Abstract
Obesity is an escalating global pandemic posing a serious threat to human health. The intervention therapy using weight-reducing drugs, accompanied by lifestyle modification, is a strategy for the treatment of obesity. In the present study, we explored the role of fucoidan, a seaweed compound, on high-fat diet (HFD)-induced obesity in mice. We found that fucoidan treatment significantly reduced the body fat and caused redistribution of visceral and subcutaneous fat in HFD-fed mice. Meanwhile, fucoidan treatment inhibited adipocyte hypertrophy and inflammation in adipose tissue. Collectively, these results suggest that fucoidan may be a promising treatment for obesity and obesity-induced complications.
Keywords: obesity, adipose tissue, fucoidan, inflammation
Introduction
Obesity, an escalating global pandemic featuring the accumulation of excess body fat[1-2], is closely related to the development of cardiovascular disease, insulin resistance (IR), type 2 diabetes, and nonalcoholic fatty liver disease (NAFLD)[3]. The excess energy stored in adipocytes leads to adipocyte hypertrophy and macrophages infiltration in adipose tissues[4-5]. Hypertrophic adipocyte secrets adipokines including adiponectin, leptin, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which can change insulin activity in adipose tissues, liver, and muscles[6-7]. Meantime, obesity also induces chronic inflammation, leading to the development of elated metabolic dysfunction such as IR, NAFLD, and cardiovascular diseases[4]. Thus, both fat accumulation and inflammation are key contributors to the pathogenesis of obesity and its related metabolic dysfunction.
As a complex sulfated polysaccharide, fucoidan has been widely investigated for its anti-oxidative, anti-tumogenestic, and anti-inflammatory effects[8-9]. It reduces body weight gain in the mice fed with a high-fat diet (HFD) and inhibits lipid accumulation in 3T3-L1 adipocytes[10-11]. Our previous studies have shown that fucoidan prevents obesity-associated high blood pressure[12]. However, whether fucoidan has impacts on adipose tissue distribution (visceral vs. subcutaneous fat) and inflammation is still unclear. In the present study, we used fucoidan in HFD-fed mice to determine its anti-obesity potential. Our results revealed that fucoidan exerted a protective effect against obesity, hyperlipidaemia, IR, and NAFLD, and suppressed obesity-associated inflammation in mice. These results indicate that fucoidan may be an effective anti-obesity substance.
Materials and methods
Animals
Wild-type male C57BL/6 mice (Charles River, China) were housed in pathogen-free conditions under a regular 12:12-hour light/dark cycle with free access to chow and water. Eight-week-old male mice were fed HFD (60% kcal from fat; Research Diets, USA) or chow diet (CD) (10% kcal from fat; Research Diets) for 24 weeks. Fucoidan administration in wild-type male mice was initiated after 8 weeks of feeding (CD or HFD). The mice were daily treated with fucoidan (Fucus vesiculosus; Sigma-Aldrich, USA) dissolved in normal saline (2 mg/mL) or vehicle (saline) intraperitoneally at a dosage of 10 mg/(kg·day) for 16 weeks. Mice were euthanized by CO2 inhalation, and the adipose and liver tissues were isolated for experiments. This study was approved by the animal ethics and welfare committee of Nanjing Medical University (IACUC-1601142).
Intraperitoneal glucose tolerance tests
After overnight fasting, mice were given an intraperitoneal injection of glucose (1.0 g/kg) for glucose tolerance test (GTT). Glucose levels of mice were determined by tail vein blood sampling using the OneTouch Horizon Glucose Monitoring kit (LifeScan, USA).
Micro-computed tomography analysis
The murine fat mass was analyzed with a micro-computer tomography (micro-CT; SkyScan 1176, Bruker, Germany). Fat tissue regions of HFD mice were drawn on the visceral and abdominal subcutaneous adipose tissues at the level of the vertebra (L6 region).
Primary murine adipocytes isolation and culture
Primary murine adipocytes were isolated from 4- to 6-week-old male mice. Inguinal adipose tissues were cut into small pieces. Minced tissues were then transferred to the serum-free DMEM containing collagenase Ⅰ (1.5 mg/mL, Sigma-Aldrich) for 45 minutes at 37 °C. The isolated stromal cells were cultured using DMEM medium with 10% FBS. Two days post confluency, the cells were stimulated with induction media (10 μg/mL insulin [Sigma-Aldrich], 1 μmol/L dexamethasone [Sigma-Aldrich], 0.5 mmol/L isobutylmethylxanthine [IBMX, Sigma-Aldrich] in DMEM with 10% FBS). Two days later, the induction media was changed to insulin medium (DMEM with 10% FBS) containing 10 μg/mL insulin. The adipocyte medium was changed every 2 days throughout the differentiation period.
Isolation and culture of murine bone marrow-derived macrophages
Murine bone marrow was isolated from 4 to 6 weeks C57BL/6 mice and re-suspended in DMEM media with 10% FBS. Cells were treated with 20 ng/mL macrophage colony stimulating factor to induce differentiation into bone marrow derived-macrophages (BMDMs). The culture medium was changed every 2 to 3 days and the adhered BMDMs were harvested at the end of the sixth day.
Quantitative RT-PCR
Total RNA was extracted from mouse adipose tissues or cells using RNAiso plus kit (Vazyme Biotech, China) according to the instructions. Gene expression was analyzed by using SYBR Green (QuantStudio 6-Flex; Applied Biosystems, USA).
The following primer pairs were used forward 5′- mouse studies: Cd68 forward, 5′-CTTCCCACAGGCAGCACAG-3′, reverse, 5′-AATGATGAGAGGCAGCAAGAGG; Emr1 forward, 5′-CTTTGGCTATGGGCTTCCAGTC-3′, reverse, 5′-GCAAGGAGGACAGAGTTTATCGTG; Tnf forward, 5′-ACGGCATGGATCTCAAAGAC-3′, reverse, 5′-AGATAGCAAATCGGCTGACG; Il1b forward, 5′-TGTCTTGGCCGAGGACTAAGG-3′, reverse, 5′-TGGGCTGGACTGTTTCTAATGC; Il6 forward, 5′-GTTCTCTGGGAAATCGTGGA-3′, reverse, 5′-GGAAATTGGGGTAGGAAGGA; Il10 forward, 5′-CAGGGCCCTTTGCTATGG-3′, reverse, 5′-GATCTCCCTGGTTTCTCTTCC; Adipoq forward, 5′-GCACTGGCAAGTTCTACTGCAA-3′, reverse, 5′-GTAGGTGAAGAGAACGGCCTTGT-3′.
Biochemical analysis
Levels of Tnf-α, Il-6, and Il-1β in mouse plasma were measured by ELISA kits (Multi Sciences, China). Plasma levels of free fatty acid (FFA), triglycerides, cholesterol, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured by using the assay kit (Nanjing Jiancheng Bioengineering Institute, China). Cholesterol and triglycerides levels in the liver were measured by assay kit (Nanjing Jiancheng Bioengineering Institute) and normalized to tissue weight (mg lipid/g tissue).
Seahorse extracellular flux analysis
Extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) in adipocytes were detected by Seahorse XF96 Extracellular Flux Analyzer (Agilent, USA) following the manufacturer's protocols. Primary murine adipocytes were seeded at 1.5×104 cells/well in a 96-well cell culture XF microplate. The differentiated adipocytes were treated with fucoidan (50 µg/mL) for 12 hours. During the ECAR assay, cells were treated with glucose (10 mmol/L), oligomycin (Oligo, 2 μmol/L), and 2-deoxyglucose (2-DG, 100 mmol/L). For the OCR assay, cells were treated with Oligo (2 mmol/L), carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP, 1.5 μmol/L), and Rotenone and antimycin A (Rote+AA, 1 μmol/L). Each condition was performed with 8 to 10 replicates.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 7 (GraphPad, USA). The data were expressed as mean±SEM. Two-group comparisons were performed using two-tailed unpaired t-test, one-way ANOVA for comparisons between multiple groups with a single variable, and two-way ANOVA for comparisons with multiple variables. Differences with P<0.05 were considered to be statistically significant.
Results
Fucoidan treatment alleviated diet-induced obesity and insulin resistance in mice
The effect of fucoidan in obesity and obesity-related metabolic dysfunction was investigated using HFD-induced obese mouse model. Fucoidan was administered to the mice from the 8th week to the 24th week of HFD feeding continuously and the changes of body weight and blood glucose were examined (Fig. 1A). It was shown that the mouse body weight was decreased after 8 weeks of fucoidan treatment (since the 16th week of HFD feeding) and the difference was statistically significant after 12 weeks of treatment (since the 20th week) in HFD-fed mice (approximately 11%) but not in CD-fed mice (Fig. 1B). Moreover, of fucoidan showed a similar blood glucose-lowering effect in the HFD mice after receiving fucoidan treatment for 12 weeks (week 20 and week 24, Fig. 1C). Fucoidan administration also significantly improved insulin sensitivity in HFD mice after 16 weeks of fucoidan treatment (week 24) (Fig. 1D and E). Furthermore, fucoidan-treated mice showed decreased levels of FFA, triglycerides, and cholesterol in plasma, compared with HFD-fed control mice (Fig. 1F-H). These results indicated that fucoidan might alleviate HFD-induced obesity and insulin resistance.
Figure 1.
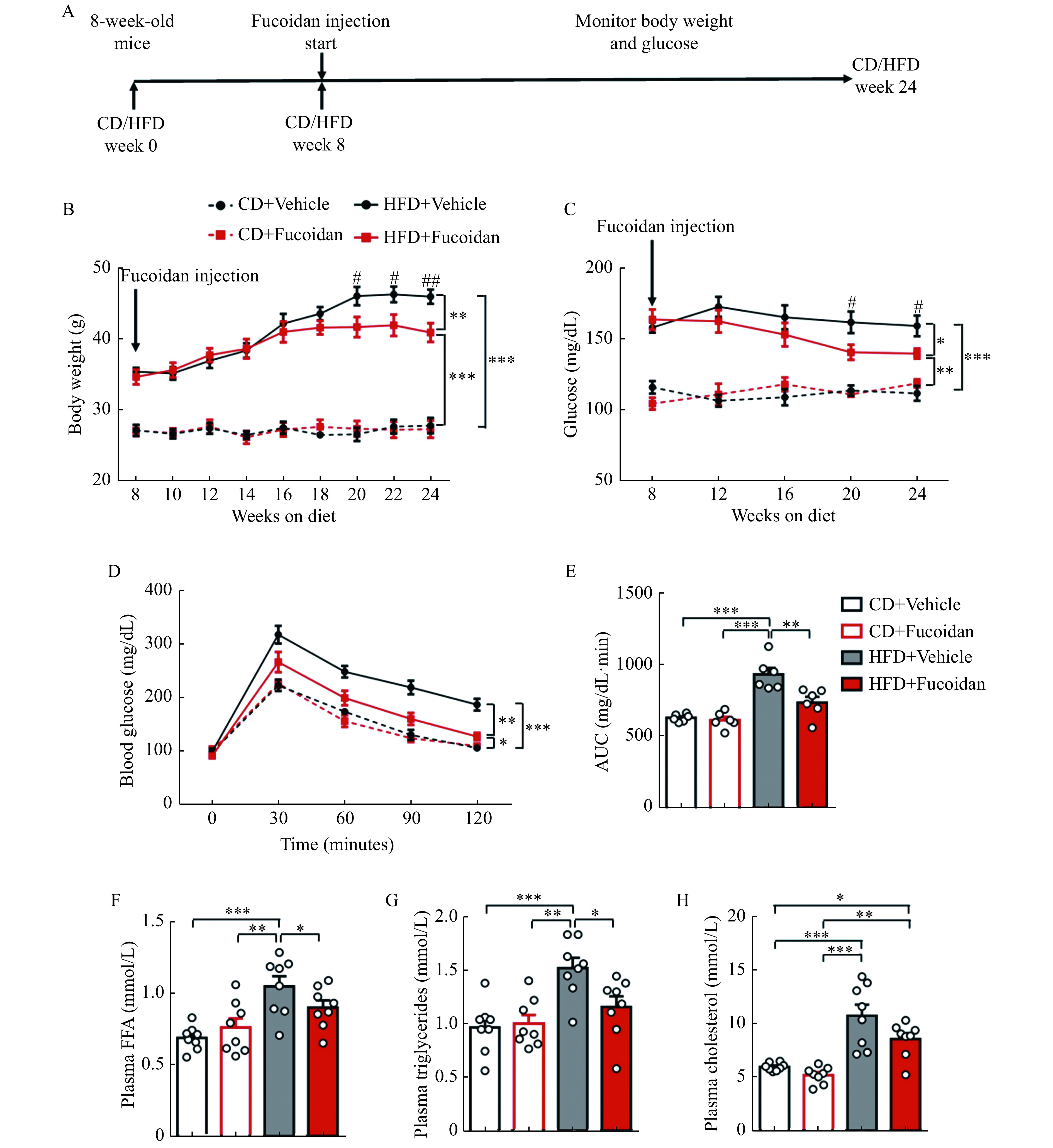
Fucoidan treatment lowered body weight and glucose in HFD-fed mice.
Mice were administered with fucoidan or vehicle after 8 weeks of feeding. A: Experimental design for fucoidan treatment [10 mg/(kg·day)]. B: Body weight of the fucoidan-treated mice, n=8. C: Glucose in the fucoidan-treated mice, n=8. D and E: GTT (D) was performed in fucoidan-treated mice after 24 weeks of feeding; AUC of GTT (E) was calculated, n=6. F–H: Plasma levels of FFA (F), triglycerides (G), and cholesterol (H) in the fucoidan-treated mice after 24 weeks of feeding,n=8. Data are expressed as mean±SEM. Statistical analyses were performed by two-way ANOVA for comparisons between multiple groups with multiple variables (B–D) and one-way ANOVA for comparisons between multiple groups with a single variable (E–H).*P<0.05;**P<0.01;***P<0.001;#P<0.05 HFD+vehicle micevs. HFD+fucoidan mice; ##P<0.01 HFD+vehicle micevs. HFD+fucoidan mice. CD: chow diet; HFD: a high-fat diet; AUC: area under the curve; GTT: glucose tolerance test; FFA: free fatty acid.
Fucoidan treatment prevented HFD-induced adipocyte hypertrophy
The murine visceral fat and subcutaneous fat mass was examined using a micro-CT system. Administration of fucoidan significantly reduced the body fat and the ratio of visceral fat, and increased the ratio of subcutaneous fat (Fig. 2A-D). Epididymal fat, a kind of visceral fat, also shrank by fucoidan treatment (Fig. 2E). Decreased lipid accumulation and adipose tissue hypertrophy were confirmed by the H&E staining of the epididymal adipose tissue (Fig. 2F and G). The whole-body oxygen consumption, carbon dioxide production, and heat production in HFD mice, as determined by metabolic cages, were comparably increased by fucoidan treatment compared with the saline treated HFD-fed mice (Fig. 2H-J). Notably, there was no change in murine food intake and drink (Fig. 2K and L).
Figure 2.
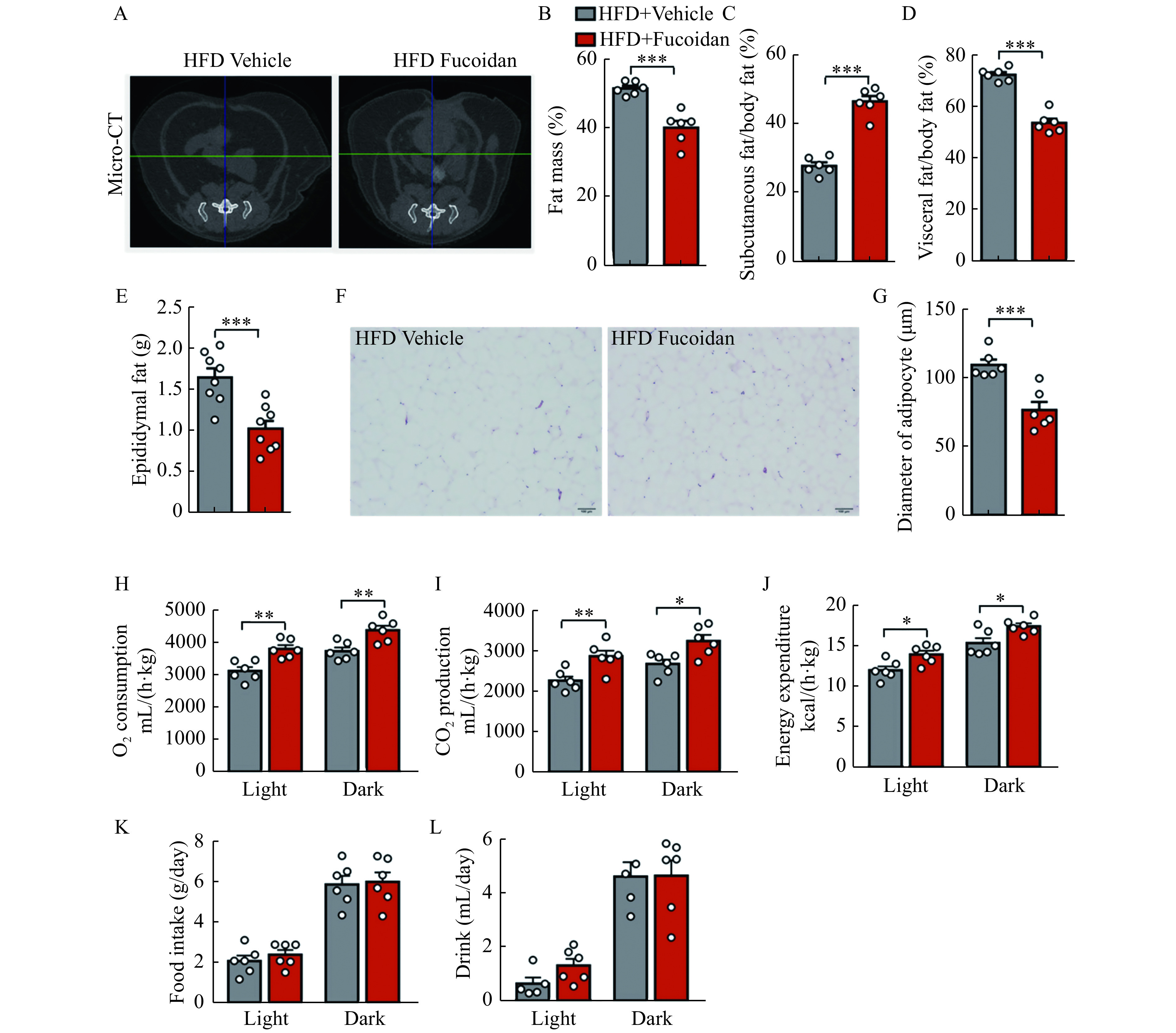
Fucoidan treatment suppressed adipose hypertrophy.
Mice were administered with fucoidan after 8 weeks of HFD feeding. A: Representative micro-CT images of the abdominal fat at L6 region in fucoidan-treated mice after 24 weeks of HFD feeding. B–D: Fat mass percentage (B), subcutaneous fat percentage (C), and visceral fat percentage (D) in the fucoidan-treated mice after 24 weeks of HFD feeding were measured by micro-CT,n=6. E: Epididymal fat weight in fucoidan-treated mice after 24 weeks of HFD feeding, n=8. F: Representative images of H&E staining of epididymal fat tissues in the fucoidan-treated mice after 24 weeks of HFD feeding. Scale bars, 100 μm. G: Average diameters of adipocytes in epididymal adipose tissue of fucoidan-treated mice after 24 weeks of HFD feeding,n=6. H–L: whole body oxygen (O2) consumption (H), carbon dioxide (CO2) production (I), energy expenditure (J), food intake (K), and drink (L) in fucoidan-treated mice after 24 weeks of HFD feeding during a 24-hour period. Data are expressed as mean±SEM. Statistical analyses were performed by two-tailed unpaired t test for two-group comparisons. *P<0.05;**P<0.01;***P<0.001. HFD: a high-fat diet; micro-CT: micro-computer tomography.
Fucoidan inhibited inflammatory response in murine adipose tissue
Chronic inflammation in fat plays a crucial role in the development of obesity and obesity-induced diseases[13-14]. We found that expression levels of Cd68 and Emr1, the major markers of inflammatory cells in the epidydimal adipose tissue, were both decreased in fucoidan-treated HFD mice (Fig. 3A), suggesting an attenuated immune cell infiltration in the adipose tissue. Moreover, the expression of pro-inflammatory cytokines (Tnf, Il1b, and Il6) decreased in fucoidan-treated mice (Fig. 3B). In concordance, plasma levels of pro-inflammatory cytokines (Tnf-α, Il-1β, and Il-6) in obese mice were also decreased by fucoidan treatment (Fig. 3C-E). Consistently, fucoidan treatment decreased expressional levels of Cebpa, Cebpb, and Ppary, crucial genes for adipogenesis, in obese adipose tissues (Fig. 3F).
Figure 3.
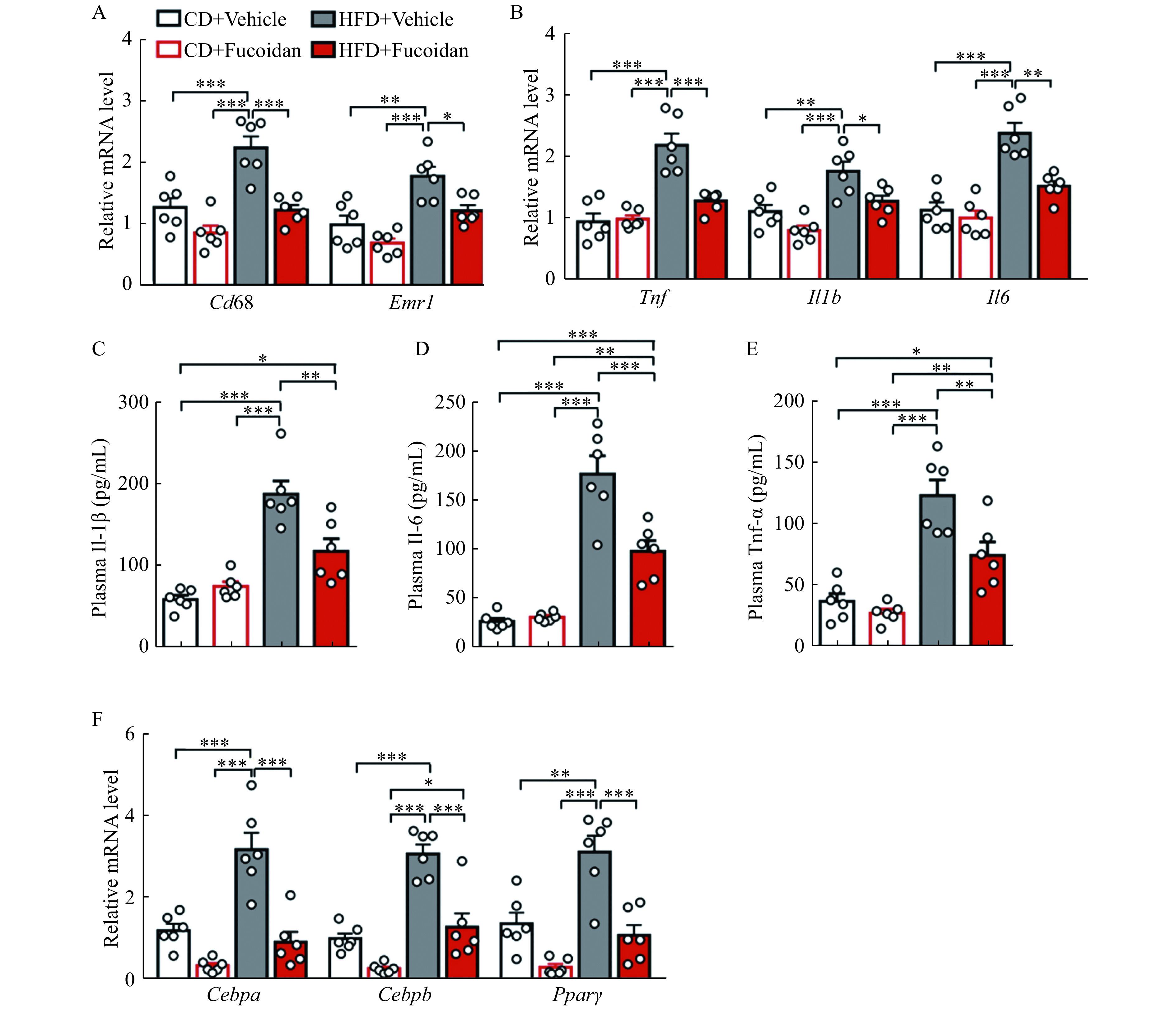
Fucoidan treatment suppressed adipose tissue inflammation.
Mice were administrated with fucoidan after 8 weeks of feeding. A and B: Epididymal adipose tissue mRNA levels of Cd68 and Emr1 (A), proinflammatory cytokines Tnf, Il1b, and Il6 (B) in fucoidan-treated mice after 24 weeks of feeding, n=6. C–E: Plasma levels of Il-1β (C), Il-6 (D), and Tnf-α (E) in fucoidan-treated mice after 24 weeks of HFD feeding,n=6. F: Epididymal adipose tissue gene expression levels of lipogenic genes Cebpa, Cebpb, and Pparγ in fucoidan-treated mice after 24 weeks of feeding, n=6. Data are expressed as mean±SEM. Statistical analyses were performed by one-way ANOVA for comparisons between multiple groups with a single variable. *P<0.05;**P<0.01;***P<0.001. CD: chow diet; HFD: a high-fat diet.
The direct effect of fucoidan on adipocytes was investigated by performing in vitro experiments. Our results showed that fucoidan could reduce the pro-inflammatory factors (Tnf, Il1b, and Il6) and increase protective cytokine Adipoq in adipocytes in the presence of palmitic acid (PA) (Fig. 4A-D). To explore the mechanism of anti-inflammatory effects of fucoidan, bioenergetics analyses were performed. As expected, ECAR, an established indicator of glycolysis, was decreased after fucoidan treatment (Fig. 4E). Meanwhile, OCR, an indicator of mitochondrial function, was slightly increased after fucoidan treatment (Fig. 4F). In cultured macrophages, fucoidan also inhibited the output of inflammatory cytokines (Tnf, Il1b, and Il6) and promoted the expression of anti-inflammatory cytokines Il10 in the PA-treated BMDMs (Fig. 4G-J). These data revealed that fucoidan treatment might suppress inflammation in the obese adipose tissues.
Figure 4.
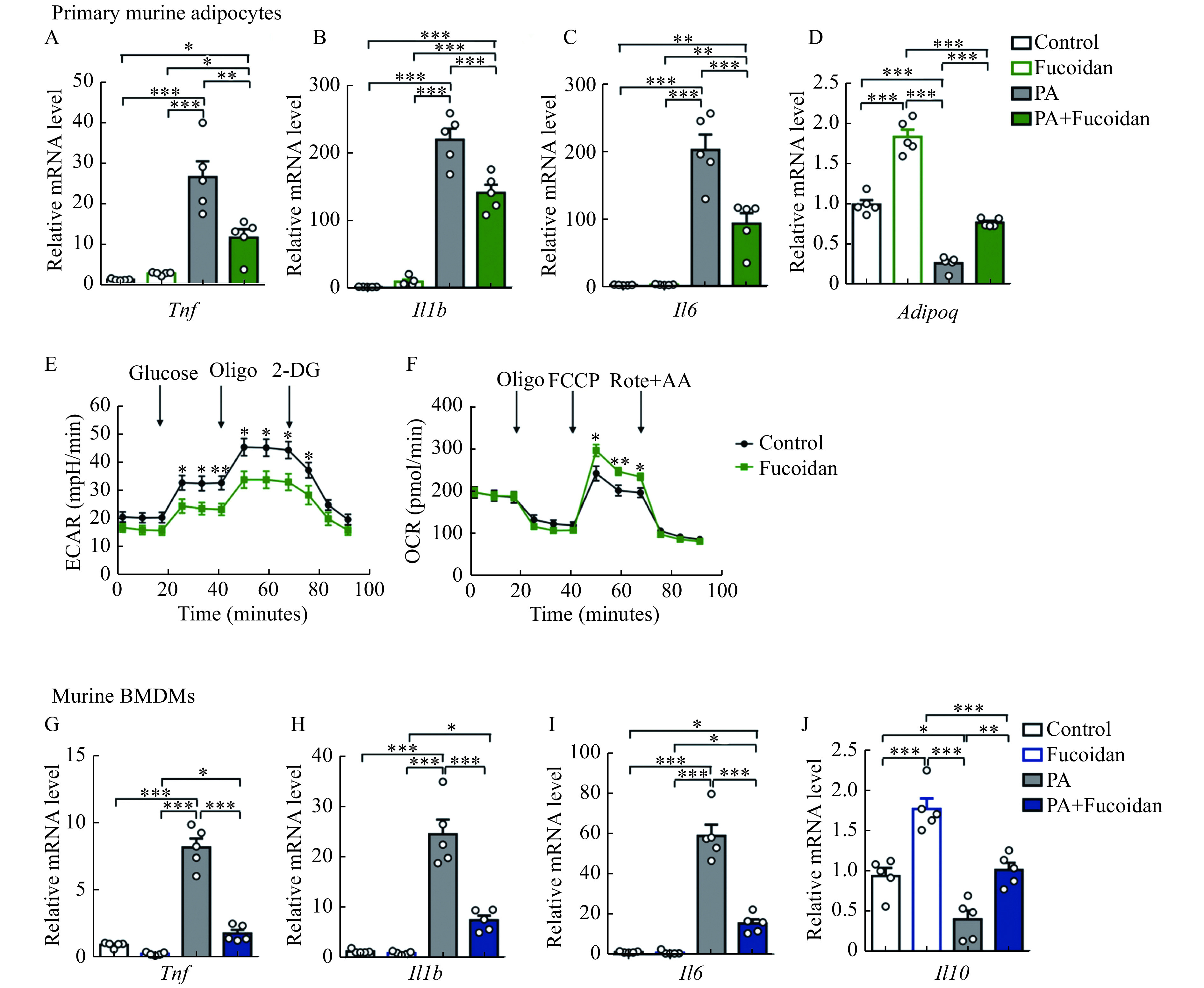
Fucoidan treatment suppressed inflammation of adipocytes and macrophages.
A–D: Primary murine adipocytes were treated by fucoidan (50 µg/mL), PA (100 µmol/L), or PA (100 µmol/L) plus fucoidan (50 µg/mL) for 12 hours. mRNA levels ofTnf (A), Il1b (B), Il6 (C), and Adipoq (D) were measured by qRT-PCR, n=5. E and F: ECAR and OCR of primary murine adipocytes with fucoidan (50 µg/mL) treatment were measured by Seahorse (n=8–10). G–J: Murine BMDMs were treated by fucoidan (50 µg/mL), PA (100 µmol/L), or PA (100 µmol/L) plus fucoidan (50 µg/mL) for 12 hours. mRNA levels ofTnf (G), Il1b (H), Il6 (I), and Il10 (J) were measured by qRT-PCR, n=5. Data are expressed as mean±SEM. Statistical analyses were performed by one-way ANOVA for comparisons between multiple groups with a single variable (A–D, G–J) and two-tailed unpairedt test for two-group comparisons (E and F). *P<0.05;**P<0.01;***P<0.001. PA: palmitic acid; ECAR: Extracellular acidification rate; OCR: oxygen consumption rate; Oligo: oligomycin; 2-DG: 2-deoxyglucose; FCCP: carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone; Rote+AA: Rotenone and antimycin A; BMDMs: bone marrow derived-macrophages.
Fucoidan administration inhibited ectopic fat accumulation in the liver
Extreme energy intake results in lipid deposition not only in adipose tissues but also in the liver, which might accelerate the progress toward steatohepatitis, a serious complication of obesity. We found that fucoidan treatment led to a decrease in liver weight accompanied with reduced levels of hepatic triglycerides and cholesterol (Fig. 5A-C). Fucoidan treatment also attenuated liver injury in HFD-fed mice (Fig. 5D and E). Histological examination of liver confirmed that the hepatic lipid droplets caused by HFD feeding were dramatically decreased by fucoidan treatment (Fig. 5F and G). Therefore, fucoidan could act as a protector against HFD-induced obesity.
Figure 5.
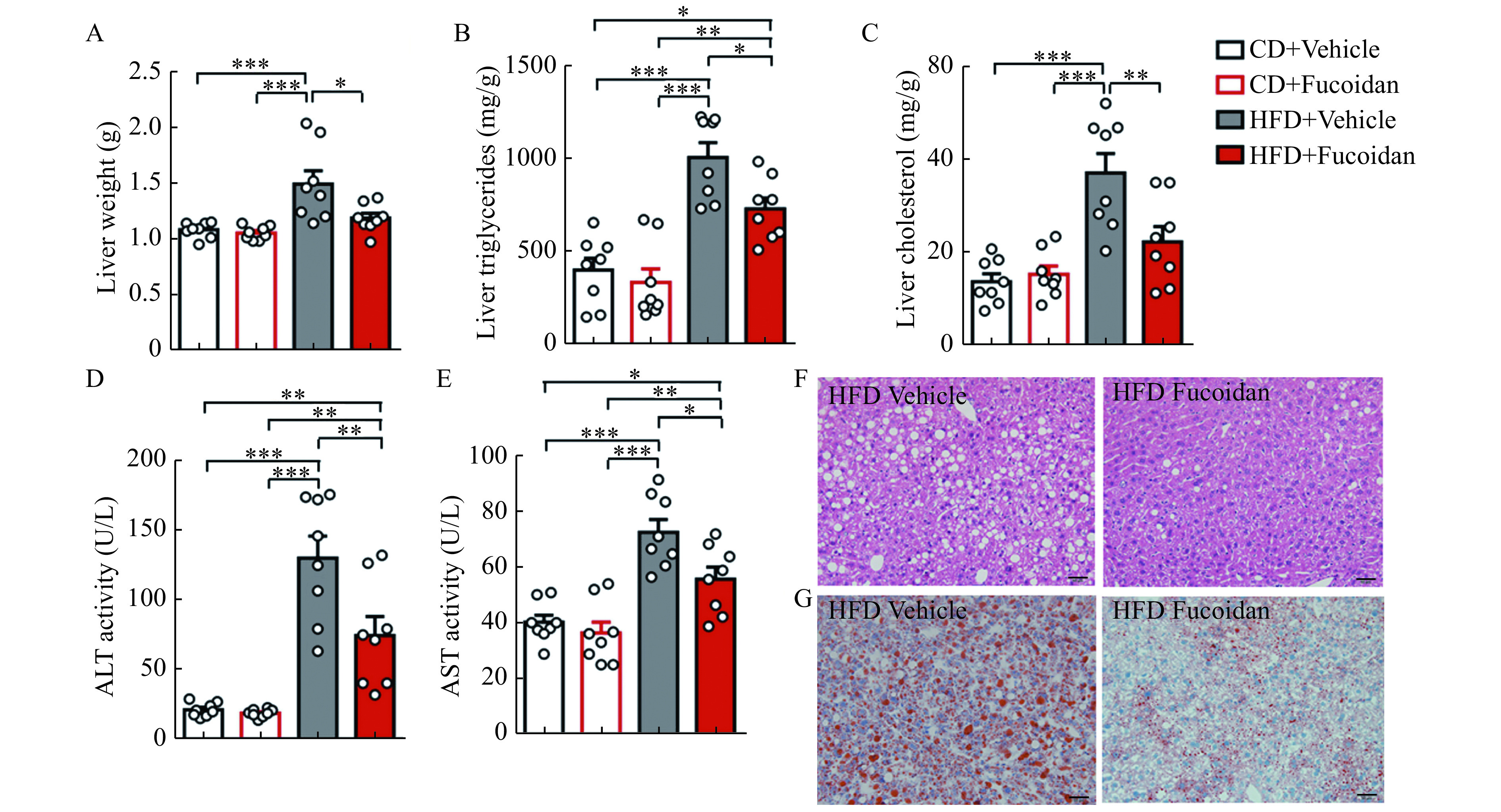
Fucoidan treatment alleviated liver adipogenesis.
Mice were administered with Fucoidan after 8 weeks of feeding. A: Average liver weights in fucoidan-treated mice after 24 weeks of feeding, n=8. B and C: Triglycerides (B) and cholesterol (C) in liver tissues of fucoidan-treated mice after 24 weeks of feeding, n=8. D and E: ALT and AST in plasma of fucoidan-treated mice after 24 weeks of feeding, n=8. F: Representative images of H&E staining of liver tissues in fucoidan-treated mice after 24 weeks of HFD feeding. Scale bars, 100 μm. G: Representative images of Oil red O staining of liver tissues in fucoidan-treated mice after 24 weeks of HFD feeding. Scale bars, 100 μm. Data are expressed as mean±SEM. Statistical analyses were performed by one-way ANOVA for comparisons between multiple groups with a single variable.*P<0.05;**P<0.01;***P<0.001. CD: chow diet; HFD: a high-fat diet; AST: aspartate aminotransferase; ALT: alanine aminotransferase.
Discussion
Despite efforts in lifestyle modifications such as improved dietary quality, food intake restriction, and physical exercises, the rate of obesity has been increasing in the world[15], and its severity also rises disproportionately[16]. The rapid prevalence of obesity is closely related to a variety of related diseases. Thus, developing noninvasive anti-obesity drugs to treat or prevent obesity and its complications would be of high clinical benefit. In the present study, we demonstrated that the administration of fucoidan decreased the HFD-induced body fat accumulation and inhibited inflammation in obese murine adipose tissue. Our results indicate that fucoidan may be effective to fight against obesity.
Fucoidan, a highly sulfated polysaccharide extracted from brown seaweed, has been found to possess antioxidant, anti-tumogenestic, and immunoregulatory properties[17-19]. Recently, an anti-obesity effect of fucoidan has been reported in HFD-induced mice as evidenced by the inhibition of fat accumulation in adipocytes and blood[10-11,20]. Besides confirming this bioactivity, we revealed a redistribution of fat in adipose tissues and increased oxygen consumption in fucoidan-treated HFD mice. Moreover, we further showed that fucoidan treatment could suppress ectopic fat deposition in the liver. However, whether fucoidan has an impact on lipid absorption is not yet known. The potential effect of fucoidan on multiple targets warrants further investigation.
Adipose tissue has been considered as important sites for inflammation in obesity[4,21]. The pathogenesis process of many obesity-related metabolic dysfunction, including IR, steatohepatitis, and cardiovascular diseases, are orchestrated by chronic low level inflammation[22-24]. Therefore, anti-inflammation constitutes an important therapeutic approach for treating these devastating diseases. The anti-inflammation effects of fucoidan have been reported in several cell types. For example, fucoidan inhibits lipopolysaccharide (LPS)-induced inflammation in macrophages[25] and microglial cells[26]. Our results indicated that fucoidan ameliorated inflammation in both adipocytes and macrophages insulted by PA. Macrophages in adipose tissues can affect insulin signaling in adipocytes via a paracrine of pro-inflammatory cytokines in obesity, promoting the progression of IR and obesity-related metabolic diseases[27-28]. Adipocyte hypertrophy causes local hypoxia, which drives the inflammatory responses in both macrophages and adipocytes. Meantime, innate immune signaling in adipocytes is activated by FFA or other cytokines. This process triggers the infiltration of inflammatory cells[29-32]. Preadipocytes in obese adipose fat have increased pro-inflammatory cytokines[33], indicating the role of adipocytes in the obesity-induced inflammation. Thus, the suppressing effect of fucoidan on inflammatory responses in both macrophages and adipocytes provides an aggravating beneficial effect against obesity. Petrus et al have been reported that reduced glycolytic rates could inhibit pro-inflammatory gene expression in adipocytes[34]. Fucoidan has been reported to improve mitochondrial TCA cycle in rats[35]. In our study, fucoidan also inhibited the inflammation of adipocytes by reducing glycolytic level and increasing TCA level.
Larger adipocytes tend to secrete more pro-inflammatory adipokines, exacerbating obesity-related metabolic dysfunction. However, increased adipogenesis in the adipose tissue is accompanied by reduction in pro-inflammatory adipokines[36]. Furthermore, large fat cells are under hypoxic stress, which augments inflammatory cell infiltration. The smaller fat cells produced in adipogenesis can decrease the inflammation in adipose tissues[37]. In our study, the decrease in fat mass was accompanied by shrinking diameter of the adipocyte and inhibition of anti-adipogenesis effects in fucoidan-treated HFD mice. In conclusion, pro-adipogenesis may be a possible mechanism for fucoidan to prevent obesity and obesity-related metabolic dysfunction, but more experiments are needed to prove it.
In this study, we show that fucoidan inhibits murine body fat accumulation and adipose inflammation induced by HFD. Combined with our previous discovery that fucoidan inhibits obesity-associated high blood pressure[12], fucoidan may be used as an new anti-obesity agent. Certainly, more studies on its efficacy in human beings are needed in the future.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (81830011, 81670418, and 91739304 to Q.C., 81770417 to X.Z., 81870371 to J.B., and 81670263 to X.L.); and the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJA310003 to J.B. and 15KJA310001 to X.L.).
Footnotes
The authors reported no conflict of interests.
CLC number: R966, Document code: A
Contributor Information
Qi Chen, Email: qichen@njmu.edu.cn.
Xudong Zhu, Email: zhuxudong@njmu.edu.cn.
References
- 1.Wang YC, McPherson K, Marsh T, et al Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 2.Chu DT, Nguyet NTM, Dinh TC, et al An update on physical health and economic consequences of overweight and obesity. Diabetes Metab Syndr Clin Res Rev. 2018;12(6):1095–1100. doi: 10.1016/j.dsx.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 3.NCD Risk Factor Collaboration Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19•2 million participants. Lancet. 2016;387(10026):1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zatterale F, Longo M, Naderi J, et al Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2020;10:1607. doi: 10.3389/fphys.2019.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ni YH, Ni LY, Zhuge F, et al Adipose tissue macrophage phenotypes and characteristics: the key to insulin resistance in obesity and metabolic disorders. Obesity. 2020;28(2):225–234. doi: 10.1002/oby.22674. [DOI] [PubMed] [Google Scholar]
- 6.Guilherme A, Henriques F, Bedard AH, et al Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat Rev Endocrinol. 2019;15(4):207–225. doi: 10.1038/s41574-019-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faraj M LDL, LDL receptors, and PCSK9 as modulators of the risk for type 2 diabetes: a focus on white adipose tissue. J Biomed Res. 2020;34(4):251–259. doi: 10.7555/JBR.34.20190124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kan JT, Hood M, Burns C, et al A novel combination of wheat peptides and fucoidan attenuates ethanol-induced gastric mucosal damage through anti-oxidant, anti-inflammatory, and pro-survival mechanisms. Nutrients. 2017;9(9):978. doi: 10.3390/nu9090978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu HH, Ko EC, Chang CL, et al Fucoidan inhibits radiation-induced pneumonitis and lung fibrosis by reducing inflammatory cytokine expression in lung tissues. Mar Drugs. 2018;16(10):392. doi: 10.3390/md16100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MJ, Jeon J, Lee JS Fucoidan prevents high-fat diet-induced obesity in animals by suppression of fat accumulation. Phytother Res. 2014;28(1):137–143. doi: 10.1002/ptr.4965. [DOI] [PubMed] [Google Scholar]
- 11.Sim SY, Shin YE, Kim HK Fucoidan from Undaria pinnatifida has anti-diabetic effects by stimulation of glucose uptake and reduction of basal lipolysis in 3T3-L1 adipocytes . Nutr Res. 2019;65:54–62. doi: 10.1016/j.nutres.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Zhu XD, Wang Y, Zhu L, et al Class A1 scavenger receptor prevents obesity-associated blood pressure elevation through suppressing overproduction of vascular endothelial growth factor B in macrophages. Cardiovasc Res. 2020:cvaa030. doi: 10.1093/cvr/cvaa030. [DOI] [PubMed] [Google Scholar]
- 13.Weisberg SP, McCann D, Desai M, et al Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chawla A, Nguyen KD, Goh YPS Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11(11):738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The GBD 2015 Obesity Collaborators Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flegal KM, Kruszon-Moran D, Carroll MD, et al Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luthuli S, Wu SY, Cheng Y, et al Therapeutic effects of fucoidan: a review on recent studies. Mar Drugs. 2019;17(9):487. doi: 10.3390/md17090487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang H, Li X, Zhuang Y, et al Class A scavenger receptor activation inhibits endoplasmic reticulum stress-induced autophagy in macrophage. J Biomed Res. 2014;28(3):213–221. doi: 10.7555/JBR.28.20130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aleissa MS, Alkahtani S, Eldaim MAA, et al Fucoidan ameliorates oxidative stress, inflammation, DNA damage, and hepatorenal injuries in diabetic rats intoxicated with aflatoxin B1. Oxid Med Cell Longev. 2020;2020:9316751. doi: 10.1155/2020/9316751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuong HD, Thuy TTT, Huong TT, et al Structure and hypolipidaemic activity of fucoidan extracted from brown seaweed Sargassum henslowianum . Nat Prod Res. 2015;29(5):411–415. doi: 10.1080/14786419.2014.948436. [DOI] [PubMed] [Google Scholar]
- 21.Reilly SM, Saltiel AR Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 22.Hotamisligil GS Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 23.Metrakos P, Nilsson T Non-alcoholic fatty liver disease--a chronic disease of the 21st century . J Biomed Res. 2018;32(5):327–335. doi: 10.7555/JBR.31.20160153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumeng CN, Saltiel AR Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J, Cha JD, Choi KM, et al Fucoidan inhibits LPS-induced inflammation in vitro and during the acute response in vivo . Int Immunopharmacol. 2017;43:91–98. doi: 10.1016/j.intimp.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Park HY, Han MH, Park C, et al Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem Toxicol. 2011;49(8):1745–1752. doi: 10.1016/j.fct.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Xu HY, Barnes GT, Yang Q, et al Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han MS, Jung DY, Morel C, et al JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339(6116):218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YS, Kim JW, Osborne O, et al Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity . Cell. 2014;157(6):1339–1352. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trayhurn P Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93(1):1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen MTA, Favelyukis S, Nguyen AK, et al A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways . J Biol Chem. 2007;282(48):35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 32.Cui XB, Chen SY White adipose tissue browning and obesity. J Biomed Res. 2016;31(1):1–2. doi: 10.7555/JBR.31.20160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang N, Zhao TT, Li SM, et al Fibroblast growth factor 21 exerts its anti-inflammatory effects on multiple cell types of adipose tissue in obesity. Obesity. 2019;27(3):399–408. doi: 10.1002/oby.22376. [DOI] [PubMed] [Google Scholar]
- 34.Petrus P, Lecoutre S, Dollet L, et al Glutamine links obesity to inflammation in human white adipose tissue. Cell Metab. 2020;31(2):375–390. doi: 10.1016/j.cmet.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 35.Veena CK, Josephine A, Preetha SP, et al Mitochondrial dysfunction in an animal model of hyperoxaluria: a prophylactic approach with fucoidan. Eur J Pharmacol. 2008;579(1-3):330–336. doi: 10.1016/j.ejphar.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 36.Skurk T, Alberti-Huber C, Herder C, et al Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92(3):1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 37.Ghaben AL, Scherer PE Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20(4):242–258. doi: 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]


