. , & Helmich, R. C. (2018). The etiology of essential tremor: Genes versus environment. Parkinsonism & Related Disorders, 46, S92–S96. 10.1016/j.parkreldis.2017.07.014 [DOI] [PubMed] [Google Scholar]
- Hua, S. E. , & Lenz, F. A. (2005). Posture‐related oscillations in human cerebellar thalamus in essential tremor are enabled by voluntary motor circuits. Journal of Neurophysiology, 93(1), 117–127. 10.1152/jn.00527.2004 [DOI] [PubMed] [Google Scholar]
- Huang, X., Gong, Q., Sweeney, J. A., Biswal, B. B. (2019). Progress in psychoradiology, the clinical application of psychiatric neuroimaging. British Journal of Radiology, 92(1101), 20181000. 10.1259/bjr.20181000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, H. I. , Radua, J. , Luckmann, H. C. , & Sack, A. T. (2013). Meta‐analysis of functional network alterations in Alzheimer's disease: Toward a network biomarker. Neuroscience and Biobehavioral Reviews, 37(5), 753–765. 10.1016/j.neubiorev.2013.03.009 [DOI] [PubMed] [Google Scholar]
- Jenkins, I. H. , Bain, P. G. , Colebatch, J. G. , Thompson, P. D. , Findley, L. J. , Frackowiak, R. S. , … Brooks, D. J. (1993). A positron emission tomography study of essential tremor: Evidence for overactivity of cerebellar connections. Annals of Neurology, 34(1), 82–90. 10.1002/ana.410340115 [DOI] [PubMed] [Google Scholar]
- Koch, S. B. , van Zuiden, M. , Nawijn, L. , Frijling, J. L. , Veltman, D. J. , & Olff, M. (2016). Aberrant resting‐state brain activity in posttraumatic stress disorder: A meta‐analysis and systematic review. Depression and Anxiety, 33(7), 592–605. 10.1002/da.22478 [DOI] [PubMed] [Google Scholar]
- Kuhn, S. , & Gallinat, J. (2013). Resting‐state brain activity in schizophrenia and major depression: A quantitative meta‐analysis. Schizophrenia Bulletin, 39(2), 358–365. 10.1093/schbul/sbr151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. Y. , Suo, X. L. , Li, N. N. , Lei, D. , Lu, Z. J. , Wang, L. , … Peng, R. (2020). Altered spontaneous brain activity in essential tremor with and without resting tremor: A resting‐state fMRI study. Magma. 10.1007/s10334-020-00865-1 [DOI] [PubMed] [Google Scholar]
- Lin, C. Y. , Louis, E. D. , Faust, P. L. , Koeppen, A. H. , Vonsattel, J. P. , & Kuo, S. H. (2014). Abnormal climbing fibre‐Purkinje cell synaptic connections in the essential tremor cerebellum. Brain, 137 (Pt 12), 3149–3159. 10.1093/brain/awu281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, A. M. , Trujillo, P. , Hernandez, A. B. , Lin, Y. C. , Kang, H. , Landman, B. A. , … Claassen, D. O. (2020). Structural correlates of the sensorimotor cerebellum in Parkinson's disease and essential tremor. Movement Disorders, 35(7), 1181–1188. 10.1002/mds.28044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, E. D. (2005). Essential tremor. Lancet Neurology, 4(2), 100–110. 10.1016/s1474-4422(05)00991-9 [DOI] [PubMed] [Google Scholar]
- Louis, E. D. (2010). Essential tremor: Evolving clinicopathological concepts in an era of intensive post‐mortem enquiry. Lancet Neurology, 9(6), 613–622. 10.1016/s1474-4422(10)70090-9 [DOI] [PubMed] [Google Scholar]
- Louis, E. D. (2018). The evolving definition of essential tremor: What are we dealing with? Parkinsonism & Related Disorders, 46, S87–S91. 10.1016/j.parkreldis.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, E. D. , Faust, P. L. , & Vonsattel, J. P. (2011). Purkinje cell loss is a characteristic of essential tremor. Parkinsonism & Related Disorders, 17(6), 406–409. 10.1016/j.parkreldis.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, E. D. , & Ferreira, J. J. (2010). How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Movement Disorders, 25(5), 534–541. 10.1002/mds.22838 [DOI] [PubMed] [Google Scholar]
- Louis, E. D. , Frucht, S. J. , & Rios, E. (2009). Intention tremor in essential tremor: Prevalence and association with disease duration. Movement Disorders, 24(4), 626–627. 10.1002/mds.22370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, E. D. , Shungu, D. C. , Chan, S. , Mao, X. , Jurewicz, E. C. , & Watner, D. (2002). Metabolic abnormality in the cerebellum in patients with essential tremor: A proton magnetic resonance spectroscopic imaging study. Neuroscience Letters, 333(1), 17–20. 10.1016/s0304-3940(02)00966-7 [DOI] [PubMed] [Google Scholar]
- Moro, E. , Schwalb, J. M. , Piboolnurak, P. , Poon, Y. Y. , Hamani, C. , Hung, S. W. , … Lozano, A. M. (2011). Unilateral subdural motor cortex stimulation improves essential tremor but not Parkinson's disease. Brain, 134 (Pt 7), 2096–2105. 10.1093/brain/awr072 [DOI] [PubMed] [Google Scholar]
- Müller, V. I. , Cieslik, E. C. , Serbanescu, I. , Laird, A. R. , Fox, P. T. , & Eickhoff, S. B. (2017). Altered brain activity in unipolar depression revisited: Meta‐analyses of neuroimaging studies. JAMA Psychiatry, 74(1), 47–55. 10.1001/jamapsychiatry.2016.2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, M. , Kishi, K. , & Kato, S. (2007). Insular cortex and neuropsychiatric disorders: A review of recent literature. European Psychiatry, 22(6), 387–394. 10.1016/j.eurpsy.2007.02.006 [DOI] [PubMed] [Google Scholar]
- Neely, K. A. , Kurani, A. S. , Shukla, P. , Planetta, P. J. , Wagle Shukla, A. , Goldman, J. G. , … Vaillancourt, D. E. (2015). Functional brain activity relates to 0‐3 and 3‐8 Hz force oscillations in essential tremor. Cerebral Cortex, 25(11), 4191–4202. 10.1093/cercor/bhu142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, P. , Zhang, Y. , Liu, Y. , Zhang, H. , Guan, D. , & Xu, Y. (2017). Abnormalities of regional brain function in Parkinson's disease: A meta‐analysis of resting state functional magnetic resonance imaging studies. Scientific Reports, 7, 40469. 10.1038/srep40469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti, L. , Novellino, F. , Cerasa, A. , Chiriaco, C. , Rocca, F. , Matina, M. S. , … Quattrone, A. (2011). Altered cortical‐cerebellar circuits during verbal working memory in essential tremor. Brain, 134 (Pt 8), 2274–2286. 10.1093/brain/awr164 [DOI] [PubMed] [Google Scholar]
- Pelzer, E. A. , Nelles, C. , Pedrosa, D. J. , Eggers, C. , Burghaus, L. , Melzer, C. , … Timmermann, L. (2017). Structural differences in impaired verbal fluency in essential tremor patients compared to healthy controls. Brain and Behavior: A Cognitive Neuroscience Perspective, 7(7), e00722. 10.1002/brb3.722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pico‐Perez, M. , Moreira, P. S. , de Melo Ferreira, V. , Radua, J. , Mataix‐Cols, D. , Sousa, N. , … Morgado, P. (2020). Modality‐specific overlaps in brain structure and function in obsessive‐compulsive disorder: Multimodal meta‐analysis of case‐control MRI studies. Neuroscience and Biobehavioral Reviews, 112, 83–94. 10.1016/j.neubiorev.2020.01.033 [DOI] [PubMed] [Google Scholar]
- Popa, T. , Russo, M. , Vidailhet, M. , Roze, E. , Lehericy, S. , Bonnet, C. , … Gallea, C. (2013). Cerebellar rTMS stimulation may induce prolonged clinical benefits in essential tremor, and subjacent changes in functional connectivity: An open label trial. Brain Stimulation, 6(2), 175–179. 10.1016/j.brs.2012.04.009 [DOI] [PubMed] [Google Scholar]
- Radua, J. , Borgwardt, S. , Crescini, A. , Mataix‐Cols, D. , Meyer‐Lindenberg, A. , McGuire, P. K. , & Fusar‐Poli, P. (2012). Multimodal meta‐analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neuroscience and Biobehavioral Reviews, 36(10), 2325–2333. 10.1016/j.neubiorev.2012.07.012 [DOI] [PubMed] [Google Scholar]
- Radua, J. , Grau, M. , van den Heuvel, O. A. , Thiebaut de Schotten, M. , Stein, D. J. , Canales‐Rodriguez, E. J. , … Mataix‐Cols, D. (2014). Multimodal voxel‐based meta‐analysis of white matter abnormalities in obsessive‐compulsive disorder. Neuropsychopharmacology, 39(7), 1547–1557.doi: 10.1038/npp.2014.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua, J. , & Mataix‐Cols, D. (2009). Voxel‐wise meta‐analysis of grey matter changes in obsessive‐compulsive disorder. The British Journal of Psychiatry, 195(5), 393–402. 10.1192/bjp.bp.108.055046 [DOI] [PubMed] [Google Scholar]
- Radua, J. , Mataix‐Cols, D. , Phillips, M. L. , El‐Hage, W. , Kronhaus, D. M. , Cardoner, N. , & Surguladze, S. (2012). A new meta‐analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European Psychiatry, 27(8), 605–611. 10.1016/j.eurpsy.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Raethjen, J. , & Deuschl, G. (2012). The oscillating central network of essential tremor. Clinical Neurophysiology, 123(1), 61–64. 10.1016/j.clinph.2011.09.024 [DOI] [PubMed] [Google Scholar]
- Raethjen, J. , Govindan, R. B. , Kopper, F. , Muthuraman, M. , & Deuschl, G. (2007). Cortical involvement in the generation of essential tremor. Journal of Neurophysiology, 97(5), 3219–3228. 10.1152/jn.00477.2006 [DOI] [PubMed] [Google Scholar]
- Raichle, M. E. , & Mintun, M. A. (2006). Brain work and brain imaging. Annual Review of Neuroscience, 29(1), 449–476. 10.1146/annurev.neuro.29.051605.112819 [DOI] [PubMed] [Google Scholar]
- Rossi, A. F. , Pessoa, L. , Desimone, R. , & Ungerleider, L. G. (2009). The prefrontal cortex and the executive control of attention. Experimental Brain Research, 192(3), 489–497. 10.1007/s00221-008-1642-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxo, M. R. , Franceschini, P. R. , Zubaran, C. , Kleber, F. D. , & Sander, J. W. (2011). The limbic system conception and its historical evolution. Scientific World Journal, 11, 2428–2441. 10.1100/2011/157150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi‐Khorshidi, G. , Smith, S. M. , Keltner, J. R. , Wager, T. D. , & Nichols, T. E. (2009). Meta‐analysis of neuroimaging data: A comparison of image‐based and coordinate‐based pooling of studies. NeuroImage, 45(3), 810–823. 10.1016/j.neuroimage.2008.12.039 [DOI] [PubMed] [Google Scholar]
- Sengul, Y. , Temur, H. O. , Corakci, Z. , Sengul, H. S. , Dowd, H. , Ustun, I. , … Louis, E. D. (2020). Brain microstructural changes and cognitive function in non‐demented essential tremor patients: A diffusion tensor imaging study. The International Journal of Neuroscience, 1–11. 10.1080/00207454.2020.1803859 [DOI] [PubMed] [Google Scholar]
- Sharifi, S. , Nederveen, A. J. , Booij, J. , & van Rootselaar, A.‐F. (2014). Neuroimaging essentials in essential tremor: A systematic review. Neuroimage: Clinical, 5, 217–231.doi: 10.1016/j.nicl.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Fox, P. T. , Miller, K. L. , Glahn, D. C. , Fox, P. M. , Mackay, C. E. , … Beckmann, C. F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106(31), 13040–13045. 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, I.‐U. , Park, J.‐W. , Chung, S.‐W. , & Chung, Y.‐A. (2013). Differences in cerebral perfusion according to phenotypes of essential tremor: Brain perfusion SPECT study using SPM analysis. Neurological Sciences, 35(5), 767–772. 10.1007/s10072-013-1600-9 [DOI] [PubMed] [Google Scholar]
- Stoodley, C. J. , & Schmahmann, J. D. (2010). Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex, 46(7), 831–844. 10.1016/j.cortex.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H., Chen, Y., Huang, Q., Lui, S., Huang, X., Shi, Y., … Gong, Q. (2018). Psychoradiologic Utility of MR Imaging for Diagnosis of Attention Deficit Hyperactivity Disorder: A Radiomics Analysis. Radiology, 287(2), 620–630. 10.1148/radiol.2017170226 [DOI] [PubMed] [Google Scholar]
- Suo, X., Lei, D., Li, W., Li, L., Dai, J., Wang, S., … Gong, Q. (2021). Altered white matter microarchitecture in Parkinson’s disease: a voxel‐based meta‐analysis of diffusion tensor imaging studies. Frontiers of Medicine, 15(1), 125–138. 10.1007/s11684-019-0725-5 [DOI] [PubMed] [Google Scholar]
- Wang, J. , Zhang, J. R. , Zang, Y. F. , & Wu, T. (2018). Consistent decreased activity in the putamen in Parkinson's disease: A meta‐analysis and an independent validation of resting‐state fMRI. Gigascience, 7(6), 1–13. 10.1093/gigascience/giy071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Lei, D. , Suo, X. , Li, N. , Lu, Z. , Li, J. , … Peng, R. (2018). Resting‐state fMRI study on drug‐naive patients of essential tremor with and without head tremor. Scientific Reports, 8(1), 10580. 10.1038/s41598-018-28778-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. , Luo, C. , Dong, L. , Bin, Y. , Ma, S. , Yao, D. , … Yang, Z. (2015). Altered intrinsic brain activity in patients with familial cortical myoclonic tremor and epilepsy: An amplitude of low‐frequency fluctuation study. Journal of the Neurological Sciences, 351(1–2), 133–139. 10.1016/j.jns.2015.03.005 [DOI] [PubMed] [Google Scholar]
- Wang, P. , Luo, X. , Zhong, C. , Yang, L. , Guo, F. , & Yu, N. (2018). Resting state fMRI reveals the altered synchronization of BOLD signals in essential tremor. Journal of the Neurological Sciences, 392, 69–76. 10.1016/j.jns.2018.07.008 [DOI] [PubMed] [Google Scholar]
- Wang, T. , Liu, J. , Zhang, J. , Zhan, W. , Li, L. , Wu, M. , … Gong, Q. (2016). Altered resting‐state functional activity in posttraumatic stress disorder: A quantitative meta‐analysis. Scientific Reports, 6, 27131. 10.1038/srep27131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills, A. J. , Jenkins, I. H. , Thompson, P. D. , Findley, L. J. , & Brooks, D. J. (1994). Red nuclear and cerebellar but no olivary activation associated with essential tremor: A positron emission tomographic study. Annals of Neurology, 36(4), 636–642. 10.1002/ana.410360413 [DOI] [PubMed] [Google Scholar]
- Yin, W. , Lin, W. , Li, W. , Qian, S. , & Mou, X. (2016). Resting state fMRI demonstrates a disturbance of the cerebello‐cortical circuit in essential tremor. Brain Topography, 29(3), 412–418. 10.1007/s10548-016-0474-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Quality assessment checklist.
Table S2. Quality assessment details.
Table S3. Meta‐analysis results of differences in resting state brain activity between ET and HC after excluding Ha et al., 2015.
Table S4. Subgroup meta‐analysis results in studies in ET patients with head tremor showing hyper‐ and hypoactivity of brain regions.
Table S5. Subgroup meta‐analysis results in medication‐naïve ET patients showing hyper‐ and hypoactivity of brain regions.
Table S6. Subgroup meta‐analysis results in studies with threshold correction showing hyper‐ and hypoactivity of brain regions.
Table S7. Subgroup meta‐analysis results in studies using fMRI methods showing hyper‐ and hypoactivity of brain regions.
Table S8. Jackknife sensitivity analysis of subgroup meta‐analysis in ET patients with head tremor.
Table S9. Jackknife sensitivity analysis of subgroup meta‐analysis in medication‐naïve ET patients.
Table S10. Jackknife sensitivity analysis of subgroup meta‐analysis in studies with correction threshold.
Table S11. Jackknife sensitivity analysis of subgroup meta‐analysis in studies using fMRI methods.
Table S12. Jackknife sensitivity analysis of meta‐analysis results after excluding Ha et al., 2015.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.


