Abstract
Background
The neurovascular unit (NVU) is emerging as a potential therapeutic target in neurological conditions, such as stroke, brain injury, Alzheimer's disease, and Parkinson's disease; meanwhile, stroke is the second leading cause of death globally. The purpose of the study is to analyze the most influential articles, authors, countries, and topics in the role of NVU in stroke.
Methods
The Web of Science (WoS) database was used for bibliometric analysis using the search terms “Stroke” and “Neurovascular unit” on January 1st, 2021. Data were extracted from the WoS database to identify collaborations between authors, countries, organizations, and keywords using VOSviewer (1.6.16 mac). Two bibliometric indicators, the activity index (AI) and category normalized citation impact (CNCI), were computed. The keywords of bursts were also identified by CiteSpace.
Results
A total of 770 articles were analyzed by VOSviewer. AIs and CNCIs were computed of the eighteen countries according to VOSviewer co‐authorship analysis results. The majority of authors mainly came from the United States and Japan. Romania, Hungary, and Poland have emerged as rising‐star countries. In the 100 most‐cited articles, the number of citations ranged from 1873 to 69, with a total of 15,758 citations. Most articles were published in 2011 and 2012 (n = 13 each), followed by 2009 (n = 11) and 2013, 2014, and 2015 (n = 8 each). Stroke and Journal of Cerebral Blood Flow and Metabolism were the two top journals. EH Lo from Harvard University/ Massachusetts General Hospital was the top first author and corresponding author. Harvard University/Massachusetts General Hospital was the most productive affiliated institution with 15 publications.
Conclusion
There has been growing attention and efforts made in the field of stroke and NVU. The merit of the above findings may help to shape the research policy in ischemic stroke both at the country and institutional level.
Keywords: bibliometric analysis, blood‐brain barrier, neuroprotection, neurovascular unit, stroke
We performed VOSviewer analysis of collaborations between authors and countries and found two different clusters of authors mainly from USA and Japan had a broad collaboration with authors from other countries. The Lo EH research group and the Abe K research group showed most extensive collaboration with other research groups.

1. INTRODUCTION
Stroke is the second leading cause of death globally 1 and has become the fifth leading cause of death in the United States, which leaves an enormous burden on patients' families and the society at large. 2 The incidence and mortality of stroke decreased in high‐income countries between 1990 and 2010. However, no significant change has been seen in low‐ and middle‐income countries. 1 , 3 , 4 Stroke can be divided into ischemic stroke and hemorrhagic stroke, and our studies include both stroke types. Nowadays, with standard treatments such as tissue plasminogen activator (tPA) or thrombolytic therapy and mechanical thrombectomy, 5 more ischemic stroke patients survive the stroke but with complications such as hemorrhagic transformation, cerebral edema, epilepsy, and pneumonia. 6 , 7 , 8 , 9 Microvascular reperfusion injury following mechanical thrombectomy is also an urgent problem to be solved. Thrombolytic therapy requires a narrow time window and is only used in about 7% of patients after an acute ischemic stroke in the United States. 10 One novel thrombolytic, desmoteplase, has been reported to have failed the Phase IIb/III trial with no significant improvement in functional outcome at 3 months, 11 but other thrombolytics and reperfusion agents, such as tenecteplase and stachybotrys microspore triprenyl phenol‐7 (SMTP‐7), remain in development. 12 Basic studies and clinical trials may uncover next‐generation thrombolytic drugs or agents that could reduce the risk of thrombolysis‐associated hemorrhagic transformation that may allow an extension of the current narrow therapeutic window and reduce the risk of catastrophic brain hemorrhage after stroke. The emergence of the neurovascular unit (NVU) concept provides a new foothold for that research in this regard.
The concept of the NVU, which is comprised of neurons, astrocytes, pericytes, microglia, endothelial cells, and vascular smooth muscle cells (VSMCs), was formalized at the 2001 Stroke Progress Review Group meeting of the National Institute of Neurological Disorders and Stroke (https://www.ninds.nih.gov/About‐NINDS/Strategic‐Plans‐Evaluations/Strategic‐Plans/Stroke‐Progress‐Review‐Group) and demonstrated that components of the NVU in the central nervous system communicated dynamically with each other and maintained hemostasis of the brain. 13 The interaction and signaling between different components of the NVU are very complicated, and latest advances have shown that the NVU might regulate cerebral blood flow and maintain brain homeostasis through neurovascular coupling or vasculoneuronal coupling. 13 , 14 , 15 Meanwhile, it was recently suggested that astrocytes or microglia, which have multiple phenotypes, have controversial effects on the regulation of neuroinflammation or neurovascular coupling and require further investigation. 16 , 17 , 18 , 19 Due to the complexity of the NVU, there has been an increasing number of studies on the NVU in stroke. However, currently, none of these studies have translated into new stroke treatments. 20 , 21 , 22 , 23 , 24 Considerable efforts have been devoted to finding effective therapeutic targets and pathways in the NVU for not only ischemic stroke, but also a number of other neurological diseases, such as Alzheimer's disease and Parkinson's disease.
The latest progress and the most vital contributions in stroke and the NVU can be found in the surging number of publications in the field of NVU, including reviews, basic studies and clinical trials. However, there is no bibliometric analysis of stroke and NVU. It is of great importance to look into the distribution of articles and authors in this field, which would give readers a vivid overview of the most important works in the past two decades.
2. METHODS
We performed a bibliometric analysis on January 1st, 2021 by using the Web of Science (WoS) database using the search terms “Stroke” and “Neurovascular unit” in the title/abstract/keywords between 2001 and 2020. Total citation number and average number of citations for each report of all results were collected in the WoS analysis tool.
Data were extracted from the WoS database to identify collaborations between authors, countries, organizations and keywords within the boundaries of the aforementioned search terms using VOSviewer (1.6.16 mac). Co‐authorship analysis and co‐occurrence analysis were performed, and the number of authors, countries, organizations, and keywords was based on the choice of a minimum number of documents or occurrences. Two bibliometric indicators, the activity index (AI) and category normalized citation impact (CNCI), were computed. AI indicates the relative research effort of a country to a research field based on publications. The CNCI of an article is the ratio of the observed number of citations to the expected number of citations. If AI or CNCI was greater than 1, then the research power or the academic influence of a country was higher than the global average, and vice versa.
The initial results were then filtered in descending order of the number of citations in WoS to find the 100 most‐cited articles. The article title, number of citations, publication year, journal, journal impact factor (IF), first author, corresponding author, country/institution of the corresponding author, and study type were collected. Eight articles were excluded after reviewing the title, abstract, and whole text of these papers by two experienced anesthesiologists in our group. Study type was accessed by the two doctors mentioned above reading the full text. These data were then imported to Microsoft Excel for analysis manually. The keywords of bursts were also identified by CiteSpace.
3. SEARCH RESULTS
We found 774 articles in the initial search of the WoS database under the search terms “Stroke” and “Neurovascular unit.” Of these papers, 20 176 reports had cited these articles, with a total citation number of 28141. The average number of citations for each report was 36.26.
Seven‐hundred and seventy articles were analyzed by VOSviewer after excluding one withdrawn article and three patent documents. The result of co‐authorship analysis, which includes 27 authors with >9 reports, is shown in Figure 1A. Among the 27 authors, two of them were not connected to each other. Two different clusters of authors were identified, which represented American and Japanese authors. The result of country co‐authorship analysis is shown in Figure 1B. A minimum of 8 publications per country was required for the country to be included in the analysis. Three different clusters of countries were identified, which represented the USA, European, and East Asian countries. We also performed organization co‐authorship analysis and keywords co‐occurrence analysis. Nineteen organizations and 20 keywords that met the threshold are shown in Figure S1. The size of the circle is weighted by documents or occurrences of the author, country, organization, and keyword. The thickness of the connecting lines reflects the strength of collaborations. The color of each circle represents the order of time, the more yellow the color is the closer the time, whereas purple is the farthest in time.
FIGURE 1.
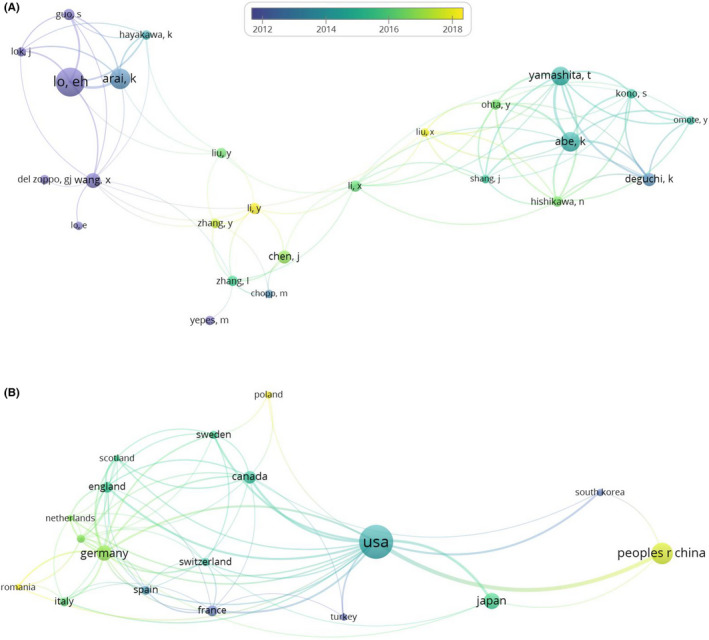
VOSviewer analysis of collaborations between authors and countries based on the co‐authorship analysis. (A) Two different clusters of authors were identified which represented American and Japanese authors. A total of 2,662 authors were counted, and we set the minimum number of documents of an author for 9 and 27 authors meet the threshold. 25 authors were shown as 2 authors were not connected to each other. (B) Three different clusters of countries were identified which represented USA, European, and East Asian countries. A total of 50 countries were counted, and we set the minimum number of documents of a country for 8 and 18 countries meet the threshold. The size of the circle is weighed by documents of the author and country. The thickness of the connecting lines reflects the strength of collaborations. The color of each circle represents the order of time, the more yellow the color is, the closer the time, and purple farther
We computed AIs and CNCIs of the eighteen countries according to VOSviewer co‐authorship analysis results for the periods 2003–2008, 2009–2014, and 2015–2020. At the country level, AIs and CNCIs varied across study periods and from one country to another (Table 1). To interpret the trends better, AIs were plotted against CNCIs for each of the eighteen countries for the periods 2003–2008, 2009–2014, and 2015–2020 (Figure 2, Figure S2). The size of the bubbles represents the number of articles one country published. Romania, Hungary, and Poland have emerged as rising‐star countries. Switzerland increased in CNCI, but decreased in AI. Canada, Sweden, Turkey, and Australia had a sharp decline in influence in CNCI. The USA had steady improvement in CNCI but decreased in AI. China showed great increase in AI, which indicates research effort, and the number of articles from China increased sharply from 2003–2008 to 2015–2020. France declined in both AI and CNCI. Spain and South Korea had a sharp decline in AI. Other countries showed relatively little change.
TABLE 1.
AIs and CNCIs for the 18 Countries for three 6‐year time periods, listed in descending order of the total number of citations
| Total | 2003–2008 | 2009–2014 | 2015–2020 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | SI | CNCI | N | SI | CNCI | N | SI | CNCI | ||
| World | 770 | 66 | 270 | 434 | ||||||
| USA | 303 | 42 | 1.62 | 1.00 | 125 | 1.18 | 1.17 | 136 | 0.80 | 1.21 |
| China | 125 | 4 | 0.37 | 0.98 | 19 | 0.43 | 0.58 | 102 | 1.45 | 0.73 |
| Japan | 59 | 0 | 0.00 | 0.00 | 28 | 1.35 | 0.45 | 31 | 0.93 | 0.51 |
| Germany | 52 | 0 | 0.00 | 0.00 | 17 | 0.93 | 0.99 | 35 | 1.19 | 0.85 |
| France | 38 | 4 | 1.23 | 0.35 | 23 | 1.73 | 1.13 | 11 | 0.51 | 0.16 |
| Canada | 29 | 3 | 1.21 | 3.37 | 9 | 0.89 | 0.89 | 17 | 1.04 | 0.43 |
| England | 21 | 2 | 1.11 | 0.87 | 8 | 1.09 | 1.38 | 11 | 0.93 | 0.82 |
| Spain | 18 | 3 | 1.94 | 0.35 | 9 | 1.43 | 0.57 | 6 | 0.59 | 0.69 |
| Italy | 14 | 0 | 0.00 | 0.00 | 5 | 1.02 | 0.41 | 9 | 1.14 | 0.51 |
| Australia | 10 | 0 | 0.00 | 0.00 | 4 | 1.14 | 2.24 | 6 | 1.06 | 0.98 |
| Turkey | 9 | 1 | 1.30 | 1.61 | 3 | 0.95 | 0.84 | 5 | 0.99 | 0.54 |
| Switzerland | 9 | 1 | 1.30 | 0.00 | 4 | 1.27 | 0.94 | 4 | 0.79 | 2.52 |
| Poland | 9 | 0 | 0.00 | 0.00 | 2 | 0.63 | 0.49 | 7 | 1.38 | 1.26 |
| Sweden | 8 | 1 | 1.46 | 3.58 | 2 | 0.71 | 0.13 | 5 | 1.11 | 0.66 |
| South Korea | 7 | 2 | 3.33 | 0.44 | 2 | 0.81 | 0.79 | 3 | 0.76 | 0.70 |
| Hungary | 6 | 0 | 0.00 | 0.00 | 1 | 0.48 | 0.01 | 5 | 1.48 | 0.54 |
| Romania | 3 | 0 | 0.00 | 0.00 | 0 | 0.00 | 0.00 | 3 | 1.77 | 1.55 |
| Scotland | 3 | 0 | 0.00 | 0.00 | 2 | 1.90 | 0.38 | 1 | 0.59 | 0.18 |
| Netherlands | 1 | 0 | 0.00 | 0.00 | 0 | 0.00 | 0.00 | 1 | 1.77 | 3.80 |
FIGURE 2.
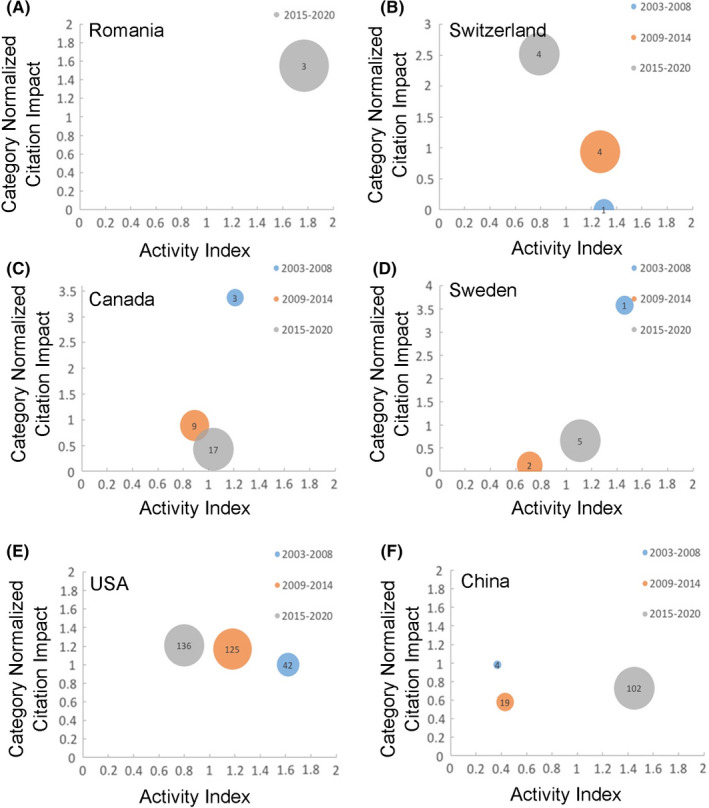
Changes in Activity Index and Category Normalized Citation Impact for the periods 2003–2008, 2009–2014 and 2015–2020 for the 6 countries in the research of stroke and neurovascular unit. (A) Romania has emerged as new‐star country. (B) Switzerland increased in CNCI, but decreased in AI. (C, D) Canada and Sweden had a sharp decline in influence in CNCI. (E) USA had steady improvement in CNCI but decreased in AI. (F) China shown great increase in AI which indicates research effort and the number of articles from China increased sharply from 2003–2008 to 2015–2020. If AI or CNCI was greater than 1, then the research power or the academic influence of a country was higher than the global average, and vice versa. The size of the bubbles represented the number of articles one country published in the time period
3.1. Characteristics of the 100 most‐cited reports
The top 10 most‐cited articles‐reviews and the top 10 most‐cited articles‐basic studies are listed in Tables 2 and 3. Details of the 100 most‐cited articles are listed in descending order of the total number of citations (Table S1). These articles were published between 2003 and 2019. The distribution of the number of publications is shown in Figure 3A. Most articles were published in 2011 and 2012 (n = 13 each), followed by 2009 (n = 11) and 2013, 2014, and 2015 (n = 8 each). In the 100 most‐cited articles, the number of citations ranged from 1873 to 69, with a total of 15758 citations. “Vascular Contributions to Cognitive Impairment and Dementia A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association” published in the journal Stroke had 1873 citations, with five articles having >400 citations and eight articles having >200 citations. The trend in the total number of citations by year is shown in Figure 3B. The 100 most‐cited articles were published in 58 journals. Journals that published two or more articles are listed in Table 4. The top journals included Stroke with fourteen reports, followed by Journal of Cerebral Blood Flow and Metabolism with eleven articles.
TABLE 2.
The Top‐10 most‐cited articles‐reviews about the role of neurovascular unit in stroke ranked in order of the number of citations
| Rank | Year | Article title | Number of citations | First author |
|---|---|---|---|---|
| 1 | 2011 | Vascular contributions to cognitive impairment and dementia a statement for healthcare professionals from the American Heart Association/American Stroke Association | 1873 | PB Gorelick |
| 2 | 2008 | Blood‐brain barrier tight junction permeability and ischemic stroke | 562 | KE Sandoval |
| 3 | 2011 | Central nervous system pericytes in health and disease | 497 | EA Winkler |
| 4 | 2006 | Perivascular nerves and the regulation of cerebrovascular tone | 432 | E Hamel |
| 5 | 2011 | Blood‐brain barrier breakdown in acute and chronic cerebrovascular disease | 421 | Y Yang |
| 6 | 2017 | The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease | 410 | C Iadecola |
| 7 | 2012 | Tight junctions at the blood‐brain barrier: physiological architecture and disease‐associated dysregulation | 244 | AC Luissint |
| 8 | 2013 | Hemorrhagic transformation after ischemic stroke in animals and humans | 227 | GC Jickling |
| 9 | 2004 | Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke | 225 | XY Wang |
| 10 | 2014 | The impact of microglial activation on blood‐brain barrier in brain diseases | 223 | ACC da Fonseca |
TABLE 3.
The Top‐10 most‐cited articles‐basic studies about the role of neurovascular unit in stroke ranked in order of the number of citations
| Rank | Year | Article title | Number of citations | First author |
|---|---|---|---|---|
| 1 | 2008 | Activation of PDGF‐CC by tissue plasminogen activator impairs blood‐brain barrier integrity during ischemic stroke | 293 | EJ Su |
| 2 | 2004 | Reperfusion‐induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia | 216 | Y Gursoy‐Ozdemir |
| 3 | 2008 | Neuroprotection via matrix‐trophic coupling between cerebral endothelial cells and neurons | 182 | SZ Guo |
| 4 | 2012 | Macrophages prevent hemorrhagic infarct transformation in murine stroke models | 163 | M Gliem |
| 5 | 2011 | Transplanted stem cell‐secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair | 156 | N Horie |
| 6 | 2014 | MicroRNA‐155 negatively affects blood‐brain barrier function during neuroinflammation | 146 | MA Lopez‐Ramirez |
| 7 | 2009 | Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain | 134 | T Yamashita |
| 8 | 2015 | Neuronal interleukin‐4 as a modulator of microglial pathways and ischemic brain damage | 131 | XR Zhao |
| 9 | 2012 | Paeoniflorin protects against ischemia‐induced brain damages in rats via inhibiting MAPKs/NF‐kappa B‐mediated inflammatory responses | 131 | RB Guo |
| 10 | 2013 | The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (PMN) infiltration into the brain after ischemic injury | 120 | Gaby Enzmann |
FIGURE 3.
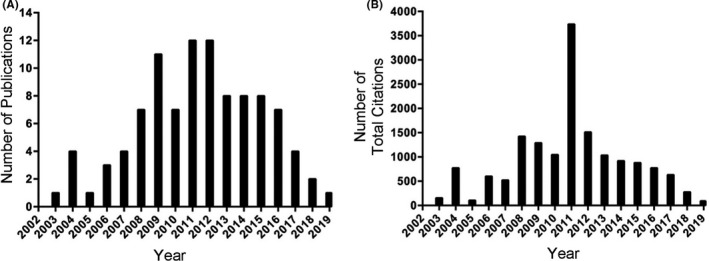
(A) Annual number of publications of the 100 most‐cited articles in Stroke and Neurovascular Unit from 2001 to 2020 in Web of Science. (B) Annual number of total citations of the 100 most‐cited articles in Stroke and Neurovascular Unit
TABLE 4.
Ranking of journals publishing the 100 most‐cited articles in stroke and neurovascular unit
| Rank | Journal | IF | Number of articles |
|---|---|---|---|
| 1 | Stroke | 7.19 | 14 |
| 2 | Journal of Cerebral Blood Flow and Metabolism | 5.681 | 11 |
| 3a | Acta Neuropathologica | 14.256 | 3 |
| 3b | Innate Inflammation and Stroke | 4.728 | 3 |
| 5a | British Journal of Pharmacology | 7.73 | 2 |
| 5b | Current Opinion in Investigational Drugs | 3.553 | 2 |
| 5c | Current Pharmaceutical design | 2.208 | 2 |
| 5d | Febs Journal | 4.392 | 2 |
| 5e | Frontiers in Cellular Neuroscience | 3.921 | 2 |
| 5f | International Journal of Stroke | 4.882 | 2 |
| 5g | Journal of Pineal Research | 14.528 | 2 |
| 5h | Proceedings of the National Academy of Sciences of the United States of America | 9.412 | 2 |
| 5i | Progress in Neurobiology | 9.371 | 2 |
| 5j | Stem Cells | 6.002 | 2 |
| 5k | Trends in Neurosciences | 12.891 | 2 |
A total of 90 authors had been listed as the first author. EH Lo (n = 3), Y Yang (n = 3), K Arai (n = 2), J Badaut (n = 2), GJ del Zoppo (n = 2), U Dirnagl (n = 2), SZ Guo (n = 2), and XY Wang (n = 2) published the greatest number of studies. A total of 74 authors had been listed as the corresponding author. EH Lo (n = 9) from Harvard University/Massachusetts General Hospital, GJ del Zoppo (n = 3) from Harborview Medical Center/University of Washington Seattle, and GA Rosenberg (n = 3) from the University of New Mexico authored the most articles as the corresponding author. As for the geographical distribution, the articles originated from eighteen countries as shown in Table 5. The majority of the publications were from the United States (n = 60), followed by France (n = 7), Germany (n = 6), and China (n = 5). A total of 71 affiliated institutions were listed in the reports depending on the corresponding author's affiliation. Harvard University/Massachusetts General Hospital produced fifteen publications, followed by the University of New Mexico with 6 articles and the Henry Ford Hospital with 4 articles.
TABLE 5.
Countries of origin publishing the 100 most‐cited articles in stroke and neurovascular unit
| Country | Number of articles |
|---|---|
| USA | 60 |
| France | 7 |
| Germany | 6 |
| China | 5 |
| England | 4 |
| Australia | 2 |
| Canada | 2 |
| Colombia | 2 |
| Japan | 2 |
| Turkey | 2 |
| Austria | 1 |
| Brazil | 1 |
| Finland | 1 |
| Ireland | 1 |
| Netherlands | 1 |
| Spain | 1 |
| Sweden | 1 |
| Switzerland | 1 |
In terms of the publication form, the articles could be divided into the following categories (Table 6): review (n = 70, ten of which were proceeding papers), basic study (n = 27), clinical trial (n = 1), book chapter (n = 1), and editorial material (n = 1). In the 70 reviews, seven articles discussed the topic of blood‐brain barrier (BBB), followed by tPA with six articles, pericytes with five articles, angiogenesis/neurovascular coupling with four articles, Alzheimer's disease/astrocyte/matrix metalloproteinase (MMP) with three articles each, and up to 25 articles mentioning therapeutic strategies. In the 27 basic studies, four were about the BBB, followed by stem cell with three publications, and melatonin/MMP/pericytes with 2 articles each. Fourteen articles tried to find therapeutic strategies for stroke. The clinical trial “Reparative Therapy for Acute Ischemic Stroke with Allogeneic Mesenchymal Stem Cells from Adipose Tissue: A Safety Assessment A Phase II Randomized, Double‐blind, Placebo‐controlled, Single‐center, Pilot Clinical Trial” was just a protocol.
TABLE 6.
Study types of the 100 most‐cited articles in stroke and neurovascular unit
| Study type | Number of articles |
|---|---|
| Review | 70 |
| Basic study | 27 |
| Book chapter | 1 |
| Editorial material | 1 |
| Clinical trial | 1 |
CiteSpace was used to capture burst keywords, the indicators of frontier topics over time. A total of 20 bursts keywords were obtained, together with their strength and the duration, as shown in Figure 4.
FIGURE 4.
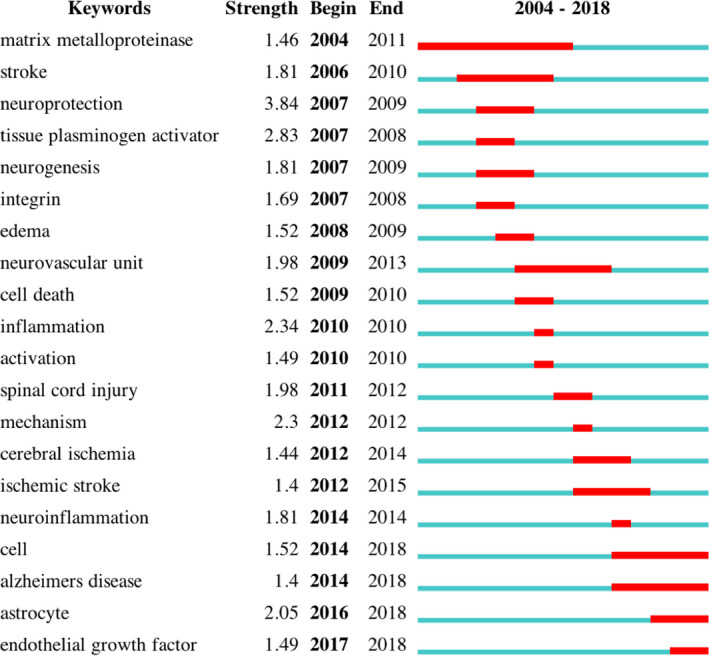
CiteSpace bursts detection was used to capture burst keywords. A total of 20 keywords with strong citation burst in the 100 most‐cited articles published about the role of neurovascular unit in stroke, together with their strength and duration. The strength value reflected the frequency of citation. The green blocks represented the year 2004–2018. The red blocks represented the begin, the end, and the duration of citation bursts
4. DISCUSSION
The neurovascular unit had been proposed in 2001 as a set of cells and structures, including neurons, pericytes, astrocytes, and microglia that should be investigated in an integrated context. Herein, we analyzed the time period between 2001 and 2020. Most articles were published between 2009 and 2012, about a decade after the concept of the “neurovascular unit” was proposed. Given the fact that citations usually need 3–10 years to accumulate after publication, 25 the trend in the total number of citations by year peaked in 2011 with a total number of 3,736 citations, as the most‐cited article with 1,873 citations was published in 2011.
VOSviewer showed a visualized view of the relationship between each item. In this study, EH Lo was foremost among American authors, while K Abe was foremost among Japanese researchers. These two clusters are connected by some Chinese and Japanese researchers. This overlap can be explained by overseas post‐doctoral researchers or further education program scholars returning to their home countries and continuing to collaborate within the framework of international networks, thereby increasing the connections between countries. Besides, the USA maintains a dominant position worldwide and has strong connection with China, Japan, Canada, Sweden, and England. These countries also have relatively large bubbles, which are in accordance with the result of the AIs and CICNs analyses. There are also close ties among European countries. Trends in collaborations between authors and countries, which can be illustrated by VOSviewer, are required to highlight changes over time.
AIs and CNCIs analyses provide different perspectives and allow identification of different trends in research output. We found that there was an overall increase in research output. Romania, Hungary, and Poland have emerged as rising‐star countries but with only a few papers. CNCI is an average value, which is affected by sample size and baseline value. Romania, Hungary, and Poland had highly cited papers in recent years, which could have had a huge impact on their CNCI. The USA showed steady improvement in the CNCI, which indicates its great influence in this field but had a decrease in AI. China showed a great increase in research effort, and the number of articles from China increased sharply from 2003–2008 to 2015–2020; however, little change has been seen in its CNCI. Meanwhile, VOSviewer co‐author country analysis of China suggests a relatively big bubble, but VOSviewer co‐author analysis only showed American and Japanese authors with articles >9, which indicates that researchers in China might be scattered and that influential authors still need time to repatriate. AIs and CNCIs combined with VOSviewer could give readers a more in depth understanding of current development of the field.
The article “Neuroinflammation: friend and foe for ischemic stroke” written by RL Jayaraj, and corresponded by Rosenberg GA in 2019, emerged as a “Hot Paper,” which is defined as ranking among the top 0.1% of papers in the academic field of clinical medicine in WoS. In this article, 26 the authors proposed that neuroinflammation had both beneficial and detrimental roles in stroke, while the NVU, including microglia, astrocytes, endothelial cells, BBB, and leukocytes participated in the pathogenesis of stroke. Understanding the temporal dynamics of immune cells during stroke and the involvement of the pathological mediators, such as oxidative stress, excitotoxicity, matrix metalloproteinases (MMPs), transcriptional modifications, mitogen‐activated protein kinase (MAPK), and high‐mobility group box protein family (HMGB), might provide time‐defined diagnostic, prognostic, and therapeutic neuroprotective strategies and ideas for future studies. This hot paper led to a deeper understanding of the close relationship between the NVU and neuroinflammation, and proposed divergent roles of immune responses on stroke outcome. Five papers had >400 citations, which is defined as “citation classics.” These papers are of high quality and have had wide and significant impact on subsequent stroke research.
Stroke with an IF of 7.19 and the Journal of Cerebral Blood Flow and Metabolism with 5.681 are two top journals in this field. Researchers were likely to contribute their articles to more influential journals in one field. Thus, we searched the latest papers in these two journals for progress in stroke and the NVU. One paper suggested that caveolin‐1 might have a potential protective role in neovascularization, astrogliosis, and scar formation. 27 Meanwhile, nitroxide radical‐containing nanoparticles (RNPs) could preserve the endothelial tight junctions and BBB integrity, improving oxygen species scavenging capacity. 28 The total citation number of “stroke” and “neurovascular unit” related publications in these two journals adds up to 5161 between 2004 and 2016.
Since the majority of the publications related to “stroke” and “neurovascular unit” were from the United States, we also analyzed the specialties of these authors. We found that most of the authors were also prominent in the field of traumatic brain injury, 29 vascular cognitive impairment, 30 , 31 and neuroimaging, 32 , 33 suggesting that the NVU is a field that is getting multidisciplinary attention.
In terms of the publication types, most of the 100 most‐cited articles were reviews, and one‐third were basic studies. There was increasing research effort into mechanisms underlying the role the NVU plays after stroke in the pursuit of novel therapeutic strategies for stroke patients. In these 29 basic studies, components of the NVU such as the BBB, tight junction, pericytes, astrocyte, endothelial cells, macrophages, and microglia were widely discussed. Stem cell therapy was mentioned in three articles. Hemorrhagic transformation, oxidative/reperfusion injury, and tPA were still hot topics. Potential therapeutic drugs and targets including melatonin, adenosine, curcumin, paeoniflorin (PF), pinocembrin, tissue inhibitor of MMP, bioactive lipids, stromal cell, polymorphonuclear granulocytes (PMN), (4‐phenoxyphenylsulfinyl) methylthiirane (SB‐3CT), purinergic receptor (P2RY12), brain‐derived neurotrophic factor (BDNF), platelet‐derived growth factor (PDGF), 12/15 lipoxygenase (12/15‐LOX), Rho kinase, sphingosine kinase (SphK), IL‐4, cascade‐3, microRNA, and PPAR‐γ were also investigated in these basic studies, providing pre‐clinical insights into the development of potential therapeutic strategies.
Although many of the above treatments have proved effective in animal stroke models, none have proved effective in treating stroke patients in clinical trials until now, 34 perhaps due to differences between animal models and humans. Meanwhile, stroke patients often have comorbid disease, such as hypertension, diabetes, hyperlipemia, and even aging, 2 , 14 , 35 , 36 which might impact the effectiveness of the therapy. Accurate animal models that better match with the stroke population should be established and the role of NVU in stroke requires further investigation. The above findings may help to shape research policy both at the country and institutional level.
4.1. Limitations
One of the major limitations of the current study is that we used only “stroke” and “neurovascular unit” as search terms; thus, a lot of studies that did not use the NVU term may have been missed. Another limitation is that given the fact that citations gradually reach a peak in 3–10 years after publication, the current analysis could not underscore the recently published articles. Our study had small sample size, which could have huge impact on CNCI.
CONFLICT OF INTEREST
We declare there is no conflict of interest among all the authors.
Supporting information
App S1
ACKNOWLEDGEMENTS
P.L. is supported by the National Natural Science Foundation of China (NSFC, 81722017, 91957111, 81971096, 82061130224), New Frontier Technology Joint Research sponsored by Shanghai Shenkang Hospital Development Center (SHDC12019102), Shanghai Municipal Education Commission‐Gaofeng Clinical Medical Grant Support (20181805), “Shuguang Program” supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (20SG17), Shanghai Outstanding Academic Leaders' Program from Shanghai Municipal Science and Technology Committee (20XD1422400), the Newton Advanced Fellowship grant provided by the UK Academy of Medical Sciences (NAF\R11\1010).
Lv Xie and Bingwei Lu contributed equally to this work.
Contributor Information
Li Zheng, Email: sunny2011313@163.com.
Peiying Li, Email: peiying.li@qq.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics‐2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38‐e360. [DOI] [PubMed] [Google Scholar]
- 2. Guzik A, Bushnell C. Stroke epidemiology and risk factor management. Continuum (Minneap Minn). 2017;23(1):15‐39. [DOI] [PubMed] [Google Scholar]
- 3. Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first‐ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1(5):e259‐e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Global Burden of Disease Study 2013 Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lyden PD. Measuring outcome after stroke: more lessons learned again. Stroke. 2020;51(4):1053‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu G, He Q, Shen Y, Cao F, et al. Potential biomarkers for predicting hemorrhagic transformation of ischemic stroke. Int J Neurosci. 2018;128(1):79‐89. [DOI] [PubMed] [Google Scholar]
- 7. Castro P, Azevedo E, Serrador J, Rocha I, Sorond F. Hemorrhagic transformation and cerebral edema in acute ischemic stroke: Link to cerebral autoregulation. J Neurol Sci. 2017;372:256‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang H, Rajah G, Guo A, Wang Y, Wang Q. Pathogenesis of epileptic seizures and epilepsy after stroke. Neurol Res. 2018;40(6):426‐432. [DOI] [PubMed] [Google Scholar]
- 9. Hoffmann S, Harms H, Ulm L, et al. Stroke‐induced immunodepression and dysphagia independently predict stroke‐associated pneumonia ‐ The PREDICT study. J Cereb Blood Flow Metab. 2017;37(12):3671‐3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damani RH, Anand S, Asgarisabet P, Bissell C, Savitz S, Suarez JI. Regional intervention of stroke care to increase thrombolytic therapy for acute ischemic stroke. Stroke. 2018;49(8):2008‐2010. [DOI] [PubMed] [Google Scholar]
- 11. von Kummer R, Mori E, Truelsen T, et al. Desmoteplase 3 to 9 hours after major artery occlusion stroke: the DIAS‐4 trial (efficacy and safety study of desmoteplase to treat acute ischemic stroke). Stroke. 2016;47(12):2880‐2887. [DOI] [PubMed] [Google Scholar]
- 12. Shibata K, Hashimoto T, Miyazaki T, Miyazaki A, Nobe K. Thrombolytic therapy for acute ischemic stroke: past and future. Curr Pharm Des. 2019;25(3):242‐250. [DOI] [PubMed] [Google Scholar]
- 13. Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Presa JL, Saravia F, Bagi Z, Filosa J. Vasculo‐neuronal coupling and neurovascular coupling at the neurovascular unit: impact of hypertension. Front Physiol. 2020;11:584135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lok J, Wang XS, Xing CH, et al. Targeting the neurovascular unit in brain trauma. CNS Neurosci Ther. 2015;21(4):304‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ronaldson PT, Davis TP. Regulation of blood‐brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J Cereb Blood Flow Metab. 2020;40(1_suppl):S6‐S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eldahshan W, Fagan SC, Ergul A. Inflammation within the neurovascular unit: focus on microglia for stroke injury and recovery. Pharmacol Res. 2019;147:104349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen J, Zhang DM, Feng X, et al. TIGAR inhibits ischemia/reperfusion‐induced inflammatory response of astrocytes. Neuropharmacology. 2018;131:377‐388. [DOI] [PubMed] [Google Scholar]
- 19. Shindo A, Takase H, Hamanaka G, et al. Biphasic roles of pentraxin 3 in cerebrovascular function after white matter stroke. CNS Neurosci Ther. 2020;27(1):60‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haupt M, Zechmeister B, Bosche B et al. Lithium enhances post‐stroke blood‐brain barrier integrity, activates the MAPK/ERK1/2 pathway and alters immune cell migration in mice. Neuropharmacology. 2020;181:108357. [DOI] [PubMed] [Google Scholar]
- 21. Li P, Stetler RA, Leak RK, et al. Oxidative stress and DNA damage after cerebral ischemia: potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology. 2018;134(Pt B):208‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lyden PD, Lamb J, Kothari S, Toossi S, Boitano P, Rajput PS. Differential effects of hypothermia on neurovascular unit determine protective or toxic results: toward optimized therapeutic hypothermia. J Cereb Blood Flow Metab. 2019;39(9):1693‐1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Zhu ZY, Huang TT, et al. The peripheral immune response after stroke‐A double edge sword for blood‐brain barrier integrity. CNS Neurosci Ther. 2018;24(12):1115‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang X, Xuan W, Zhu ZY, et al. The evolving role of neuro‐immune interaction in brain repair after cerebral ischemic stroke. CNS Neurosci Ther. 2018;24(12):1100‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pepe A, Kurtz MJ. A measure of total research impact independent of time and discipline. PLoS ONE. 2012;7(11):e46428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blochet C, Buscemi L, Clément T, Gehri S, Badaut J, Hirt L. Involvement of caveolin‐1 in neurovascular unit remodeling after stroke: Effects on neovascularization and astrogliosis. J Cereb Blood Flow Metab. 2020;40(1):163‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hosoo H, Marushima A, Nagasaki Y, et al. Neurovascular unit protection from cerebral ischemia‐reperfusion injury by radical‐containing nanoparticles in mice. Stroke. 2017;48(8):2238‐2247. [DOI] [PubMed] [Google Scholar]
- 29. Takase H, Washida K, Hayakawa K, et al. Oligodendrogenesis after traumatic brain injury. Behav Brain Res. 2018;340:205‐211. [DOI] [PubMed] [Google Scholar]
- 30. Rosenberg GA. Vascular cognitive impairment: biomarkers in diagnosis and molecular targets in therapy. J Cereb Blood Flow Metab. 2016;36(1):4‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosenberg GA. Binswanger's disease: biomarkers in the inflammatory form of vascular cognitive impairment and dementia. J Neurochem. 2018;144(5):634‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raja R, Rosenberg GA, Caprihan A. MRI measurements of blood‐brain barrier function in dementia: a review of recent studies. Neuropharmacology. 2018;134(Pt B):259‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xing C, Hayakawa K, Lo EH. Mechanisms, imaging, and therapy in stroke recovery. Transl Stroke Res. 2017;8(1):1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55(3):363‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo S, Deng W, Xing C, Zhou Y, Ning M, Lo EH. Effects of aging, hypertension and diabetes on the mouse brain and heart vasculomes. Neurobiol Dis. 2019;126:117‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cai W, Zhang K, Li P, et al. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: an aging effect. Ageing Res Rev. 2017;34:77‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


