Highlights
-
•
The ultrasound can release of bioactive compounds of the crystallized matrix of honey.
-
•
Some honeys liquefied with ultrasound showed increases in the bioactive compounds.
-
•
The treatment with ultrasound increased inhibitory effect against S. Typhimurium in some honeys.
Keywords: Honey, Bacterial inhibition, Phenolic compounds, Ultrasound
Abstract
The effect of ultrasound on the crystal size, phenols, flavonoids, Maillard products and antibacterial activity of crystallized honeys was studied. Three multifloral honeys (M), one monofloral (MO) and one honeydew (HD) honey were used. Ultrasound was performed at 42 kHz for different times (0, 5, 10 and 15 min). The antibacterial activities were tested against Salmonella typhimurium, Bacillus subtilis, Pseudomonas aeruginosa, Listeria monocytogenes, Staphylococcus aureus and Escherichia coli. In all honeys, the parameters analyzed had significant differences ((P < 0.05)). After 15 min of ultrasound the HD had increments of 44 mg of gallic acid/100 g of honey in phenols, and some M showed increase in flavonoids (5.64 mg of quercitin /100 g of honey) and improvement in inhibition against Salmonella typhimurium was 13.1%. In some honeys the correlation between phenols or flavonoids and antibacterial activity were significant ((P < 0.05)). No correlation was found between Maillard products and antibacterial activity. The ultrasound treatment effect on the crystal size, phenols, flavonoid, Maillard products, and antibacterial activity of crystallized honeys were different in each honey.
1. Introduction
Honey has biological properties such as antibacterial activity. Voidarou et al. [1] reported that all honeys had antibacterial activity regardless of botanical origin (coniferous, citrus, thyme or multifloral). Alvarez-Suarez et al. [2] found that honey has varying antibacterial effects, which can be attributed to the phenolic content of each honey. Noori et al. [3] reported the antimicrobial effects of honeys with different concentrations of phenols, flavonoids and hydrogen peroxide. The honeys show a strong correlation exists between the bacterial inhibitory capacity and polyphenol content in honey [4], [5].
The crystallization of honey is an undesirable process because it affects the texture properties and the appearance of honey as a liquid and transparent product [6], [7]. Furthermore, crystallization has a negative influence on honey because it promotes the growth of fungi and yeast [8]. Pimentel-Gonzalez et al. [9] found that the effect of thermal treatment on the antibacterial activity of honey depends on its botanical origin and the type of bacteria to be inhibited. Bucekova et al. [10] found that the thermal liquefaction of crystallized honeys sometimes increases the antibacterial activities depending on their botanical origin.
In recent years, the use of emerging technology and its application in the food industry has been studied. The ultrasound processing slows honey crystallization and removes most of the yeasts present in honey [11]. Kuś and Jerković [12] mentions that the ultrasound process is very suitable for the extraction of volatile and semi-volatile compounds present in honey. In addition, the bioactive compounds in honey are not affected during ultrasound processing [13]. Kabbani, et al. [6] showed that ultrasound treatment accelerates the liquefaction of the honey, especially at temperatures below 50° C. Quintero-Lira et al. [14] and Önür et al. [15] found that the liquefaction of crystallized honey by an ultrasound process does not produce a significant increase in hydroxymethylfurfural.
The objective of the present study was to determine if ultrasound at different times influences the characteristics of crystallized honeys, such as phenols, total flavonoids, maillard reaction products, crystal size and antibacterial activities
2. Materials and methods
2.1. Samples of honey
The crystalized honeys were recollected from different regions of Hidalgo State, Mexico, in August of 2018. The multiflower honeys were from Acaxochitlan (M1), Arenal (M2), Huhuetla (M3), monoflower from Orizatlan (MO) and honeydew from Tasquillo (HD). (Table 1). These honeys were analyzed by microscopy and frequency determination of the classes of pollen. Each sample was collected in a sterile container and weighing 500 g were packet and sealed in amber glass bottles and stored at 5 0C in the dark until tested [16].
Table 1.
Botanical source of the honeys from Hidalgo State, Mexico.
| Honey | Geographical zone of Hidalgo, Mexico | Botanical source |
|---|---|---|
| Multifloral 1 (*M1) | Acaxochitlan | Gramineae sp 29.1%, Mirtaceae: Eucalyptus 5.4%, Mercurialis sp 6.9%, Cupressaceae 7%: Cupressus lusitánica, Juniperus fláccida, and Ericacea sp. 7.2% |
| Multifloral 2 (M2) | Arenal | Arecaceae sp. 21.5%, Convolvulaceae sp.13.9%. |
| Multifloral 3 (M3) | Huehuetla | Quercus sp.11.2%, Ricinus communis 10.4%, Rubiceas sp. 12.9%. |
| Monofloral (M0) | Orizatlan | Citrus sinesis 12%.: Quercus sp, Ricinus communis, Papilionoideae sp, |
| Honeydew (HD) | Tasquillo | Juglans sp. 20.9%-others: Crucifers sp, Ericacea sp. and Mercurialis sp. |
2.2. Ultrasound treatment
3 g of each of the honey samples were placed in a 20 mL test tube at 20 °C. After, they were processed with ultrasound during 5, 10 or 15 min into an ultrasonic bath (Branson 3510, Connecticut, USA) at 42 kHz.
2.3. Crystal size
The crystals were measure using the technique described by Kabbani et al. [6] with some modifications. One gram of honey was put on slid at Olympus CX31 microscope (JP) coupled to camera Infinity 1-2C, the size of 30 crystals of each honey were measured in µm2, 30 images were captured for sample and the measurements were realized with Image-pro plus (USA).
2.4. Phenolic compounds
The technique Folin-Ciocalteu [17] was used to quantify the contained of total phenols. Five grams of honey were diluted with distilled water (1:5 w/v), homogenized and centrifuged at 17,500 × g for 15 min at 4 °C in a centrifuge Z 36 HK (HERMLE Labortechnik GmbH, Wehingen, Germany). From supernatant, 0.5 mL was mixed with 2.5 mL of the Folin-Ciocalteu reagent 0.2 N, after 5 min at rest, 2 mL of a sodium carbonate solution (7.5% w/v) were added. The mixture was mixed vigorously and allowed to stand for 2 h in the dark. The mixture was read at 760 nm in a spectrophotometer (Jenway, 6715 UV/vis.). The results were expressed as mg of gallic acid equivalents (Fermont, MX) per 100 g of honey (mg GAE / 100 g).
2.5. Total flavonoids
The flavonoids were analyzed according the technique reported by Sancho et al. [18] with some modifications Medina-Pérez et al. [19]. One gram of honey was diluted (1:10) with methanol (Femont, MX), homogenized and centrifuged at 17,500 × g for 15 min at 4 °C. Two mL of supernatant were mixed with two mL of aluminum trichloride (2% with methanol) (Femont, MX). The mix was rested for 15 min in the dark and read at 415 nm in spectrophotomer (Jenway, 6715 UV/vis.). The results were expressed as mg of quercetin equivalents (Sigma-Aldrich, USA) per 100 g of honey (mg QE/100 g).
2.6. Products of the Maillard reaction
The melanoidin content was determined in 50% honey aqueous solution using a spectrophotometry technique measured the net absorbance. The read (absorbance) was done at 450 nm [20]. The melanoidin content was expressed in absorption units.
2.7. Antibacterial activity
Three gram-negative bacteria (Escherichia coli ATCC 25922; Salmonella typhimurium ATCC 43971; and Pseudomonas aeruginosa, ATCC 27853), and three gram-positive bacteria (Bacillus subtilis ATCC 6630, Staphylococcus aureus ATCC 13709; and Listeria monocytogenes ATCC 15313) were used the antibacterial activity of honey.
The bacteria were activated in nutrient broth at 37 °C, the activation time was as follows: E. coli for 9 h, S. aureus 20 h, while B. subtilis, P. aeruginosa and L. monocytogenes were incubated for 24 h. Obtaining a final concentration of 107 colony forming units per mL (CFU / mL). An 8.5% (w/v) honey solution was prepared with deionized water. In a tube, 1 mL of the activated culture (106 CFU/mL) was placed in 9 mL of the honey solution and they were homogenized. The time of exposition was fifteen minutes. In a sterile petri dish (100 × 15 mm), 1 mL of the honey dilution was placed with the culture, and 20 mL of the culture medium, they were homogenized with horizontal movements and left to rest until they solidified. All strains were incubated at 37 °C for 24 h. Each strain was grown in specific medium. The eosin methylene blue agar for E. coli, agar S. aureus for S. aureus, agar Salmonella and Shigella with S. typhimurium and agar nutritive for B. subtillis, L. monocytogenes and P. aeruginosa were used. Sterile saline solution was used as negative control and ciprofloxacin as positive control. All tests were done in triplicate. The inhibition percentage was calculated as follows:
% inhibition = ((Concentration initial (CFU/mL) - Concentration final (CFU/mL) / Concentration initial (CFU/mL)) * 100.
2.8. Statistical analysis
The experimental design was completely randomized. All tests were performed in triplicate. When significant differences were found (P < 0.05) between treatment, the technique of Tukey’s was used to compare the means with a significantly of (P < 0.05). The correlation was done using Pearson correlation. The analyses were done with the statistical program SPSS (version 20) software.
3. Results and discussion
3.1. Crystal size
In table 2, it is observed that the crystal size presents a significant difference (P < 0.05) between the honey samples, the M2 honey sample is the largest (276.3 µm). Bakier [21] reported that the crystal size depends on the type of honey. The reduction of crystal size by ultrasound was different in each honey. The greatest reduction percentage was observed in the honey HD (68.3%). Kabbani et al. [6] and Quintero et al. [14] found a reduction in the crystal size of different honeys upon ultrasound treatment. Ultrasound treatment reduces crystal sizes through the application of sound waves [22].
Table 2.
Effect of ultrasound treatments (5, 10, 15 min) on the size of the crystallized honey crystal size.
| Crystal size (µm2) | |||||
|---|---|---|---|---|---|
| Ultrasound time (minutes) | M1 | M2 | M3 | MO | HD |
| 0 | 138.6 ± 18.2a,A | 276.3 ± 21.3a,C | 202.8 ± 18.4a,B | 233.6 ± 18.4a,B | 132.9 ± 19.3a,A |
| 5 | 128.9 ± 17.7ab,A | 118.7 ± 19.7b,C | 185.4 ± 19.4a,B | 211.9 ± 17.9ab,BC | 110.9 ± 17.2a,A |
| 10 | 114.9 ± 19.3ab,B | 181.8 ± 16.5c,C | 142.1 ± 13.3b,B | 200.7 ± 16.1ab,C | 71.4 ± 15.3b,A |
| 15 | 95.4 ± 19.3ab,B | 162.1 ± 15.4c,C | 109.3 ± 14.2bB | 184.3 ± 17.6b,C | 42.1 ± 9.4b,A |
Multifloral 1 (M1), multifloral 2 (M2), multifloral 3 (M3), monofloral (MO) and honeydew (HD). different lowercase letters represent a significant difference (P < 0.05) within the column (among time) as determined by tukey’s comparison of averages. different uppercase letters represent a significant difference (P < 0.05) within the row (among honeys) as determined by tukey’s comparison of averages. all determinations were performed in triplicate.
3.2. Bioactive compounds
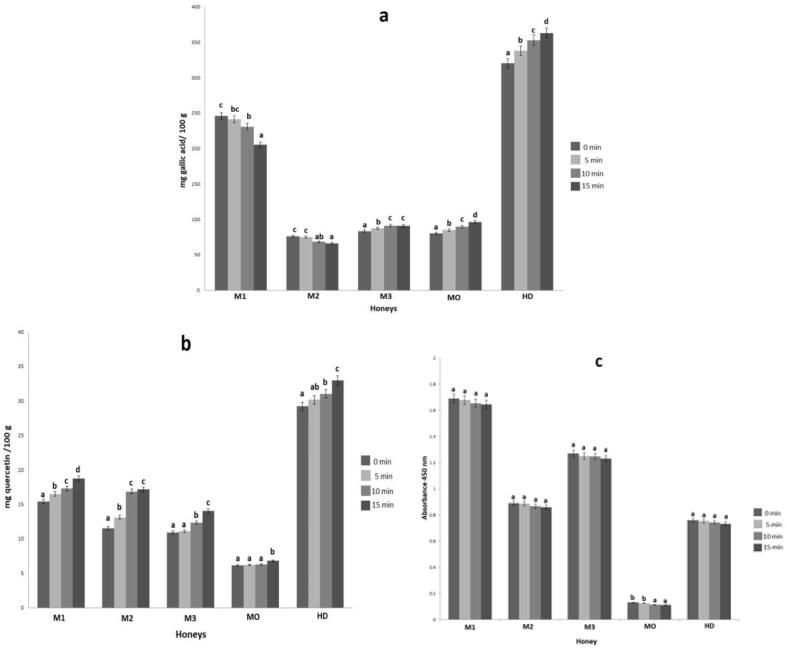
The concentration of total phenols in the crystallized honeys and the honeys processed with ultrasound showed significant differences ((P < 0.05)) as evident in Fig. 1. The M3, MO and HD honeys showed an increase of total phenols since 5 min (Fig. 1a). Stojkovic et al. [23] mentions that ultrasound treatment increases the content of total phenols and the antioxidant capacity of honey, compared to conventional heat treatment. The flavonoid content increased in all samples since 15 min (Fig. 1b). Chaikman et al. [24] also observed that the ultrasound process increased the contents of phenols and flavonoids in honey. The ultrasound process increased the amounts of organic compounds extracted, such as volatile compounds [25]. The Maillard products showed a reduction in all honeys, but the differences were not significant (P > 0.05) (Fig. 1c). Shirahashi et al. [26] reported that the final stages of the Maillard reaction produce a melanoidin polymer. Ultrasound reduces the organic polymer [27]; as a consequence, the application of ultrasound to honey reduces Maillard products.
Fig. 1.
Effect of the ultrasound a different time (0. 5, 10 and 15 min) in different crystallized honeys (M1, M2, M3, MO and HD) in their antibacterial compounds: a) total phenols, b) total flavonoids and c) Maillard products.
3.3. Antibacterial activities
The crystallized honey solution to 8.5%, showed significant differences (P < 0.05) in the inhibition of Escherichia coli. Honey HD had the greatest effect (Table 3). Moussa et al. [28] reported the inhibitory effect of different honeys against E. coli. The effect of the ultrasound on each honey was different. The honey M3 showed a significant (P < 0.05) increase in inhibitory effect after ultrasound treatment. Plaza et al. [29] reported the extraction of active compounds that inhibit the growth of E. coli by ultrasound. All the crystallized honeys to 8.5% inhibited Salmonella typhimurium, and the MO and HD honeys had the strongest effect, achieving 94% inhibition (Table 3). These results are similar to those of Taormina et al.[30]; Mundo et al. [31] who found that honeys of different botanical origins exhibited antibacterial activity against S. typhimurium. Only the honey HD did not show a significant increase (P > 0.05) inhibition with ultrasound treatment. Damyeh et al. [32] reported the use of ultrasound to extract essential oils from Prangos ferulácea and Satureja macrosiphonia that had an inhibitory effect on Salmonella typhimurium.
Table 3.
Effect of the time in ultrasound treatment (5, 10, 15 min) in different crystallized honey in the % inhibition of negative gram bacteria.
| Ultrasound Time (minutes)/Honey | M1 | M2 | M3 | MO | HD |
|---|---|---|---|---|---|
| Escherichia coli | |||||
| 0 | 83.1 ± 1.7a,A | 90.1 ± 3.4a,BC | 87.1 ± 1.7a,AB | 90.1 ± 1.7a,BC | 95.05 ± 3.4a,C |
| 5 | 83.2 ± 1.2a,A | 90.0 ± 2.0a,B | 89.5 ± 1.6ab,B | 90.7 ± 3.3a,B | 94.7 ± 1.5a,B |
| 10 | 81.7 ± 2.5a,A | 89.1 ± 1.3a,B | 92.7 ± 1.3bc,B | 90.9 ± 1.5a,BC | 94.4 ± 1.8ª,B |
| 15 | 79.5 ± 1.3a,A | 89.3 ± 2.2a,B | 93.9 ± 0.9c,CD | 91.5 ± 3.3a,B | 95.1 ± 1.0a,D |
| Salmonella typhimurium | |||||
| 0 | 80.8 ± 1.2a,A | 89.3 ± 1.7a,BC | 86.2 ± 1.8a,AB | 94.7 ± 1.8a,C | 94.6 ± 1.7a,C |
| 5 | 81.3 ± 1.5a,A | 98.3 ± 1.4b,B | 97.6 ± 1.1b,B | 96.7 ± 1.4ab,B | 94.3 ± 1.5a,B |
| 10 | 86.2 ± 1.4b,A | 99.1 ± 1.5b,B | 98.4 ± 1.4b,B | 98.4 ± 1.4ab,B | 95.1 ± 1.6a,B |
| 15 | 88.2 ± 1.9b,A | 99.2 ± 1.3b,B | 99.2 ± 1.5b,B | 99.2 ± 1.3b,B | 95.9 ± 1.8a,B |
| Pseudomona aeruginosa | |||||
| 0 | 96.9 ± 1.1a,B | 99.2 ± 0.9a,B | 97.6 ± 1.5a,B | 98.6 ± 0.7a,B | 78.8 ± 1.1a,A |
| 5 | 98.8 ± 0.9ab,B | 99.4 ± 1.4a,B | 97.8 ± 0.8a,B | 98.3 ± 1.1a,B | 78.1 ± 0.9a,A |
| 10 | 99.4 ± 1.2ab,B | 99.5 ± 0.9a,B | 98.0 ± 0.9a,B | 98.9 ± 1.2a,B | 78.6 ± 1.2a,A |
| 15 | 99.8 ± 0.9b,B | 99.7 ± 0.8a, B | 98.3 ± 1.2a,B | 99.1 ± 0.9a,B | 79.7 ± 0.8a,A |
Multifloral 1 (M1), multifloral 2 (M2), multifloral (M3), monofloral (MO) and honeydew (HD). Different lowercase letters represent a significant difference ((P < 0.05)) within the column (among time) as determined by Tukeýs comparison of average. Different uppercase letters represent a significant difference ((P < 0.05)) within the row (among honeys) as determined by Tukeýs comparison of averages.
In contrast, the crystallized honey to 8.5% with the least inhibition of Pseudomonas aeruginosa was the HD (Table 3). Honeys of diverse countries have shown the inhibitory effect of these bacteria [33], [34], [35]. Only the honey M1 had a significant increase (P < 0.05) in antibacterial activity after 15 min of ultrasound treatment. All crystallized honeys to 8.5% had an effect against Listeria monocytogenes, and M1 honey had the greatest inhibitory effect (99%). Elbanna et al. [36] and Rodriguez et al. [37] also reported the effect of different honeys on Listeria monocytogenes. The M2, M3, MO and HD honeys showed significant increases (P < 0.05) in antibacterial activity against Listeria monocytogenes after ultrasound treatment. Salarbashi et al. [38] found that the application of ultrasound to essential oils from Achillea biebersteinii and Achillea wilhelmsii increased the bioactive compounds and the inhibitory effect on these bacteria.
The inhibitory effect of Staphylococcus aureus differed significantly (P < 0.05) between the honeys. The HD honey had the least effect (Table 4), and the same antibacterial activity was found by Bueno-Costa et al. [39] with honeys of different floral origins. The ultrasound treatment had no significant effect (P > 0.05) on the M1, M2, MO and HD honeys. Again, there are reports of the increased extraction of bioactive compounds with inhibitory effects on Staphylococcus aureus using ultrasound [40]. All multifloral M1, M2 and M3 honeys had significant increases (P < 0.05) in the inhibitory effect with the ultrasound process in Bacillus subtilis (Table 4). Honeys from New Zealand [41] exhibited similar inhibitory effects against Bacillus subtilis. The Ultrasounds assistance increased the extraction of antibacterial compounds in Cyclocarya paliurus against Bacillus subtilis [42].
Table 4.
Effect of the time in ultrasound treatment (5, 10, 15 min) in different crystallized honey in the % inhibition of positive gram bacteria.
| Ultrasound Time (minutes)/Honey | M1 | M2 | M3 | MO | HD |
|---|---|---|---|---|---|
| Listeria monocytogenes | |||||
| 0 | 99.0 ± 1.7a,C | 82.7 ± 1.1a,A | 94.2 ± 0.9a,B | 84.6 ± 1.7ab,A | 93.3 ± 1.6a,B |
| 5 | 99.2 ± 1.5a,C | 87.1 ± 1.2b,B | 97.6 ± 1.1b,D | 84.1 ± 1.4a,A | 94.3 ± 1.8a,C |
| 10 | 97.5 ± 1.5a,B | 88.7 ± 1.3b,A | 98.4 ± 1.3b,B | 87.3 ± 1.3b,A | 96.3 ± 1.9ab,B |
| 15 | 97,1 ± 1.9a,B | 90.3 ± 1.4bAB | 98.5 ± 1.1b,C | 88.1 ± 1.6b,A | 98.4 ± 1.4b,C |
| Staphylococcus aureus | |||||
| 0 | 98.1 ± 1.8a,B | 98.6 ± 1.5a,B | 99.1 ± 1.7a,AB | 99.5 ± 1.1a,B | 77.3 ± 1.7a,A |
| 5 | 97.6 ± 1.6a,B | 99.0 ± 1.4aB | 97.2 ± 1.4ab,B | 98.1 ± 1.4a,B | 77.5 ± 1.5a,A |
| 10 | 97.7 ± 1.3a,BC | 99.1 ± 1.6a,C | 94.4 ± 1.4bc,B | 98.7 ± 1.4a,C | 78.4 ± 1.6a,A |
| 15 | 97.1 ± 1.9a,C | 98.9 ± 1.4a,C | 92.5 ± 1.6cB | 99.1 ± 1.3a,C | 79.4 ± 1.9a,A |
| Bacillus subtillis | |||||
| 0 | 94.4 ± 1.5a,C | 73.2 ± 1.5a,A | 81.7 ± 1.2a,B | 95.8 ± 2.1a,C | 71.1 ± 1.8,A |
| 5 | 96.8 ± 1.2ab,C | 74.3 ± 1.2a,A | 84.8 ± 1.1b,B | 96.1 ± 1.9a,C | 72.2 ± 1.9a,A |
| 10 | 98.7 ± 1.2b,D | 79.2 ± 1.8b,B | 87.4 ± 0.9c,C | 98.7 ± 1.2a,D | 70.1 ± 2.2a,A |
| 15 | 99.7 ± 1.1b,D | 81.3 ± 2.0b,B | 89.4 ± 1.2c,C | 99.3 ± 1.8a,D | 68.9 ± 1.8a,A |
Multifloral 1 (M1), multifloral 2 (M2), multifloral (M3), monofloral (MO) and honeydew (HD). Different lowercase letters represent a significant difference (P < 0.05) within the column (among time) as determined by Tukeýs comparison of average. Different uppercase letters represent a significant difference (P < 0.05) within the row (among honeys) as determined by Tukeýs comparison of averages.
3.4. Correlations
The Pearsońs correlations did not show significant correlation (P < 0.05) between phenols, flavonoids, and Maillard products and the effect on gram-negative and gram-positive bacteria of M1, MO and HD honeys (Table 5). The M2 honey showed significant correlations (P < 0.05) between phenols and Salmonella typhimurium and between flavonoids and Bacillus subtilis. The M3 honey showed significant correlations (P < 0.05) between phenols and Salmonella typhimurium and Listeria monocytogenes and between flavonoids and Escherichia coli. Shan et al. [43] reported correlations between the phenols of medical plants and their effects on Listeria monocytogenes, Escherichia coli, and Salmonella.
Table 5.
Correlations between phenols, flavonoids, maillard products and the inhibitory effect against negative and positive gram bacteria.
| Salmonella typhimurium | Escherichia coli | Pseudomona aeruginosa | Bacillus subtilis | Staphylococcus aureus | Listeria monocytogenes | |
|---|---|---|---|---|---|---|
| M1 Correlation coefficients | ||||||
| Phenols | 0.532 | −0.37 | 0.866 | 0.834 | −0.834 | −0.331 |
| Flavonoids | 0.942 | −0.993 | 0.834 | 0.68 | −0.103 | −0.99 |
| Maillard products | −0.985 | 0.902 | −0.944 | −0.911 | 0.209 | 0.87 |
| M2 Correlation coefficients | ||||||
| Phenols | 0.980* | −0.471 | 0.943 | 0.524 | 0.679 | 0.743 |
| Flavonoids | 0.834 | −0.952 | 0.896 | 0.959* | 0.232 | 0.934 |
| Maillard products | −0.996 | 0.649 | −0.992 | −0.698 | −0.563 | −0.839 |
| M3 Correlation coefficients | ||||||
| Phenols | 0.997* | 0.829 | 0.546 | 0.852 | −0.779 | 0.977* |
| Flavonoids | 0.88 | 0.978* | 0.903 | 0.836 | −0.984 | 0.712 |
| Maillard products | −0.882 | −0.705 | −0.677 | −0.544 | 0.708 | −0.79 |
| MO Correlation coefficients | ||||||
| Phenols | 0.916 | 0.817 | 0.297 | 0.703 | −0.437 | 0.582 |
| Flavonoids | −0.38 | −0.656 | 0.383 | −0.1 | 0.944 | 0.041 |
| Maillard products | −0.822 | −0.704 | −0.12 | −0.549 | 0.539 | −0.411 |
| HD Correlation coefficients | ||||||
| Phenols | 0.393 | −0.479 | 0.114 | −0.255 | 0.651 | 0.738 |
| Flavonoids | 0.49 | −0.388 | 0.228 | −0.363 | 0.729 | 0.806 |
| Maillard products | −0.864 | 0.149 | −0.661 | 0.77 | −0.973 | −0.993 |
Multifloral 1 (M1), multifloral 2 (M2), multifloral (M3), monofloral (MO) and honeydew (HD). (*) Represent significant correlation (P < 0.05)-.
4. Conclusions
All crystallized honeys had different inhibitory effects in each bacterial assay. The ultrasound process reduced the size of the crystals and the content of Maillard products in all honeys. Some honeys showed increased contents of bioactive compounds (phenols or flavonoids) and increased antibacterial activity due the release of bioactive compounds of the crystallized matrix of honey by the ultrasound process.
Author contributions
Campos-Montiel R.G. coordinated the work, Peláez-Acero A. participating in the writing of the manuscript, Cobos-Velazco J.E. conducted experimentation, Gonzales-Lemus U. analysis of results, Espino-Manzano S.O helped draft the manuscript, Gonzalez-Montiel L. carried out the methodology and Aguirre-Alvarez G. editing the manuscript. All authors read and approved the manuscript.
CRediT authorship contribution statement
Campos-Montiel R.G. coordinated the work, Peláez-Acero A. participating in the writing of the manuscript, Cobos-Velazco J.E. conducted experimentation, Gonzales-Lemus U. analysis of results, Espino-Manzano S.O helped draft the manuscript, Gonzalez-Montiel L. carried out the methodology and Aguirre-Alvarez G. editing the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
A. Peláez-Acero, Email: pelaeza@uaeh.edu.mx.
G. Aguirre-Álvarez, Email: aguirre@uaeh.edu.mx.
L. González-Montiel, Email: luciogonzalez@unca.edu.mx.
A.C. Figueira, Email: afiguei@ualg.pt.
R.G. Campos-Montiel, Email: rcampos@uaeh.edu.mx.
References
- 1.Voidarou C., Alexopoulos A., Plessas S., Karapanou A., Mantzourani I., Stavropoulou E., Fotou K., Tzora A., Skoufos I., Bezirtzoglou E. Antibacterial activity of different honeys against pathogenic bacteria. Anaerobe. 2011;17(6):375–379. doi: 10.1016/j.anaerobe.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Suarez J.M., Tulipani S., Díaz D., Estevez Y., Romandini S., Giampieri F., Damiani E., Astolfi P., Bompadre S., Battino M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol. 2010;48(8-9):2490–2499. doi: 10.1016/j.fct.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 3.AL-Waili N., Al Ghamdi A., Ansari M.J., Al-Attal Y., Al-Mubarak A., Salom K. Differences in composition of honey samples and their impact on the antimicrobial activities against drug multiresistant bacteria and pathogenic fungi. Arch. Med. Res. 2013;44(4):307–316. doi: 10.1016/j.arcmed.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Isla M.I., Craig A., Ordoñez R., Zampini C., Sayago J., Bedascarrasbure E., Alvarez A., Salomón V., Maldonado L. Physico chemical and bioactive properties of honeys from Northwestern Argentina. Food Sci. Technol. 2011;44(9):1922–1930. doi: 10.1016/j.lwt.2011.04.003. [DOI] [Google Scholar]
- 5.Tenore G.C., Ritieni A., Campiglia P., Novellino E. Nutraceutical potential of monofloral honeys produced by the Sicilian black honeybees (Apis mellifera ssp. sicula) Food Chem. Toxicol. 2012;50(6):1955–1961. doi: 10.1016/j.fct.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 6.Kabbani D., Sepulcre F., Wedekind J. Ultrasound-assisted liquefaction of rosemary honey: Influence on rheology and crystal content. J. Food Eng. 2011;107(2):173–178. doi: 10.1016/j.jfoodeng.2011.06.027. [DOI] [Google Scholar]
- 7.Rahima D.K. Ultrasound-assisted liquefaction of honey (Doctoral dissertation. Universitat Politècnica de Catalunya (UPC)) 2014 [Google Scholar]
- 8.Tosi E.A., Ré E., Lucero H., Bulacio L. Effect of honey high-temperature short-time heating on parameters related to quality, crystallization phenomena and fungal inhibition. Food Sci. Technol. 2004;37:669–678. doi: 10.1016/j.lwt.2004.02.005. [DOI] [Google Scholar]
- 9.Pimentel-González D.J., Basilio-Cortes U.A., Hernández-Fuentes A.D., Figueira A.C., Quintero-Lira A., Campos-Montiel R.G. Effect of thermal processing on antibacterial activity of multifloral honeys. J. Food Process Eng. 2017;40(1):e12279. doi: 10.1111/jfpe.2017.40.issue-110.1111/jfpe.12279. [DOI] [Google Scholar]
- 10.Bucekoba M., Juricova V., Di Marco G., Gismondi A., Leonardi D., Canini A., Majtan J. Effect of thermal liquefying of crystallized honeys on their antibacterial activities. Food Chem. 2018;269:335–341. doi: 10.1016/j.foodchem.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Subramanian R., Umesh Hebbar H., Rastogi N.K. Processing of honey: a review. Int. J. Food Prop. 2007;10(1):127–143. doi: 10.1080/10942910600981708. [DOI] [Google Scholar]
- 12.Kuś P., Jerković I. New Sample preparation method for honey volatiles fingerprinting based on dehydration homogeneous liquid-liquid extraction (DHLLE) Molecules. 2018;23:1769. doi: 10.3390/molecules23071769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaikham P., Prangthip P. Alteration of antioxidative properties of longan flower-honey after high -pressure, ultra-sonic and thermal processing. Food Biosci. 2015;10:1–7. doi: 10.1016/j.fbio.2015.01.002. [DOI] [Google Scholar]
- 14.Quintero-Lira A., Ángeles Santos A., Aguirre-Álvarez G., Reyes-Munguía A., Almaraz-Buendía I., Campos-Montiel R.G. Effects of liquefying crystallized honey by ultrasound on crystal size, 5-hydroxymethylfurfural, colour, phenolic compounds and antioxidant activity. Eur. Food Re. Technol. 2017;243(4):619–626. doi: 10.1007/s00217-016-2775-0. [DOI] [Google Scholar]
- 15.Önür İ., Misra N.N., Barba F.J., Putnik P., Lorenzo J.M., Gökmen V., Alpas H. Effects of ultrasound and high pressure on physicochemical properties and HMF formation in Turkish honey types. J. Food Eng. 2018;219:129–136. doi: 10.1016/j.jfoodeng.2017.09.019. [DOI] [Google Scholar]
- 16.Suarez-Vargas A., Pimentel-González D.J., Quintero-Lira A., Hernández-Fuentes A.D., Campos-Montiel R.G. Análisis Polínico de Diferentes Mieles del Estado de Hidalgo. Rev biológico agropecuaria Tuxpan. 2013;2:136–141. [Google Scholar]
- 17.Ainsworth E.A., Gillespie K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007;2(4):875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- 18.Sancho M.T., Pascual-Maté A., Rodríguez-Morales E.G., Osés S.M., Escriche I., Periche Á., Fernández-Muiño M.A. Critical assessment of antioxidant-related parameters of honey. Int. J. Food Sci. Tech. 2016;51(1):30–36. doi: 10.1111/ijfs.12988. [DOI] [Google Scholar]
- 19.Medina-Pérez G., Estefes-Duarte J.A., Afanador-Barajas L.N., Fernández-Luqueño F., Zepeda-Velázquez A.P., Franco-Fernández M.J., Peláez-Acero A., Campos-Montiel R.G. Encapsulation preserves antioxidant and antidiabetic activities of cactus acid fruit bioactive compounds under simulated digestion conditions. Molecules. 2020;25:5736–5753. doi: 10.3390/molecules25235736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramonaitytė D.T., Keršienė M., Adams A.n., Tehrani K.A., Kimpe N.D. De Kimpe; The interaction of metal ions with Maillard reaction products in a lactose–glycine model system. Food Res. Int. 2009;42(3):331–336. doi: 10.1016/j.foodres.2008.12.008. [DOI] [Google Scholar]
- 21.Bakier S. Influence of glucose changes on water activity in selected honeys. Acta Agrophys. 2007;9:7–19. [Google Scholar]
- 22.D'Arcy B. Rural Industries Research and Development Corporation; Australian: 2007. High-Power Ultrasound To Control Of Honey Crystallization; pp. 21–28. [Google Scholar]
- 23.Stojković M., Cvetković D., Savić A., Topalić-Trivunović L., Velemir A., Papuga S., Žabić M. Changes in the physicochemical, antioxidant, and antibacterial properties of honeydew honey subjected to heat and ultrasound pretreatments. J. Food Sci. Technol. 2020:1–12. doi: 10.1007/s13197-020-04762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaikam P., Kemsawasd V., Apichartsrangkoon A. Effects of conventional and ultrasound treatments on physicochemical properties and antioxidant capacity of floral honeys from Northern Thailand. Food Biosci. 2016;15:19–26. doi: 10.1016/j.fbio.2016.04.002. [DOI] [Google Scholar]
- 25.Alissandrakis E., Daferera D., Tarantilis P.A., Polissiou M., Harizanis P.C. Ultrasound-assisted extraction of volatile compounds from citrus flowers and citrus honey. Food Chem. 2003;82:575–582. doi: 10.1016/S0308-8146(03)00013-X. [DOI] [Google Scholar]
- 26.Shirahashi Y., Watanabe H., Hayase F. Identification of red pigments formed in a D-xylose-glycine reaction system. Biosci. Biotechnol. Biochem. 2009;73:2287–2292. doi: 10.1271/bbb.90382. [DOI] [PubMed] [Google Scholar]
- 27.Grönroos A., Pirkonen P., Ruppert O. Ultrasonic depolymerization of aqueous carboxymethylcellulose. Ultrason. Sonochem. 2004;11(1):9–12. doi: 10.1016/S1350-4177(03)00129-9. [DOI] [PubMed] [Google Scholar]
- 28.Moussa A., Noureddine D., Abdelmelek M., Saad A. Antibacterial activity of various honey types of Algeria against Pathogenic Gram-Negative Bacilli: Escherichia coli and Pseudomonas aeruginosa. Asian Pac. J. Trop. Dis. 2012;2(3):211–214. doi: 10.1016/S2222-1808(12)60048-6. [DOI] [Google Scholar]
- 29.Plaza M., Santoyo S., Jaime L., Avalo B., Cifuentes A., Reglero G., García-Blairsy Reina G., Señoráns F.J., Ibáñez E. Comprehensive characterization of the functional activities of pressurized liquid and ultrasound-assisted extracts from Chlorella vulgaris. Food Sci. Technol. 2012;46(1):245–253. doi: 10.1016/j.lwt.2011.09.024. [DOI] [Google Scholar]
- 30.Taormina P.J., Niemira B.A., Beuchat L.R. Inhibitory activity of honey against foodborne pathogens as influenced by the presence of hydrogen peroxide and level of antioxidant power. Int. J. Food Microbiol. 2001;69(3):217–225. doi: 10.1016/S0168-1605(01)00505-0. [DOI] [PubMed] [Google Scholar]
- 31.Mundo M.A., Padilla-Zakour O.I., Worobo R.W. Growth inhibition of foodborne pathogens and food spoilage organisms by select raw honeys. Int. J. Food Microbiol. 2004;97(1):1–8. doi: 10.1016/j.ijfoodmicro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Damyeh M.S., Niakousari M., Saharkhiz M.J. Ultrasound pretreatment impact on Prangos ferulacea Lindl. and Satureja macrosiphonia Bornm. essential oil extraction and comparing their physicochemical and biological properties. Ind Crops. Prod. 2016;87:105–115. doi: 10.1016/j.indcrop.2016.04.025. [DOI] [Google Scholar]
- 33.Al-Naama R.T. Evaluation of in-vitro inhibitory effect of honey on some microbial isolate. Afr. J. Bacteriol. 2009;1:64–67. doi: 10.5897/JBR.9000020. [DOI] [Google Scholar]
- 34.Melliou E., Chinou I. Chemical constituents of selected unifloral Greek bee-honeys with antimicrobial activity. Food Chem. 2011;129(2):284–290. doi: 10.1016/j.foodchem.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 35.Al-Nahari A.A.M., Almasaudi S.B., Abd El-Ghany E.S.M., Barbour E., Al Jaouni S.K., Harakeh S. Antimicrobial activities of Saudi honey against Pseudomonas aeruginosa. Saudi. J. Biol. Sci. 2015;22(5):521–525. doi: 10.1016/j.sjbs.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elbanna K., Attalla K., Elbadry M., Abdeltawab A., Gamal-Eldin H., Fawzy Ramadan M. Impact of floral sources and processing on the antimicrobial activities of different unifloral honeys. Asian Pac. J. Trop Dis. 2014;4(3):194–200. doi: 10.1016/S2222-1808(14)60504-1. [DOI] [Google Scholar]
- 37.Rodriguez B.A., Mendoza S., Iturriga M.H., Castaño-Tostado E. Quality parameters and antioxidant and antibacterial properties of some Mexican honeys. J. Food Sci. 2012;77:121–127. doi: 10.1111/j.1750-3841.2011.02487.x. [DOI] [PubMed] [Google Scholar]
- 38.Salarbashi D., Bazzaz B.S.F., Karimkhani M.M., Noghabi Z.S., Khanzadeh F., Sahebkar A. Oil stability index and biological activities of Achillea biebersteinii and Achillea wilhelmsii extracts as influenced by various ultrasound intensities. Ind. Crops Prod. 2014;55:163–172. doi: 10.1016/j.indcrop.2014.01.044. [DOI] [Google Scholar]
- 39.Bueno-Costa F.M., Zambiazi R.C., Bohmer B.W., Chaves F.C., da Silva W.P., Zanusso J.T., Dutra I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul. Brazil. Food Sci. Technol. 2016;65:333–340. doi: 10.1016/j.lwt.2015.08.018. [DOI] [Google Scholar]
- 40.Tekin K., Akalın M.K., Şeker M.G. Ultrasound bath-assisted extraction of essential oils from clove using central composite design. Ind. Crops Prod. 2015;77:954–960. doi: 10.1016/j.indcrop.2015.09.071. [DOI] [Google Scholar]
- 41.Brudzynski K., Abubaker K., Miotto D. Unraveling a mechanism of honey antibacterial action: Polyphenol/H2O2-induced oxidative effect on bacterial cell growth and on DNA degradation. Food Chem. 2012;133(2):329–336. doi: 10.1016/j.foodchem.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 42.Xie J.-H., Shen M.-Y., Xie M.-Y., Nie S.-P., Chen Y.i., Li C., Huang D.-F., Wang Y.-X. Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (Batal.) Iljinskaja polysaccharides. Carbohydr. 2012;89(1):177–184. doi: 10.1016/j.carbpol.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 43.Shan B., Cai Y.-Z., Brooks J.D., Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007;117(1):112–119. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]