Abstract
Summary
We developed the MicrobiomeExplorer R package to facilitate the analysis and visualization of microbial communities. The MicrobiomeExplorer R package allows a user to perform typical microbiome analytic workflows and visualize their results, either through the command line or an interactive Shiny application included with the package. In addition to applying common analytical workflows, the application enables automated analysis report generation.
Availability and implementation
Available at https://github.com/zoecastillo/microbiomeExplorer.
Supplementary information
Supplementary data are available at Bioinformatics online.
1 Introduction
High-throughput DNA-sequencing technologies, including targeted marker-gene sequencing, are providing unprecedented insight into the composition and population structure of microbial communities. Researchers are rapidly applying these new technologies in a variety of clinical settings, to understand the structure and function of bacteria ranging varied communities (Chopyk et al., 2017). Although it may seem that DNA sequencing should reduce many scientific questions to simply counting the representative sequence of the organism, there are subtle but significant challenges associated with sampling, counting, and representational statistics. As a consequence, many microbiome-specific analytical pipelines and workflows have been developed.
Although tools and packages exist to answer many of the questions asked of this exciting new data type, analyses remain inaccessible to researchers without the appropriate skill-set. The MicrobiomeExplorer R package allows users to analyze microbiome data either from the R command line or through an interactive Shiny application. It aims to address the needs of computational scientists by combining different analysis methods in one package, and also the needs of bench scientists with a limited coding background. In addition, it delivers a set of powerful and well-designed interactive visualizations based on the plotly package (Sievert, 2020). This distinguishes it from previous approaches such as phyloseq and metaviz which are command line or do not contain all the visualization capabilities featured (Wagner et al., 2018).
2 Microbiomeexplorer R package
As input, MicrobiomeExplorer accepts several different input formats such as raw count tables, BIOM-formatted files, and MRexperiment objects from the metagenomeSeq R package (McMurdie and Paulson, 2016; Paulson et al., 2013). Raw counts and BIOM files can be annotated using tabular phenotype information on the samples as well as a feature table describing the taxonomic features identified.
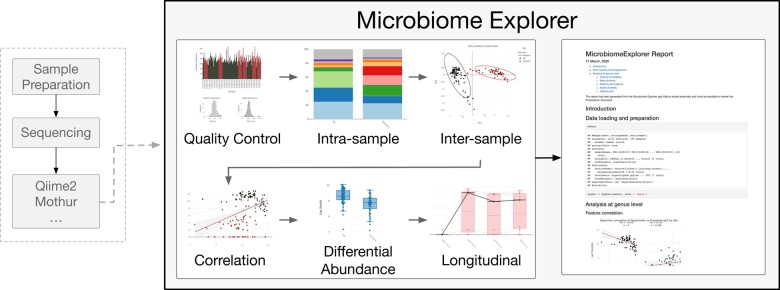
For data pre-processing, users can perform filtering of poor quality samples through exploratory data analysis of the sequencing depth and number of features observed. Normalization is enabled to calculate proportions or by using cumulative sum scaling. Subsetting of samples to exclude certain phenotypes or modifying the phenotype data by combining different columns or adjusting the data types is available. Additionally, provided feature data can be updated by rolling down information from higher taxonomy levels if an operational taxonomic unit (OTU) cannot be uniquely identified (Fig. 1).
Fig. 1.
Flow diagram for the MicrobiomeExplorer R package. Following sample preparation, sequencing and post-processing of reads by tools like Qiime2 and Mothur, counts, taxonomy and associated metadata can be uploaded into the MicrobiomeExplorer explorer. Modules include (i)quality control, (ii) intra-samples, (iii) inter-sample/intra-feature, (iv) correlation, (v) differential abundance and (vi) longitudinal analyses. Downstream of this, the MicrobiomeExplorer tool allows for downloadable reports
2.1 Analysis workflow and reproducibility reporting
Organization of the analysis workflow consists of multiple analysis types separated into: overview and pre-processing, intra-, inter-sample, intra-feature, feature correlation, differential abundance and longitudinal analyses.
The first step of any analysis within the application is to pre-process the data. Initial visualizations help in assessing the quality and characteristics of sequencing depth and feature presence.
The second step of an analysis is to choose a taxonomy level and aggregate data at the chosen level for downstream analyses.
Intra-sample analysis contains functions that aid in the investigation of the microbial composition within a sample or a group of samples. The user can explore the relative abundance, feature abundance or alpha diversity. Alpha diversity is implemented through the vegan package and includes diversity metrics such as diversity (Oksanen et al., 2007).
Inter-sample analyses focus on differences between samples or groups of samples. This is accomplished through the use of heatmaps of variable or differentially abundant OTUs, or features and beta diversity calculations. Beta diversity distance metrics vary within the package and include Bray–Curtis. Within an analysis session of the application, computed distance matrices are cached for improved efficiency. Color annotations can be added for higher taxonomy levels or specific phenotypes.
Feature correlation analysis modules provide scatterplots of abundance between features and correlation statistics, both of which can be faceted by phenotypes. A test for significance within each facet can be performed. Phenotype correlation is similar, except it analyzes the relationship between a feature and a continuous phenotype.
Differential abundance testing can be performed using DESeq2, Kruskal–Wallis, limma or a zero-inflated log normal model. DESeq2 and limma are widely used methods for comparisons in microarray and RNA-sequencing data that have been adapted for microbiome data. Kruskal–Wallis is a non-parametric test for any differences in distribution between groups. The zero-inflated log normal model is implemented in the metagenomeSeq package to account for zero-inflation in microbiome data.
Longitudinal analysis provides the option to visualize feature abundances across specific levels of a phenotype, such as dates or tissue types. Optionally, a separate phenotype can be used to link a common data point across the different levels with lines.
A key feature includes the ability to report select analyses and export them in HTML, PDF, Word document or Powerpoint with R markdown code for reproducibility and adjustments.
3 Conclusion
MicrobiomeExplorer, available at https://github.com/zoecastillo/microbiomeExplorer, is an R package specifically meant to help facilitate the analysis and visualization of microbial communities from marker-gene surveys or whole metagenomic shotgun sequencing. The tool provides a user-friendly interactive interface through Shiny with downloadable R Markdown scripts and reports.
Supplementary Material
Acknowledgements
We would like to acknowledge Elizabeth Skippington, Kelly Storek, Allyson Byrd, Fiona Tamburini, Jordan Mar and Connie Peng for their feedback and suggestions.
Conflict of Interest: none declared.
Contributor Information
Janina Reeder, Department of OMNI Bioinformatics, Genentech, Inc, South San Francisco, CA 94080, USA.
Mo Huang, Department of Statistics, The Wharton School, University of Pennsylvania, Philadelphia, PA 19104, USA; Department of Biostatistics, Genentech, Inc, South San Francisco, CA 94080, USA.
Joshua S Kaminker, Department of OMNI Bioinformatics, Genentech, Inc, South San Francisco, CA 94080, USA.
Joseph N Paulson, Department of Biostatistics, Genentech, Inc, South San Francisco, CA 94080, USA.
References
- Chopyk J. et al. (2017) Mentholation affects the cigarette microbiota by selecting for bacteria resistant to harsh environmental conditions and selecting against potential bacterial pathogens. Microbiome, 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie P., Paulson J. (2016) biomformat: an interface package for the biom file format. R/Bioconductor package version 1.0.
- Oksanen J. et al. (2007) The vegan package. Community ecology package, 10, 719. [Google Scholar]
- Paulson J.N. et al. (2013) Differential abundance analysis for microbial marker-gene surveys. Nat. Methods, 10, 1200–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert C. (2020) Interactive Web-Based Data Visualization with R, Plotly, and Shiny. Chapman and Hall/CRC, London, United Kingdom. [Google Scholar]
- Wagner J. et al. (2018) Metaviz: interactive statistical and visual analysis of metagenomic data. Nucleic Acids Res., 46, 2777–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.