Abstract
Mitochondrial transplantation emerges as a novel therapeutic solution for ischemia/reperfusion injury (IRI) in various tissues. Platelets have recently been used in mitochondrial transplantation as readily-available donors of small-size platelet mitochondria (plt-mito). Interestingly, FUN14 Domain Containing 2 (FUNDC2), a protein highly-expressed in the outer membrane (OMM) of plt-mito, has been identified to maintain platelet survival under hypoxic condition. The current study determined whether and how FUNDC2 contributed to the therapeutic effect of plt-mito transplantation for hypoxia/reoxygenation (HR) injury. The results showed that incorporation of human plt-mito into SH-SY5Y cells rescued HR-induced mitochondrial malfunction and mitochondrial apoptotic pathway. Mechanistically, plt-mito transplantation led to an increased expression of FUNDC2 in the recipient cells. This protein induced mitochondrial translocation of phosphatidylinositol-3,4,5-trisphosphate (PIP3) via its N-term, resulting in the stimulation of the protein kinase B (Akt)/forkhead box O3a (FOXO3a) pathway, which inhibited HR-induced mitochondrial accumulation of a mitochondrial target of FOXO3a, Bim, also known as a pro-apoptotic protein. Therefore, the FUNDC2/PIP3/Akt/FOXO3a axis may facilitate the incorporated plt-mito to restore mitochondrial function and cell viability of the recipient cells, and platelets may serve as readily-available sources of donor mitochondria that afford therapeutic benefits against IRI.
Keywords: mitochondrial transplantation, platelet mitochondria, hypoxia/reoxygenation, FUN14 Domain Containing 2, neuronal apoptosis
Introduction
Mitochondria are important cell organelles that supply energy, generate reactive oxidative species (ROS), and mediate cell apoptosis, and therefore serve as the primary subcellular target of IRI 1 . Impaired bioenergetic efficiency is considered to be the most rapid response of mitochondria to the sudden reduced glucose/oxygen delivery during ischemic period 1 . Oxidative stress has been implicated in the secondary injury induced by the re-supply of glucose/oxygen following ischemia 1 –5 . Since mitochondria are both targets and sources of ROS 1 –5 , reperfusion may cause the secondary injury of mitochondria.
Mitochondrial transplantation, an innovative therapeutic solution for mitochondria-associated disorders, has been validated by clinical trials and animal studies to be effective in treating IRI 6 –9 . Through replacing the damaged mitochondria with exogenous healthy mitochondria, this method supplements the compromised cells with mtDNA, ATP and antioxidants and promotes their self-repair 6 –9 .
In clinical trials, skeletal muscles have been commonly used as mitochondrial donors 9,10 . However, acquirement of muscular tissues is invasive, which limits the clinical application of this approach 9,10 . Platelets are anucleate cells abundant in blood. Platelet collection by venous venipuncture is minimally-invasive 11 . More significantly, platelets contain small-size mitochondria and possess neuron-like biochemical properties 11 . Consequently, platelets may be employed as mitochondrial donor cells 11 . In a recent study, delivery of plt-mito into the brain of diabetic mice restored hippocampal mitochondrial function and improved the cognitive ability 11 .
Interestingly, FUNDC2 has been identified as a protein highly-expressed in the OMM of plt-mito, and offers significant protection against hypoxia-induced platelet apoptosis 12 . Mechanistically, FUNDC2 has two extra-mitochondrial parts, that is, N- and C-term 12 . Its N-term recruits the lipid PIP3 to plt-mito. PIP3 is required for Akt phosphorylation, which activates a variety of pro-survival substrates to facilitate platelet survival under hypoxic stress 12 . On the contrary, FUNDC2 knockout diminished PIP3 in platelets, suppressed Akt activation, and led to platelet apoptosis 12 . Since FUNDC2 is specifically high in plt-mito 12 , it would be interesting to determine whether FUNDC2 helps the transplanted plt-mito to confer neuroprotection following HR exposure. A neuronal HR model in vitro was built in this study by using retinoic acid (RA)-differentiated human SH-SY5Y cells, which has been validated to be reliable in vitro models for human systems 13 and are commonly used to establish in vitro neuronal IR models 14 –16 . The current study elucidated whether and how FUNDC2 contributed to the favorable effect of plt-mito transplantation for HR injury.
Methods
Plt-Mito Preparation
Blood samples were obtained from 9 healthy subjects (M/F: 4 of 9; Age range: 24-35 years; Average age 27.9 years) at Outpatient Center of our Hospital, with written informed consent acquired. Plt-mito were prepared within 5 h after the whole blood was drained into the Vacutainer® (BD, US) containing K2EDTA. Blood samples was spinned for 15 min at 300× g to obtain platelet-rich plasma (PRP). The PRP containing 1 µM prostaglandin E1 (Sigma, US) was then spinned for 5 min at 4600× g to yield platelet pellets. The platelets in the donor’s own plasma (5 × 105/ml) were dyed with Mitotracker-green (100 nM, 20 min, Beyotime, China). Mitochondria isolation kit (MITOISO2, sigma, US) was applied to extract plt-mito from platelets.
To block the PI3P-binding motif of the N-term of FUNDC2, plt-mito was incubated with rabbit anti-human FUNDC2 (N-term) antibody (Invitrogen, Themofisher scientific., US, 10 μg/ml, 1 h). As a control, the C-term of FUNDC2 was blocked with rabbit anti-human FUNDC2 (C-term) antibody (Invitrogen, Themofisher scientific, US, 10 μg/ml, 1 h). To visualize the FUNDC2-labelled plt-mito in the recipient cells, they were stained, prior to transplantation, with anti-rabbit secondary antibody with Alexa Fluor Plus-488 (1:1000, 1 h, Invitrogen, Themofisher scientific., US), which was pre-cross absorbed against human IgG.
HR Model in Vitro and PLT-Mito Transplantation
SH-SY5Y cells were maintained and differentiated with RA (20 μM, Sigma, US) by a previously-available method 17 . The cells were exposed for 8 h to ischemia-mimetic condition (140 mM NaCl, 3.5 mM KCl, 0.43 mM KH2PO4, 1.25 mM MgSO4, 1.7 mM CaCl2, 5 mM NaHCO3, 20 mM HEPES, pH 7.2–7.4, 95% N2 and 5% CO2). For reoxygenation, the cells were washed and cultured in regular medium with 95% air and 5% CO2 for 48 h. For plt-mito transplantation, plt-mito was added at the onset of reoxygenation to the host cells at 1 × 107/5000 cells. The co-incubation lasted for 48 h. To verify if the incorporation of plt-mito into the recipient cells stimulated the PIP3/Akt pathway, PI3 K inhibitor (LY294002, 5 μM, Sigma, US) was applied in combination with plt-mito. To collect the host cells after coculture with plt-mito, the cells were rinsed three times with PBS, harvested using trypsin-EDTA, and spinned at 600 g for 5 min to remove extracellular plt-mito.
Fluorescent Microscopy
For visualization of the innate mitochondria, cells were seeded onto a 15-mm glass-bottom dish and dyed with Mitotracker-red (100 nm, 40 min, Beyotime, China). Subsequently, cells were washed and subjected to a 48-h coculture with plt-mito. Stained cells were imaged using a fluorescent microscope (AXIOVERT, Carl Zeiss, GER) equipped with MShot image analysis system.
Colorimetric Assay
MTT assay kit (C0009, Beyotime, China) was applied to evaluate cell viability. The absorbance values were read by an ELISA reader (ELX808 BIOTEK, US) and normalized to the corresponding cell number, which was counted by an automatic cell counter (Countstar IC1000, China).
Mitochondrial ATP content was determined using a colorimetric kit (ab83355, Abcam, UK). Mitochondrial PIP3 was extracted and assessed using PIP3 Mass ELISA Kit (Echelon Biosciences Inc., US). Both ATP and PIP3 were standardized to corresponding mitochondrial protein concentration, which was determined by a BCA Assay kit (PC0020, Solarbio, China).
Fluorescence Assay
A JC-1 kit (C2006, Beyotime, China) was used to assess the mitochondrial membrane potential ΔΨm. The fluorescent intensities of JC-1 aggregates and monomers were quantified by a multi-Plate Reader (Biotek SynergyH4, US) and imaged by a fluorescent microscope.
Mitochondrial ROS levels were measured using mitochondrial ROS detection kit (Caymen Chem., US), and standardized to the corresponding mitochondrial protein concentration.
Western Blotting
Mitochondrial leakage of cytochrome c (Cyto C) was analyzed by Western blotting with Cyto C-releasing assay kit (ab65311, Abcam, UK).
Western blotting was conducted on whole-cell, cytoplasmic and mitochondrial lysates with primary antibodies at 1:1000 (Abcam, UK) following our published protocol 18 . The band intensities of p-Akt (Ser473/474), t-Akt, p-FOXO3a (Thr32 and Ser253), t-FOXO3a, Bim, FUNDC2, and Cyto C were quantified by Amersham Imager 600 (Cytiva, US). Porin, β-actin and platelet factor 4 (PF4, Sigma, US) were analyzed as mitochondrial, cytoplasmic and platelet markers, respectively.
Luciferase Reporter Assay
FOXO luciferase reporter vector and FOXO3 expression vector provided by FOXO Reporter Kit (Bpsbioscience, China) were transfected into the cells. Following plt-mito transplantation, FOXO reporter activity was determined by dual luciferase assay following the kit’s protocol, and standardized to corresponding mitochondrial protein concentration.
Statistical Analysis
Data (Mean ± SD) were analyzed by SPSS 21.0 with Student’s t test, or One-way ANOVA followed by Tukey’s post-hoc tests.
Results
Internalization of PLT-Mito and FUNDC2-Labelled Plt-Mito by SH-SY5Y Cells
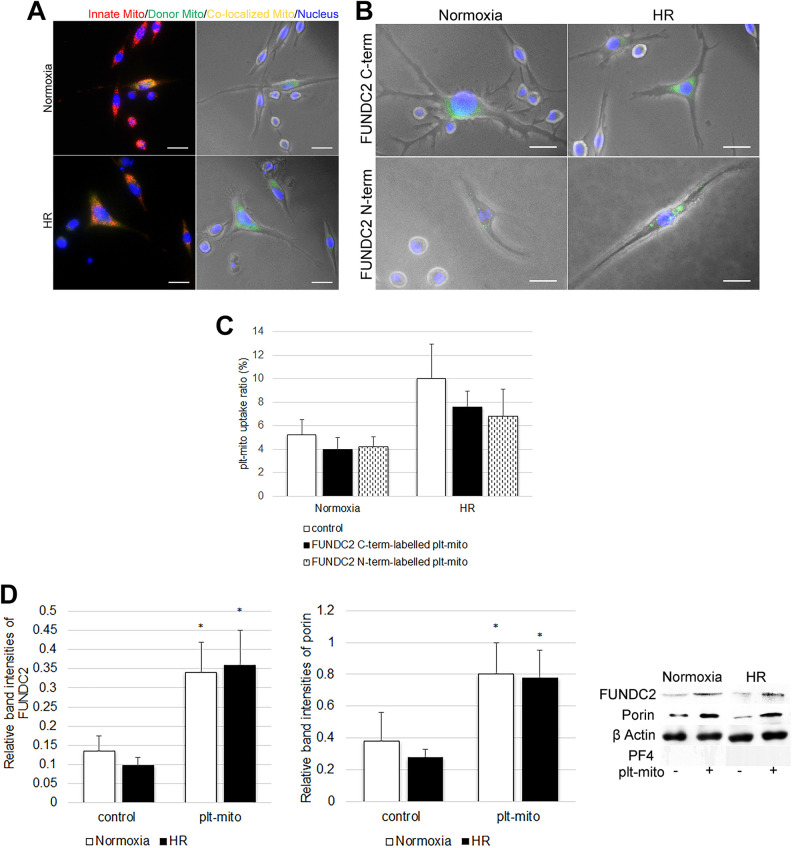
To verify the uptake of exogenous plt-mito by SH-SY5Y cells under normoxic or HR condition, plt-mito labeled with Mitotracker-green were added to SH-SY5Y cells pre-stained with Mitotracker-red. After 48 h of coculture, these exogenous organelles (green fluorescence) were found inside the cells exposed to normoxia or HR (Fig. 1A). To block the two extra-mitochondrial terminals of FUNDC2, plt-mito were pre-treated with antibodies against C- or N-term of FUNDC2, and then cocultured with the normoxic or HR-treated cells. Following coculture for 48 h, the internalization of FUNDC2-labelled plt-mito (green fluorescence) by the cells could be detected by fluorescent microscopy (Fig. 1B). Of noted, blockage of the C- or N-term of plt-mito FUNDC2 showed no significant impact on the uptake ratio of plt-mito under normoxia (F = 1.824; df1 = 2, df2 = 12; P > .05, Fig. 1C) or HR stress (F = 2.684; df1 = 2, df2 = 12; P > .05, Fig. 1C). Western blot analysis further showed that plt-mito transplantation led to elevated expression of FUNDC2 and porin in the host cells exposed to normoxia (FUNDC2: t = −7.282; porin: t = −4.624; v = 4; P < .05, Fig. 1D) or HR (FUNDC2: t = −8.934; porin: t = −8.895; v = 4; P < .05, Fig. 1D), indicating an elevation in mitochondrial density. No PF4 was detected in the protein extracts of host cells (Fig. 1D), suggesting no contamination by cytoplasmic remnants of platelets.
Figure 1.
Internalization of plt-mito and FUNDC2-labelled plt-mito by SH-SY5Y cells. (A) Internalization of plt-mito by SH-SY5Y cells under normoxic and HR conditions was observed by fluorescent microscopy. Scale: 200 nm. Red (Mitotracker Red), green (Mitotracker Green) and yellow fluorescence represent plt-mito, innate mitochondria and co-localized mitochondria, respectively. Nuclei were dyed with DAPI (blue fluorescence). (B) Uptake of FUNDC2-labelled plt-mito by the cells under normoxic or HR condition was observed by fluorescent microscopy. Scale: 200 nm. Green (Alexa Fluor Plus-488) fluorescence represents plt-mito labelled with anti-FUNDC2 N- or C-term antibodies. (C) Quantification of plt-mito uptake ratio, n = 5. (D) Following plt-mito transplantation, FUNDC, porin, and PF4 levels were evaluated in the recipient cells under normoxic and HR conditions, n = 5. *P < .05 vs. control.
Plt-Mito Transplantation Rescues HR-Induced Mitochondrial Malfunction and Mitochondria-Mediated Apoptosis
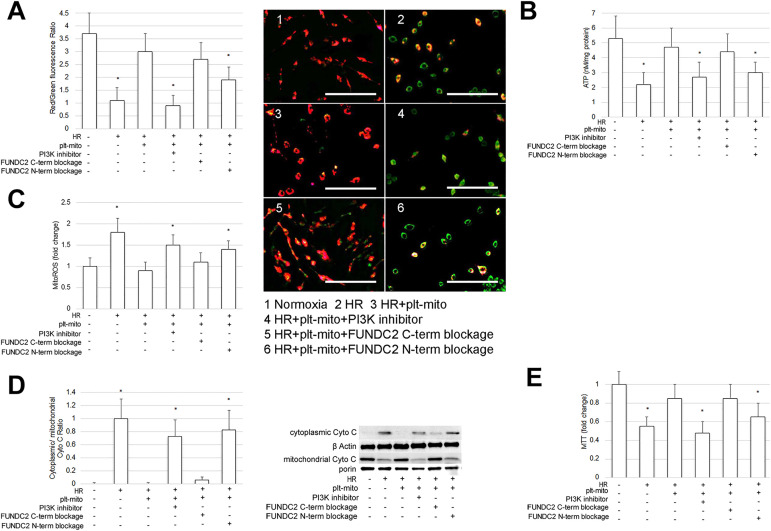
Mitochondria have been validated as subcellular targets of IRI 1,2 and its dysfunction can trigger cell apoptosis 1,2 . We therefore evaluated the mitochondrial function of SH-SY5Y cells exposed to HR. As expected, when compared to normoxic cells, HR-treated cells displayed mitochondrial malfunction and mitochondria-mediated apoptosis, as indicated by ΔΨm depolarization (F = 9.091; df1 = 5, df2 = 24; P < .05, Fig. 2A), mitochondrial ATP deficit (F = 12.153; df1 = 5, df2 = 24; P < .05, Fig. 2B), ROS overproduction (F = 15.746; df1 = 5, df2 = 24; P < .05, Fig. 2C), mitochondrial leakage of Cyto C (F = 37.738; df1 = 5, df2 = 24; P < .05, Fig. 2D) and decrease of cell viability (F = 10.154; df1 = 5, df2 = 24; P < .05, Fig. 2E).
Figure 2.
Plt-mito transplantation prevents HR-induced mitochondrial malfunction and mitochondria-mediated apoptosis. Plt-mito were cocultured with the cells under the normoxic and HR condition. To inhibit FUNDC2-evoked PIP3/Akt pathway, LY294002 was applied at the onset of plt-mito transplantation. To block the extra-mitochondrial terminals of FUNDC2, plt-mito were treated with antibodies to N- or C-term of FUNDC2 prior to transplantation. (A) ΔΨm of the recipient cells was assessed by JC-1 assay and observed by fluorescent microscopy. Scale: 500 nm. n =5. *P < .05 vs. control. Fluorescence changes from red to green as ΔΨm decreases. (B-E) Mitochondrial ATP, ROS, Cyto C leakage, and cell viability were analyzed as specified in the Methods, n = 5. *P < .05 vs. control.
To determine whether plt-mito transplantation afforded neuroprotection following HR induction, plt-mito were added to SH-SY5Y cells at the onset of oxygenation and coculture for 48 h with the cells. The results observed that incorporation of plt-mito into the cells prevented HR-induced ΔΨm decrease (Fig. 2A), mitochondrial ATP reduction (Fig. 2B), ROS overgeneration (Fig. 2C), mitochondrial release of Cyto C (Fig. 2D), and loss of cell viability (Fig. 2E), indicating the neuroprotective effect of plt-mito transplantation.
FUNDC2/PIP3/AKT/FOXO3a/Bim Axis Mediates the Protective Effect of Plt-Mito Transplantation on Recipient Cells Exposed to HR
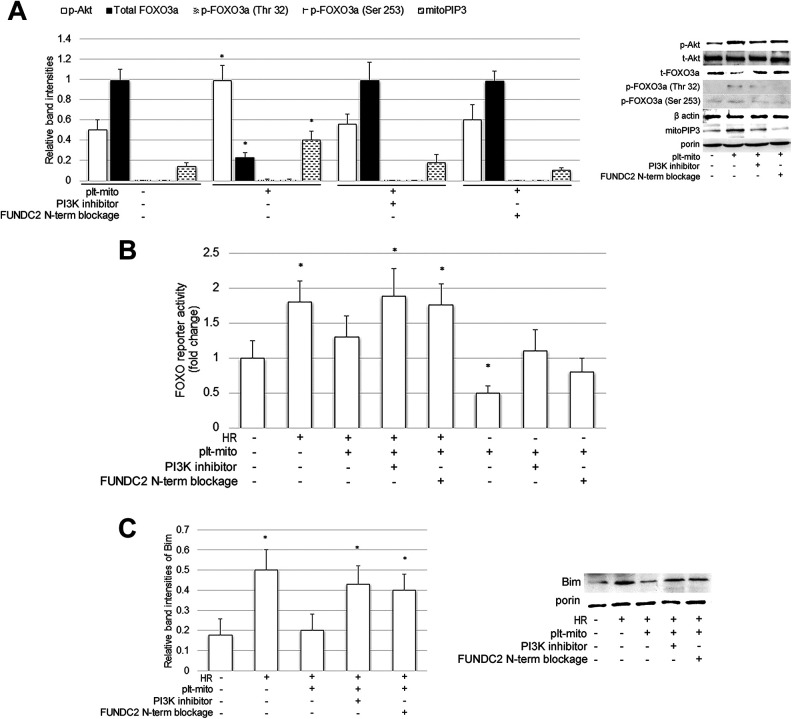
FUNDC2, a protein highly-expressed in the OMM of plt-mito, has been identified to recruit PIP3 to mitochondria via its N-term, which activated the pro-survival Akt pathway 12 . To elucidate whether plt-mito exerted its neuroprotective effect via FUNDC2 and its substrate PI3P, two extra-mitochondrial parts of FUNDC2 were blocked, prior to plt-mito transplantation, with antibodies specific for C- or N-term of FUNDC2, and a PI3 K inhibitor LY294002 was applied in combination with plt-mito to inhibit PIP3 generation and Akt activity. As illustrated in Fig. 2, blockage of FUNDC2 N-term or administration of the PI3 K inhibitor abrogated the positive effects of plt-mito transplantation on ΔΨm (Fig. 2A), mitochondrial ATP (Fig. 2B), ROS production (Fig. 2C), mitochondrial Cyto C (Fig. 2D), and cell viability (Fig. 2E) of the host neuronal cells exposed to HR. To confirm that incorporation of plt-mito into the host cells evoked the FUNDC2/PIP3/Akt pathway, we also analyzed mitochondrial PIP3 levels and the phosphorylation status of Akt following plt-mito transplantation. It was observed that plt-mito transplantation promoted mitochondrial accumulation of PIP3 and Akt phosphorylation, which could be prevented by either blockage of FUNDC2 N-term or the PI3 K inhibitor (PIP3: F = 29.944; p-Akt: F = 23.173; df1 = 3, df2 = 16; P < .05, Fig. 3A).
Figure 3.
FUNDC2/PIP3/AKT/FOXO3a/Bim axis mediates the protective effect of plt-mito on the recipient cells under HR stress. Plt-mito pre-treated with or without anti-FUNDC2 N-term antibody were cocultured with the cells under the normoxic and/or HR condition. PI3 K inhibitor LY294002 was added to inhibit FUNDC2-induced PIP3/Akt pathway. (A) Mitochondrial PIP3, p-Akt, p-FOXO3a, and t-FOXO3a were assessed in the recipient cells, n = 5. *P < .01 vs. control. (B) Cells were transfected with FOXO3 and FOXO reporter prior to plt-mito transplantation. FOXO reporter activity was determined by dual luciferase assay, n = 5. *P < .05 vs. control. (C) Mitochondrial Bim was analyzed in the recipient cells, n = 5. *P < .05 vs. control.
Next, we investigated the possible mechanism whereby the FUNDC2/PIP3/Akt pathway promoted the survival of the host cells exposed to HR. FOXO3a, a substrate of the PI3K/Akt pathway 19 , can regulate mitochondrial ROS and mitochondrial apoptotic pathway by acting on its mitochondrial targets, such as Bim 20 –22 . Under normoxia, incorporation of plt-mito into the recipient cells decreased FOXO3a levels (F = 99.243; df1 = 3, df2 = 16; P < .05, Fig. 3A) and inhibited FOXO3-driven reporter activity (F = 29.864; df1 = 7, df2 = 32; P < .05, Fig. 3B), which could be blocked by blockage of FUNDC2 N-term or the PI3 K inhibitor. When exposed to HR, plt-mito transplantation prevented HR-induced enhancement of FOXO3-driven reporter activity (Fig. 3B) and mitochondrial localization of Bim (F = 27.866; df1 = 4, df2 = 20; P < .05, Fig. 3C), which could be abrogated by blockage of FUNDC2 N-term or the PI3 K inhibitor. Therefore, delivery of plt-mito into the recipient cells might evoke the FUNDC2/PIP3/Akt/FOXO3a axis to mediate its favorable effects.
Discussion
Given the role of mitochondrion in energy supply, ROS generation, and cell apoptosis, it serves as the major subcellular target for cerebral IRI and neuroprotective interventions 1 –5 . Ischemia can cause dysfunction of mitochondrial energy metabolism, which is indicated by ΔΨm loss, respiratory dysfunction and ATP reduction 1–2 . Since the mitochondria are also known as the primary intracellular source and targets of ROS 1–2 , over-activation of mitochondrial respiration by reperfusion leads to excessive ROS, which induces the secondary injury of mitochondria 1–2 . Mitochondrial destruction induced by either ischemia or reperfusion can initiate apoptotic cascade, indicated by the leakage of pro-apoptotic inducer from mitochondria, such as cyto C 1–2 . In consistent with these views, our results showed that HR caused mitochondrial malfunction and mitochondria-mediated apoptosis, displayed as ΔΨm depolarization, ATP decrease, ROS overproduction, release of Cyto C, COXIV downregulation, and impaired cell viability.
Mitochondria has been validated to be transferred between different cell types, including neurons, mesenchymal stem cells (MSCs), astrocytes, and endothelial progenitor cells, as a “help-me” response to cerebral injury, such as IRI 11 , 23 –25 . Intercellular transfer of functional mitochondria promotes mitochondrial repair by replenishing the recipient cells with ATP, mtDNA, and antioxidants 23 –25 . Transplantation of MSC with hypoxic tolerance into the recipient tissues has been reported to donate mitochondria to facilitate mitochondrial recover from IRI 25,26 . Therefore, mitochondria transplantation may be a promising therapeutic solution for cerebral IRI 6 –10 . In previous clinical studies using mitochondrial transplantation, skeletal muscles, such as pectoralis major, rectus abdominis, and gastrocnemius, have been commonly selected as mitochondrial donors, but acquisition of muscular tissues is invasive 9,11 . Although MSC possess the potential as mitochondrial-repair stem cells for stroke 25,26 , the procedure for isolation, sorting and culture expand of transplantable MSC can be time-consuming and cumbersome. Another concern is that the therapeutic effect of mitochondrial transplantation may be mitochondrial source-specific. For example, high glucose-induced oxidative injury in rat renal proximal tubular cells could be mitigated by MSC-derived mitochondria, but not by NIH-3T3-derived mitochondria 27 . Therefore, it is necessary to find readily-available sources of donor mitochondria that offer therapeutic benefits against IRI and to elucidate whether mitochondria from various donors exert differential protective mechanisms.
Platelets are enucleated cells abundant in blood and contains small-size mitochondria. More significantly, acquisition of platelets is minimally-invasive 11 . Therefore, platelets may serve as novel donors of mitochondria 11 . In a previous study, injection of plt-mito into the brain of diabetic mice restored mitochondrial number and function, suppressed oxidative stress and neuronal apoptosis, and improved the cognitive ability 11 . However, this study used a diabetic mouse model to elucidate the effect of plt-mito transplantation under high-glucose stress. The therapeutic effect of plt-mito transplantation for HR injury is undetermined. After all, the cellular responses to plt-mito transplantation under HR and high-glucose conditions may not be exactly the same. Moreover, there may be significant differences in gene expression and transcription factor pathway between murine and human 17 , and to elucidate the pathways that are conserved between rodent and human is necessary. In addition, investigation of the plt-mito-specific neuroprotective mechanism was lacking in this study. Using RA-differentiated human SH-SY5Y cells, the present study determined whether transplantation of human plt-mito benefited neuronal survival and mitochondrial function under HR stress, and found that plt-mito transplantation rescued HR-induced mitochondrial malfunction, ROS overproduction and mitochondrial apoptotic pathway. Moreover, plt-mito transplantation increased mitochondrial density of the host cells, which might be associated not only with the supplement of plt-mito, but with enhanced mitochondrial biogenesis following mitochondrial transplantation 28 .
Mitochondria from various donors may provide diverse mitochondrial protective proteins, which facilitate the donor mitochondria to exert their specialized protective mechanisms. FUNDC2 is a OMM protein highly-expressed in plt-mito 11 . In a recent study, FUNDC2 was essential for platelet survival under hypoxic condition. N-term of FUNDC2 can bind PIP3, which activates Akt-mediated pro-survival pathway 11 . Given the important role of FUNDC2 in platelet survival, the current study investigated if plt-mito transplantation brought extra FUNDC2 to the recipient cells and if FUNDC2 exerted its favorable action within the host neuronal cells. The results revealed increased FUNDC2 in the recipient cells after plt-mito transplantation, which promoted mitochondrial accumulation of PIP3 and Akt phosphorylation. Blockage of FUNDC2 N-term or inhibition of PIP3/Akt abrogated the pro-survival effect of plt-mito on the host cells exposed to HR. In addition, the finding that FUNDC2 N-term blockage did not affected the uptake ratio of plt-mito excluded the possibility that FUNDC2 N-term blockage abrogated the favorable effect of plt-mito through inhibiting its internalization by the host cells.
FOXO3a is a downstream protein of the PI3K/Akt pathway 19 . Active Akt can phosphorylate FOXO3a, which evokes its cytoplasmic accumulation and subsequent degradation 19 . Akt thus inhibits FOXO3a transcriptional activity 19 . More importantly, FOXO3a can regulate mitochondrial ROS production and mitochondrial apoptotic pathway via its mitochondrial targets, such as Bim 20 –22 . In the current study, plt-mito transplantation promoted FOXO3a degradation, inhibited FOXO3-driven FOXO transcriptional activity and prevented HR-induced mitochondrial translocation of Bim, which were in a FUNDC2/PIP3/Akt-dependent manner. Thus, FOXO3a might be one of the downstream proteins of the FUNDC2/PIP3/Akt pathway evoked by plt-mito transplantation.
Of note, based on previous reports, mitochondrial uptake ratio of cardiac myocytes/heart was only 3–7% under IR condition, albeit the beneficial effect of mitochondrial supplement observed in these studies 23,29 . Moreover, in a previous study, transplantation of plt-mito into the brain of diabetic mice benefited the cognitive ability, with a plt-mito uptake ratio of 9% 11 . In the current study, plt-mito transplantation afforded neuroprotection following HR induction although the uptake ratio was 7–14%. Such a small quantity of incorporated mitochondria maintains ATP production and cell viability, probably via certain rapid specialized pro-survival signal transduction mechanism, not merely because of replenishing of the recipient cells with ATP, mtDNA and mitochondrial proteins. As presented in this study, the FUNDC2/PIP3/Akt/FOXO3a axis might greatly amplify the positive effects of plt-mito transplantation by inhibition of mitochondrial apoptotic pathway following HR stress.
To sum up, using SH-SY5Y cells, we provided evidences that plt-mito transplantation rescued HR-induced mitochondrial malfunction and mitochondrial apoptotic pathway. Mechanistically, plt-mito transplantation increased FUNDC2, a OMM protein highly-expressed in plt-mito, in the recipient cells. This protein recruited PIP3 to mitochondria via its N-term, leading to the activation of the pro-survival Akt/FOXO3a pathway, which prevented HR-induced pro-apoptotic Bim, a mitochondrial target of FOXO3. Thus, the FUNDC2/PIP3/Akt/FOXO3a axis may amplify the favorable effects of plt-mito transplantation, and platelets may serve as readily-available sources of donor mitochondria that offer protection against IRI.
Footnotes
Ethical Approval: The ethical approval to report this work was obtained from the ethics committee of Guangdong second provincial general Hospital (Approval NO. DG2H-KY IRB-AF-SC.08-01.1).
Statement of Human and Animal Rights: All procedures in this study were conducted following the protocol approved by “the ethics committee of Guangdong second provincial general Hospital” and complied with the recommendations of the Declaration of Helsinki.
Statement of Informed Consent: The written informed consents were obtained from the subjects for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Natural Science Foundation of China (No. 81960264), PhD workstation start-up project of Guangdong second provincial general Hospital (2020BSGZ029) and Science and Technology Program of Guangzhou (202102080507).
ORCID iD: Chun Shi  https://orcid.org/0000-0001-6391-9100
https://orcid.org/0000-0001-6391-9100
References
- 1. Andrabi SS, Parvez S, Tabassum H. Reperfusion promotes mitochondrial dysfunction following focal cerebral ischemia in rats. Dis Model Mech. 2017;10(6):787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christophe M, Nicolas S. Mitochondria: a target for neuroprotective interventions in cerebral ischemia-reperfusion. Curr Pharm Des. 2006;12(6):739–757. [DOI] [PubMed] [Google Scholar]
- 3. Wu L, Xiong X, Wu X, Ye Y, Jian Z, Zhi Z, Gu L. Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Front Mol Neurosci. 2020;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roushandeh AM, Kuwahara Y, Roudkenar MH. Mitochondrial transplantation as a potential and novel master key for treatment of various incurable diseases. Cytotechnology. 2019;71(2):647–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shin B, Cowan DB, Emani SM, Del Nido PJ, McCully JD. Mitochondrial transplantation in myocardial ischemia and reperfusion injury. Adv Exp Med Biol. 2017;982:595–619. [DOI] [PubMed] [Google Scholar]
- 8. Gollihue JL, Rabchevsky AG. Prospects for therapeutic mitochondrial transplantation. Mitochondrion. 2017;35:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamada Y, Ito M, Arai M, Hibino M, Tsujioka T, Harashima H. Challenges in promoting mitochondrial transplantation therapy. Int J Mol Sci. 2020;21(17):6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakamura Y, Lo EH, Hayakawa K. Placental mitochondria therapy for cerebral ischemia-reperfusion injury in mice. Stroke. 2020;51(10):3142–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma H, Jiang T, Tang W, Ma Z, Pu K, Xu F, Chang H, Zhao G, Gao W, Li Y, Wang Q. Transplantation of platelet-derived mitochondria alleviates cognitive impairment and mitochondrial dysfunction in db/db mice. Clin Sci (Lond). 2020;134(16):2161–2175. [DOI] [PubMed] [Google Scholar]
- 12. Ma Q, Zhu C, Zhang W, Ta N, Zhang R, Liu L, Feng D, Cheng H, Liu J, Chen Q. Mitochondrial PIP3-binding protein FUNDC2 supports platelet survival via AKT signaling pathway. Cell Death Differ. 2019;26(2):321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kovalevich J, Langford D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol. 2013;1078:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Piccirillo S, Castaldo P, Macrì ML, Amoroso S, Magi S. Glutamate as a potential “survival factor” in an in vitro model of neuronal hypoxia/reoxygenation injury: leading role of the Na+/Ca2+ exchanger. Cell Death Dis. 2018;9(7):731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng F, Ma C, Sun L, Zhang X, Zhai C, Li C, Zhang S, Ren B, Liu S, Liu S, Yin X, et al. Synergistic neuroprotective effects of Geniposide and ursodeoxycholic acid in hypoxia-reoxygenation injury in SH-SY5Y cells. Exp Ther Med. 2018;15(1):320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu L, Jiang C, Kang Y, Dai Y, Fang W, Huang P. urcumin exerts protective effects against hypoxia reoxygenation injury via the enhancement of apurinic/apyrimidinic endonuclease 1 in SH SY5Y cells: Involvement of the PI3K/AKT pathway. Int J Mol Med. 2020;45(7):993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shipley MM, Mangold CA, Szpara ML. Differentiation of the SH-SY5Y human neuroblastoma cell line. J Vis Exp. 2016;108:53193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi C, Zhu X, Wang J, Long D. Intromitochondrial IκB/NF-κB signaling pathway is involved in amyloid β peptide-induced mitochondrial dysfunction, J Bioenerg Biomembr. 2014;46(5):371–376. [DOI] [PubMed] [Google Scholar]
- 19. Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813(11):1978–1986. [DOI] [PubMed] [Google Scholar]
- 20. Hagenbuchner J, Ausserlechner MJ. Mitochondria and FOXO3: breath or die. Front Physiol. 2013;4:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hagenbuchner J, Kuznetsov A, Hermann M, Hausott B, Obexer P, Ausserlechner MJ. FOXO3-induced reactive oxygen species are regulated by BCL2L11 (Bim) and SESN3. J Cell Sci. 2012;125(5):1191–1203. [DOI] [PubMed] [Google Scholar]
- 22. Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A, Johnson EM. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron. 2001;29(3):615–628. [DOI] [PubMed] [Google Scholar]
- 23. Chang JC, Hoel F, Liu KH, Wei YH, Cheng FC, Kuo SJ, Tronstad KJ, Liu CS. Peptide-mediated delivery of donor mitochondria improves mitochondrial function and cell viability in human cybrid cells with the MELAS A3243G mutation. Sci Rep. 2017;7(1):10710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535(7613):551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Russo E, Lee JY, Nguyen H, Corrao S, Anzalone R, La Rocca G, Borlongan CV. Energy metabolism analysis of three different mesenchymal stem cell populations of umbilical cord under normal and pathologic conditions. Stem Cell Rev Rep. 2020;16:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nguyen H, Lee JY, Sanberg PR, Napoli E, Borlongan CV. Eye opener in stroke. Stroke. 2019;50:2197–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamada Y, Ito M, Arai M, Hibino M, Tsujioka T, Harashima H. Challenges in promoting mitochondrial transplantation therapy. Int J Mol Sci. 2020;21(17):6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun X, Gao R, Li W, Zhao Y, Yang H, Chen H, Jiang H, Dong Z, Hu J, Liu J, Zou Y, et al. Alda-1 treatment promotes the therapeutic effect of mitochondrial transplantation for myocardial ischemia-reperfusion injury. Bioact Mater. 2021;6(7):2058–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bertero E, Maack C, O’Rourke B. Mitochondrial transplantation in humans: “magical” cure or cause for concern. J Clin Invest. 2018;128(12):5191–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]