Abstract
Study Objectives
The purpose of this study was to examine how rest-activity (RA) rhythm stability may be associated with white matter microstructure across the lifespan in healthy adults free of significant cardiovascular risk.
Methods
We analyzed multi-shell diffusion tensor images from 103 healthy young and older adults using tract-based spatial statistics (TBSS) to examine relationships between white matter microstructure and RA rhythm stability. RA measures were computed using both cosinor and non-parametric methods derived from 7 days of actigraphy data. Fractional anisotropy (FA) and mean diffusivity (MD) were examined in this analysis. Because prior studies have suggested that the corpus callosum (CC) is sensitive to sleep physiology and RA rhythms, we also conducted a focused region of interest analysis on the CC.
Results
Greater rest-activity rhythm stability was associated with greater FA across both young and older adults, primarily in the CC and anterior corona radiata. This effect was not moderated by age group. While RA measures were associated with sleep metrics, RA rhythm measures uniquely accounted for the variance in white matter integrity.
Conclusions
This study strengthens existing evidence for a relationship between brain white matter structure and RA rhythm stability in the absence of health risk factors. While there are differences in RA stability between age groups, the relationship with brain white matter was present across both young and older adults. RA rhythms may be a useful biomarker of brain health across both periods of adult development.
Keywords: aging, sleep and the brain, circadian rhythms, actigraphy, neuroimaging
Statement of Significance.
Our study investigated the relationship between rest-activity rhythm stability and white matter integrity in healthy aging. We addressed three primary limitations of previous studies on this topic. (1) We used actigraphy recordings in addition to self-reported questionnaire data to assess rest-activity patterns. (2) We enrolled healthy adults free of significant disease burden to reduce the mediation effect of cardiovascular disease and sub-threshold cardiovascular pathology. (3) We included a young adult group to evaluate whether the association between rest-activity rhythm stability and white matter changes with age. Our results support the importance of day-to-day stability in rest-activity rhythms as a biomarker of white matter integrity not only within older adults, but across the healthy adult lifespan.
Introduction
Circadian rhythms are endogenously generated patterns responsible for controlling cyclical physiological processes, such as thermoregulation, metabolism, and rest-activity patterns. In healthy aging, older adults often report changes in their rest-activity rhythms, for example, increased morningness preference, earlier wake times, increased sleep latency, and decreased total sleep time [1]. Rest-activity rhythms can be assessed through self-report questionnaires, which can be a useful clinical tool but remain subject to a high level of recall bias [2]. Wearable electronics, such as wrist-based actigraphy devices, offer an attractive alternative for acquiring objective, non-invasive, real-world data about individuals’ rest-activity behaviors [3]. Wrist-based actigraphy has enabled both researchers and clinicians to passively collect activity data with less participant burden and high ecological validity [4, 5].
Measures of rest-activity patterns can be calculated from actigraphy data to provide a quantitative snapshot of an individual’s rest-activity behavior. Actigraphy studies of developmental changes in rest-activity patterns show increased variability with aging. In general, compared to young adults, older adults demonstrate increased interdaily stability, or greater day-to-day activity stability indicating coupling of activity patterns to fixed markers such as sunlight, as well as earlier acrophase, or time of peak activity [6]. However, within-group studies of older adults have shown that increased age is associated with increased fragmentation within a 24-h period and decreased rhythm amplitude [7, 8]. The extent to which such age-related changes occur as a result of alterations in the biological clock mechanism and associated neurobiological pathways, increased disease burden, or as a result of lifestyle changes remains an important question, and likely varies across individuals [9]. Nevertheless, numerous studies point to rest-activity rhythm changes as useful clinical markers of various health-related outcomes. Disturbances in rest-activity cycles have been linked to cognitive dysfunction [10–12], dementia [7, 13], cerebrovascular changes [14, 15], and depression [16] independent of the contribution of total sleep time. Developing a greater knowledge base around changes in rest-activity profiles and their neurobehavioral correlates in healthy aging may reveal an important biomarker for distinguishing healthy aging from those with increased risk for illness and cognitive dysfunction, facilitating the earlier detection of health changes.
Aging is associated with a number of structural and functional brain changes [17]. One domain that has been examined is white matter. White matter microstructure can be examined using diffusion tensor MRI (DTI), which measures the diffusion of water molecules to characterize brain white matter. From DTI, scalar metrics reflecting the underlying micro-architecture of white matter have been developed. These metrics, fractional anisotropy (FA), which reflects the level of directionality associated with the diffusion, and mean diffusivity (MD), which indicates the degree of diffusion, offer quantitative information about the structural integrity of the white matter. Higher FA is typically associated with greater white matter integrity, such that water diffusion is constrained along the direction of the white matter tract. Studies in non-human animals have indicated that FA is relatively non-specific in that it is sensitive to multiple aspects of white matter integrity, including local differences in myelination, axonal density, and crossing fiber orientation [18]. MD is typically associated with lower white matter integrity, specifically tissue degeneration [19]. Aging is commonly characterized by widespread decreases in FA [20–23] and increases in MD [22–24], with some studies (but not all: see Barrick et al. [25]) noting greater susceptibility in more anterior brain regions to white matter microstructural changes [26].
Some studies suggest that regional patterns of age-related changes in white matter may show distinct relationships with cognitive abilities. In a cross-sectional study, Kennedy and Raz [27] found age-related degradation in anterior white matter corresponded to less efficient processing speed and working memory, whereas posterior changes were associated with worse performance on task switching and inhibition. Intervention studies have also found white matter diffusion metrics to correspond to changes in cognitive performance over time. Engvig et al. [28] found that healthy older adults randomly assigned to an 8-week memory training intervention showed greater memory performance related to controls, and that this improvement in memory was associated with greater FA in the left anterior thalamic radiation, inferior fronto-occipital fasciculus, uncinate fasciculus, and superior longitudinal fasciculus.
Prior DTI studies have examined neural correlates of sleep behavior, and in general have found that white matter microstructure is sensitive to sleep quality. Using actigraphy to obtain objective sleep assessments, Kocevska et al. [29] found that among middle-aged and older individuals, greater sleep onset latency, lower sleep efficiency, and shorter total sleep times were associated with differences in white matter microstructure, particularly in the projection and association tracts, the cingulum, and the anterior forceps of the corpus callosum (CC). However, studies regarding how the day-to-day stability of rest-activity behaviors may be related to white matter integrity are relatively few. Existing studies have reported that disruptions in rest-activity cycles may be associated with a higher prevalence of white matter hyperintensities [30, 31] and widespread reductions in white matter integrity [32] in older adults. However, the extent to which these results may be accounted for by other factors, such as cardiovascular risk, remains an important area of inquiry.
In a recent study, Baillet et al. [32] reported a significant correlation between 24-h rhythm amplitude and white matter microstructure among older adults, such that greater 24-h amplitude, or robustness, in rest-activity patterns was associated with greater FA and lower diffusivity (MD and radial diffusivity) in a number of brain regions. While the study sample was relatively healthy, a majority endorsed cardiovascular risk factors, such as hypertension, diabetes, and/or showed ischemic lesions on the MRI. Furthermore, after removing four subjects with significant white matter hyperintensity burden from their dataset, the effect of 24-h rhythm amplitude on FA was substantially reduced from widespread non-localized clusters to a circumscribed effect in the CC and right frontal region. The inclusion of individuals with risk factors and hyperintensities in their sample infers significant limitations on the conclusions that can be drawn about the link between rhythm amplitude and white matter microstructure, which rather than being a primary relationship, could be wholly a consequence of cardiovascular burden.
In this study, we aimed to remove the mediating influence of cardiovascular risk on the relationship between rest-activity rhythm stability and white matter integrity in older adults. We hypothesized that greater rest-activity rhythm stability would correspond to greater white matter integrity. Our sample is comprised of healthy community-dwelling young adults and older adults free of uncontrolled hypertension, diabetes, and positive cardiovascular history to discern whether the relationship between rest-activity rhythm stability and white matter microstructure was present in older adults free of significant cardiovascular risk. We explored different measures of rest-activity stability such as cosinor rhythm amplitude and fit (F-statistic), as well as non-parametric measures used in previous studies. Based on Baillet et al.’s findings suggesting the sensitivity of the CC to rest-activity rhythms in healthy older adults without significant white matter hyperintensity burden, we conducted a focused analysis on the effect of rhythm stability in this region. Additionally, our study is the first to include a young adult comparison group. In doing so, we sought to better understand the rest-activity rhythm and white matter relationships across the lifespan.
Methods
Participants
Participants included right-handed healthy older and younger adults from central Texas, in and around Austin. The study was advertised through posters, online forums, and recruitment events at aging conferences and senior recreation centers. Candidate participants were invited to participate in the study if they met the following inclusion criteria: (1) were between the ages of 18–30 or 60–90, (2) endorsed fewer than eight items on the Pittsburgh Sleep Quality Inventory (PSQI), (3) endorsed fewer than 16 items on the Center for Epidemiological Studies Depression Scale (CESD) or fewer than 15 items on the Geriatric Depression Scale (GDS). Participants were excluded if they (1) met criteria for significant sleep disturbance or disorder, neurological or psychiatric disorders, or cardiovascular disease, including uncontrolled hypertension and diabetes, (2) were currently taking sleep medication or psychoactive substances, or (3) scored below two standard deviations from the age-adjusted norm on an abbreviated neuropsychological battery assessing memory and executive functions. Participants who endorsed sleep apnea were considered eligible for the study if apnea was controlled with continuous positive airway pressure (CPAP) machine or behavioral therapy (e.g. cognitive behavioral therapy for insomnia). Participants were compensated for their participation in the study. Ethical approval was received from The University of Texas at Austin Institutional Review Board and prior written consent was obtained from all participants.
Neuropsychological assessments
All participants were administered an abbreviated neuropsychological battery, including the following assessments: the Wechsler Adult Intelligence Scale IV (WAIS-IV) Vocabulary and Digit Span subtest, and Trail Making Test A and B. Older adults were additionally administered the California Verbal Learning Test-II (CVLT-II) and Controlled Oral Word Association Test (COWAT-FAS). Age-adjusted z-scores were retained for statistical analysis.
Psychomotor vigilance test
Participants were administered the psychomotor vigilance test (PVT) on a Windows computer (Lenovo Intel® 2015 Core™ i5-2410M CPU 2.30 GHz 64-bit Windows 10) using freely available PC-PVT 1.0 software [33]. The PVT is a widely used measure of alertness which is highly sensitive to sleep deprivation. Participants are asked to attend to a dark screen and respond by clicking a mouse as soon as a stimulus appears on the screen center. The stimulus appears at random inter-stimulus intervals over the course of ten minutes. We collected data on participants’ mean response times, number of false starts, and number of response lapses for analysis.
Actigraphy
Physical activity levels were continuously recorded for 10–14 days from wrist-based actigraphs (Actiwatch 2.0, Philips Respironics, Bend, OR) in zero-crossings mode using 30-s epochs. Participants were instructed to maintain their normal routines to refrain from using any sleep medications. The most recent 7 days of actigraphy data were used for all participants in order to maximize our study sample size while retaining sufficient data for computation of rest-activity measures. Activity counts were linearly interpolated for 5-min periods in which activity data was missing. Participants who removed the watch for more than 20% of the recording time or who had less than 7 days of actigraphy data were excluded from the analysis. Participants also completed daily sleep surveys through a REDCap [34] online survey instrument to document bedtime, wake time, sleep quality, and any instances in which they removed the actigraph.
Rest-activity measures
Measures calculated from actigraphy data allow researchers to measure the timing and consistency of rest-activity patterns. Rest-activity measures can be derived using either variations on a cosinor model or non-parametric approaches. The extended cosinor model assumes either a sinusoidal or square-like shape for activity data and enables the computation of quantitative, descriptive metrics summarizing global rest-activity patterns [35]. These include measures of robustness (amplitude, F-statistic), phase (up-mesor, acrophase, down-mesor), rate of transition from low activity to high activity (beta), and relative duration of peak activity (width). While this model can offer important quantitative insights into one’s rest-activity behavior, it is not “one-size-fits-all.” Some individuals have rest-activity patterns that deviate from the extended cosinor pattern resulting in poor model fit, which can limit the informativeness of these parameters.
Non-parametric approaches to rest-activity analysis offer an alternative for deriving useful measures that are not contingent on assumptions about the shape of human activity behavior [36, 37]. We used the nparACT R package [37] to calculate non-parametric measures including interdaily stability, or how coupled an individual’s activity is to stable markers, such as sunlight; intradaily variability, or how much an individual’s activity varies within a 24-h cycle regardless of the “shape” of that pattern; and relative amplitude, or a ratio of daytime activity to activity during rest. In this study, we examined rhythm stability measures derived from both the five-parameter extended cosinor model (amplitude, F-statistic) and non-parametric approach similar to that used in Baillet et al. [32] (interdaily stability, intradaily variability, relative amplitude) to assess rest-activity relationships with white matter integrity in aging.
Sleep measures
Actiware 6.0.9 software (Philips Respironics, Bend, OR) was used for data retrieval and sleep scoring, in which each data point was coded as either sleep or wake based on adjacent activity values using a sensitivity threshold of 40 counts [38]. We retained total sleep duration, sleep efficiency, and physical activity during the active interval for correlation analyses with rest-activity rhythm measures. Sleep duration is given as the number of minutes scored as “sleep” by the algorithm between the estimated sleep onset time and wake onset time. Sleep efficiency is the ratio of minutes sleeping to minutes in bed given as a percentage. Physical activity is the total activity counts during the interval coded as “active” by the Actiware algorithm. These measures were averaged across the seven most recent days of actigraphy recording before being included in statistical analyses.
MRI acquisition
Imaging data were collected using a Siemens Skyra 3T scanner (TIM Systems, Siemens Medical Solutions, Erlangen, Germany) with a 32-channel head coil at the Biomedical Imaging Center at The University of Texas at Austin. Anatomical MRI volumes were acquired using a 3D MPRAGE T1-weighted (T1w) sequence with the following parameters: TR = 2530.0 ms, TE = 1.69, 3.55, 5.41, and 7.27 ms, T1 = 1100 ms, FOV = 256 mm2, 176 coronal slices, voxel size 1.0 mm3. Two diffusion-weighted images were acquired using a multi-echo EPI sequence with the following parameters: TR = 4836 ms, TE = 120.60 ms, 78 slices, voxel size of 1.8 mm3. Images were collected in an axial/horizontal orientation with alignment to maximize brain coverage beginning at the dorsal surface. Two b0 images were acquired per image and diffusion gradients were applied in 71 directions within an interwoven b = 1000 and b = 3000 multi-shell acquisition. For each of the two scan protocols, an equivalent set was acquired with reversed phase-encode blips anterior to posterior (AP) and posterior to anterior (PA) separately, resulting in pairs of images with opposite direction distortions which when processed increase SNR and allow for distortion correction.
Gray matter volume analysis
Anatomical preprocessing was conducted using fMRIPrep version 1.5.0 [39]. The T1w MPRAGE image was corrected for intensity non-uniformity (INU) with N4BiasFieldCorrection [40], distributed with ANTs 2.2.0 [41], and used as T1w-reference throughout the workflow. The T1w-reference was then skull-stripped with a Nipype implementation of the antsBrainExtraction.sh workflow (from ANTs), using OASIS30ANTs as a target template. Brain tissue segmentation of cerebrospinal fluid, white matter, and gray matter was performed on the brain-extracted T1w using fast (FSL 5.0.9) [42]. Brain surfaces were reconstructed using recon-all (FreeSurfer 6.0.1) [43]. Volume-based spatial normalization to fsaverage space, which is a standard coordinate system approximately registered to MNI space, was performed through nonlinear registration with antsRegistration (ANTs 2.2.0) using brain-extracted versions of both T1w reference and the T1w template.
White matter microstructure analysis
Whole-brain voxelwise analysis
Diffusion tensor images were processed using the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (http://www.fmrib.ox.au.uk/fsl), or FSL. Susceptibility-induced off-resonance field was estimated using a method described by Andersson [44] as implemented in FSL [45] and the two multi-direction, multi-B value images were combined into a single corrected one. FMRIB BET [46] was applied to eliminate nonbrain tissue. Images were corrected for eddy currents and registered to the b = 0 s/mm2 volume using FMRIB FLIRT [47]. A diffusion tensor model was constructed using the FMRIB DTIFIT algorithm to produce FA at each voxel, and data were visually inspected for model fit.
Tract-based spatial statistics (TBSS) was then used to conduct voxelwise statistical analysis. Images from all subjects were aligned to all other subject images within their age group to identify the best registration candidate for each group. That subject’s image was identified as the target image, and then was aligned to the standard MNI template. Every remaining subject was then aligned to MNI space by combining the nonlinear transform to the target FA image with the affine transform from that target to MNI152 space. FA images were then averaged to produce a group mean image. For each subject, an FA threshold of 0.20 was used before projecting the aligned FA map onto the skeleton [48]. MD maps were also generated using the same steps outlined above. TBSS analysis was conducted independently for both age groups to validate our results.
To assess the relationship between rest-activity rhythm amplitude and white matter microstructure, design matrices were constructed. Missing values (amplitude N = 4) were interpolated before de-meaning. FSL randomize was used to conduct threshold-free cluster enhancement (TFCE)-based nonparametric permutation inference (k = 500) [49]. TFCE is a method to correct for multiple comparisons that enhance areas of signal with spatial contiguity which allows for improved discrimination of voxels within-cluster regions from background noise. The mean FA image was used as a mask to restrict analyses to white matter regions. Clusters were assessed for significance at p < 0.05. The same procedures were executed for MD.
TBSS analyses examining age group effects were conducted by first concatenating FA maps from the two age groups and generating a mask from FA averaged across all subjects. This was then used to generate a skeleton for all subjects before running voxelwise statistics. Using a skeleton-based approach reduces the number of statistical tests performed and ensures that only actual white matter voxels are included thereby reducing the likelihood of Type I error. In addition, the skeleton-based approach is less biased to volumetric differences, which are a known challenge to comparing white matter properties across age groups. By concatenating all participants’ FA maps after they had undergone within-age group registration, we were able to test for interaction effects while preserving the unique within-group registration results. For better visibility, areas of significant results were augmented using tbss_fill.
Region of interest analysis
Because the CC has previously been suggested as a region that may be sensitive to rest-activity rhythms, we conducted a focused ROI analysis to test for correlations between white matter diffusion metrics and rhythm amplitude in this region. The CC was defined using the Johns Hopkins University (JHU) atlas. Mean FA and MD values for the CC were extracted using fslmeants. Analyses were adjusted for age and CC volume. Volume measurements were extracted from the FreeSurfer segmentation and adjusted for total intracranial volume (ICV) as follows: adjusted CC volume = raw CC volume – b × (ICV – average ICV), where b is the slope of the regression of the raw CC volume on ICV [50].
Statistical analyses
Statistical analyses were performed using R version 3.6.1. Welch’s t tests were conducted to compare group means for behavioral variables. Linear regression with leave-one-out cross-validation was used to assess relationships among CC structural properties and rest-activity rhythm stability [51]. Separate models were used for non-parametric and cosinor rest-activity measures. Interaction analyses were performed using moderated multiple regression for which the rest-activity measure, binary age group, and their cross-product were included in the model. We also included CC volume as a covariate. If the interaction was not significant, we evaluated the main effect of the rest-activity measure on CC FA, again covarying for CC volume. We performed leave-one-out cross-validation to estimate the robustness of the model to changes in the subject pool. We retained the models with the most conservative estimated effect size of the rest-activity measure for this manuscript (see Supplementary Material for additional details). Correlations among rest-activity rhythm measures, sleep measures, and cognitive assessment scores were evaluated using bootstrapped confidence intervals with a jackknife adjustment [52]. Data are available on request.
Results
Demographic and health information
Of the 124 participants enrolled in the study, we analyzed data from a total of 103 participants, including 57 older adults (45 females, age = 68.11 ± 5.64 years) and 46 younger adults (28 females, age = 21.39 ± 3.78 years; Table 1). The main reasons for excluding participants from the analysis were: issues with DTI preprocessing (N = 2), unable to collect MRI (N = 3), issues with MRI acquisition (N = 10), issues with actigraphy acquisition (N = 3), failure to fit a cosinor rest-activity model (N = 4), unable to obtain non-parametric rest-activity values (N = 10), and voluntary withdrawal from the study (N = 5).
Table 1.
Participant Demographics and Health Characteristics
| Young adults | Older adults | |
|---|---|---|
| N | 46 | 57 |
| Age (mean [SD]) | 21.39 (3.78) | 68.11 (5.64) |
| Sex = female (%) | 28 (60.9) | 45 (78.9) |
| Education (mean [SD]) | 14.64 (2.33) | 17.06 (3.06) |
| Vascular disease = positive (%) | 0 (0.0) | 1 (1.8) |
| Diabetes = positive (%) | 0 (0.0) | 0 (0.0) |
| Cancer = positive (%) | 0 (0.0) | 3 (5.3) |
| Head injury = positive (%) | 0 (0.0) | 3 (5.3) |
| Neurological disorder = positive (%) | 0 (0.0) | 3 (5.3) |
| Stroke = positive (%) | 0 (0.0) | 0 (0.0) |
| Psychiatric disorder = positive (%) | 0 (0.0) | 3 (5.3) |
| Sleep disorder = positive (%) | 0 (0.0) | 6 (10.5) |
Age group differences in rest-activity rhythms
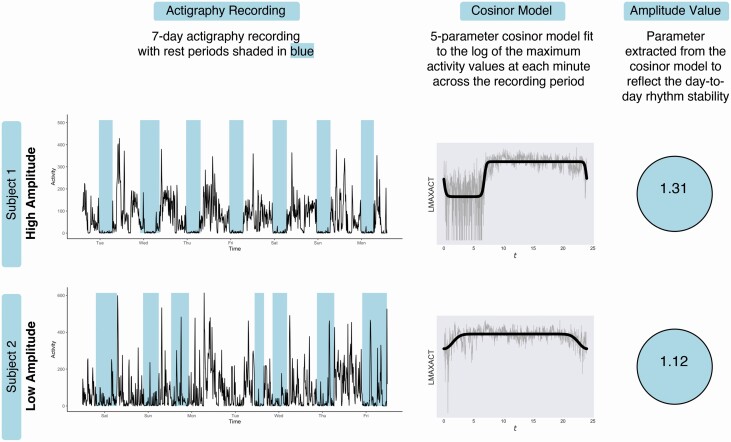
Correlations between rest-activity measures and mean sleep duration, sleep efficiency, mean daily physical activity, and global PSQI scores are presented in Table 2. To illustrate how rhythm amplitude manifests in terms of real-world behavior, a visual comparison of subjects with differing amplitude values is provided in Figure 1. Participants with a higher amplitude tend to exhibit greater day-to-day stability across the recording period, whereas participants with low amplitude exhibit more variability in activity across days. Comparison of sleep and activity metrics derived from the Actiware program showed that participants with high rhythm amplitude determined by a median split had greater sleep efficiency (t = −2.161, p = 0.033) and shorter sleep onset latency (t = 2.863, p = 0.005), but no observable differences in mean sleep duration or mean daily physical activity.
Table 2.
Correlations among Rest-Activity Rhythm Stability Measures and Sleep Duration, Sleep Efficiency, Physical Activity, and Global PSQI
| Variable | Correlate | Young adults | Older adults | ||
|---|---|---|---|---|---|
| R | 95% CI | R | 95% CI | ||
| Amplitude | Sleep duration | −0.04 | [−0.61, 0.56] | 0.20 | [−0.14, 0.53] |
| Sleep efficiency | 0.23 | [−0.37, 0.65] | 0.33 | [0.15, 0.52]* | |
| Physical activity | −0.12 | [−0.47, 0.59] | 0.25 | [−0.19, 0.56] | |
| Global PSQI | −0.16 | [−0.51, 0.25] | 0.14 | [−0.11, 0.34] | |
| F-statistic | Sleep duration | 0.21 | [−0.41, 0.73] | 0.15 | [−0.18, 0.38] |
| Sleep efficiency | 0.43 | [−0.03, 0.68] | 0.43 | [0.24, 0.62]* | |
| Physical activity | −0.27 | [−0.55, 0.30] | 0.35 | [0.00, −0.57] | |
| Global PSQI | −0.19 | [−0.47, 0.07] | −0.02 | [−0.25, 0.22] | |
| Interdaily stability | Sleep duration | 0.03 | [−0.54, 0.59] | 0.20 | [−0.05, 0.44] |
| Sleep efficiency | 0.23 | [−0.25, 0.52] | 0.30 | [0.05, 0.53]* | |
| Physical activity | −0.11 | [−0.33, 0.18] | 0.17 | [−0.16, 0.40] | |
| Global PSQI | −0.01 | [−0.33, 0.21] | 0.22 | [−0.09, 0.43] | |
| Intradaily variability | Sleep duration | −0.39 | [−0.67, −0.06] | −0.26 | [−0.49, 0.02] |
| Sleep efficiency | −0.11 | [−0.48, 0.27] | −0.22 | [−0.49, 0.07] | |
| Physical activity | 0.00 | [−0.25, 0.20] | −0.11 | [−0.33, 0.18] | |
| Global PSQI | 0.09 | [−0.27, 0.45] | −0.25 | [−0.45, −0.04]* | |
| Relative amplitude | Sleep duration | 0.62 | [0.41, 0.81]* | 0.56 | [0.36, 0.70]* |
| Sleep efficiency | 0.70 | [0.49, 0.86]* | 0.58 | [0.28, 0.75]* | |
| Physical activity | −0.52 | [−0.78, −0.06]* | −0.13 | [−0.47, 0.18] | |
| Global PSQI | −0.18 | [−0.52, 0.16] | −0.02 | [−0.33, 0.27] |
Correlations were performed using bootstrapped confidence intervals with jackknife adjustment. All neuropsychological measures were reported as standardized z-scores. * indicates p < 0.05.
Figure 1.
Calculation of rest-activity rhythm amplitude for two example older adult participants with high and low rhythm amplitudes. Subject 1. Examination of activity level data for the high amplitude participant shows well-defined rest intervals (in blue). These rest and active intervals repeat in a similar way from day to day, indicating greater day-to-day stability in rest-activity rhythm, which results in a greater rhythm amplitude value. Subject 2. In contrast, the low amplitude participant shows less defined rest intervals with greater variation across in both rest interval duration and frequency, resulting in a lower rhythm amplitude value.
Within our study sample, there were a number of age group differences in sleep and rest-activity pattern characteristics (Table 3). Compared to young adults, older adults had significantly reduced total activity during the active period, but no significant differences in mean sleep duration, sleep efficiency, or sleep onset latency were observed. Older adults also had earlier phase onset, indicating earlier transitions from rest to active periods, and from active periods to rest (acrophase, up-mesor, and down-mesor). Using the extended cosinor model, we found that older adults showed a nonsignificant trend toward greater width values, indicating shorter durations of high activity periods (alpha), as well as greater slope values, meaning faster transitions between low and high activity periods. There was no significant difference in rest-activity rhythm amplitude across age groups. Non-parametric rest-activity analysis indicated that older adults had greater day-to-day stability in rest-activity patterns (interdaily stability).
Table 3.
Sleep and Rest-Activity Rhythm Characteristics of Young and Older Adults
| Young adults | Older adults | p | |
|---|---|---|---|
| N | 46 | 57 | |
| Sleep measures | |||
| Sleep time (mean [SD]) | 400.33 (94.99) | 403.49 (84.68) | 0.860 |
| Sleep efficiency (mean [SD]) | 81.72 (6.78) | 80.49 (8.91) | 0.442 |
| Onset latency (mean [SD]) | 20.48 (13.59) | 28.92 (20.20) | 0.018* |
| Total activity (mean [SD]) | 322,947.97 (183,583.88) | 252,918.64 (89,505.82) | 0.014* |
| Cosinor rest-activity measures | |||
| Amplitude (mean [SD]) | 1.61 (0.29) | 1.58 (0.34) | 0.598 |
| Width (alpha) (mean [SD]) | −0.48 (0.16) | −0.40 (0.26) | 0.050 |
| Slope (beta) (mean [SD]) | 6.63 (2.74) | 14.02 (26.63) | 0.070 |
| Mesor (mean [SD]) | 0.94 (0.16) | 0.95 (0.19) | 0.768 |
| Acrophase (phi) (mean [SD]) | 16.45 (1.43) | 14.76 (1.63) | <0.001*** |
| Up-mesor (mean [SD]) | 8.58 (1.73) | 7.10 (1.78) | <0.001*** |
| Down-mesor (mean [SD]) | 24.31 (1.41) | 22.43 (1.83) | <0.001*** |
| Minimum (mean [SD]) | 0.14 (0.18) | 0.16 (0.17) | 0.444 |
| F-statistic (mean [SD]) | 3216.56 (1613.86) | 3612.04 (1624.19) | 0.230 |
| Non-parametric rest-activity measures | |||
| Interdaily stability (IS) (mean [SD]) | 0.42 (0.11) | 0.54 (0.12) | <0.001*** |
| Intradaily variability (IV) (mean [SD]) | 0.87 (0.25) | 0.85 (0.27) | 0.746 |
| Relative amplitude (RA) (mean [SD]) | 0.84 (0.14) | 0.86 (0.11) | 0.341 |
| L5 (mean [SD]) | 16.46 (16.77) | 11.97 (12.07) | 0.143 |
| M10 (mean [SD]) | 172.61 (46.04) | 158.76 (49.12) | 0.177 |
* indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
Rest-activity rhythms and cognitive performance
The results of the participants’ performance on neuropsychological measures are summarized in Table 4. In general, our study sample’s age-adjusted scores were above average relative to population norms, particularly with respect to performance on the WAIS-IV Vocabulary subtest. Older adults exhibited comparatively better performance on Trails Making Test (TMT) A and B for their age relative to young adults. Performance on other assessments was similar across age groups.
Table 4.
Comparison of Performance on Cognitive Assessments by Age Group
| Young adults | Older adults | p | |
|---|---|---|---|
| N | 46 | 57 | |
| Neuropsychological assessments | |||
| Controlled Oral Word Association Test (mean [SD]) | — | 0.27 (1.00) | — |
| California Verbal Learning Test (CVLT; mean [SD]) | — | 0.23 (0.73) | — |
| CVLT Short Delay (mean [SD]) | — | 0.59 (1.05) | — |
| CVLT Long Delay (mean [SD]) | — | 0.52 (0.95) | — |
| Trails Making Test A (mean [SD]) | —0.28 (1.10) | 0.43 (1.33) | 0.005** |
| Trails Making Test B (mean [SD]) | −0.39 (1.43) | 0.25 (1.40) | 0.026* |
| WAIS-IV Digit Span (mean [SD]) | 0.33 (1.07) | 0.46 (1.03) | 0.521 |
| WAIS-IV Vocabulary (mean [SD]) | 1.35 (1.00) | 1.29 (0.98) | 0.756 |
| Psychomotor vigilance task | |||
| Mean response time (mean [SD]; ms) | 284.65 (54.90) | 328.51 (222.42) | 0.195 |
| False starts (mean [SD]) | 1.59 (1.72) | 4.19 (10.84) | 0.110 |
| Response lapses (mean [SD]) | 0.00 (0.00) | 0.04 (0.26) | 0.372 |
All neuropsychological measures are reported as age-adjusted standardized z-scores. Young adults were not administered the Controlled Oral Word Association Test or the California Verbal Learning Test. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
Results from a correlation analysis using standardized z-scores for neuropsychological test measures and PVT results with rhythm amplitude are shown in Table 5. There was a weak positive correlation between rhythm amplitude and performance on TMT B. No other correlations with cognitive measures were statistically significant. Due to the weak evidence for a relationship between the selected rest-activity rhythm measures and cognitive performance in this sample, we did not further explore voxelwise white matter associations with cognitive measures.
Table 5.
Correlations between Rhythm Amplitude and Cognitive Measures
| Variable | Amplitude | |
|---|---|---|
| r | 95% CI | |
| Neuropsychological assessments | ||
| California Verbal Learning Test+ | −0.16 | [−0.4, 0.11] |
| Controlled Oral Word Association Test+ | −0.15 | [−0.45, 0.19] |
| Trails Making Test A | −0.02 | [−0.18, 0.13] |
| Trails Making Test B | 0.29 | [0.04, 0.47] |
| WAIS-IV Vocabulary | 0.13 | [−0.04, 0.29] |
| WAIS-IV Digit Span | −0.01 | [−0.19, 0.16] |
| Psychomotor vigilance task | ||
| Mean response time | 0.08 | [−0.05, 0.30] |
| False starts | −0.04 | [−0.19, 0.23] |
Correlations were performed using bootstrapped confidence intervals with jackknife adjustment. All neuropsychological measures were reported as standardized z-scores. + indicates results from the older adult group only.
Age group differences in white matter diffusion metrics
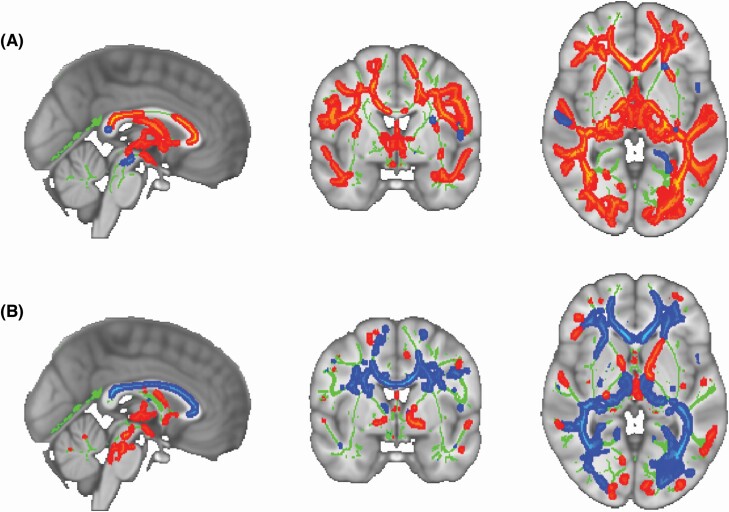
TBSS analyses showed widespread decreases in FA and increases in MD in older adults compared to younger adults (p < 0.05, TFCE corrected, Figure 2), a finding that is consistent with the existing literature [20–24].
Figure 2.
Results of TBSS analyses examining age group differences in (A) FA and (B) MD between healthy young and older adults superimposed on the MNI standard. The average white-matter skeleton is presented in green. Red and blue colored areas indicate regions of the skeleton in which significant age group differences in diffusion metrics were observed at p < 0.05 (TFCE; corrected for multiple comparisons). Areas where younger adults showed greater values for diffusion metrics are shown in warm colors, whereas areas where younger adults showed lower values are shown in cool colors. Surrounding voxels were augmented for visual purposes.
Rest-activity rhythms and white matter microstructure
Whole-brain analysis
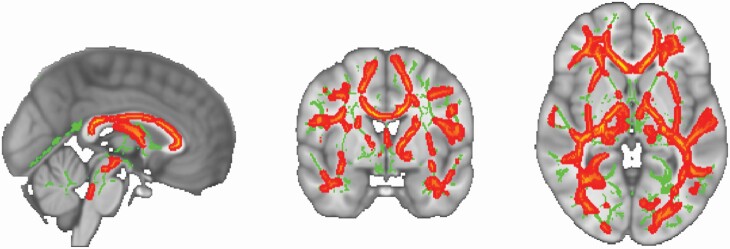
TBSS analysis showed significant clusters for a positive association between rest-activity rhythm amplitude and FA across both age groups (Figure 3). A single significant cluster was observed spanning the CC and bilateral anterior corona radiata (Table 6). There was not a significant interaction with age group for the relationship between rhythm amplitude and FA. Given ongoing debate about the use of an FA skeleton in TBSS analysis, we sought to validate our results through statistical analysis of mean FA values extracted from 6 mm spherical ROIs selected from overlapping and non-overlapping regions from the skeletonized statistical map. Consistent with our TBSS analysis, we did not see evidence of an interaction effect of age group at any of the 12 ROIs. Some previous research [53] supports that there should be between group differences in this relationship, therefore because this study was of modest sample size, we explored some of these potential differences with less statistical rigor in order to generate hypotheses for future studies. These analyses are included in Supplementary Material.
Figure 3.
Results of TBSS analyses examining correlations between rhythm amplitude and FA superimposed on the MNI standard. The average white-matter skeleton is presented in green. Warm colors indicate areas of the white matter skeleton for which there was a positive correlation between rhythm amplitude and FA across all subjects at p < 0.05 (TFCE; corrected for multiple comparisons). Surrounding voxels were augmented for visual purposes.
Table 6.
Clusters Showing Significant Association between Rhythm Amplitude and FA
| Cluster index | Voxels | 1–p MAX | MAX X (mm) | MAX Y (mm) | MAX Z (mm) | Locations (>2% probability) |
|---|---|---|---|---|---|---|
| 1 | 57,839 | 0.998 | 13 | 43 | -14 | Genu of corpus callosum: 2.82 |
| Body of corpus callosum: 3.94 | ||||||
| Splenium of corpus callosum: 2.50 | ||||||
| Anterior corona radiata L: 2.07 |
No significant correlations with white matter FA were found for either the cosinor F-statistic or any non-parametric rest-activity measures, including relative amplitude, interdaily stability, or intradaily variability.
Analysis of MD maps revealed no significant clusters associated with any of the selected rest-activity measures, including rhythm amplitude. T-statistic maps showed some evidence of a moderating effect of age group on the relationship between intradaily variability and MD. However, this effect did not reach statistical significance at p < 0.05, TFCE-corrected (p = 0.058). Again, because we did not find robust between group differences in the relationship between intradaily variability and MD, we present these analyses in the Supplementary Material.
CC ROI analysis
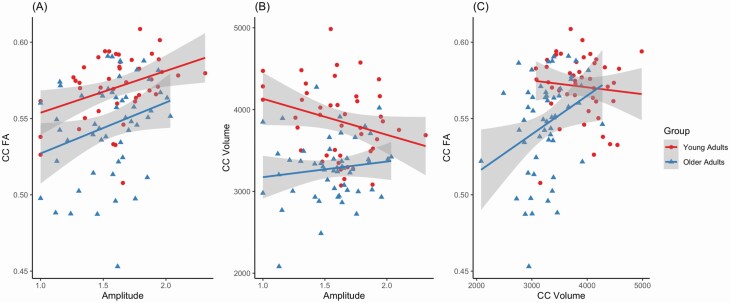
Results of linear regression analyses were consistent with the whole brain analysis. There was no significant interaction with age group for the relationship between rhythm amplitude and CC FA adjusting for CC volume. There was a significant main effect of rhythm amplitude adjusting for age group and CC volume (Table 7, Figure 4). Neither CC MD nor volume showed a significant relationship with rhythm amplitude. Because of the significant correlation between sleep efficiency and rhythm amplitude, sleep efficiency was also examined as a candidate predictor of CC FA in separate models controlling for age and CC volume, but was not significant (see Supplementary Material).
Table 7.
Results of Regression Analyses Predicting Corpus Callosum FA
| β coef | 95% CI | p | F | df | p | R 2 | CV R2 | |
|---|---|---|---|---|---|---|---|---|
| Interaction model | 10.15 | 4.92 | <0.001*** | 0.306 | 0.246 | |||
| Age group | −0.28 | [−0.34, −0.22] | 0.571 | |||||
| Amplitude | 0.34 | [0.32, 0.37] | 0.020* | |||||
| CC volume | 0.23 | [0.23, 0.23] | 0.040* | |||||
| Age group * amplitude | 0.01 | [−0.02, 0.05] | 0.978 ns | |||||
| Main effect model | 13.69 | 3.93 | <0.001*** | 0.306 | 0.254 | |||
| Age group | −0.26 | [−0.28, −0.25] | 0.015* | |||||
| Amplitude | 0.35 | [0.33, 0.36] | <0.001*** | |||||
| CC volume | 0.23 | [0.23, 0.23] | 0.036* |
Variance explained is given by R2 and cross-validated (CV) R2.
* indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
Figure 4.
Scatter plots showing relationships between (A) rhythm amplitude and corpus callosum (CC) FA, (B) rhythm amplitude and CC volume, and (C) CC volume and CC FA.
Discussion
The goal of this study was to investigate the relationship between rest-activity rhythms and white matter microstructure in healthy young and older adults free of cardiovascular and other significant health risk factors. Up-to-date, this is the only study where this relationship has been examined in both a healthy young and older adult sample. Our results showed that rest-activity rhythm amplitude was associated with brain white matter microstructure (FA) in both healthy young and older adults, primarily in the CC and anterior corona radiata. The CC is the predominant interhemispheric pathway in the brain, and in young adults CC diffusion metrics have been found to be sensitive to acute sleep effects, such as sleep deprivation vulnerability [54] and sleep spindle power [55]. Our study suggests that white matter microstructure is not only associated with acute sleep effects, but that subtle, global patterns of rest and activity occurring over an extended period may also have an important relationship with white matter microstructure. The use of rest-activity measures derived from non-intrusive, wearable technology may therefore be a useful biomarker of brain health.
Age-related differences in rest-activity rhythms
While many older adults report changes in rest-activity behavior, there is mixed evidence regarding associations between later life and rest-activity stability assessed through actigraphy [56]. In our study, we found that older adults exhibited significantly greater coupling of rest-activity rhythms to stable 24-h makers (interdaily stability), as well as an earlier phase onset (acrophase, up-mesor, and down-mesor) compared to young adults. The latter finding is consistent with a number of other studies documenting increased rigidity in rest-activity rhythms as well as earlier chronotypes, including earlier waketimes and bedtimes, among older adults [6, 56–58].
These age group differences in rest-activity rhythms may highlight the potential relative roles of age-related changes in both biological function and lifestyle factors which contribute to rest-activity profiles. Age-related decreases in rest-activity rhythm stability are often attributed to the degradation of the biological clock, or the suprachiasmatic nucleus (SCN), and its associated pathways. The extent to which rest-activity disturbances in these populations are attributable to direct interference with the SCN or indirect interference through either upstream or downstream effects remains an important area of investigation. Other health-related concerns can also significantly impact rest-activity patterns. Studies in clinical samples have found that compared to healthy older adult controls, individuals with Alzheimer’s disease [7, 36], Parkinson’s disease [59, 60], depression [16, 61], and schizophrenia [62], show greater disruption in rhythm stability. Additionally, lifestyle factors, such as employment status, are another important contributor to age-related differences in rest-activity rhythm behaviors [57].
Rest-activity rhythms and cognition
Prior studies suggest that brain regions underlying important aspects of learning and memory are sensitive to aspects of sleep physiology [53, 63]. However, in our study, while we did find brain white matter correlates of rest-activity rhythm stability, we did not find significant correlations between brain white matter and cognitive performance. This contrasts with research showing correspondence between greater fragmentation of the rest-activity rhythm and poorer executive function [10, 64]. Our selective enrollment criteria limiting our study sample to very healthy older adults is consistent with the skewed distribution of neuropsychological assessment scores we observed, with the mean scores for the older adult group in the average to high average range. While the participants’ relatively high scores on these standardized tests are evidence for the integrity of our healthy sample, this may have limited the sensitivity of our analysis to detecting cognitive associations with rest-activity rhythms. Therefore, the extent to which rest-activity measures could provide important measures associated with cognitive decline will require future work in an older adult sample with greater functional variability.
Rest-activity rhythms and white matter in healthy aging
This study disambiguates the role of cardiovascular risk in a prior study [32] showing a positive effect of 24-h rhythm amplitude on white matter integrity in older adults. This is important because cardiovascular risk, comprising chronic hypertension, diabetes, and ischemic lesions, has been associated with greater fragmentation of rest-activity rhythms [14, 15], as well as age-related changes in white matter integrity [65]. Older adults with chronic hypertension are at greater risk for white matter lesions [66, 67], and demonstrate reduced FA and increased MD compared to healthy controls [68]. Here, in a sample of healthy young and older adults free of significant cardiovascular risk, we sought to clarify whether rest-activity rhythms have a unique relationship with white matter independent of cardiovascular risk factors. In our study, greater rhythm amplitude was associated with greater FA, but was not associated with MD. Greater FA is generally reflective of greater myelination, although it can also reflect the degree of fiber crossing, axonal density, and average axonal diameter [69–71]. In compact white matter structures, such as the CC, greater FA may better reflect a higher degree of fiber organization, or a greater number of axons involved in interhemispheric communication, rather than myelin content [72]. In contrast, MD reflects the rate of diffusion independent of directionality [73]. With this in mind, is interesting that in our study rhythm amplitude was associated with CC FA independent of CC volume, suggesting that rhythm amplitude may account for unique variance in the fiber organization of the CC.
Our study also examined whether the relationship between rhythm stability and white matter is moderated by age group. We did not find a significant interaction effect with age group on the relationship between rhythm amplitude and white matter integrity. These findings align with findings from Kocevska et al. [74] which showed that poor sleep quality predicted lower FA in middle-age and older adults up to 7 years later but was not associated with changes in white matter over time. In line with these findings, our results suggest that individual differences in rest-activity rhythm stability appear to be an important associate of brain white matter irrespective of age. This conclusion remains based on our relatively modest sample size. For that reason, we have explored some of the less robust findings of potential age group differences in the relationship between rest-activity rhythms and white matter in the Supplementary Material.
It is important to draw attention to the fact that while we did not find evidence of an interaction effect of age group on the relationship between rhythm stability and white matter integrity, the manner in which this relationship unfolds with aging will vary across individuals depending on other lifestyle and health-related factors. Given the cross-sectional nature of our study, it is possible that due to cohort effects, we were unable to detect a true interaction effect of age group. Because our older adult participants were selected to represent healthy aging, the way the relationship between rest-activity rhythms and white matter unfolds in this group may differ from how it is expressed in typical aging. For example, it is likely that participants in our healthy young adult group will go on to develop various health and lifestyle changes as they age. These changes are likely to impact rest-activity behaviors and consequently brain health, or vice versa. The causal sequence of how changes in either white matter integrity or rest-activity rhythms may produce changes in the other remains an important area of inquiry. However, it is interesting that even the young adult group, which likely had a greater variety of lifestyle and sub-clinical health risk factors, showed a robust relationship between rhythm amplitude and FA in multiple brain regions. This suggests that rest-activity rhythm stability may be expected a similarly important role in white matter integrity in older adults with health profiles more typical of “normal” aging.
Cosinor rhythm amplitude as a unique predictor of white matter microstructure
Among the rest-activity rhythm measures we explored, cosinor rhythm amplitude was uniquely important for white matter integrity across age groups. Rhythm amplitude was correlated with greater sleep efficiency, but was not related to sleep duration, daily physical activity, or global PSQI scores. In contrast, relative amplitude derived from the non-parametric method was associated with sleep duration and daily physical activity in addition to sleep efficiency. Of particular interest, follow-up regression analyses focusing on the CC did not reveal a significant relationship between sleep efficiency and white matter integrity. This indicates that the stability of daily activity patterns, comprised of not only rest but also active periods, is as a whole important for white matter integrity in this region. Furthermore, in accordance with Kocevska et al. [75], we did not find a relationship between white matter and subjective sleep complaints ascertained from participants’ global PSQI scores. However, we restricted our eligibility criteria to only include participants who endorsed eight or fewer items on the PSQI, so we were unable to test this relationship with a sample that expressed a fuller range of sleep complaints. Rest-activity measures derived from objective actigraphic assessments may therefore offer greater sensitivity to the state of an organism, thereby providing greater utility beyond that of subjective sleep assessments as biomarkers of brain health.
Strengths and limitations of the study
This study advanced the existing literature on this topic by addressing three main limitations: We enrolled healthy adults selected to be free of significant disease burden to reduce the mediation effect of cardiovascular disease and sub-threshold cardiovascular pathology, included a young adult group to evaluate whether the association between rest-activity rhythm stability and white matter changes with age, and used actigraphy recordings in addition to self-reported questionnaire data to assess rest-activity patterns. However, our study is not without its limitations. A priori power analysis on the question of circadian rhythm–white matter relationships was not performed, which hindered our ability to understand whether the sample size used in this study was sufficient to detect a true effect. While the TBSS approach to analyzing DTI data offers several methodological advantages over other approaches, including increased statistical power from dimensionality reduction and removing the need for spatial smoothing, there is ongoing debate about the FA skeleton’s effects on anatomical localizability and interpretability of effects, as well as its sensitivity to the quality of image registration [76]. In order to address this limitation, we conducted follow-up ROI analyses which reconfirmed our TBSS findings. Finally, while many cross-sectional studies report white matter microstructure is an important neurobiological marker of age-related cognitive changes, longitudinal studies allowing for evaluation of a causal relationship between white matter and cognition are somewhat more sparse [77]. Given the observational nature of our study, future longitudinal work is needed to clarify whether the current findings are reflective of microstructure alterations arising as a consequence of lifelong rest-activity behaviors as opposed to a non-causal relationship driven by other age-related processes.
Conclusion
In summary, this study examined how subtle differences in rest-activity rhythms are associated with brain white matter and whether those relationships are reflected in cognitive functioning. We found that greater rest-activity rhythm stability as indicated by cosinor rhythm amplitude was associated with greater FA across both young and older adults. This effect was primarily observed in the CC and anterior corona radiata, and is consistent with prior literature documenting associations between diffusion metrics in these regions and acute sleep effects. Given that we now understand that there are relationships in healthy elderly adults, future studies can better examine how the relationship between rhythm amplitude and white matter microstructure is affected in older adults with significant health risk factors, such as cardiovascular risk.
Supplementary Material
Acknowledgments
We would like to offer our thanks to Dr. Andreana Haley and Dr. Kimberly Ray for their manuscript comments, Derek Pisner for his programming guidance, and to Laura Gandy for her assistance with study recruitment.
Funding
This research is supported by the National Institute on Aging of the National Institutes of Health under award number R01AG043425. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement. None declared.
Disclosure statement
Financial disclosure: none.
Non-financial disclosure: none.
References
- 1. Mander BA, et al. Sleep and human aging. Neuron. 2017;94(1):19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lockley SW, et al. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8(3):175–183. [DOI] [PubMed] [Google Scholar]
- 3. Sallis JF, et al. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(Suppl 2):1–14. [DOI] [PubMed] [Google Scholar]
- 4. Ginexi EM, et al. The promise of intensive longitudinal data capture for behavioral health research. Nicotine Tob Res. 2014;16(Suppl 2):S73–S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin JL, et al. Wrist actigraphy. Chest. 2011;139(6):1514–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoon IY, et al. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51(8):1085–1091. [DOI] [PubMed] [Google Scholar]
- 7. Musiek ES, et al. Circadian rest-activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol. 2018;75(5):582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang YL, et al. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol Behav. 2002;76(4–5):597–603. [DOI] [PubMed] [Google Scholar]
- 9. Lieberman HR, et al. Circadian rhythms of activity in healthy young and elderly humans. Neurobiol Aging. 1989;10(3):259–265. [DOI] [PubMed] [Google Scholar]
- 10. Walsh CM, et al. Weaker circadian activity rhythms are associated with poorer executive function in older women. Sleep. 2014;37(12):2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lim AS, et al. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep. 2012;35(5): 633–40B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luik AI, et al. Associations of the 24-h activity rhythm and sleep with cognition: a population-based study of middle-aged and elderly persons. Sleep Med. 2015;16(7):850–855. [DOI] [PubMed] [Google Scholar]
- 13. Tranah GJ, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70(5):722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kadono M, et al. Various patterns of disrupted daily rest-activity rhythmicity associated with diabetes. J Sleep Res. 2016;25(4):426–437. [DOI] [PubMed] [Google Scholar]
- 15. Paudel ML, et al. Rest/activity rhythms and cardiovascular disease in older men. Chronobiol Int. 2011;28(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smagula SF, et al. Circadian rest-activity rhythms predict future increases in depressive symptoms among community-dwelling older men. Am J Geriatr Psychiatry. 2015;23(5):495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raz N, et al. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30(6):730–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15(7–8):435–455. [DOI] [PubMed] [Google Scholar]
- 19. Concha L, et al. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage. 2006;32(3):1090–1099. [DOI] [PubMed] [Google Scholar]
- 20. Teipel SJ, et al. Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study. J Alzheimers Dis. 2010;22(2):507–522. [DOI] [PubMed] [Google Scholar]
- 21. McLaughlin NC, et al. Diffusion tensor imaging of the corpus callosum: a cross-sectional study across the lifespan. Int J Dev Neurosci. 2007;25(4):215–221. [DOI] [PubMed] [Google Scholar]
- 22. Lebel C, et al. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340–352. [DOI] [PubMed] [Google Scholar]
- 23. Sexton CE, et al. Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. J Neurosci. 2014;34(46):15425–15436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sullivan EV, et al. Longitudinal study of callosal microstructure in the normal adult aging brain using quantitative DTI fiber tracking. Dev Neuropsychol. 2010;35(3):233–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barrick TR, et al. White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage. 2010;51(2):565–577. [DOI] [PubMed] [Google Scholar]
- 26. Gunning‐Dixon FM, et al. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24(2):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kennedy KM, et al. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47(3):916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Engvig A, et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp. 2012;33(10):2390–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kocevska D, et al. The prospective association of objectively measured sleep and cerebral white matter microstructure in middle-aged and older persons. Sleep. 2019;42(10). doi: 10.1093/sleep/zsz140 [DOI] [PubMed] [Google Scholar]
- 30. Oosterman J, et al. Distortions in rest-activity rhythm in aging relate to white matter hyperintensities. Neurobiol Aging. 2008;29(8):1265–1271. [DOI] [PubMed] [Google Scholar]
- 31. Zuurbier LA, et al. Cerebral small vessel disease is related to disturbed 24-h activity rhythms: a population-based study. Eur J Neurol. 2015;22(11):1482–1487. [DOI] [PubMed] [Google Scholar]
- 32. Baillet M, et al. Activity/rest cycle and disturbances of structural backbone of cerebral networks in aging. Neuroimage. 2017;146:814–820. [DOI] [PubMed] [Google Scholar]
- 33. Khitrov MY, et al. PC-PVT: a platform for psychomotor vigilance task testing, analysis, and prediction. Behav Res Methods. 2014;46(1):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harris P, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marler MR, et al. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25(22):3893–3904. [DOI] [PubMed] [Google Scholar]
- 36. Witting W, et al. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 1990;27(6):563–572. [DOI] [PubMed] [Google Scholar]
- 37. Blume C, et al. ‘nparACT’ package for R: a free software tool for the non-parametric analysis of actigraphy data. MethodsX. 2016;3:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kushida CA, et al. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. [DOI] [PubMed] [Google Scholar]
- 39. Esteban O, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16(1):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tustison NJ, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Avants BB, et al. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Y, et al. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. [DOI] [PubMed] [Google Scholar]
- 43. Dale AM, et al. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- 44. Andersson JL, et al. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870–888. [DOI] [PubMed] [Google Scholar]
- 45. Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. [DOI] [PubMed] [Google Scholar]
- 46. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jenkinson M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. [DOI] [PubMed] [Google Scholar]
- 48. Smith SM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. [DOI] [PubMed] [Google Scholar]
- 49. Winkler AM, et al. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brehmer Y, et al. The importance of the ventromedial prefrontal cortex for associative memory in older adults: a latent structural equation analysis. Neuroimage. 2020;209:116475. [DOI] [PubMed] [Google Scholar]
- 51. Shumake J. Beset: Best Subset Predictive Modeling; 2020. https://github.com/jashu/beset. Accessed November 7, 2020.
- 52. Shumake J. Corxplor: Correlation Exploration; 2017. https://github.com/jashu/corxplor. Accessed November 7, 2020.
- 53. Mander BA, et al. White matter structure in older adults moderates the benefit of sleep spindles on motor memory consolidation. J Neurosci. 2017;37(48):11675–11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rocklage M, et al. White matter differences predict cognitive vulnerability to sleep deprivation. Sleep. 2009;32(8):1100–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Piantoni G, et al. Individual differences in white matter diffusion affect sleep oscillations. J Neurosci. 2013;33(1):227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Monk TH. Aging human circadian rhythms: conventional wisdom may not always be right. J Biol Rhythms. 2005;20(4):366–374. [DOI] [PubMed] [Google Scholar]
- 57. Luik AI, et al. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int. 2013;30(10):1223–1230. [DOI] [PubMed] [Google Scholar]
- 58. Duffy JF, et al. Aging and Circadian rhythms. Sleep Med Clin. 2015;10(4):423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Whitehead DL, et al. Circadian rest-activity rhythm is altered in Parkinson’s disease patients with hallucinations. Mov Disord. 2008;23(8):1137–1145. [DOI] [PubMed] [Google Scholar]
- 60. Niwa F, et al. Circadian rhythm of rest activity and autonomic nervous system activity at different stages in Parkinson’s disease. Auton Neurosci. 2011;165(2):195–200. [DOI] [PubMed] [Google Scholar]
- 61. Luik AI, et al. 24-hour activity rhythm and sleep disturbances in depression and anxiety: a population-based study of middle-aged and older persons. Depress Anxiety. 2015;32(9):684–692. [DOI] [PubMed] [Google Scholar]
- 62. Martin JL, et al. Older schizophrenia patients have more disrupted sleep and circadian rhythms than age-matched comparison subjects. J Psychiatr Res. 2005;39(3):251–259. [DOI] [PubMed] [Google Scholar]
- 63. Van Someren EJW, et al. Medial temporal lobe atrophy relates more strongly to sleep-wake rhythm fragmentation than to age or any other known risk. Neurobiol Learn Mem. 2019;160:132–138. [DOI] [PubMed] [Google Scholar]
- 64. Luik AI, et al. Associations of the 24-h activity rhythm and sleep with cognition: a population-based study of middle-aged and elderly persons. Sleep Med. 2015;16(7):850–855. [DOI] [PubMed] [Google Scholar]
- 65. Maillard P, et al. Cooccurrence of vascular risk factors and late-life white-matter integrity changes. Neurobiol Aging. 2015;36(4):1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. de Leeuw FE, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125(Pt 4):765–772. [DOI] [PubMed] [Google Scholar]
- 67. Duanping L, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. Stroke. 1996;27(12):2262–2270. [DOI] [PubMed] [Google Scholar]
- 68. Gons Rob AR, et al. Hypertension and cerebral diffusion tensor imaging in small vessel disease. Stroke. 2010;41(12):2801–2806. [DOI] [PubMed] [Google Scholar]
- 69. Budde MD, et al. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57(4):688–695. [DOI] [PubMed] [Google Scholar]
- 70. Song SK, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. [DOI] [PubMed] [Google Scholar]
- 71. Song SK, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–140. [DOI] [PubMed] [Google Scholar]
- 72. Mädler B, et al. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging. 2008;26(7):874–888. [DOI] [PubMed] [Google Scholar]
- 73. Madden DJ, et al. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822(3):386–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kocevska D, et al. The prospective association of objectively measured sleep and cerebral white matter microstructure in middle-aged and older persons. Sleep. 2019;42(10). doi: 10.1093/sleep/zsz140 [DOI] [PubMed] [Google Scholar]
- 75. Kocevska D, et al. Sleep complaints and cerebral white matter: a prospective bidirectional study. J Psychiatr Res. 2019;112:77–82. [DOI] [PubMed] [Google Scholar]
- 76. Bach M, et al. Methodological considerations on tract-based spatial statistics (TBSS). Neuroimage. 2014;100:358–369. [DOI] [PubMed] [Google Scholar]
- 77. Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol Bull. 2011;137(5):753–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.