Abstract
Study Objectives
Depression is among the most prevalent perinatal complications, yet modifiable risk factors remain elusive. Over half of perinatal women endorse clinical insomnia symptoms, which are etiologically implicated in depression in nonperinatal samples. Yet, prospective data on perinatal insomnia and depression are mixed. We sought to clarify temporal associations of insomnia and depression during peripartum, and to investigate cognitive arousal as a potential mechanism facilitating this relationship.
Methods
Seventy pregnant women completed sociodemographic information and baseline sleep and mood symptoms between gestational weeks 25 and 30. Beginning at gestational week 30, participants completed 17 weekly online surveys assessing insomnia, depression, and three cognitive arousal indices (nocturnal cognitive arousal, perseverative thinking, and perinatal-focused rumination). Mixed effects models were conducted to test hypotheses.
Results
Women were at risk for depression when experiencing insomnia (odds ratio [OR] = 2.36, 95% confidence interval [CI] = 1.28 to 4.35), nocturnal cognitive arousal (OR = 3.05, 95% CI = 1.60 to 5.79), perinatal-focused rumination (OR = 2.05, 95% CI = 1.11 to 3.79), and perseverative thinking (OR = 7.48, 95% CI = 3.90 to 14.32). Prospective analyses revealed bidirectional effects between insomnia and cognitive arousal, and both predicted future depression. Nocturnal cognitive arousal mediated 23–43% of the effect of insomnia on depression. Insomnia mediated 12%–18% of the effect of nocturnal cognitive arousal on depression. A similar pattern was observed with perinatal-focused rumination. Depression did not predict insomnia.
Conclusion
Nocturnal cognitive arousal, including ruminating on perinatal concerns while trying to fall asleep, fuels insomnia. In turn, lying awake at night provides an opportunity for nocturnal cognitive arousal. This cycle feeds perinatal depression. Daytime cognitive arousal may indirectly disrupt sleep as perseverating during the day persists into the night.
Keywords: worry, pregnancy, postpartum, sleep, prospective, cognitive, emotional, hyperarousal
Statement of Significance.
Depression is a serious perinatal complication. In nonperinatal samples, insomnia and cognitive arousal are implicated in depression etiology, yet their temporal associations with perinatal depression are poorly characterized. We collected health reports from 70 women across late pregnancy and early postpartum. We observed a toxic cycle between insomnia and cognitive arousal wherein cognitive arousal at night produced insomnia, and insomnia provided an opportunity for cognitive arousal at night. This cycle fueled perinatal depression. Critically, depression risk was highest when women experienced insomnia and high cognitive arousal at the same time. Depression fed into cognitive arousal, but did not directly impact sleep. Our results suggest that insomnia and cognitive arousal are key therapeutic targets for the prevention and maximal treatment of perinatal depression.
Introduction
Perinatal depression refers to major and minor depression that occurs in pregnancy and/or the first postpartum year. Estimates suggest that 10%–19% of women experience major or minor depression at some point during pregnancy and postpartum [1–3], thereby supporting depression as one of the most highly prevalent perinatal complications. Perinatal depression is a serious and debilitating condition that affects both mother and child. Depressed mothers are more likely to have suicidal thoughts and struggle with caregiving, resulting in fewer well-child visits and vaccinations, more infant sleep problems, and poorer adherence to safety practices [4, 5]. To help detect women at risk for perinatal depression, researchers have identified factors influencing depressive symptoms during pregnancy and postpartum. Some factors are perinatal-specific, such as prior prenatal loss [6], pregnancy-related anxiety [7], and concerns about fetal health [8]. Other etiological factors have been firmly established first in the broader depression literature, such as genetics [9] and cognitive arousal, particularly in the form of perseverative/repetitive thinking [10]. In the broader depression population, insomnia precedes half of all incident and relapse depression cases [11]. And yet, despite evidence characterizing insomnia as a robust risk factor for depression [12], the role of insomnia in perinatal depression remains poorly characterized.
Perinatal insomnia is a public health crisis. Over half of pregnant women meet diagnostic criteria for insomnia disorder or screen positive for clinically significant insomnia symptoms [13, 14]. Despite this high prevalence, perinatal insomnia is not typically assessed in routine obstetric evaluation. Historically, sleep disturbance has been considered a normative experience in pregnancy, dismissed by healthcare providers as unworthy of attention beyond reassurance [15]. However, insomnia during pregnancy is often not merely a transient disruption in sleep, and women with clinically significant insomnia in early pregnancy consistently report disturbed sleep throughout pregnancy [16]. Importantly, insomnia symptoms frequently persist long after delivery [14]. The course of perinatal insomnia appears chronic as half of all postpartum women complain of symptoms 2 years after childbirth [17], and many do not recover fully until 6 years postpartum [18]. Given the high rates of insomnia and depression in pregnancy and postpartum, coupled with robust literature supporting insomnia as a risk factor for depression in the general population, the role of insomnia in perinatal depression development has received considerable attention.
Depression rates are estimated at 19%–26% in pregnant and postpartum women with insomnia compared to just 4% among perinatal women without insomnia [5, 14, 19]. Despite strong support for an association between insomnia and depression in peripartum [20], prospective data regarding the influence of insomnia on depressive symptoms have yielded contradictory findings. Some studies offer support for insomnia as a risk factor for postpartum depression. In a sample of 142 perinatal women, Sedov and Tomfohr-Madsen [16] recently showed clinical insomnia in early pregnancy augured a chronic insomnia course and greater depression levels in early postpartum. Notably, 42% of pregnant women with clinical insomnia developed postpartum depression compared to just 8% of those without insomnia symptoms in pregnancy. In a prospective study of 382 women, Marques et al. [21] showed that insomnia in late pregnancy predicts postpartum depressive symptoms. In two other studies, Okun et al. [22] and Dorheim et al. [23] showed that sleep disturbances and insomnia symptoms in late pregnancy predicted postpartum depression, but only in women with histories of depression.
On the other hand, several findings suggest that insomnia may not exert a direct influence on perinatal depression. In Dorheim et al.’s [23] aforementioned population study, insomnia in late pregnancy predicted postpartum depression in women with histories of depression, but not in women who had never been depressed. In another population-based study, insomnia during middle pregnancy was associated with concurrent depression, but did not predict postpartum depression [24]. Another study published by Okun’s team [25] showed that sleep complaints in late pregnancy did not increase the risk for postpartum depression, but rather predicted the timing of depression onset (a later onset, rather than earlier, curiously). In Marques et al.’s aforementioned study, the prospective association of prenatal insomnia and postpartum depression became nonsignificant when controlling for negative and positive mood ratings. The authors proposed that insomnia may influence perinatal depression via an indirect path involving cognitive–emotional regulation. Overall, prospective studies provide modest and inconsistent evidence that insomnia may increase symptoms of perinatal depression.
If perinatal insomnia does predict depression, the extent to which perinatal insomnia affects depression directly and indirectly via an influence on cognitive–emotional regulation is unknown. Cognitive arousal, particularly regarding negative perseverative thought, is an aspect of cognitive–emotional regulation that is especially germane to insomnia and depression [26–28]. Epidemiological data show that insomnia directly increases the risk for incident depression [12, 29], but also increases depression risk via cognitive arousal [30, 31]. This insomnia → cognitive arousal → depression pathway may explain why Marques et al. [21] found that insomnia’s effect on depression diminished when accounting for mood; mood disturbances and cognitive arousal are fundamentally and inextricably linked [32]. Early evidence suggests that cognitive arousal is strongly associated with insomnia and depression during pregnancy and postpartum.
Most pregnant women, irrespective of insomnia or depression status, endorse high levels of cognitive arousal; levels commensurate with those observed in individuals with mental illness [5]. Importantly, many pregnant women report concerns relating to pregnancy, childbirth, and motherhood, and focusing on these concerns is linked to maternal depression [7, 33]. As a result, the manner in which ruminating on perinatal concerns may influence maternal sleep and mental well-being has drawn recent attention. Unsurprisingly, perinatal women with insomnia report higher levels of both broadly focused nocturnal cognitive arousal and perinatal-focused rumination (PFR) than their good-sleeping counterparts [5, 34].
Although insomnia and cognitive arousal are both highly elevated and closely related in peripartum, they share independent associations with perinatal depression. Cross-sectional data offer preliminary support that they may work together to fuel depression. In an analysis of 257 pregnant women, the highest depression rates were observed among women with insomnia and high cognitive arousal (36%) as compared to those with only insomnia (4%), only high cognitive arousal (11%), or neither (1%) [5]. The same study showed similar patterns when examining the combined effects of insomnia and PFR. These cross-sectional findings are consistent with prospective epidemiological data showing that adults with insomnia and high cognitive arousal have a higher risk for 2-year incident depression (13%) than those with only insomnia (4%), only high cognitive arousal (8%), or neither (3%) [35]. These findings, combined with prospective data from perinatal and nonperinatal insomnia samples [21, 30, 31], suggest that cognitive arousal may link insomnia to depression in peripartum. However, no studies to date have examined prospective associations among insomnia, cognitive arousal, and depression in pregnant or postpartum women.
Prior prospective studies of perinatal insomnia and depression have been methodologically limited in a critical way: They have focused exclusively on the effects of prenatal insomnia on postpartum depression. This limited scope is problematic for two reasons: First, although insomnia symptoms remain highly prevalent after childbirth, sleep symptoms and challenges drastically change from pregnancy to early postpartum (e.g. greater sleep deprivation, more external wake-promoting stimuli such as poor infant sleep and nighttime feedings) [17, 18, 23]. Notably, insomnia symptoms, while persistent, tend to trend downward in early postpartum [16], perhaps at least partly due to reduced sleep opportunity related to nighttime infant caretaking, thus further complicating the perinatal insomnia–depression relationship. Second, the postpartum period is simply not a robust trigger for depression development. A review of large perinatal population studies shows that depression rates are similar between pregnancy and postpartum [3]. Some population studies show that depression is actually lower in postpartum than in pregnancy [1, 36]. Therefore, studies testing only for effects of prenatal insomnia on postpartum depression fail to account for sleep changes after childbirth and miss critical prenatal changes in depressive symptoms, thereby diminishing the ability to detect effects of perinatal insomnia on depression. Prospective studies of insomnia and depression should test temporal associations within pregnancy and postpartum, while accounting for any potential effects of childbirth on symptom changes.
In the present study, we investigated whether insomnia and cognitive arousal predict future depression across late pregnancy and early postpartum. To accomplish this goal, we assessed depressive symptoms, insomnia symptoms, and three cognitive arousal indices (nocturnal cognitive arousal, nocturnal PFR, and broadly focused perseverative thinking) for 17 consecutive weeks across late pregnancy and early postpartum. In this report, the term “cognitive arousal” in reference to study outcomes applies to all three measures. Yet, different indices can elucidate the importance of the timing of cognitive arousal symptoms (e.g. night) and the cognitive content on which perinatal women perseverate (e.g. perinatal concerns). Our study differed from prior investigations in two critical ways: (1) We examined temporal associations during pregnancy and postpartum at the same time, rather than restrict analyses to testing effects of prenatal sleep on postpartum depression. (2) We accounted for cognitive arousal, which perinatal women endorse at pathological levels [5], and have been posited to mediate effects of insomnia on depression in perinatal and nonperinatal populations [21, 30, 31].
Study Goal 1: Determine prospective effects of insomnia and cognitive arousal indices on perinatal depression
Hypothesis: We predicted that insomnia, nocturnal cognitive arousal, PFR, and perseverative thinking would independently predict future depressive symptoms.
Study Goal 2: Test mediation models characterizing the effects of insomnia and cognitive arousal on depression
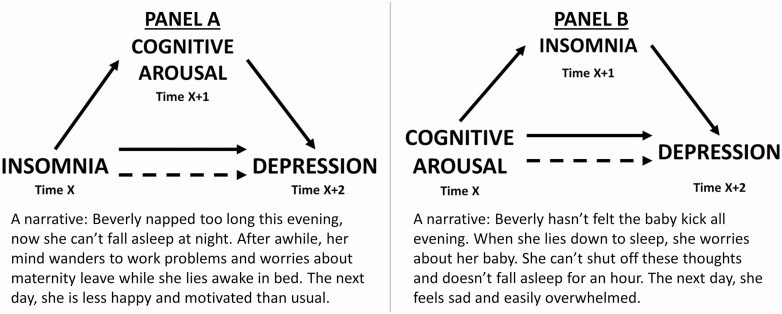
Prior studies in the broader insomnia population show that insomnia increases cognitive arousal [31, 37] and that cognitive arousal increases insomnia [38–41]. Given that insomnia and cognitive arousal each predict future depression, we hypothesized the following mediation models. Hypothesis 1: We predicted that insomnia would lead to depression, but that this would be partly mediated by cognitive arousal (Figure 1, Panel A). Hypothesis 2: We predicted that cognitive arousal would lead to depression, but that this effect would also be partly mediated by insomnia (Figure 1, Panel B).
Figure 1.
Panel A: Hypothesized model wherein cognitive arousal mediates the effect of insomnia on future depression. Panel B: Hypothesized model wherein insomnia mediates the effect of cognitive arousal on future depression. Narratives are included to demonstrate how these processes may work in the real world.
Study Goal 3: Examine whether insomnia and high cognitive arousal have compounding effects on odds for depression
Hypothesis: We predicted that depression risk would be highest when women experienced high levels of insomnia and cognitive arousal at the same time, relative to when experiencing only insomnia, only high cognitive arousal, or neither. This hypothesis was based on prior data showing that the combination of insomnia and high cognitive arousal is more depressogenic than experiencing one without the other [5, 35].
Methods
Study setting and design
The study was approved by the Internal Review Board at the Henry Ford Health System. Pregnant women receiving care in a multi-hospital health system centrally located in Metropolitan Detroit, Michigan, USA were invited to participate in an observational study on perinatal sleep and health. Once enrolled, participants were asked to complete 17 weekly online surveys (approximately 15–20 min, hosted by Qualtrics) beginning at gestational week 30. All participants received routine sleep hygiene tips via email (note: tips were not tailored to pregnancy or postpartum, but rather were broadly focused on caffeine, light, electronic devices, etc.). Although childbirth is typically estimated to occur at gestational week 40, the actual timing of delivery varies considerably. Even so, this data collection schedule was designed to collect health information across most of the third trimester of pregnancy and approximately the first 1.5 months of postpartum.
Study population
The inclusion criterion for the present study was gestational age between 25 and 30 weeks at the time of eligibility screening. Exclusion criteria included high-risk pregnancy per self-report (e.g. preeclampsia diagnosis, age >40 years), being in the care of the maternal-fetal medicine team for high-risk pregnancy (any reason) per electronic medical records, multiple pregnancy, use of prescription or over-the-counter sleep aids or any other sedating medications at the time of screening, alcohol or recreational drug use at the time of screening, rotating and/or night shift work, epilepsy or seizures, bipolar disorder, diagnosis of a sleep disorder that is untreated (other than insomnia), and severe depression (see the Measures section).
This study was part of a larger parent study on perinatal health wherein some participants were randomized to treatment and some were randomized to naturalistic observation. This study included eligible participants who were randomized to observation. Invitations advertising a study on perinatal sleep (without mentioning that we were focused on poor sleep as we were recruiting good sleepers and those with insomnia) were sent via email and phone calls to 3,585 patients. A total of 535 women contacted us with interest in our study. Of these patients, 272 women consented to participate in the study, 267 of whom provided sufficient data for full eligibility determination, which were collected between September 12, 2018 and March 9, 2019. A total of 70 participants (44 with clinical insomnia and 26 without insomnia) were randomized into this observational study. All participants provided written informed consent.
Measures
Baseline data
Baseline data were collected during eligibility screening between gestational weeks 25 and 30. Sociodemographic information included age, annual household income, race, prior birth history, and history of miscarriages. In addition, we assessed current medication use, body mass index (derived from electronic medical records), snoring (“Do you snore?” Yes/No), and sleep duration (“During the past month, how many hours of sleep did you get at night?” from the Pittsburgh Sleep Quality Index) [42].
Depressive symptoms were measured using the Edinburgh Postnatal Depression Scale (EPDS) [43], a 10-item self-report measure of depressive symptoms. EPDS scores range from 0 to 30, with higher scores indicating greater severity. A cut-point of EPDS of at least 10 favors sensitivity over specificity to detect both minor and major depression and is commonly used in clinical practice to initiate treatment for perinatal depression. Women who scored at least 18 at baseline were excluded for severe depression.
Insomnia symptoms were measured using the Insomnia Severity Index (ISI) [44, 45]. Scores range from 0 to 28 with higher scores indicating greater severity. ISI of at least 10 indicates clinically significant insomnia symptoms in community samples [45]. Prior research supports this cutoff for pregnant women as those who score at least 10 on the ISI report elevated levels of depression, cognitive arousal, and risk for suicidal ideation [5].
The first aspect of cognitive arousal measured was nocturnal cognitive arousal, which was measured using the Presleep Arousal Scale—Cognitive factor (PSASC) [46]. Specifically, the PSASC measures trait tendency for cognitive arousal while trying to fall asleep at night. Example items from the PSASC are “review or ponder events of the day” and “can’t shut off thoughts.” Scores range from 8 to 40 with higher scores indicating greater nocturnal cognitive arousal. PSASC scores of at least 20 represent high nocturnal cognitive arousal and suggest clinically significant sleep and mood pathology [47].
The second aspect of cognitive arousal measured was nocturnal PFR, which was measured using a single item that we appended to the PSASC. Specifically, participants were asked how intensely they “worried or had stressful thoughts about your pregnancy or new infant” when attempting to fall asleep. Women who scored 1 (not at all) or 2 (slightly) were considered low on PFR, whereas women who scored 3 (moderately) through 5 (extremely) were considered high on PFR (notably, the median was 2).
Repeated measures
Repeated measures were collected weekly starting at gestational week 30 and for 17 consecutive weeks thereafter. All measures were modified to assess symptoms over “the prior week” for two reasons: (1) ensure that assessment windows are consistent with our weekly repeated measures study design and (2) depression was our primary outcome and the EPDS, our depression measure, is validated for a 1-week assessment window. Most repeated measures were included in the baseline assessment. The EPDS assessed weekly depression symptoms (unchanged assessment window). The ISI assessed weekly insomnia symptoms (typically measures 2 weeks of symptoms). The PSASC and the added PFR item assessed nocturnal cognitive arousal and nocturnal PFR, respectively (originally, the PSASC measures trait tendencies). The third aspect of cognitive arousal that we measured was perseverative thinking, which was measured using the Perseverative Thinking Questionnaire (PTQ) which is a 15-item transdiagnostic measure of repetitive thinking [48]. The PTQ was not administered at baseline, but was administered weekly. The valence of many PTQ items is neutral (e.g. My thoughts repeat themselves and Thoughts just pop into my mind), although the valence of some items is negative (e.g. I think about many problems without solving them). Higher scores on the PTQ indicate a greater tendency to engage in transdiagnostic, broadly focused repetitive thinking. Notably, the PTQ is content-independent. In other words, it does not measure perseverative thinking specific to depression, anxiety, insomnia, and so on. We are not aware of any empirically validated PTQ scores to indicate high levels of repetitive thinking, particularly when assessing over the prior week. Even so, we operationalized PTQ scores of at least 10 as high perseverative thinking as it represented levels above the grand mean for this sample (M ± SD: 9.77 ± 11.04), which identified 35.31% PTQ observations as high (399/1,130).
Analysis plan
Descriptive analyses and baseline group comparisons were run using SPSS 25. Linear and logit mixed effects models were run using STATA/SE 15.1. PRODCLIN estimates were performed using R 4.0.2 and the RMediation package. Descriptive data analysis involved presenting frequency rates of dichotomous variables (snoring, obesity, positive insomnia status, etc.) and means and standard deviations for continuous variables. As this project primarily focused on the prospective effects of insomnia and high cognitive arousal, we compared women with insomnia versus without insomnia, and with high versus low nocturnal cognitive arousal, on sociodemographic characteristics and baseline sleep and mood symptoms. These analyses were performed to provide an impression of how these groups differ in late pregnancy.
Our primary study outcome was weekly reports of depressive symptoms in the third trimester of pregnancy and early postpartum. To account for the time-nested structure of the outcome variable, we used linear mixed modeling (also known as hierarchical linear modeling) when estimating depression as a repeated measures continuous outcome, and mixed effects logit modeling when estimating depression as a repeated measures binary outcome. This approach permits the examination of both covariance and lagged effects that may exist between independent variables (IVs) and dependent variables (DVs), revealing both concurrent and temporal associations. Mixed effects models allow the time-nested DV to be regressed onto interindividual factors (variables that represent differences between participants, which are baseline differences between participants in the present study) and intraindividual factors (variables that represent changes within participants; fluctuations in symptoms as observed across repeated measures). Importantly, mixed effects modeling is robust to the presence of missing data, which are common in longitudinal studies [49].
For our mixed effects models, we regressed the DV (measured repeatedly, thus an intraindividual factor) on baseline IVs (measured once, thus an interindividual factor) and repeatedly measured IVs (intraindividual factors). Due to the study design, results from these models as described here demonstrate (1) prospective effects of baseline factors on depression (i.e. baseline factors predicting differences in depression levels across peripartum between participants) and (2) concurrent associations between weekly depression scores and other weekly factors (i.e. weekly symptoms associate with weekly changes in depression). To test temporal associations among intraindividual factors (repeated measures), we lagged the repeatedly measured IVs (i.e. lagged refers to intraindividual factors only). Lagged models show temporal associations between intraindividual IVs and DVs (e.g. does insomnia this week predict depression a week later?). All lagged models control for the previous week’s value of the DV. For more details and example equations demonstrating our covariance and lagged effects mixed models, please refer to Supplementary Materials.
Mediation models were first tested with three primary linear mixed models. The direct effect (also known as the tau (τ) path) refers to the IV → DV effect. As mediation analyses must demonstrate directionality ideally utilizing assessments across at least three time points, we regressed the DV onto the IV lagged by 2 weeks (IVit−2). Next, we tested the indirect effect, which comprises the alpha (α) path (IV → M) and the beta (β) path (M → DV). For the α path, we regressed the mediator onto the IV lagged by 1 week (IVit−1). For the β path, we regressed the DV onto the mediator lagged by 1 week (Mit−1), while controlling for the IV lagged by 2 weeks (IVit−2; i.e. the τ′ path, which is the new estimate of the direct effect after controlling for the effects of the proposed mediator). Ultimately, our mediation models evaluated IVit−2 → Mit−1 → DVit, while controlling for appropriate covariates (see Figure 1 at the end of Introduction for hypothesized models).
From these regression models, the product of the α and β parameter estimates represented the indirect (i.e. mediated) effect. The confidence intervals (CIs) of the mediated effects were estimated using the PRODCLIN method in R 4.0.2 using the RMediation library [50]. This method does not assume a normal distribution, yields asymmetric CIs, and is more accurate than traditional significance tests. If the 95% CI for the indirect effect does not include zero, then significant mediation is inferred. Proportions of the unadjusted direct effect (τ) accounted for by the indirect effect (αβ) can be estimated in two simple ways: (1) αβ/τ and (2) (τ − τ′)/t.
Results
Sample characteristics, baseline clinical symptoms, and depression rates
Participant ages ranged from 20 to 39 years. Most participants self-identified racially as non-Hispanic white (57.1%) or non-Hispanic black (24.3%). Twenty-six women (37.1%) screened positive for minor or major depression at baseline. Forty-one women (58.6%) screened positive for depression at least once over the following 17 weekly assessments (23 of 26 participants who screened positive at baseline, plus 18 of 44 participants who initially screened negative at baseline). As this project focused on the effects of insomnia and cognitive arousal, we compared participants by baseline insomnia status and by nocturnal cognitive arousal status on demographic and baseline symptoms for descriptive purposes. See Table 1 for details on sociodemographics and group comparisons.
Table 1.
Sample demographics and characteristics for all patients and by insomnia status at study entry screening
| All participants | ISI ≥10 | ISI <10 | PSASC ≥20 | PSASC <20 | |||
|---|---|---|---|---|---|---|---|
| Sample size | 70 | 44 | 26 | 33 | 37 | ||
| Age (M ± SD), years | 29.21 ± 4.09 y | 29.14 ± 4.15 | 29.35 ± 4.07 | t(68) = −0.21, p = 0.838 | 28.85 ± 4.49 | 29.54 ± 3.73 | t(68) = −0.70, p = 0.484 |
| Gestational week (M ± SD) | 27.83 ± .96 | 27.61 ± .97 | 28.19 ± .85 | t(68) = −2.52, p = 0.014 | 27.85 ± .94 | 27.81 ± 1.00 | t(68) = 0.16, p = 0.872 |
| Poverty (n; %) | 12/68; 17.1% | 8/43; 18.6% | 4/25; 16.0% | χ 2 = 0.07, p = 0.786 | 9/32; 28.1% | 3/36; 8.3% | χ 2 = 4.57, p = 0.033 |
| BMI ≥35 (n; %) | 10/67; 14.9% | 3/41; 7.3% | 7/26; 26.9% | χ 2 = 4.82, p = 0.028 | 4/31; 12.9% | 6/36; 16.7% | χ 2 = 0.19, p = 0.666 |
| Multiparous (n; %) | 58/70; 58.6% | 29/44; 65.9% | 12/26; 46.2% | χ 2 = 2.62, p = 0.105 | 23/33; 69.7% | 18/37; 48.6% | χ 2 = 3.19, p = 0.074 |
| Snoring (n; %) | 12/70; 17.1% | 9/44; 20.5% | 3/26; 11.5% | χ 2 = 0.92, p = 0.339 | 5/33; 15.2% | 7/37; 18.9% | χ 2 = 0.17, p = 0.676 |
| Antidepressants | 2/70; 2.9% | 1/44; 2.3% | 1/26; 3.8% | — | 2/36; 6.1% | 0/37; 0.0% | — |
| Race (n; %) | |||||||
| White | 40; 57.1% | 23; 52.3% | 17; 65.4% | 18; 54.5% | 22; 59.5% | ||
| Black | 17; 24.3% | 13; 29.5% | 4; 15.4% | 10; 30.3% | 7; 18.9% | ||
| Asian | 3; 4.3% | 3; 6.8% | 0; 0.0% | 2; 6.1% | 1; 2.7% | ||
| Middle Eastern or Arabic | 2; 2.9% | 2; 4.5% | 0; 0.0% | 0; 0.0% | 2; 5.4% | ||
| Hispanic or Latino | 4; 5.7% | 1; 2.3% | 3; 11.5% | 3; 9.1% | 1; 2/7% | ||
| Multiracial | 4; 5.7% | 2; 4.5% | 2; 7.7% | 0; 0.0% | 4; 10.8% | ||
| Baseline symptoms | |||||||
| ISI | 11.01 ± 5.12 | 14.11 ± 3.39 | 5.77 ± 2.66 | t(68) = 10.74, p < 0.001 | 12.58 ± 5.09 | 9.62 ± 4.79 | t(68) = 2.50, p = 0.015 |
| ISI ≥10 | 44/70; 62.9% | — | — | — | 18/33; 54.5% | 8/37; 21.6% | χ 2 = 8.10, p = 0.004 |
| Sleep duration (h) | 6.72 ± 1.16 | 6.31 ± 1.19 | 7.42 ± .66 | t(67) = -4.49, p < 0.001 | 6.52 ± 1.40 | 6.91 ± .86 | t(68) = −1.42, p = 0.160 |
| Short sleep (≤6 h) | 21/70; 30.0% | 21/44; 47.7% | 0/26; 0.0% | χ 2 = 17.73, p < 0.001 | 14/33; 42.4% | 7/37; 18.9% | χ 2 = 4.59, p = 0.032 |
| EPDS (M ± SD) | 7.84 ± 4.83 | 9.45 ± 4.67 | 5.12 ± 3.81 | t(68) = 4.01, p < 0.001 | 9.85 ± 5.00 | 6.05 ± 3.93 | t(68) = 3.55, p < 0.001 |
| EPDS ≥10 | 26/70; 37.1% | 21/44; 47.7% | 5/26; 19.2% | χ 2 = 5.68, p = 0.017 | 18/33; 54.5% | 8/37; 21.6% | χ 2 = 8.10, p = 0.004 |
| PSASC (M ± SD) | 20.39 ± 7.07 | 22.70 ± 7.10 | 16.46 ± 5.08 | t(68) = 3.93, p < 0.001 | 26.24 ± 5.34 | 15.16 ± 3.30 | t(68) = 10.56, p < 0.001 |
| PSASC ≥20 | 33/70; 47.1% | 39/44; 88.6% | 16/26; 61.5% | χ 2 = 7.13, p = 0.008 | — | — | — |
| PFR (M ± SD) | 2.24 ± 1.00 | 2.45 ± 1.04 | 1.88 ± .82 | t(68) = 2.38, p = 0.020 | 2.58 ± 1.06 | 1.95 ± .85 | t(68) = 2.76, p = 0.008 |
| PFR ≥3 | 26/70; 37.1% | 21/44; 47.7% | 5/26; 19.2% | χ2=5.68, p=.017 | 16/33; 48.5% | 10/37; 27.0% | χ 2 = 3.44, p = 0.064 |
M ± SD = mean and standard deviation; n; % = number of participants, percentage of sample. Gestational week reflects the status at study entry. Poverty is operationalized as less than $20,000 annual household income. BMI = body mass index before pregnancy, BMI values 35 and higher indicate class II obesity. Snoring indicates whether patients endorsed snoring at study entry baseline. Antidepressants were reported at baseline, only sertraline was reported. Race was self-reported. ISI = insomnia severity index, a cutoff score represents clinically significant insomnia symptoms relative to a community sample. Sleep duration represents average nightly sleep over the prior month. Short sleep was operationalized as sleeping 6 h or fewer on average over the past month. EPDS = Edinburgh Postnatal Depression Scale. EPDS scores 10 and higher suggest probably minor or major depression. PSASC = Presleep Arousal Scale—Cognitive factor. PSASC scores 20 and higher indicate cognitive hyperarousal. PFR = perinatal-focused rumination, scores of 3 and higher indicate high rumination on perinatal concerns.
Predicting perinatal depression symptoms: Identifying clinical signs
A null linear mixed model regressing depression on an intercept yielded 1,133 observations, indicating the mean number of completed assessments was 16.19 per participant, reflecting a 4.8% missing data rate. An estimated 56% of the variance in depression was due to interindividual differences, whereas 44% was due to weekly fluctuations within participants.
To test our hypotheses, we used linear mixed effects regression to estimate depressive symptoms as predicted by sociodemographic factors and baseline depression, insomnia, nocturnal cognitive arousal, and PFR (interindividual factors), while controlling for effects of time and changes from pre- to postpartum (intraindividual factors; Table 2, Model 1). By using clinical cutoffs, we can identify women in late pregnancy who may be at risk for perinatal depression. Women who reported high nocturnal cognitive arousal and PFR were more depressed across peripartum than women low in cognitive arousal and rumination. Baseline depression status and snoring, as well as study week and childbirth, were also associated with depressive symptoms. Significant predictors in this model were entered as covariates in subsequent models.
Table 2.
Identifying insomnia and cognitive arousal factors associated with perinatal depression using linear and logit mixed effects modeling
| Model 1, Predicting depression symptoms: Wald χ 2 = 144.25, p < 0.001 (obs = 1,051) | ||||
|---|---|---|---|---|
| b | 95% CI | z | p | |
| Interindividual factors | ||||
| Prior miscarriage | −0.90 | −2.76 to 0.96 | −0.94 | 0.345 |
| Age | −0.03 | −0.21 to 0.15 | −0.36 | 0.716 |
| Poverty | −1.64 | −3.73 to 0.46 | −1.53 | 0.125 |
| BMI ≥35 | −1.08 | −3.27 to 1.11 | −0.97 | 0.333 |
| Snoring | 2.64 | 0.80 to 4.49 | 2.81 | 0.005 |
| EPDS ≥10 | 3.81 | 2.34 to 5.29 | 5.07 | <0.001 |
| ISI ≥10 | 0.18 | −0.65 to 1.01 | 0.43 | 0.667 |
| Short sleep (≤6 h/night) | 0.56 | −1.14 to 2.25 | 0.64 | 0.519 |
| PSASC ≥20 | 1.51 | 0.02 to 3.00 | 1.99 | 0.046 |
| PFR ≥3 | 2.00 | 0.52 to 3.47 | 2.66 | 0.008 |
| Intraindividual factors | ||||
| Time | 2.44 | −0.26 to −0.13 | −6.03 | <0.001 |
| Prenatal vs Postpartum | 0.75 | 0.09 to 1.40 | 2.24 | 0.025 |
| Model 2, Predicting depression symptoms: Wald χ 2 = 541.81, p < 0.001 (obs = 1,130) | ||||
| b | 95% CI | z | p | |
| Interindividual factors | ||||
| Snoring | 0.88 | −0.16 to 1.93 | 1.66 | 0.096 |
| EPDS ≥10 | 1.86 | 0.98 to 2.74 | 4.15 | <0.001 |
| PSASC ≥20 | 0.13 | −0.69 to 0.95 | 0.30 | 0.761 |
| PFR ≥3 | 0.75 | −0.05 to 1.54 | 1.84 | 0.066 |
| Intraindividual factors | ||||
| Time | −0.09 | −0.15 to −0.04 | −3.54 | <0.001 |
| Prenatal vs Postpartum | 0.70 | 0.15 to 1.26 | 2.48 | 0.013 |
| ISI | 0.12 | 0.07 to 0.16 | 5.10 | <0.001 |
| Sleep duration | 0.03 | −0.13 to 0.18 | 0.36 | 0.717 |
| PSASC | 0.09 | 0.04 to 0.13 | 3.68 | <0.001 |
| PFR | 0.33 | 0.16 to 0.51 | 3.68 | <0.001 |
| PTQ | 0.17 | 0.14 to 0.19 | 14.44 | <0.001 |
| Model 3, Predicting positive depression status: Wald χ 2 = 128.83, p < 0.001 (obs = 1,130) | ||||
| OR | 95% CI | z | p | |
| Interindividual factors | ||||
| Snoring | 0.84 | — | −0.26 | 0.791 |
| EPDS ≥10 | 13.85 | 4.27 to 44.93 | 4.38 | <0.001 |
| PSASC ≥20 | 1.12 | — | 0.20 | 0.844 |
| PFR ≥3 | 2.04 | — | 1.35 | 0.178 |
| Intraindividual factors | ||||
| Time | 0.91 | 0.83 to 0.99 | −2.04 | 0.041 |
| Prenatal vs Postpartum | 1.68 | — | 1.12 | 0.264 |
| ISI ≥10 | 2.36 | 1.28 to 4.35 | 2.76 | 0.006 |
| PSASC ≥20 | 3.05 | 1.60 to 5.79 | 4.03 | <0.001 |
| PFR ≥3 | 2.05 | 1.11 to 3.79 | 2.30 | 0.021 |
| PTQ ≥10 | 7.48 | 3.90 to 14.32 | 6.06 | <0.001 |
The Wald χ 2 statistic reflects the difference between the tested model and a null model with no predictors. Thus, a significant χ 2 is desirable as it indicates a model that accounts for significant variance in the outcome. obs = observations; b = unstandardized parameter estimate; SE = standard error; 95% CI = 95% confidence interval; z = z-statistic; p = significance value. Interindividual factors were recorded at the study baseline, and significant effects represent between-subject differences. Intraindividual factors were recorded weekly across peripartum, and significant effects represent within-subject associations. Age represents age in years reported at baseline. Poverty was operationalized as less than $20,000 of annual household income. BMI = body mass index; BMI ≥ 35 represents obesity class II. Snoring was recorded at study entry (Yes = 1, No = 0). EPDS = Edinburgh postnatal depression scale, scores 10 and higher indicate probable minor or major depression. ISI = Insomnia Severity Index, scores 10 and higher indicate clinically significant symptoms. Short sleep operationalized as averaging ≤6 h per night over the prior month. Time represents study week. Prenatal vs Postpartum coded as prenatal = 0 and postpartum = 1. ISI as an intraindividual factor represents insomnia symptom severity over the prior week. Sleep duration as an intraindividual factor represents the average nightly sleep duration over the prior week. PSASC as an interindividual factor represents trait nocturnal cognitive arousal; scores of 20 and higher indicate high nocturnal cognitive arousal. PSASC as an intraindividual factor represents nocturnal cognitive arousal over the prior week. PFR represents nocturnal perinatal-focused rumination; scores of 3 and higher indicate high perinatal-focused rumination. PFR as an intraindividual factor represents nocturnal perinatal-focused rumination over the prior week. PTQ represents perseverative thinking over the prior week; scores of 10 and higher indicate elevated perseverative thinking levels.
Next, we regressed depression on concurrent insomnia symptoms, sleep duration, nocturnal cognitive arousal, PFR, and perseverative thinking (all intraindividual factors), while controlling for covariates (Table 2, Model 2). Results showed that women were more depressed when experiencing greater insomnia, nocturnal cognitive arousal, PFR, and perseverative thinking. Sleep duration was not independently associated with depressive symptoms.
To offer more readily interpretable estimates of the magnitude of associations, we ran a posthoc logit mixed effects regression model, which showed that the odds of screening positive for depression doubled when women reported clinically significant insomnia (odds ratio [OR] = 2.36) or high PFR at night (OR = 2.05); see Table 2, Model 3. Odds for positive depression screens were even greater when women endorsed high nocturnal cognitive arousal (OR = 3.05) and perseverative thinking (OR = 7.48).
Does nocturnal cognitive arousal mediate the effects of insomnia on depression?
Next, we conducted a series of linear mixed models to test whether nocturnal cognitive arousal mediated the effects of insomnia on depression (see Table 3 for full results).
Table 3.
Linear mixed models to evaluate nocturnal cognitive arousal as a mediator of insomnia and depression
| Tau Path, Insomnia predicting depression: Wald χ 2 = 116.58, p < 0.001 (obs = 987) | ||||
|---|---|---|---|---|
| b | 95% CI | z | p | |
| Interindividual factors | ||||
| Snoring | 2.42 | 0.52 to 4.32 | 2.50 | 0.012 |
| EPDS ≥10 | 3.29 | 1.71 to 4.87 | 4.08 | <0.001 |
| PSASC ≥20 | 1.28 | −0.21 to 2.77 | 1.69 | 0.092 |
| PFR ≥3 | 1.55 | 0.11 to 3.00 | 2.11 | 0.035 |
| Intraindividual factors | ||||
| Time | −0.09 | −0.16 to −0.01 | −2.36 | 0.018 |
| Prenatal vs Postpartum | 0.15 | −0.49 to 0.79 | 0.46 | 0.645 |
| ISI, lagged 2 weeks | 0.07 | 0.02 to 0.11 | 2.65 | 0.008 |
| EPDS, lagged 2 weeks | 0.13 | 0.06 to 0.19 | 3.88 | <0.001 |
| Alpha Path, Insomnia predicting nocturnal cognitive arousal: Wald χ 2 = 244.39, p < 0.001 (obs = 1,051) | ||||
| b | 95% CI | z | p | |
| Interindividual factors | ||||
| Snoring | 3.65 | 1.28 to 6.02 | 3.02 | 0.003 |
| EPDS ≥10 | 1.89 | −0.09 to 3.87 | 1.87 | 0.061 |
| PSASC ≥20 | 2.29 | 0.43 to 4.15 | 2.41 | 0.016 |
| PFR ≥3 | 1.19 | −0.62 to 3.00 | 1.29 | 0.198 |
| Intraindividual factors | ||||
| Time | −0.11 | −0.20 to −0.03 | −2.67 | 0.008 |
| Prenatal vs Postpartum | −0.56 | −1.36 to 0.24 | −1.37 | 0.171 |
| ISI, lagged 1 week | 0.08 | 0.02 to 0.15 | 2.58 | 0.010 |
| PSASC, lagged 1 week | 0.26 | 0.19 to 0.33 | 7.68 | <0.001 |
| Beta Path, Nocturnal cognitive arousal predicting depression: Wald χ 2 = 208.62, p < 0.001 (obs = 978). | ||||
| b | 95% CI | z | p | |
| Interindividual factors | ||||
| Snoring | 1.80 | 0.19 to 3.41 | 2.19 | 0.029 |
| EPDS ≥10 | 2.69 | 1.34 to 4.03 | 3.91 | <0.001 |
| PSASC ≥20 | .82 | −0.44 to 2.08 | 1.27 | 0.203 |
| PFR ≥3 | 1.17 | −0.06 to 2.39 | 1.86 | 0.063 |
| Intraindividual factors | ||||
| Time | −0.05 | −0.13 to 0.02 | −1.47 | 0.140 |
| Prenatal vs Postpartum | 0.24 | −0.40 to 0.87 | 0.73 | 0.463 |
| ISI, lagged 2 weeks | 0.04 | −0.01 to 0.09 | 1.59 | 0.112 |
| EPDS, lagged 2 weeks | 0.10 | 0.03 to 0.16 | 2.90 | 0.004 |
| PSASC, lagged 1 week | 0.20 | 0.15 to 0.25 | 8.45 | <0.001 |
The Wald χ 2 statistic reflects the difference between the tested model and a null model with no predictors. Thus, a significant χ 2 is desirable as it indicates a model that accounts for significant variance in the outcome. obs = observations. Tau path represents the direct path from insomnia to future depression. Alpha path represents part of the indirect (mediated) effect wherein insomnia influences future cognitive arousal. Beta path represents the other part of the indirect (mediated) effect wherein cognitive arousal influences future depression. b = unstandardized parameter estimate; SE = standard error; 95% CI = 95% confidence interval; z = z-statistic; p = significance value. Interindividual factors were recorded at the study baseline, and significant effects represent between-subject differences. Intraindividual factors were recorded weekly across peripartum, and significant effects represent within-subject associations. Lagged 2 weeks indicates Xit−2. Lagged 1 week indicates Xit−1. Snoring was recorded at study entry (Yes = 1, No = 0). EPDS ≥10 indicates clinically significant depression. PSASC ≥20 indicates high nocturnal cognitive arousal. PFR ≥3 indicates high nocturnal perinatal-focused rumination. Time represents study week. Prenatal vs Postpartum coded as prenatal = 0 and postpartum =1. ISI (insomnia severity index) as an intraindividual factor represents insomnia symptom severity over the prior week. PSASC as an intraindividual factor represents nocturnal cognitive arousal over the prior week.
τ path (direct effect)
We first regressed depression on insomnia (lagged by 2 weeks), while controlling for prior depression and relevant covariates (Table 3). Results showed that higher levels of insomnia predicted higher levels of depression 2 weeks later (τ: b = 0.07, p = 0.005). In other words, we observed a direct effect of insomnia on future depression. Next, we tested the α path (IV → Mediator) and the β path (Mediator → DV) of the indirect effect.
α path
Insomnia predicted nocturnal cognitive arousal a week later (α: b = 0.08, SE = 0.03, p < 0.001; Table 3).
β path
Nocturnal cognitive arousal predicted depression a week later (β: b = 0.20, SE = 0.02, p < 0.001; Table 3). Notably, insomnia was no longer a significant predictor in the model (τ′: b = 0.04, p = 0.112).
αβ path (indirect effect)
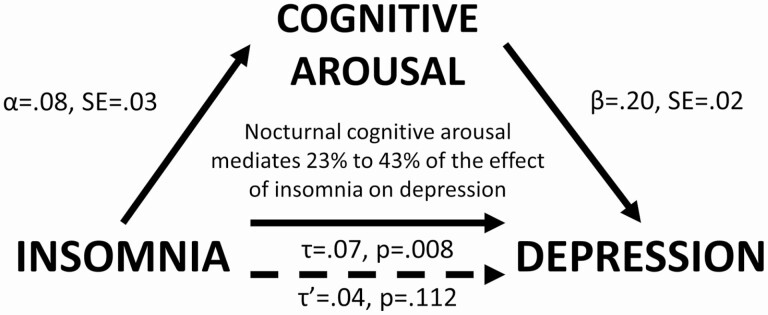
Using the PRODCLIN method, the αβ path was estimated to be 0.016 (SE = .007, 95% CI = 0.004 to 0.031). Since the CI did not overlap with zero, mediation was supported. Nocturnal cognitive arousal was estimated to mediate 23% (αβ/τ = 0.016/0.07) to 43% (τ−τ′/τ = [0.07−0.04]/0.07) of the effect of insomnia on future depression (Figure 2).
Figure 2.
Nocturnal cognitive arousal mediates the effect of insomnia on depression.
Does insomnia mediate the effects of nocturnal cognitive arousal on depression?
We then conducted a series of linear mixed models to test whether insomnia mediated prospective effects of nocturnal cognitive arousal on depression (see Table 4 for full results).
Table 4.
Linear mixed models to evaluate insomnia as a mediator of nocturnal cognitive arousal and depression
| Tau Path, Nocturnal cognitive arousal predicting depression: Wald χ 2 = 137.57, p < 0.001 (obs = 987) | ||||
|---|---|---|---|---|
| b | 95% CI | z | p | |
| Interindividual factors | ||||
| Snoring | 2.21 | 0.45 to 3.99 | 2.45 | 0.014 |
| EPDS ≥10 | 3.10 | 1.63 to 4.58 | 4.13 | <0.001 |
| PSASC ≥20 | 1.06 | −0.33 to 2.46 | 1.49 | 0.135 |
| PFR ≥3 | 1.54 | 0.20 to 2.88 | 2.25 | 0.025 |
| Intraindividual factors | ||||
| Time | −0.07 | −0.14 to 0.003 | −1.88 | 0.060 |
| Prenatal vs Postpartum | 0.18 | −0.46 to 0.82 | 0.55 | 0.581 |
| PSASC, lagged 2 weeks | 0.11 | 0.06 to 0.16 | 4.37 | <0.001 |
| EPDS, lagged 2 weeks | 0.10 | 0.03 to 0.17 | 2.98 | .003 |
| Alpha Path, Nocturnal cognitive arousal predicting insomnia: Wald χ 2 = 246.80, p < 0.001 (obs = 1,051). | ||||
| b | 95% CI | z | p | |
| Interindividual factors | ||||
| Snoring | 2.92 | 0.82 to 5.02 | 2.72 | 0.007 |
| EPDS ≥10 | 0.78 | −0.98 to 2.54 | 0.87 | 0.383 |
| PSASC ≥20 | 0.88 | −0.77 to 2.54 | 1.05 | 0.295 |
| PFR ≥3 | 2.33 | 0.73 to 3.93 | 2.86 | 0.004 |
| Intraindividual factors | ||||
| Time | −0.03 | −0.11 to 0.06 | −0.59 | 0.554 |
| Prenatal vs Postpartum | −0.11 | −0.91 to 0.68 | −0.28 | 0.778 |
| PSASC, lagged 1 week | 0.11 | 0.05 to 0.17 | 3.37 | 0.001 |
| ISI, lagged 1 week | 0.36 | 0.29 to 0.42 | 10.91 | <0.001 |
| Beta Path, Insomnia predicting depression: Wald χ 2 = 155.82, p < .001 (obs = 978). | ||||
| b | 95% CI | z | p | |
| Interindividual factors | ||||
| Snoring | 2.01 | 0.28 to 3.75 | 2.27 | 0.023 |
| EPDS ≥10 | 2.89 | 1.44 to 4.33 | 3.91 | <0.001 |
| PSASC ≥20 | 0.98 | −0.39 to 2.34 | 1.40 | 0.160 |
| PFR ≥3 | 1.18 | −0.14 to 2.50 | 1.75 | 0.080 |
| Intraindividual factors | ||||
| Time | −0.07 | −0.14 to 0.01 | −1.79 | 0.073 |
| Prenatal vs Postpartum | 0.14 | −0.50 to 0.78 | 0.42 | 0.678 |
| PSASC, lagged 2 weeks | 0.09 | 0.04 to 0.14 | 3.40 | 0.001 |
| EPDS, lagged 2 weeks | 0.09 | 0.03 to 0.16 | 2.76 | 0.006 |
| ISI, lagged 1 week | 0.11 | 0.06 to 0.15 | 4.49 | <0.001 |
The Wald χ 2 statistic reflects the difference between the tested model and a null model with no predictors. Thus, a significant χ 2 is desirable as it indicates a model that accounts for significant variance in the outcome. obs = observations. Tau path represents the direct path from cognitive arousal to future depression. Alpha path represents part of the indirect (mediated) effect wherein cognitive arousal influences future insomnia. Beta path represents the other part of the indirect (mediated) effect wherein insomnia influences future depression. b = unstandardized parameter estimate; SE = standard error; 95% CI = 95% confidence interval; z = z-statistic; p = significance value. Interindividual factors were recorded at the study baseline, and significant effects represent between-subject differences. Intraindividual factors were recorded weekly across peripartum, and significant effects represent within-subject associations. Lagged 2 weeks indicates Xit−2. Lagged 1 week indicates Xit−1. Snoring was recorded at study entry (Yes = 1, No = 0). EPDS = Edinburgh postnatal depression scale, scores 10 and higher indicate probable minor or major depression. Time represents study week. Prenatal vs Postpartum coded as prenatal = 0 and postpartum =1. EPDS as an intraindividual factor represents depression severity over the prior week. ISI (insomnia severity index) as an intraindividual factor represents insomnia symptom severity over the prior week. PSASC as an intraindividual factor represents nocturnal cognitive arousal over the prior week.
τ path (direct effect)
We regressed depressive symptoms onto nocturnal cognitive arousal (lagged by 2 weeks), while controlling for past depressive symptoms and covariates (Table 4). Results supported a direct effect of nocturnal cognitive arousal on depression 2 weeks later (τ: b = 0.11, p < 0.001). Next, we examined the α and β paths of the indirect effect.
α path
Nocturnal cognitive arousal predicted insomnia a week later (α: b = 0.11, SE = 0.03, p < 0.001).
β path
Insomnia predicted depression a week later (β: b = 0.11, SE = 0.02, p < 0.001). Nocturnal cognitive arousal remained a significant predictor of depression (τ′: b = 0.09, p = 0.001).
αβ path (indirect effect)
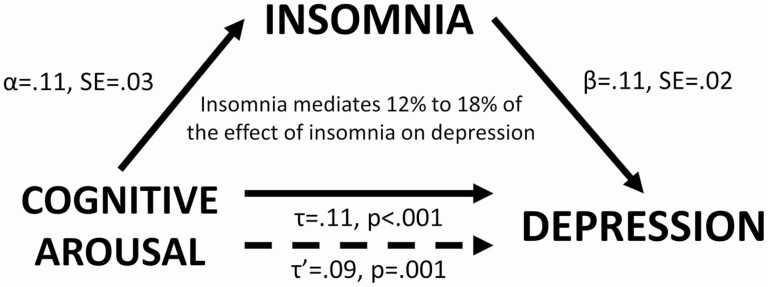
Using the PRODCLIN method, the αβ path was estimated to be 0.013 (SE = 0.013, 97.5% CI = 0.003 to 0.024). Since the CI did not overlap with zero, mediation was supported. Insomnia was estimated to mediate 12% (αβ/τ = 0.013/0.11) to 18% (τ−τ′/τ = [0.11−0.09]/0.09) of the effect of nocturnal cognitive arousal on depression (Figure 3)
Figure 3.
Insomnia mediates the effect of nocturnal cognitive arousal on depression.
Mediation models with perinatal rumination, insomnia, and depression
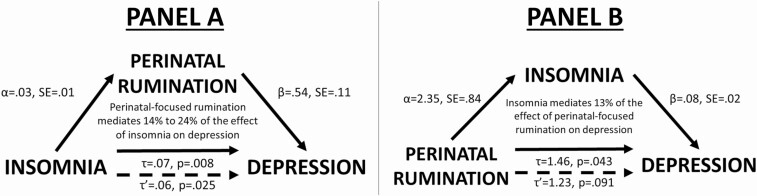
We repeated these analytic steps, replacing nocturnal cognitive arousal with nocturnal PFR (see Supplementary Materials for step-by-step results). PFR was estimated to mediate 14%–24% of the effect of insomnia on depression (Figure 4, Panel A; Supplementary Table S1). In the other direction, results showed that insomnia mediated approximately 13% of the effect of baseline PFR on depression (Figure 4, Panel B; Supplementary Table S2).
Figure 4.
Panel A: Perinatal-focused rumination mediates the effect of insomnia on depression. Panel B: Insomnia mediates the effect of perinatal-focused rumination on depression.
Mediation models with perseverative thinking, insomnia, and depression
We repeated these steps a final time testing perseverative thinking as the index for cognitive arousal (Supplementary Tables S3 and S4). Although perseverative thinking reliably predicted future depression, no temporal associations were observed between insomnia and perseverative thinking. Thus, mediation models were not supported for perseverative thinking.
Do insomnia and cognitive arousal have compounding effects on depression?
Our last hypothesis predicted that odds of depression would be highest when women endorse clinically significant insomnia and high cognitive arousal at the same time. We conducted mixed effects logit models wherein depression status was regressed on three dummy coded variables per cut-points detailed in the Measures section: (1) insomnia only, (2) high cognitive arousal only, and (3) combined insomnia and high cognitive arousal, plus covariates; see Supplementary Materials for step-by-step results.
Nocturnal cognitive arousal and insomnia.
Depression risk increased when women experienced insomnia or high nocturnal cognitive arousal alone, but were highest when experiencing insomnia and high nocturnal cognitive arousal simultaneously.
PFR and insomnia.
When re-running these analyses with PFR, we observed a similar pattern wherein insomnia or rumination alone increased depression risk, but depression risk was greatest when women endorsed insomnia and PFR simultaneously.
Posthoc: Does depression predict cognitive arousal or insomnia? Does bidirectionality exist between daytime and nocturnal cognitive arousal?
Finally, we conducted a series of lagged linear mixed models, which broadly showed that: (1) nocturnal cognitive arousal predicts future insomnia, but depression does not predict future insomnia; (2) depression predicts both future nocturnal and daytime cognitive arousal; and (3) evidence supports a bidirectional relationship between daytime perseverative thinking and nighttime cognitive arousal. Please see Supplementary Materials and Supplementary Table S5 for walkthrough and data.
Discussion
In a sample of 70 women, insomnia and cognitive arousal predicted future depression across late pregnancy and early postpartum. Results showed that insomnia, nocturnal cognitive arousal, PFR, and perseverative thinking were each independently associated with perinatal depression. Critically, bidirectionality was observed between insomnia and nocturnal cognitive arousal, and this toxic cycle fueled perinatal depression. The combination of clinical insomnia and high cognitive arousal corresponded to higher depression risk than insomnia or high cognitive arousal alone. Notably, depression did not directly impact sleep, but did increase cognitive arousal during the day and night. Overall, this study reveals a complex interplay between insomnia and cognitive arousal in their influences on perinatal depression. Cognitive arousal symptoms and insomnia may serve as key targets for the prevention and intervention of perinatal depression.
The role of cognitive arousal in perinatal insomnia
Before delving into the effects of insomnia and cognitive arousal on depression, it is important to first characterize study results that inform our understanding of how insomnia and cognitive arousal relate to one another. Spielman’s 3-P model identifies predisposing factors (i.e. vulnerabilities), precipitating events (e.g. stress exposure), and perpetuating behaviors that lead to the development and maintenance of insomnia [51]. Pregnancy itself has been posited as a precipitating event for insomnia, whereas the tendency for cognitive arousal and perseverative thinking has been proposed as a predisposing factor [15]. That is, while pregnancy is stressful for many women, it elicits especially strong responses in women prone to perseverate on their own health, health of the fetus, finances, lifestyle changes, and so on, during periods of high stress. This cognitive arousal disrupts nightly sleep and contributes to the development of insomnia. The effects of cognitive arousal on insomnia are especially well-documented [28, 52], with evidence showing that cognitive arousal in the evening and night disrupts the onset and maintenance of sleep [38, 39, 41, 53].
The effect of sleep disturbance on nighttime cognitive arousal has received less attention, but even so, prospective data suggest insomnia can trigger cognitive arousal [31]. Results from the present study showing bidirectionality between insomnia and cognitive arousal are significant for two key reasons: (1) The present study is the first to support bidirectionality between insomnia and cognitive arousal in a single sample, perinatal or otherwise. (2) This study is the first to prospectively examine the temporal associations between insomnia and cognitive arousal in perinatal women. Our findings suggest that nocturnal cognitive arousal in peripartum interferes with falling and staying asleep, which includes ruminating on concerns about pregnancy and fetal/infant health. In addition, our data suggest that insomnia can trigger cognitive arousal. That is, when perinatal women lie in bed at night and struggle to sleep, nighttime thoughts during this idleness may wander to concerns and stressful thoughts from earlier in the day (supported by daytime cognitive arousal predicting nocturnal cognitive arousal). Women who worry and ruminate on perinatal- and fetal/infant-related distressing thoughts may be especially vulnerable to difficulty sleeping.
This bidirectionality between insomnia and cognitive arousal may support nocturnal cognitive arousal as a perpetuating behavior in the 3-P model of insomnia. That is, increased cognitive arousal at night in response to insomnia also serves to perpetuate insomnia, thereby creating a toxic cycle. Notably, evidence from this study suggests that cognitive arousal does not appear to have a stronger effect on insomnia than vice versa. Future research is needed to further explore the development and evolution of the insomnia–cognitive arousal cycle and to better characterize the magnitude of effects in each direction. Even so, currently available data, including from this study, are clear that this cycle contributes to perinatal depression.
Does insomnia predict future perinatal depression?
Previous studies offered mixed results regarding the influence of prenatal insomnia on postpartum depression [16, 21–23, 25]. Unlike prior investigations, our prospective analysis examined temporal associations during pregnancy and postpartum at the same time, rather than restrict analyses to testing effects of prenatal sleep on postpartum depression. We observed that higher levels of insomnia augured higher levels of depression, irrespective of whether it was during pregnancy or postpartum. Importantly, the influence of insomnia on future depression was independent of the effects of prior depression, thereby highlighting the robustness of this effect.
Past prospective studies have treated childbirth or the postpartum period as a depression-triggering stressor and insomnia as a trait vulnerability: That is, women with insomnia during pregnancy are more vulnerable to depression triggered during postpartum. Our data offer a simpler characterization: Poor sleep leads to mood disturbances at any point in peripartum. Notably, depression did not increase insomnia, which is consistent with reports showing that the effect of sleep on mood is more robust than in the opposite direction [11, 54–56]. Taken together, these data suggest that insomnia may be a prodrome to depression during peripartum. Yet, given the toxic cycle of insomnia and cognitive arousal outlined in the previous section, cognitive arousal appears to play a critical role in the transaction from insomnia to depression. Thus, our inclusion of cognitive arousal within the perinatal insomnia–depression risk relationship offers a more complex path from insomnia to depression in peripartum.
The depressogenic cycle of insomnia and cognitive arousal
Directionality
Not only do insomnia and cognitive arousal feed into one another, they mediate each other’s effect on depression. These data suggest that when perinatal women have difficulty sleeping, they experience increases in depressive symptoms; however, this may be partly because women spend much of their nocturnal wakefulness perseverating on stressors and perinatal-related concerns. We also observed that nocturnal cognitive arousal and PFR disrupt sleep, and the resulting sleep disturbance partly accounted for the effect of cognitive arousal symptoms on depression. These results are consistent with prospective data from perinatal and nonperinatal samples suggesting that the effects of insomnia on depression are mediated by cognitive–emotional dysregulation [21, 30, 31], but also add to the literature by showing that the effects of cognitive–emotional dysregulation are also mediated by sleep disturbances. Importantly, we have highlighted that cognitive arousal that occurs specifically at night is most germane to the insomnia–depression risk relationship. Taken together, the present study depicted a vicious cycle between insomnia and cognitive arousal that corrodes perinatal mood and fuels depression.
Magnitude
Temporal associations simply show directionality of effects, but do not necessarily lend easy interpretation of magnitude. Important to emphasize here is that mild elevations in nocturnal cognitive arousal can produce transient insomnia, just as a bad night of sleep can trigger ruminative thoughts while lying in bed, which may persist after waking. Combined, a bad night of sleep and a mild episode of ruminating at night will likely negatively influence mood, but have no real lasting effects if the cycle is not sustained. More simply stated, transient elevations can be normal day-to-day experiences. The insomnia–cognitive arousal cycle is not likely to produce clinical consequences until these symptoms reach severe levels that are sustained over time. When the cycle is weak (e.g. transient insomnia triggers mild rumination for one night), the transaction has minimal to no clinical significance. However, when insomnia and cognitive arousal are severe and persist over time, then the cycle becomes highly depressogenic. For a visual depiction of this cycle, please refer to the conceptual model in Supplementary Figure S1.
Women were at a 20-fold increased odds for depression when experiencing clinically significant insomnia symptoms and high nocturnal cognitive arousal, relative to when they reported sleeping well and low levels of arousal. The cycle appears somewhat weakened when only sleep or cognitive–emotional regulation is perturbed. Overall, when experiencing only insomnia or only high cognitive arousal, women were less likely to be depressed than when experiencing high levels of both. Even so, experiencing just insomnia or high cognitive arousal was associated with increased depression risk relative to when sleeping well and having low levels of cognitive arousal. These data are consistent with evidence showing that the prevalence of depression and suicidality is highest among pregnant women with insomnia and high cognitive arousal [5], and with prospective epidemiological data showing that risk for incident major depression is highest among insomniacs with high cognitive arousal, relative to those with only insomnia, only high cognitive arousal, or neither [35].
Clinical implications for prevention and treatment
Women in prenatal care represent a critically important and highly accessible patient population, given the regular appointments throughout pregnancy and early postpartum. If insomnia and high cognitive arousal are prodromes to perinatal depression, then early detection of these symptoms may offer the opportunity for prevention or early intervention. To maximize treatment outcomes for perinatal women receiving insomnia therapy, treatment may need to be bolstered with greater emphasis on cognitive therapy, rumination-focused cognitive behavioral therapy, mindfulness-based, or emotion regulation interventions that successfully defuse cognitive arousal. Please refer to Supplementary Materials for more clinical implications for prevention and treatment.
Limitations
These results should be interpreted in light of important methodological limitations. Chiefly, we collected data across just 4 months of the perinatal period. Following women for longer periods, from early pregnancy to 12 months postpartum, would provide a more thorough depiction of general trends in symptom levels across peripartum and allow for exploring depression incidence and recurrence. Similarly, the sample size is another limitation. While we had sufficient power to detect intraindividual associations, we were likely underpowered for detecting interindividual differences.
The timing between assessments is an important limitation to consider. As discussed in the introduction, testing temporal associations between prenatal sleep and postpartum depression is not optimal. Along these lines, weekly assessments may not be optimal either, even if more sensitive. Rather, these processes likely occur both within a night (I ruminate tonight, so I have difficulty falling asleep tonight) and day-to-day (I slept poorly last night and ruminated in bed, so my mood is more negative today), with compounding effects across longer periods when cycles are sustained. Thus, although we showed that weekly assessments provide sufficient resolution to capture these temporal associations, we may have underestimated the magnitude of these effects. Prospective observational studies with nightly/daily assessments would likely better capture nuances and association strength.
Somewhat along these lines, our study findings should be replicated in the laboratory to better understand causation, characterize the timing of effects, and explore psychophysiological mechanisms that may facilitate these effects. Inducing cognitive arousal or insomnia in the laboratory can demonstrate how these experiences affect one another and even influence mood upon waking the next day. Important to emphasize here, however, is that multiple components of our model (but not all) have been tested and supported in laboratory experiments in nonperinatal samples (e.g. inducing cognitive–emotional dysregulation triggers symptoms of insomnia [39, 53, 57] and depression [58]).
Lastly, the measurement of PFR in the present study consisted of a single appended item to the PSASC. This has several methodological limitations, which include limited variance inherent to single-item measures, an inability to separate shared construct and methodological variance between PFR and PSASC in the present study, and an exclusive focus on PFR that occurs at night. Measurement of PFR deserves its own line of inquiry to develop valid and reliable measures of this construct with emphasis on nocturnal and daytime phenomena. While we are reasonably confident in our study findings pertaining to PFR as they are rather consistent with patterns observed when analyzing more broadly focused nocturnal cognitive arousal in the present study and prior research [5], these results should nevertheless be considered tentative in nature and further explored in future studies using more sophisticated measures.
Conclusions
Pregnancy and early postpartum are stressful periods. Perinatal women endorse high levels of cognitive arousal, which includes ruminating on pregnancy and fetal/infant health. Cognitive arousal symptoms persist into the night and disrupt sleep. Moreover, difficulty falling and staying asleep also provides ample opportunity for nocturnal cognitive arousal. This cycle between insomnia and cognitive arousal fuels perinatal depressive symptoms. Fortunately, insomnia and cognitive arousal are modifiable with psychotherapy. Interventions to prevent or maximally treat perinatal depression should improve sleep quality while teaching patients adaptive strategies for coping with stress and defusing negative repetitive thoughts, particularly while trying to sleep in bed at night.
Supplementary Material
Institution where the work was performed: Thomas Roth Sleep Disorders & Research Center, Henry Ford Health System, Detroit, Michigan, USA.
Funding
This study was funded by the American Academy of Sleep Medicine (198-FP-18, PI: Kalmbach). P.C.’s effort was supported by the National Heart, Lung, and Blood Institute (K23-HL13866, PI: Cheng).
Disclosure Statement
Financial disclosure: P.C. has received research support from Harmony Biosciences. C.L.D. has received research support from Merck & Co. and has served on the speaker’s bureau for Harmony Biosciences. No other financial or nonfinancial interests exist.
Nonfinancial disclosure: The authors have no nonfinancial disclosures to report.
Conflict of interest statement. None declared.
References
- 1. Banti S, et al. From the third month of pregnancy to 1 year postpartum. Prevalence, incidence, recurrence, and new onset of depression. Results from the perinatal depression-research & screening unit study. Compr Psychiatry. 2011;52(4):343–351. [DOI] [PubMed] [Google Scholar]
- 2. Woody CA, et al. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86–92. [DOI] [PubMed] [Google Scholar]
- 3. Gavin NI, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–1083. [DOI] [PubMed] [Google Scholar]
- 4. Field T. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav Dev. 2010;33(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalmbach DA, et al. Depression and suicidal ideation in pregnancy: exploring relationships with insomnia, short sleep, and nocturnal rumination. Sleep Med. 2020;65:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blackmore ER, et al. Previous prenatal loss as a predictor of perinatal depression and anxiety. Br J Psychiatry. 2011;198(5):373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blackmore ER, et al. Pregnancy-related anxiety: evidence of distinct clinical significance from a prospective longitudinal study. J Affect Disord. 2016;197:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dayan J, et al. Developmental model of depression applied to prenatal depression: role of present and past life events, past emotional disorders and pregnancy stress. PLoS One. 2010;5(9):e12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Viktorin A, et al. Heritability of perinatal depression and genetic overlap with nonperinatal depression. Am J Psychiatry. 2016;173(2):158–165. [DOI] [PubMed] [Google Scholar]
- 10. DeJong H, et al. Rumination and postnatal depression: a systematic review and a cognitive model. Behav Res Ther. 2016;82:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohayon MM, et al. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37(1):9–15. [DOI] [PubMed] [Google Scholar]
- 12. Baglioni C, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–19. [DOI] [PubMed] [Google Scholar]
- 13. Kalmbach DA, et al. Insomnia, short sleep, and snoring in mid-to-late pregnancy: disparities related to poverty, race, and obesity. Nat Sci Sleep. 2019;11:301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D⊘rheim SK, et al. Insomnia and depressive symptoms in late pregnancy: a population-based study. Behav Sleep Med. 2012;10(3):152–166. [DOI] [PubMed] [Google Scholar]
- 15. Swanson LM, et al. Perinatal insomnia and mental health: a review of recent literature. Curr Psychiatry Rep. 2020;22(12):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sedov ID, Tomfohr-Madsen LM. Trajectories of insomnia symptoms and associations with mood and anxiety from early pregnancy to the postpartum. Behav Sleep Med. 2020:1–12. doi: 10.1080/15402002.2020.1771339 [DOI] [PubMed] [Google Scholar]
- 17. Sivertsen B, et al. Trajectories of maternal sleep problems before and after childbirth: a longitudinal population-based study. BMC Pregnancy Childbirth. 2015;15:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Richter D, et al. Long-term effects of pregnancy and childbirth on sleep satisfaction and duration of first-time and experienced mothers and fathers. Sleep. 2019;42(4). doi: 10.1093/sleep/zsz015 [DOI] [PubMed] [Google Scholar]
- 19. Dørheim SK, et al. Sleep and depression in postpartum women: a population-based study. Sleep. 2009;32(7):847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Emamian F, et al. Link between insomnia and perinatal depressive symptoms: a meta-analysis. J Sleep Res. 2019;28(6):e12858. [DOI] [PubMed] [Google Scholar]
- 21. Marques M, et al. Is insomnia in late pregnancy a risk factor for postpartum depression/depressive symptomatology? Psychiatry Res. 2011;186(2–3):272–280. [DOI] [PubMed] [Google Scholar]
- 22. Okun ML, et al. Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. J Affect Disord. 2011;130(3):378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dørheim SK, et al. Can insomnia in pregnancy predict postpartum depression? A longitudinal, population-based study. PLoS One. 2014;9(4):e94674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osnes RS, et al. Mid-pregnancy insomnia and its association with perinatal depressive symptoms: a prospective cohort study. Behav Sleep Med. 2020:1–18. doi: 10.1080/15402002.2020.1743705 [DOI] [PubMed] [Google Scholar]
- 25. Okun ML, et al. Sleep complaints in late pregnancy and the recurrence of postpartum depression. Behav Sleep Med. 2009;7(2):106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Samtani S, et al. Assessing maladaptive repetitive thought in clinical disorders: a critical review of existing measures. Clin Psychol Rev. 2017;53:14–28. [DOI] [PubMed] [Google Scholar]
- 27. Harvey AG, et al. Cognitive approaches to insomnia. Clin Psychol Rev. 2005;25(5):593–611. [DOI] [PubMed] [Google Scholar]
- 28. Pillai V, et al. Sleep and repetitive thought: the role of rumination and worry in sleep disturbance. Sleep and Affect. London, UK: Elsevier; 2015: 201–225. [Google Scholar]
- 29. Drake CL, et al. Stress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomnia. Sleep. 2014;37(8):1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Batterham PJ, et al. Sleep disturbance, personality and the onset of depression and anxiety: prospective cohort study. Aust N Z J Psychiatry. 2012;46(11):1089–1098. [DOI] [PubMed] [Google Scholar]
- 31. Danielsson NS, et al. Sleep disturbance and depressive symptoms in adolescence: the role of catastrophic worry. J Youth Adolesc. 2013;42(8):1223–1233. [DOI] [PubMed] [Google Scholar]
- 32. Moberly NJ, et al. Ruminative self-focus and negative affect: an experience sampling study. J Abnorm Psychol. 2008;117(2):314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martini J, et al. A prospective-longitudinal study on the association of anxiety disorders prior to pregnancy and pregnancy- and child-related fears. J Anxiety Disord. 2016;40:58–66. [DOI] [PubMed] [Google Scholar]
- 34. Swanson LM, et al. Relationships among depression, anxiety, and insomnia symptoms in perinatal women seeking mental health treatment. J Womens Health (Larchmt). 2011;20(4):553–558. [DOI] [PubMed] [Google Scholar]
- 35. Kalmbach DA, et al. Nocturnal insomnia symptoms and stress-induced cognitive intrusions in risk for depression: a 2-year prospective study. PLoS One. 2018;13(2):e0192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Evans J, et al. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323(7307):257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kalmbach DA, et al. Insomnia symptoms and short sleep predict anxiety and worry in response to stress exposure: a prospective cohort study of medical interns. Sleep Med. 2019;55:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pillai V, et al. A seven day actigraphy-based study of rumination and sleep disturbance among young adults with depressive symptoms. J Psychosom Res. 2014;77(1):70–75. [DOI] [PubMed] [Google Scholar]
- 39. Zoccola PM, et al. Rumination predicts longer sleep onset latency after an acute psychosocial stressor. Psychosom Med. 2009;71(7):771–775. [DOI] [PubMed] [Google Scholar]
- 40. Galbiati A, et al. Repetitive thought is associated with both subjectively and objectively recorded polysomnographic indices of disrupted sleep in insomnia disorder. Sleep Med. 2018;45:55–61. [DOI] [PubMed] [Google Scholar]
- 41. Kalmbach DA, et al. Nocturnal cognitive arousal is associated with objective sleep disturbance and indicators of physiologic hyperarousal in good sleepers and individuals with insomnia disorder. Sleep Med. 2020;71:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 43. Cox JL, et al. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782–786. [DOI] [PubMed] [Google Scholar]
- 44. Bastien CH, et al. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 45. Morin CM, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nicassio PM, et al. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther. 1985;23(3):263–271. [DOI] [PubMed] [Google Scholar]
- 47. Puzino K, et al. Am I (hyper)aroused or anxious? Clinical significance of pre-sleep somatic arousal in young adults. J Sleep Res. 2019;28(4):e12829. [DOI] [PubMed] [Google Scholar]
- 48. Ehring T, et al. The Perseverative Thinking Questionnaire (PTQ): validation of a content-independent measure of repetitive negative thinking. J Behav Ther Exp Psychiatry. 2011;42(2):225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Singer JD, et al. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- 50. Tofighi D, et al. RMediation: an R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43(3):692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spielman AJ, et al. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541–553. [PubMed] [Google Scholar]
- 52. Harvey AG. Pre‐sleep cognitive activity: a comparison of sleep‐onset insomniacs and good sleepers. Br J Clin Psychol. 2000;39(3):275–286. [DOI] [PubMed] [Google Scholar]
- 53. Wuyts J, et al. The influence of pre-sleep cognitive arousal on sleep onset processes. Int J Psychophysiol. 2012;83(1):8–15. [DOI] [PubMed] [Google Scholar]
- 54. Kalmbach DA, et al. Effects of sleep, physical activity, and shift work on daily mood: a prospective mobile monitoring study of medical interns. J Gen Intern Med. 2018;33(6):914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jansson-Fröjmark M, et al. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J Psychosom Res. 2008;64(4):443–449. [DOI] [PubMed] [Google Scholar]
- 56. Johnson EO, et al. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700–708. [DOI] [PubMed] [Google Scholar]
- 57. Chen IY, et al. Investigating psychological and physiological responses to the Trier Social Stress Test in young adults with insomnia. Sleep Med. 2017;40:11–22. [DOI] [PubMed] [Google Scholar]
- 58. Nolen-Hoeksema S, et al. Rethinking rumination. Perspect Psychol Sci. 2008;3(5):400–424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.