Abstract
Mitochondrial encephalomyopathies are disorders caused by mitochondrial and nuclear DNA mutations which affect the nervous and muscular systems. Current therapies for mitochondrial encephalomyopathies are inadequate and mostly palliative. However, stem cell‐derived mitochondria transplantation has been demonstrated to play an key part in metabolic rescue, which offers great promise for mitochondrial encephalomyopathies. Here, we summarize the present status of stem cell therapy for mitochondrial encephalomyopathy and discuss mitochondrial transfer routes and the protection mechanisms of stem cells. We also identify and summarize future perspectives and challenges for the treatment of these intractable disorders based on the concept of mitochondrial transfer from stem cells.
Keywords: mitochondria, mitochondria quality control, mitochondrial dynamics, mitochondrial encephalomyopathy, stem cell, tunneling nanotube
This review is mainly about the present status of stem cell therapy for mitochondrial encephalomyopathy, the triggering mechanisms for mitochondrial release, pathways of mitochondrial delivery, and the protection mechanisms on recipient cells.

1. INTRODUCTION
Mitochondria are multifunctional cellular organelles that have a critical role in energy production via oxidative phosphorylation. 1 , 2 Mitochondria not only generate adenosine triphosphate (ATP, Table 1) during the oxidative phosphorylation (OXPHOS) process, but also contribute to many other processes, such as cell survival and autophagy. 3 The OXPHOS complexes in mitochondria are dually encoded by the mitochondrial DNA (mtDNA) and the nuclear DNA (nDNA). Mutations in mtDNA or mitochondrial nDNA can cause fatal or severely debilitating disorders, 4 such as mitochondrial encephalomyopathies, which occur in the neuromuscular system.
TABLE 1.
The list of abbreviations in the paper
| Abbreviations | |
|---|---|
| ATP | adenosine triphosphate |
| BNIP3 | Bcl2 interacting protein 3 |
| Cx43 | connexin 43 |
| Drp 1 | dynamin‐related protein 1 |
| EVs | extracellular vesicles |
| Fis1 | fission 1 protein |
| IL | cytokines interleukin‐1 |
| LC3 | light chain 3 |
| MERRF | myoclonic epilepsy with ragged red fibers |
| Mfn1 | mitofusin 1 |
| Miro | mitochondrial Rho‐GTPase |
| MNGIE | mitochondrial neurogastrointestinal encephalopathy |
| MSC | mesenchymal stem cell |
| mtDNA | mitochondrial DNA |
| nDNA | nuclear DNA |
| OPA1 | optic atrophy 1 |
| OXPHOS | oxidative phosphorylation |
| PINK1 | putative kinase 1 |
| TNFα | tumor necrosis factor alpha |
| TNT | tunneling nanotube |
| TP | thymidine phosphorylase |
| TRAK | trafficking kinesin protein |
The traditional “mitochondrial cocktail” has little therapeutic effect on mitochondrial encephalomyopathies. 2 Mitochondria dysfunction have unusual characteristics that may be treated from the cell level to the molecular level. 2 Gene therapy prior to conception and reproductive technology to uncouple the inheritance of mtDNA from nDNA may offer possible solutions for mitochondrial encephalomyopathies. However, treatments for current mitochondrial patients still face numerous challenges. By understanding the molecular pathogenesis of mitochondrial diseases, it has been possible to develop some targeting therapy approaches, such as DNA manipulation and small‐molecule pharmaceuticals. 2 , 5 , 6 However, the heterogeneity of mitochondrial encephalomyopathies and double‐membrane structure make these therapy approaches difficult to materialize. 2 , 7 The development of stem cell therapy may offer great promise for mitochondrial encephalomyopathies. 2 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 The therapeutic mechanism includes paracrine cytokines, modulation of the immune system, and transdifferentiation effects. 2 , 16 Recently, stem cells have been found to donate healthy mitochondria to injured cells to rescue aerobic respiration and recover their metabolism capability. This is being considered as a new therapeutic strategy for tissue damage, 17 , 18 especially for mitochondrial diseases. 15 Here, we summarize and discuss the current research on mitochondrial transfers and their protection mechanisms to provide an update on stem cell therapy targeting mitochondrial encephalomyopathy.
2. CLINICAL AND PRECLINICAL EVIDENCE FOR STEM CELL THERAPIES IN MITOCHONDRIAL ENCEPHALOMYOPATHY
More and more research evidence supports the effects of stem cell therapy in some neurological diseases with mitochondrial dysfunction. 19 One of the most representative mitochondrial diseases that benefits from stem cells therapy is mitochondrial neurogastrointestinal encephalopathy (MNGIE), which is due to thymidine phosphorylase (TP) gene mutations, leading to secondary mitochondrial DNA damage. 20 Stem cell therapies can recover TP enzyme function and improve the prognosis of MNGIE patients, which provides the initial evidence to support the effects of stem cell therapy in mitochondrial encephalomyopathy. 20 , 21 , 22 , 23 The relative lack of mtDNA‐based animal models limits stem cell research on mtDNA‐related mitochondrial encephalomyopathies. 24 Encouragingly, some in vitro studies have demonstrated stem cells donate healthy mitochondria to replace dysfunctional mitochondria and recover energy metabolism in different types of recipient cells. 18 , 36 Mesenchymal stem cells (MSCs) are shown to transfer their own mitochondria into mtDNA‐depleted cells. 37 Moreover, Wharton's jelly MSCs can also transfer mitochondria to stressed mitochondrial encephalopathy fibroblasts to eliminate mutation burden, rescue mitochondrial functions, and resist against apoptotic stress, which demonstrates the protective effects of stem cell‐derived mitochondria transplantation in an in vitro model of mitochondrial encephalomyopathy. 15 , 38 Recently, in an in vivo study, transplanted pluripotent stem cell‐derived MSCs can transfer their own mitochondria to recipient cells to protect against damaged retinal ganglion cell. 35 These findings pave the way for clinical therapy study on mtDNA‐related mitochondrial encephalomyopathies through stem cell‐derived mitochondrial transplantation.
3. TRIGGERING MECHANISMS FOR MITOCHONDRIAL RELEASE
The transfer of stem cell mitochondria is a complex and intriguing phenomenon. The intercellular communication between recipient cells and stem cells may set up a specific "find‐me" and "rescue me" signal connection in the local injured regions. 18 Mitochondrial damage appears to be the main trigger for release of the mitochondria. 39 For the mitochondrial encephalomyopathies, injured mitochondrial components and other molecules are secreted to the periplasmic space as triggering signals by stressed cells. 40 , 41 Mahrouf‐Yorgov et al. found MSCs engulfed the mtDNA released by the co‐cultured cells with mitochondrial dysfunction. They reported there were subsequently increases in cytoprotective enzyme heme oxygenase‐1 expression that potentially enhanced the mitochondrial donation capability of MSCs. 42 The loss of cytochrome c can trigger mitochondrial transport from stem cells to the injured cells. 43 In addition, mitochondrial components and mtDNA also play a role in damage‐associated molecular patterns. 44 , 45 , 46 , 47 , 48 The cytokines interleukin‐1 (IL‐1), IL‐4, IL‐10, and tumor necrosis factor alpha (TNFα) can be perceived by stem cells and can also act as triggering signals. 49 Jiang et al. found that the high production of TNFα from the retinal ganglion cells results in mitochondrial release from stem cells. 35 The translocation of p53 in neurotoxic recipient cells sends out a danger signal to donor MSCs to prompt healthy mitochondrial transfer. 50 When stem cells receive these triggering signals from cells with mitochondrial dysfunction, the intrinsic mechanisms in stem cell begin to regulate the mitochondria transfer. 49 , 50 , 51 The CD38/CADPR/Ca2+ pathway is also shown to mediate astrocytes to provide their mitochondria to the damaged neurons. 52 It is necessary to explore whether the CD38/CADPR/Ca2+ pathway also works in stem cells. These findings suggest that stem cells might perceive some degree of metabolic dysfunction in adjacent cells with mitochondrial disorders and prepare to initiate mitochondrial transfer.
4. PATHWAYS OF MITOCHONDRIAL DELIVERY
Several different routes have been found to participate in mitochondrial transmission from stem cells to recipient cells, which include tunneling nanotube (TNT) formation, release of extracellular microvesicles, cellular fusion, and mitochondrial extrusion (Figure 1A). The molecular mechanisms mediating different intercellular transmission routes are complex. A clear understanding of these routes and mechanisms will be a benefit to stem cell therapy in mitochondrial diseases.
FIGURE 1.
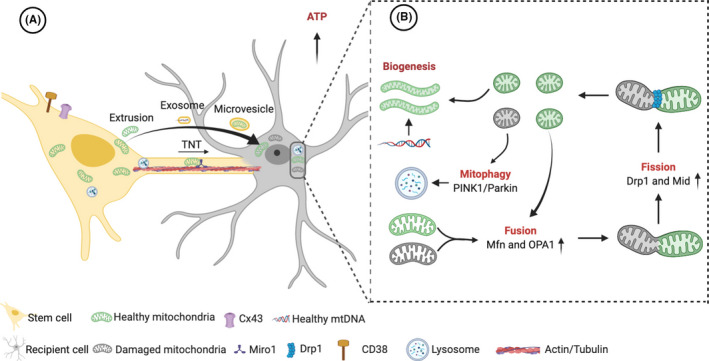
Transfer routes and protection mechanisms of stem cell‐derived mitochondria. (A) The routes of healthy mitochondria transfer from stem cells to recipient cells with dysfunctional mitochondria include TNT formation, release of extracellular microvesicles and mitochondrial extrusion. Exosomes might transfer organelle fragments (such as protein complexes of the mitochondrial electron transfer chain), mtDNA and ribosomes. The permanent cell fusion and formation of synkaryons are scarce in co‐culture conditions and in vivo, which are not drawn in the figure. (B) Stem cell‐derived mitochondria might rescue aerobic respiration and energy metabolism directly, regulate mitophagy and mitochondrial biogenesis, optimize mitochondrial fission and fusion, and decrease the mtDNA mutation load. Cx43, connexin 43; Drp 1, dynamin‐related protein 1; Miro1, Mitochondrial Rho‐GTPase 1; mtDNA, mitochondrial DNA; Mfn, mitofusin; Mid, mitochondrial dynamics protein; OPA1, optic atrophy 1; PINK1, putative kinase 1; TNT, Tunneling nanotube. (Figure Created with BioRender.com)
4.1. Tunneling nanotubes
Tunneling nanotubes are actin‐based cytoplasmic extensions connecting cells as intercellular channels 50–1000 nm in diameter in a wide variety of cell types. Ramírez‐Weber initially described a kind of membrane nanotube when studying drosophila wing imaginal disks. 53 The tunneling nanotubes were then defined by Rustom et al. in a rat PC12 cell‐human 293 cell co‐culture. 54 As a new mechanism of intercellular communication, TNTs promote the exchange of signaling molecules and cellular components between cells such as Ca2+, nucleic acids, pathogens, organelles, and plasma membrane components, including mitochondria. 18 , 54 , 55 A variety of motor proteins enable efficient transport of mitochondria between connected cells, 56 like mitochondrial Rho‐GTPase 1 (Miro1) and Miro2, 57 , 58 KLF 5 kinesin motor protein, 59 trafficking kinesin protein 1 (TRAK 1) and TRAK 2, 60 , 61 and Myo 19 62 and Myo 10. 63 MSCs have been shown to rescue damaged cells through TNT‐mediated mitochondrial transmission. MSCs detect a "find‐me" signal gradient, form membrane protrusions, and extend to create TNT with adjacent injured cells. 18 , 64 Meanwhile, the injured cells can also form membrane protrusions, subsequently extending the adjacent stem cells to establish TNT structures. The activation of tumor suppressor molecule p53 is a necessary mechanism for the formation of TNTs, and the downstream pathway (Akt/PI3K/mTOR signaling) upregulated by p53 is also involved in nanotube formation. 65 , 66 Connexin 43 (Cx43) has also been demonstrated to mediate intercellular communication through TNTs. 67 Overexpression of Cx43 facilitated mitochondrial transmission from stem cells to epithelial cells through the upregulation of tunnel tube formation. 68 , 69 The stress caused by rotenone or TNFα has also been shown to induce nanotube formation. 57 The TNFα/NF‐κB/TNFαip2 pathway is upregulated in response to TNFα 70 ; then, stem cells further promote the formation of TNT. 71 Inflammation by interferon‐γ or lipopolysaccharide has also been shown to promote the expression of M‐Sec proteins associated with TNT formation. 72 , 73 The Rho‐GTPase Miro1 can facilitate the migration of mitochondria via TNTs between two cells. A high level of Miro1 can enhance the ability of mitochondrial transfer and the rescue potential of MSCs via TNT. 57
4.2. Extracellular vesicles
Extracellular vesicles (EVs), ranging from 40 to 1000 nm, are divided into microvesicles, exosomes, and apoptotic bodies according to their size, molecular composition, and source. 39 , 56 , 74 , 75 Exosomes are small extracellular vesicles with a diameter from 30 to 150 nm. 76 , 77 , 78 , 79 , 80 Due to the small size, exosomes are unlikely to carry intact mitochondria. 56 Instead, they are able to transfer organelle fragments and genetic components. 56
As larger EVs (50–1000 nm), microvesicles can contain both intact mitochondria and mtDNA. 52 , 81 Microvesicles are more heterogeneous structures independent of cell origin. 56 The mechanisms of microvesicle biogenesis are associated with TSG101 protein recruitment to the cell surface. 82 Islam et al. discovered the phenomenon of microvesicle‐mediated mitochondrial transmission from stem cells to pulmonary alveoli protects against acute lung injury. 30 The mitochondria are packaged into vesicles containing light chain 3 (LC3) in the cytoplasm of stem cells and then integrated into outward budding in the plasma membrane. 49 Stem cells can depolarize mitochondria to the plasma membrane via arrestin domain‐containing protein 1‐mediated microbubbles, thereby controlling intracellular oxidative stress and enhanced bioenergetics. 78 Another study has shown that astrocytes can produce extracellular mitochondria that enter neurons to improve neuronal activity after ischemic stroke. 52 These studies suggest that intercellular mitochondria transmission through microvesicles is an important route to rescue mitochondrial function in the damaged cells. 3
Recently, apoptotic bodies generated from cells undergoing apoptosis have been demonstrated to be rich in mitochondria and mitochondrial components. 83 Galleu et al. have demonstrated that the presence of labilized cytotoxic cells in patients can induce apoptosis in stem cells and predict therapeutic efficacy of stem cells. 56 , 84
4.3. Cellular fusion
Aberrant mitochondrial function and endoplasmic reticulum stress enables upregulation of cell fusion events, which might represent the adaptive mechanism that promotes cell plasticity and survival in oxidative injury. 85 Cell fusion is a multistep process, including a cellular stress response, activation of autophagy, rearrangement of cellular cytoskeleton structure, expression of fusion protein, intercellular communication, and exchange of cytoplasm. 39 , 85 , 86 It has been reported that stem cells can fuse with cardiomyocytes, airway epithelial cells, neurons, and hepatocytes. 25 , 87 Adrien et al. have demonstrated that stem cells reprogram myocytes into an immature state through cell fusion and mitochondrial transfer. 32 However, the synkaryons and permanent cell fusion are scarce both in vivo and in co‐cultures, so cell fusion does not seem to be the main route of mitochondrial transfer and stem cell therapy. 32 , 39
4.4. Mitochondrial extrusion
Emerging data speculate that naked mitochondrial extrusion is another route for mitochondrial transfer. Akihito Nakajima et al. show that cytoplasmic vacuoles engulf and subsequently extrude naked mitochondria into the extracellular medium when undergoing acute TNFα‐induced apoptosis. 88 Unuma et al. also indicate that the extrusion of mitochondria and mitochondrial contents in this process, which can provoke the inflammatory response of the immune cells. 89 Both basophils 90 and eosinophils 91 can eject their mtDNA to bind and kill infectious bacteria in an ROS‐dependent manner. Boudreau et al. show that platelets can release naked mitochondria leading to inflammatory responses both in vitro and in vivo. 92 Although the above data are supported, whether stem cells are able to release naked mitochondria into the extracellular medium as a route for therapeutic mitochondria transfer is yet to be shown.
5. PROTECTION MECHANISMS ON RECIPIENT CELLS
After the process of intercellular delivery, stem cell‐derived healthy mitochondria able to enter the recipient cells with defective mitochondria and corporate with the endogenous energy metabolism network. 39 Existent data suggest that stem cell‐derived mitochondria might improve survival of recipient cells through rescuing aerobic respiration and energy metabolism, regulating mitophagy and mitochondrial biogenesis, optimizing mitochondrial dynamics, and decreasing the mtDNA mutation load (Figure 1B).
5.1. Rescuing aerobic respiration and energy metabolism directly
Spees et al. firstly show that the mitochondria transfer from adult stem cells can directly rescue aerobic respiration in recipient cells. 25 It has also been demonstrated that the stem cells able to rescue cybrid cells of myoclonic epilepsy with ragged red fibers (MERRF) through providing intact mitochondria and improving mitochondrial bioenergetics. 15 Likewise, bone marrow‐derived MSCs able to rescue energy metabolism of the cells under oxidative stress through transporting healthy mitochondria in vitro. 93 MSCs can also transfer intact mitochondria to protect against acute central nervous system 17 or lung injury in vivo. 30 Therefore, it is a quick and direct way that stem cell‐derived mitochondria incorporate into the endogenous mitochondrial network to repair metabolic machinery.
5.2. Regulating mitochondrial biogenesis and mitophagy
For mitochondrial encephalomyopathy, stem cell‐derived healthy mitochondria are also important pathways for intracellular quality control of mitochondria. Mitophagy and biogenesis are coordinated and opposing pathways that regulate mitochondrial quality control and metabolism. 94 Mitochondrial biogenesis is intricate process that includes transcription and translation of nuclear and mitochondrial genomes, recruitment, and import of mitochondrial proteins and lipids. 94 , 95 , 96
Mitochondrial biogenesis is rigorously controlled by intracellular signaling pathways and the activation of nuclear transcription factors, such as peroxisome proliferator‐activated receptor gamma, coactivator 1 α, nuclear respiratory factors, and transcription factor A. 96 The kinase pathways, second messenger molecules, and hormones participate in regulating the complex process. 95 , 96 The import of stem cell‐derived mitochondria and components may provide a quick supplement for mitochondrial biogenesis, and the exogenous mitochondria also require coordination with intracellular network in recipient cells.
Dysfunctional mitochondria are also deleterious in mitochondrial encephalomyopathy. 97 , 98 , 99 Mitophagy, the selective degradation of mitochondria, is also crucial to maintain cell homeostasis. Parkin‐dependent process, which is regulated by induced putative kinase 1 (PINK1), is a well‐studied mechanism of mitophagy. 100 , 101 , 102 Besides, FUN14 domain containing 1, Bcl2 interacting protein 3 (BNIP3) or BNIP3L can directly recruit autophagosomes to injured mitochondria via interaction with LC3. 100 In addition to canonical mitochondrial degradation, mitochondrial‐derived vesicle is one important kind of mitophagy pathway. 100 , 103 Mitochondrial spheroid also distincts from other autophagy pathways and represents the structural remodel of mitochondria in response to oxidative stresses. 100 , 104 , 105 , 106 , 107 The transmitophagy has also been observed in stem cells that mitochondria released from damaged cells are engulfed by stem cells, 108 , 109 then stem cells can degrade the damaged mitochondria and produce healthy mitochondria against programmed cell death. 42 Except the mitochondrial transfer, the transport of progenitor cell‐derived lysosomes via TNT is also observed and involved in the autophagy process of stressed cells. 110 Therefore, stem cells may involve in regulating both intra‐ and intercellular mitophagy and biogenesis process of mitochondria in host cells with mitochondrial disorders.
5.3. Optimizing mitochondrial dynamics
As high dynamic organelles, mitochondria fission and fusion are crucial for quality control. The fission can segregate dysfunctional mitochondria, while fusion can share healthy mitochondrial components. 100 , 111 , 112 Mitochondrial encephalomyopathies always have the imbalance of mitochondrial dynamics in skeletal muscle or nervous system. 113 It has the possibility that stem cell‐derived mitochondria may involve in the mitochondria dynamics in recipient cells of mitochondrial encephalomyopathies, especially the fission and fusion process. 108 A 3D imaging of the cells shows that the MSC mitochondria are spread out throughout the endogenous recipient mitochondria network. 114
Mitochondrial fusion is controlled by three dynamin‐related GTPase proteins. The fusion of the outer mitochondrial membrane is regulated by mitofusin 1 (Mfn1) and Mfn2, 103 , 115 , 116 and the fusion of the inner mitochondrial membrane is regulated by optic atrophy 1 (OPA1). 103 , 117 , 118 Recently, another study demonstrates that stem cells play protective role in a mitochondrial dysfunction mice model through the mechanisms of transferring mitochondria and increasing the fusion gene expression of OPA1, Mfn1, and Mfn2 in host cells. 119
Mitochondrial fission is mediated by the dynamin‐related protein 1 (Drp 1), which binds to four receptors: fission 1 protein (Fis1), mitochondria fission factor, mitochondrial dynamics protein of 49 and 51 kDa. 100 , 103 , 120 Chuang et al. show that the stem cells able to rescue MERRF cybrid cells and optimize mitochondrial dynamics through donating healthy mitochondria. 15 The MERRF cybrid cells present decreased OPA1 and increased Fis1, which cause the imbalance of mitochondrial fusion and fission. 15 It is demonstrated that stem cells can recapture the dysfunctional mitochondria network and abnormal fusion/fission protein in MERRF cybrid cells through mitochondria transfer. 15
5.4. Decreasing the mtDNA mutation load
Majority of mitochondrial encephalomyopathies is caused by the pathogenic mtDNA mutations. Previous research find that the level of the mtDNA mutations will also influence disease severity and is reliable measure for clinical assessment. 121 , 122 Therefore, reducing mutation load through intercellular exchange of mtDNA may have a certain effect on alleviating clinical severity of mitochondrial encephalomyopathy. The transfer of low copy number of mtDNA can recover the mitochondrial function of immunoincompetent mice. 123 Jayaprakash et al. find the horizontal transfer of mitochondrial DNA between different cell lines in the co‐cultures. 124 Tumor cells can repair the transcription and translation of mtDNA and improve mitochondrial bioenergetics through acquiring healthy mtDNA from ambient cells. 39 It is also proposed that stem cells can partly reduce mtDNA mutation load via providing healthy mtDNA and mitochondria, which is sufficient to recover the mitochondrial respiration in MERRF cybrid cells long term. 15 The routes of mtDNA transfer not only restrict to TNTs, microvesicles, cellular fusion, and mitochondrial extrusion, 125 , 126 , 127 but also including more tiny structures such as exosomes and gap junction. 127 , 128 However, emerging data challenge the potential therapeutic use of EV‐based delivery systems for mtDNA‐based diseases. 129 After exposure to the donor mtDNA in EV fractions for years, there is little transfer of the donor mtDNA to the host mtDNA fraction in subjects tissues. 129
6. FUTURE PERSPECTIVES AND CHALLENGES
An increasing number of diseases are being found to have the pathogenesis of mitochondrial dysfunction. 130 However, the treatments for mitochondrial disorders are inadequate, especially in the therapies of mitochondrial encephalomyopathies. The main strategy employed in the treatment of nDNA‐based mitochondrial encephalomyopathy, while current capacity to repair or replace mDNA in somatic cells is inadequate. Stem cells have the characteristic of lower immunogenicity and the ability for long‐term proliferation to amplify the quantity of mitochondria. 49 Thus, stem cells are an ideal choice as potential mitochondrial donors.
The transplantation of stem cell‐derived mitochondria to somatic cells has been demonstrated not only in vitro but also in vivo and is recognized as a novel and promising strategy for treating mitochondrial dysfunction. However, there are still several major challenges and concerns remaining. Time, number, efficiency, and route of mitochondrial transfer are important for the activity restoration of the recipient cells bearing mitochondria dysfunction. 39 , 72 , 73 Various transfer routes of stem cell‐derived mitochondria include TNTs, extracellular microvesicles, cellular fusion, and mitochondrial extrusion, which have significant differences in the dosage and efficiency of mitochondria transfer and may directly affect the rescue effect of stem cells. 39 , 72 , 73 TNTs are known as the highway for intercellular organelle transport, 54 which is much more efficient than other modes of intercellular mitochondria delivery, such as the extracellular microvesicles and mitochondrial extrusion. 43 In spite of this, it is possible that different transfer routes might complement each other in different pathophysiological stages and microenvironments, and even cooperate with each other to promote the therapeutic effect of stem cells. On the other hand, various mitochondria transfer routes have respective signaling pathways. The identification of these signaling pathways and the mechanisms of intercellular mitochondria delivery will improve the potential applications of cell therapy‐based mitochondrial restoration. 131 , 132
Future therapeutic investigations should consider strategies to pharmacologically enhance or control the transfer of stem cell‐derived mitochondria, 39 especially in stem cell therapy for mitochondrial encephalomyopathy.
Moreover, the mechanisms through which stem cell‐derived mitochondria can be incorporated into the endogenous energy metabolism network remain to be elucidated. 39
Furthermore, mitochondrial damage and ROS are considered to be probably involved in the inflammation. 133 The mitochondrial dysfunction could also play an important role in chronic inflammation of the neurodegenerative disorders and mitochondrial diseases. 134 The transplantation of stem cell‐derived mitochondria may be used as an effective treatment for these pathologies, which may attenuate production of ROS and have immunomodulatory effects. 133 , 134
Future research on the molecular mechanisms underlying the improvement of aerobic respiration, dynamics, and the quality control of transplanted mitochondria will accelerate the development of stem cell treatment in mitochondrial encephalomyopathies. However, most current studies on mitochondrial disease remain in the in vitro stage due to a lack of mtDNA‐based animal models of mitochondrial encephalomyopathies. Meanwhile, novel tracking technologies are necessary to unravel the mechanism of intercellular mitochondria transmission in vivo. The development of cell heteroplasmy in single cells and mitoception to detect mitochondrial transfer 114 are useful to unambiguously assess mitochondria transmission and the effects of stem cell mitochondria on cell metabolism and function studies. 39
7. CONCLUSION
Currently, the treatment of mitochondrial encephalomyopathy faces serious challenges. 39 Restoring the function of mitochondria and rescuing damaged mitochondria are crucial for treating mitochondrial disorders. Stem cell‐derived mitochondria transplantation has been demonstrated to play a significant role not only in metabolic rescue but also in mitochondrial dynamics, quality control, and reduction of mutation load, which may eventually prevent cell apoptosis. Thus, the therapy offers great promise for mitochondrial encephalomyopathies. Meanwhile, mitochondrial integrity and mitochondrial dynamics also become dysfunctional during some neurodegenerative diseases. 135 , 136 , 137 , 138 The application of stem cell‐derived mitochondria transplantation has also attracted attention for its potential to treat many diseases with the pathogenesis of mitochondrial dysfunction, such as cerebral vascular disease, 139 , 140 Parkinson's disease, dementia, amyotrophic lateral sclerosis, myocardial ischemia–reperfusion injury, and acute lung injury. 30 , 71 , 74 , 108 , 141 , 142 , 143 , 144 However, as one of the most representative and intractable mitochondrial diseases, mitochondrial encephalomyopathies should be the first to see a breakthrough and benefit from this novel treatment strategy.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
Study Funded by the Shandong Provincial Natural Science Foundation of China (Grant No. ZR2013HQ050), Zhejiang Provincial Natural Science Foundation of China (Grant No. LY19H090025, Grant No. LQ15H090003), National Natural Science Foundation of China (Grant No. 81101157).
Liu K, Zhou Z, Pan M, Zhang L. Stem cell‐derived mitochondria transplantation: A promising therapy for mitochondrial encephalomyopathy. CNS Neurosci Ther. 2021;27:733–742. 10.1111/cns.13618
Kaiming Liu and Zhijian Zhou contributed equally to this work.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482‐1488. [DOI] [PubMed] [Google Scholar]
- 2. Nightingale H, Pfeffer G, Bargiela D, et al. Emerging therapies for mitochondrial disorders. Brain. 2016;139:1633‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakamura Y, Park JH, Hayakawa K. Therapeutic use of extracellular mitochondria in CNS injury and disease. Exp Neurol. 2020;324:113114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma H, Folmes CDL, Wu J, et al. Metabolic rescue in pluripotent cells from patients with mtDNA disease. Nature. 2015;524:234‐238. [DOI] [PubMed] [Google Scholar]
- 5. Yang YI, Wu H, Kang X, et al. Targeted elimination of mutant mitochondrial DNA in MELAS‐iPSCs by mitoTALENs. Protein Cell. 2018;9:283‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang J‐C, Hoel F, Liu K‐H, et al. Peptide‐mediated delivery of donor mitochondria improves mitochondrial function and cell viability in human cybrid cells with the MELAS A3243G mutation. Sci Rep. 2017;7:10710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341‐355. [DOI] [PubMed] [Google Scholar]
- 8. Hanbali A, Rasheed W, Peedikayil MC, et al. Mitochondrial neurogastrointestinal encephalomyopathy syndrome treated with stem cell transplant: a case series and literature review. Exp Clin Transplant. 2018;16:773‐778. [DOI] [PubMed] [Google Scholar]
- 9. Yadak R, Sillevis Smitt P, van Gisbergen MW, et al. Mitochondrial neurogastrointestinal encephalomyopathy caused by thymidine phosphorylase enzyme deficiency: from pathogenesis to emerging therapeutic options. Front Cell Neurosci. 2017;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hirano M, Marti R, Casali C, et al. Allogeneic stem cell transplantation corrects biochemical derangements in MNGIE. Neurology. 2006;67:1458‐1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halter J, Schüpbach WMM, Casali C, et al. Allogeneic hematopoietic SCT as treatment option for patients with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a consensus conference proposal for a standardized approach. Bone Marrow Transplant. 2011;46:330‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hussein E. Non‐myeloablative bone marrow transplant and platelet infusion can transiently improve the clinical outcome of mitochondrial neurogastrointestinal encephalopathy: a case report. Transfus Apher Sci. 2013;49:208‐211. [DOI] [PubMed] [Google Scholar]
- 13. Filosto M, Scarpelli M, Tonin P, et al. Course and management of allogeneic stem cell transplantation in patients with mitochondrial neurogastrointestinal encephalomyopathy. J Neurol. 2012;259:2699‐2706. [DOI] [PubMed] [Google Scholar]
- 14. Sicurelli F, Carluccio MA, Toraldo F, et al. Clinical and biochemical improvement following HSCT in a patient with MNGIE: 1‐year follow‐up. J Neurol. 2012;259:1985‐1987. [DOI] [PubMed] [Google Scholar]
- 15. Chuang Y‐C, Liou C‐W, Chen S‐D, et al. Mitochondrial transfer from Wharton's jelly mesenchymal stem cell to MERRF cybrid reduces oxidative stress and improves mitochondrial bioenergetics. Oxid Med Cell Longev. 2017;2017:5691215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu K, Chi L, Guo L, et al. The interactions between brain microvascular endothelial cells and mesenchymal stem cells under hypoxic conditions. Microvasc Res. 2008;75:59‐67. [DOI] [PubMed] [Google Scholar]
- 17. Liu K, Guo L, Zhou Z, et al. Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc Res. 2019;123:74‐80. [DOI] [PubMed] [Google Scholar]
- 18. Liu K, Ji K, Guo L, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia‐reperfusion model via tunneling nanotube like structure‐mediated mitochondrial transfer. Microvasc Res. 2014;92:10‐18. [DOI] [PubMed] [Google Scholar]
- 19. Lunn JS, Sakowski SA, Hur J, et al. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011;70:353‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Giorgio R, Pironi L, Rinaldi R, et al. Liver transplantation for mitochondrial neurogastrointestinal encephalomyopathy. Ann Neurol. 2016;80:448‐455. [DOI] [PubMed] [Google Scholar]
- 21. Halter JP, Michael W, Schüpbach M, et al. Allogeneic haematopoietic stem cell transplantation for mitochondrial neurogastrointestinal encephalomyopathy. Brain. 2015;138:2847‐2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scarpelli M, Todeschini A, Volonghi I, et al. Mitochondrial diseases: advances and issues. Appl Clin Genet. 2017;10:21‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Torres‐Torronteras J, Cabrera‐Pérez R, Barba I, et al. Long‐term restoration of thymidine phosphorylase function and nucleoside homeostasis using hematopoietic gene therapy in a murine model of mitochondrial neurogastrointestinal encephalomyopathy. Hum Gene Ther. 2016;27:656‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farrar GJ, Chadderton N, Kenna PF, et al. Mitochondrial disorders: aetiologies, models systems, and candidate therapies. Trends Genet. 2013;29:488‐497. [DOI] [PubMed] [Google Scholar]
- 25. Spees JL, Olson SD, Whitney MJ, et al. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331‐e339. [DOI] [PubMed] [Google Scholar]
- 27. Kalogeris T, Baines CP, Krenz M, et al. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cho YM, Kim JH, Kim M, et al. Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PLoS One. 2012;7:e32778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Figeac F, Lesault P‐F, Le Coz O, et al. Nanotubular crosstalk with distressed cardiomyocytes stimulates the paracrine repair function of mesenchymal stem cells. Stem Cells. 2014;32:216‐230. [DOI] [PubMed] [Google Scholar]
- 30. Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone‐marrow‐derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang D, Gao F, Zhang Y, et al. Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 2016;7:e2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Acquistapace A, Bru T, Lesault P‐F, et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor‐like state through partial cell fusion and mitochondria transfer. Stem Cells. 2011;29:812‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Borlongan CV, Nguyen H, Lippert T, et al. May the force be with you: transfer of healthy mitochondria from stem cells to stroke cells. J Cereb Blood Flow Metab. 2019;39:367‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayakawa K, Chan SJ, Mandeville ET, et al. Protective effects of endothelial progenitor cell‐derived extracellular mitochondria in brain endothelium. Stem Cells. 2018;36:1404‐1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang D, Xiong G, Feng H, et al. Donation of mitochondria by iPSC‐derived mesenchymal stem cells protects retinal ganglion cells against mitochondrial complex I defect‐induced degeneration. Theranostics. 2019;9:2395‐2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moschoi R, Imbert V, Nebout M, et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood. 2016;128:253‐264. [DOI] [PubMed] [Google Scholar]
- 37. Lin H‐Y, Liou C‐W, Chen S‐D, et al. Mitochondrial transfer from Wharton's jelly‐derived mesenchymal stem cells to mitochondria‐defective cells recaptures impaired mitochondrial function. Mitochondrion. 2015;22:31‐44. [DOI] [PubMed] [Google Scholar]
- 38. Lin T‐K, Chen S‐D, Chuang Y‐C, et al. Mitochondrial transfer of Wharton's jelly mesenchymal stem cells eliminates mutation burden and rescues mitochondrial bioenergetics in rotenone‐stressed MELAS fibroblasts. Oxid Med Cell Longev. 2019;2019:9537504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Torralba D, Baixauli F, Sanchez‐Madrid F. Mitochondria know no boundaries: mechanisms and functions of intercellular mitochondrial transfer. Front Cell Dev Biol. 2016;4:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maeda A, Fadeel B. Mitochondria released by cells undergoing TNF‐alpha‐induced necroptosis act as danger signals. Cell Death Dis. 2014;5:e1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grazioli S, Pugin J. Mitochondrial damage‐associated molecular patterns: from inflammatory signaling to human diseases. Front Immunol. 2018;9:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mahrouf‐Yorgov M, Augeul L, Da Silva CC, et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 2017;24:1224‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015;22:1181‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Q, Raoof M, Chen YU, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol. 2012;13:780‐788. [DOI] [PubMed] [Google Scholar]
- 46. West AP, Khoury‐Hanold W, Staron M, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oka T, Hikoso S, Yamaguchi O, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang J, Li H, Yao Y, et al. Stem cell‐derived mitochondria transplantation: a novel strategy and the challenges for the treatment of tissue injury. Stem Cell Res Ther. 2018;9:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boukelmoune N, Chiu GS, Kavelaars A, et al. Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin. Acta Neuropathol Commun. 2018;6:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Davis C‐ha O, Kim K‐Y, Bushong EA, et al. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci U S A. 2014;111:9633‐9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hayakawa K, Esposito E, Wang X, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ramirez‐Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599‐607. [DOI] [PubMed] [Google Scholar]
- 54. Rustom A, Saffrich R, Markovic I, et al. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007‐1010. [DOI] [PubMed] [Google Scholar]
- 55. Belting M, Wittrup A. Nanotubes, exosomes, and nucleic acid‐binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease. J Cell Biol. 2008;183:1187‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Murray LMA, Krasnodembskaya AD. Concise review: intercellular communication via organelle transfer in the biology and therapeutic applications of stem cells. Stem Cells. 2019;37:14‐25. [DOI] [PubMed] [Google Scholar]
- 57. Ahmad T, Mukherjee S, Pattnaik B, et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33:994‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fransson S, Ruusala A, Aspenstrom P. The atypical Rho GTPases Miro‐1 and Miro‐2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun. 2006;344:500‐510. [DOI] [PubMed] [Google Scholar]
- 59. Falnikar A, Tole S, Baas PW. Kinesin‐5, a mitotic microtubule‐associated motor protein, modulates neuronal migration. Mol Biol Cell. 2011;22:1561‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stowers RS, Megeath LJ, Górska‐Andrzejak J, et al. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063‐1077. [DOI] [PubMed] [Google Scholar]
- 61. Glater EE, Megeath LJ, Stowers RS, et al. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bohil AB, Robertson BW, Cheney RE. Myosin‐X is a molecular motor that functions in filopodia formation. Proc Natl Acad Sci U S A. 2006;103:12411‐12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nawaz M, Fatima F. Extracellular vesicles, tunneling nanotubes, and cellular interplay: synergies and missing links. Front Mol Biosci. 2017;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ravichandran KS. Find‐me and eat‐me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207:1807‐1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang Y, Cui J, Sun X, et al. Tunneling‐nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 2011;18:732‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sun X, Wang Y, Zhang J, et al. Tunneling‐nanotube direction determination in neurons and astrocytes. Cell Death Dis. 2012;3:e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang X, Veruki ML, Bukoreshtliev NV, et al. Animal cells connected by nanotubes can be electrically coupled through interposed gap‐junction channels. Proc Natl Acad Sci U S A. 2010;107:17194‐17199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yao Y, Fan X‐L, Jiang D, et al. Connexin 43‐mediated mitochondrial transfer of iPSC‐MSCs alleviates asthma inflammation. Stem Cell Reports. 2018;11:1120‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Otsu K, Das S, Houser SD, et al. Concentration‐dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113:4197‐4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ohno H, Hase K, Kimura S. M‐Sec: emerging secrets of tunneling nanotube formation. Commun Integr Biol. 2010;3:231‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang Y, Yu Z, Jiang D, et al. iPSC‐MSCs with high intrinsic MIRO1 and sensitivity to TNF‐alpha yield efficacious mitochondrial transfer to rescue anthracycline‐induced cardiomyopathy. Stem Cell Reports. 2016;7:749‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hase K, Kimura S, Takatsu H, et al. M‐Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol. 2009;11:1427‐1432. [DOI] [PubMed] [Google Scholar]
- 73. Bagheri HS, Bani F, Tasoglu S, et al. Mitochondrial donation in translational medicine; from imagination to reality. J Transl Med. 2020;18:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Z, Sheng H, Liao LI, et al. Mesenchymal stem cell‐conditioned medium improves mitochondrial dysfunction and suppresses apoptosis in Okadaic Acid‐treated SH‐SY5Y cells by extracellular vesicle mitochondrial transfer. J Alzheimers Dis. 2020;78(3):1161‐1176. [DOI] [PubMed] [Google Scholar]
- 75. Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lai RC, Tan SS, Yeo RWY, et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;5:29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581‐593. [DOI] [PubMed] [Google Scholar]
- 79. Zhang B, Yin Y, Lai RC, et al. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23:1233‐1244. [DOI] [PubMed] [Google Scholar]
- 80. Valadi H, Ekström K, Bossios A, et al. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654‐659. [DOI] [PubMed] [Google Scholar]
- 81. Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nabhan JF, Hu R, Oh RS, et al. Formation and release of arrestin domain‐containing protein 1‐mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A. 2012;109:4146‐4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Atkin‐Smith GK, Tixeira R, Paone S, et al. A novel mechanism of generating extracellular vesicles during apoptosis via a beads‐on‐a‐string membrane structure. Nat Commun. 2015;6:7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Galleu A, Riffo‐Vasquez Y, Trento C, et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient‐mediated immunomodulation. Sci Transl Med. 2017;9(416):eaam7828. [DOI] [PubMed] [Google Scholar]
- 85. Filippova N, Nabors LB. ELAVL1 role in cell fusion and tunneling membrane nanotube formations with implication to treat glioma heterogeneity. Cancers (Basel). 2020;12(10):3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Aguilar PS, Baylies MK, Fleissner A, et al. Genetic basis of cell‐cell fusion mechanisms. Trends Genet. 2013;29:427‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Alvarez‐Dolado M, Pardal R, Garcia‐Verdugo JM, et al. Fusion of bone‐marrow‐derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968‐973. [DOI] [PubMed] [Google Scholar]
- 88. Nakajima A, Kurihara H, Yagita H, et al. Mitochondrial Extrusion through the cytoplasmic vacuoles during cell death. J Biol Chem. 2008;283:24128‐24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Unuma K, Aki T, Funakoshi T, et al. Extrusion of mitochondrial contents from lipopolysaccharide‐stimulated cells: involvement of autophagy. Autophagy. 2015;11:1520‐1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Morshed M, Hlushchuk R, Simon D, et al. NADPH oxidase‐independent formation of extracellular DNA traps by basophils. J Immunol. 2014;192:5314‐5323. [DOI] [PubMed] [Google Scholar]
- 91. Yousefi S, Gold JA, Andina N, et al. Catapult‐like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949‐953. [DOI] [PubMed] [Google Scholar]
- 92. Boudreau LH, Duchez A‐C, Cloutier N, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA‐secreted phospholipase A2 to promote inflammation. Blood. 2014;124:2173‐2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Han H, Hu J, Yan Q, et al. Bone marrow‐derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol Med Rep. 2016;13:1517‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Palikaras K, Tavernarakis N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol. 2014;56:182‐188. [DOI] [PubMed] [Google Scholar]
- 95. Herrmann JM, Longen S, Weckbecker D, et al. Biogenesis of mitochondrial proteins. Adv Exp Med Biol. 2012;748:41‐64. [DOI] [PubMed] [Google Scholar]
- 96. Zhu J, Wang KZ, Chu CT. After the banquet: mitochondrial biogenesis, mitophagy, and cell survival. Autophagy. 2013;9:1663‐1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ariosa AR, Klionsky DJ. Autophagy core machinery: overcoming spatial barriers in neurons. J Mol Med (Berl). 2016;94:1217‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kiriyama Y, Nochi H. The function of autophagy in neurodegenerative diseases. Int J Mol Sci. 2015;16:26797‐26812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ni HM, Williams JA, Ding WX. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem. 2012;393:547‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kiriyama Y, Nochi H. Intra‐ and intercellular quality control mechanisms of mitochondria. Cells. 2017;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ding W‐X, Guo F, Ni H‐M, et al. Parkin and mitofusins reciprocally regulate mitophagy and mitochondrial spheroid formation. J Biol Chem. 2012;287:42379‐42388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ding W‐X, Li M, Biazik JM, et al. Electron microscopic analysis of a spherical mitochondrial structure. J Biol Chem. 2012;287:42373‐42378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yin XM, Ding WX. The reciprocal roles of PARK2 and mitofusins in mitophagy and mitochondrial spheroid formation. Autophagy. 2013;9:1687‐1692. [DOI] [PubMed] [Google Scholar]
- 107. Ni H‐M, Williams JA, Jaeschke H, et al. Zonated induction of autophagy and mitochondrial spheroids limits acetaminophen‐induced necrosis in the liver. Redox Biol. 2013;1:427‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Han D, Zheng X, Wang X, et al. Mesenchymal stem/stromal cell‐mediated mitochondrial transfer and the therapeutic potential in treatment of neurological diseases. Stem Cells Int. 2020;2020:8838046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase‐1. Annu Rev Pharmacol Toxicol. 2010;50:323‐354. [DOI] [PubMed] [Google Scholar]
- 110. Yasuda K, Khandare A, Burianovskyy L, et al. Tunneling nanotubes mediate rescue of prematurely senescent endothelial cells by endothelial progenitors: exchange of lysosomal pool. Aging (Albany NY). 2011;3:597‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. van der Bliek AM, Shen Q, Kawajiri S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol. 2013;5(6):a011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Twig G, Elorza A, Molina AJA, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bartsakoulia M, Pyle A, Troncoso‐Chandía D, et al. A novel mechanism causing imbalance of mitochondrial fusion and fission in human myopathies. Hum Mol Genet. 2018;27:1186‐1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Caicedo A, Fritz V, Brondello J‐M, et al. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci Rep. 2015;5:9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Koshiba T, Detmer SA, Kaiser JT, et al. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858‐862. [DOI] [PubMed] [Google Scholar]
- 116. Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535‐6546. [DOI] [PubMed] [Google Scholar]
- 117. Ehses S, Raschke I, Mancuso G, et al. Regulation of OPA1 processing and mitochondrial fusion by m‐AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187:1023‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ishihara N, Fujita Y, Oka T, et al. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966‐2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Maremanda KP, Sundar IK, Rahman I. Protective role of mesenchymal stem cells and mesenchymal stem cell‐derived exosomes in cigarette smoke‐induced mitochondrial dysfunction in mice. Toxicol Appl Pharmacol. 2019;385:114788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hu C, Huang Y, Li L. Drp1‐dependent mitochondrial fission plays critical roles in physiological and pathological progresses in mammals. Int J Mol Sci. 2017;18(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chinnery PF, Howell N, Lightowlers RN, et al. Molecular pathology of MELAS and MERRF. The relationship between mutation load and clinical phenotypes. Brain. 1997;120(Pt 10):1713‐1721. [DOI] [PubMed] [Google Scholar]
- 122. Grady JP, Pickett SJ, Ng YS, et al. mtDNA heteroplasmy level and copy number indicate disease burden in m.3243A>G mitochondrial disease. EMBO Mol Med. 2018;10(6):e8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Dickinson A, Yeung KY, Donoghue J, et al. The regulation of mitochondrial DNA copy number in glioblastoma cells. Cell Death Differ. 2013;20:1644‐1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jayaprakash AD, Benson EK, Gone S, et al. Stable heteroplasmy at the single‐cell level is facilitated by intercellular exchange of mtDNA. Nucleic Acids Res. 2015;43:2177‐2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lin HP, Zheng DJ, Li YP, et al. Incorporation of VSV‐G produces fusogenic plasma membrane vesicles capable of efficient transfer of bioactive macromolecules and mitochondria. Biomed Microdevices. 2016;18:41. [DOI] [PubMed] [Google Scholar]
- 126. Barteneva NS, Maltsev N, Vorobjev IA. Microvesicles and intercellular communication in the context of parasitism. Front Cell Infect Microbiol. 2013;3:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Paliwal S, Chaudhuri R, Agrawal A, et al. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci. 2018;25:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Guescini M, Genedani S, Stocchi V, et al. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm (Vienna). 2010;117:1‐4. [DOI] [PubMed] [Google Scholar]
- 129. Tarnopolsky MA, Kerkhof J, Stuart A, et al. Bone marrow‐derived mitochondrial DNA has limited capacity for inter‐tissue transfer in vivo. FASEB J. 2020;34:9297‐9306. [DOI] [PubMed] [Google Scholar]
- 130. Patananan A, Wu T‐H, Chiou P‐Y, et al. Modifying the mitochondrial genome. Cell Metab. 2016;23:785‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Abe T, Kiyonari H, Shioi G, et al. Establishment of conditional reporter mouse lines at ROSA26 locus for live cell imaging. Genesis. 2011;49:579‐590. [DOI] [PubMed] [Google Scholar]
- 132. Pham AH, McCaffery JM, Chan DC. Mouse lines with photo‐activatable mitochondria to study mitochondrial dynamics. Genesis. 2012;50:833‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Filippov MA, Tatarnikova OG, Pozdnyakova NV, et al. Inflammation/bioenergetics‐associated neurodegenerative pathologies and concomitant diseases: a role of mitochondria targeted catalase and xanthophylls. Neural Regen Res. 2021;16:223‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Nair S, Rocha‐Ferreira E, Fleiss B, et al. Neuroprotection offered by mesenchymal stem cells in perinatal brain injury: role of mitochondria, inflammation, and reactive oxygen species. J Neurochem. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Cowan K, Anichtchik O, Luo S. Mitochondrial integrity in neurodegeneration. CNS Neurosci Ther. 2019;25:825‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wang Y, Xu E, Musich PR, et al. Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci Ther. 2019;25:816‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zheng YR, Zhang XN, Chen Z. Mitochondrial transport serves as a mitochondrial quality control strategy in axons: Implications for central nervous system disorders. CNS Neurosci Ther. 2019;25:876‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wang Y, Liu N, Lu B. Mechanisms and roles of mitophagy in neurodegenerative diseases. CNS Neurosci Ther. 2019;25:859‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Tseng N, Lambie SC, Huynh CQ, et al. Mitochondrial transfer from mesenchymal stem cells improves neuronal metabolism after oxidant injury in vitro: the role of Miro1. J Cereb Blood Flow Metab. 2021;41:761‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Park J‐H, Nakamura Y, Li W, et al. Effects of O‐GlcNAcylation on functional mitochondrial transfer from astrocytes. J Cereb Blood Flow Metab. 2020;271678X20969588. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li X, Zhang Y, Yeung SC, et al. Mitochondrial transfer of induced pluripotent stem cell‐derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke‐induced damage. Am J Respir Cell Mol Biol. 2014;51:455‐465. [DOI] [PubMed] [Google Scholar]
- 142. Calabria E, Scambi I, Bonafede R, et al. ASCs‐exosomes recover coupling efficiency and mitochondrial membrane potential in an in vitro model of ALS. Front Neurosci. 2019;13:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Babenko VA, Silachev DN, Zorova LD, et al. Improving the post‐stroke therapeutic potency of mesenchymal multipotent stromal cells by cocultivation with cortical neurons: the role of crosstalk between cells. Stem Cells Transl Med. 2015;4:1011‐1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Onyango IG. Modulation of mitochondrial bioenergetics as a therapeutic strategy in Alzheimer's disease. Neural Regen Res. 2018;13:19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


