Abstract
The ability to perceive speech in noise (SPiN) declines with age. Although the etiology of SPiN decline is not well understood, accumulating evidence suggests a role for the dorsal speech stream. While age‐related decline within the dorsal speech stream would negatively affect SPiN performance, experience‐induced neuroplastic changes within the dorsal speech stream could positively affect SPiN performance. Here, we investigated the relationship between SPiN performance and the structure of the arcuate fasciculus (AF), which forms the white matter scaffolding of the dorsal speech stream, in aging singers and non‐singers. Forty‐three non‐singers and 41 singers aged 20 to 87 years old completed a hearing evaluation and a magnetic resonance imaging session that included High Angular Resolution Diffusion Imaging. The groups were matched for sex, age, education, handedness, cognitive level, and musical instrument experience. A subgroup of participants completed syllable discrimination in the noise task. The AF was divided into 10 segments to explore potential local specializations for SPiN. The results show that, in carefully matched groups of singers and non‐singers (a) myelin and/or axonal membrane deterioration within the bilateral frontotemporal AF segments are associated with SPiN difficulties in aging singers and non‐singers; (b) the structure of the AF is different in singers and non‐singers; (c) these differences are not associated with a benefit on SPiN performance for singers. This study clarifies the etiology of SPiN difficulties by supporting the hypothesis for the role of aging of the dorsal speech stream.
Keywords: aging, brain plasticity, diffusion tensor imaging, magnetic resonance imaging, music, singing, speech perception, white matter
The arcuate fasciculus (AF) was divided into ten segments to explore potential local specializations for speech perception in noise (SPiN) in young and older singers and non‐singers. Our results show that the structure of the AF differs in young and older adults, especially in frontotemporal segments, and that these differences affect SPiN performance. The structure of the AF was also different in singers and non‐singers, but these differences were not associated with a better SPiN performance.
1. INTRODUCTION
Elderly adults often struggle to perceive speech‐in‐noise (SPiN), which is associated with a decline in communication efficiency and pleasantness. Several hypotheses have been proposed to account for the age‐related SPiN decline, but a consensus about the underlying mechanisms is still lacking. Several studies have suggested that normal aging of the peripheral auditory system, called presbycusis, is the main contributing factor to SPiN difficulties in aging (e.g., Akeroyd, 2008; Divenyi & Haupt, 1997; Humes & Christopherson, 1991; Humes & Roberts, 1990; Jerger, Jerger, & Pirozzolo, 1991; Souza & Turner, 1994), but evidence suggesting that SPiN decline cannot be explained solely by presbycusis is accumulating (Bilodeau‐Mercure, Lortie, Sato, Guitton, & Tremblay, 2015; Fostick, Ben‐Artzi, & Babkoff, 2013; Jin, Liu, & Sladen, 2014; Tremblay et al., 2019).
An alternative hypothesis is that SPiN decline is associated with brain aging. Specifically, structural and functional aging within the dorsal speech stream has been associated with SPiN decline in aging (Bilodeau‐Mercure et al., 2015; Du, Buchsbaum, Grady, & Alain, 2016; Hwang, Li, Wu, Chen, & Liu, 2007; Manan, Yusoff, Franz, & Mukari, 2017; Sheppard, Wang, & Wong, 2011; Wong et al., 2009; Wong, Ettlinger, Sheppard, Gunasekera, & Dhar, 2010). The dorsal speech stream is an important network for sublexical speech perception and auditory‐motor integration (Hickok & Poeppel, 2007; Rauschecker & Scott, 2009). It connects the ventral premotor cortex (PMv), which contains speech motor programs (Guenther, Ghosh, & Tourville, 2006), and posterior inferior frontal gyrus (pIFG), involved in phonological processing (e.g., Gough, Nobre, & Devlin, 2005; Hartwigsen et al., 2010) to the posterior superior temporal gyrus (pSTG) via the inferior parietal lobule, involved in sensorimotor transformations and phonological working memory (e.g., Deschamps, Baum, & Gracco, 2014; Deschamps, Courson, Dick, & Tremblay, 2020; Kirschen, Davis‐Ratner, Jerde, Schraedley‐Desmond, & Desmond, 2006; Romero, Walsh, & Papagno, 2006). The arcuate fasciculus (AF) forms the white matter scaffolding of the dorsal speech stream. A recent study from our group using High Angular Resolution Diffusion Imaging (HARDI) techniques has found that the structure of the bilateral AF is related to SPiN performance in young and older adults (Tremblay et al., 2019), particularly in terms of sensitivity to phonetic details.
Because of the lack of consensus regarding underlying SPiN mechanisms, treatment options for SPiN difficulties are scarce. A growing body of evidence suggests that playing a musical instrument (Alain, Zendel, Hutka, & Bidelman, 2014; Bidelman & Alain, 2015; Fleming, Belleville, Peretz, West, & Zendel, 2019; Fostick, 2019; Parbery‐Clark, Strait, Anderson, Hittner, & Kraus, 2011; White‐Schwoch, Woodruff Carr, Anderson, Strait, & Kraus, 2013; Zendel & Alain, 2012; Zendel, West, Belleville, & Peretz, 2019) or singing in a choir (Dubinsky, Wood, Nespoli, & Russo, 2019) is associated with SPiN benefits in aging, but the mechanism of action for this effect is unclear. Playing a musical instrument and singing strongly rely on auditory‐motor integration, whereby accurate mapping between a sound and the motor commands used to produce that sound is necessary to monitor performance and perform fine sensory‐motor adjustments. Importantly, auditory‐motor integration is also key to speech perception. Further, it has been suggested that the dorsal speech stream is involved in auditory‐motor integration. It is therefore possible that musical activities influence SPiN through their effect on auditory‐motor integration. Consistent with this idea, several studies have shown that singing engages a frontotemporal network akin to the dorsal speech stream, including the inferior parietal lobule, dorsal premotor cortex, PMv, pSTG, and IFG, as well as other regions involved in vocalization (e.g., Brown, Martinez, Hodges, Fox, & Parsons, 2004; Kleber & Zarate, 2014; Ozdemir, Norton, & Schlaug, 2006; Perry et al., 1999; Segado, Hollinger, Thibodeau, Penhune, & Zatorre, 2018). Moreover, one study has shown that professional musical training is associated with better SPiN performance via strengthening of the functional connectivity between right auditory associative regions (pSTG and the planum temporale) and right IFG and PMv (Du & Zatorre, 2017). According to the OPERA hypothesis (Patel, 2011, 2012, 2014), since singing and SPiN are processed within overlapping brain networks, in this case the AF, singing would induce experience‐dependent plasticity in the AF, which could lead to lesser SPiN decline in older singers compared to non‐singers. The notion of a relationship between musical training and white matter plasticity is long‐standing. Indeed, differences have been observed between professional musicians, amateur musicians and non‐musicians in several fasciculi, including the corticospinal tract (Imfeld, Oechslin, Meyer, Loenneker, & Jancke, 2009; Ruber, Lindenberg, & Schlaug, 2015), the inferior longitudinal fasciculus (Schmithorst & Wilke, 2002), the inferior and middle cerebellar peduncles (Abdul‐Kareem et al., 2011), the corpus callosum (Ozturk, Tascioglu, Aktekin, Kurtoglu, & Erden, 2002; Schlaug, Jancke, Huang, Staiger, & Steinmetz, 1995; Schmithorst & Wilke, 2002; Steele, Bailey, Zatorre, & Penhune, 2013), and the internal and external capsule (Bengtsson et al., 2005; Han et al., 2009; Schmithorst & Wilke, 2002). Importantly, microstructural differences between musicians and non‐musicians have been found in the AF (Halwani, Loui, Ruber, & Schlaug, 2011) as defined by Glasser and Rilling (2008) and the AF/superior longitudinal fasciculus (Oechslin, Imfeld, Loenneker, Meyer, & Jancke, 2009) as defined by Catani, Jones, and ffytche (2005).
The objectives of the present study were fourfold: (a) to compare SPiN performance between singers and non‐singers, (b) to examine the effect of aging on the macrostructure and microstructure of the bilateral AF, (c) to examine the relationship between the structure of the AF and SPiN decline in aging, and (d) to determine whether differences in the structure of the AF between singers and non‐singers are associated with a benefit in SPiN performance for singers. Because the anatomy of the AF is still debated in terms of the number of sub‐components it contains and their roles (Dick & Tremblay, 2012), here we examined it through two different approaches. First, we examined the structure of the AF as a whole, and then we divided it into 10 segments to identify (a) potential spatial heterogeneity in the effect of age and singing on the structure of the AF and (b) potential local specializations for SPiN. Our main hypotheses were that (a) a SPiN advantage would be found for aging singers, (b) age differences in the structure of the bilateral AF would be more pronounced in non‐singers than in singers, reflecting a protective role for singing on brain structure, (c) the structure of the bilateral AF would be positively correlated with SPiN performance, especially within frontal segments believed to host speech representations, and (d) the SPiN advantage for aging singers would be explained by the structure of the bilateral AF. To test these hypotheses, we used HARDI anatomically constrained probabilistic tractography based on crossing‐fiber robust fiber orientation distribution function and automatic fiber bundle extraction of the AF. Specifically, we combined conventional diffusion tensor imaging (DTI) measures, track‐based measures and more robust measures to crossing and kissing fibers provided by fiber orientation distributions (FOD) computed using spherical deconvolution, such as apparent fiber density (AFD) (Raffelt et al., 2012) and number of fiber orientations (NuFO) (Dell'Acqua, Simmons, Williams, & Catani, 2013).
2. MATERIAL AND METHODS
2.1. Participants
A non‐probabilistic sample of 85 native speakers of Quebec French aged 20–87 years (M = 54.11 ± 19.47, 50 females) was assembled. Participants were recruited through emails, Facebook messages and posters distributed in the community and at Université Laval, as well as through emails and Facebook messages sent directly to choirs in the Quebec City area. Eligibility criteria to participate in this study were to be either a choral singer or a non‐singer, to have no history of hearing, speech, language, psychological, neurological, or neurodegenerative disorders, and to have little or no experience with a musical instrument. Amateur singers were defined as individuals singing in a choir since at least 2 years with a minimal weekly practice of 60 min. Non‐singers were defined as individuals not participating in any form of amateur or professional singing. Eligibility criteria were verified through telephone interviews. One participant was excluded a posteriori because he played a musical instrument regularly in addition to singing (see Section 2.2 for more information). The remaining 84 participants were divided into two groups: 43 non‐singers and 41 choral singers.
The general cognitive functioning of the participants was evaluated using the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005). All participants were right‐handed according to the Edinburgh Handedness Inventory (score ≥ 60%) (Oldfield, 1971). Participants' characteristics are provided in Table 1A. As detailed in the table, non‐singers and singers did not differ in age, education, handedness and cognition (all p > .05). Both groups were also matched for sex (χ2 = 1.864, p = .172, φ = .149). The study was approved by the Comité d'éthique de la recherche sectoriel en neurosciences et santé mentale, Institut Universitaire en Santé Mentale de Québec (#192–2017 and #1495–2018). All participants provided informed consent.
TABLE 1.
Group characteristics
| A. All participants | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Non‐singers (N = 43, 22 females) | Singers (N = 41, 27 females) | t test | |||||||
| Mean | SD | Min | Max | Mean | SD | Min | Max | t | p | |
| Age | 54.02 | 19.53 | 20.00 | 86.00 | 54.95 | 19.25 | 22.00 | 87.00 | −0.22 | .83 |
| Education (years) a | 15.26 | 2.58 | 11.00 | 21.00 | 15.15 | 2.78 | 6.00 | 23.00 | 0.19 | .85 |
| Handedness (Oldfield) b | 93.58 | 9.97 | 60.00 | 100.00 | 95.77 | 8.63 | 66.67 | 100.00 | −1.08 | .28 |
| MoCA (/30) c | 27.56 | 2.14 | 21.00 | 30.00 | 27.54 | 1.92 | 23.00 | 30.00 | 0.05 | .96 |
| Health (/7) d | 5.16 | 0.88 | 3.00 | 7.00 | 5.10 | 0.97 | 3.00 | 7.00 | 0.32 | .75 |
| Right ear PTA e | 14.78 | 11.94 | −5.00 | 56.67 | 11.02 | 7.93 | 0.00 | 33.33 | 1.69 | .10 |
| Left ear PTA | 12.40 | 8.89 | −3.33 | 31.67 | 7.94 | 7.18 | −3.33 | 25.00 | 2.53 | .01 |
| B. Participants who have completed the SPiN task | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Non‐singers (N = 36, 19 females) | Singers (N = 36, 22 females) | t test | |||||||
| Mean | SD | Min | Max | Mean | SD | Min | Max | t | p | |
| Age | 53.19 | 18.78 | 20.00 | 86.00 | 55.00 | 18.27 | 22.00 | 87.00 | −0.41 | .68 |
| Education (years) a | 15.17 | 2.43 | 11.00 | 21.00 | 15.17 | 2.83 | 6.00 | 23.00 | 0.00 | 1.00 |
| Handedness (Oldfield) b | 93.44 | 9.86 | 60.00 | 100.00 | 95.74 | 8.71 | 66.67 | 100.00 | −1.05 | .30 |
| MoCA (/30) c | 27.50 | 2.09 | 21.00 | 30.00 | 27.53 | 1.81 | 23.00 | 30.00 | −0.06 | .95 |
| Health (/7) d | 5.14 | 0.88 | 3.00 | 7.00 | 5.19 | 0.98 | 3.00 | 7.00 | −0.25 | .80 |
| Right ear PTA e | 13.57 | 10.72 | −5.00 | 40.00 | 10.74 | 8.01 | 0.00 | 33.33 | 1.27 | .21 |
| Left ear PTA | 11.81 | 9.10 | −3.33 | 31.67 | 7.83 | 7.24 | −3.33 | 25.00 | 2.05 | .04 |
Note: Independent t tests were conducted to compare groups of participant (A) who have completed the MRI only and (B) who have completed the MRI and the SPiN task. Values in bold indicate significant differences between the two groups.
Abbreviations: SD, standard deviation of the mean; N, number of participants per group.
Education = Number of years of education based on the highest degree obtained in Quebec.
Handedness = The handedness was measured with the Edinburgh Handedness Inventory. A lateralization quotient of 60% or more indicates laterality on the right.
MoCA = Montreal cognitive assessment. Higher scores indicate better cognitive functions. A cutoff of 20/30 has been proposed to avoid false positive (Waldron‐Perrine & Axelrod, 2012).
Health = self‐reported general health status on a scale of 0 to 7 (0 being lowest health level).
PTA = pure tone average thresholds measured in decibels at 0.5, 1, and 2 kHz for each ear.
2.2. Information on past and present musical activities
All participants answered a questionnaire on past and present musical experiences. The questionnaire is available on the Scholar Portal Dataverse: https://doi.org/10.5683/SP2/8IX6QZ. For singing, the choral singers had between 2 and 62 years of continuous choral singing experience (M = 17.68 ± 14.14 years). All singers practiced at least once a week for 1 hr in a choir. In addition to singing in a choir, 16 singers (39%) practiced at home every day, 17 at least once a week (41%), 1 at least once a month (2%) and 2 less than once month (5%). Five singers did not practice outside of their weekly choir (12%). Finally, 11 singers (27%) had received formal singing training. Among the non‐singers, 11 had previous experience of group singing, including six who stopped singing 30 to 60 years prior to the experiment, three who stopped singing 7 to 15 years prior to the experiment and 1 who stopped singing 1 year prior to the experiment after only 3 months of singing experience. The others had between 5 months to 12 years of singing experience (M = 4.00 ± 3.58 years). Those with the most years of experience were those who stopped singing decades prior to the study.
In terms of musical instrument experience, of the 84 participants included in this study, 48 (28 non‐singers, 20 singers) reported having never played a musical instrument or having only taken mandatory music lessons in elementary school. 28 participants (12 non‐singers, 16 singers) reported having played a musical instrument in the past, with experience ranging from 1 month to 20 years (non‐singers: M = 5.15 ± 6.12, range = .25–.20 years; singers: M = 6.27 ± 3.77, range = 1–15 years), and having stopped playing between 6 months and 65 years (non‐singers: M = 26.46 ± 17.92, range = .50–.52 years; singers: M = 27.94 ± 15.91, range = 10–65 years). The participant who stopped playing 6 months before the study had only 1 month of experience. Information on the number of years of previous practice is missing for two participants. Finally, eight participants (three non‐singers, five singers) reported irregularly and infrequently playing a musical instrument at the time of the study, with the frequency of such practice ranging from once every other week to a few times a year. Those who played at the time of the study reported playing an instrument for 1 to 38 years (non‐singers: M = 1.00 ± .00, range = 1–1 years; singers: M = 13.00 ± 14.46, range = 2–38 years). The participant with 38 years of practice reported playing only a few times a year since childhood. The participant who was excluded on the basis of musical instrument experience (see Section 2.1) reported playing 4 hr per week for 12 years. The proportion of participants with no experience, past or present, of musical instrument playing did not differ between the two groups (χ2 = 2.358, p = .308, Cramér's V = .168).
2.3. Experimental design
The experiment included three visits on three separate days. The first and third visits took place at the CERVO research centre in a double‐walled soundproof room. The second visit took place at the Clinic IRM Québec‐Mailloux in Quebec City. During the first visit, participants completed questionnaires and underwent an audiometric evaluation. They also completed several other tests (articulation, prosody, and voice evaluations) that are not reported here. The second visit was the MRI session. Finally, during the third visit, a subgroup of participants (n = 72) completed a SPiN task. The characteristics of the participants who completed the SPiN task are detailed in Table 1B. Of the 12 participants that did not complete the SPiN task, 10 participants (six non‐singers, four singers) were among those who reported having never played a musical instrument and 2 participants (one non‐singer, one singer) were among those who reported having played a musical instrument in the past. The subgroups of singers and non‐singers were matched in musical experience (χ2 = 2.063, p = .357, Cramér's V = .169). The MRI visit had an average duration of 1 hour and the lab visits had an average duration of 2–3 hr.
2.4. Audiometric evaluation
Peripheral hearing was evaluated with pure tone audiometry using a clinical audiometer (AC40, Interacoustic, Danemark) in a double‐walled soundproof room. Each ear was tested separately at 0.5, 1, and 2 kHz, and a standard pure tone average (PTA) was computed (Stach, 2008). The PTAs of both ears were strongly correlated (r = .747, p ≤ .001). Eleven participants showed signs of mild hearing loss (PTA between 25 and 40 dB) and one participant showed signs of moderately severe hearing loss (PTA = 56 dB) in at least one ear (Stach, 2008). Of the 12 participants, 2 were singers aged 80, 1 was a non‐singer aged 43 and the others (9 participants) were non‐singers aged over 60. None of the participants wore hearing aids. Independent t‐tests were carried out to compare hearing levels across groups. The results showed significantly lower left ear PTA in singers than non‐singers (t[82] = 2.527, p = .013), with no difference for the right ear PTA (t[82] = 1.691, p = .095). The difference in left ear PTA was related to a difference in thresholds at .5 kHz (t[70.74] = 2.695, p = .009), with singers exhibiting lower thresholds than non‐singers. No difference was observed for the other frequencies. Results of the pure tone audiometry are illustrated in Supporting Information S1.
2.5. SPiN task
Participants completed a syllable discrimination in noise task that took place in a double‐walled soundproof room on a third visit. Only 72 (36 singers, 36 non‐singers) out of 84 participants were available for this visit. The task consisted in the discrimination of 300 minimal pairs (150 identical, 150 different) of monosyllabic Quebec‐French Consonant‐Vowel‐Consonant (CVC) syllables created using SyllabO+ (Bedard et al., 2017). The pairs differed by only one phonemic trait, which was located in the onset (e.g., /kas–gas/) (50%) or coda position of the syllable (e.g., /vɛʃ–vɛʒ/) (50%). The syllables were produced by a native 22 years‐old male Quebec French speaker and recorded (sampling rate = 44,000 Hz, 16 bits of quantization), using a Shure headset microphone (Microflex Beta 53) connected to a Quartet USB audio interface (Apogee Electronics, Santa Monica) connected to an iMac computer. Syllables were produced at the end of a complete sentence (Now I say ___) to ensure that prosody was constant and neutral. The sound files were saved directly to disk using Sound Studio 4.8.14 (Felt Tip Software, New York) and edited using a PRAAT (Boersma & Weenink, 2001) script to normalize root‐mean‐square (RMS) intensity at 70 dB SPL. Each syllable was recorded three times and the best trial was selected independently by two native Quebec French speakers. To validate the selected sounds, a phonetic transcription was performed by a native Quebec French student with training in phonetics. The syllables had an average duration (mean ± SD) of 496 ± 48 ms. To ensure the absence of a syllable duration effect, an ANOVA on syllables duration (dependent variable) with Condition and Type (Identical, Different) as within‐subject variables was conducted. No effect of Condition (F[2,98] = 2.096, p = .128, ηp 2 = .041) or type (F[1,49] < .001, p = .994, ηp 2 < .001) and no Condition x Type interaction (F[2,98] = .230, p = .795, ηp 2 = .005) were found.
During the task, the syllables were presented simultaneously with a multi‐talker's babble noise. The noise was created by Perrin and Grimault (2005). It was generated by mixing four French‐talker voices (2 females, 2 males, 25 to 45 years old) reading newspaper news independently in a soundproof booth. Three signal‐to‐noise ratios (SNR; Pressuresignal/Pressurenoise) were used (Quiet, SNR +3 dB, SNR −3 dB). The noise files were edited to normalize RMS intensity at either 67 dB SPL (SNR +3) or 73 dB SPL (SNR −3).
The experiment started with a practice session of 16 trials (50% identical). The sound level was adjusted for each participant by the experimenter to a comfortable level based on the participant's feedback, before and/or after the practice session, if necessary, and was kept constant thereafter. Sound level adjustments were made to ensure that the participants were able to hear the syllables and that the task actually measured discrimination, not hearing. The main task was divided in two runs of 15 min each with a short break between the runs. The order of presentation of the two runs was randomized across participants. In each trial, two syllables were presented diotically with an inter‐stimuli interval of 300 ms, through high quality headphones (Beyer, DT 770 Pro), using Presentation® Software (Version 20.0, Neurobehavioral Systems, Inc., Berkeley, CA, https://www.neurobs.com). 100 different pairs of syllables (50 identical and 50 different) were presented for each condition. Ten percent of the pairs (five identical and five different) in each condition were repeated to calculate intra‐speaker reliability. The intraclass correlation coefficient (two‐way mixed effects, single measurement, absolute agreement) for the task was .688 with a 95% confidence interval from .665 to.710 (F[2,159,2,159] = 5.415, p < .001), indicating a significant moderate reliability (Koo & Li, 2016).
During the presentation of the syllables, a white fixation cross centered on a dark gray background was presented on a 27‐in. monitor (HP EliteDisplay, E272q) that was located ~45 cm from the participant. Following the presentation of the syllables, a green question mark (?) was presented to indicate to the participants to answer. Participants were asked to determine whether the syllables were identical or different using a response box (Cedrus, Model RB‐530). Participants were given a maximum of 3 s to respond. The inter‐trial interval was 1,000 ms. All stimuli and experiment files are available on the Scholar Portal Dataverse: https://doi.org/10.5683/SP2/8IX6QZ.
For each condition of noise, sensitivity was calculated. Sensitivity is a measure of the capacity to correctly recognize whether pairs are different or not (sensitivity = z(Probability[“different” | DIFFERENT]) – z(Probability[“different” | IDENTICAL])) (Macmillan & Creelman, 2004). The quiet condition was removed because of a strong ceiling effect.
2.6. MRI data acquisition
The MRI data were acquired on a whole‐body Philips 3.0 Tesla Achieva TX using an 8‐channel head coil at the Clinic IRM Québec‐Mailloux in Quebec City. Structural MR images were acquired with a 3D T1‐weighted MPRAGE sequence (TR = 8.3 ms, TE = 4.0 ms, FOV = 240 mm, flip angle = 8°, 240 × 240 acquisition matrix, 180 slices/volume, no gap, voxel size = 1 mm3). Diffusion weighted (DW) images were acquired with a HARDI acquisition (TR = 7,200 ms, TE = 86 ms, flip angle = 90°, 112 × 112 acquisition matrix, 60 slices/volume, voxel size = 2 mm3), including a HARDI sequence with 2 b‐values (b = 1,000 s/mm2, 25 directions; b = 2000 s/mm2, 50 directions, where directions are uniformly distributed on the sphere accounting both b‐values), a non‐diffusion‐weighted volume (b = 0 s/mm2, 5 directions) and a non‐diffusion‐weighted sequence with reversed phase encoding (b = 0 s/mm2). Throughout the procedure, the participants' head was immobilized using a set of cushions and pads.
2.7. MRI data processing
The DW images processing was performed using TractoFlow‐ABS (atlas‐based segmentation) pipeline (Theaud et al., 2020), a robust processing pipeline including 14 steps using Nextflow (Di Tommaso et al., 2017) and Singularity (Kurtzer, Sochat, & Bauer, 2017). The analysis codes used in the current study are available online (refer to the Data availability statement). The processing included denoising of the DW images using principal‐component‐analysis‐based denoising algorithm (Veraart et al., 2016) from MRtrix3 (Tournier et al., 2019), correction for susceptibility artifacts with the TOPUP procedure (Andersson, Skare, & Ashburner, 2003) implemented in FMRIB Software Library (FSL) (Smith et al., 2004) and correction for eddy‐current distortions and subject motion with the eddy tool (Andersson & Sotiropoulos, 2016) implemented in FSL. Next, FSL's brain extraction tool (bet) (Smith, 2002) was used to extract the brain mask. The image intensities were normalized using a N4 correction procedure (Tustison et al., 2010) from the advanced normalization tools (ANTs) (Avants et al., 2011) and the resulting DW images were resampled to 1 mm isotropic as implemented in Diffusion Imaging in Python (DIPY) toolbox (Garyfallidis et al., 2014). Next, the b‐values 0 mm 2/s, 1000 mm 2/s were extracted from the HARDI sequence to compute the tensor model and extract the DTI measures (fractional anisotropy [FA], radial diffusivity [RD], axial diffusivity [AD], and mean diffusivity [MD]) using DIPY. The b‐values 0, 1000, 2000 mm2/s were used to compute the function of ODF (fODF) and its measures: AFD (Raffelt et al., 2012) and NuFO (Dell'Acqua et al., 2013). Here, the AFD total (AFDtot), which represents the sum of all the fODF in the sphere, has been computed. The fODF were computed using constrained spherical deconvolution (Descoteaux, Deriche, Knosche, & Anwander, 2009; Tournier, Calamante, & Connelly, 2007), with a spherical harmonic order eight, as implemented in DIPY.
The T1 images were denoised using a Non‐Local Means filter robust to Rician noise (Coupe et al., 2008), corrected with a N4 correction procedure from ANTs, resampled with DIPY and the brain mask was extracted with ANTs. Subsequently, the T1 image was registered on the b = 0 mm2/s and the FA image using the symmetric image normalization registration algorithm available in ANTs. Finally, white matter mask was extracted using wmparc and aparc+aseg labels from Freesurfer software version 6.0.0 (http://freesurfer.net) (Dale, Fischl, & Sereno, 1999; Fischl & Dale, 2000; Fischl, Sereno, & Dale, 1999). The whole brain tractograms were generated using probabilistic local tracking with 10 seeds per voxel and the white matter mask extracted from Freesurfer as seeding and tracking mask. The outputs from all steps of Tractoflow (DW and T1 images) and Freesurfer were visualized and interventions were performed when required.
2.8. AF extraction
For each participant, the bilateral AF were extracted from the whole brain tractogram using a modified multi‐atlas and multi‐parameter version of RecoBundles (Garyfallidis et al., 2018) (RecoBundlesX), which has been shown to be robust to pathological brains (Garyfallidis et al., 2018). RecoBundles uses tract‐based registration and clustering methods to extract tracks that have similar shapes to prior bundle models generated from manual virtual dissection (Garyfallidis et al., 2018). The AF were generated from manual virtual dissection on 5 participants from the Human Connectome Project (HCP) dataset (subjects ID: 193441, 219231, 286650, 486759, and 615441) (Van Essen et al., 2013). The AF was filtered from the diffusion RGB‐color map of each subject using MI‐Brain software (https://www.imeka.ca/mi-brain) with a two‐regions‐of‐interest (ROIs) approach as described in Catani, Howard, Pajevic, and Jones (2002) including a ROI in the frontal lobe and another one in the temporal lobe.
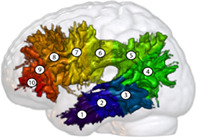
Finally, tractometry was performed using Nextflow and The Sherbrooke Connectivity Imaging Lab PYthon (SCILPY) dMRI processing toolbox as described in Cousineau et al. (2017) to compute the number of tracks, volume, DTI and ODF measures of the AF. All DTI and fODF measures were weighted for the local track density and track‐based measures were normalized by the individual total brain volume. Next, the bilateral AF of each participant was divided into 10 segments per hemisphere (Figure 1) based on the centroid of one HCP subjects (193441) resampled to 10 points. The resulting AF underwent visual inspection to ensure the accuracy of the extraction. The bundles that failed the visual inspection were excluded.
FIGURE 1.
Example of a left arcuate fasciculus (participant ID: S029) divided into 10 segments from the temporal lobe (1) to the frontal lobe (10). The fasciculus is displayed on the MNI152c 2009 nonlinear symmetrical template (Fonov, Evans, McKinstry, Almli, & Collins, 2009), which was warped to the individual anatomical scan (T1) of the participant
2.9. Statistical analyses
All statistical analyses were conducted with SPSS 25 for Mac (IBM). Each dependent variable was visually inspected using histograms and boxplots to verify normality, and Levene's test was used to verify the assumption of equality of variances across groups (p > .05). One participant was removed for the SPiN task because of a sensitivity below 0. Any diffusion data more than 2.5 box length from the edge of the boxplots was removed. NuFO (logarithm), AFDtot (squared) and RD (squared) were transformed.
To test the first hypothesis of the study—that a SPiN advantage would be found for aging singers—a linear mixed model (LMM) analysis was conducted with sensitivity as dependent variable. Group (singers, non‐singers) was used as a between‐subject categorical variable, SNR (+ 3, −3 dB)) was used as a within‐subject (repeated) categorical fixed factor, and Age (grand‐mean centered) and left ear PTA were used as continuous between‐subject fixed factors. Participants were included as a random factor (random intercept model).
To test the second hypothesis—that age differences in the structure of the bilateral AF would be more pronounced in non‐singers than in singers—LMM analyses were conducted for each diffusion measures (FA, RD, MD, AD, NuFO, and AFDtot) and track‐based measures (number of tracks, volume) of each fasciculus/segment (1–10). In all analyses, Group (singers, non‐singers) was used as a between‐subject categorical variable, Hemisphere (Left, Right) was used as a within‐subject (repeated) categorical fixed factor, and Age (grand‐mean centered) and left ear PTA were used as continuous between‐subject fixed factors. Participants were included as a random factor (random intercept models).
To test the third hypothesis—that the structure of the bilateral AF would be positively correlated with SPiN performance, especially within frontal segments—simple mediation analyses (model #4) were conducted with PROCESS for SPSS (Hayes, 2017), separately for each metric that showed age effects. Age (grand‐mean centered) was used as the predictor variable (X), sensitivity was used as dependent variable (Y), and diffusion measures were used as continuous mediators (M).
To test the fourth hypothesis—that a SPiN advantage would be found for aging singers, which would be explained (at least in part) by the structure of the bilateral AF—two sets of analyses were conducted. For each diffusion metric showing age‐independent group differences, simple mediation (model #4) analyses were conducted. For each diffusion metric showing age‐dependent group differences, moderated mediation (model #58) analyses were conducted. The conceptual diagrams of the simple and the moderated mediations are presented in Supporting Information S2. In the simple mediation models, Group was used as a dichotomous predictor variable (X), Sensitivity was used as dependent variable, diffusion measures were used as mediators (M) and Age (grand‐mean centered) as a continuous between‐subject covariate. For the moderated mediation analyses, Age (grand‐mean centered) was used as a continuous predictor variable (X), Sensitivity was used as dependent variables (Y), diffusion measures were used as continuous mediators (M) and Group was used as a dichotomous moderator (W).
In all analyses, hearing ‐operationalized as the left ear PTA‐ was included as a between‐subject covariate. A bootstrapping approach was used to test for the significance of the indirect effects (i.e., effect of age on sensitivity through white matter) using percentile bootstrapping with 20,000 samples.
3. RESULTS
3.1. SPiN performance
A linear mixed model analysis was conducted to compare behavioral performance between groups and SNRs. Controlling for hearing, the results revealed significant main effects of age (F[1,69.0] = 79.05, p ≤ .001) and hearing (F[1,67.5] = 5.60, p = .021) on sensitivity, revealing a decrease in performance with an increase in age and decline in hearing. A significant main effect of SNR was also found (F[1,67.3] = 682.45, p ≤ .001), revealing a lower performance in the SNR −3 dB condition compared with the SNR +3 dB condition. No other significant effect or interaction was found. The detailed results are provided in Supporting Information S3. The singers had a sensitivity of 2.75 ± .69 (SNR +3) and 1.26 ± .55 (SNR −3), and non‐singers had a sensitivity of 2.63 ± .58 (SNR +3) and 1.17 ± .56 (SNR −3).
Additional regression analyses were performed on the singers to investigate the relationship between the number of continuous years of singing and sensitivity. Holding hearing and age constant, no significant relationship between sensitivity and number of years of singing was found in both SNRs (SNR +3 dB: β = −.010, t = −1.680, p = .103; SNR −3 dB: β = −.005, t = −1.242, p = .223).
Since no Age x SNR interaction and no relationship between singing and sensitivity in both SNRs were observed, SPiN performance was operationalized as the average sensitivity of the two SNRs in all subsequent analyses. The singers had an average sensitivity of 2.00 ± .58 and non‐singers had an average sensitivity of 1.92 ± .50.
3.2. AF extraction
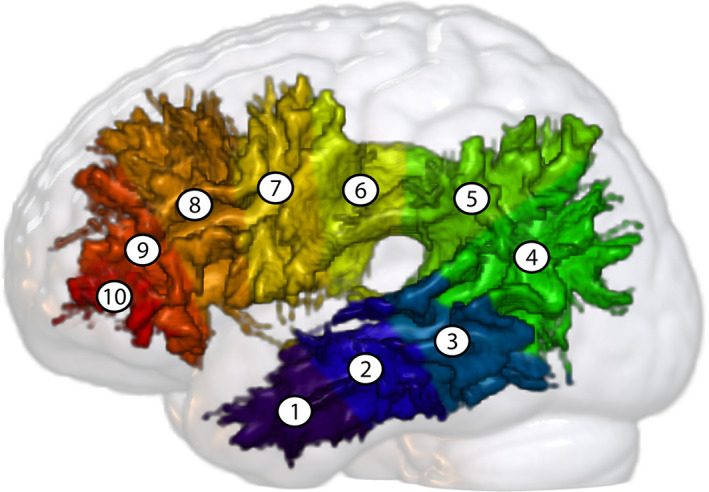
For each participant except one (a young non‐singer, representing 1.19% of all participants), we were able to extract the bilateral AF. In addition, two bundles (right, left) for another subject failed the visual inspection and were excluded. For three other participants, the segment 10 of the left AF was not found, but they were not excluded from analyses. The resulting groups included 42 non‐singers and 40 singers. The average of the right and left AF of all participants are illustrated in Figure 2 and all individual bundles are shown in Supporting Information S4.
FIGURE 2.
Average of the right and left AF of all participants. The average AF are displayed on the MNI152c 2009 nonlinear symmetrical template (Fonov et al., 2009) in standard space
3.3. Age effects and group differences in the AF
3.3.1. Age effects
The analyses revealed that the AF undergoes important changes with age. Specifically, holding hearing constant, age effects on FA, RD, MD, and AD were found, with a decrease in FA and an increase in RD, MD, and AD with increasing age. Hemispheric differences were also found, indicating that the left whole AF had greater MD, AD, RD, volume, and number of tracks and lower AFDtot and NuFO compared with the right AF. No other effect was significant. The detailed results are provided in Supporting Information S5.
The next analyses focus on the 10‐part AF segmentation. Inferential statistics are provided in Supporting Information S6 to S8. Here again, age effects were widespread (Figure 3a). As summarized in Figure 3a, an increase in age was associated with lower FA in all segments except for the fourth and fifth, higher RD in all segments, higher MD in all segments except for the third, higher AD (segments 1, 5, 7, 8, 9, and 10), higher AFDtot (segments 3, 5, and 6), lower AFDtot (segment 9), higher NuFO (segments 3 and 6) and lower volume (segments 4, 5, and 10). In addition, hemispheric differences (Figure 3b) were found for each metric. Finally, Age x Hemisphere interactions were found for RD (segment 10), AD (segments 5, 6, 7, 9, and 10), MD (segments 5, 7, 9, and 10), AFDtot (segment 8) and volume (segment 9) (Figure 3c). To decompose these interactions, linear regression analyses were conducted for each hemisphere with diffusion measures as dependent variables, and age (grand‐mean centered) as regressor. The detailed results are provided in Supporting Information S9. Overall, negative age effects were slightly more pronounced in the right compared with the left hemisphere.
FIGURE 3.
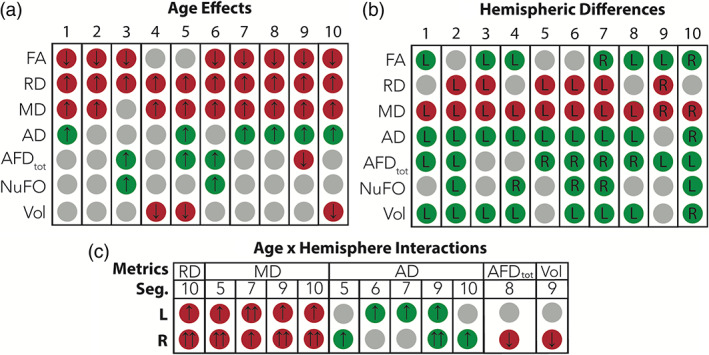
Age‐related and hemispheric effects for the 10 segments of the bilateral AF. (a) Age effects. An upward‐pointing arrow indicates that age is associated with an increased value while a downward‐pointing arrow indicates that age is associated with a decreased value. A green circle signals a beneficial age effect while a red circle signals a detrimental age effect. (b) Hemispheric differences. The letter in the circles indicates the hemisphere (L: left; R: right) with the highest value. A green circle signals a beneficial effect on the white matter while a red circle signals a detrimental effect. (c) Results of the linear regression analyses conducted to decompose the Age x Hemisphere interactions. An upward‐pointing arrow indicates that age is associated with an increased value while a downward‐pointing arrow indicates that age is associated with a decreased value. A double arrow indicates the hemisphere with the strongest age effect. A green circle signals a beneficial age effect on the white matter while a red circle signals a detrimental age effect
3.3.2. Group differences in AF
Holding hearing and age constant, the LMM analysis revealed no group difference. However, Group x Hemisphere interactions were found for FA (segment 3, 5, and 6), RD (segments 1, 5, and 8), MD (segments 1 and 4), AD (segment 4), and volume (segment 6). To decompose these interactions, independent‐t tests were conducted on asymmetry indexes calculated using the following formula: [(left–right)/(left+right)], which is widely used in diffusion MRI studies (e.g., O'Muircheartaigh et al., 2013; Ruber et al., 2015; Shu, Liu, Duan, & Li, 2015; Wilde et al., 2009). Positive values indicate leftward asymmetry, and negative values indicate rightward asymmetry. All effects are summarized in Figure 4a. Singers showed significantly greater leftward FA asymmetry in segment 3 (t[80] = −2.085, p = .040), RD asymmetry in segment 5 (t[80] = −2.510, p = .014), MD asymmetry in segment 4 (t[80] = −2.625, p = .010), AD asymmetry in segment 4 (t[80] = −2.446, p = .017) and nonsignificant greater leftward volume asymmetry in segment 6 (t[80] = −1.949, p = .055). The singers also showed significant rightward FA asymmetry in segments 5 (t[80] = 2.648, p = .010) and 6 (t[80] = 2.148, p = .035) and nonsignificant greater rightward RD asymmetry in segments 1 (t[77] = 1.465, p = .147) and 8 (t[79] = 1.797, p = .076), and MD asymmetry in segment 1 (t[73] = .955, p = .343). All effects except that for volume asymmetry were significant before removing extreme values. The box plots illustrating the interhemispheric asymmetry differences between singers and non‐singers are provided in Figure 5. Pairwise comparisons controlled for age and hearing were also conducted to compare each hemisphere between Groups. Singers had higher FA in the right segment 6 (mean difference = .028, p = .024), and higher AD (mean difference = 0.00005, p = .005) and MD (mean difference = .00002, p = .045) in the left segment 4 compared with non‐singers.
FIGURE 4.
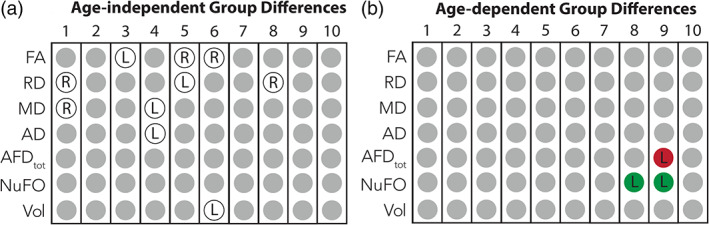
Summary of the interhemispheric asymmetry differences found between groups. (a) Age‐independent differences are represented in white circles. The letter in the circles indicates the direction of the asymmetry for singers (L: leftward; R: rightward). In 60% of these cases, singers presented greater white matter structure in the left hemisphere compared with the right (FA, segment 3; RD, segments 1 and 8; MD, segment 1; AD, segment 4; volume, segment 6). In the other cases, singers presented greater white matter structure in the right hemisphere compared with the left (FA, segments 5 and 6; RD, segment 5, MD, segment 4). (b) Age‐dependent asymmetry differences. The letter in the circles indicates the direction of the age‐related increasing asymmetry for singers (L: leftward; R: rightward). A green circle signals a beneficial age effect for singers and a red circle signals a detrimental age effect for singers (see results and discussion sections for explanation)
FIGURE 5.
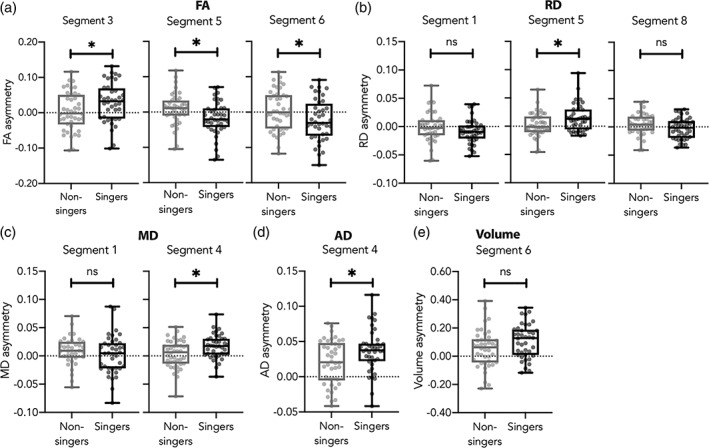
The box plots illustrate the interhemispheric asymmetry differences between singers and non‐singers for (a) FA, (b) RD, (c) MD, (d) AD, and (e) volume. A value of zero (dotted line) represents a perfect inter‐hemispheric symmetry. A negative value indicates a rightward asymmetry, and a positive value indicates a leftward asymmetry. Each gray dot represents a participant. Asterisks indicate significance at p < .05
Finally, Group x Hemisphere x Age interactions were found for AFDtot in segment 9 and NuFO in segments 8 and 9. These effects are summarized in Figure 4b. Post hoc linear regression analyses revealed that singing was associated with an age‐related increase in leftward AFDtot asymmetry in segment 9 (β = .00027, adj. R 2 = .139, p = .010) and an increase in leftward NuFO asymmetry in segments 8 and 9 (8: β = .002: adj. R 2 = .107, p = .022; 9: β = .003, adj. R 2 = .236, p = .001), with no significant effect for non‐singers. The age‐related increase in leftward asymmetry was related to a decrease in AFDtot in the right segment 9 (β = −.0005, adj. R 2 = .147, p = .015) and an increase in NuFO in both left segments for singers (8: β = .001: adj. R 2 = .098, p = .028; 9: β = .002, adj. R 2 = .109, p = .021).
Additional regression analyses were performed on singers to investigate the relationship between the number of continuous years of singing and white matter asymmetry. Holding hearing and age constant, only RD asymmetry in segment 5 was positively associated with the number of years of singing (β = .001, t = 2.612, p = .037). This association revealed that an increase in the number of years of singing was associated with an increase in leftward RD asymmetry.
3.4. Relationship between AF and SPiN in aging
A series of mediation analyses were conducted to investigate the relationship between age, SPiN performance (measured as sensitivity) and the AF. Consistent with the behavioral analysis described in Section 3.1, in all analyses, an increase in age was associated with a decrease in sensitivity (Supporting Information S10). In most analyses, age effects on the micro‐ and macrostructure of the AF were also found (Supporting Information S11). There were also age‐independent effects of white matter microstructure on sensitivity (Supporting Information S12). A better sensitivity was associated with lower RD and MD in the left whole AF. The segment‐based analysis showed that a better sensitivity was associated with lower RD in the bilateral segment 4, left segments 6 and 7, lower MD in the left segments 4, 6, and 7, and lower volume in the right segment 4. Importantly, mediation analyses revealed negative indirect age effects on sensitivity through the FA, RD, and MD of the left whole AF (Figure 6a and Supporting Information S13). The segment‐based analysis revealed negative age effects on sensitivity through the RD and MD of the left segments 4 (Figure 6b) and 7 (Figure 6c), RD of the left segment 6 (Figure 6d) and AD of the right segment 9 (Figure 6e). All indirect effects were negative, indicating that cases higher on age are estimated to have lower SPiN through white matter microstructure. Specifically, an increase in age was associated with lower sensitivity because of the effect of age on white matter microstructure (lower FA and higher RD, MD, and AD). The effect of age on white matter was then negatively associated with SPiN performance.
FIGURE 6.
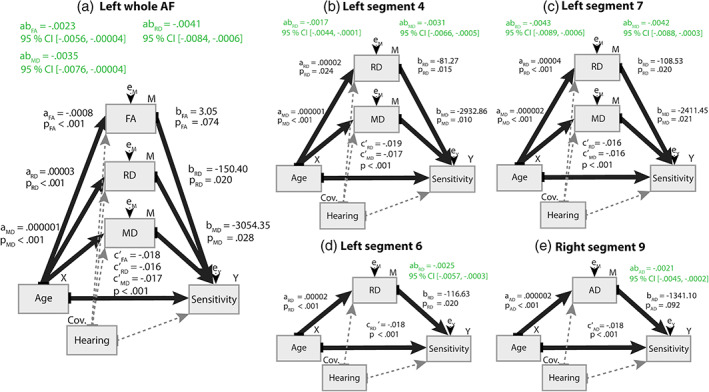
Results of the simple mediation analyses. The statistical diagrams illustrate each of the indirect effects (ab) of Age on sensitivity through white matter of the whole AF (a) and through specific segments (b‐eE). For each path in the graphs, the unstandardized coefficients and the probability value are reported. The bootstrapped 95% confidence interval is provided for the indirect effects (ab)
3.5. Relationship between AF, singing and SPiN
To investigate whether group differences in white matter were related to differences in SPiN performance (Supporting Informations S14 and S15), mediation, and moderated mediations were conducted. These analyses revealed no relationship between asymmetry and sensitivity, and no difference in this relationship between groups.
4. DISCUSSION
This is the first study to decompose the AF into different segments to examine how aging in this tract affects SPiN performance in amateur singers and non‐singers. The four main findings of this study are that, in groups of amateur singers and non‐singers carefully matched on sex, age, education, handedness, cognitive level, and musical instrument experience, (a) singers did not outperform non‐singers in SPiN; (b) myelin and/or axonal membrane deterioration within the bilateral frontal and posterior temporal AF segments was associated with SPiN difficulties in aging singers and non‐singers; (c) the structure of the AF was different in singers and non‐singers; (d) these differences were not associated with a SPiN benefit for amateur singers. These findings are discussed in the following sections.
4.1. SPiN, aging, and musical activities
One important finding of the present study is that, contrary to our hypothesis, singers did not outperform non‐singers in a SPiN task, and there was no relationship between the number of years of singing and SPiN performance. These results suggest that choral singing may not be beneficial to SPiN performance. This finding is consistent with a study showing no benefit of a 6‐month piano training in older adults (Fleming et al., 2019). However, it is in contrast to previous aging studies showing that playing a musical instrument as amateurs (Fostick, 2019; Zendel & Alain, 2012) or professionals (Bidelman & Alain, 2015; Parbery‐Clark et al., 2011) have small but positive effects on SPiN. It is also at odds with longitudinal studies that have shown that attending a short amateur training, such as a 6‐month piano‐based training (Zendel et al., 2019) or a 10 weeks of choral singing (Dubinsky et al., 2019), positively affect SPiN.
The literature on the benefits of musical activities on SPiN performance in children, adolescents and adults is heterogeneous (for a review, see Coffey, Mogilever, & Zatorre, 2017). Such heterogeneity could be related to participant factors such as personality (Corrigall, Schellenberg, & Misura, 2013), auditory predispositions towards music (e.g., Mankel & Bidelman, 2018) or to differences in the SPiN tasks that were used. Here we used a syllable discrimination task and we found no global singing effect on SPiN. The studies showing a behavioral effect of musical activities on SPiN used sentences or word repetition tasks (Dubinsky et al., 2019; Fostick, 2019; Parbery‐Clark et al., 2011; Zendel et al., 2019; Zendel & Alain, 2012), with the exception of Bidelman and Alain (2015), which used a vowel‐continuum categorization task. The other previous study that did not find musical advantage used a sentences‐picture‐naming task (Fleming et al., 2019). Given that a sub‐lexical speech discrimination task is more demanding perceptually that tasks using words or sentences, as no lexical or semantic cues are available to facilitate recognition, it seems unlikely that our SPiN task was so easy that it masked a potential singer's advantage. It also appears unlikely that our task was so difficult that it masked a potential singer's advantage. Indeed, the average sensitivity of each group (singers: 2.00 ± 0.58; non‐singers: 1.92 ± 0.50) was well above chance level.
Another potentially explanatory factor is that different types and amount of musical practice including vocal training may differently affect SPiN capacity. In the current study, singers were individuals with at least 2 years of choir‐singing experience. Musicians are often defined more stringently as those with a minimum of 6 years of musical experience (for a review, see Zhang, Susino, McPherson, & Schubert, 2020). Here we chose a less strict definition because of our interest in speech rehabilitation. Yet, although our singers were less strictly defined, our sample still included 32 singers (out of 41) who had 6 years of experience or more.
Another possibility is that singing impacts SPiN differently than does musical instrument playing. The majority of studies that examined the impact of musical activities on SPiN in aging have focused on instrumentalists (Alain et al., 2014; Bidelman & Alain, 2015; Fleming et al., 2019; Fostick, 2019; Parbery‐Clark et al., 2011; White‐Schwoch et al., 2013; Zendel et al., 2019; Zendel & Alain, 2012). Only one study has examined the impact of choral training (Dubinsky et al., 2019). In that study, Dubinsky et al. (2019) showed SPiN performance improves after 10 weeks of 3‐hr choral training, including 2 hr of group choral practice and up to 1 hr of individual online pitch discrimination and vocal production exercises. In the present study, in contrast, not all participants practiced up to 3 hr weekly and most did not have formal singing training. It is therefore possible that short but intense practice with formal training is a more effective approach to induce behavioral gains. Singing, like instrument playing, may influence SPiN performance but only under certain conditions of practice.
The current finding of an absence of relationship between the number of years of singing experience and SPiN performance is in line with another aging study showing that the number of years of musical instrument practice did not correlate with SPiN performance (Parbery‐Clark et al., 2011). However, this is at odds with other studies showing a positive relationship between the number of years of musical practice and SPiN performance in young adults. For example, Skoe, Camera, and Tufts (2019) used a measure combining both singing experience and musical instrument experience and showed that more years of musical practice in young adults was associated with better SPiN performance. Here we only studied the effect of singing, but our participants had very limited, experience playing musical instruments.
Other factors may have decreased the effect of singing on SPiN. One of them is our sample of well‐educated participants, with an average of 15.2 ± 2.7 years of education, which corresponds approximately to 2 years of university (45 participants [23 non‐singers, 22 singers] reported having completed a university degree), the idea being that more educated people would benefit less from singing because their education is already protecting them. However, previous studies have found SPiN benefits in samples of older instrumentalists with approximately the same or more years of education (Bidelman & Alain, 2015; Zendel et al., 2019; Zendel & Alain, 2012). Other studies have identified neurobiological differences related to the practice of musical instruments in speech processing in samples of well‐educated participants (Fleming et al., 2019; White‐Schwoch et al., 2013). Though it remains possible that amateur singing may have a greater effect in less educated adults, this hypothesis awaits empirical validation. Another potential factor is the level of noise exposure. Skoe et al. (2019) showed that the relationship between musical instrument playing and SPiN performance was negatively mediated by the level of noise exposure, suggesting that noise exposure may reduce the benefits of musical practice on SPiN. However, it is not known whether this effect extends to amateur singing as well.
In sum, further studies are needed to replicate the results of the present study and to compare the impact of different musical expertise and experiences on SPiN performance in different populations (e.g., singers with formal singing/voice training, different levels of noise exposure or lower education levels) in order to determine whether, and to what extent, amateur singing is associated with a protective effect on SPiN.
4.2. Aging of the AF and SPiN
Our study shows that the AF undergoes important decline with age in healthy participants. In general, the DTI and track‐based measures suggest that aging is associated with a decrease in the macrostructure and microstructure of all frontotemporal segments. The fODF measures, moreover, suggest that aging is associated with an increase in the microstructure at the temporal extremity and middle segments of the AF, except for the right frontal segment 8 for which fODF measures suggest the opposite. While aging of the AF has only been sparsely investigated, our results are consistent with our previous results (Tremblay et al., 2019), showing beneficial and detrimental age effects on the structure of the three segments of the AF defined by Catani, Jones, and ffytche (2005) using a completely independent sample. In that previous study, we found age‐related decrease in volume and increase in MD in the left AF (detrimental age effects), as well as age‐related increase in volume and AFDtot in the right AF and NuFO in the left AF (beneficial age effects). The current results are also in line with other neuroimaging studies showing an age‐related decrease in FA in the AF (Ikuta, Gollnick, & Rutledge, 2020; Voineskos et al., 2010; Voineskos et al., 2012).
The most dominant pattern that was found in the present study is a decrease in FA with a concomitant increase in RD and MD, with or without an increase in AD. Animal studies have shown that increased RD without changes in AD is indicative of a loss in myelin integrity (Song et al., 2002; Song et al., 2005). When increased in RD is also accompanied by an increase in AD, human research suggests that this is indicative of a loss in both myelin and axonal membrane integrity (for a review, see Madden et al., 2012). These types of age‐related loss within the structure of the white matter can alter the conduction of neural signals in the central nervous system, leading to slower or incomplete transmission of neural information (Bartzokis, 2004).
Controlling for peripheral hearing, we found that the aging of the AF—predominantly the left AF—was associated with age‐related SPiN difficulties, which is consistent with our second hypothesis. This is also in line with our previous study (Tremblay et al., 2019) and with the notion that the dorsal speech stream is relatively left‐lateralized (Hickok & Poeppel, 2007; Rauschecker & Scott, 2009). Specifically, we found that age‐related decrease in FA and increase in RD and MD in the left whole AF was negatively associated with SPiN performance, suggesting that SPiN difficulties are related to a loss of myelin sheath which would reduce the transmission of neural information within the AF, as described above. These results provide new and important evidence to support the notion that age‐related SPiN difficulties cannot be entirely accounted for by presbycusis or central auditory processing (e.g., Bilodeau‐Mercure et al., 2015; Fostick et al., 2013; Jin et al., 2014; Tremblay et al., 2019) and instead involve a decline in higher‐order functions such as phonological processing and auditory‐motor integration, two functions that are supported by the AF, as detailed below.
By dividing the AF into 10 segments, we were able to identify local heterogeneity in the relationship between the AF and SPiN performance in aging. Specifically, age‐related structural decline of a right prefrontal segment ending in the pIFG/middle frontal gyrus and left frontotemporal segments connecting posterior pSTG to PMv were associated with age‐related decrease in SPiN performance. While the role of right prefrontal cortex in speech perception has not been extensively studied, Hartwigsen, Price, et al. (2010) were the first to identify a role for the right pIFG in phonological judgments using transcranial magnetic stimulation. More recently Moliadze et al. (2019) showed that stimulation over the right prefrontal cortex facilitated phonological decisions for words in terms of response speed. Together, these studies suggest a role for the right prefrontal areas in phonological processing. The finding that aging of a right prefrontal segment can have a deleterious effect on SPiN during a syllable discrimination task that required phonological processing is thus consistent with these studies. However, the right pIFG has also been extensively involved in attentional control and inhibition (e.g., Aron, Robbins, & Poldrack, 2014; Hampshire, Chamberlain, Monti, Duncan, & Owen, 2010), which are important functions for SPiN. However, to our knowledge, no studies have shown a link between the right AF and attention in adults. We do not rule out the possibility that the AF is involved in attention, but further studies are needed to understand the role of this tract in both speech and auditory cognitive processes.
The left segments connecting pSTG to PMv could be particularly important for auditory‐motor integration by mapping the auditory speech sounds into auditory‐motor speech representations, a mechanism involved in both speech perception and singing. According to the Directions Into Velocities of Articulators (DIVA) model of speech production (Guenther et al., 2006), articulatory‐motor speech representations are stored in the left PMv. During SPiN, articulatory‐motor speech representations could provide top‐down information to resolve ambiguity in the noisy incoming speech sounds (for a review, see McGettigan & Tremblay, 2018). This idea is supported by several neuroimaging studies and meta‐analyses showing that speech perception in difficult listening conditions activates the PMv (e.g., Adank, 2012; Callan, Callan, Gamez, Sato, & Kawato, 2010; Du, Buchsbaum, Grady, & Alain, 2014; Osnes, Hugdahl, & Specht, 2011). For example, Du et al. (2014) showed that left PMv activation in young adults is positively associated with performance in a phonemic identification task only when speech is highly degraded. Similarly, another functional MRI study has shown that correct phonemic identification in noise was associated with increased activation in PMv compared with incorrect phonemic identification (Callan et al., 2010). Inhibitory TMS over the left PMv disrupts speech perception in young adults when it is accompanied by noise (Meister, Wilson, Deblieck, Wu, & Iacoboni, 2007) and even in quiet, though to a lesser extent (Sato, Tremblay, & Gracco, 2009). Finally, other studies have shown that age‐related decline in the functioning and structure of the precentral gyrus, which contains the PMv, is associated with age‐related decline in SPiN performance (Manan et al., 2017; Rudner, Seeto, Keidser, Johnson, & Rönnberg, 2019). Taken together, these results support a role for the PMv in speech processing, particularly when speech is degraded, at all ages.
In sum, controlling for peripheral hearing, our results suggest that aging of the bilateral AF could lead to a less efficient neural transmission through myelin damages and potentially affect phonological processing and/or cognitive functions in the right frontal AF and auditory‐motor integration for speech in the left frontotemporal AF, revealing local specialization for SPiN in the AF and supporting the notion that brain aging is an important factor in the etiology of SPiN difficulties in aging.
4.3. Amateur singing and the AF
Our analyses of the decomposed AF revealed, for the first time, that the relationship between amateur singing and the structure of the AF varies from anterior to posterior segments. Some of these group differences were age‐dependent, but most were age‐independent.
Few age‐dependent effects of singing on AF were observed. These effects were both beneficial and detrimental to white matter. The beneficial pattern, found in NuFO, suggests that amateur singing is associated with an increase in the number of distinct fiber orientations in left frontal segments. This indicates an increase in tissue complexity in aging, which could represent a structural compensatory mechanism or reflect age‐related experience. In contrast, the negative pattern, found in AFDtot, suggests that amateur singing is associated with a decrease in white matter microstructure in aging in the right frontal segment. These findings suggest that the practice of amateur singing may not be sufficient in itself to maintain and protect the global structure of the white matter of the AF in aging.
In contrast to age‐dependent group differences, age‐independent group differences were numerous. In particular, the microstructure of the right parietal and left parieto‐temporal segments was greater (higher FA, higher AD) in singers compared with non‐singers, indicating beneficial singing effects on the AF. However, these results are at odds with those of Halwani et al. (2011) who showed that young professional choral singers had higher track volume but lower FA in the right dorsal and ventral AF segments, as defined by Glasser and Rilling (2008), compared with non‐musicians. In our study, we did not find such effects using the segmentation protocol of Catani et al. (2002) and dividing the AF into several frontotemporal segments. It is possible that these differences could be explained by the AF models used across the two studies. Glasser and Rilling (2008) divide the AF into only two segments with different hypothesized roles: one ending in the STG involved in phonological processing, and another ending in the middle temporal gyrus involved in lexical‐semantic processing. Another possibility is that the differences in study samples in our study and that of Halwani et al. (2011) can account for the different findings. Here we studied amateur singers of all ages, while Halwani et al. (2011) studied young professional singers. It is possible that the effects of singing on white matter are dynamic rather than stable, and that they differ for amateur vs. trained professional singers. Additional research on singing is needed to fully understand how singing experience can shape the AF throughout the lifespan.
Our finding of group differences in hemispheric asymmetry is consistent with those of Oechslin et al. (2009) who did not show overall structural difference in bilateral AF between young professional musicians and non‐musicians, but did show group hemispheric asymmetry differences, using a similar AF model than the one used in the current study. Hemispheric asymmetry differences are long‐standing and well documented in the literature (e.g., Galaburda, Sanides, & Geschwind, 1978; Geschwind & Levitsky, 1968; for a review for the AF, see Ocklenburg, Friedrich, Gunturkun, & Genc, 2016; Wada, Clarke, & Hamm, 1975). Prior neuroimaging studies have indeed shown different patterns of gray and white matter asymmetry between young musicians and non‐musicians in the primary auditory cortex (Schneider et al., 2002), the AF/superior longitudinal fasciculus (Oechslin et al., 2009), the corticospinal tract (Ruber et al., 2015), the planum temporale (Schlaug, Jancke, Huang, & Steinmetz, 1995), and the precentral gyrus (Amunts et al., 1997). Differences in white matter asymmetry suggest that segments with higher structural integrity carry neural information more efficiently.
Contrary to our third hypothesis that group differences in the structure of the bilateral AF would be associated with a benefit in SPiN for singers, none of these age‐dependent and age‐independent group differences in white matter were associated with SPiN performance. These group differences may be linked with other functions which have been attributed to the AF such as vocabulary knowledge (Teubner‐Rhodes et al., 2016), prosodic processing for language (Glasser & Rilling, 2008) and more recently intelligence (Ikuta et al., 2020). In particular, hemispheric symmetry in the AF has been found to be a predictor of better episodic verbal learning in young adults (Catani et al., 2007) and leftward asymmetry in the AF has been related to better phonological processing in children (Lebel & Beaulieu, 2009). Interestingly, Oechslin et al. (2009) found a leftward FA asymmetry in the AF/superior longitudinal fasciculus in musicians with absolute pitch that was positively correlated with the performance on an absolute pitch test. Here we did not document absolute pitch, but since our singers are amateur choral singers, it is unlikely that many had absolute pitch.
Another possibility is that the age‐independent group differences, specifically in the parietal parts of the AF, that we found in the present study could be related to phonological decision for words (Hartwigsen et al., 2010) and phonological working memory (e.g., Deschamps et al., 2014; Deschamps et al., 2020; Kirschen et al., 2006; Romero et al., 2006), two functions that have been associated with the inferior parietal lobule. These functions, however, were minimized in the present study by using a short inter‐stimulus interval and sublexical stimuli. Further studies are needed to clarify the impact of AF asymmetry on cognitive functions in amateur singers.
Finally, only RD asymmetry in segment 5 was positively associated with the number of years of choral singing. There was no association between the number of years of choral singing and any of the other group differences in AF. Other variables related to the amount and type of practice, such as the age of onset of musical practice (Amunts et al., 1997; Imfeld et al., 2009; Kleber et al., 2016; Schlaug, Jancke, Huang, & Steinmetz, 1995; Steele et al., 2013; Vaquero et al., 2016), the number of hours of practice at different times of life (Bengtsson et al., 2005) and the number of hours of practice per day (Hutchinson, Lee, Gaab, & Schlaug, 2003), have been associated with music‐induced neuroplasticity. It is therefore possible that other variables could be related to the structural differences in white matter found between singers and non‐singers in the present study.
4.4. Limits
The main limitation of this study is its cross‐sectional design, which does not warrant causal interpretations of the relationship between musical training, age and SPiN. Indeed, white matter asymmetry differences found in this study could be related to pre‐existing differences between singers and non‐singers (e.g., genetic predisposition towards music for singers). They could also be related to the heterogeneous degree of lateralization of the AF identified in the normal population (Catani et al., 2007). Further studies are needed to clarify the impact of different type and amount of amateur singing on the aging brain. In particular, longitudinal studies are needed to clarify the causal effects of age and singing on white matter over the course of the lifespan. Another limitation is the relatively small sample size (N = 84), of highly educated participants. Despite these limitations, the validity of the study is reinforced by the sophisticated and robust diffusion pipeline used, the rigorous data quality controls that were implemented for behavioral, diffusion and structural data, our ability to track the bilateral AF on all but one participant and the strict matching of the groups on sex, age, education, handedness, cognition, and musical instrument experience. In addition, all analyses were controlled for peripheral hearing in the low frequency range.
5. CONCLUSION
This study demonstrates the importance of decomposing the AF into different segments to identify specific age and singing effects that cannot be identified at the whole‐tract level. By decomposing the AF into 10 frontotemporal segments, this study is the first to observe that aging and singing differently impact the structure of the AF. Specifically, local specializations in the right frontal AF and left frontal and posterior temporal AF for SPiN in aging singers and non‐singers were found. This study also shows that the relationship between amateur singing and the structure of the AF varies from the anterior to the posterior AF, especially in terms of hemispheric asymmetry. None of these differences were related to a SPiN benefit in singers. The lack of an effect of singing on SPiN performance suggests that not all musical activities are equally beneficial to speech processing, and that specific training conditions may be required to positively influence communicative outcomes including speech processing. Despite not being associated with a SPiN performance gain, the current results show that amateur choral singing has the potential to promote structural neuroplasticity throughout the lifespan.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Maxime Perron: Conceptualization, methodology, investigation, project administration, formal analysis, visualization, writing‐original draft preparation, and data curation. Guillaume Theaud: Methodology, writing‐reviewing, and editing. Maxime Descoteaux: Methodology, writing‐reviewing and editing. Pascale Tremblay: Conceptualization, funding acquisition, methodology, investigation, supervision, resources, project administration, writing‐reviewing and editing, data curation.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
We thank Valérie Brisson for her contribution to the development of the task and to the recruitment and testing of participants. We also thank Julie Poulin, Emilie Belley, Lisa‐Marie Deschênes, Anne‐Christine Bricaud, Elena Vaccaro, and Antoine Halbaut for their contributions to the recruitment and testing of participants. This study was supported by a grant from the Drummond Foundation (2016RFA proposal #27) to PT, a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC grant # RGPIN‐2019‐06534) also to PT, and two Globalink research internships from MITACS. PT holds a Career award from the Fonds de la Recherche en Santé du Québec (FRQ‐S, #35016). MP was funded by graduate scholarships from the Fonds de la Recherche en Santé du Québec (FRQS) and the Canadian Institutes of Health Research (CIHR). Technical support for MRI data acquisition was provided by the Centre intégré en neuroimagerie et neurostimulation de Québec (CINQ).
Perron M, Theaud G, Descoteaux M, Tremblay P. The frontotemporal organization of the arcuate fasciculus and its relationship with speech perception in young and older amateur singers and non‐singers. Hum Brain Mapp. 2021;42:3058–3076. 10.1002/hbm.25416
Funding information Drummond Foundation, Grant/Award Number: 2016RFA proposal #27; Fonds de Recherche du Québec ‐ Santé, Grant/Award Number: 35016; Natural Sciences and Engineering Research Council of Canada, Grant/Award Number: RGPIN‐2019‐06534
DATA AVAILABILITY STATEMENT
All stimuli and experiment files are publicly available on the Scholar portal Dataverse: https://doi.org/10.5683/SP2/8IX6QZ. Individual data are not available since participants did not consent to full data sharing. The codes used for this study are available online: https://github.com/scilus/scilpy?fbclid=IwAR2xgpYuNW2Q6CoC06‐6FnlLNVqu8SnIa6KzLS1QrBt4ztcMgLUoL3mLOf4. The multi‐atlas bundle segmentation is available on Zenodo: https://zenodo.org/record/3613688?fbclid=IwAR0SNxO8LVgkYYAgKtfg9ZeshbfTvyXMeggqHnoRou_a2MmAaQsvxhroeC0#.XoStbNNKg0o.
REFERENCES
- Abdul‐Kareem, I. A. , Stancak, A. , Parkes, L. M. , Al‐Ameen, M. , Alghamdi, J. , Aldhafeeri, F. M. , … Sluming, V. (2011). Plasticity of the superior and middle cerebellar peduncles in musicians revealed by quantitative analysis of volume and number of streamlines based on diffusion tensor tractography. Cerebellum, 10(3), 611–623. 10.1007/s12311-011-0274-1 [DOI] [PubMed] [Google Scholar]
- Adank, P. (2012). The neural bases of difficult speech comprehension and speech production: Two activation likelihood estimation (ALE) meta‐analyses. Brain and Language, 122(1), 42–54. 10.1016/j.bandl.2012.04.014 [DOI] [PubMed] [Google Scholar]
- Akeroyd, M. A. (2008). Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing‐impaired adults. International Journal of Audiology, 47(Suppl 2), S53–S71. 10.1080/14992020802301142 [DOI] [PubMed] [Google Scholar]
- Alain, C. , Zendel, B. R. , Hutka, S. , & Bidelman, G. M. (2014). Turning down the noise: The benefit of musical training on the aging auditory brain. Hearing Research, 308, 162–173. 10.1016/j.heares.2013.06.008 [DOI] [PubMed] [Google Scholar]
- Amunts, K. , Schlaug, G. , Jancke, L. , Steinmetz, H. , Schleicher, A. , Dabringhaus, A. , & Zilles, K. (1997). Motor cortex and hand motor skills: Structural compliance in the human brain. Human Brain Mapping, 5(3), 206–215. [DOI] [PubMed] [Google Scholar]
- Andersson, J. L. , Skare, S. , & Ashburner, J. (2003). How to correct susceptibility distortions in spin‐echo echo‐planar images: Application to diffusion tensor imaging. NeuroImage, 20(2), 870–888. 10.1016/s1053-8119(03)00336-7 [DOI] [PubMed] [Google Scholar]
- Andersson, J. L. R. , & Sotiropoulos, S. N. (2016). An integrated approach to correction for off‐resonance effects and subject movement in diffusion MR imaging. NeuroImage, 125, 1063–1078. 10.1016/j.neuroimage.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron, A. R. , Robbins, T. W. , & Poldrack, R. A. (2014). Inhibition and the right inferior frontal cortex: One decade on. Trends in Cognitive Sciences, 18(4), 177–185. 10.1016/j.tics.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Avants, B. B. , Tustison, N. J. , Song, G. , Cook, P. A. , Klein, A. , & Gee, J. C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage, 54(3), 2033–2044. 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis, G. (2004). Age‐related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiology of Aging, 25(1), 5–18. 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bedard, P. , Audet, A. M. , Drouin, P. , Roy, J. P. , Rivard, J. , & Tremblay, P. (2017). SyllabO+: A new tool to study sublexical phenomena in spoken Quebec French. Behavior Research Methods, 49(5), 1852–1863. 10.3758/s13428-016-0829-7 [DOI] [PubMed] [Google Scholar]
- Bengtsson, S. L. , Nagy, Z. , Skare, S. , Forsman, L. , Forssberg, H. , & Ullen, F. (2005). Extensive piano practicing has regionally specific effects on white matter development. Nature Neuroscience, 8(9), 1148–1150. 10.1038/nn1516 [DOI] [PubMed] [Google Scholar]
- Bidelman, G. M. , & Alain, C. (2015). Musical training orchestrates coordinated neuroplasticity in auditory brainstem and cortex to counteract age‐related declines in categorical vowel perception. The Journal of Neuroscience, 35(3), 1240–1249. 10.1523/JNEUROSCI.3292-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau‐Mercure, M. , Lortie, C. L. , Sato, M. , Guitton, M. J. , & Tremblay, P. (2015). The neurobiology of speech perception decline in aging. Brain Structure & Function, 220(2), 979–997. 10.1007/s00429-013-0695-3 [DOI] [PubMed] [Google Scholar]
- Boersma, P. , & Weenink, D. (2001). PRAAT, a system for doing phonetics by computer. Glot International, 5, 341–345. [Google Scholar]
- Brown, S. , Martinez, M. J. , Hodges, D. A. , Fox, P. T. , & Parsons, L. M. (2004). The song system of the human brain. Brain Research. Cognitive Brain Research, 20(3), 363–375. 10.1016/j.cogbrainres.2004.03.016 [DOI] [PubMed] [Google Scholar]
- Callan, D. , Callan, A. , Gamez, M. , Sato, M. A. , & Kawato, M. (2010). Premotor cortex mediates perceptual performance. NeuroImage, 51(2), 844–858. 10.1016/j.neuroimage.2010.02.027 [DOI] [PubMed] [Google Scholar]
- Catani, M. , Allin, M. P. , Husain, M. , Pugliese, L. , Mesulam, M. M. , Murray, R. M. , & Jones, D. K. (2007). Symmetries in human brain language pathways correlate with verbal recall. Proceedings of the National Academy of Sciences of the United States of America, 104(43), 17163–17168. 10.1073/pnas.0702116104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani, M. , Howard, R. J. , Pajevic, S. , & Jones, D. K. (2002). Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage, 17(1), 77–94. 10.1006/nimg.2002.1136 [DOI] [PubMed] [Google Scholar]
- Catani, M. , Jones, D. K. , & Ffytche, D. H. (2005). Perisylvian language networks of the human brain. Annals of Neurology, 57(1), 8–16. 10.1002/ana.20319 [DOI] [PubMed] [Google Scholar]
- Coffey, E. B. J. , Mogilever, N. B. , & Zatorre, R. J. (2017). Speech‐in‐noise perception in musicians: A review. Hearing Research, 352, 49–69. 10.1016/j.heares.2017.02.006 [DOI] [PubMed] [Google Scholar]
- Corrigall, K. A. , Schellenberg, E. G. , & Misura, N. M. (2013). Music training, cognition, and personality. Frontiers in Psychology, 4, 222. 10.3389/fpsyg.2013.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupe, P. , Yger, P. , Prima, S. , Hellier, P. , Kervrann, C. , & Barillot, C. (2008). An optimized blockwise nonlocal means denoising filter for 3‐D magnetic resonance images. IEEE Transactions on Medical Imaging, 27(4), 425–441. 10.1109/TMI.2007.906087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousineau, M. , Jodoin, P. M. , Morency, F. C. , Rozanski, V. , Grand'Maison, M. , Bedell, B. J. , & Descoteaux, M. (2017). A test‐retest study on Parkinson's PPMI dataset yields statistically significant white matter fascicles. Neuroimage Clinical, 16, 222–233. 10.1016/j.nicl.2017.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale, A. M. , Fischl, B. , & Sereno, M. I. (1999). Cortical surface‐based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Dell'Acqua, F. , Simmons, A. , Williams, S. C. , & Catani, M. (2013). Can spherical deconvolution provide more information than fiber orientations? Hindrance modulated orientational anisotropy, a true‐tract specific index to characterize white matter diffusion. Human Brain Mapping, 34(10), 2464–2483. 10.1002/hbm.22080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps, I. , Baum, S. R. , & Gracco, V. L. (2014). On the role of the supramarginal gyrus in phonological processing and verbal working memory: Evidence from rTMS studies. Neuropsychologia, 53, 39–46. 10.1016/j.neuropsychologia.2013.10.015 [DOI] [PubMed] [Google Scholar]
- Deschamps, I. , Courson, M. , Dick, A. S. , & Tremblay, P. (2020). The phonological loop: Is speech special? Experimental Brain Research, 238, 2307–2321. 10.1007/s00221-020-05886-9 [DOI] [PubMed] [Google Scholar]
- Descoteaux, M. , Deriche, R. , Knosche, T. R. , & Anwander, A. (2009). Deterministic and probabilistic tractography based on complex fibre orientation distributions. IEEE Transactions on Medical Imaging, 28(2), 269–286. 10.1109/TMI.2008.2004424 [DOI] [PubMed] [Google Scholar]
- Di Tommaso, P. , Chatzou, M. , Floden, E. W. , Barja, P. P. , Palumbo, E. , & Notredame, C. (2017). Nextflow enables reproducible computational workflows. Nature Biotechnology, 35(4), 316–319. 10.1038/nbt.3820 [DOI] [PubMed] [Google Scholar]
- Dick, A. S. , & Tremblay, P. (2012). Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain, 135(12), 3529–3550. 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Divenyi, P. L. , & Haupt, K. M. (1997). Audiological correlates of speech understanding deficits in elderly listeners with mild‐to‐moderate hearing loss. I. Age and lateral asymmetry effects. Ear and Hearing, 18(1), 42–61. 10.1097/00003446-199702000-00005 [DOI] [PubMed] [Google Scholar]
- Du, Y. , Buchsbaum, B. R. , Grady, C. L. , & Alain, C. (2014). Noise differentially impacts phoneme representations in the auditory and speech motor systems. Proceedings of the National Academy of Sciences of the United States of America, 111(19), 7126–7131. 10.1073/pnas.1318738111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Y. , Buchsbaum, B. R. , Grady, C. L. , & Alain, C. (2016). Increased activity in frontal motor cortex compensates impaired speech perception in older adults. Nature Communications, 7, 12241. 10.1038/ncomms12241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Y. , & Zatorre, R. J. (2017). Musical training sharpens and bonds ears and tongue to hear speech better. Proceedings of the National Academy of Sciences of the United States of America, 114(51), 13579–13584. 10.1073/pnas.1712223114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky, E. , Wood, E. A. , Nespoli, G. , & Russo, F. A. (2019). Short‐term choir singing supports speech‐in‐noise perception and neural pitch strength in older adults with age‐related hearing loss. Frontiers in Neuroscience, 13, 1153. 10.3389/fnins.2019.01153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , & Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–11055. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Sereno, M. I. , & Dale, A. M. (1999). Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. NeuroImage, 9(2), 195–207. 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- Fleming, D. , Belleville, S. , Peretz, I. , West, G. , & Zendel, B. R. (2019). The effects of short‐term musical training on the neural processing of speech‐in‐noise in older adults. Brain and Cognition, 136, 103592. 10.1016/j.bandc.2019.103592 [DOI] [PubMed] [Google Scholar]
- Fonov, V. S. , Evans, A. C. , McKinstry, R. C. , Almli, C. R. , & Collins, D. L. (2009). Unbiased nonlinear average age‐appropriate brain templates from birth to adulthood. NeuroImage, 47, S102. 10.1016/S1053-8119(09)70884-5 [DOI] [Google Scholar]
- Fostick, L. (2019). Card playing enhances speech perception among aging adults: Comparison with aging musicians. European Journal of Ageing, 16(4), 481–489. 10.1007/s10433-019-00512-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fostick, L. , Ben‐Artzi, E. , & Babkoff, H. (2013). Aging and speech perception: Beyond hearing threshold and cognitive ability. Journal of Basic and Clinical Physiology and Pharmacology, 24(3), 175–183. 10.1515/jbcpp-2013-0048 [DOI] [PubMed] [Google Scholar]
- Galaburda, A. M. , Sanides, F. , & Geschwind, N. (1978). Human brain. Cytoarchitectonic left‐right asymmetries in the temporal speech region. Archives of Neurology, 35(12), 812–817. 10.1001/archneur.1978.00500360036007 [DOI] [PubMed] [Google Scholar]
- Garyfallidis, E. , Brett, M. , Amirbekian, B. , Rokem, A. , van der Walt, S. , Descoteaux, M. , … Dipy, C. (2014). Dipy, a library for the analysis of diffusion MRI data. Frontiers in Neuroinformatics, 8, 8. 10.3389/fninf.2014.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garyfallidis, E. , Cote, M. A. , Rheault, F. , Sidhu, J. , Hau, J. , Petit, L. , … Descoteaux, M. (2018). Recognition of white matter bundles using local and global streamline‐based registration and clustering. NeuroImage, 170, 283–295. 10.1016/j.neuroimage.2017.07.015 [DOI] [PubMed] [Google Scholar]
- Geschwind, N. , & Levitsky, W. (1968). Human brain: Left‐right asymmetries in temporal speech region. Science, 161(3837), 186–187. 10.1126/science.161.3837.186 [DOI] [PubMed] [Google Scholar]
- Glasser, M. F. , & Rilling, J. K. (2008). DTI tractography of the human brain's language pathways. Cerebral Cortex, 18(11), 2471–2482. 10.1093/cercor/bhn011 [DOI] [PubMed] [Google Scholar]
- Gough, P. M. , Nobre, A. C. , & Devlin, J. T. (2005). Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. Journal of Neuroscience, 25(35), 8010–8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther, F. H. , Ghosh, S. S. , & Tourville, J. A. (2006). Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and Language, 96(3), 280–301. 10.1016/j.bandl.2005.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halwani, G. F. , Loui, P. , Ruber, T. , & Schlaug, G. (2011). Effects of practice and experience on the arcuate fasciculus: Comparing singers, instrumentalists, and non‐musicians. Frontiers in Psychology, 2, 156. 10.3389/fpsyg.2011.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire, A. , Chamberlain, S. R. , Monti, M. M. , Duncan, J. , & Owen, A. M. (2010). The role of the right inferior frontal gyrus: Inhibition and attentional control. NeuroImage, 50(3), 1313–1319. 10.1016/j.neuroimage.2009.12.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Y. , Yang, H. , Lv, Y. T. , Zhu, C. Z. , He, Y. , Tang, H. H. , … Dong, Q. (2009). Gray matter density and white matter integrity in pianists' brain: A combined structural and diffusion tensor MRI study. Neuroscience Letters, 459(1), 3–6. 10.1016/j.neulet.2008.07.056 [DOI] [PubMed] [Google Scholar]
- Hartwigsen, G. , Baumgaertner, A. , Price, C. J. , Koehnke, M. , Ulmer, S. , & Siebner, H. R. (2010). Phonological decisions require both the left and right supramarginal gyri. Proceedings of the National Academy of Sciences of the United States of America, 107(38), 16494–16499. 10.1073/pnas.1008121107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen, G. , Price, C. J. , Baumgaertner, A. , Geiss, G. , Koehnke, M. , Ulmer, S. , & Siebner, H. R. (2010). The right posterior inferior frontal gyrus contributes to phonological word decisions in the healthy brain: Evidence from dual‐site TMS. Neuropsychologia, 48(10), 3155–3163. 10.1016/j.neuropsychologia.2010.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A. F. (2017). Introduction to mediation, moderation, and conditional process analysis, second edition: A regression‐based approach. New York, NY: The Guilford Press. [Google Scholar]
- Hickok, G. , & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews. Neuroscience, 8(5), 393–402. 10.1038/nrn2113 [DOI] [PubMed] [Google Scholar]
- Humes, L. E. , & Christopherson, L. (1991). Speech identification difficulties of hearing‐impaired elderly persons: The contributions of auditory processing deficits. Journal of Speech and Hearing Research, 34(3), 686–693. 10.1044/jshr.3403.686 [DOI] [PubMed] [Google Scholar]
- Humes, L. E. , & Roberts, L. (1990). Speech‐recognition difficulties of the hearing‐impaired elderly: The contributions of audibility. Journal of Speech and Hearing Research, 33(4), 726–735. 10.1044/jshr.3304.726 [DOI] [PubMed] [Google Scholar]
- Hutchinson, S. , Lee, L. H. , Gaab, N. , & Schlaug, G. (2003). Cerebellar volume of musicians. Cerebral Cortex, 13(9), 943–949. 10.1093/cercor/13.9.943 [DOI] [PubMed] [Google Scholar]
- Hwang, J. H. , Li, C. W. , Wu, C. W. , Chen, J. H. , & Liu, T. C. (2007). Aging effects on the activation of the auditory cortex during binaural speech listening in white noise: An fMRI study. Audiology & Neuro‐Otology, 12(5), 285–294. 10.1159/000103209 [DOI] [PubMed] [Google Scholar]
- Ikuta, T. , Gollnick, H. M. , & Rutledge, A. N. (2020). Age associated decline in the arcuate fasciculus and IQ. Brain Imaging and Behavior, 14(2), 362–367. 10.1007/s11682-019-00154-z [DOI] [PubMed] [Google Scholar]
- Imfeld, A. , Oechslin, M. S. , Meyer, M. , Loenneker, T. , & Jancke, L. (2009). White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. NeuroImage, 46(3), 600–607. 10.1016/j.neuroimage.2009.02.025 [DOI] [PubMed] [Google Scholar]
- Jerger, J. , Jerger, S. , & Pirozzolo, F. (1991). Correlational analysis of speech audiometric scores, hearing loss, age, and cognitive abilities in the elderly. Ear and Hearing, 12(2), 103–109. 10.1097/00003446-199104000-00004 [DOI] [PubMed] [Google Scholar]
- Jin, S. H. , Liu, C. , & Sladen, D. P. (2014). The effects of aging on speech perception in noise: Comparison between normal‐hearing and cochlear‐implant listeners. Journal of the American Academy of Audiology, 25(7), 656–665. 10.3766/jaaa.25.7.4 [DOI] [PubMed] [Google Scholar]
- Kirschen, M. P. , Davis‐Ratner, M. S. , Jerde, T. E. , Schraedley‐Desmond, P. , & Desmond, J. E. (2006). Enhancement of phonological memory following transcranial magnetic stimulation (TMS). Behavioural Neurology, 17(3–4), 187–194. 10.1155/2006/469132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber, B. , & Zarate, J. M. (2014). The Neuroscience of Singing. In Welch G. F., Howard D. M. & John N. (Eds.) The Oxford Handbook of Singing, Oxford, England: Oxford University Press. 10.1093/oxfordhb/9780199660773.013.015. [DOI] [Google Scholar]
- Kleber, B. , Veit, R. , Moll, C. V. , Gaser, C. , Birbaumer, N. , & Lotze, M. (2016). Voxel‐based morphometry in opera singers: Increased gray‐matter volume in right somatosensory and auditory cortices. NeuroImage, 133, 477–483. 10.1016/j.neuroimage.2016.03.045 [DOI] [PubMed] [Google Scholar]
- Koo, T. K. , & Li, M. Y. (2016). A guideline of selecting and reporting Intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15(2), 155–163. 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzer, G. M. , Sochat, V. , & Bauer, M. W. (2017). Singularity: Scientific containers for mobility of compute. PLoS One, 12(5), e0177459. 10.1371/journal.pone.0177459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel, C. , & Beaulieu, C. (2009). Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Human Brain Mapping, 30(11), 3563–3573. 10.1002/hbm.20779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan, N. , & Creelman, D. (2004). Detection Theory: A User's Guide (Vol. xix).
- Madden, D. J. , Bennett, I. J. , Burzynska, A. , Potter, G. G. , Chen, N. K. , & Song, A. W. (2012). Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochimica et Biophysica Acta, 1822(3), 386–400. 10.1016/j.bbadis.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manan, H. A. , Yusoff, A. N. , Franz, E. A. , & Mukari, S. Z. M. S. (2017). Effects of aging and background babble noise on speech perception processing: An fMRI study. Neurophysiology, 49(6), 441–452. 10.1007/s11062-018-9707-5 [DOI] [Google Scholar]
- Mankel, K. , & Bidelman, G. M. (2018). Inherent auditory skills rather than formal music training shape the neural encoding of speech. Proceedings of the National Academy of Sciences of the United States of America, 115(51), 13129–13134. 10.1073/pnas.1811793115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGettigan, C. , & Tremblay, P. (2018). Links Between Perception and Production: Examining the roles of motor and premotor cortices in understanding speech. In Rueschemeyer S. A. & Gaskell G. (Eds.), The Oxford Handbook of Psycholinguistics (2nd ed.), Oxford, England: Oxford University Press. 10.1093/oxfordhb/9780198786825.013.14. [DOI] [Google Scholar]
- Meister, I. G. , Wilson, S. M. , Deblieck, C. , Wu, A. D. , & Iacoboni, M. (2007). The essential role of premotor cortex in speech perception. Current Biology, 17(19), 1692–1696. 10.1016/j.cub.2007.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moliadze, V. , Sierau, L. , Lyzhko, E. , Stenner, T. , Werchowski, M. , Siniatchkin, M. , & Hartwigsen, G. (2019). After‐effects of 10 Hz tACS over the prefrontal cortex on phonological word decisions. Brain Stimulation, 12, 1464–1474. 10.1016/j.brs.2019.06.021 [DOI] [PubMed] [Google Scholar]
- Nasreddine, Z. S. , Phillips, N. A. , Bedirian, V. , Charbonneau, S. , Whitehead, V. , Collin, I. , … Chertkow, H. (2005). The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Ocklenburg, S. , Friedrich, P. , Gunturkun, O. , & Genc, E. (2016). Intrahemispheric white matter asymmetries: The missing link between brain structure and functional lateralization? Reviews in the Neurosciences, 27(5), 465–480. 10.1515/revneuro-2015-0052 [DOI] [PubMed] [Google Scholar]
- Oechslin, M. S. , Imfeld, A. , Loenneker, T. , Meyer, M. , & Jancke, L. (2009). The plasticity of the superior longitudinal fasciculus as a function of musical expertise: A diffusion tensor imaging study. Frontiers in Human Neuroscience, 3, 76. 10.3389/neuro.09.076.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- O'Muircheartaigh, J. , Dean, D. C., 3rd , Dirks, H. , Waskiewicz, N. , Lehman, K. , Jerskey, B. A. , & Deoni, S. C. (2013). Interactions between white matter asymmetry and language during neurodevelopment. The Journal of Neuroscience, 33(41), 16170–16177. 10.1523/JNEUROSCI.1463-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osnes, B. , Hugdahl, K. , & Specht, K. (2011). Effective connectivity analysis demonstrates involvement of premotor cortex during speech perception. NeuroImage, 54(3), 2437–2445. 10.1016/j.neuroimage.2010.09.078 [DOI] [PubMed] [Google Scholar]
- Ozdemir, E. , Norton, A. , & Schlaug, G. (2006). Shared and distinct neural correlates of singing and speaking. NeuroImage, 33(2), 628–635. 10.1016/j.neuroimage.2006.07.013 [DOI] [PubMed] [Google Scholar]
- Ozturk, A. H. , Tascioglu, B. , Aktekin, M. , Kurtoglu, Z. , & Erden, I. (2002). Morphometric comparison of the human corpus callosum in professional musicians and non‐musicians by using in vivo magnetic resonance imaging. Journal of Neuroradiology, 29(1), 29–34. [PubMed] [Google Scholar]
- Parbery‐Clark, A. , Strait, D. L. , Anderson, S. , Hittner, E. , & Kraus, N. (2011). Musical experience and the aging auditory system: Implications for cognitive abilities and hearing speech in noise. PLoS One, 6(5), e18082. 10.1371/journal.pone.0018082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, A. D. (2011). Why would musical training benefit the neural encoding of speech? The OPERA hypothesis. Frontiers in Psychology, 2, 142. 10.3389/fpsyg.2011.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, A. D. (2012). The OPERA hypothesis: Assumptions and clarifications. Annals of the New York Academy of Sciences, 1252, 124–128. 10.1111/j.1749-6632.2011.06426.x [DOI] [PubMed] [Google Scholar]
- Patel, A. D. (2014). Can nonlinguistic musical training change the way the brain processes speech? The expanded OPERA hypothesis. Hearing Research, 308, 98–108. 10.1016/j.heares.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Perrin, F. , & Grimault, N. (2005). Fonds sonores. Laboratoire Unités Mixtes de Recherche, Centre National de la Recherche Scientifique 5020, Lyon, France.
- Perry, D. W. , Zatorre, R. J. , Petrides, M. , Alivisatos, B. , Meyer, E. , & Evans, A. C. (1999). Localization of cerebral activity during simple singing. Neuroreport, 10(18), 3979–3984. 10.1097/00001756-199912160-00046 [DOI] [PubMed] [Google Scholar]
- Raffelt, D. , Tournier, J. D. , Rose, S. , Ridgway, G. R. , Henderson, R. , Crozier, S. , … Connelly, A. (2012). Apparent fibre density: A novel measure for the analysis of diffusion‐weighted magnetic resonance images. NeuroImage, 59(4), 3976–3994. 10.1016/j.neuroimage.2011.10.045 [DOI] [PubMed] [Google Scholar]
- Rauschecker, J. P. , & Scott, S. K. (2009). Maps and streams in the auditory cortex: Nonhuman primates illuminate human speech processing. Nature Neuroscience, 12(6), 718–724. 10.1038/nn.2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, L. , Walsh, V. , & Papagno, C. (2006). The neural correlates of phonological short‐term memory: A repetitive transcranial magnetic stimulation study. Journal of Cognitive Neuroscience, 18(7), 1147–1155. 10.1162/jocn.2006.18.7.1147 [DOI] [PubMed] [Google Scholar]
- Ruber, T. , Lindenberg, R. , & Schlaug, G. (2015). Differential adaptation of descending motor tracts in musicians. Cerebral Cortex, 25(6), 1490–1498. 10.1093/cercor/bht331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner, M. , Seeto, M. , Keidser, G. , Johnson, B. , & Rönnberg, J. (2019). Poorer speech reception threshold in noise is associated with lower brain volume in auditory and cognitive processing regions. Journal of Speech, Language, and Hearing Research, 62(4S), 1117–1130. 10.1044/2018_JSLHR-H-ASCC7-18-0142 [DOI] [PubMed] [Google Scholar]
- Sato, M. , Tremblay, P. , & Gracco, V. L. (2009). A mediating role of the premotor cortex in phoneme segmentation. Brain and Language, 111(1), 1–7. 10.1016/j.bandl.2009.03.002 [DOI] [PubMed] [Google Scholar]
- Schlaug, G. , Jancke, L. , Huang, Y. , Staiger, J. F. , & Steinmetz, H. (1995). Increased corpus callosum size in musicians. Neuropsychologia, 33(8), 1047–1055. 10.1016/0028-3932(95)00045-5 [DOI] [PubMed] [Google Scholar]
- Schlaug, G. , Jancke, L. , Huang, Y. , & Steinmetz, H. (1995). In vivo evidence of structural brain asymmetry in musicians. Science, 267(5198), 699–701. 10.1126/science.7839149 [DOI] [PubMed] [Google Scholar]
- Schmithorst, V. J. , & Wilke, M. (2002). Differences in white matter architecture between musicians and non‐musicians: A diffusion tensor imaging study. Neuroscience Letters, 321(1–2), 57–60. 10.1016/s0304-3940(02)00054-x [DOI] [PubMed] [Google Scholar]
- Schneider, P. , Scherg, M. , Dosch, H. G. , Specht, H. J. , Gutschalk, A. , & Rupp, A. (2002). Morphology of Heschl's gyrus reflects enhanced activation in the auditory cortex of musicians. Nature Neuroscience, 5(7), 688–694. 10.1038/nn871 [DOI] [PubMed] [Google Scholar]
- Segado, M. , Hollinger, A. , Thibodeau, J. , Penhune, V. , & Zatorre, R. J. (2018). Partially overlapping brain networks for singing and cello playing. Frontiers in Neuroscience, 12, 351. 10.3389/fnins.2018.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard, J. P. , Wang, J. P. , & Wong, P. C. (2011). Large‐scale cortical functional organization and speech perception across the lifespan. PLoS One, 6(1), e16510. 10.1371/journal.pone.0016510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu, N. , Liu, Y. , Duan, Y. , & Li, K. (2015). Hemispheric asymmetry of human brain anatomical network revealed by diffusion tensor tractography. BioMed Research International, 2015, 908917. 10.1155/2015/908917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoe, E. , Camera, S. , & Tufts, J. (2019). Noise exposure may diminish the musician advantage for perceiving speech in noise. Ear and Hearing, 40(4), 782–793. 10.1097/aud.0000000000000665 [DOI] [PubMed] [Google Scholar]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckmann, C. F. , Behrens, T. E. , Johansen‐Berg, H. , … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Song, S. K. , Sun, S. W. , Ramsbottom, M. J. , Chang, C. , Russell, J. , & Cross, A. H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage, 17(3), 1429–1436. 10.1006/nimg.2002.1267 [DOI] [PubMed] [Google Scholar]
- Song, S. K. , Yoshino, J. , Le, T. Q. , Lin, S. J. , Sun, S. W. , Cross, A. H. , & Armstrong, R. C. (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage, 26(1), 132–140. 10.1016/j.neuroimage.2005.01.028 [DOI] [PubMed] [Google Scholar]
- Souza, P. E. , & Turner, C. W. (1994). Masking of speech in young and elderly listeners with hearing loss. Journal of Speech and Hearing Research, 37(3), 655–661. 10.1044/jshr.3703.655 [DOI] [PubMed] [Google Scholar]
- Stach, B. A. (2008). Clinical audiology: An introduction: Cengage Learning.
- Steele, C. J. , Bailey, J. A. , Zatorre, R. J. , & Penhune, V. B. (2013). Early musical training and white‐matter plasticity in the corpus callosum: Evidence for a sensitive period. The Journal of Neuroscience, 33(3), 1282–1290. 10.1523/JNEUROSCI.3578-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teubner‐Rhodes, S. , Vaden, K. I., Jr. , Cute, S. L. , Yeatman, J. D. , Dougherty, R. F. , & Eckert, M. A. (2016). Aging‐resilient associations between the arcuate fasciculus and vocabulary knowledge: Microstructure or morphology? The Journal of Neuroscience, 36(27), 7210–7222. 10.1523/jneurosci.4342-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theaud, G. , Houde, J.‐C. , Boré, A. , Rheault, F. , Morency, F. , & Descoteaux, M. (2020). TractoFlow‐ABS (atlas‐based segmentation). bioRxiv , 197384. doi: 10.1101/2020.08.03.197384 [DOI] [PubMed]
- Tournier, J. D. , Calamante, F. , & Connelly, A. (2007). Robust determination of the fibre orientation distribution in diffusion MRI: Non‐negativity constrained super‐resolved spherical deconvolution. NeuroImage, 35(4), 1459–1472. 10.1016/j.neuroimage.2007.02.016 [DOI] [PubMed] [Google Scholar]
- Tournier, J. D. , Smith, R. , Raffelt, D. , Tabbara, R. , Dhollander, T. , Pietsch, M. , … Connelly, A. (2019). MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 202, 116137. 10.1016/j.neuroimage.2019.116137 [DOI] [PubMed] [Google Scholar]
- Tremblay, P. , Perron, M. , Deschamps, I. , Kennedy‐Higgins, D. , Houde, J. C. , Dick, A. S. , & Descoteaux, M. (2019). The role of the arcuate and middle longitudinal fasciculi in speech perception in noise in adulthood. Human Brain Mapping, 40(1), 226–241. 10.1002/hbm.24367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison, N. J. , Avants, B. B. , Cook, P. A. , Zheng, Y. , Egan, A. , Yushkevich, P. A. , & Gee, J. C. (2010). N4ITK: Improved N3 bias correction. IEEE Transactions on Medical Imaging, 29(6), 1310–1320. 10.1109/TMI.2010.2046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen, D. C. , Smith, S. M. , Barch, D. M. , Behrens, T. E. , Yacoub, E. , Ugurbil, K. , & Consortium, W. U.‐M. H. (2013). The WU‐Minn human connectome project: An overview. NeuroImage, 80, 62–79. 10.1016/j.neuroimage.2013.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero, L. , Hartmann, K. , Ripolles, P. , Rojo, N. , Sierpowska, J. , Francois, C. , … Altenmuller, E. (2016). Structural neuroplasticity in expert pianists depends on the age of musical training onset. NeuroImage, 126, 106–119. 10.1016/j.neuroimage.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Veraart, J. , Novikov, D. S. , Christiaens, D. , Ades‐Aron, B. , Sijbers, J. , & Fieremans, E. (2016). Denoising of diffusion MRI using random matrix theory. NeuroImage, 142, 394–406. 10.1016/j.neuroimage.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos, A. N. , Lobaugh, N. J. , Bouix, S. , Rajji, T. K. , Miranda, D. , Kennedy, J. L. , … Shenton, M. E. (2010). Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain, 133(Pt 5), 1494–1504. 10.1093/brain/awq040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos, A. N. , Rajji, T. K. , Lobaugh, N. J. , Miranda, D. , Shenton, M. E. , Kennedy, J. L. , … Mulsant, B. H. (2012). Age‐related decline in white matter tract integrity and cognitive performance: A DTI tractography and structural equation modeling study. Neurobiology of Aging, 33(1), 21–34. 10.1016/j.neurobiolaging.2010.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, J. A. , Clarke, R. , & Hamm, A. (1975). Cerebral hemispheric asymmetry in humans. Cortical speech zones in 100 adults and 100 infant brains. Archives of Neurology, 32(4), 239–246. 10.1001/archneur.1975.00490460055007 [DOI] [PubMed] [Google Scholar]
- Waldron‐Perrine, B. , & Axelrod, B. N. (2012). Determining an appropriate cutting score for indication of impairment on the Montreal cognitive assessment. International Journal of Geriatric Psychiatry, 27(11), 1189–1194. 10.1002/gps.3768 [DOI] [PubMed] [Google Scholar]
- White‐Schwoch, T. , Woodruff Carr, K. , Anderson, S. , Strait, D. L. , & Kraus, N. (2013). Older adults benefit from music training early in life: Biological evidence for long‐term training‐driven plasticity. The Journal of Neuroscience, 33(45), 17667–17674. 10.1523/JNEUROSCI.2560-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde, E. A. , McCauley, S. R. , Chu, Z. , Hunter, J. V. , Bigler, E. D. , Yallampalli, R. , … Levin, H. S. (2009). Diffusion tensor imaging of hemispheric asymmetries in the developing brain. Journal of Clinical and Experimental Neuropsychology, 31(2), 205–218. 10.1080/13803390802098118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, P. C. , Ettlinger, M. , Sheppard, J. P. , Gunasekera, G. M. , & Dhar, S. (2010). Neuroanatomical characteristics and speech perception in noise in older adults. Ear and Hearing, 31(4), 471–479. 10.1097/AUD.0b013e3181d709c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, P. C. M. , Jin, J. X. , Gunasekera, G. M. , Abel, R. , Lee, E. R. , & Dhar, S. (2009). Aging and cortical mechanisms of speech perception in noise. Neuropsychologia, 47(3), 693–703. 10.1016/j.neuropsychologia.2008.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendel, B. R. , & Alain, C. (2012). Musicians experience less age‐related decline in central auditory processing. Psychology and Aging, 27(2), 410–417. 10.1037/a0024816 [DOI] [PubMed] [Google Scholar]
- Zendel, B. R. , West, G. L. , Belleville, S. , & Peretz, I. (2019). Musical training improves the ability to understand speech‐in‐noise in older adults. Neurobiology of Aging, 81, 102–115. 10.1016/j.neurobiolaging.2019.05.015 [DOI] [PubMed] [Google Scholar]
- Zhang, J. D. , Susino, M. , McPherson, G. E. , & Schubert, E. (2020). The definition of a musician in music psychology: A literature review and the six‐year rule. Psychology of Music, 48(3), 389–409. 10.1177/0305735618804038 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
All stimuli and experiment files are publicly available on the Scholar portal Dataverse: https://doi.org/10.5683/SP2/8IX6QZ. Individual data are not available since participants did not consent to full data sharing. The codes used for this study are available online: https://github.com/scilus/scilpy?fbclid=IwAR2xgpYuNW2Q6CoC06‐6FnlLNVqu8SnIa6KzLS1QrBt4ztcMgLUoL3mLOf4. The multi‐atlas bundle segmentation is available on Zenodo: https://zenodo.org/record/3613688?fbclid=IwAR0SNxO8LVgkYYAgKtfg9ZeshbfTvyXMeggqHnoRou_a2MmAaQsvxhroeC0#.XoStbNNKg0o.


