Abstract
The role of guanidinoacetic acid (GAA) and its relationship with arginine was reviewed in order to define a replacement ratio between GAA and arginine for broiler diet formulation, the ratio being of how much arginine could be spared, or replaced by GAA. Guanidionoacetic acid, the precursor of creatine, can be synthesized de novo from the amino acids arginine and glycine, whereby 1 mol of arginine creates 1 mol of GAA; that is a weight:weight (w:w) ratio of 1.49:1 (arginine:GAA). Guanidinoacetic acid exerts a growth effect through its primary physiological fate to form creatine, and additionally spares dietary arginine from GAA synthesis; so that it contributes to protein accretion and other functions. Creatine is critical in energy metabolism as a carrier and reservoir of phosphate for adenosine triphosphate (ATP) formation. Arginine deficiency causes reduced growth and can lead to disrupted levels of blood and muscle energy metabolites (phosphocreatine and creatine). Supplementing GAA into the diet restores these metabolites. At severe arginine deficiency, GAA addition cannot fully compensate the arginine deficit, as measured by growth performance. As arginine becomes nearer to sufficiency, the effect of GAA becomes more pronounced. When using growth rate or FCR as an indicator in broilers, a ratio in the range of 0.77 to 1.3:1 (w:w arginine:GAA) was seen, with one study noting a ratio of 2:1 when using FCR as an indicator. Higher ratios of up to 2.7:1 are achieved when using muscle creatine and phosphocreatine measurements. A recommendation of 1:1 (w:w) is proposed, which takes a conservative approach. Large scale studies with practical diets would be helpful to confirm that a ratio of 1:1 (w:w) or higher may be used in the field for broilers.
Key words: guanidinoacetic acid, creatine, arginine-sparing, broiler, feed conversion efficiency, bodyweight gain
INTRODUCTION
Guanidinoacetic acid (GAA), the common name of N-(aminoimino-methyl)-glycine, also known as glycocyamine or guanidinoacetate, is the precursor to creatine, which, together with phosphocreatine, is intrinsically involved in cellular energy metabolism through adenosine triphosphate (ATP) regeneration. Creatine has a direct role in protein accretion by improving the availability of ATP for myosin. It has been proposed as an indicator of muscle quality due to the finding of a constant relationship (23 mg creatine per g net muscle protein) in different cuts of pork, beef, veal, and lamb (Dvořák, 1981). Because of its storage in skeletal muscle, animal-derived feed ingredients are natural sources of creatine. To meet the bird's requirement for creatine, especially in diets with reduced animal protein, GAA may be supplemented into diets as a source of creatine. A GAA feed additive is approved in several regions of the world and is widely used in commercial diets. In the European Union, GAA is authorized for use in broilers as a nutritional additive (EC/ 904/2009 updated by Commission Regulation EU/2016/1768 of 4th October, 2016), and in the United States it is listed by the FDA to spare arginine and as a precursor of creatine (Federal Register 81, 30th November 2016). The effects of GAA on growth performance, energy utilization and muscle development (protein accretion) have recently been reviewed (Khajali et al., 2020). In addition to functioning directly in muscle accretion as the precursor to creatine, dietary GAA can also effectively “spare” arginine from being used for GAA synthesis, so that the arginine may be used for muscle accretion and other physiological functions. The extent of the substitution is important to determine when GAA is applied as a source of arginine to broiler diets. A number of animal studies have been carried out to date to explore the application of GAA to broilers; whilst most of these investigated the effect of adding GAA on-top to a complete diet, several have specifically looked at the question of arginine substitution with GAA. The studies were reviewed with consideration to physiological processes and inter-relationships at the metabolic level.
GAA AS A SOURCE OF CREATINE
Role of Creatine
The creatine/ phosphocreatine system functions as a rapidly mobilizable reserve of high-energy phosphate in cells that have high and variable energy demands (particularly skeletal muscle, the brain, macrophages and sperm cells) to recycle ATP. Creatine carries the high-energy phosphate from mitochondria to myosin filaments, acting as a reservoir of phosphate that can regenerate ATP from ADP directly at the site of ATP need. Regeneration of ATP from the creatine and phospho-creatine system is particularly important in fast-growing species such as broiler chickens and piglets because of their higher demand for creatine to supply growing muscles (Brosnan et al., 2009). The energy for amino acid incorporation by the ribosomes is provided by ATP and guanosine triphosphate (GTP). In skeletal muscle, creatine phosphate provides a relatively large proportion of the phosphate groups which regenerate the ATP and GTP used in ribosomal amino acid incorporation, and can stimulate microsomal protein synthesis more effectively than ATP and GTP alone (Savabi et al., 1988). Creatine also plays a significant role in cardiac energy management (Nain et al., 2008).
Synthesis of Creatine; End-Product Regulation of Amidinotransferase Activity
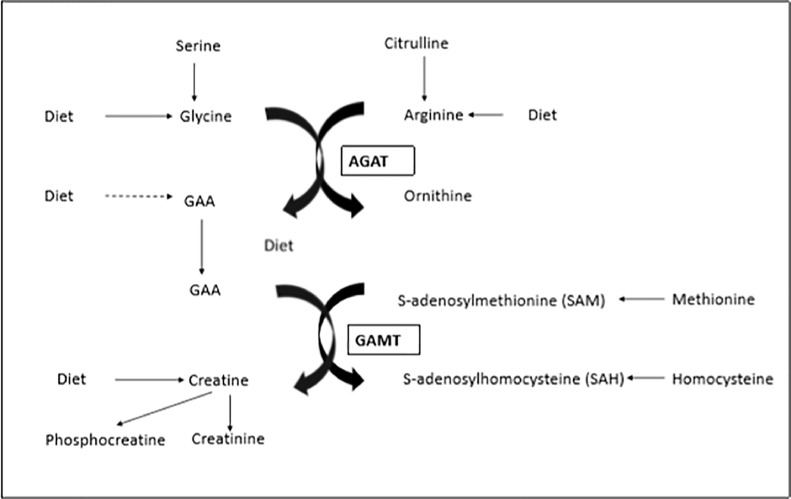
Guanidionoacetic acid is synthesised de novo from the amino acids glycine and arginine (Figure 1); in most vertebrates the synthesis takes place mainly in the kidneys and pancreas, then the GAA is transported to the liver where it is methylated to creatine. The transfer of the amidino group of arginine to glycine is catalyzed by the enzyme L-arginine glycine amidinotransferase (AGAT) commonly called transamidinase. This is the principal regulatory site and rate-limiting step in the biosynthesis of creatine, as demonstrated in chickens and other species (Walker, 1963, 1979). In some lower vertebrates such as poultry, AGAT is also present in the kidney. High blood concentrations of the end product, creatine, have been demonstrated to alter the level of kidney AGAT activity (Walker, 1979). Rats fed a diet containing 0.3% creatine had 26% of the kidney AGAT activity compared to rats fed a creatine-free diet (0.107 enzyme units/mg compared to 0.412 enzyme units/mg); the functional AGAT mRNA in the creatine-fed rats was correspondingly reduced to 37% of the amount in the control animals (McGuire et al., 1984). Other studies have also demonstrated that creatine regulates AGAT expression by a feedback mechanism (Van Pilsum et al., 1992; Guthmiller et al., 1994). Guanidionacetic acid is methylated to creatine with S-adenosylmethionine as the methyl group donor and by the action of S-adenosyl-L-methionine:N-guanidino acetate methyltransferase as the mediating enzyme, which, in poultry, is also expressed in the kidney as well as the liver (Van Pilsum et al., 1972).
Figure 1.
Synthesis of GAA from glycine and arginine and conversion to creatine.1 Adapted from Ostojic (2015) GAA is synthesized from the amino acids glycine and L-arginine; the reaction is catalyzed by the enzyme L-arginine–glycine amidinotransferase (AGAT). AGAT is located mainly in the kidneys and pancreas. After transport mainly to the liver GAA is methylated to form creatine. This reaction is catalyzed by the enzyme guanidinoacetate N-methyltransferase (GAMT) and requires transfer of a methyl group from S-adenosylmethionine (SAM) to GAA, to form creatine and S-adenosylhomocysteine (SAH).
Requirement for Creatine
Creatine degradation to creatinine is a spontaneous reaction and cannot be prevented. In vertebrates an average of 1.7% of the total body creatine and phosphocreatine (PCr) pool is irreversibly converted to creatinine and excreted in the urine, daily (Walker, 1979; Kan et al., 2006), although this figure is likely to be different in broilers. Although creatine can be endogenously synthesized, birds rely on intake to fully meet their needs. In typical poultry diets, dietary creatine is scarce. It is absent in plant and grain ingredients, and the inclusion of meat or fishmeal may not always be sufficient, especially as the creatine content in animal protein varies widely. Creatine content of Menhaden, Peruvian and South-East Asian fishmeal was analyzed at (respectively) 59.2, 127.0 and 14.0 mg/kg (Li and Wu, 2020), while a value of 89 mg/kg has been established in meat and bone meal (Dobenecker and Braun, 2015). Requirements of creatine have been estimated based on factorial calculations as 98.3 mg/d for a 1,000 g bird, increasing to 137.6 mg/d for a 2,050 g bird, corresponding to an optimum dietary level of 0.077 and 0.068%, respectively (Tossenberger et al., 2016). A diet containing 2.5% fishmeal and 2.5% meat and bonemeal will contain only around 3.7 mg creatine/kg feed (0.00037%) which will not supply sufficient creatine to meet the birds’ demands. As arginine is a precursor of creatine, and cannot be synthesised de novo by birds, creatine balance is normally dependent on dietary arginine (Chamruspollert et al, 2002). Therefore, factors that influence creatine supply or synthesis influencing the amount of arginine used in creatine synthesis, also have the potential to influence the amount of arginine that is available as a building block directly for protein synthesis and muscle accretion. Creatine itself is not practical to use as a feed additive as it is relatively unstable and has not been authorized as a feed additive. Broiler diets are increasingly supplemented with GAA as a source of creatine, and so the GAA can spare arginine and glycine for growth and other biological functions, including cell signaling and hormone release. Additionally, a three-way interaction between arginine, lysine and methionine has been demonstrated in broiler chicks (P = 0.075) indicating that all 3 amino acids may be important in determining responses and requirements to any of the three (Chamruspollert et al., 2002).
GAA Effects on Glucose Metabolism
Guanidionacetic acid acts as a moderate glucagon secretagogue (Marco et al., 1976) although the overall effect on glucose metabolism is not yet fully defined. When GAA was fed to rodents there was an immediate increase in plasma insulin persisting for approximately 20 min (Aynsley-Green and Alberti, 1974). The insulin secretion from the rat pancreas in response to GAA was shown to be greater than the release in response to creatine or arginine (Alsever et al., 1970). Although a reduction in plasma glucose has been demonstrated in mice (Meglasson et al., 1993), this was not observed by De Groot et al. (2018) in chicks. In their study, neither GAA nor arginine directly affected blood glucose, although there was an interaction between GAA and arginine on blood glucose level (P = 0.011). Additional effects of GAA have been reported, such as reduction in gamma amino butyric acid levels in healthy men (Ostojic and Stojanovic, 2015), although the relevance of these findings are not fully understood.
Tissue Deposition of GAA, Creatine, and Creatinine
Apparent digestibility of GAA has been determined as being close to 100%; measuring 99.4% at 0.6g GAA/kg diet and 98.77% at 6 g GAA/kg diet. Utilization of dietary GAA was 77.1% at 0.6 g/kg diet, reducing to 46.4% at 6 g/kg diet (Tossenberger et al., 2016).
Our understanding of the metabolic fate of GAA is helped by tissue deposition studies which have been reviewed by the European Food Safety Authority (EFSA, 2009). Normal creatine concentration in grower/ finisher broiler breast muscle ranges from 4,000 to 5,000 mg/kg. When dietary GAA is increased, the creatine and GAA levels in the breast muscle, liver, and kidney respond differently. Creatine level in the breast muscle and liver increase with increasing dietary GAA (P < 0.050). Conversely, increasing dietary GAA causes a reduction in GAA in breast muscle and in the liver (P < 0.050). Creatinine content in these tissues does not correlate with dietary intake of GAA.
Effect of Guanidionoacetic Acid on broiler Performance
Published growth performance trials in which GAA was used as the test treatment as an on-top treatment to nutritionally adequate diets were collated and are presented in Table 1. Several of the trials had used dose-rates or treatments for which pair-wise statistical evaluation had not been performed; therefore, results are presented as means. Increases of less than 1.0 % were arbitrarily considered for the purpose of this analysis of “no positive effect” (percentage improvement = 0). Of the 42 individual data sets, 6 showed no positive effect of GAA on liveweight gain; 22 showed improvement of up to 5%, 10 showed improvement of between 5.1% and 10% and 4 showed improvement above 10.1%. When feed conversion efficiency was analyzed, 3 data sets reported no positive effect; 26 showed improvement of up to 5%, 10 showed improvement of between 5.1% and 10% and 3 showed improvement greater than 10.1%. The data support that GAA at its recommended dose rate of 0.6 to 1.2 g/kg can support improvements in feed conversion efficiency and increases in daily liveweight gain of 5% and above. The most consistent improvements are seen on feed conversion efficiency, which are apparent even where arginine level in the diet meets requirement i.e. there is no arginine deficiency. Birds receiving GAA convert feed to body weight more efficiently than control birds.
Table 1.
Effect of dietary addition of GAA as on-top treatment to nutritionally adequate diets on growth performance of broiler chickens.
| Reference | n (reps) | Level of GAA | % Improvement |
|
|---|---|---|---|---|
| in diet g/kg | BWG | FCR | ||
| Esser et al., 2018 | 315 (9×35) | 0.8 | 0.0 | 1.0 |
| Emami et al., 2017 | 90 (6×15) | 0.6, 1.2 | 0.0, 1.6 | 1.3, 1.3 |
| Borges, 2017 | 84 (7×12) | 0.1, 0.2 | 0.0, 0.0 | 0.0, 1.6 |
| Cordova-Noboa et al., 2018a | 320 (16×20) | 0.6, 0.6§ | 0.0, 1.1 | 1.0, 0.6 |
| Cordova-Noboa et al., 2018b | 200 (10×20) | 0.6 | 2.2 | 2.4 |
| Abudabos et al., 2014 | 20 (5×4) | 0.6 | 0.0 | 4.9 |
| Zelenka et al., 2015 | 840 (6×140) | 0.6 | 1.6 | 4.1 |
| Yazdia et al., 2017 | 75 | 0.6, 1.2 | 1.9, 1.3 | 1.8, 1.2 |
| Jiang et al., 2012 | 60 (3×20) | 0.2, 0.4 | 1.4, 4.4 | 0.0, 3.0 |
| Majdeddin et al., 2017 | 240 (12×20) | 0.6, 1.2 | 1.9, 1.5 | 2.7, 3.4 |
| Ale Sahib Fosoul et al., 2018 | 65 (5×13) | 0.6, 1.2 | 1.9. 6.5 | 4.9, 7.6 |
| 0.6, 1.2 | 5.4, 3.2 | 4.2, 0 | ||
| Lemme et al., 2011 | 90 (10×9) | 0.6, 0.6# | 2.0, 4.6 | 1.9, 2.6 |
| Pradeep et al., 2016 (exp.1) | 270 (9×30) | 0.6 | 5.2 | 2.5 |
| Pradeep et al., 2016 (exp.2) | 90 (9×10) | 0.6, 0.6# | 2.0, 4.6 | 1.9, 2.6 |
| Michiels et al., 2012 | 192 (6×32) | 0.6, 1.2 | 2.7, 2.2 | 1.6, 3.3 |
| Zhang et al., 2016 (exp.1) | 108(6×18) | 0.2#, 0.4# | 5.1, 3.0 | 3.6, 3.0 |
| Zhang et al., 2016 (exp.2) | 15,000 | 0.6 | 16.0 | 6.0 |
| Ringel et al., 2007 | 280 (7×40) | 0.3, 0.6, 0.9, 1.3 | 5.0, 4.2, 7.0, 4.0 | 6.1, 4.6, 6.0, 6.4 |
| He et al., 2019 | 108 (6×18) | 0.6, 0.8, 1.0, 1.2 | 10.2, 9.7, 9.8, 7.9 | 10.9, 9.1, 9.1, 7.3 |
| Ahmadipour et al., 2018 | 60 (4×15) | 0.5, 1.0, 1.5, 2.0 | 8.2, 11.3, 15.3, 9.8 | 5.1, 10.2, 10.7, 6.6 |
All diets were vegetable-based except where indicated:§ Poultry By-Product # Fishmeal.
It has also been proposed that the enhanced ATP buffering capacity in the muscles created by GAA affects energy metabolism at low dietary energy (Ale Sahib Fosoul et al., 2018). Adding GAA at 1.2 g/kg to a standard energy (12.56 MJ/kg starter; 12.97 MJ/kg grower) or reduced energy diet (11.93 MJ/kg starter; 12.33 MJ/kg grower), reversed the negative effects of the energy reduction on weight gain and feed conversion efficiency (P < 0.050). Energy retention, both as fat, and total retention, were increased (P < 0.050).
Requirement of Broilers for Arginine
Arginine is an essential amino acid in birds. They rely on dietary intake of arginine, which must be sufficient to support protein synthesis, growth, feathering, and other biological processes. Dietary arginine is absorbed through the intestinal epithelium; post digestion, the metabolism is complex and there is an incomplete picture, particularly of homeostasis. Arginine is also described as a functional amino acid (Wu, 2013), that is, one that regulates key metabolic pathways to improve health, growth and other physiological processes. As well as being a precursor of creatine, arginine is a precursor to nitric oxide, which regulates blood flow as a vasodilator. It may therefore be of relevance to prevent pulmonary hypertension, which is a common problem in birds reared at high altitudes and/ or under temperature challenged conditions. Arginine is also involved in hormone production and release (Barbul, 1986) and plays a key role in immunity (Kwak et al., 1999; Lee et al., 2002; Abdukalykova et al., 2008).
Recommendations of dietary arginine requirements put forward by the National Research Council (NRC, 1994) are 1.25% of the diet to 3 wk, reducing to 1.10 % from 3 to 6 wk, and thereafter to 1.0 % from 6 to 8 wk of age. However, these are in need of updating and are no longer adequate, especially at high altitudes and cold conditions. More recent standards have been adopted by the Brazilian Poultry Sector (Rostagno et al., 2017). For high performance females, 1.40% SID/ 1.50% total at 0 to 7 d, reducing to 1.0% SID/ 1.06% total at 34 to 42 d are recommended. For high-performance males, 1.43% SID/ 1.53% total at 0 to 7 d, reducing to 1.10% SID/1.18% total at 34 to 42 d are recommended. In Europe, a ratio of SID arginine to lysine of 107% for maximum bodyweight and 112% for maximum feed conversion efficiency has been established (CVB, 2018). Ross 308 Nutrient specifications (2019) for broiler chickens up to 2.4 kg are 1.37% digestible arginine (1.52% total arginine) at days 0 to 10 reducing to 1.10% digestible arginine (1.22% total arginine) at day 25 onward. In wheat-based diets, arginine can be limiting and this is particularly the case when low crude protein diets are formulated, as arginine is usually the fourth or fifth limiting amino acid.
ARGININE SPARING WITH GAA
The observation that both creatine and GAA could spare arginine and glycine in broilers was put forward by Almquist et al. (1941). The arginine-sparing effect means that arginine can be used for other functions than creatine formation, such as directly in muscle accretion, cell signaling, and hormone release. It is therefore important to know the extent to which arginine may be replaced by GAA. The relationship between arginine and GAA has been explored in a series of bioassays carried out with male New Hampshire × Columbian chicks using semisynthetic diets singly and severely deficient in arginine, to which graded levels of arginine and GAA were added (Dilger et al., 2013). The first assay (Assay 1) was carried out with birds from 8 to 17 days post-hatch. Dietary treatments were an unsupplemented arginine-deficient basal diet to which added either 0.06% or 0.12% GAA. A fourth diet containing 1% supplemented arginine was used as a positive control diet. Birds receiving the arginine-deficient basal diet containing 0.86% total arginine showed significant reductions in body weight gain and gain: feed ratio, which were overcome by the addition of 1% arginine, but not fully overcome by the addition of 0.12% GAA. It appeared that the arginine deficiency was too great to be compensated by GAA. This would be expected, however, since the recommended maximum dose-rate of GAA (0.12%) could not be expected to compensate for 8 times the dose-rate of arginine (1.0%). It should be noted that the deficiency level achieved would not be seen in practical formulations. Adding creatine was also unable to overcome the marked arginine deficiency (Table 2). In a second assay (Assay 2), the basal diet (0.86% total arginine) was supplemented with 0.12% GAA or an equal concentration of arginine. A fourth diet contained the combination of 0.12% GAA and 0.12% arginine. The addition of 0.12% arginine and 0.12% GAA yielded similar increases in weight gain and feed efficiency; suggesting a 1:1 ratio of arginine:GAA.
Table 2.
Performance of chicks fed graded doses of guanidino acetic acid (GAA) when added to an arginine-deficient dextrose-casein diet (Dilger et al., 2013, Assay 1).
| Diet | Treatment | BWG (g) | Feed Intake (g) | Gain/Feed g/kg |
|---|---|---|---|---|
| 1 | Basal Diet 0.86% Arg | 111e | 195g | 569d |
| 2 | As 1 + 1.0% L Arg | 212a | 275a | 773a |
| 3 | As 1 + 0.06% GAA | 108e | 195g | 553d |
| 4 | As 1 + 0.12% GAA | 145d | 233de | 622c |
| 5 | As 1 + 0.39% GAA | 139d | 220de | 631c |
| 6 | As 1 + 0.78% GAA | 134d | 209fg | 641c |
| 7 | As 2 + 0.39% GAA | 209ab | 265abc | 789a |
| 8 | As 2 + 0.78% GAA | 197b | 249cd | 792a |
| 9 | As 1 + 1% Creatine.H2O | 173c | 254bc | 680b |
| 10 | As 2 + 1% Creatine.H2O | 212a | 268ab | 794a |
| SEM | 5.0 | 6.2 | 12.5 |
a-gMeans in columns with no common superscript letters are significantly different (P < 0.050).
Influence of Deficiency Level on Response to GAA
The recommended dose rate of GAA addition has been defined as 600 to 1,200 mg/ kg. Few studies have been carried out to test whether a further response may be expected at dietary GAA greater than 1,200 mg/kg and it may be that no further response is achieved. Therefore, in diets, which are markedly deficient in arginine, use of GAA even at a high dose may not fully overcome the arginine deficiency. As arginine becomes nearer to the bird's dietary requirement, the addition of GAA has a more measurable effect. A further assay by Dilger's group (Assay 3) used a practical corn-soybean meal diet to achieve levels of arginine which were more representative of typical deficiencies. Birds were fed the an arginine - deficient diet containing 1% total arginine (20% crude protein) supplemented with 0.25% arginine, to reach arginine requirement, or supplemented with 0.12% GAA. A methionine-fortified corn-soybean meal diet (23% crude protein, 1.25% arginine) was used as a positive control. There were no differences in body weight gain between the treatments but the addition of GAA or arginine resulted in improved feed conversion efficiency (P < 0.050). The positive control diet showed a further improvement in feed conversion efficiency (P < 0.050). Although the assay had a limited sensitivity, it could be concluded that 0.12% GAA gave an equivalent feed conversion response to 0.25% arginine; which would suggest a replacement factor of 2:1 (w:w arginine:GAA). A final assay in this series (Assay 4) used the semisynthetic diet singly deficient in arginine with graded doses of supplemental arginine (0, 0.12, 0.24, 0.36, 0.48, 0.60 and 0.72% to provide 0.86–1.58% total arginine) with or without 0.12% added GAA. Growth rate and feed:gain increased quadratically in response to arginine (P < 0.010). At dietary arginine levels lower than 1.22%, body weight gains achieved with GAA were consistently lower than could be achieved with the addition of arginine, suggesting that 0.12% arginine could not be replaced by an equal amount of GAA, whereas at dietary arginine levels greater than 1.22%, a 1:1 ratio was seen. When using feed conversion efficiency as the indicator, 0.12% arginine could be replaced by 0.12% GAA; achieving a 1:1 replacement ratio. A subsequent regression analysis of the data (Lemme et al., 2018) calculated that an equivalence of 1.25:1 (w:w arginine:GAA) could be assumed for body weight gain and an equivalence of 1.65:1 for FCR. However, the use of regression analysis is constrained by the limited data points.
Influence of Biological Indicator on Equivalence Value
Further studies have found that differences in equivalence values are obtained, depending on the indicator used. Ringel et al. (2013) formulated a basal diet which was singly arginine deficient, containing 0.92% total arginine (0.80%, SID) to which arginine was added in increments at 0, 0.05, 0.10, 0.17, 0.25, and 0.35 % of the diet, with or without GAA at 0.06%. Diets were fed to Ross 308 birds from d 14 to 27. According to a further regression analysis, when body weight gain was used as the indicator, the equivalence was 1.16:1 (w:w arginine:GAA) however, when FCR was used, an equivalence of 0.77:1 was seen (Lemme et al., 2018). When GAA was added to the diet, breast muscle creatine concentrations were consistently greater than the corresponding arginine level without GAA. When the values were calculated to reflect the total amount of creatine in the breast muscle, a ratio of up to 1.67:1 (w:w arginine:GAA) was derived (Scharch et al., 2019). It should be noted that the level of GAA used in this study was the lowest recommended level whereas other studies showed that improved responses could be achieved at higher levels.
De Groot et al. (2018) looked at the effect of replacing arginine with GAA on muscle and blood creatine levels using Ross 708 birds from d 8 to 22. A corn/soybean meal diet which was singly deficient in arginine, containing 0.96% total (0.84% SID) arginine, was used as a negative control diet. A second diet was prepared by supplementing the control diet with 0.16% arginine to provide 1.12% total (1.00% SID) arginine. Both diets were supplemented with 0, 0.06, or 0.12% GAA. A positive control diet was obtained by adding 0.32% arginine to the negative control diet to obtain 1.28% total (1.16% SID) arginine. Body weight gain of birds receiving the 0.84% SID arginine (deficient) diet indicated that 0.12% GAA could replace 0.16% arginine, suggesting a replacement factor of 1.3:1 (w:w arginine:GAA), this was confirmed by the FCR response which suggested that a replacement factor of more than 1.3:1. No main effects on BWG were observed for either arginine or GAA during d 8 to 15. Again, it was observed that at the less severe deficiency, GAA could replace a higher level of arginine. Muscle PCr measurements indicated that a replacement factor of 2.7:1 could be exceeded. Total creatine content of the muscle of birds receiving the 0.84% arginine plus 0.12% GAA was greater than that of birds receiving 1.16% arginine. Although this difference was statistically nonsignificant, it indicates that a ratio of greater than 2.7:1 (arginine:GAA) may be derived when total creatine is used as the indicator (Table 3). Results of this study demonstrate that while arginine deficiency can disrupt levels of blood and muscle energy metabolites, supplementation with GAA can restore the levels in blood and tissue.
Table 3.
Muscle phosphocreatine (P-creatine) and muscle total creatine content of broilers fed graded levels or arginine with or without GAA (De Groot et al., 2018).
| Muscle analysis mmol/kg DW |
Serum analysis µg/Kg |
Growth performance |
|||||
|---|---|---|---|---|---|---|---|
| Treatment | P-creatine | Creatine | Arg | Creatine | BWG (g) | FI (g) | G:F |
| 0.84% SID Arg (control) | 37.61 | 106.6a,1 | 155.8a,b,1 | 14.21 | 528.6 | 815.6 | 647.31 |
| + 0.06%GAA | 56.1 | 112.4a,1 | 136.9a,1 | 22.3 | 526.8 | 797.7 | 661.5 |
| + 0.12% GAA | 91.0 | 150.6b | 143.8a,1 | 37.1 | 541.6 | 791.0 | 684.3 |
| 1.0% SID Arg | 61.7 | 116.5a | 199.1b,c,1 | 19.8 | 542.7 | 791.5 | 685.1 |
| +0.06% GAA | 82.4 | 158.9b | 244.6c,d,1 | 26.0 | 556.8 | 813.8 | 686.9 |
| +0.12 % GAA | 108.71 | 176.0b | 274.0d,1 | 43.21 | 562.7 | 791.3 | 710.9 |
| 1.16% SID Arg | 74.4 | 139.2 | 364.9 | 28.3 | 569.2 | 810.8 | 700.5 |
| SEM | 8.14 | 7.13 | 15.35 | 3.31 | 14.39 | 20.98 | 13.63 |
| Probability | |||||||
| Arg | <0.001 | <0.001 | <0.001 | 0.006 | 0.037 | NS | 0.004 |
| GAA | <0.001 | <0.001 | NS | <0.001 | NS | NS | 0.047 |
| Arg × GAA | NS | <0.050 | 0.019 | NS | NS | NS | NS |
a-d Means separation analysis carried out on GAA × Arg interaction; means within a column lacking a common superscript letter differ (P < 0.050)
Mean value for this treatment was different from the positive control treatment (P < 0.050); SEM Standard error of the mean that applies to all 7 dietary treatments. P-values apply only to the first 6 treatments that were included in the 2-way ANOVA. Abbreviations: NS, Nonsignificant; P-Creatine, Phosphocreatine; Arg, Arginine; SID Arg, Standard Ileal Digestible Arginine; BWG, Body weight Gain; FI, Feed Intake; G:F, Gain:Feed.
A further study (De Groot et al., 2019) used dietary arginine levels which were nearer to practical conditions than the modeling studies which had been carried out. The diets, which were singly deficient in arginine were an arginine-deficient basal diet containing 0.97% SID (1.08% total) arginine in the starter phase and 0.84% SID (0.95% total) arginine in the grower phase. The basal diet was supplemented with GAA at 0, 0.06, 0.12, or 0.18%. An arginine-sufficient diet, containing an additional 0.37% arginine in the starter and 0.32 % arginine in the grower phase, was used as a positive control. Ross 708 birds were fed the diets from day 0 to 28. Body weight gain and feed conversion efficiency at 0.12% GAA were not significantly different from the positive control diet although numerically the growth performance did not reach that of the positive control (Table 5), suggesting that a 3:1 (w:w) ratio was not appropriate for growth performance parameters. On the other hand, muscle phosphocreatine levels did indicate a ratio of 3:1. This study is noteworthy because it provides a link between the modeling studies and a more practical study, which, although lacking the sensitivity of some of the previously reported assays, allows the ratios to be tested using practical diets. A further study (Ale Sahib Fosoul et al., 2019) again used a more practical level of arginine with the singly reduced – arginine control diet formulated to contain 12.10 g total arginine/kg feed in the starter phase and 11.30 g/kg in the grower phase. This was supplemented with arginine to achieve requirement levels (15.40 g/kg starter/ 13.5 g/kg grower) or with GAA at 0.06, 1.2 or 1.8%. Broiler chicks were allocated to one of the 5 diets and fed from d 0 to 35. Addition of arginine to the negative control diet resulted in an increased daily liveweight gain (P < 0.050). Increased growth rate was also achieved with the addition of GAA at 0.12% and 0.18% but not at 0.06% of the diet. Numerically, the increases achieved with GAA were smaller than achieved with arginine. When FCR was used as the indicator the improvement at 0.06% GAA was not significantly different to the improvement achieved with arginine; increasing the level of GAA led to further improvements in FCR. However, reliable ratios could not be calculated because of contradictory results.
Table 5.
Zero to 28 d performance and tissue measurement of broilers (De Groot et al., 2019).
| Treatment | BW gain | FI | Gain: Feed | Phospho-creatine | Total creatine |
|---|---|---|---|---|---|
| g/day/bird | mmol/kg DW | ||||
| 1.08, 0.95 total Arg | 32.8a | 49.4 | 646.0a | 52.81a | 88.45a |
| 1.08, 0.95 Arg + 0.06 GAA | 34.1 a-c | 48.2 | 663.7a | 62.42a | 102.29a |
| 1.08, 0.95 Arg + 0.12 GAA | 34.9 bc | 48.5 | 696.6b,c | 87.69b | 131.54c |
| 1.08, 0.95 Arg + 0.18 GAA | 33.9ab | 47.0 | 695.3a,b | 108.17c | 155.28d |
| 1.45, 1.27 total Arg | 36.0c | 49.5 | 712.6c | 87.60b | 139.33c |
| POOLED SEM | 0.71 | 0.80 | 8.79 | 4.25 | 4.33 |
| P overall | <0.050 | NS | <0.001 | <0.001 | <0.001 |
a–dMeans within a column lacking a common superscript letter are significantly different.
Abbreviation: NS, Nonsignificant.
Comparison of Equivalence Values
The lower arginine:GAA equivalence value obtained from body weight gain measurements in comparison with other indicators appears to be consistent across the data sets (Table 4). The improvements in FCR are also consistent and support the suggestion that arginine is spared from serving as a precursor for creatine synthesis (Almquist et al., 1941) and is therefore available for protein accretion (lean body mass). In general, GAA was able to improve, and restore growth performance of birds receiving arginine deficient diets (as long as the deficiency was not too large; as can only be achieved by the use of synthetic diets). The combined results indicate that the amount of arginine that may be spared by GAA is in the region of 0.77 to 1.3:1 (w:w basis) when taking body weight gain or FCR as indicators. The metabolic relationship, whereby one mol of arginine creates one mol of GAA, would give a weight:weight ratio of 1.49:1 (arginine:GAA) if it were replicated at the whole-body level. However, due to the multiple functions and pathways of arginine, whereby only a proportion is involved in creatine metabolism, it may not be expected that the metabolic relationship is translated and measurable to the same value at the whole-body level, using growth and FCR indicators. Nonetheless, the fact that different arginine/GAA equivalence values are obtained depending on the parameter that is measured (growth rate, feed conversion or muscle creatine/ phosphocreatine level) deserves further attention. Protein accretion requires not only the amino acid building blocks to be present but additionally requires an energy component of protein accretion. The more closely the metabolic energy component is met, the more enriched the newly formed muscle would be, and the better able to withstand stress conditions and support growth. Arginine, by its involvement in GAA synthesis, is an integral part of the energy component of muscle accretion. In addition, it is directly a building block of muscle protein. Selection for fast growth may have resulted in arginine being diverted from the “energy component” of muscle accretion directly to the muscle protein. This mechanism may be through enhanced negative feedback regulation of creatine on AGAT, which would inhibit the production of GAA from arginine and glycine, reduce creatine synthesis and lead to suboptimal muscle integrity (especially under stress conditions). Adding GAA directly not only potentially spares arginine directly for muscle accretion growth but could also over-ride the negative feedback regulation and allow creatine to be produced in greater quantities than would normally be supported by the addition of arginine. Increased arginine equivalence values obtained when tissue measurements are used, compared to growth measurements, suggest that muscle creatine is not at its optimum, since when there is an opportunity to increase creatine levels in the muscle (as provided by GAA) the bird appears to utilize this.
Table 4.
Arginine: GAA equivalence values derived from broiler studies.
| Reference | Diet type /Bird breed & Age | Arg, GAA in diet | Arg:GAA ratio w:w5 | |
|---|---|---|---|---|
| Dilger et al., 20131 | Semisynthetic / New Hampshire × Columbian/ d 8-17 |
0.86 Total Arg + 0.06% GAA + 0.12% GAA + 1.0% Arg |
n/a | |
| Dilger et al., 20132 | Semisynthetic / New Hampshire × Columbian/ d 8-17 |
0.86 Total Arg + 0.12% Arg + 0.12% GAA + 0.12% Arg + 0.12% GAA |
BWG FCR |
1:1 1.1 |
| Dilger et al., 20133 | Practical / New Hampshire × Columbian/ d 8-17 |
1.0% Total Arg + 0.12% GAA + 0.25% Arg + 0.12% GAA + 0.25% Arg + 1.25% Arg/ Met suppl. |
BWG FCR |
n/a 2:1 |
| Dilger et al., 20134 | Semisynthetic / New Hampshire × Columbian/ d 8-17 |
0.88% Total Arg + 0.12, 0.24, 0.36, 0.48, 0.60, 0.72% Arg +/- GAA 0.12% |
BWG FCR |
<1:1 (1.256) 1:1 (1.656) |
|
Ringel et al., 2013 Scharch et al., 2019 |
Practical / Ross 308 / d 14-28 |
0.92 Total Arg (0.80% SID) +/- 0.06% GAA + 0.05, 0.10, 0.17, 0.25, 0.35% Arg +/- 0.06% GAA |
BWG FCR Total PCr in breast muscle |
1.16:16 0.77:16 1.67:1 |
| De Groot et al., 2018 | Practical / Ross 708 / d 8-22 |
0.96% Total Arg (0.84% SID) + 0.06 / 0.12% GAA 1.0% Arg + 0.06 / 0.12% GAA 1.16% Arg |
BWG FCR Muscle PCr |
1.3:1 >1.3:1 2.7:1 |
| De Groot et al., 2019 | Practical / Ross 708/ d 2-28 | 1.08% Total Arg (0.97 SID) Starter 0.95% Total Arg (0.84% SID) Grower + 0.06, 0.12, 0.18% GAA + 0.37% 0.32% Arg |
BWG FCR Muscle PCr |
<3:1 <3:1 3:1 |
| Ale Sahib Fosoul et al., 2019 | Practical / Ross 308/ d 1-35 |
1.21/ 1.12 Total Arg Starter & Grower + 0.06, 0.12, 0.18% GAA + 0.33%, 0.23% Arg |
BWG FCR Blood Nitric oxide Blood creatinine |
n/a |
Study 1.
Study 2.
Study 3.
Study 4.
Arg:GAA (w:w) interpretation of current authors derived from published results.
interpretation of original authors derived by further regression analysis (Lemme et al, 2018).
CONCLUSIONS
Addition of guanidinoacetic acid to the diet of broilers exerts a growth performance effect (5% and greater increases in body weight gain) even in arginine-sufficient diets. It also acts to spare a part of arginine, releasing it from creatine formation to protein accretion and other metabolic functions. When GAA is synthesised de novo from arginine and glycine the theoretical w:w relationship is 1 g of GAA replaces 1.49 g of arginine. However, combined tissue and growth performance results indicate that the replacement value obtained depends on the parameter measured. A recommendation of 1:1 (w:w) is proposed for practical diets, which takes a conservative approach and may be increased on the basis of further practical testing. The ratio is influenced by the level of arginine deficiency; at a less severe deficiency, GAA can replace a higher level of arginine. A higher ratio is supported by muscle creatine and PCr measurements (up to 2.7–3:1). The ratios are proposed for broiler chickens; separate values would need to be generated for other species. The studies carried out thus far have mainly used models relying on severe deficiencies to allow assay sensitivity. Further studies using diets with more typical arginine deficiencies as well as field trials would be helpful to confirm that w:w ratios of 1:1 for body weight gain and FCR are appropriate in broiler chickens under practical field conditions.
DISCLOSURES
The review was commissioned by Alzchem Trostberg GmbH through the independent organisation, Feed Food & Future. The review was drafted independently of Alzchem GmbH. Ulrike Braun of Alzchem has extensive knowledge on the biochemistry and metabolism of GAA and provided critique of the work. Analysis of the information and data remained independent of commercial influence.
REFERENCES
- Abdukalykova S.T., Zhao X., Ruiz-Feria C.A. Arginine and vitamin E modulate the subpopulations of T lymphocytes in broiler chickens. Poult. Sci. 2008;87:50–55. doi: 10.3382/ps.2007-00315. [DOI] [PubMed] [Google Scholar]
- Abudabos A.M., Saleh F., Lemme A., Zakaria H.A.H. The relationship between guanidino acetic acid and metabolisable energy level of diets on performance of broiler chickens. Ital. J. Anim. Sci. 2014;13:548–556. [Google Scholar]
- Ahmadipour B., Khajali F., Sharifi M.R. Effect of guanidinoacetic acid supplementation on growth performance and gut morphology in broiler chickens. Poult. Sci. J. 2018;6:19–24. doi: 10.2141/jpsa.0170044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almquist H.J., Mecchi E., Kratzer F.H. Creatine formation in the chick. J. Biol. Chem. 1941;141:365–373. [Google Scholar]
- Alsever R.N., Georg R.H., Sussman K.E. Stimulation of insulin secretion by guanidinoacetic acid and other guanidine derivatives. Endocrinology. 1970;86:332–336. doi: 10.1210/endo-86-2-332. [DOI] [PubMed] [Google Scholar]
- Aynsley-Green A., Alberti K.G. In vivo stimulation of insulin secretion by guanidine derivatives in the rat. Horm. Metab. Res. 1974;6:115–120. doi: 10.1055/s-0028-1093873. [DOI] [PubMed] [Google Scholar]
- Barbul A. Arginine: biochemistry, physiology, and therapeutic implications. J. Parenteral and Enteral Nutr. 1986;10:227–238. doi: 10.1177/0148607186010002227. [DOI] [PubMed] [Google Scholar]
- Borges K.M. Universidade Federal de Goiás; 2017. Ácido guanidinoacético em dieta pré-inicial para frangos. Thesis. [Google Scholar]
- Brosnan J.T., Wijekoon E.P., Warford-Woolgar L., Trottier N.L., Brosnan M.E., Brunton J.A., Bertolo R.F P. Creatine synthesis is a major metabolic process in neonatal piglets and has important implications for amino acid metabolism and methyl balance. J. Nutr. 2009;139:1292–1297. doi: 10.3945/jn.109.105411. [DOI] [PubMed] [Google Scholar]
- Centraal Veevoederbureau (CVB) CVB; The Netherlands: 2018. Standardized Ileal Digestible Arginine Requirement for Broilers. [Google Scholar]
- Chamruspollert M., Pesti G.M., Bakalli R.I. Dietary interrelationships among arginine, lysine and methionine in young broiler chicks. Br. J. Nutr. 2002;88:655–660. doi: 10.1079/BJN2002732. [DOI] [PubMed] [Google Scholar]
- Cordova-Noboa H.A., Oviedo-Rondon E.O., Sarsour A.H., Barnes J., Sapcota D., Lopez D., Gross L., Rademacher-Heilshorn M., Braun U. Effect of guanidinoacetic acid supplementation on live performance, meat quality, pectoral myopathies and blood parameters of male broilers fed corn-based diets with or without poultry by-products. Poult. Sci. 2018;97:2494–2505. doi: 10.3382/ps/pey097. [DOI] [PubMed] [Google Scholar]
- Cordova-Noboa H.A., Oviedo-Rondon E.O., Sarsour A.H., Barnes J., Ferzola P., Rademacher-Heilshorn M., Braun U. Performance, meat quality, and pectoral myopathies of broilers fed either corn or sorghum based diets supplemented with guanidinoacetic acid. Poult. Sci. 2018;97:2479–2493. doi: 10.3382/ps/pey096. [DOI] [PubMed] [Google Scholar]
- Dilger R.N., Bryant-Angeloni K., Payne R.L., Lemme A., Parsons C.M. Dietary guanidino acetic acid is an efficacious replacement for arginine in young chicks. Poult. Sci. 2013;92:171–177. doi: 10.3382/ps.2012-02425. [DOI] [PubMed] [Google Scholar]
- Dobenecker B., Braun U. Creatine and creatinine contents in different diet types for dogs – effects of source and processing. J. Anim. Physiol. and Anim. Nutr. 2015;99:1017–1024. doi: 10.1111/jpn.12383. [DOI] [PubMed] [Google Scholar]
- Dvořák Z. Creatine as an indicator of net muscle proteins. J. Sci. Food Agric. 1981;32:1033–1036. [Google Scholar]
- Emami N.K, Golian A., Rhoads D.D., Danesh M. Interactive effects of temperature and dietary supplementation of arginine or guanidinoacetic acid on nutritional and physiological responses in male broiler chickens. Br. Poult. Sci. 2017;58:87–94. doi: 10.1080/00071668.2016.1257779. [DOI] [PubMed] [Google Scholar]
- Esser A.F.G., Taniguti T.L., da Silva A.M., Vanroo E., Kaneko I.N., dos Santos T.C., Mueller Fernandes J.I. Effect of supplementation of guanidinoacetic acid and arginine in vegetable diets for broiler on performance, carcass yield and meat quality. Sem Ciências Agrárias. 2018;39:S1307–S1318. [Google Scholar]
- European Food Safety Authority (EFSA) Scientific opinion - Safety and efficacy of guanidinoacetic acid as feed additive for chickens for fattening. EFSA J. 2009;988:1–30. [Google Scholar]
- Ale Sahib Fosoul S.S., Azarfar A., Gheisari A., Khosravinia H. Energy utilisation of broiler chickens in response to guanidinoacetic acid supplementation in diets with various energy contents. Br. J. Nutr. 2018;120:131–140. doi: 10.1017/S0007114517003701. [DOI] [PubMed] [Google Scholar]
- Ale Sahib Fosoul S.S., Azarfar A., Gheisari A., Khosravinia H. Performance and physiological responses of broiler chickens to supplemental guanidinoacetic acid in arginine-deficient diets. Br. Poult. Sci. 2019;60:161–168. doi: 10.1080/00071668.2018.1562156. [DOI] [PubMed] [Google Scholar]
- De Groot A.A., Braun U., Dilger R.N. Efficacy of guanidinoacetic acid on growth and muscle energy metabolism in broiler chicks receiving arginine-deficient diets. Poult. Sci. 2018;97:890–900. doi: 10.3382/ps/pex378. [DOI] [PubMed] [Google Scholar]
- De Groot A.A., Braun U., Dilger R.N. Guanidinoacetic acid is efficacious in improving growth performance and muscle energy homeostasis in broiler chicks fed arginine-deficient or arginine-adequate diets. Poult. Sci. 2019;98:2896–2905. doi: 10.3382/ps/pez036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthmuller P., Van Pilsum J.F., Boen J.R., McGuire D.M. Cloning and sequencing of rat kidney L-arginine:glycine amidinotransferase. Studies on the mechanism of regulation by growth hormone and creatine. J. Biol. Chem. 1994;269:17556–17560. [PubMed] [Google Scholar]
- He D., Yang L., Li J., Dong B., Lai W., Zhang L. Effects of guanidinoacetic acid on growth performance, creatine metabolism and plasma amino acid profile in broilers. J. Anim. Physiol. Anim. Nutr. 2019;103:766–773. doi: 10.1111/jpn.13081. [DOI] [PubMed] [Google Scholar]
- Jiang T., Dai C., Li X.Y., Cheng M. Effects of guanidinoacetic acid on growth performance and slaughter performance of AA broilers. Feed Res. 2012;4:8–10. [Google Scholar]
- Kan H.E., van der Graaf M., Klomp D.W.J., Vlak M.H.K., Padber G.W., Heerschap A. Intake of 13C-4 Creatine enables simultaneous assessment of creatine and phosphocreatine pools in human skeletal muscle by 13C MR Spectroscopy. Magn. Reson. Med. 2006;56:953–957. doi: 10.1002/mrm.21068. [DOI] [PubMed] [Google Scholar]
- Khajali F., Lemme A., Rademacher-Heilshorn M. Guanidinoacetic acid as a feed supplement for poultry. Worlds Poul. Sci. J. 2020 doi: 10.1080/00439339.2020.1716651. [DOI] [Google Scholar]
- Kwak H., Austic R.E., Dietert R.R. Influence of dietary arginine concentration on lymphoid organ growth in chickens. Poult. Sci. 1999;78:1536–1541. doi: 10.1093/ps/78.11.1536. [DOI] [PubMed] [Google Scholar]
- Lee J.E., Austic R.E., Naqi S.A., Golemboski K.A., Dietert R.R. Dietary arginine intake alters avian leukocyte population distribution during infectious bronchitis challenge. Poult. Sci. 2002;81:793–798. doi: 10.1093/ps/81.6.793. [DOI] [PubMed] [Google Scholar]
- Lemme A., Elwert C., Gobbi C.R., Rademacher M. Application of the guanidino acetic acid as creatine source in broilers fed diets with or without fish meal. Proc. 18th Eur. Symp. Poult. Nutr; Izmir, Turkey; 2011. [Google Scholar]
- Lemme A., Rademacher-Heilshorn M., Dilger R.N., Scharch C., Braun U. Arginine sparing potential of guanidinoacetic acid in broiler nutrition. PSA Latin American Scientific Conference; São Paulo, Brazil; 2018. [Google Scholar]
- Li P., Wu G. Composition of amino acids and related nitrogenous nutrients in feedstuffs for animal diets. Amino Acids. 2020;52:523–542. doi: 10.1007/s00726-020-02833-4. [DOI] [PubMed] [Google Scholar]
- Majdeddin M., Braun U., Lemme A., Golian A., Kermanshahi H., De Smet S., Michiels J. Guanidinoacetic acid supplementation improves feed conversion in broilers subjected to chronic cyclic heat stress in the finishing phase associated with improved energy and arginine metabolism. 21st European Symposium on Poultry Nutrition; Salou/Vila-seca, Spain. 8-11th May, 2017; 2017. [Google Scholar]
- Marco J., Calle C., Hedo J.A., Villanueva M.L. Glucagon releasing activity of guanidine compounds in mouse pancreatic islets. FEBS Lett. 1976;64:52–54. doi: 10.1016/0014-5793(76)80246-3. [DOI] [PubMed] [Google Scholar]
- McGuire D.M., Gross M.D., Van Pilsum J.F., Towle H.C. Repression of rat kidney L-arginine:glycine amidinotransferase synthesis by creatine at a pretranslational level. J. Biol. Chem. 1984;259:12034–12038. [PubMed] [Google Scholar]
- Meglasson M.D., Wilson J.M., Yu J.H., Robinson D.D., Wyse B.M., de Souza C.J. Antihyperglycemic action of guanidinoalkanoic acids: 3-guanidinopropionic acid ameliorates hyperglycemia in diabetic KKAy and C57BL6Job/ob mice and increases glucose disappearance in rhesus monkeys. J. Pharmacol. Exp. Ther. 1993;266:1454–1462. [PubMed] [Google Scholar]
- Michiels J., Maertens L., Buyse J., Lemme A., Rademacher M., Dierick N.A., De Smet S. Supplementation of guanidinoacetic acid to broiler diets: effects on performance, carcass characteristics, meat quality, and energy metabolism. Poult. Sci. 2012;91:402–412. doi: 10.3382/ps.2011-01585. [DOI] [PubMed] [Google Scholar]
- Nain S., Ling B., Alcorn J., Wojnarowicz C.M., Laarveld B., Olkowski A.A. Biochemical factors limiting myocardial energy in a chicken genotype selected for rapid growth. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2008;149:36–43. doi: 10.1016/j.cbpa.2007.10.001. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) 9th ed. National Academy Press; Washington DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Ostojic S.M. Advanced physiological roles of guanidinoacetic acid. Eur. J. Nutr. 2015;54:211–1215. doi: 10.1007/s00394-015-1050-7. [DOI] [PubMed] [Google Scholar]
- Ostojic S.M., Stojanovic M.D. Guanidinoacetic acid loading affects plasma ƴ-aminobutyric acid in healthy men. Eur. J. Nutr. 2015;54:855–858. doi: 10.1007/s00394-015-0858-5. [DOI] [PubMed] [Google Scholar]
- Pradeep K.R., Rademacher M., Girish C.K. Meeting creatine needs of modern broilers via guanidinoacetic acid supplementation in diets with or without animal protein. Austr. Poult. Sci. Symp. 2016;27:240–243. [Google Scholar]
- Ringel J., Lemme A., Knox A., McNab J., Redshaw M.S. Effects of graded levels of creatine and guanidino acetic acid in vegetable-based diets on performance and biochemical parameters in muscle tissue. Proc.10th Eur. Symp. Poult. Nutr; Strasbourg, France; 2007. [Google Scholar]
- Ringel J., Rademacher M., Elwert C. Arginine sparing effect of guanidinoacetic acid in broilers. Proc. 19th Eur. Symp. Poult. Nutr; Potsdam, Germany; 2013. [Google Scholar]
- Rostagno H.S., Albino L.F.T., Donzele J.L., Gomes P.C., de Oliviera R.F., Lopes D.C., Ferreira A.S., de Toledo Barreto S.L., Euclides R.F., editors. Brazilian Tables for Poultry and Swine Composition of Feedstuffs and Nutritional Requirements. Universidade Federal de Viçosa; Brazil: 2017. p. 488. [Google Scholar]
- Savabi F., Carpenter C.L., Mohan C., Bessman S.P. The polysome as a terminal for the creatine phosphate energy shuttle. Biochem. Med. Metab. Biol. 1988;40:291–298. doi: 10.1016/0885-4505(88)90131-4. [DOI] [PubMed] [Google Scholar]
- Ross 308 Broiler Nutrient Specifications. 2019. Aviagen, UK.
- Scharch C., Rademacher M., Braun U., Thomson J. The effect of guanidinoacetic acid supplementation on the chemical composition of breast meat in broilers. Poult.Sci. 2019;98:158. [Google Scholar]
- Tossenberger J., Rademacher M., N'emeth K., Halas V., Lemme A. Digestibility and metabolism of dietary guanidino acetic acid fed to broilers. Poult. Sci. 2016;95:2058–2067. doi: 10.3382/ps/pew083. [DOI] [PubMed] [Google Scholar]
- Van Pilsum J.F., Grover C., Stephens C., Taylor D. Distribution of creatine, guanidinoacetate and the enzymes for their biosynthesis in the animal kingdom; implications for phylogeny. Biochem. J. 1972;126:325–345. [PubMed] [Google Scholar]
- Van Pilsum, J.F., D.M. Mcguire, and C.A. Miller. 1992 The antagonistic action of creatine and growth hormone on the expression of the gene for rat kidney L-arginine:glycine amidinotransferase. In: Guanidino Compounds in Biology and Medicine, edited by P.P. De Deyn, B. Marescau, V. Stalon, and I.A. Qureshi. London: p. 147–151.
- Walker J.B. End product repression in the creatine pathway of the developing chick embryo. Adv. Enzyme Regulation. 1963;1:151–168. doi: 10.1016/0065-2571(63)90015-3. [DOI] [PubMed] [Google Scholar]
- Walker J.B. Creatine: biosynthesis, regulation, and function. Adv Enzymol. 1979;50:177–242. doi: 10.1002/9780470122952.ch4. [DOI] [PubMed] [Google Scholar]
- Wu G. Functional amino acids in nutrition and health. Amino acids. 2013;45:407–411. doi: 10.1007/s00726-013-1500-6. [DOI] [PubMed] [Google Scholar]
- Yazdia F.T., Goliana A., Zarghia H., Varidi M. Effect of wheat-soy diet nutrient density and guanidine acetic acid supplementation on performance and energy metabolism in broiler chickens. Ital. J. Anim. Sci. 2017;16:593–600. [Google Scholar]
- Zelenka J., Heger J., de la Cruz C., Machander V., Hampel D. Guanidinoacetic acid as an additive to broiler diets. Proc. 20th European Symp. Poult. Nutr; Prague, Czech Republic; 2015. [Google Scholar]
- Zhang D., Tian Y., Ma J., Zhang J.L., Guo S.G., Yang L. Effect of guanidinoacetic acid on growth performance and economic benefit of AA broilers. Feed Res. 2016;17:32–35. [Google Scholar]