Abstract
Study Objectives
Neighborhood disadvantage is associated with poor sleep, which may contribute to and exacerbate racial and socioeconomic health disparities. Most prior work has been cross-sectional and thus it has not been possible to estimate causal effects.
Methods
We leveraged a natural experiment opportunity in two low-income, predominantly African American Pittsburgh, PA neighborhoods, following a randomly selected cohort of households (n = 676) between 2013 and 2016. One of the neighborhoods received substantial public and private investments (housing, commercial) over the study period, while the other socio-demographically similar neighborhood received far fewer investments. Primary analyses used a difference-in-difference analysis based on neighborhood, to examine changes in actigraphy-assessed sleep duration, efficiency, and wakefulness after sleep onset (WASO), and self-reported sleep quality. Secondary analyses examined whether residents’ proximity to investments, regardless of neighborhood, was associated with changes in sleep outcomes.
Results
Resident sleep worsened over time in both neighborhoods with no significant differences among residents between the two neighborhoods. Secondary analyses, including covariate adjustment and propensity score weighting to improve comparability, indicated that regardless of neighborhood, those who lived in closer proximity to investments (<0.1 mile) were significantly less likely to experience decreases in sleep duration, efficiency, and quality, or increases in WASO, compared to those who lived farther away.
Conclusions
While we did not observe sleep differences among residents between neighborhoods, living closer to a neighborhood investment was associated with better sleep outcomes. Findings have relevance for public health and policy efforts focused on investing in historically disinvested neighborhoods.
Keywords: sleep, natural experiment, social determinants, disparities, socioeconomic status, neighborhoods
Statement of Significance.
Neighborhood disadvantage is associated with poor sleep health, which may contribute to and intensify racial and socioeconomic health disparities. The current study leverages an ongoing natural experiment in two urban, low-income, African American neighborhoods, in which one neighborhood has experienced dramatic reinvestment, whereas the other has experienced significantly less investment. Although we did not find significant differences in sleep over a 3-year period between the two neighborhoods, residents living in closer proximity to neighborhood investments (<0.1 mile) showed better sleep outcomes over time, compared to those who lived farther away. These findings suggest that investment in structural sources of disparities (i.e. the social and built neighborhood environment) may have positive impacts on sleep among African Americans living in historically disinvested neighborhoods.
Introduction
The historical legacy that has influenced the sociodemographic compositions and built environments of United States cities and their neighborhoods is complex. Housing and city planning policies (e.g. redlining, urban renewal, mortgage lending) have created and reinforced unequal distribution of resources. The resulting racial and income residential segregation has created a foundation of differences in conditions from housing quality, to transportation access, to retail, to crime [1]. In fact, a large body of evidence has linked current social and built environment conditions of neighborhoods to health outcomes of residents [2]. Whether birth outcome, overweight and obesity, or cardiovascular disease, the health of African Americans (AAs) in the United States is substantially worse than that of white Americans [3]. From top-down policies to individual-level experiences of discrimination and unfair treatment, racial inequities in economic and social opportunity are purported to be a key contributor to health disparities.
Sleep is a critical, understudied pathway that may underlie links between socioeconomic and racial/ethnic inequities and health. AAs and individuals from low-socioeconomic status (SES) backgrounds have significantly higher rates of sleep disorders, such as obstructive sleep apnea (OSA), as well as insufficient sleep duration, poor sleep quality, and lower sleep efficiency compared to whites and higher SES individuals [4–7]. Sleep is a critical contributor to health and well-being, and is associated with adverse health outcomes, including depression, cardiovascular disease, and mortality [8–10]. Cross-sectional studies suggest that neighborhood socioeconomic disadvantage contributes to the elevated risk of sleep problems in AAs and individuals of low SES, even after accounting for individual SES [11–17]. For instance, perceived neighborhood disadvantage (based on perceptions of noise, cleanliness, and crime) is associated with poor self-reported sleep quality, independent of individual-level sociodemographics [13, 15, 16, 18]. With few exceptions [19–21], prior studies have primarily focused on self-reported sleep, which provides a limited assessment of overall sleep patterns and is subject to reporting bias. In contrast, actigraphic assessment of sleep provides valid and reliable measures of habitual sleep continuity (i.e. sleep efficiency and wakefulness after sleep onset) and duration, which are key dimensions of sleep that have previously been associated with physical health outcomes [22, 23].
Further, most neighborhood and sleep research to date has been based on cross-sectional data. In one of the few prior longitudinal studies of changes in housing conditions and sleep, Simonelli and colleagues [24] found improvements in self-reported sleep quality among Argentinians living in slums following improvements in housing conditions (i.e. a move to prefabricated modular house) 18 months later.
While improvements in housing quality and conditions as well as overall aesthetic upgrades in the neighborhood may positively impact residents, improvements may also have adverse consequences on residents [25, 26]. Housing upgrades or landscape remodeling, for example, may result in safer streets, better mobility, and better day-to-day conditions in the long-term for some residents, but can also result in rent increases, the relocation and displacement of residents, or short-term disruptions. To date, however, there is limited longitudinal evidence linking changing neighborhood conditions on health outcomes, and most of the existing longitudinal work has focused on obesity-related outcomes [27]. For example, Mehdipanah et al. examined the relationship between urban renewal and health outcomes in Barcelona, Spain with the Neighbourhoods Law (NL) program which sought to improve physical infrastructure, social integration and economic gains, and found that the intervention improved self-rated health [25] among residents. Another recent study [28] examined the rapid creation and occupancy of East Village (formerly the London 2012 Olympic and Paralympic Athletes’ Village, London (UK)), a purpose-built mixed-use residential development specifically designed to encourage healthy active living. The team found that changes in a range of residential built environment features were associated with changes in measures of physical activity in adults. Longitudinal work by our own team has shown mixed effects of neighborhood change on resident health outcomes. We found improved dietary behavior and neighborhood satisfaction following receipt of a supermarket in a neighborhood that previously lacked access to one [29], and greater park use resulting from improved walkability and neighborhood aesthetics [30, 31]. However, we also found that neighborhood investments did not change physical activity, psychological distress, or perceptions of the neighborhood [31].
Our current research took advantage of the same unique natural experiment to evaluate whether improving neighborhood conditions impacts sleep in a randomly selected cohort of residents from two low-income neighborhoods in Pittsburgh, PA. We conducted objective assessments of sleep, using wrist actigraphy, before and after one neighborhood received substantial investments resulting in a variety of improvements to the neighborhood (intervention neighborhood), whereas the other neighborhood (comparison) received investments of much smaller scope and magnitude during the same time period. We hypothesized that residents of the neighborhood that underwent extensive improvements would experience improvements in key indicators of sleep health, including objectively measured sleep duration, sleep efficiency, and wakefulness after sleep onset (WASO) and self-reported sleep quality, relative to the comparison neighborhood.
Methods
Study design
Data for this study came from the PHRESH Zzz Study (Pittsburgh Hill/Homewood Research on Neighborhoods, Sleep, and Health), part of an ongoing longitudinal study designed to examine the effect of changes in the built and social environment on health behaviors and risk factors in two low-income predominantly AA neighborhoods, the Hill District and Homewood. Both neighborhoods are located in Pittsburgh, PA, separated by approximately 4 miles and several other distinct neighborhoods. In 2011, a random sample of households was enrolled from each neighborhood to examine the aforementioned impact of opening a full-service supermarket in the ‘intervention’ neighborhood (the Hill District) on diet and food purchasing behaviors, relative to the comparison neighborhood, Homewood, where no supermarket opening took place. The two neighborhoods were sociodemographically matched and the primary food shopper in the household was enrolled into the study. Following 2011, households were re-interviewed at four follow-up waves (2013, 2014, 2016, 2018). The present analyses are based on data collected in 2013 and 2016, when objectively measured sleep data were collected.
During this time period, the Hill District neighborhood, with an area of approximately 1.5 square miles, received approximately $194 million [31] in investments that were funded at least partially by public funding, including the full-service grocery store, multiple public housing developments, a community center, and an energy innovation center dedicated to workforce development and incubation of businesses. These investments also changed the streetscape surrounding the developments, providing improved aesthetics (e.g. trees, grass) and walkability (e.g. sidewalks, street crossings). During this same period, Homewood (similar-sized comparison neighborhood) also received publicly-funded investments, but they were almost exclusively in housing developments and totaled $48 million. In total, Homewood received about a quarter of the investments made in the intervention neighborhood. Data on investments were collected from interviews with and requests from the following four public agencies: Housing Authority of the City of Pittsburgh (HACP), the Urban Redevelopment Authority (URA), the Pennsylvania Housing Finance Agency (PHFA), and the City of Pittsburgh. They provided details (dates, funding, funder, location) on investments within both neighborhoods between 1999 and 2017 [32]. We included all investments that were at least partially funded by public dollars. We then geocoded each investment using ArcView GIS and calculated the distance from each respondent’s location to the closest point of each development’s footprint.
The primary food shopper in each household, based on original enrollment in 2011, completed an in-person interviewer-administered survey, measurements of height and weight, and wore a wrist actigraph for 7 days after completing the survey. Participants were also asked to complete daily sleep logs and diaries for 7 days, concurrent with actigraphy. Survey participants received an incentive of $25 for completing the survey and up to an additional $50 for completing 7 days of actigraphy and a daily sleep diary. Further details of the study design, including recruitment and data collection procedures, are described in depth elsewhere [18, 20, 33, 34]. Study protocols were approved by our institution’s Institutional Review Board. All participants provided signed informed consent for all aspects of the study.
Sleep outcomes
The Actigraph GT3x+, a wrist-worn device that has been validated to measure sleep/wake rhythms relative to both polysomnography and Actiwatch, was used to obtain objective assessments of sleep duration and continuity (efficiency and WASO) [35, 36]. Participants with fewer than four nights of actigraphy data were excluded from analyses, consistent with recommendations for the minimum nights required to establish reliable sleep-wake patterns via actigraphy [37]. Sleep outcomes were averaged across all available nights to provide an assessment of habitual sleep duration, efficiency, and WASO. The average number of nights of actigraphy for the analytic sample was 5.9 (SD = 0.9, range = 4–7) in 2013 and 6.8 (SD = 0.5, range = 4–7) in 2016. Bedtimes and waketimes were taken from sleep diaries to define the sleep interval, which was further verified by visual inspection of the actigraphy tracings. Actigraphic sleep data were scored using GGIR.
Sleep duration.
Sleep duration is the total amount of time spent sleeping, as assessed by actigraphy, during the participant’s time in bed.
Sleep efficiency.
Sleep efficiency is calculated as the total duration of actigraphy-measured sleep divided by the total time in bed as reported in sleep diaries and visual inspection of actigraphy records. Higher values (expressed in percent) indicate better sleep efficiency.
WASO is the total number of minutes scored as wake after sleep onset, with higher values indicating longer WASO.
Sleep quality.
Participants completed sleep diaries each morning upon awakening, to provide assessments of sleep quality and to report their bedtimes and waketimes, which were used to calculate the sleep interval for actigraphy processing. Sleep quality scores were based on responses to a question asking participants to rate “how well you slept last night” on a 5-point Likert scale from “very poorly” to “very well,” averaged across available nights. The average number of nights of sleep diary data was 6.7 (SD = 0.7, range = 4–7) in 2013 and 6.8 (SD = 0.4, range = 4–7) in 2016.
Covariates (assessed at baseline).
Age, sex, household income, marital status, educational attainment, children in the household, years in the neighborhood, psychological distress, and BMI, were included as covariates in all models. Height was measured to the nearest eighth inch using a carpenter’s square and an 8-foot folding wooden ruler marked in inches. The weight of each participant was measured to the nearest tenth of a pound using the SECA Robusta 813 digital scale. Body mass index (BMI) was calculated as the ratio of objectively-measured weight (kg) divided by squared height (m2), and was also included as a covariate given previously reported associations between increased BMI and sleep disturbances [38].
Analytic sample
Of the 1,051 participants who were part of the study cohort in 2013, we excluded residents who no longer lived in one of the two study neighborhoods (n = 48), and participants with less than four nights of actigraphy-measured sleep (N = 262) or missing their sleep diary (n = 161). Therefore, the baseline (2013) sample size with valid actigraphy outcomes was 741, and with diary-assessed sleep quality was 842. Of those, 475 (64.1%) people had sufficient actigraphy data and 544 (64.6%) had diary data at follow-up in 2016. Those with a baseline value with missing or insufficient sleep data at follow-up were more likely to be male, to have children living in the household, to have lived fewer years in the neighborhood, to have lower BMI, and to be living in Homewood rather than Hill District at baseline. The analytic sample did not differ significantly from the excluded sample on any other study variables.
Statistical analysis
We compared baseline (2013) characteristics of study participants in the two neighborhoods, and computed t-tests or chi-squared tests to identify statistically significant differences. Next, we computed (1) the average difference between baseline and follow-up values in the intervention group, (2) the average difference between baseline and follow-up values in the comparison group, and (3) a difference-in-difference estimator indicating changes in the intervention group between 2013 and 2016 compared with those in the comparison group for each of the outcomes. Each value was tested to determine if it was significantly different from zero. The analyses employed an intention-to-treat approach based on neighborhood/location of residence in 2013 [39, 40]. Analyses were adjusted for all of the individual-level covariates listed above and weighted to account for sample attrition between baseline and follow-up to ensure that results generalized to the baseline sample. Attrition weights were derived as the inverse probability of response at follow-up, estimated using a logistic regression model with socio-demographics and additional baseline characteristics as predictors. We performed sensitivity analyses (not shown) restricting the analytic sample to only African Americans (or, 96% of the analytic sample) and found similar results as with the full sample. Thus, findings are reported only for the full sample.
In a natural experiment, researchers cannot control whether the “intervention” (in this case, neighborhood improvements in housing, greenspace, and commercial development) is implemented as planned, in the intervention neighborhood, and completely absent in the comparison. While at study onset, investments were planned for only the intervention neighborhood (Hill District), some investments also occurred in the comparison neighborhood (Homewood), as noted above. To guard against false positives, or potential error due to individuals in the comparison neighborhood exposed to investments, we supplemented our neighborhood-level intervention with an individual-level measure capturing exposure to intervention. In this approach, we designated study participants, regardless of neighborhood, as exposed (or unexposed) to investments based on their proximity to a new investment or development that occurred.
To create an individual-level indicator of exposure to neighborhood investments we coded a household as ‘closer to an investment’ (=1), if the household was within one-tenth of a mile of any neighborhood investment project (funded, at least in-part, publicly, as described above) that occurred after baseline data collection and before follow-up data collection and coded as ‘farther from investment’ (=0) otherwise. We chose a proximal distance because both neighborhoods are relatively small geographic areas (each approximately 1.5 miles squared) and because our prior research showed that this distance best captured associations between another neighborhood characteristic (number of recent crimes) and sleep [41]. We compared participants in the two exposure groups and observed statistically significant differences at baseline in several characteristics. Participants who lived closer to investments were, on average, older (60 years, compared to 54 years for those farther from investment), less likely to be married or living with a partner (9% of those who lived close to investments were married, versus 24% of those who lived further from investments), more likely to have less than high school education (20% versus 10% who lived farther away from investments); fewer had some college (29%) or had completed college (11%) compared with those who lived further from the investments (35% had some college and 16% had completed college).
To account for these baseline differences between the two exposure groups defined by differential proximity to investments, we employed a doubly robust approach [42], where we adjusted for both covariates as well as propensity scores. The R package twang (Toolkit for Weighting and Analysis of Nonequivalent Groups) was used to create propensity score weights [43]. Adequate balance (absolute standardized mean difference <0.25) was achieved for all covariates. The assessment of covariate balance included attrition weighting. The analytic weights used in modeling were a product of attrition and propensity score weights. All analyses were performed in R, version 3.4.2, and SAS, version 9.4. Difference-in-difference modeling accounted for correlations among repeated measurements of each participant.
Results
Table 1 shows characteristics of the analysis sample overall and stratified by neighborhood. By design, the sociodemographic characteristics were similar across neighborhoods. In both neighborhoods, 96% of the sample were African American, and just over 81% had a household income below $20,000/year. About a quarter or less were married or living with a partner, while about half had a high school diploma or less. Compared to residents in Homewood, those in the Hill District included fewer males, lived in the neighborhood longer, and were more likely to reside close to an investment.
Table 1.
Baseline characteristics of analytic samples by neighborhood
| Characteristic | Overall (n = 570); % or Mean (SD) | Hill District (intervention neighborhood, n = 403); % or Mean (SD) | Homewood (comparison neighborhood, n = 167); % or Mean (SD) | p-valuea |
|---|---|---|---|---|
| Age (years) | 54.7 (14.8) | 55.3 (15.0) | 53.7 (14.4) | .25 |
| Male | 22.3% | 19.9% | 27.2% | .08 |
| Annual Household Income ($) | 21,700 (19,700) | 21,200 (19,200) | 22,700 (20,800) | .41 |
| Married/living with partner | 22.0% | 19.7% | 26.8% | .12 |
| Education | .07 | |||
| < High school | 11.3% | 12.5% | 8.7% | |
| High school | 40.0% | 42.3% | 35.3% | |
| Some college | 33.6% | 32.8% | 35.4% | |
| College | 15.2% | 12.4% | 20.7% | |
| Any children in household | 28.1% | 26.2% | 31.8% | .25 |
| Years in neighborhood | 31.4 (22.3) | 35.2 (22.8) | 24.0 (19.2) | <.0001 |
| Psychological Distress | 4.3 (4.6) | 4.1 (4.5) | 4.6 (4.9) | .29 |
| Body Mass Index | 31.1 (7.5) | 30.9 (7.2) | 31.5 (8.2) | .41 |
| Proximity to investment < 0.1 mile | 17.1% | 22.3% | 6.6% | <.0001 |
Includes all participants in the analytic sample for either the sleep actigraphy (N = 475) or sleep quality (N = 544) analyses. Means and percentages weighted to adjust for sample attrition between baseline (2013) and follow-up (2016).
a p from two-sided significance testing using t-tests for continuous variables and chi-squared tests for binary variables; SD = standard deviation.
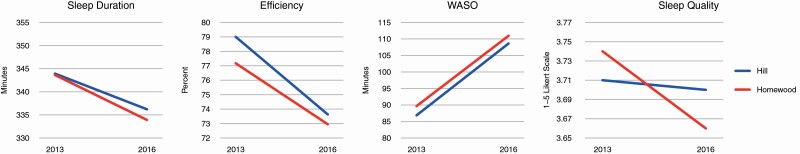
Table 2 shows results of the difference-in-difference estimates of changes in sleep duration, sleep efficiency, WASO and perceived sleep quality between Hill District and Homewood residents from 2013 to 2016. Each sleep outcome was modeled individually, adjusted for baseline covariates including: age, sex, income, marital status, education, any children in the household, years living in the neighborhood, body mass index, and psychological distress, and included attrition weighting. We observed no statistically significant difference-in-difference estimates for any of the sleep outcomes. That is, there were no changes over time in one neighborhood that proved significantly different from changes over time in the other neighborhood. However, there were some significant changes within each of the neighborhoods. Sleep efficiency decreased by 5.4 percentage points in the Hill District and 4.2 percentage points in Homewood, each demonstrating a statistically significant decrease at p < .001. WASO increased by 22 minutes in the Hill District and 21 minutes in Homewood, also showing significant increases within each neighborhood at p < .001.
Table 2.
Changes in sleep outcomes for study participants between 2013 and 2016, by neighborhood
| Hill district (intervention neighborhood) (N = 336)a | Homewood (comparison neighborhood) (N = 139)a | Difference-in-difference estimate | |||
|---|---|---|---|---|---|
| Baseline mean (95% CI) | Post - Pre (95% CI) | Baseline mean (95% CI) | Post - Pre (95% CI) | Hill district change - homewood change (95% CI) | |
| Sleep duration, minutes | 343.93 (335.73, 352.13) | −7.72 (−16.04, 0.60) | 343.63 (331.16, 356.09) | −9.72 (−21.53, 2.09) | 2.00 (−12.44, 16.45) |
| Sleep efficiency, percent | 79.00 (77.83, 80.18) | −5.37 (−6.67, −4.07)*** | 77.17 (75.39, 78.94) | −4.22 (−6.06, −2.38)*** | −1.15 (−3.40, 1.10) |
| WASO, minutes | 86.87 (80.63, 93.12) | 21.75 (14.06, 29.44)*** | 89.66 (80.36, 98.96) | 21.29 (10.37, 32.21)*** | 0.46 (−12.90, 13.81) |
| Perceived Sleep quality | 3.71 (3.63, 3.79) | −0.01 (−0.09, 0.08) | 3.74 (3.63, 3.86) | −0.08 (−0.20, 0.04) | 0.07 (−0.07, 0.22) |
HD = Hill District, HW = Homewood. All models are covariate adjusted for age, sex, income, marital status, education, any children in the household, years in the neighborhood, body mass index, and psychological distress, and included attrition weights.
aSample size reported in the table are for actigraphy measures. For perceived sleep quality, collected via sleep diaries, sample sizes were 387 for the Hill District and 157 for Homewood.
***p < .001 from two-sided significance testing using a t-test; statistically significant results are shown in bold font.
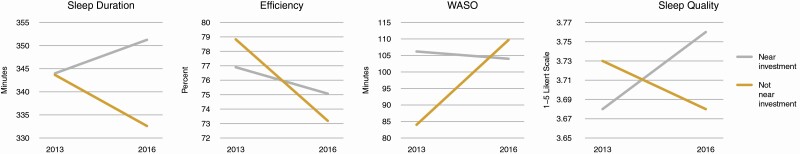
Table 3 shows the difference-in-difference analysis comparing participants living closer to investments to those living further from investments. The difference-in-difference analysis showed statistically significant differences in participants’ sleep duration, sleep efficiency, WASO and perceived sleep quality over time. We observed a 7-minute increase in sleep duration among those who lived closer to investments (not statistically significant), and a decrease of about 11 minutes among those who lived further from investments (p < .05). The difference-in-difference was 18.3 minutes (p < .01). Both groups also experienced a decrease in sleep efficiency; for those closer to investments, the decrease was approximately 2 percentage points (not statistically significant) and for those further away, the decrease was approximately 6 percentage points (p < .001). This resulted in a difference-in-difference of 3.8 percentage points (p < .001). Those who lived closer to an investment had an average decrease of about 2 minutes of WASO (not statistically significant) while those who lived further had an increase of almost 26 minutes (p < .001). This resulted in a large difference-in-difference of 28 minutes for WASO (p < .001). Finally, perceived quality of sleep slightly increased for those closer to investments and slightly decreased for those further from investments (difference-in-difference p < .05).
Table 3.
Changes in sleep outcomes for study participants between 2013 and 2016, by proximity to neighborhood investment
| Intervention (proximity to investment < 0.1 mile) (N = 82)a | Comparison (proximity to investment 0.1 mile or more) (N = 391)a | Difference-in-difference | |||
|---|---|---|---|---|---|
| Baseline mean (95% CI) | Post - Pre (95% CI) | Baseline mean (95% CI) | Post - Pre (95% CI) | Intervention change - Comparison change (95% CI) | |
| Sleep duration, minutes | 343.98 (330.45, 357.51) | 7.26 (−2.71, 17.23) | 343.65 (335.53, 351.76) | −11.05 (−19.93, −2.17)* | 18.31 (4.96, 31.66)** |
| Sleep efficiency | 76.90 (75.04, 78.75) | −1.83 (−3.40, −0.27)* | 78.83 (77.65, 80.02) | −5.65 (−7.04, −4.25)*** | 3.82 (1.72, 5.91)*** |
| Wakefulness after sleep onset, minutes | 106.20 (95.80, 116.61) | −2.20 (−12.51, 8.11) | 84.01 (76.74, 91.28) | 25.69 (16.50, 34.87)*** | −27.88 (−41.70, −14.07)*** |
| Perceived sleep quality | 3.68 (3.56, 3.80) | 0.08 (−0.01, 0.18) | 3.73 (3.65, 3.81) | −0.05 (−0.14, 0.04) | 0.13 (0.002, 0.27)* |
All models are covariate adjusted for age, sex, income, marital status, education, any children in the household, years in the neighborhood, body mass index, and psychological distress, and are weighted using product weights (propensity score weights × attrition weights).
aSample size reported in the table are for actigraphy measures. For perceived sleep quality, collected via sleep diaries, sample sizes were 101 for the intervention group (proximity to investment < 0.1 mile) and 440 for the comparison group (proximity to investment 0.1 mile or more).
*p < .05, **p < .01, ***p < .001 from two-sided significance testing using a t-test; statistically significant results are shown in bold font.
Figure 1 illustrates the differences over time in—sleep duration, sleep efficiency, WASO, and perceived quality of sleep comparing the two neighborhoods. Figure 2 shows changes over the 3-year period by resident proximity to investment.
Figure 1.
Changes in sleep duration, efficiency, WASO, and sleep quality between the Hill District neighborhood (intervention) and Homewood neighborhood (comparison).
Figure 2.
Changes in sleep duration, efficiency, WASO, and sleep quality near and farther from investments between 2013 and 2016.
Discussion
This natural experiment is the first study to longitudinally examine the potential impacts of neighborhood development on sleep outcomes. We compared objectively and subjectively assessed sleep over time in residents from two sociodemographically similar neighborhoods in the context of a natural experiment. We also examined changes in sleep among residents who were more likely to have been exposed to these investments to those less likely to have been exposed, by virtue of their differential geographic proximity to them.
Overall, our findings showed no statistically significant difference-in-differences in sleep outcomes in the intervention versus comparison neighborhoods. However, when we considered the individual-level indicator of exposure to investments by relative proximity, we found significant differences in sleep. Specifically, there was a large and statistically significant (28-minute) difference in WASO for those residents who lived closer to investments versus those who lived farther away. Those residents who lived closer to investments, on average, decreased WASO by 2 minutes while residents who lived farther away from investments had an increase of 26 minutes of WASO. For total sleep duration, residents who lived closer to investments increased their sleep time by 7 minutes per night, on average, while those who lived farther from investments decreased their sleep time by 11 minutes for a difference-in-difference of 18 minutes. We observed declines in sleep outcomes over time in both neighborhoods, which may be attributable to aging, particularly in a sample that has a high prevalence of comorbidities, including cardiometabolic risk factors [44].
In this analysis, we used the one-tenth of a mile distance based on both urban planning literature as well as prior work of our team that indicated stronger associations between neighborhood crime and sleep when exposure to crime was measured as crimes within 1/10th mile of participants’ residences than when measured as longer distances [41]. Although the one-tenth of a mile is a reasonable and practical distance to expect that residents will be aware of changes to the landscape of their surrounds, we note that the distance is meant as an indicator of likely exposure, rather than a specific geographic metric. Results indicate that residents who are living in closer proximity to places that are undergoing improvement receive greater benefits, but not the mechanism or the degree of proximity required.
We controlled for a number of factors, including psychological distress, that could explain why residents who lived closer to investments experienced improvement, or at least lesser amounts of decline in their sleep duration and sleep quality. However, other unmeasured factors may also play a role. For example, it is possible that living closer to investments fostered positive emotions, including hope and sense of community in residents, which could benefit sleep via affective pathways and by reducing stress. Although we did not assess these pathways in the 2013 and 2016 waves of data collection, our future longitudinal work in this cohort is focused, in part, on how these constructs (hope and sense of community) as well as changes in individual- and neighborhood-level socioeconomic status may explain observed health benefits of neighborhood revitalization efforts. Alternatively, it is possible that the residents who lived closer to investments were qualitatively different from those residents who lived further away. We implemented a doubly-robust modeling strategy of using both covariate adjustment and propensity score weighting to account for such differences. This approach is not as rigorous as the quasi-experimental analysis comparing our intervention and comparison neighborhood. Yet, it is more rigorous than the more typical method of covariate adjustment alone.
There are limitations to this work. Although our research was designed as a difference-in-difference analysis to compare an ‘intervention’ and ‘control’ neighborhood, as is the case with many natural experiments, the comparison neighborhood also experienced changes throughout the study period, though to a lesser extent than the intervention neighborhood. This may have limited the ability to detect statistically significant differences in sleep over time. Furthermore, these two neighborhoods are only 4 miles apart. We considered the geographic proximity of the neighborhoods in the study design, and the possibility of “spillover” effects. However, there are multiple neighborhoods between the two neighborhoods and distinct topographic features (e.g. hills) that makes “spillover” unlikely. Nevertheless, it is possible that residents of the comparison neighborhood may have benefited from the improvements in the intervention neighborhood. Recognizing these limitations, we employed the additional ‘proximity to investment’ analysis, which captured residents’ individual-level exposure to investments, regardless of their neighborhood of residence.
Another limitation is that we did not characterize investments by size or scope. Thus, different types and sizes of investments (housing versus greenspace versus commercial) might have differential impacts on residents’ sleep. Further, in our proximity to investment analysis, we found that the residents who lived closer to investments were generally older and less well educated than residents who lived further from investments. Propensity scores allowed for a more robust comparison and decreased the standard errors. However, a general limitation of propensity score matching is that unmeasured factors may have contributed to differences between the groups and ultimately influenced the results [45]. Finally, our study occurred in a low-income predominantly AA urban setting, which limits generalizability. Yet, this is a critically important population to study in terms of upstream determinants of sleep health disparities [46].
These findings, in the context of prior analyses which looked at changes in diet and food purchasing following the opening of a new full-service supermarket [29], and lack of changes in physical activity and psychological distress following investments including greenspace [31], indicate there may be some benefits (although dependent on outcome) to residents from residing closer to neighborhood investments.
Our longitudinal difference-in-difference analysis, which examined a randomly selected group of residents from two neighborhoods, is the first study to demonstrate that investing in neighborhoods may ultimately have a positive impact on the sleep of residents. Although the initial neighborhood difference-in-difference analysis did not show significant change, we found evidence of difference-in-difference in our proximity to investment analysis. Therefore, these mixed findings suggest that additional work is needed to more firmly establish causality and to identify potential mechanisms. For policymakers, practitioners, city planners, and health departments, these findings suggest that investment in structural sources of disparities (i.e. the social and built neighborhood environment) may have localized positive impacts on sleep.
Acknowledgments
The authors express sincere appreciation and gratitude to La’Vette Wagner, study field coordinator, the data collection staff, our project coordinator, Jennifer Sloan, and our research assistant, Alvin Nugroho. The authors thank our community partners, including Hill House Association, Operation Better Block, and Homewood Children’s Village and most importantly, our participants, who make this work possible. All authors contributed to and provided written feedback on the manuscript and approve of the manuscript in its final form.
Funding
Funding was provided by the National Heart Lung Blood Institute (Grant No. R01 HL122460 and HL131531) and the National Cancer Institute (Grant No. R01CA164137).
Conflict of interest statement. Dr. Buysse has served as a paid consultant to Bayer, BeHealth Solutions, Emmi Solutions, Weight Watchers International, and Pear Therapeutics. He has served as a paid consultant for professional educational programs developed by the American Academy of Physician Assistants, CME Institute and Emmi Solutions, and received payment for a professional education program sponsored by Eisai. Dr. Dr. Buysse is an author of the Pittsburgh Sleep Quality Index, Pittsburgh Sleep Quality Index Addendum for PTSD (PSQI-A), Brief Pittsburgh Sleep Quality Index (B-PSQI), Daytime Insomnia Symptoms Scale, Pittsburgh Sleep Diary, Insomnia Symptom Questionnaire, and RU_SATED (copyright held by University of Pittsburgh). These instruments have been licensed to commercial entities for fees. He is also co-author of the Consensus Sleep Diary (copyright held by Ryerson University), which is licensed to commercial entities for a fee. Dr. Hale receives an honorarium from the National Sleep Foundation for her role as the Editor in Chief of Sleep Health. There are no non-financial conflicts of interest to disclose relevant to the work under review.
Data Availability
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. However, data can be shared on reasonable request to the corresponding author.
References
- 1. Greene S, et al. Racial Residential Segregation and Neighborhood Disparities. Washington, DC: US Partnership on Mobility from Poverty; 2017. [Google Scholar]
- 2. Diez-Roux AV. Neighborhoods and Health. Oxford University Press; 2003. [Google Scholar]
- 3. Centers for Disease Control and Prevention. Health disparities experienced by Black or African Americans--United States. Morb Mortal Wkly Rep. 2005;54(1):1–3. [PubMed] [Google Scholar]
- 4. Adenekan B, et al. Sleep in America: role of racial/ethnic differences. Sleep Med Rev. 2013;17(4):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baldwin CM, et al. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010;6(2):176–183. [PMC free article] [PubMed] [Google Scholar]
- 6. Hall MH, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 7. Olafiranye O, et al. Obstructive sleep apnea and cardiovascular disease in blacks: a call to action from the Association of Black Cardiologists. Am Heart J. 2013;165(4):468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paunio T, et al. Poor sleep predicts symptoms of depression and disability retirement due to depression. J Affect Disord. 2015;172:381–389. [DOI] [PubMed] [Google Scholar]
- 9. Yin J, et al. Relationship of sleep duration with all‐cause mortality and cardiovascular events: a systematic review and dose‐response meta‐analysis of prospective cohort studies. J Am Heart Assoc. 2017;6(9):e005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Troxel WM, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33(12):1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riedel N, et al. ; Heinz Nixdorf Recall Study Group. Insomnia and urban neighbourhood contexts–are associations modified by individual social characteristics and change of residence? Results from a population-based study using residential histories. BMC Public Health. 2012;12:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steptoe A, et al. Positive affect, psychological well-being, and good sleep. J Psychosom Res. 2008;64(4):409–415. [DOI] [PubMed] [Google Scholar]
- 13. Hale L, et al. Perceived neighborhood quality, sleep quality, and health status: evidence from the Survey of the Health of Wisconsin. Soc Sci Med. 2013;79:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hill TD, et al. Neighborhood disorder, sleep quality, and psychological distress: testing a model of structural amplification. Health Place. 2009;15(4):1006–1013. [DOI] [PubMed] [Google Scholar]
- 15. Hale L, et al. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hale L, et al. Does sleep quality mediate the association between neighborhood disorder and self-rated physical health? Prev Med. 2010;51(3-4):275–278. [DOI] [PubMed] [Google Scholar]
- 17. Spilsbury JC, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149(3):342–347. [DOI] [PubMed] [Google Scholar]
- 18. DeSantis A, et al. Is the association between neighborhood characteristics and sleep quality mediated by psychological distress? An analysis of perceived and objective measures of 2 Pittsburgh neighborhoods. Sleep Health. 2016;2(4):277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simonelli G, et al. Neighborhood factors as predictors of poor sleep in the Sueno ancillary study of the Hispanic community health study/study of Latinos. Sleep. 2016;40(1). doi: 10.1093/sleep/zsw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Troxel WM, et al. Neighborhood disadvantage is associated with actigraphy-assessed sleep continuity and short sleep duration. Sleep. 2019;42(3). doi: 10.1093/sleep/zsy250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson DA, et al. The neighborhood social environment and objective measures of sleep in the multi-ethnic study of atherosclerosis. Sleep. 2017;40(1). doi: 10.1093/sleep/zsw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang T, et al. Cross-sectional and prospective associations of actigraphy-assessed sleep regularity with metabolic abnormalities: the multi-ethnic study of atherosclerosis. Diabetes Care. 2019;42(8):1422–1429. 10.2337/dc19-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramos AR, et al. Sleep patterns and hypertension using actigraphy in the Hispanic community health study/study of Latinos. Chest. 2018;153(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simonelli G, et al. Sleep and quality of life in urban poverty: the effect of a slum housing upgrading program. Sleep. 2013;36(11):1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehdipanah R, et al. Exploring complex causal pathways between urban renewal, health and health inequality using a theory-driven realist approach. Soc Sci Med. 2015;124:266–274. [DOI] [PubMed] [Google Scholar]
- 26. Gelormino E, et al. From built environment to health inequalities: an explanatory framework based on evidence. Prev Med Rep. 2015;2:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chandrabose M, et al. Built environment and cardio-metabolic health: systematic review and meta-analysis of longitudinal studies. Obes Rev. 2019;20(1):41–54. [DOI] [PubMed] [Google Scholar]
- 28. Clary C, et al. Longitudinal impact of changes in the residential built environment on physical activity: findings from the ENABLE London cohort study. Int J Behav Nutr Phys Act. 2020;17(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dubowitz T, et al. Diet and perceptions change with supermarket introduction in a food desert, but not because of supermarket use. Health Aff (Millwood). 2015;34(11):1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richardson AS, et al. Improved street walkability, incivilities, and esthetics are associated with greater park use in two low-income neighborhoods. J Urban Health. 2020;97(2):204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dubowitz T, et al. Results from a natural experiment: initial neighbourhood investments do not change objectively-assessed physical activity, psychological distress or perceptions of the neighbourhood. Int J Behav Nutr Phys Act. 2019;16(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baird M, et al. Does large-scale neighborhood reinvestment work? Effects of public-private real estate investment on local sales prices, rental prices, and crime rates. Hous Policy Debate. 2020;30(2):164–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dubowitz T, et al. A natural experiment opportunity in two low-income urban food desert communities: research design, community engagement methods, and baseline results. Health Educ Behav. 2015;42(1 Suppl):87S–96S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dubowitz T, et al. Healthy food access for urban food desert residents: examination of the food environment, food purchasing practices, diet and BMI. Public Health Nutr. 2015;18(12):2220–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cellini N, et al. Free-living cross-comparison of two wearable monitors for sleep and physical activity in healthy young adults. Physiol Behav. 2016;157:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quante M, et al. Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. Nat Sci Sleep. 2018;10:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ancoli-Israel S, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 38. Vgontzas AN, et al. Obesity and sleep: a bidirectional association? Sleep. 2010;33(5):573–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol. 1992;21(5):837–841. [DOI] [PubMed] [Google Scholar]
- 40. Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richardson AS, et al. Violent crime, police presence and poor sleep in two low-income urban predominantly Black American neighbourhoods. J Epidemiol Community Health. 2020;0:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Funk MJ, et al. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173(7):761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ridgeway G, et al. Toolkit for Weighting and Analysis of Nonequivalent Groups: A Tutorial for the R TWANG Package. Santa Monica, CA: RAND Corporation; 2014. TL-136/1-NIDA. [Google Scholar]
- 44. Ohayon MM, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. [DOI] [PubMed] [Google Scholar]
- 45. Haukoos JS, et al. The Propensity Score. JAMA. 2015;314(15):1637–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jackson CL, et al. A workshop report on the causes and consequences of sleep health disparities. Sleep. 2020;43(8). doi: 10.1093/sleep/zsaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. However, data can be shared on reasonable request to the corresponding author.