Abstract
The present study describes the generation of Salmonella enteritidis (SE) ghosts with a surface decorated Salmonella Typhimurium (ST) flagellin (FliC) antigen for immune enhancement and strain-specific protection. The ghosts were generated by biological means using pJHL184::fliC temperature inducible plasmid where the lysis occurs by phage PhiX174 lysis gene E expression. Being an inactivated strain, no environmental contamination was observed by fecal shedding upon inoculation into the chicken. To test the protective immune responses, ghost vaccination was conducted via the intramuscular route using chicken as the model organism. The development of antigen-specific humoral, cell-mediated, and protective immune responses was assessed. Compared to vector alone and phosphate-buffered saline (PBS) control groups, pJHL184::fliC ghost could generate significantly high antigen-specific IgY and cell-mediated immune (CMI) responses measured by a peripheral blood mononuclear cell proliferation, flow cytometer, and cytokine responses elicited by stimulated splenic T-cells (P < 0.05). The adjuvant effect induced by FliC was demonstrated by elicitation of Toll-like receptor 5 (TLR5). To test the protection efficacy, chickens were challenged with both SE and ST wild type (WT) strains, and the protection efficacy was assessed by determining the presence of challenging strains in the spleen and liver, and by assessing the histopathological alterations. Complete clearance of the challenged strain and least inflammatory signs were evident in the SE ghosts vaccinated group compared to the vector and PBS control. The elimination of both SE and ST in chicken organs ensures the intramuscular immunization of the present SE ghost vaccine can reduce SE and ST contamination levels in chicken that can be beneficial to prevent enteric infections in humans.
Key words: Salmonella enteritidis, flagellin, phage PhiX174, gene E, wild type challenge
INTRODUCTION
Nontyphoidal salmonellosis (NTS) is one of the major causes of acute gastroenteritis among humans (Kirk et al., 2015). The global incidence of NTS is estimated to be 94 million annual cases, with over 155,000 deaths. Out of it, approximately more than 94% of incidences are due to foodborne transmission (Majowicz et al., 2010; CDC, 2021). Salmonella infections in the USA alone are 1.35 million human cases with 26,500 hospitalizations and 420 deaths annually (CDC, 2021). Both domestic and wild animals are colonized by Salmonella species often without signs of infection. Hence these animals act as carriers causing contamination of the environment and subsequently human food sources. Up to date, over 2500 different Salmonella serotypes have been identified (Eng et al., 2015), and 2 of the serotypes are responsible for the majority of human incidences namely Salmonella enteritidis (SE), responsible for 24.7 %, and Salmonella Typhimurium, responsible for 23.5 % of total human cases in the globe (CDC, 2003; de Freitas Neto et al., 2010). Both these serotypes are prevalent in poultry as the poultry industry is one of the top culprits in the human acquisition of these enteric infections (de Freitas Neto et al., 2010; Antunes et al., 2016). To prevent Salmonella in poultry products, vaccination is one of the most promising strategies available (Nandre et al., 2012; Jawale and Lee, 2016; Jia et al., 2020). In animal vaccination programs, the safety of vaccine candidates is of utmost importance as the vaccine strains should not cause environmental contamination or disease in immunized animals. According to safety perspectives, inactivated vaccines are the safest, however, their immunogenic capacity can be lower than live vaccines (Singh, 2009). An inactivated vaccine that can compensate for the reduction in immune responses could be utterly important to provide both efficacy and safety in a single vaccine design.
In a previous study, we created a SE ghost with surface-displayed Salmonella Typhimurium (ST) flagellin (FliC) antigen as an adjuvant and assessed safety and protective immunity developed in mice (Senevirathne et al., 2020a). This study has been set out to investigate the environmental safety and real protection that the vaccination can be delivered in the most appropriate host, chicken, one of the predominant culprits for human salmonellosis. Intramuscular immunization of chicken with a single booster application could generate sufficient immunity to eliminate both SE and ST, while no environmental contamination of the live vaccine strain. Our findings propose SE ghost JOL2435 with surface-displayed FliC is not only safe but also delivers strong humoral and cell-mediated immune responses in chicken, preventing salmonellosis caused by 2 major serotypes responsible for the highest number of human incidences.
MATERIALS AND METHODS
Bacterial Strains, Plasmids, and Primers
Supplementary Table 1 contains the bacterial strains and plasmids used in the present study. All cultures were grown and maintained in Luria Bertani (LB; BD, Sparks, MD) medium or Brilliant Green Agar (BGA; BD, Sparks, MD) or in LB broth at 37 °C with vigorous shaking. The Salmonella strain hosting ghost plasmid was routinely propagated at 30 °C in the presence of 20 mM L-arabinose. All bacteria were stored in a freezing medium containing 30 % glycerol and stored in -80 °C for future use.
Construction and Characterization of Salmonella Ghosts
The construction of SE carrier strain by deleting lon, cpxR, and asd genes is described in a previous study (Nandre et al., 2013). The temperature-induced SE ghost generation procedure was detailed by Jawale et al. and Hajam et al. (Jawale et al., 2015; Hajam et al., 2017b). Briefly, biological generation of SE ghosts was achieved by employing the lysis gene E of bacteriophage PhiX174 (Halfmann et al., 1993). The lysis gene E was kept under the control of temperature inducible λPR promoter which is suppressed by cI857 suppressor at temperatures lower than 30 °C. The abrupt lysis of the host Salmonella by leaky expression of gene E was effectively prevented by arabinose inducible antisense ParaBAD promoter. The Salmonella Typhimurium FliC open reading frame was amplified by a polymerase chain reaction (PCR) and cloned into multiple cloning sites of the ghost 184 plasmids using EcoRI and HindIII restriction enzymes (Senevirathne et al., 2020a). The plasmid construct was transformed into aspartate semialdehyde dehydrogenase (asd) mutant SE strain JOL1087 (Δlon ΔcpxR Δasd) and designated as JOL2435. A detailed description of SE ghost construction and characterization by cell lysis can be found in a previously published report (Senevirathne et al., 2020a). As a control, the ghost plasmid without fliC was transformed into SE strain JOL1087 and was designated as JOL2436.
Western Blot Analysis
Temporal expression of fliC during the ghost generation was assessed by western blot. The SE strains, JOL 2435, JOL2436 were grown in the presence of 20 mM arabinose at 30 °C with vigorous shaking until the cultures reach 0.6 absorbances at 600 nm (OD600) absorbance. Cells were collected by centrifugation and once washed with PBS and re-suspended in LB. Then 2 mL portions were taken and incubated at 42 °C. At 1, 2, 6, and 8 h time points, cells were collected by centrifugation and dissolved in SDS sample buffer and heated at 95 °C for 10 min. The lysate was resolved in SDS PAGE proceeded with western blot using rabbit hyper-immune serum raised against FliC antigen (Senevirathne et al., 2020b). The intensity of the expected band was quantified by Fiji Image J software (Rueden et al., 2017).
Animal Immunization and Challenge Study
A day-old Brown Nick Layer hens (N=60, n=15) were purchased from a Korean joint hatchery and raised for 1 mo with an ad libitum supply of food and water. All animal experiments were approved by the Jeonbuk National University animal ethics committee (CBNU-2018-00264), in accordance with the guidelines of the Korean Council on Animal Care and Korean Animal Protection Law, 2007: Article 13. At the age of 1 mo, all birds were randomly divided into 4 groups and intra-muscularly immunized with A: JOL2435 (pJHL184:: fliC), B: JOL2436 (pJHL184 vector alone), C: phosphate-buffered saline (PBS) control, and D: naïve control. The dose of ghost inoculation was adjusted to 1 Χ 108 CFU/bird/200 µL in PBS. Booster immunization was carried out 3 wk after the primary inoculation following the same doses and methods as with primary inoculation. Collection of feces to assess the environmental contamination of live Salmonella was assessed in cloacal swab collected on 1st, 3rd, 5th, 7th, 14th, and 21st d post-immunization. Blood collection was done from 8 birds per group by using the wing vein on the 7th, 14th, and 35th d of primary inoculation (Kelly and Alworth, 2013). Two weeks after the booster application, blood collection was done for peripheral blood mononuclear cells (PBMC) isolation from 8 birds per group (Kaiser et al., 2006). The PBMC was used for the assessment of cell-mediated immune responses (CMI) by PBMC proliferation assay, T-cell responses by fluorescent assisted cell sorting (FACS) analysis, and cytokine expression by quantitative real-time PCR (qRT-PCR). After 2 wk of boosting, all groups were challenged with oral inoculation of SE wild type (WT) JOL860 (n=10) and ST WT JOL401 (n=5) at 2 Χ 108 CFU/ bird. Challenged birds were euthanized after 2 wk and 3 wk of the challenge. Fecal shedding of challenged bacteria was assessed in cloacal swab collected on 1st, 3rd, 5th, 7th, and 14th d post-challenge. On the 14th d post-challenge, their spleen and liver samples were aseptically collected and 1 gram of tissue was homogenized in 3 mL of peptone buffered water. Specimens were serially diluted and 100 µL of each homogenate was spread on BGA for colony counting. Colonies on BGA plates were confirmed by PCR using serotype-specific primers or fliC specific primers (Alvarez et al., 2004). A portion from the remaining tissue was processed by eosin and hematoxylin for histopathological assessment (Wan et al., 2019). The challenge study was conducted 2 times and the averages of trials were used in the efficacy assessments.
Assessment of Fecal Shedding
Bacterial presence in fecal shedding was conducted by enrichment study. Collected cloacal swabs were put into 10 mL of Rappaport-Vassiliadis broth (RV broth; BD, Sparks, MD) in 100 mL sterile glass conical flasks and incubated at 42 °C for 24 h (Daquigan et al., 2016). The resulted broth was swirled for mixing and 100 µL was withdrawn, serially diluted, and plated on Brilliant Green Agar (BGA) plates and incubated at 37 °C. Resultant colonies were confirmed by polymerase chain reaction (PCR) using strain-specific primers (Supplementary Table 1).
Assessment of Humoral Responses
The humoral antibody responses were evaluated in immunized chicken. Withdrawal of blood and serum preparation from the chicken was conducted on 7th, 14th, and 35th-d post-primary immunization. The prominent avian antibody subtype IgY levels were determined using an indirect Enzyme-Linked Immunosorbent Assay (ELISA) platform. The outer membrane proteins from SE wild-type challenge strain JOL860 were isolated according to a previous publication (Hamid and Jain, 2008) and the ST FliC was purified by Ni-NTA affinity chromatography (Chandrapala et al., 2014). Proteins were buffer exchanged to PBS using PD midiTrap G-25 ion exchange columns (GE Healthcare, Buckinghamshire, UK) and concentrated by Amicon Ultra-4 10K columns (Millipore, Tullagreen, Ireland). Protein concentrations were measured by the Bradford method (He, 2011). Antibody reactivity was assessed against the purified SE outer membrane proteins or ST FliC antigen by coating at 500 ng/well in 0.5 M Na2CO3/ NaHCO3 coating buffer, overnight at 4 °C. The ELISA plates were washed with PBS 3 times and blocked with 5% Bovine Serum Albumin (BSA; GeneAll, Songpa-gu, Seoul, Korea) for 1 h under room conditions. The serum samples were diluted to a 1:50 ratio and incubated for 2 h at 37 °C. The ELISA plates were washed 3 times with PBS and incubated with horseradish peroxidase (HRP) labeled anti-chicken IgY at 1:3000 dilution (Southern Biotech, Birmingham, AL). After further incubation for 1 h, the reactivity of the immune sera was determined by adding the substrate O-phenylenediamine dichloride (Sigma, St. Louis, MO). The reaction was continued for 10 min at 37 °C at room temperature in the dark. Then the reaction was stopped by adding 2 N H2SO4 50 µL/ well. The final concentrations of antibody were determined by developing a standard curve between known concentrations of purified chicken IgY (Southern Biotechnology, Birmingham, AL) and absorbance values obtained at 492 nm (Hajam et al., 2018). The experiments were repeated in 2 independent trials.
Cell-Mediated Immune Responses
The CMI responses against antigen stimulation were assessed by PBMC proliferation by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay (Nagarajan et al., 2011). The PBMCs were separated by Histopaque 1077 (Sigma, St. Louis, MO) density gradient centrifugation. Collected PBMC was washed with Roswell Park Memorial Institute medium 1640 (RPMI-1640; Lonza, Walkersville, MD) medium and seeded at 1 Χ 106 Cells/well in RPMI medium supplemented with 10 % fetal bovine serum (FBS; Serana, Pessin, Germany). Cells were stimulated with SE outer membrane proteins or FliC purified proteins, 500 ng/well, and continued to incubate for 72 h at 37 °C in a humidified 5 % CO2 atmosphere. In the end, ELISA plates for briefly centrifuged, and the supernatant was aspirated. Developed formazan was dissolved by adding 100 µL of dimethyl sulfoxide and further incubated for 10 min at 37 °C in an aluminum wrap. The development of formazan was measured at 570 nm. Further, changes in T-cell populations were measured using flow cytometry (Miltenyi, Bergisch Gladbach, Germany). Harvested PBMC was seeded in 96-well plates and incubated with purified SE outer membrane proteins or FliC at 500 ng/well concentration. After 72 h, all cells were harvested by centrifugation. Then, cells were once washed with PBS and twice with fluorescence assisted cell sorting (FACS) running buffer and labeled with fluorescence-tagged antibodies, anti-chicken CD3a-PE, CD8-FITC, and anti-chicken CD4-perCP-vio700 antibodies (Miltenyi Biotec, Bergisch Gladbach, Germany) for 30 min on ice. Cells were washed with FACS running buffer 3 times and finally suspended in 200 μl of FACS running buffer and analyzed in the flow cytometer (Macsquant, Miltenyi, Germany). The assay was carried out by gating out appropriate CD3+, CD3+CD4+, and CD3+CD8+ populations using MacsQuant software. The averages of independent 2 trials were considered (Won et al., 2017).
Induction of TLR5 Responses
Human Embryonic Kidney (HEK293T) cells were grown in RPMI-1640 supplemented with 10% FBS at 1 Χ 106 cells/well in 24-well plates and treated with SE ghosts with and without FliC on the surface at 100 multiplicity of infection (MOI). After 4 h, cells were then harvested washed 1 time with PBS and twice with FACS running buffer (Macsquant; Miltenyi, Bergisch Gladbach, Germany). The level of Toll-like receptor-5 (TLR5) expression was assessed by labeling with an anti-human TLR5 antibody (Abcam. Cambridge, UK) as previously described (Won and Lee, 2018). Further chicken PBMC was obtained from 3 chickens and seeded at 1 Χ 106 cell/ well in a 24-well plate. The PBMCs were isolated as described elsewhere using histopaque density separation procedure followed by cells exposing to JOL2435 (184:: fliC ghosts) and JOL2436 (184 ghost vector alone) and media alone for 5 h. Total RNA was extracted from cells using a commercial kit (GenAll R; HybriR, GenAll, Seoul, Korea) as per the manufacturer's instructions. The purified RNA was immediately converted into cDNA using a kit (Toyobo, Tokyo, Japan) according to the manufacturer's instructions. The expression of TLR5 at the mRNA transcript level was quantified using a quantitative real-time polymerase chain reaction (qRT-PCR) (Applied Biosystems; Waltham, MA) Real-Time PCR machine following a previous report and the relative fold change was determined (Hajam et al., 2015). This experiment was repeated twice.
Statistical Analysis
Data analysis was statistically analyzed using the Prism 6.00 GraphPad software (San Diego, CA). Mean comparisons were conducted by 1-way analysis of variance (ANOVA) with Tukey's multiple comparison test. All experiments were conducted at least twice. The mean with ± standard deviation (SD) was demonstrated. The significant difference was determined if P < 0.05. *, **, *** indicates different levels of significance & probability values.
RESULTS
Characterization of SE Ghosts
Bacterial ghost generation was assessed by exposing cells at an elevated temperature at 42 °C without adding L-arabinose. The viability of cells after exposing different time intervals at elevated temperature could be assessed by plating on BGA which confirmed the lysis of bacterial cells. Furthermore, simultaneous expression of FliC antigen could be detected on western blot by detecting the band with a size of 52 kDa (Supplementary Figure 1). Despite the close genomic proximity, the fliC sequence between SE and ST is significantly different (approximately 29% amino acid sequence identity). Amino acid sequence comparison was also revealed that the FliC antigen is highly conserved in the ST genome with more than 99% amino acid sequence identity, however, in SE, the same antigen demonstrates considerable diversity between 162-425 amino acid positions (Supplementary Figure 2).
Environmental Contamination
Enrichment of cloacal swab assessment confirms the complete absence of live Salmonella vaccine strain in the immunized chicken. Assessment of challenged bacterial counts revealed the presence of challenge Salmonella in cloacal swab remained until the 14th-d post-challenge. Recovered strains were confirmed by PCR (Supplementary Table 2).
SE Specific Antibody Responses
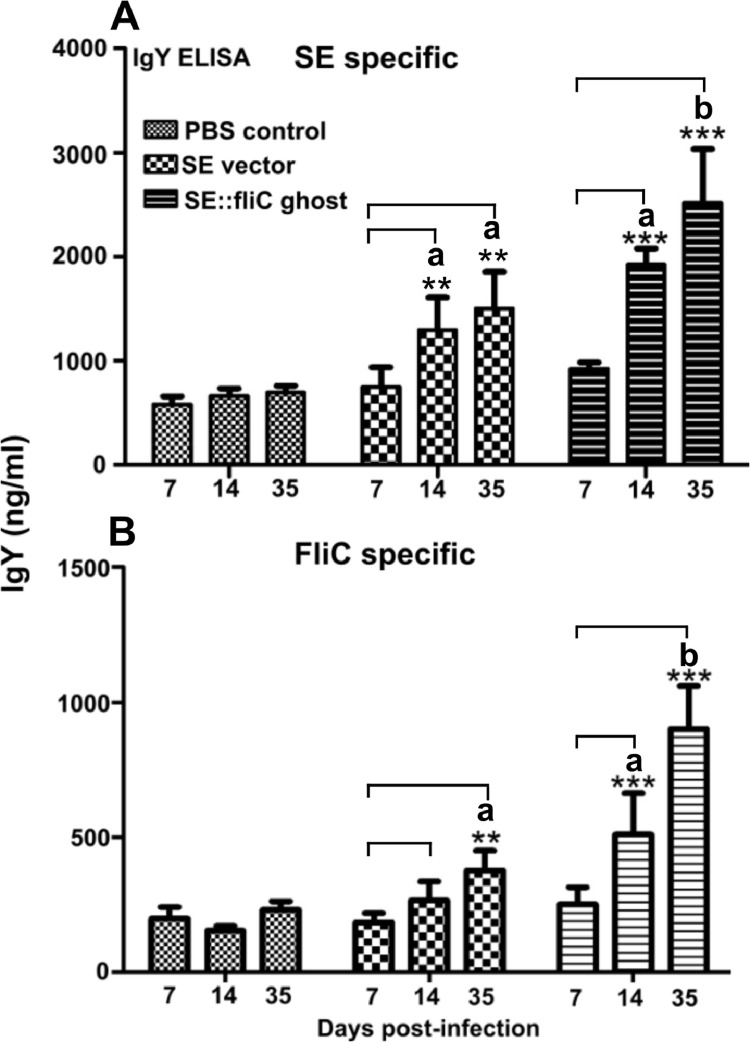
The SE-specific systemic antibody responses in ghost immunized chicken were evaluated in serum collected on 7th, 14th, and 35th-d post-primary immunization. The IgY antibody responses were peaked at 5 wk of primary immunization with JOL2435 ghosts (pJHL184:: fliC). At the end of the second wk, chicken immunized with bacterial ghost vector control also resulted in a significantly high IgY response than the PBS group. However, the response was plateaued after 2 wk and no significant increase could be observed in the 35-d serum IgY response (Figure 1A).
Figure 1.
Humoral responses. A month-old chicken (n = 8) was intramuscularly immunized either with PBS, SE:: fliC ghost, or vector alone control. Three wk after the primary immunization, all groups were boosted using the same inoculation doses used for priming. The IgY antibody responses against SE outer membrane proteins (A) and FliC purified proteins (B) were depicted. Stars indicate a significant increase in antibody responses against the day 7 reaction. Different letters indicate a significant difference for multiple comparisons. Significant differences were determined at P < 0.05.
FliC Specific Antibody Responses
The FliC induced adjuvant effect on chicken immunization was evaluated in serum samples collected on the day 7th, 14th, and 35th after the primary inoculation. The serum antibody levels against the FliC were determined by indirect ELISA using ST-FliC purified proteins. The results indicate a gradual increase in FliC specific antibody response in JOL2435 immunized chicken than the vector alone group (JOL2436) on the 14th d of primary immunization. The IgY peak could be observed after the booster immunization (Figure 1B).
Peripheral Blood Mononuclear Cell Proliferation Assay
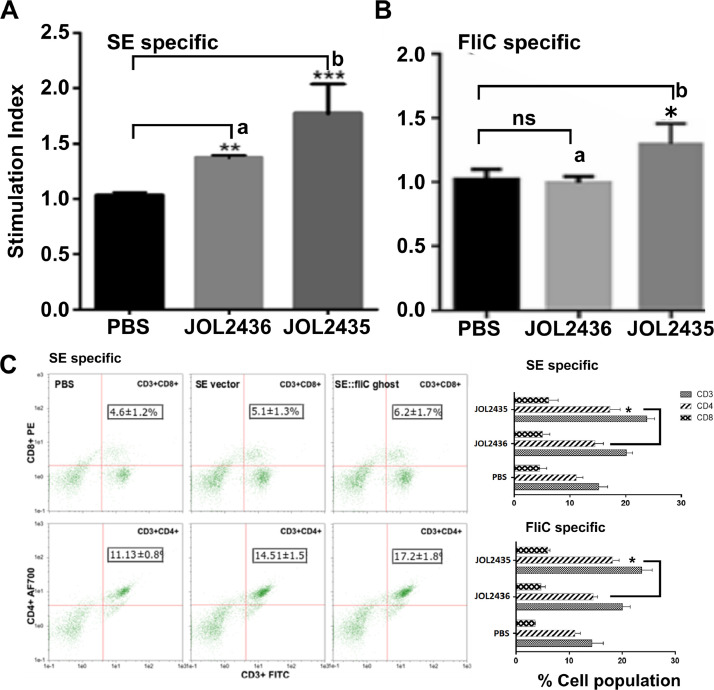
The antigen-specific cell proliferative responses were assessed on PBMC collected from each test group 2 wk of booster immunization and re-stimulated with either SE outer membrane proteins or purified FliC antigens. The PBMC were cultured in 96-well plates at a culture density of 1 Χ 105cells/well and exposed to each antigen for 72 h. After 72 h incubation, antigen-specific cell proliferative responses were quantified by MTT assay. The results demonstrate a significant increase in the PBMC proliferative responses of chicken immunized with JOL2435 compared to the vector alone control (Figure 2A). Both JOL2435 and JOL2436 ghosts significantly induced SE-specific proliferative response, while the FliC specific response was only found with the JOL2435 vaccine strain (Figure 2B).
Figure 2.
Antigen-specific cell-mediated immune responses. Antigen-specific CMI responses were assessed by PBMC proliferative responses by MTT assay and T-cell responses by flow cytometry analysis. (A) demonstrates proliferative responses against SE outer membrane proteins, (B) demonstrates proliferative responses against FliC purified proteins. Mean comparisons were done against the PBS control. Different letters indicate a significant difference in each treatment. (C) Demonstrates flow cytometric analysis for CD3+CD4+ and CD3+CD8+ cell subsets after stimulation either with SE outer membrane proteins or FliC purified proteins. The level of significant difference was determined at P < 0.05.
Flow Cytometry Analysis of T-Cell Populations
The immunization-derived T-cell responses were assessed in PBMC collected after 2 wk of booster immunization. Cells were propagated in a 96-well plate at a concentration of 1 × 105cells/well and exposed to SE outer membrane proteins FliC purified proteins for 27 h. Changes in T-cell populations were quantified by flow cytometry analysis. Results demonstrated a significant increase in CD3+CD4+ and CD3+CD8+ T-cells in immunized chicken with both JOL2435 and JOL2436 immunized chicken compared to the PBS control. However, both SE proteins and FliC specific responses show the significant engagement of CD4+ T-cell responses in JOL2435 immunized chicken than the vector control (P < 0.05) (Figure 2C). Between JOL2535 and JOL2436, there were no significant difference in CD8+ T-cell responses were generated.
Cytokine Response
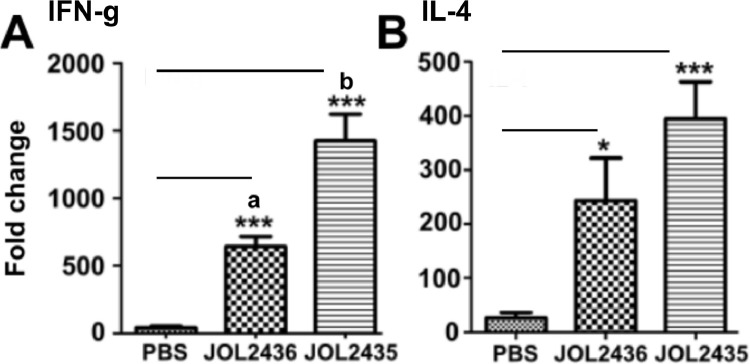
The CMI responses were evaluated by assessing the cytokine responses in immunized chicken. Herein, both Th1 and Th2 type immune responses were evaluated by quantifying the expression of IFN- γ and IL-4 at mRNA level by qRT-PCR. The responses derived from stimulated PBMC demonstrated both IFN- γ and IL-4 cytokine induction occur in chicken immunized with JOL2435 than the PBS control group. The vector alone group too resulted in a significant expression of IFN- γ than the PBS control. These results corroborate the engagement of both Th1 and Th2 type immune responses in JOL2435 ghost immunized chicken that may comply with the previous observations in T-cell responses (Figure 3A and 3B).
Figure 3.
Cytokine responses. The PBMC from immunized chicken groups was harvested 2 wk after the booster application (n = 8). The collected PBMC was washed and seeded in 24-well plates at a seeding density of 1 × 106 cells/well. Upon stimulation with Salmonella outer membrane proteins for 24 h, the total RNA was extracted and converted into cDNA. The elicitation of cytokine responses (A) IFN-γ and (B) IL-4 levels were quantified using qRT-PCR. The housekeeping gene GAPDH was kept as the internal quality control for expression normalization. The ΔΔ−CT values were determined. *** indicates the significant difference against the PBS control. The level of significance was determined at P < 0.05 level. The experiment was repeated twice.
Ghost Induced TLR5 Responses
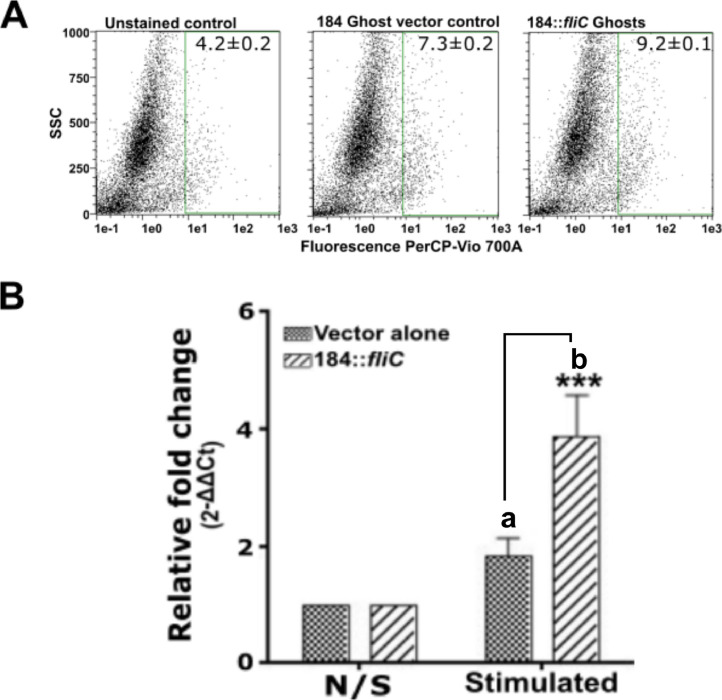
Flagellin interacts with TLR5 receptors on eukaryotic cells during immune elicitation. Ghost-induced TLR5 expression was evaluated using HEK293T human cell line and chicken PBMC by flow cytometry analysis and qRT-PCR respectively. Both HEK293T cells and PBMCs were co-incubated at 1:100 multiplicity of infection with JOL2435, FliC ghosts, and JOL2436 vector alone strains in 24 well plates. After 4 h of co-incubations, HEK293T cells were assessed for surface expression of TLR5 using flow cytometry analysis (Figure 4A), and the PBMC was used for total RNA isolation and qRT-PCR assessment (Figure 4B). The levels of TLR5 expression in both HEK293T and chicken PBMC were significantly augmented by JOL2435 infection as confirmed by both flow cytometric and qRT-PCR analysis.
Figure 4.
Flow cytometric and qRT-PCR analysis of TLR5 expression. HEK cells and chicken PBMCs were stimulated with either SE ghosts, SE:: fliC ghosts, or left unstimulated for 4 h and then analyzed by flow cytometry (A) and qRT-PCR (B), respectively. The qRT-PCR results are expressed as fold change in mRNA transcription after stimulation with either SE ghosts (vector alone) or SE:: fliC ghosts (184:: fliC). The expression values were normalized against the internal quality control gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). *** indicates a significant difference against the vector control. P < 0.05. The average of the 2 trials was demonstrated.
Protective Efficacy
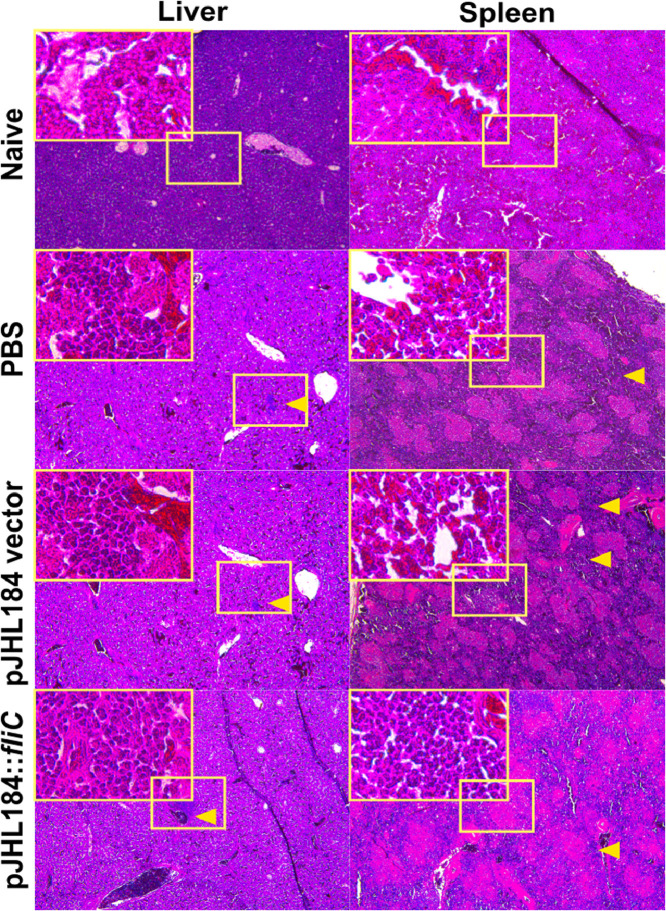
After 3 wk of the challenge, there was no chicken positive for SE or ST in spleen and liver tissues of JOL2435 ghosts immunized groups. A significant reduction in challenged bacterial load could be observed in spleen and liver tissues of chicken immunized with JOL2435 ghosts, as compared to that in the vector control (Supplementary Table 3). The level of pathogen-induced inflammatory responses was also low in JOL2435 ghost immunized groups than the vector or PBS control. Splenic pathological signs also demarcated an expansion of red pulp regions and heavy infiltration of macrophages in the PBS group compared to the vector control and SE ghost treated groups (Figure 5).
Figure 5.
Histopathological examinations. Spleen and liver specimens from immunized and challenged chicken were collected 2 wk after the challenge using 5 birds/ group. Tissues were fixed in a 19% formaldehyde solution for 3 d, washed, and embedded in paraffin for sectioning. Specimens were processed for hematoxylin and eosin staining and examined under a light microscope (Leica, Wetzlar, Germany). The yellow arrow indicates the areas of profound inflammation marked with heavy macrophage infiltration. In spleen sections, expanded and diffused red pulp also visible compared to the PBS control group.
DISCUSSION
Under field conditions, SE and ST infections in chickens remain subclinical and remain without obvious symptoms. This increases the risk of food contamination with Salmonella species. Immunization of poultry flocks may be the most effective way of preventing salmonellosis and thereby preventing human transmission through poultry products (Filho et al., 2009). Giving priority towards safety and efficacy aspects, we developed a SE ghost vaccine that surfaces display ST-FliC antigen. The scope of this study is to evaluate vaccine efficacy against SE and ST infection in the chicken model to assess the potential of the novel biologically generated ghost vaccine candidate. As a strategy to induce strain-specific protective immune responses, here SE ghosts were created with a surface decorated ST-FliC antigen (Honko et al., 2006; Hajam et al., 2017a). The whole FliC antigen's amino acid sequence is highly conserved in the ST genome, whereas in the SE, it is highly variable between 161 and 425 amino acid residues (Supplementary Figure 2). Furthermore, the FliC amino acid sequence between the 2 serotypes was significantly different. Hence, the selection of ST FliC can be a reasonable choice to induce adaptive immune responses against both SE and ST serotypes. During bacterial inactivation approaches, most immunogens degrade their potency due to adverse conditions employed in the inactivation process (Supplementary Figure 1). However, deliberate expression of FliC on the bacterial ghost surface may compensate for any reduction in immunogenicity that can occur during the ghost generation by cellular lysis. The data acquired from in vivo chicken study and in vitro studies demonstrate SE ghosts with surface ST-FliC antigen significantly improve antigen-specific humoral and cell-mediated immune responses by activating proinflammatory pathways such as FliC induced TLR5 responses (Mizel and Bates, 2010) and completely safe with no environmental contaminations with live Salmonella.
The temperature-induced switching mechanism of ghost plasmids pJHL184::fliC eliminates the requirement of lethal heat or chemical inactivators, thus generating bacterial ghosts by simple temperature shift up to 42 °C in the absence of L-arabinose. Temperature induction expresses both the lysis gene E and the target antigen FliC at the same time due to inducible λPr promoter. At 42 °C, the repressor cI857 is eliminated, thus the hindrance for gene E expression is removed. The complete lysis could be achieved within 36 h of induction under a non-permissive temperature. Due to the absence of harsh conditions, native antigenic properties are well preserved (Makrides, 1996; Rabea et al., 2018). The leaky expression of gene E was minimized by placing it between 2 convergent promoters λPR and ParaBAD. The ParaBAD promoter encodes the antisense RNA in the presence of L-arabinose (Huang et al., 2015), thus, abrupt lysis of the host bacterium is eliminated until the required bacterial cell density is reached (Jawale et al., 2014). The efficient export of FliC on the surface of the bacterial cell envelope was facilitated by the outer membrane protein A signal sequence that was derived from the Escherichia coli genome (Senevirathne et al., 2020a).
The JOL2435, SE:: fliC bacterial ghosts were well safe to be inoculated at >107 CFU/mice via IM route without any observable adverse reactions in the mice model (data not are shown). During preliminary studies, we inoculated chicken via IM route with 107, 108, and 109 CFU/bird and found 1 Χ 108 dose as the appropriate inoculum level for immune elicitation. Therefore, for chicken immunization, 1 Χ 108 CFU/bird/ 200 µL of PBS via IM route was determined as optimum for the prime-booster immunization approach. No commercial adjuvant was incorporated in the formulation, to evaluate the effect of FliC adjuvant function. The immunization of chicken with JOL2435 SE:: fliC ghosts elicited higher antigen-specific IgY responses than the vector control, which demonstrates the potential influence of the FliC mediated adjuvant effect. There also can be hyper-flagellation, where the JOL2435 bacterial ghosts display both SE and ST flagellin on the cell surface (Legnani-Fajardo et al., 1991; Campodónico et al., 2010). Flagellin is a pathogen-associated molecular pattern that interacts with the Toll-like receptor 5 (TLR-5) (Figure 5A) and elicits a pro-inflammatory immune response in the host. Besides, vaccination with SE JOL2435 ghosts eliminated both SE and ST serotypes that are the most prominent etiological agents for human salmonellosis. The intramuscular route of SE ghost inoculation is particularly advantageous, because of the ability to bypass the harsh gastric environment. Further, bacterial ghosts are dead cells that cannot actively invade the intestinal epithelium.
Systemic humoral responses are an important segment of adaptive immunity. The antibody-mediated opsonization makes efficient uptake by macrophages thus enabling rapid elimination (Oh et al., 2014;Won and Lee, 2017). Immunization of SE ghosts demarcates significant IgY antibody responses that ensure antibody-mediated protection after immunization. The PBMC proliferation assay demarcates cell-mediated immune responses that visualize the responsiveness of T-cells upon exposure to the same immunized antigens. Here we observed both vectors alone JOL2436 and SE JOL2435 ghosts derive significant proliferative response than the PBS control, whereas the significant hike represented in SE JOL2435 ghost can be attributed to surface-displayed FliC antigen. Further, flow cytometric analysis of splenic T-cell populations also corroborates antibody-mediated immune responses showing a particular skewed pattern towards the CD3+CD4+ T-cell subset. Compared to the PBS control both JOL2435 and JOL2436 ghosts revealed significant engagement of CD4+ and CD8+ T cell responses that are essential to eliminate intracellular pathogens (Buchmeier and Heffron, 1989). To further confirm CMI responses, both IFN- γ and IL-4 cytokine levels were measured in stimulated PBMC as indicators for Th1 and Th2 type immune responses. Cytokine responses demonstrate Th1 oriented immune responses which can be connected with CD4+ immune responses, as helper T-cells, they can differentiate either towards Th1 or Th2 type T-cells. Here too, both vector alone and SE JOL2435 ghosts resulted in a significantly high response than the PBS control whereas the significant difference between the vector alone and SE JOL2435 can be attributed to surface-displayed FliC. We also observed, SE JOL2435 ghosts were efficiently uptake and presented by macrophage cells as one of the professional antigen-presenting cell types in the body (Senevirathne et al., 2020a). More importantly, SE JOL2435 immunization in chicken derived complete protection against SE and ST oral challenge resulting in the complete absence of challenged bacteria in spleen and liver tissues 14-d after the challenge. These observations are further supported by histopathological examination of spleen and liver tissues demonstrated by reduced inflammation in FliC ghosts immunized groups upon the virulent challenge.
Our results demonstrate that the incorporation of FliC on SE ghosts augments humoral and CMI responses in immunized chicken and eliminates the systemic presence of SE and ST wild-type strains. Thus, ghost immunization may prevent contamination levels of the most prominent Salmonella serotypes SE and ST in poultry farms that could be useful to prevent horizontal transfer into human communities.
ACKNOWLEDGEMENTS
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through Animal Disease Management Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (grant number. 121006-01-1-HD020).
Disclosures
The authors declare that they have no competing interests.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2021.101205.
Appendix. Supplementary materials
Supplementary Figure 1 Temporal expression of fliC on bacterial ghosts. To evaluate the expression of foreign antigen FliC during the bacterial ghost generation, cells were collected at different time intervals after exposing to non-permissible growth conditions at 42 °C, without L-arabinose. Collected cells were lysed in SDS sample buffer, heated for denaturation, and resolved in SDS PAGE. Western blot was conducted using FliC specific rabbit hyperimmune sera. The specific increase of the expected band at 52 kDa was demonstrated by the arrow. The band intensity was also measured.
Supplementary Figure 2 Sequence comparison of Salmonella enteritidis and Salmonella typhimurium FliC antigen. The FliC antigen is highly conserved in the ST genome with more than 99% amino acid sequence identity, whereas, in the SE genome, the same antigen show high variability between 16-425 amino acid residues. The sequence logo was generated using Web logo software to demonstrate the sequence conservation in the variable region.
REFERENCES
- Alvarez J., Sota M., Vivanco A.B., Perales I., Cisterna R., Rementeria A., Garaizar J. Development of a multiplex PCR technique for detection and epidemiological typing of Salmonella in human clinical samples. J. Clin. Microbiol. 2004;42:1734–1738. doi: 10.1128/JCM.42.4.1734-1738.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes P., Mourão J., Campos J., Peixe L. Salmonellosis: the role of poultry meat. Clin. Microbiol. Infect. 2016;22:110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Buchmeier N.A., Heffron F. Intracellular survival of wild-type Salmonella Typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect. Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campodónico V.L., Llosa N.J., Grout M., Döring G., Maira-Litrán T., Pier G.B. Evaluation of flagella and flagellin of Pseudomonas aeruginosa as vaccines. Infect. Immun. 2010;78:746–755. doi: 10.1128/IAI.00806-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Reptile-associated salmonellosis–selected states, 1998-2002. MMWR Morb. Mortal. Wkly. Rep. 2003;52:1206–1209. [PubMed] [Google Scholar]
- CDC. Salmonella Homepage. 2021 Accessed Apr. 2021. https://www.cdc.gov/salmonella/index.html#:~:text=CDC%20estimates%20Salmonella%20bacteria%20cause,%2C%20fever%2C%20and%20stomach%20cramps. [Google Scholar]
- Chandrapala D., Kim K., Choi Y., Senevirathne A., Kang D.-H., Ryu S., Kim K.-P. Putative inv is essential for basolateral invasion of Caco-2 cells and acts synergistically with OmpA to affect in vitro and in vivo virulence of Cronobacter sakazakii ATCC 29544. Infect. Immun. 2014;82:1755–1765. doi: 10.1128/IAI.01397-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daquigan N., Grim C.J., White J.R., Hanes D.E., Jarvis K.G. Early recovery of Salmonella from food using a 6-hour non-selective pre-enrichment and reformulation of tetrathionate broth. Front. Microbiol. 2016;7:1–12. doi: 10.3389/fmicb.2016.02103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng S.K., Pusparajah P., Ab Mutalib N.S., Ser H.L., Chan K.G., Lee L.H. Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015;8:184–193. [Google Scholar]
- Filho R.A.C.P., de Paiva J.B., Argüello Y.M.S., da Silva M.D., Gardin Y., Resende F., Junior A.B., Sesti L. Efficacy of several vaccination programmes in commercial layer and broiler breeder hens against experimental challenge with Salmonella enterica serovar enteritidis. Avian Pathol. 2009;38:367–375. doi: 10.1080/03079450903183645. [DOI] [PubMed] [Google Scholar]
- de Freitas Neto O.C., Penha Filho R.A.C., Barrow P., Berchieri J. Sources of human non-typhoid salmonellosis: a review. Rev. Bras. Cienc. Avic. 2010;12:01–11. [Google Scholar]
- Hajam I.A., Dar P.A., Appavoo E., Kishore S., Bhanuprakash V., Ganesh K. Bacterial ghosts of Escherichia coli drive efficient maturation of bovine monocyte-derived dendritic cells. PLoS One. 2015;10:e0144397. doi: 10.1371/journal.pone.0144397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajam I.A., Dar P.A., Shahnawaz I., Jaume J.C., Lee J.H. Bacterial flagellin—a potent immunomodulatory agent. Exp. Mol. Med. 2017;49:e373. doi: 10.1038/emm.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajam I.A., Dar P.A., Won G., Lee J.H. Bacterial ghosts as adjuvants: mechanisms and potential. Vet. Res. 2017;18:37. doi: 10.1186/s13567-017-0442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajam I.A., Kim J.H., Lee J.H. Incorporation of membrane-anchored flagellin into Salmonella gallinarum bacterial ghosts induces early immune responses and protection against fowl typhoid in young layer chickens. Vet. Immunol. Immunopathol. 2018;199:61–69. doi: 10.1016/j.vetimm.2018.03.011. [DOI] [PubMed] [Google Scholar]
- Halfmann G., Götz F., Lubitz W. Expression of bacteriophage PhiX174 lysis gene E in Staphylococcus carnosus TM300. FEMS Microbiol. Lett. 1993;108:139–143. doi: 10.1111/j.1574-6968.1993.tb06089.x. [DOI] [PubMed] [Google Scholar]
- Hamid N., Jain S.K. Characterization of an outer membrane protein of Salmonella enterica serovar Typhimurium that confers protection against typhoid. Clin. Vaccine Immunol. 2008;15:1461–1471. doi: 10.1128/CVI.00093-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F. Bradford Protein Assay. Bio-Protocol. 2011;101 [Google Scholar]
- Honko A.N., Sriranganathan N., Lees C.J., Mizel S.B. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect. Immun. 2006;74:1113–1120. doi: 10.1128/IAI.74.2.1113-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Yuan Z., Liu P., Zhou T. Effects of promoter leakage on dynamics of gene expression. BMC Syst. Biol. 2015;9:16. doi: 10.1186/s12918-015-0157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawale C.V., Kim S.W., Lee J.H. Tightly regulated bacteriolysis for production of empty Salmonella enteritidis envelope. Vet. Microbiol. 2014;169:179–187. doi: 10.1016/j.vetmic.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Jawale C.V., Lee J.H. Evaluation of immunogenicity and protective efficacy of adjuvanted Salmonella Typhimurium ghost vaccine against salmonellosis in chickens. Vet. Q. 2016;36:130–136. doi: 10.1080/01652176.2016.1138248. [DOI] [PubMed] [Google Scholar]
- Jawale C.V., Somsanith N., Eo S.K., Park S.Y., Lee J.H. Evaluation of Salmonella Gallinarum ghost formulated with MontanideTM ISA 70 VG adjuvant as a vaccine against fowl typhoid. Acta Vet. Hung. 2015;63:401–412. doi: 10.1556/004.2015.038. [DOI] [PubMed] [Google Scholar]
- Jia S., McWhorter A.R., Andrews D.M., Underwood G.J., Chousalkar K.K. Challenges in vaccinating layer hens against Salmonella typhimurium. Vaccines. 2020;8:1–12. doi: 10.3390/vaccines8040696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M.G., Cheeseman K., Kaise J.H., P., Lamonth S.J. Cytokine expression in chicken peripheral blood mononuclear cells after in vitro exposure to Salmonella enterica serovar Enteritidis. Poult. Sci. 2006;85:1907–19011. doi: 10.1093/ps/85.11.1907. [DOI] [PubMed] [Google Scholar]
- Kelly L.M., Alworth L.C. Techniques for collecting blood from the domestic chicken. Lab. Anim. (NY). 2013;42:359–361. doi: 10.1038/laban.394. [DOI] [PubMed] [Google Scholar]
- Kirk M.D., Pires S.M., Black R.E., Caipo M., Crump J.A., Devleesschauwer B., Döpfer D., Fazil A., Fischer-Walker C.L., Hald T., Hall A.J., Keddy K.H., Lake R.J., Lanata C.F., Torgerson P.R., Havelaar A.H., Angulo F.J. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legnani-Fajardo C., Zunino P., Algorta G., Laborde H.F. Antigenic and immunogenic activity of flagella and fimbriae preparations from uropathogenic Proteus mirabilis. Can. J. Microbiol. 1991;37:325–328. doi: 10.1139/m91-052. [DOI] [PubMed] [Google Scholar]
- Majowicz S.E., Musto J., Scallan E., Angulo F.J., Kirk M., O'Brien S.J., Jones T.F., Fazil A., Hoekstra R.M. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- Makrides S.C. Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol. Rev. 1996;60:512–538. doi: 10.1128/mr.60.3.512-538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S.B., Bates J.T. Flagellin as an adjuvant: cellular mechanisms and potential. J. Immunol. 2010;185:5677–5682. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan G., Ravikumar P., Kumar C.A., Reddy G.R., Dechamma H.J., Suryanarayana V.V.S. Self replicating gene vaccine carrying P1-2A gene of FMDV serotype O and its effects on the immune responses of cattle. Indian J. Virol. 2011;22:50–58. doi: 10.1007/s13337-011-0032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandre R.M., Jawale C.V., Lee J.H. Adjuvant effect of Escherichia coli heat labile enterotoxin B subunit against internal egg contamination in domestic fowl immunised with a live Salmonella enterica serovar Enteritidis vaccine. Vet. J. 2013;197:361–367. doi: 10.1016/j.tvjl.2013.03.024. [DOI] [PubMed] [Google Scholar]
- Nandre R.M., Matsuda K., Chaudhari A.A., Kim B., Lee J.H. A genetically engineered derivative of Salmonella enteritidis as a novel live vaccine candidate for salmonellosis in chickens. Res. Vet. Sci. 2012;93:596–603. doi: 10.1016/j.rvsc.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Oh J.Z., Ravindran R., Chassaing B., Carvalho F.A., Maddur M.S., Bower M., Hakimpour P., Gill K.P., Nakaya H.I., Yarovinsky F., Sartor R.B., Gewirtz A.T., Pulendran B. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014;41:478–492. doi: 10.1016/j.immuni.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabea S., Salem-Bekhit M.M., Alanazi F.K., Yassin A.S., Moneib N.A., Hashem A.E.M. A novel protocol for bacterial ghosts’ preparation using tween 80. Saudi Pharm. J. 2018;26:232–237. doi: 10.1016/j.jsps.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueden C.T., Schindelin J., Hiner M.C., DeZonia B.E., Walter A.E., Arena E.T., Eliceiri K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18:1–26. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senevirathne A., Hewawaduge C., Lee J.H. Salmonella enterica serovar Enteritidis ghosts displaying a surface FliC adjuvant elicit a robust immune response and effective protection against virulent challenge. Vet. Microbiol. 2020;243 doi: 10.1016/j.vetmic.2020.108633. [DOI] [PubMed] [Google Scholar]
- Senevirathne A., Hewawaduge C., Lee J.H. Live vaccine consisting of attenuated Salmonella secreting and delivering Brucella ribosomal protein L7/L12 induces humoral and cellular immune responses and protects mice against virulent Brucella abortus 544 challenge. Vet. Res. 2020;51:6. doi: 10.1186/s13567-020-0735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B.R. Salmonella vaccines for animals and birds and their future perspective. Open Vaccine J. 2009;2:100–112. [Google Scholar]
- Wan Z., Wang X., Zhang X., Chen H. Intrasplenic transplantation of cytotoxic T-lymphocyte associated protein 4-Fas ligand–modified hepatic oval cells for acute liver injury in rats. Transplant. Proc. 2019;51:942–950. doi: 10.1016/j.transproceed.2019.01.060. [DOI] [PubMed] [Google Scholar]
- Won G., Hajam I.A., Lee J.H. Improved lysis efficiency and immunogenicity of Salmonella ghosts mediated by co-expression of λ phage holin-endolysin and ɸX174 gene E. Nat. Publ. Gr. 2017;7:45139. doi: 10.1038/srep45139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won G., Lee J.H. Salmonella Typhimurium, the major causative agent of foodborne illness inactivated by a phage lysis system provides effective protection against lethal challenge by induction of robust cell-mediated immune responses and activation of dendritic cells. Vet. Res. 2017;48:1–12. doi: 10.1186/s13567-017-0474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won G., Lee J.H. Antigenic and functional profiles of a Lawsonia intracellularis protein that shows a flagellin-like trait and its immuno-stimulatory assessment. Vet. Res. 2018;49:17. doi: 10.1186/s13567-018-0515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Temporal expression of fliC on bacterial ghosts. To evaluate the expression of foreign antigen FliC during the bacterial ghost generation, cells were collected at different time intervals after exposing to non-permissible growth conditions at 42 °C, without L-arabinose. Collected cells were lysed in SDS sample buffer, heated for denaturation, and resolved in SDS PAGE. Western blot was conducted using FliC specific rabbit hyperimmune sera. The specific increase of the expected band at 52 kDa was demonstrated by the arrow. The band intensity was also measured.
Supplementary Figure 2 Sequence comparison of Salmonella enteritidis and Salmonella typhimurium FliC antigen. The FliC antigen is highly conserved in the ST genome with more than 99% amino acid sequence identity, whereas, in the SE genome, the same antigen show high variability between 16-425 amino acid residues. The sequence logo was generated using Web logo software to demonstrate the sequence conservation in the variable region.