Abstract
The global obesity pandemic is among the most significant public health crises today. Furthermore, obesity remains a major risk factor for many weight-related comorbid conditions including cardiovascular disease, type 2 diabetes mellitus, liver disease, and cancer. Endoscopic bariatric therapies are currently on the rise as a new tool in the fight against the obesity epidemic, offering patients an alternative to more invasive surgery and a more effective option than diet and lifestyle modifications. The aim of this review article is to summarize the current literature regarding endoscopic bariatric therapies and their impact on obesity and its associated metabolic complications.
Keywords: endobariatric therapy, endoscopic sleeve gastroplasty, obesity
Introduction
Obesity is an epidemic and a major risk factor for many weight-related comorbid conditions. 1 In the United States alone, the Centers for Disease Control and Prevention (CDC) estimates that 93.3 million (39.8%) adults are classified as obese. 2 Furthermore, the World Health Organization (WHO) predicts that over the next decade, the rising prevalence of obesity will also contribute to a reduction in life expectancy of approximately 7 years in patients with obesity compared with normal-weight adults.1,3
Many of the leading causes of preventable death among adults are obesity-related comorbidities; most notably, these include nonalcoholic fatty liver disease (NAFLD), type 2 diabetes mellitus (T2DM), coronary heart disease, chronic renal disease, and some types of cancer (e.g. endometrial, breast, and colon).4–6 Moreover, it is well established that obesity is associated with an increase in all-cause mortality independent of age, race, and sex. 7 Bariatric surgery is an effective option in the management of obesity and its related comorbidities. There is clear evidence that type 2 diabetes can be cured by bariatric surgery. 8 In fact, the American Diabetes Association and Diabetes Surgery Summit (DSS-II) consensus conference advises that bariatric surgery should be recommended to treat type 2 diabetes in select patients and be included in the overall diabetes treatment algorithm.9,10 However, only a very small percentage of patients with obesity who qualify undergo bariatric surgery. 11
Endoscopic bariatric therapies (EBTs) are currently on the rise as a new tool in the fight against the obesity epidemic, offering patients an alternative to more invasive surgery and a more effective option than diet and lifestyle modifications. The aim of this review article was to summarize the current literature regarding EBTs and their impact on obesity and its associated metabolic complications.
Gastric endobariatric therapies
Intragastric balloon therapy
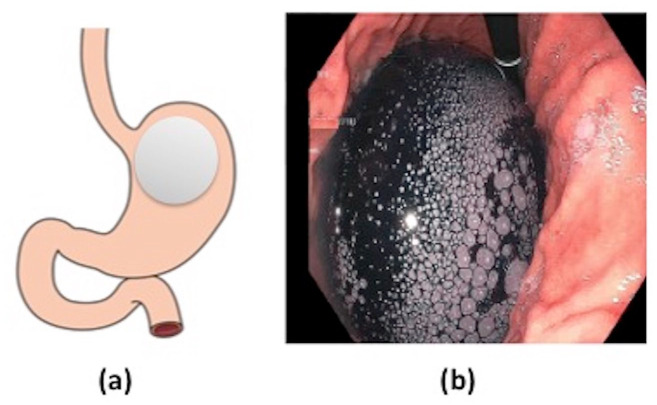
Among the most widely available endobariatric procedures is the intragastric balloon (IGB). The IGB is a minimally invasive endoscopic weight loss procedure that reduces gastric volume, suppresses intake by vagal signaling through stimulation of gastric mechanoreceptors, and delays gastric emptying (Figure 1).12–14 Furthermore, it is a temporary and completely reversible intervention, with balloons usually being removed 6 months after placement. In a meta-analysis by the American Society for Gastrointestinal Endoscopy (ASGE) Bariatric Endoscopy Task Force, IGB therapy was found to achieve greater than 5% total body weight loss (TBWL) in patients with a body mass index (BMI) above 30 kg/m2 who previously failed diet and lifestyle interventions. 15 The multicenter, open-label randomized control trial conducted by Courcoulas and colleagues 16 found that patients in the group who underwent behavioral medication with changes in lifestyle in addition to the IGB achieved a TBWL of 10.2% compared with 3.3% in the lifestyle modification-only group at 6 months after implantation (Table 1).
Figure 1.
(a) Intragastric balloon and (b) endoscopic image of Orbera gastric balloon after inflation in gastric body.
Table 1.
Summary of weight loss and metabolic disease outcomes for various endobariatric interventions.
| Procedure | TBWL at 12 months | HbA1c (%) | HOMA-IR | ALT (IU/l) | Improved hepatic steatosis | Improved liver fibrosis |
|---|---|---|---|---|---|---|
| Intragastric balloon15–20 | 7.6–9.2% | −1.2% | N/A | −52 | + | + |
| Endoscopic sleeve gastroplasty21–29 | 16.5% | −0.4% | −3.8 | −5.0 | + | + |
| Aspiration therapy30–32 | 14.2% | −0.3% | N/A | −15.5 | N/A | N/A |
| Duodenal mucosal resurfacing33,34 | 2–3% (at 6 months) | −1.0% | −3.3 | −10.0 | N/A | N/A |
| Duodenal-jejunal bypass liner35–38 | 14.6% | −1.3% | −4.6 | N/A | N/A | N/A |
| Endoluminal magnetic partial jejunal diversion 39 | 14.6% | −1.9% | N/A | N/A | N/A | N/A |
ALT, alanine transaminase; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; TBWL, total body weight loss.
All values reported as mean difference.
A number of recent studies have further evaluated the impact of IGB therapy on the metabolic complications of obesity (Table 1). A 2016 systematic review and meta-analysis by Popov and colleagues found that following IGB therapy, there was a mean decrease in alanine transaminase (ALT) of 10.02 U/l and in gamma-glutamyl transferase (GGT) of 9.98 U/l, as well as a mean decrease in BMI of 4.98 kg/m2. The changes in transaminases following IGB therapy represented a 29% decrease from baseline in ALT and 27.3% decrease in GGT. They also found that the decreases in ALT and GGT correlated with improvement in measured liver fat by magnetic resonance imaging (MRI) or abdominal ultrasound. 17 A follow-up meta-analysis in 2017 by Popov and colleagues evaluated the impact of IGB therapy on metabolic syndrome. They found that studies with a mean baseline fasting blood glucose (FBG) greater than 100 mg/dl had a 15% decrease in FBG; HbA1c decreased by 17% from baseline; triglycerides and ALT decreased by 23% and 29%, respectively, from baseline. 18
A recent prospective, single-blinded observational study by Bazerbachi and colleagues 19 further characterized the impact of IGB therapy on NAFLD. They evaluated 21 patients with biopsy-proven nonalcoholic steatohepatitis (NASH) and evidence of early hepatic fibrosis and found that there was significant decrease in aspartate aminotransferase (AST) (mean difference of −36.2 IU/l ± 9.8) and ALT (mean difference of −52 IU/l ± 17.3) at the time of balloon removal. The nonalcoholic fatty liver disease activity score (NAS) improved in 90% of patients, with a median decrease of 3 points. Post-intervention, histologic fibrosis improved by 1.17 stages in 15% of patients. Half of the patients in the cohort reached endpoints approved by the Food and Drug Administration (FDA) for NASH resolution and fibrosis improvement. Interestingly, they found that the degree of weight loss achieved did not correlate with reductions in NAS or liver fibrosis. They also found that IGB therapy had significant impact on insulin resistance, resulting in a significant reduction in HbA1c (mean difference of −1.2%) and FBG (mean difference of −22.9 mg/dl). 19 Taken together, these results suggest that the IGB may provide another treatment option for patients with significant metabolic complications of obesity.
Endoscopic sleeve gastroplasty
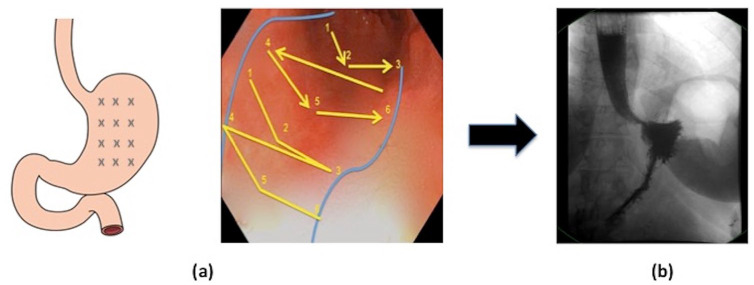
Endoscopic sleeve gastroplasty (ESG) is a minimally invasive procedure involving endoscopic full-thickness suturing to reduce the gastric volume and change digestive physiology (Figure 2). A number of prior studies have demonstrated the weight loss efficacy and procedural safety of ESG in patients with obesity.21–24 Studies report the TBWL at 12 months following ESG to be between 15.0% and 17.6%.21–24 A recent systematic review and meta-analysis by Hedjoudje and colleagues 25 similarly found that the mean TBWL at 12 months following ESG was 16.5% (Table 1). Sharaiha and colleagues 26 recently reported long-term outcomes following ESG and showed that weight loss was sustained up to 5 years post procedure, with a mean TBWL at 5 years of 14.5%.
Figure 2.
Endoscopic sleeve gastroplasty (ESG) (a) stitch pattern for sleeve formation. (b) Gastric emptying study following ESG demonstrating reduced gastric volume.
A 2017 observational study by Sharaiha and colleagues 22 showed that ESG induces favorable changes to obesity-related comorbidities and metabolic complications. They found that at 12 months following ESG there were significant reductions in HbA1c, transaminases, triglyceride level, and systolic blood pressure (SBP). 22 A recent follow-up study by Hajifathalian and colleagues 27 evaluated the impact of ESG on insulin resistance and NAFLD. They found that ESG resulted in significant improvement in insulin resistance through reduced Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) scores that were sustained at 2-year post-procedure (mean difference of −3.8). The biochemical loss of insulin resistance was mirrored by the clinical effect, with a significant decrease in mean A1c at 2 years following ESG (mean difference of −0.4%). They also found significant improvements in liver-related parameters, with a significant decrease in aminotransferase levels, as well as decreases in the mean hepatic steatosis index (HSI) and NAFLD fibrosis score over 2 years of follow-up after ESG (Table 1). In fact, 20% of patients with risk of severe or indeterminate fibrosis at baseline achieved sustained resolution at 2-year post procedure. 27
The mechanisms driving the weight loss and improved metabolic parameters following ESG remain poorly understood. A recent study by Lopez-Nava and colleagues also found that at 6 months following ESG there was a trend toward decreased fasting insulin levels and a significant improvement of HOMA-IR. The authors further examined the impact of ESG on gut hormone pathways and found that at 6 months post-procedure, there was a significant decline in serum leptin levels. They did not observe any change in fasting ghrelin levels, Glucagon-like peptide-1 (GLP-1), and Peptide YY (PYY). 28 The 2020 study by Hajifathalian and colleagues 27 also evaluated the effect of ESG on leptin and observed a statistically significant decrease in leptin levels from baseline at 2 years post procedure. The sustained decrease in leptin following ESG may play a role in improved insulin sensitivity following ESG and warrants further study. Still, these findings suggest that gut hormone (ghrelin, GLP-1, PYY) changes play little role in weight loss or metabolic improvements after ESG. Interestingly, studies show that ESG significantly delays the gastric emptying process, increasing the time for 50% emptying of solid by 90 min and promoting early satiety and decreased caloric intake. 29 Ultimately, it may be these changes in gastric emptying and time to satiation that best explain the weight loss and metabolic effects of ESG and also warrants further study.
Aspiration therapy
The AspireAssist System (Aspire Bariatrics, King of Prussia, PA, USA) is a percutaneous endoscopic gastrostomy tube (A tube) with an external port to allow the partial drainage of gastric contents (Figure 3). Via the device, patients can aspirate 20–30 min after a meal up to 3 meals per day. 30 The U.S. Food and Drug Administration (FDA) recently approved the AspireAssist System for adults ⩾ 22 years, with a BMI of 35–55 kg/m2 who have not responded to lifestyle therapy.
Figure 3.
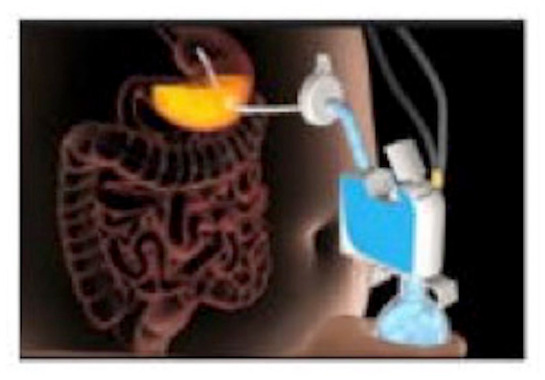
Aspiration therapy. Photo courtesy of Jirapinyo and Thompson. 40
In the initial pilot study on aspiration therapy (AT) by Sullivan and colleagues, 31 patients in the AT group had a TBWL of approximately 19% compared with 6% in the lifestyle therapy group. Thompson and colleagues 30 recently reported the 4-year data from their Pivotal Aspiration Therapy with Adjusted Lifestyle (PATHWAY) Study. They found that AT patients experienced 14.2%, 15.3%, 16.6%, and 18.7% TBWL at 1, 2, 3, and 4 years, respectively. They also reported improvement in obesity-related comorbidities following AT, including decreased SBP (mean difference of −10.5 mm Hg), increased high-density lipoprotein (HDL) (mean difference of 7.7 mg/dl), ALT (mean difference of −15.5 IU/l), and HbA1c (mean difference of −0.3%). 30 A recent systematic review and meta-analysis by Jirapinyo and colleagues 32 further characterized the impact of AT on obesity-related comorbidities. They found that at 1 year following AT, patients had decreased SBP (mean difference of −7.8 mm Hg), triglycerides (mean difference of −15.8 mg/dL), HbA1c (mean difference of −1.3%), ALT (mean difference of −7.5 IU/l), and increased HDL (3.6 mg/dl) (Table 1). Still, there remains insufficient evidence to suggest AT has a durable effect on improving cardiometabolic complications of obesity at this time.
Small-bowel endobariatric therapies
Studies suggest an important physiologic role of the small bowel in metabolic homeostasis. Rubino and colleagues 41 demonstrated in 2006 that exclusion of the proximal small bowel, as occurs in Roux-en-Y Gastric Bypass (RYGB) and similar procedures, results in improved glucose tolerance that occurs independently of effects from reductions in food intake and body weight. As a result, altering the delivery and presentation of nutrients to the small bowel has proven an attractive target for improving insulin sensitivity and glucose metabolism. A number of EBTs have been developed recently to target the small bowel with the goal of both promoting weight loss and directly treating metabolic disease. These include duodenal mucosal resurfacing (DMR), the duodenal-jejunal bypass liner (DJBL) device, and the endoluminal magnetic partial jejunal diversion.
Duodenal mucosal resurfacing
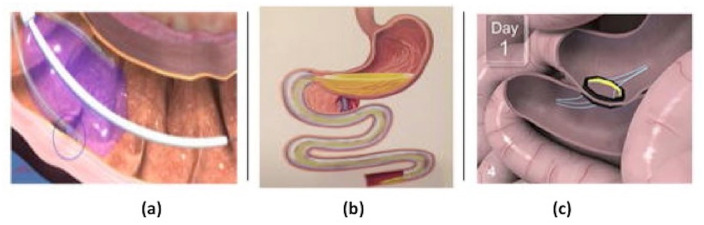
DMR is a minimally invasive endoscopic procedure using specially designed catheters (Fractyl Laboratories, Lexington, Massachusetts, USA). The procedure is performed involving the circumferential hydrothermal ablation of the duodenal mucosa, resulting in subsequent regeneration of the mucosa and alterations in gut hormone secretion (Figure 4(a)).
Figure 4.
Small bowel therapies: (a) duodenal mucosal resurfacing, (b) duodenal-jejunal bypass liner, and (c) endoluminal magnetic partial jejunal diversion. Photo courtesy of Jirapinyo and Thompson. 40
Rajagopalan and colleagues 33 conducted the first clinical study in a cohort of 39 patients with T2DM. In the first 6 months, patients’ HbA1c was reduced by a mean of 1.2%, with a positive association between the length of the ablated segment and degree of reduction in HbA1c. 33 A large multicenter, prospective observational study was recently reported by van Baar and colleagues that showed at 1-year post DMR, patients had decreased HbA1c (mean difference −10 mmol/mol), FBG (mean difference of −1.8 mmol/l), and HOMA-IR (mean difference of −3.3). The study also showed that the metabolic improvements following DMR were rapid, with decreases in HbA1c, FBG, and HOMA-IR as early as 4 weeks post procedure. While there was some initial weight loss at 4 weeks post-procedure following DMR (mean difference of −2.5 ± 0.6 kg), this weight loss effect was not sustained during the follow-up period. Finally, they did report a modest improvement in ALT at 1 year (mean difference of 10.0 IU/l) following DMR (Table 1). 34 The results suggest that DMR provides a safe and durable therapeutic option for improving glycemic control in patient with type 2 diabetes.
Duodenal-jejunal bypass liner
The EndoBarrier DJBL (GI Dynamics, Lexington, Massachusetts, USA) is an endoscopically placed impermeable fluoropolymer sleeve that extends 60 cm through the duodenum and into the jejunum. The sleeve creates a barrier between the partially digested food and the absorptive surface of the small intestine, thus preventing absorption within the foregut (Figure 4(b)). In a 2014 randomized control trial, Koehestanie and colleagues 35 evaluated the safety and efficacy of 6 months’ DJBL treatment on obesity, T2DM, and cardiovascular risk. Six months after treatment initiation, the DJBL group was found to have significantly greater TBWL (10% versus 4.7%) and decrease in HbA1c (mean difference of −1.3% versus −0.4%) compared with the diet group (Table 1). Patients in the DJBL group were also noted to have a greater reduction in both postprandial glucose levels and need for glucose-lowering medications compared with the control patients. Finally, patients in the DJBL group had a greater reduction in their estimated 10-year coronary heart disease risk compared with the control group (mean difference of −2% versus −1%). 35
A 2018 meta-analysis and systematic review by Jirapinyo and colleagues 36 analyzed the effects of DJBL on glycemic control, weight loss, and changes in gut hormones in patients with obesity and T2DM. At the time of DJBL removal, HbA1c decreased by a mean of 1.3%, HOMA-IR decreased by a mean of 4.6, fasting insulin decreased by a mean of 4.8 mU/l, and FBG decreased by a mean of 44.6 mg/dl. They also reported that at the time of DJBL removal, patients achieved a TBWL of 18.9%. Changes in gut hormones following DJBL implantation included elevations in GLP-1, PYY, and ghrelin and decreases in glucose-dependent insulinotropic polypeptide (GIP). 36 A recent study by Betzel and colleagues 37 reported long-term outcomes following 2 years of DJBL treatment. They found that at the time of DJBL explantation, patients achieved a TBWL of 14.6% and HbA1c decreased by an average of 4.9 mmol/mol (Table 1). Finally, the number of insulin users and daily dose of insulin both decreased significantly. The authors noted, however, that the largest improvements in weight and glycemic control were observed early, during the first 9–12 months after implantation. Furthermore, as in previous studies of the DJBL device, following explantation they observed relapse of metabolic disease with worsened glycemic control. While patients did also have some weight regain following explantation, body weight remained significantly improved from baseline at 48 months after removal. 37
The most common adverse events associated with the procedure were abdominal pain, nausea, and vomiting, followed by device migration. Severe adverse events, while rare, notably included hepatic abscesses. 38 In fact, the pivotal US ENDO trial was terminated in July 2015, due to a higher than expected hepatic abscess rate of 3.5% (compared with a global incidence of 0.73%). 38 Consequently, the DJBL has not yet been FDA approved in the United States.
Endoluminal magnetic partial jejunal diversion
The ability to create a permanent anastomosis endoscopically without relying on foreign materials is a potential paradigm shift in the endoscopic management of obesity and metabolic disease. The incisionless magnetic anastomosis system (IMAS), first described by Machytka and colleagues in 2017, is a self-assembling magnetic device that allows for side-to-side anastomosis to create a partial jejunal diversion (PJD). This allows foods and nutrients to bypass most of the small bowel and enter directly into the ileum (Figure 4(c)). The early delivery of food and digestive enzymes to the ileum leads to increased secretion of gut hormones like GLP-1 and PYY, which in turn promotes improved glucose homeostasis and weight loss. 39
In their prospective observational pilot study, Machytka and colleagues enrolled 10 patients with obesity. In all cases, the IMAS was delivered using a colonoscope under laparoscopic supervision. Of note, in 6 of the 10 cases, a laparoscopic grasper was used to facilitate magnet coupling. The authors found that at 12 months patients had a mean TBWL of 14.6%, a mean decrease in HbA1c of 1.9% in diabetic patients, a significant reduction in postprandial insulin and glucose levels at 2 and 6 months, and a 23% reduction in ALT measurements at 12 months (Table 1). As expected, they also found a significant increase in PYY activity at 2 months post procedure. The procedure was found to be safe, with only mild nutritional diarrhea and minor post procedure micronutrient deficiencies that were all reversible with supplementation. 39 These results showed that the PJD created via the IMAS is a technically feasible, safe, and effective procedure that may prove a viable treatment option for patients with type 2 diabetes and obesity.
Conclusion
The management of the metabolic complications of obesity remains a persistent issue, and weight loss is still the primary treatment recommendation. In this review article, we summarize the early evidence showing that EBT results not only in a significant and durable weight loss but also long-term improvement in obesity-related metabolic comorbidities. Both gastric and small bowel EBTs promote effective weight loss and can also help ameliorate hepatic steatosis, early liver fibrosis, insulin resistance, and cardiovascular disease risk. These results suggest that EBTs may prove a viable alternative treatment in the management of both obesity and its many downstream metabolic complications.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.S. has served as a consultant for Olympus, Cook Medical, and Boston Scientific. A.M. has no conflicts of interest to disclose.
ORCID iD: Amit Mehta  https://orcid.org/0000-0002-8225-8632
https://orcid.org/0000-0002-8225-8632
Contributor Information
Amit Mehta, Division of Gastroenterology and Hepatology, Weill Cornell Medicine and NewYork-Presbyterian Hospital, New York, NY, USA.
Reem Z. Sharaiha, Associate Professor of Medicine, Division of Gastroenterology and Hepatology, Weill Cornell Medicine and NewYork-Presbyterian Hospital, New York, NY 10021, USA.
References
- 1. Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011; 378: 804–814. [DOI] [PubMed] [Google Scholar]
- 2. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief 2017; 288: 1–8. [PubMed] [Google Scholar]
- 3. Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med 2003; 138: 24–32. [DOI] [PubMed] [Google Scholar]
- 4. Hales CM, Fryar CD, Carroll MD, et al. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA 2018; 319: 1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haslam DW, James WP. Obesity. Lancet 2005; 366: 1197–1209. [DOI] [PubMed] [Google Scholar]
- 6. Nimptsch K, Pischon T. Body fatness, related biomarkers and cancer risk: an epidemiological perspective. Horm Mol Biol Clin Investig 2015; 22: 39–51. [DOI] [PubMed] [Google Scholar]
- 7. Prospective Studies Collaboration, Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes – 5-year outcomes. N Engl J Med 2017; 376: 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kheniser KG, Kashyap SR. Diabetes management before, during, and after bariatric and metabolic surgery. J Diabetes Complications 2018; 32: 870–875. [DOI] [PubMed] [Google Scholar]
- 10. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Obes Surg 2017; 27: 2–21. [DOI] [PubMed] [Google Scholar]
- 11. Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient – 2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis 2013; 9: 159–191. [DOI] [PubMed] [Google Scholar]
- 12. Ozaki N, Sengupta JN, Gebhart GF. Mechanosensitive properties of gastric vagal afferent fibers in the rat. J Neurophysiol 1999; 82: 2210–2220. [DOI] [PubMed] [Google Scholar]
- 13. Cubattoli L, Barneschi C, Mastrocinque E, et al. Cardiac arrest after intragastric balloon insertion in a super-obese patient. Obes Surg 2009; 19: 253–256. [DOI] [PubMed] [Google Scholar]
- 14. Geliebter A, Melton PM, Gage D, et al. Gastric balloon to treat obesity: a double-blind study in nondieting subjects. Am J Clin Nutr 1990; 51: 584–588. [DOI] [PubMed] [Google Scholar]
- 15. ASGE Bariatric Endoscopy Task Force and ASGE Technology Committee, Abu Dayyeh BK, Kumar N, et al. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc 2015; 82: 425–438.e425. [DOI] [PubMed] [Google Scholar]
- 16. Courcoulas A, Abu Dayyeh BK, Eaton L, et al. Intragastric balloon as an adjunct to lifestyle intervention: a randomized controlled trial. Int J Obes 2017; 41: 427–433. [DOI] [PubMed] [Google Scholar]
- 17. Popov VB, Thompson CC, Kumar N, et al. Effect of intragastric balloons on liver enzymes: a systematic review and meta-analysis. Dig Dis Sci 2016; 61: 2477–2487. [DOI] [PubMed] [Google Scholar]
- 18. Popov VB, Ou A, Schulman AR, et al. The impact of intragastric balloons on obesity-related co-morbidities: a systematic review and meta-analysis. Am J Gastroenterol 2017; 112: 429–439. [DOI] [PubMed] [Google Scholar]
- 19. Bazerbachi F, Vargas EJ, Rizk M, et al. Intragastric balloon placement induces significant metabolic and histologic improvement in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2021; 19: 146–154.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fuller NR, Pearson S, Lau NS, et al. An intragastric balloon in the treatment of obese individuals with metabolic syndrome: a randomized controlled study. Obesity 2013; 21: 1561–1570. [DOI] [PubMed] [Google Scholar]
- 21. Alqahtani A, Al-Darwish A, Mahmoud AE, et al. Short-term outcomes of endoscopic sleeve gastroplasty in 1000 consecutive patients. Gastrointest Endosc 2019; 89: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 22. Sharaiha RZ, Kumta NA, Saumoy M, et al. Endoscopic sleeve gastroplasty significantly reduces body mass index and metabolic complications in obese patients. Clin Gastroenterol Hepatol 2017; 15: 504–510. [DOI] [PubMed] [Google Scholar]
- 23. Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc 2013; 78: 530–535. [DOI] [PubMed] [Google Scholar]
- 24. Sartoretto A, Sui Z, Hill C, et al. Endoscopic sleeve gastroplasty (ESG) is a reproducible and effective endoscopic bariatric therapy suitable for widespread clinical adoption: a Large, International Multicenter Study. Obes Surg 2018; 28: 1812–1821. [DOI] [PubMed] [Google Scholar]
- 25. Hedjoudje A, Abu Dayyeh BK, Cheskin LJ, et al. Efficacy and safety of endoscopic sleeve gastroplasty: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020; 18: 1043–1053.e1044. [DOI] [PubMed] [Google Scholar]
- 26. Sharaiha RZ, Hajifathalian K, Kumar R, et al. Five-year outcomes of endoscopic sleeve gastroplasty for the treatment of obesity. Clin Gastroenterol Hepatol 2021; 19: 1051–1057.e2. [DOI] [PubMed] [Google Scholar]
- 27. Hajifathalian K, Mehta A, Ang B, et al. Improvement in insulin resistance and estimated hepatic steatosis and fibrosis after endoscopic sleeve gastroplasty. Gastrointest Endosc 2021; 93: 1110–1118. [DOI] [PubMed] [Google Scholar]
- 28. Lopez-Nava G, Negi A, Bautista-Castano I, et al. Gut and metabolic hormones changes after endoscopic sleeve gastroplasty (ESG) vs. laparoscopic sleeve gastrectomy (LSG). Obes Surg 2020; 30: 2642–2651. [DOI] [PubMed] [Google Scholar]
- 29. Abu Dayyeh BK, Acosta A, Camilleri M, et al. Endoscopic sleeve gastroplasty alters gastric physiology and induces loss of body weight in obese individuals. Clin Gastroenterol Hepatol 2017; 15: 37–43.e31. [DOI] [PubMed] [Google Scholar]
- 30. Thompson CC, Abu Dayyeh BK, Kushnir V, et al. Aspiration therapy for the treatment of obesity: 4-year results of a multicenter randomized controlled trial. Surg Obes Relat Dis 2019; 15: 1348–1354. [DOI] [PubMed] [Google Scholar]
- 31. Sullivan S, Stein R, Jonnalagadda S, et al. Aspiration therapy leads to weight loss in obese subjects: a pilot study. Gastroenterology 2013; 145: 1245–1252.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jirapinyo P, de Moura DTH, Horton LC, et al. Effect of aspiration therapy on obesity-related comorbidities: systematic review and meta-analysis. Clin Endosc 2020; 53: 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajagopalan H, Cherrington AD, Thompson CC, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes: 6-month interim analysis from the first-in-human proof-of-concept study. Diabetes Care 2016; 39: 2254–2261. [DOI] [PubMed] [Google Scholar]
- 34. van Baar ACG, Holleman F, Crenier L, et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study. Gut 2020; 69: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koehestanie P, de Jonge C, Berends FJ, et al. The effect of the endoscopic duodenal-jejunal bypass liner on obesity and type 2 diabetes mellitus, a multicenter randomized controlled trial. Ann Surg 2014; 260: 984–992. [DOI] [PubMed] [Google Scholar]
- 36. Jirapinyo P, Haas AV, Thompson CC. Effect of the duodenal-jejunal bypass liner on glycemic control in patients with type 2 diabetes with obesity: a meta-analysis with secondary analysis on weight loss and hormonal changes. Diabetes Care 2018; 41: 1106–1115. [DOI] [PubMed] [Google Scholar]
- 37. Betzel B, Cooiman MI, Aarts EO, et al. Clinical follow-up on weight loss, glycemic control, and safety aspects of 24 months of duodenal-jejunal bypass liner implantation. Surg Endosc 2020; 34: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Betzel B, Drenth JPH, Siersema PD. Adverse events of the duodenal-jejunal bypass liner: a systematic review. Obes Surg 2018; 28: 3669–3677. [DOI] [PubMed] [Google Scholar]
- 39. Machytka E, Buzga M, Zonca P, et al. Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in patients with obesity and diabetes. Gastrointest Endosc 2017; 86: 904–912. [DOI] [PubMed] [Google Scholar]
- 40. Jirapinyo P, Thompson CC. Endoscopic bariatric and metabolic therapies: surgical analogues and mechanisms of action. Clin Gastroenterol Hepatol 2017; 15: 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006; 244: 741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]