Key Points
Question
What are the long-term responses with combined ibrutinib and venetoclax first-line therapy in patients with chronic lymphocytic leukemia?
Findings
In this phase 2 nonrandomized trial, 80 previously untreated patients with chronic lymphocytic leukemia received combined ibrutinib and venetoclax for 24 cycles; on an intent-to-treat analysis, bone marrow–undetectable measurable residual disease remission was achieved by 56% of the patients at 12 cycles and 66% of the patients at 24 cycles of combined treatment. Overall, 75% of the patients achieved bone marrow–undetectable measurable residual disease remission as best response, with 3-year progression-free survival noted in 93% of the patients.
Meaning
The findings of this study suggest that combination therapy with ibrutinib and venetoclax may be a useful regimen for previously untreated patients with chronic lymphocytic leukemia.
Abstract
Importance
Oral targeted therapies have advanced the treatment of chronic lymphocytic leukemia (CLL). These therapies include Bruton tyrosine kinase inhibitors, used as monotherapy, and the Bcl-2 inhibitor venetoclax, typically combined with the CD20 monoclonal antibody. Preclinical studies have shown synergy between Bruton tyrosine kinase inhibitors and the Bcl-2 inhibitor venetoclax.
Objective
To examine the rate of complete remission, complete remission with incomplete count recovery, and bone marrow–undetectable measurable residual disease (U-MRD) after treatment with the combination of ibrutinib and venetoclax.
Design, Setting, and Participants
A single-center, phase 2 nonrandomized trial enrolled patients from August 17, 2016, to June 5, 2018. Participants included previously untreated patients with CLL who met International Workshop on CLL 2008 criteria for treatment indication. Patients were required to have at least 1 of the following features: del(17p), TP53-mutated CLL, del(11q), unmutated immunoglobulin heavy-chain variable gene, or age 65 years or older.
Interventions
Therapy consisted of ibrutinib, 420 mg/d, monotherapy for 3 cycles, thereafter combined with venetoclax (standard weekly dose ramp-up to 400 mg/d) for a total of 24 cycles of combination treatment. Responses were assessed at serial points according to International Workshop on CLL 2008 criteria. Measurable residual disease (MRD) was assessed by multicolor flow cytometry with a sensitivity of 10−4.
Main Outcomes and Measures
Outcomes included complete remission, complete remission with incomplete count recovery, and bone marrow U-MRD rate.
Results
Eighty patients (57 [71%] men) were treated; median age was 65 years (range, 26-83 years). The median follow-up for all 80 patients was 38.5 months (range, 5.6-51.1 months). Five patients discontinued the study during the ibrutinib monotherapy phase; the remaining 75 patients received combination therapy. On an intent-to-treat analysis of combined treatment, 45 (56%) patients achieved bone marrow U-MRD remission at 12 cycles and 53 (66%) patients achieved bone marrow U-MRD remission at 24 cycles. Overall, 60 (75%) patients achieved bone marrow U-MRD remission as their best response. Responses were seen across all high-risk subgroups, independent of the immunoglobulin heavy-chain variable gene mutation status, fluorescence in situ hybridization category, or TP53 mutation. The 3-year progression-free survival was 93%, and 3-year overall survival was 96%. No patient had CLL progression; 2 patients developed Richter transformation.
Conclusions and Relevance
The findings of this study suggest that combination therapy with ibrutinib and venetoclax might be beneficial for previously untreated patients with CLL. Remissions appeared to be durable during a follow-up of more than 3 years, with activity seen across high-risk disease subgroups, including those with del(17p)/TP53-mutated CLL.
Trial Registration
ClinicalTrials.gov Identifier: NCT02756897
This nonrandomized phase 2 trial examines remission after combination therapy with ibrutinib and venetoclax in treatment-naive patients with chronic lymphocytic leukemia.
Introduction
Treatment of chronic lymphocytic leukemia (CLL) has undergone a substantial evolution with the development of oral targeted therapies.1 The Bruton tyrosine kinase (BTK) inhibitors ibrutinib and acalabrutinib, and venetoclax, a Bcl-2 inhibitor, are approved for treatment of CLL.2,3,4,5,6 Bruton tyrosine kinase inhibitors typically are administered as monotherapy continuously daily, with most responses being partial remission; complete remission (CR) is uncommon, and undetectable measurable residual disease (U-MRD) remissions are rare.2,3,4 Venetoclax is typically combined with the CD20 monoclonal antibody obinutuzumab and is given for 1 year in the treatment-naive setting.6 The U-MRD rate was 76% in peripheral blood and 57% in bone marrow with combined venetoclax and obinutuzumab.6
Based on the preclinical synergism of ibrutinib and venetoclax,7 complementary therapeutic activity, and a nonoverlapping toxic effects profile, we conducted a phase 2 nonrandomized trial with this combination in treatment-naive patients with CLL.8 In this report, we provide an updated analysis for this cohort with a focus on MRD and durability of remission.
Methods
Design
We designed an investigator-initiated phase 2 trial for patients with treatment-naive CLL with an International Workshop on CLL treatment indication. All patients were required to have at least 1 of the following features: del(17p), TP53-mutated CLL, del(11q), unmutated immunoglobulin heavy-chain variable gene, or age 65 years or older. Complete eligibility criteria are presented in the eMethods in the Supplement. The University of Texas MD Anderson Cancer Center Institutional Review Board approved this study. All patients provided written informed consent. No financial compensation was given. All patients were treated at The University of Texas MD Anderson Cancer Center. The study was conducted in accordance with the Declaration of Helsinki.9
Patients started treatment with ibrutinib, 420 mg/d, monotherapy for the first 3 cycles, followed by addition of venetoclax, with standard weekly dose ramp-up starting at 20 mg/d to a target dose of 400 mg/d; each cycle was 28 days. Combined ibrutinib plus venetoclax was administered for a total of 24 planned cycles. In the original protocol of the trial, patients who remained marrow MRD-positive at the end of 24 cycles of the combination therapy could continue ibrutinib monotherapy; recently, the trial protocol was amended to allow an additional 12 cycles of combination therapy for these patients.
Response assessments were performed according to International Workshop on CLL 2008 criteria.10 During combined therapy, MRD was assessed in bone marrow. After completing the combined therapy, patients were monitored for MRD in peripheral blood samples every 6 months. Measurable residual disease was assessed by multicolor flow cytometry with a sensitivity of 10−4.11
Statistical Analysis
The primary end point was best response (CR and CR with incomplete count recovery [CRi]) achieved at any time during the treatment for up to 2 months after completion of combined therapy. Time-to-event outcomes, including progression-free survival (PFS) and overall survival (OS), were estimated using the Kaplan-Meier method. Log-rank tests were used to assess the differences between subgroups of patients. All statistical analyses were performed using SAS, version 9.4 (SAS Institute). All statistical tests were 2-sided, and P < .05 was deemed statistically significant. Additional details are given in the eMethods in the Supplement.
Results
A total of 80 patients (23 women [29%] and 57 men [71%]) initiated study treatments between August 17, 2016, and June 5, 2018. Baseline characteristics are reported in Table 1. The median age was 65 years (range, 26-83 years). A total of 14 patients (18%) had del(17p) and 11 (14%) had TP53-mutated CLL; 18 (23%) patients had del(17p) and/or TP53-mutated CLL.
Table 1. Characteristics of All 80 Patients at Study Entry.
| Characteristic | No. (%) |
|---|---|
| Age, median (range), y | 65 (26-83) |
| ≥65 | 43 (54) |
| Sex | |
| Female | 23 (29) |
| Male | 57 (71) |
| Rai stage | |
| III-IV | 41 (51) |
| Baseline laboratory test results, median (range) | |
| ALC, ×109/L | 75.6 (1.1-338) |
| Hemoglobin, g/dL | 11.6 (7.7-15.8) |
| Platelet count, ×109/L | 130 (28-334) |
| Serum β2-microglobulin, mg/L | 3.5 (1.7-13.7) |
| Hierarchical fluorescence in situ hybridization | |
| Del(17p) | 14 (18) |
| Del(11q) | 20 (25) |
| Trisomy 12 | 17 (21) |
| Normal | 10 (12) |
| Del(13q) | 19 (24) |
| IGHV status | |
| No. | 76 |
| Unmutated | 63 (83) |
| Mutated | 13 (17) |
| Cytogenetics | |
| No. | 78 |
| Complex (≥3 abnormalities) | 12 (15) |
| Diploid | 32 (41) |
| Gene alteration | |
| No. | 79 |
| TP53 | 11 (14) |
| NOTCH1 | 22 (28) |
| SF3B1 | 18 (23) |
| BIRC3 | 5 (6) |
Abbreviations: ALC, absolute lymphocyte count; IGHV, immunoglobulin heavy-chain variable.
SI conversion factor: To convert hemoglobin to grams per liter, multiply by 10.
Of the 80 patients, 5 discontinued the study during the ibrutinib monotherapy phase owing to reasons other than CLL progression; the remaining 75 patients initiated combination therapy with venetoclax (eFigure 1 in the Supplement). The median follow-up for all 80 patients was 38.5 months (range, 5.6-51.1 months).
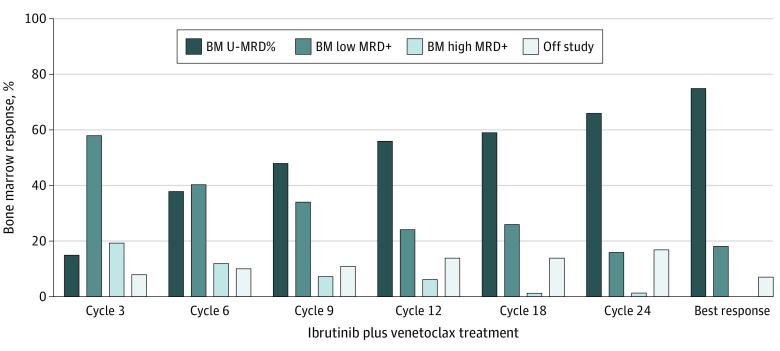
Bone marrow MRD responses on an intent-to-treat analysis including all 80 patients are shown in Figure 1. With serial assessments during combination therapy, an increasing number of patients achieved bone marrow U-MRD remission. At 12 cycles of combined therapy, 45 (56%) patients achieved bone marrow U-MRD remission, 19 (24%) had a low MRD-positive response (0.01% to <1%), 5 (6%) patients had a high MRD-positive response (≥1%), and 11 (14%) patients discontinued the study before the cycle 12 assessment. At 24 cycles of combined therapy, 53 (66%) patients achieved bone marrow U-MRD remission, 13 (16%) patients had a low MRD-positive response, 1 (1%) patient had a high MRD-positive response, and 13 (16%) patients discontinued the study before the cycle 24 assessment (eFigure 1 in the Supplement). A total of 60 (75%) patients achieved bone marrow U-MRD remission as a best response at any time during the study. Bone marrow MRD responses for evaluable patients at specific times are shown in eFigure 2 in the Supplement.
Figure 1. Bone Marrow (BM) Measurable Residual Disease (MRD) Response at Serial Points on an Intent-to-Treat Basis in 80 Patients.
Bone marrow MRD responses at serial points of combined ibrutinib and venetoclax therapy are shown; best response at any time during the study period is shown. The 5 patients who discontinued the study during ibrutinib monotherapy and never started venetoclax were included in the analysis. MRD was assessed by flow cytometry in bone marrow with a sensitivity of 10−4. Undetectable MRD (U-MRD), less than 0.01%; low MRD-positive (MRD+), 0.01% to less than 1%; and high MRD-positive, greater than or equal to 1%.
We found that patients who achieved less than 1% bone marrow MRD at the first response assessment after the initiation of combined therapy (ie, at 3 cycles of the combination) were more likely to achieve bone marrow U-MRD remission by completion of cycle 12 of combined treatment (41 of 53 [77%] vs 3 of 15 [20%]; P < .001). Similarly, patients who achieved more than a 2-log reduction in bone marrow MRD at cycle 3 of the combined treatment were more likely to achieve bone marrow U-MRD remission by the end of cycle 12 of combined treatment (40 of 48 [83%] vs 4 of 20 [20%]; P < .001). Similar results were observed for early bone marrow response and achieving U-MRD remission by the end of cycle 24 combination therapy (eMethods in the Supplement).
To assess the benefit of the second year of combined treatment, we evaluated the outcomes of the 24 patients who were bone marrow MRD–positive (low, n = 19; high, n = 5) at the completion of cycle 12 combined therapy. A total of 12 (50%) of these 24 patients (10 of 19 [53%] low MRD-positive; 2 of 5 [40%] high MRD-positive) achieved marrow U-MRD remission at the end of the 24 cycles of combined therapy (eFigure 3 and eFigure 4 in the Supplement).
A total of 53 patients achieved bone marrow U-MRD remission by the end of cycle 24 of combined therapy; 51 patients discontinued both ibrutinib and venetoclax per trial design and 2 patients continued ibrutinib monotherapy per the treating physician’s discretion. Fifty-one patients had blood MRD monitoring after completing combined cycle 24 (too early for assessment in 1 patient, 1 patient missed owing to COVID-19 travel restrictions). After a median follow-up of 12.4 months, 8 patients had MRD recurrence detected in blood samples (eTable 1 in the Supplement); notably, 5 of these 8 patients had achieved U-MRD for the first time after completion of 24 cycles of combined treatment. No patient had clinical progression and, at the time of writing, all patients continued to be observed without any active therapy.
A total of 14 patients were noted to have bone marrow MRD-positive disease (13 had low MRD-positive, 1 had high MRD-positive) at the completion of cycle 24 of combined therapy. The sole patient with high MRD-positive disease was found to have Richter transformation at that time. The 13 patients with low MRD-positive disease initially continued to receive ibrutinib monotherapy; with a recent protocol amendment, 9 of the 13 patients reinitiated venetoclax for an additional 12 cycles of combined therapy.
Bone marrow U-MRD remission at serial points by pretreatment characteristics is shown in eTable 2 in the Supplement. Responses were seen across all high-risk subgroups independent of the immunoglobulin heavy-chain variable gene, fluorescence in situ hybridization category, or TP53-mutated CLL.
A total of 18 patients had del(17p) and/or TP53-mutated CLL. Four patients discontinued the study during the ibrutinib monotherapy phase and 1 patient discontinued the study soon after completing venetoclax ramp-up (all discontinuations were due to reasons other than CLL progression). The remaining 13 patients completed 24 cycles of combined therapy. The bone marrow U-MRD rate was 69% (9 of 13 patients) at cycle 12 and 77% (10 of 13 patients) at cycle 24.
At 12 cycles of combined therapy, 55 patients (69%) achieved CR/CRi and 14 patients (18%) were in partial remission (eTable 3 in the Supplement). At 24 cycles of the combination therapy, 55 patients (69%) achieved CR/CRi and 10 patients (13%) were in partial remission. The CR/CRi as a best response during the study was noted in 62 patients (78%).
The estimated 3-year PFS rate was 93% (95% CI, 88%-99%), and estimated 3-year overall survival was 96% (95% CI, 92%-100%) (Figure 2A and B). Progression-free survival by immunoglobulin heavy-chain variable gene and del(17p)/TP53-mutated CLL are shown in Figure 2C and D. The estimated 3-year PFS for patients with del(17p)/TP53-mutated CLL is 86%. No patient had CLL progression. Two patients developed diffuse large B-cell lymphoma Richter transformation (1 during venetoclax dose escalation; 1 during cycle 24 of combination therapy); at the time of writing, both patients were alive and in remission after allogeneic stem cell transplantation (eTable 4 in the Supplement). Three patients died (eTable 5 in the Supplement); 2 patients discontinued the study during cycle 1 of ibrutinib monotherapy and died 6 and 27 months later from infection after receiving additional CLL therapy. One patient, aged 78 years, died when in bone marrow U-MRD remission during cycle 19 of combined therapy; he was found unresponsive at home. A computed tomographic scan showed pneumonia; the patient’s death was deemed possibly related to ibrutinib. Characteristics of the patients who discontinued the study are shown in eTable 6 in the Supplement.
Figure 2. Progression-Free and Overall Survival for All 80 Patients.
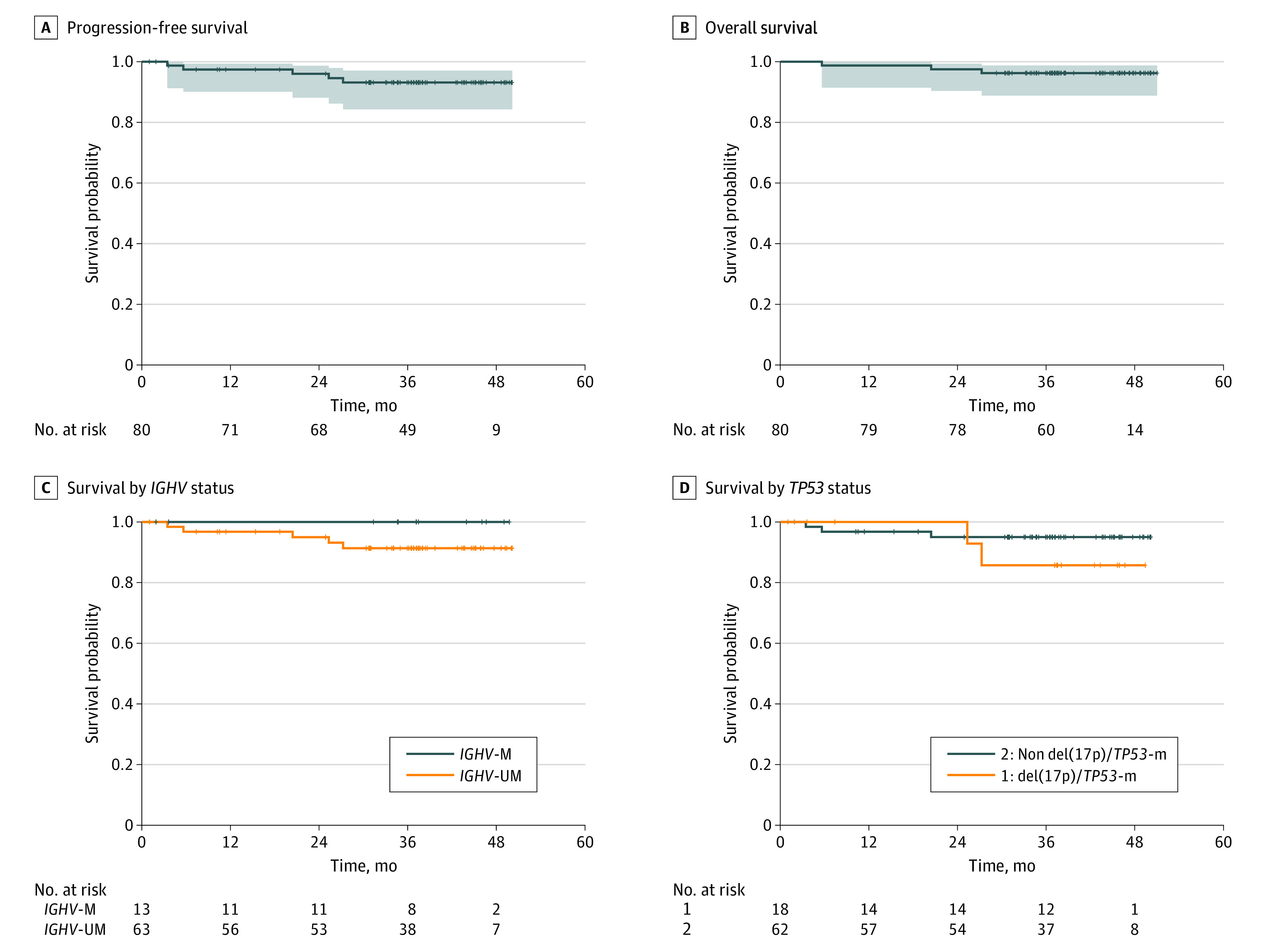
Progression-free (A) and overall (B) survival for all 80 patients. Progression-free survival by immunoglobulin heavy-chain variable (IGHV) gene (IGHV-M, IGHV mutated; IGHV-UM, IGHV unmutated) (C) and del(17p)/TP53-mutated CLL (D).
Immunoglobulin levels at serial points of the study are shown in eFigure 5 in the Supplement. Both IgG and IgM levels remained stable with an increase in IgA levels noted during the ibrutinib monotherapy phase. Absolute T-cell and natural killer–cell numbers at serial points in the study are shown in eFigure 6 in the Supplement.
Grade 3 to 4 neutropenia occurred in 41 patients (51%). Grade 3 thrombocytopenia occurred in 2 patients (2%). Nonhematologic adverse events that occurred in 5% or more of the patients are reported in Table 2. Neutropenic fever occurred in 4 patients (3 culture-negative and 1 Pneumocystis jirovecii pneumonia). Additional infectious complications leading to hospitalization included pneumonia (nonneutropenic, culture-negative) in 5 patients, cellulitis in 3 patients, and nonneutropenic fever, anaplasmosis infection, septic arthritis, disseminated cryptococcal infection, aspergillus/cryptococcal infection, and appendicitis/cholecystitis, each in 1 patient.
Table 2. Nonhematologic Adverse Events That Occurred in 5% or More of the Patients.
| Event | Patients, No. (%) | |
|---|---|---|
| Any grade | Grade ≥3 | |
| Easy bruising | 61 (76) | 0 |
| Arthralgia | 46 (58) | 2 (2) |
| Diarrhea | 42 (53) | 0 |
| Nausea/vomiting | 33 (41) | 0 |
| Myalgia | 28 (35) | 1 (1) |
| Skin rash | 19 (24) | 0 |
| Nail changes | 16 (20) | 0 |
| Fatigue | 15 (19) | 1 (1) |
| Gastroesophageal reflux disease | 14 (18) | 0 |
| Hypertension | 13 (16) | 8 (10) |
| Atrial fibrillation/flutter | 12 (15) | 8 (10) |
| Oral mucositis | 12 (15) | 0 |
| Dry skin | 11 (14) | 0 |
| Constipation | 8 (10) | 0 |
| Increased creatinine level | 7 (9) | 0 |
| Headache | 6 (8) | 0 |
| Epistaxis | 6 (8) | 0 |
| Increased alanine aminotransferase level | 5 (6) | 1 (1) |
Discussion
Oral targeted therapies have become the mainstay of CLL therapy, with a sharp decrease in the use of chemoimmunotherapy.1 The BTK inhibitors ibrutinib and acalabrutinib are approved for treatment of CLL and are indicated to be administered daily continuously. In the first-line RESONATE-2 trial, 5-year PFS with ibrutinib monotherapy was 70%.3,12 In a recent pooled analysis of 280 ibrutinib-treated patients with previously untreated CLL, the 3-year PFS was 80%.13 With a 1-year, time-limited combination of venetoclax and obinutuzumab in first-line treatment of CLL, the 3-year PFS was 82% and the 4-year PFS was 74%.14,15 We report a time-limited strategy of combined ibrutinib and venetoclax therapy with a 3-year PFS of 93% in first-line treatment of CLL.
The U-MRD responses improved with ongoing combined ibrutinib plus venetoclax. At 24 cycles of the combination, 66% of the patients achieved U-MRD remission in bone marrow. Overall, 75% of all patients achieved bone marrow U-MRD at any time during the study. These rates of U-MRD are superior to what has been reported3 with single-agent BTK inhibitors, with U-MRD remissions rarely reported. In the CLL14 trial, combined obinutuzumab (6 cycles) and venetoclax (12 cycles) led to a bone marrow U-MRD rate of 57% and a peripheral blood U-MRD rate of 76%.6,14,15 We observed a similar bone marrow U-MRD rate at 1 year (56%), with improvement in MRD noted with the second year of the combination therapy. Whether the second year of the combination therapy would translate into improved long-term PFS remains to be determined.
The results of our trial are consistent with the results of the CAPTIVATE trial, a multicenter international trial using a very similar treatment strategy.16 In the CAPTIVATE trial, similar to our trial, patients received 3 cycles of ibrutinib monotherapy followed by combined ibrutinib and venetoclax for 12 cycles. Thereafter, unlike our trial, in which all patients received a second year of the combination therapy, in the CAPTIVATE trial, patients were randomized to different treatment arms based on MRD status. In the CAPTIVATE trial, at 12 cycles of the combination therapy, the bone marrow U-MRD rate was 68% and the peripheral blood U-MRD rate was 75%. These results are similar to the 12-cycles bone marrow U-MRD rate of 56% in our trial. There are few notable differences in the patient population studied in the CAPTIVATE vs the current trial. Unlike in the CAPTIVATE trial, inclusion in our trial required a high-risk feature to be present or patients had to be aged 65 years or older, with 65 years the median age of the participants. The median age of patients in the CAPTIVATE trial was younger (58 years). Patients aged 70 years or older were excluded from the CAPTIVATE trial. More patients in our trial had unmutated immunoglobulin heavy-chain variable gene (83%) vs the CAPTIVATE trial (60%).
Responses to combined ibrutinib and venetoclax therapy were seen across all genetic subgroups in our study, including high-risk subgroups. In the CLL14 trial, among the 25 patients with del(17p)/TP53-mutated CLL enrolled in the venetoclax and obinutuzumab arm, the 3-year PFS was 60%.14 For the 18 patients with a del(17p)/TP53-mutated CLL in our trial, the 3-year PFS was 86%. These results are encouraging for a time-limited approach for this high-risk subgroup.
Because none of the pretreatment patient and disease characteristics were associated with the probability of U-MRD response, we evaluated whether early bone marrow MRD response might be indicative of a sensitive disease and thereby be associated with the probability of future MRD responses. We found that patients who achieved less than 1% bone marrow MRD or had a more than 2-log reduction in bone marrow MRD at cycle 3 of the combination therapy were more likely to achieve bone marrow U-MRD remission at a later time. This finding is in line with the recent results of the TAP CLARITY trial in patients with relapsed CLL, in which a more than 2-log reduction in the peripheral blood CLL cell count within 2 months of starting venetoclax therapy correlated with achieving U-MRD remission.17
With venetoclax-based combination therapies, an important issue is the optimal duration of therapy. In the present trial, patients received 24 cycles of combined therapy. An improved U-MRD rate was noted during the second year of combined therapy for patients who were bone marrow MRD-positive at the end of cycle 12. Therefore, we recently amended the protocol to allow an extra year of combination therapy for patients with bone marrow MRD-positive results at the end of cycle 24. Whether this additional year of therapy will lead to U-MRD remission remains to be determined. Several ongoing trials with combined ibrutinib and venetoclax are investigating different treatment durations. For example, in the CLL GLOW trial, patients received 12 cycles of combined ibrutinib and venetoclax.18 In the CAPTIVATE trial, patients received 12 cycles of the combination therapy followed by randomization based on MRD status.16 In the UK FLAIR trial, the duration of therapy is based on the time it takes for a patient to achieve U-MRD remission.19
Time-limited treatment approaches with venetoclax-based regimens (eg, venetoclax plus obinutuzumab and venetoclax plus ibrutinib) have certain advantages over continuous BTK inhibitor–based therapy in terms of reduced risk for long-term treatment-related adverse events and potential lower costs. Venetoclax-based regimens lead to higher rates of blood and bone marrow U-MRD remission compared with BTK inhibitor therapy, supporting this strategy of fixed-duration treatment. Bruton tyrosine kinase inhibitors have the advantage of the availability of long-term follow-up data, lower risk of tumor lysis syndrome and neutropenia, and durable responses in patients with del(17p)/TP53-mutated CLL. We report herein encouraging 3-year PFS of 93% with the time-limited approach of venetoclax and ibrutinib combination therapy, including durable activity in del(17p)/TP53-mutated CLL.
Several ongoing trials are investigating combined BTK inhibitors with venetoclax with and without obinutuzumab. Data from additional ongoing trials, including the CLL17 trial comparing ibrutinib vs venetoclax and obinutuzumab vs venetoclax and ibrutinib, will help to further clarify the role of this regimen. The combination of ibrutinib and venetoclax is also being explored in relapsed CLL with encouraging clinical activity.20,21,22
Limitations
Limitations of this study include its single-center, nonrandomized phase 2 design with a relatively short follow-up of 38.5 months.
Conclusions
The findings of this nonrandomized trial suggest that combined ibrutinib and venetoclax therapy may be an effective oral nonchemotherapy regimen for patients with CLL. In first-line treatment of older patients and those with high-risk CLL, during a follow-up of more than 3 years, remissions appeared to be durable, with activity seen across high-risk disease subgroups.
Trial Protocol
eMethods. Detailed Methods
eFigure 1. Patient Disposition for All Patients (N = 80)
eFigure 2. Bone Marrow MRD Response at Serial Time Points Among the Evaluable Patients
eFigure 3. Bone Marrow MRD Responses at 12 Cycles of the Combination and the Rate of Conversion of MRD-Positive Patients at Cycle 12 to U-MRD Remission at Cycle 24 Are Shown
eFigure 4. Individual Patient Level Bone Marrow MRD Levels at Serial Time Points Are Shown for the 24 Patients Who Were MRD-Positive at 12 Cycles of the Combination
eFigure 5. Immunoglobulin Levels at Serial Time Points in the Study
eFigure 6. Absolute T-Cell and NK-Cell Numbers at Serial Time Points in the Study
eTable 1. Characteristics of the Patients Who Achieved Marrow U-MRD Remission at the 24 Cycles of the Combination and Had MRD Recurrence
eTable 2. Bone Marrow Undetectable-MRD Remission by Pretreatment Characteristics
eTable 3. Clinical Responses at Serial Time Points (N = 80)
eTable 4. Characteristics of the Patients Who Developed Richter Transformation
eTable 5. Characteristics of the Patients Who Died
eTable 6. Characteristics of the Patients Who Came Off Study
eReferences
References
- 1.Burger JA. Treatment of chronic lymphocytic leukemia. N Engl J Med. 2020;383(5):460-473. doi: 10.1056/NEJMra1908213 [DOI] [PubMed] [Google Scholar]
- 2.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42. doi: 10.1056/NEJMoa1215637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger JA, Tedeschi A, Barr PM, et al. ; RESONATE-2 Investigators . Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437. doi: 10.1056/NEJMoa1509388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278-1291. doi: 10.1016/S0140-6736(20)30262-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311-322. doi: 10.1056/NEJMoa1513257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225-2236. doi: 10.1056/NEJMoa1815281 [DOI] [PubMed] [Google Scholar]
- 7.Cervantes-Gomez F, Lamothe B, Woyach JA, et al. Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res. 2015;21(16):3705-3715. doi: 10.1158/1078-0432.CCR-14-2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain N, Keating M, Thompson P, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019;380(22):2095-2103. doi: 10.1056/NEJMoa1900574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 10.Hallek M, Cheson BD, Catovsky D, et al. ; International Workshop on Chronic Lymphocytic Leukemia . Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446-5456. doi: 10.1182/blood-2007-06-093906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawstron AC, Villamor N, Ritgen M, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21(5):956-964. doi: 10.1038/sj.leu.2404584 [DOI] [PubMed] [Google Scholar]
- 12.Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787-798. doi: 10.1038/s41375-019-0602-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn IE, Tian X, Ipe D, et al. Prediction of outcome in patients with chronic lymphocytic leukemia treated with ibrutinib: development and validation of a four-factor prognostic model. J Clin Oncol. 2021;39(6):576-585. doi: 10.1200/JCO.20.00979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Sawaf O, Zhang C, Tandon M, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(9):1188-1200. doi: 10.1016/S1470-2045(20)30443-5 [DOI] [PubMed] [Google Scholar]
- 15.Al-Sawaf O, Zhang C, Robrecht S, et al. Clonal Dynamics after venetoclax-obinutuzumab therapy: novel insights from the randomized, phase 3 CLL14 trial. Blood. 2020;136(suppl 1):22-23. doi: 10.1182/blood-2020-136977 [DOI] [Google Scholar]
- 16.Wierda WG, Tam CS, Allan JN, et al. Ibrutinib (Ibr) plus venetoclax (Ven) for first-line treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): 1-year disease-free survival (DFS): results from the MRD cohort of the phase 2 CAPTIVATE Study. Blood. 2020;136(suppl 1):16-17. doi: 10.1182/blood-2020-134446 [DOI] [Google Scholar]
- 17.Munir T, Boucher RH, Webster N, et al. Continued long term responses to ibrutinib + venetoclax treatment for relapsed/refractory CLL in the Blood Cancer UK TAP Clarity Trial. Blood. 2020;136(suppl 1):17-18. [Google Scholar]
- 18.A study of the combination of ibrutinib plus venetoclax versus chlorambucil plus obinutuzumab for the first-line treatment of participants with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Updated March 5, 2021. Accessed May 4, 2021. ClinicalTrials.gov ID: NCT03462719. https://clinicaltrials.gov/ct2/show/NCT03462719
- 19.Cancer Research UK. A trial of ibrutinib with rituximab for chronic lymphocytic leukaemia (FLAIR). 2021. Accessed April 26, 2021. https://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/a-trial-ibrutinib-rituximab-chronic-lymphocytic-leukaemia-flair
- 20.Hillmen P, Rawstron AC, Brock K, et al. Ibrutinib plus venetoclax in relapsed/refractory chronic lymphocytic leukemia: The CLARITY Study. J Clin Oncol. 2019;37(30):2722-2729. doi: 10.1200/JCO.19.00894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niemann CU, Levin MD, Dubois J, et al. Venetoclax and ibrutinib for patients with relapsed/refractory chronic lymphocytic leukemia. Blood. 2021;137(8):1117-1120. doi: 10.1182/blood.2020008608 [DOI] [PubMed] [Google Scholar]
- 22.Jain N, Keating MJ, Thompson PA, et al. Combined ibrutinib and venetoclax in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). Blood. 2019;134(suppl 1):359. doi: 10.1182/blood-2019-131732 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Detailed Methods
eFigure 1. Patient Disposition for All Patients (N = 80)
eFigure 2. Bone Marrow MRD Response at Serial Time Points Among the Evaluable Patients
eFigure 3. Bone Marrow MRD Responses at 12 Cycles of the Combination and the Rate of Conversion of MRD-Positive Patients at Cycle 12 to U-MRD Remission at Cycle 24 Are Shown
eFigure 4. Individual Patient Level Bone Marrow MRD Levels at Serial Time Points Are Shown for the 24 Patients Who Were MRD-Positive at 12 Cycles of the Combination
eFigure 5. Immunoglobulin Levels at Serial Time Points in the Study
eFigure 6. Absolute T-Cell and NK-Cell Numbers at Serial Time Points in the Study
eTable 1. Characteristics of the Patients Who Achieved Marrow U-MRD Remission at the 24 Cycles of the Combination and Had MRD Recurrence
eTable 2. Bone Marrow Undetectable-MRD Remission by Pretreatment Characteristics
eTable 3. Clinical Responses at Serial Time Points (N = 80)
eTable 4. Characteristics of the Patients Who Developed Richter Transformation
eTable 5. Characteristics of the Patients Who Died
eTable 6. Characteristics of the Patients Who Came Off Study
eReferences