Abstract
Abdominal aortic aneurysm (AAA) is a chronic inflammatory degenerative aortic disease, which particularly affects older people. Nucleotide-binding oligomerization domain-like receptor family protein 3 (NLRP3) inflammasome is a multi-protein complex and mediates inflammatory responses by activating caspase 1 for processing premature interleukin (IL)-1β and IL-18. In this review, we first summarize the principle of NLRP3 inflammasome activation and the functionally distinct classes of small molecule NLRP3 inflammasome inhibitors. Next, we provide a comprehensive literature review on the expression of NLRP3 inflammasome effector mediators (IL-1β and IL-18) and components (caspase 1, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and NLRP3) in clinical and experimental AAAs. Finally, we discuss the influence of genetic deficiency or pharmacological inhibition of individual effector mediators and components of NLRP3 inflammasome on experimental AAAs. Accumulating clinical and experimental evidence suggests that NLRP3 inflammasome may be a promise therapeutic target for developing pharmacological strategies for clinical AAA management.
Keywords: Abdominal aortic aneurysm, Inflammasome, NLRP3, Interleukin-1, Interleukin-18
Introduction
Abdominal aortic aneurysm (AAA) is a life-threatening degenerative disease that is characterized by progressively localized dilatation of abdominal aorta, particularly infrarenal aorta. Advanced age, male sex, smoking, white race, hypertension, and hyperlipidemia increase the risk for AAAs, whereas female sex, black race, and diabetes reduce it 1 - 3) . There is a race difference in AAA prevalence, with high in Northern American and European and low in Asian 4 , 5) . There is currently no effective pharmacological therapy available for AAAs, and patients are mainly treated with open or endovascular surgical repair as recommended by the Society for Vascular Surgery 6) and National Institute of Health and Care Excellence guideline for abdominal aortic aneurysm: diagnosis and management (https://www.nice.org.uk/ guidance/ng156). Understanding of AAA pathogenesis will help establish nonsurgical pharmacological treatment for AAA disease.
Although AAA pathogenesis is not completely understood, inflammation has been shown to play a critical role. The major mechanisms include smooth muscle cell loss due to apoptosis 7 , 8) , accelerated extracellular matrix degradation resulting from imbalanced metalloproteinases and its tissue inhibitors 9 - 11) , increased levels and activity of renin-angiotensin (Ang) system 12 - 16) , augmented oxidative tissue damage 17) , dysregulated immune cell-derived pro- and anti-inflammatory mediators 18 - 23) , increased mural angiogenesis 24 - 30) , altered hemodynamics and homeostasis 31 - 33) , and increased genetic susceptibility 31 - 33) .
Inflammasome is a multi-protein complex that is composed of an apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), caspase 1, and nucleotide-binding oligomerization domain-like receptor family proteins (NLRPs) such as NLRP3 or HIN200 family proteins 34 , 35) . Recognizing by pathogen-associated molecular patterns or damage-associated molecular patterns, it activates caspase 1 for processing inactive interleukin (IL)-1β or IL-18 to its active form for innate immune response. NLRP3 inflammasome has been known to participate in many pathological conditions including cardiovascular diseases 36 - 39) .
NLRP3 Inflammasome
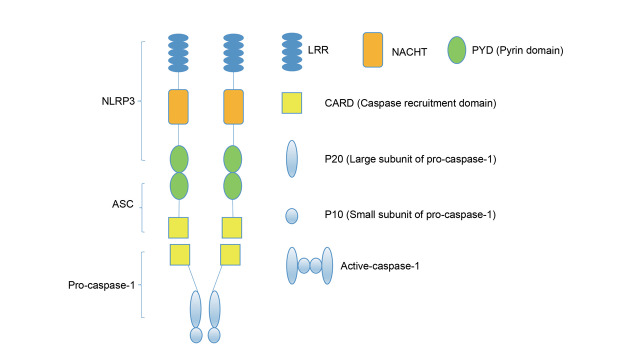
NLRP3 inflammasome is one of the well-characterized inflammasomes, with three major components: NLRP3 receptor protein, ASC adapter protein, and caspase 1 effector protein ( Fig.1 ) 34 , 35) . NLRP3 protein contains one leucine-rich repeat for ligand recognition, one nucleotide-binding oligomerization domain with ATPase activity for nucleic acid binding and self-oligomerization, and one pyrin domain for ASC binding. ASC protein composes of one pyrin domain and one caspase recruitment domain. Once NLRP3 inflammasome is activated, ASC binds to NLRP3 protein through the pyrin domain for recruiting and activating caspase 1 via caspase recruitment domain.
Fig. 1. NLRP3 inflammasome structure .
NLRP3 inflammasome is a protein complex composing of ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), pro-caspase-1, and NLRP3 protein. NLRP3 protein recruits ASC through the interaction of N-terminal PYD-PYD. ASC connects NLRP3 protein with pro-caspase-1 through PYD-PYD and CARD-CARD to form a pro-caspase-1 tetramer, which provides caspase 1 with enzyme cutting activity. LRR, leucine-rich repeat. NACHT/NOD, nucleotide-binding oligomerization domain.
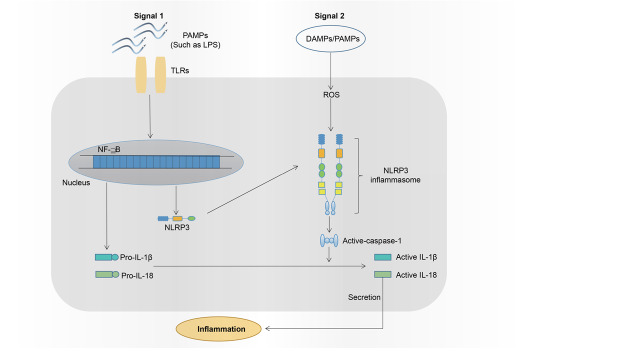
There are two signals required for NLRP3 inflammasome activation 34 , 35) ( Fig.2 ) : Signal 1 (priming) activates NF-κB pathway through toll-like receptors, leading to the mRNA expression of IL-1β, IL-18, and NLRP3-related genes, and signal 2 (activation) such as damage- and pathogen-associated molecular patterns assembles NLRP3 inflammasome and activates caspase 1. Several potential mechanisms have been proposed for NLRP3 activation. In potassium efflux model, extracellular ATP activates purine-type membrane receptor P2X7, results in potassium efflux, and consequently reduces intracellular potassium levels, thus activating NLRP3 inflammasome 40) . Alternatively, certain crystalline or particulate matter enters cells and leads to lysosome rupture, which releases cathepsin B for NLRP3 inflammasome activation 41 - 43) . Interaction of mitochondria-derived reactive oxygen species (ROS) with thioredoxin-interacting protein is also able to trigger NLRP3 inflammasome activation. In addition, calcium influx also plays a role in NLRP3 inflammasome activation. All these mechanisms are not exclusive and may work together. For example, calcium influx results in mitochondrial ROS production, while lysosomal rupture promotes calcium influx 44 , 45) .
Fig. 2. Two signals are required for NLRP3 inflammasome activation .
Signaling pathway. Signal 1: Toll-like receptors (TLRs) bind their cognate ligands such as lipopolysaccharides (LPS), trigger NF-κB signaling pathway, and ultimately lead to the expression of pro-IL-18, pro-IL-1β, and NLRP3 protein. Signal 2: DAMP (damage-associated molecular patterns) and PAMPs (pathogen-associated molecular patterns) are recognized by NLRP3, causing NLRP3-ASC-pro-caspase-1 inflammasome assembly and activation, which convert inactive caspase 1 to active caspase 1 and subsequently cleaves pro-IL-18 or pro-IL-1β to corresponding active form.
NLRP3 Inflammasome Inhibitors
Given the critical importance of NLRP3 inflammasome in a broad spectrum of inflammatory and degenerative diseases, to therapeutically target NLRP3 inflammasome, functionally distinct classes of NLRP3 inhibitors have been discovered. This has been the subject of several outstanding recent review articles 34 , 46 - 49) . NLRP3 inflammasome inhibitors target either signal 1 or signal 2. As one of signal 1 inhibitors, Bay 11-7082 inhibits transcription of IL-1β and IL-18 by blocking IKKb kinase and NF-kB signaling pathway 47 , 50) . JC124 suppresses the expression of IL-1β, NLRP3, ASC, and caspase 1 51) .
Most inhibitors have however been designed to target signal 2, including upstream inducers (ion fluxes, mitochondrial ROS, nondegradable particles, and lysosomal proteinase cathepsin B), NLRP3 complex components (ASC and caspase 1), and NLRP3 per se 47 , 48) . β-hydroxybutyrate inhibits NLRP3 inflammasome by blocking potassium efflux 52 , 53) , whereas both 5-nitro-2-(3-phenylpropylamino) benzoic acid, 4,4’-diisothiocyano-2,2’-disbenedisufonic acid, and IAA94 inhibit NLRP3 by disrupting chloride efflux 48) . Blockage of calcium mobilization by 2-APB, XeC, and U73122, the reduction of mitochondrial ROS by N-acetyl-L-cysteine and (2R,4R)-4-aminopyrro lidine-2,4-dicarboxylate, and the prevention of lysosomal rupture by a specific inhibitor to cathepsin, CA-074-Me, all inhibit NLRP3 inflammasome 48) . Inhibition of caspase 1 by VX-740 and VX-756 or the conversion of pro-caspase-1 to active caspase 1 by FC11A-2 also results in the inhibition of NLRP3 inflammasome 47 , 48) .
More important and specific inhibitors to NLRP3 inflammasome are those that directly inhibit NLRP3 activity, oligomerization, or interaction of NLRP3 with other complex components. For example, MCC950, CY-09, OLT1177/dapansutrile, MNS, and parthenolide inhibit NLRP3 by inhibiting its ATPase activity or modifying its ATPase domain 38 , 48 , 54 - 57) . Blocking NLRP3-MEK7 by oridonin via binding to NLRP3 cysteine 279 or NLRP3 oligomerization by tranilast specifically inhibits NLRP3 inflammasome 58) . Glyburide also inhibits NLRP3 inflammasome by inhibiting ATP-sensitive potassium channel and thus disturbs ASC aggregation 59) . Resveratrol inhibits the assembly of ASC and NLRP3 60) . Targeting molecules that interact with either NLRP3 or ACS represent alternative approaches for alternative inhibitor discovery 48) .
Additional avenues for targeting inhibition of NLRP3 inflammasome activity are to specifically inhibit its effector mediators. These include IL-1β, IL-18, their receptors, or receptor antagonist as represented by two humanized anti-IL-1β monoclonal antibodies (canakinumab and gevokizumab) and two IL-1 receptor antagonists (anakinra and rilonacept) 36 , 37) .
Among NLRP3 inflammasome inhibitors, some are currently used for treating certain medical conditions with proved safety. For example, glyburide and its analogs are used to treat type 2 diabetes, while tranilast is an approved drug for treating allergic diseases in China, Korea, and Japan. Resveratrol is commercially available as a nutritional supplement. A large number of clinical trials have been registered to test the safety and efficacy of functionally distinct classes of NLRP3 inflammasome inhibitors for various inflammatory diseases through https://clinicaltrials.gov or alternative clinical organizations. In one open-label, dose-adaptive, proof-of-concept phase II trial, dapansutrile has been shown to safely and effectively reduce joint pain in patients with goat flare 61) . In another trial, dapansutrile will be tested for treatment of moderate COVID-19 symptoms and early cytokine syndrome (https://clinicaltrials.gov). Several clinical trials have also completed or are undergoing to test the therapeutic efficacy of canakinumab, gevokizumab, anakinra, and rilonacept for treating several cardiovascular diseases such as certain type of heart failure and acute myocardial infraction 36) .
Expression of NLRP3 Inflammasome Effector Mediators (IL-1β and IL-18) in AAAs
In clinical AAAs, it has been reported that serum IL-1β levels were elevated in AAA patients as compared to healthy controls and patients with coronary heart disease 62) . High plasma levels of IL-1β were observed in participants who had homozygous for common C allele of NLRP3 rs35829419 but not polymorphisms of any IL-1 gene cluster 63 , 64) . However, Batra et al. reported detectable levels of serum IL-1β in non-aneurysmal patients, but not aneurysmal patients. In aortic tissues from aneurysmal patients, most studies have shown that IL-1β mRNA and protein levels were higher in aneurysmal patients than those in non-aneurysmal patients 65 - 71) . The mRNA and protein levels of IL-18, another NLRP3 inflammasome effector mediator, were also increased in the aortae of patients with AAAs as compared to non-aneurysmal controls 72 , 73) .
Consistent with the findings in clinical AAAs, IL-1β mRNA and protein levels were markedly increased in aneurysmal mice created by intra-aortic elastase infusion or adventitial calcium painting in normolipidemic mice, subcutaneous Ang II infusion, or alternative technique 65 , 74 - 77) . Elevated aortic IL-1β protein levels were also noted in hyperhomocysteinemia-accelerated experimental AAAs 78) . Both aortic macrophages and smooth muscle cells have been identified as major sources for increased expression of IL-1β 74 , 78) . IL-1β levels were also significantly higher in aneurysmal mice than those in non-aneurysmal mice in Ang II-induced AAAs 74) . In addition, increased aortic IL-18 mRNA and protein abundance have been shown in AAAs induced by combined use of Ang II and β-aminopropionitrile 79) . In summary, both clinical and experimental data support elevated levels of NLRP3 inflammasome effector mediators in AAA disease.
Expression of NLRP3 Inflammasome Components in AAAs
ASC, caspase 1, and NLRP3 are key elements in NLRP3 inflammasome complex. Analysis of 100 patients with vascular diseases, including 34 AAA patients, revealed high mRNA expression levels of ASC, caspase 1, and NLRP3 in peripheral mononuclear cells from male patients as compared to female patients; it was however unknown whether this difference was associated with increased risk for AAAs in males 68) . The mRNA levels for caspase 1 and NLRP3 were higher in male AAA patients than those in non-AAA patients, but reduced protein levels were only noted for NLRP3 68) . In another study, both NLRP3 and ASC protein levels, as assessed by flow cytometric analysis, were higher in circulating granulocytes and monocytes in AAA patients than those in non-AAA controls, but the differences did not reach statistical significance 80) .
In aneurysmal aortae, an early study by Schonbeck et al. showed increased aortic protein levels of caspase 1 in AAA patients as compared to non-AAA patient controls. NLRP3 mRNA levels were increased in the aortae of aneurysmal patients as compared to occlusive aortic disease and healthy controls 81) . In another study analyzing aortae from 46 aneurysmal patients and 40 healthy organ donors 82) , more NLRP3-positive staining was noted in the aortae from aneurysmal patients as compared to non-AAA patient controls, with no difference in ASC and caspase 1 staining between two patient groups. However, NLRP3 expression levels were inversely associated with inflammation pathological grade, implying that NLRP3 inflammasome may be an early event in AAA pathogenesis 82) . In addition, smooth muscle cells from AAA patients had an increased ability to express NLRP3 and caspase 1 in response to necrotic cell debris 83) .
In contrast, studies on the expression of NLRP3 inflammasome components are limited for experimental AAAs. In Ang II-induced AAAs, ASC was highly expressed in adventitial macrophages 74) . Increased active caspase 1 was also elevated in Ang II-induced aneurysmal aorta 84) . Furthermore, NLRP3 and active caspase 1 were also detected in aneurysmal aorta in calcium chloride AAA model and further augmented by hyperhomocysteinemia 78) . Although evidence from experimental AAAs is limited, a large body of evidence from clinical AAA studies has demonstrated increased expression of NLRP3 inflammasome components in aneurysmal lesion or immune cells.
Influence of Inflammasome Effector Mediators on Experimental AAAs
Several studies have been conducted to investigate the role of two NLRP3 inflammasome effector cytokines, IL-1β and IL-18, in AAA pathogenesis using rodents deficient for IL-1β, IL-18 or its cognate receptor, or pharmacological inhibitors ( Table 1 ) . In mice deficient for IL-1β or IL-1 receptor, or treated with IL-1 receptor blocker anakinra, Johnston et al. reported attenuation of aneurysmal aortic enlargement and accumulation of macrophages and neutrophils following intra-aortic elastase infusion 65) . In these experimental settings, AAA suppression was associated with reduced expression levels or activity of CC-motif chemokine ligand 2, IL-6, complement C5a, and matrix metalloproteinase (MMP) 9. A recent study by Meker et al. reported that IL-1β mediated AAA disease by promoting neutrophil extracellular trap formation 69) . In a special form of AAAs associated with Kawasaki disease induced by Lactobacillus casei cell wall extract injection, AAAs were suppressed in mice deficient for IL-1α, IL-1β, or IL-1 receptor or treated with an antibody against IL-1α, IL-1β, or IL-1 receptor 77) . Conversely, Ang II-induced AAAs were accelerated in high fat diet fed mice deficient for IL-1 receptor antagonist and counteracted following IL-1β antibody injection 85) .
Table 1. Influence of genetic deficiency or pharmacological inhibition of inflammasome effector mediators on experimental AAAs.
| Mouse strain | AAA model | Gene knockout or pharmacological inhibitor | Influence on experimental AAAs | References |
|---|---|---|---|---|
| C57BL/6 | Elastase infusion | IL-1β -/- , IL-1R -/- or anakinra treatment | •Attenuated AAA formation and reduced aortic macrophages and neutrophils | [65] Johnston WF et al. Arterioscler Thromb Vasc Biol 33: 294-304, 2013. |
| C57BL/6 | Lactobacillus casei cell-wall extracts | IL-1α -/- , IL-1β -/- , or treatment with an antibody to IL-1α IL-1β | •Protected AAA formation | [77] Wakita D et al. Arterioscler Thromb Vasc Biol 36: 886-897, 2016. |
| C57BL/6 | Calcium chloride | IL-1β -/- , IL-1R -/- | •No influence on AAA formation and proinflammatory macrophage polarization | [75] Batra R et al. Arterioscler Thromb Vasc Biol 38: 457-463, 2018. |
| C57BL/6 | Elastase infusion | IL-1β -/- |
•Reduced elastase-induced aortic diameter enlargement and aortic neutrophil infiltration •Transferring wild type neutrophils restored aneurysmal aortic expansion in IL-1β knockout mice |
[69] Meher AK et al. Arterioslcer Thromb Vasc Biol 38: 843-853, 2018. |
| C57BL/6 | Ang II infusion | IL-1Ra -/- |
•Increased aneurysmal aortic diameter and aneurysm rupture •Elevated inflammatory cytokines (IL-6 and TNF-α) and matrix metalloproteinases (MMP2 and MMP9) production •Promoted smooth muscle degradation and macrophage infiltration •Anti-IL-1β antibody treatment inhibited aortic dilation and inflammation in Ang II-infused IL-1Ra -/- mice |
[85] Isoda K et al. Int J Cardiol 270: 221-227, 2018. |
| C57BL/6 |
Ang II infusion and treatment with β -aminopropionitrile |
IL-18 -/- |
•Reduced aneurysm diameter and rupture •Reduced macrophage infiltration and phenotype switch towards anti- inflammatory macrophages •Inhibited OPN-induced inflammation and matrix metalloproteinases activation |
[79] Suehiro C et al. Atherosclerosis 289: 14-20, 2019. |
| ApoE-/- | Ang II infusion | NCC -/- or IL-18R -/- |
•Attenuated aneurysmal aortic dilation and reduced aneurysm rupture •Reduced inflammatory cell infiltration and the levels of MMP2, MMP9, IL-6, IFN-γ and TNF-α |
[73] Liu CL et al. Eur J Heart 41: 2456-2468, 2020. |
AAA: Abdominal aortic aneurysm. Ang II: Angiotensin II. ApoE: Apolipoprotein E. IFN: Interferon. IL: Interleukin. IL-1Ra: IL-1 receptor antagonist. MMP: Matrix metalloproteinase. R: Receptor. TNF: Tumor necrosis factor. NCC: Na-Cl co-transporter. OPN: Osteopontin. -/-: Deficient mice
Two recent studies have further demonstrated the significance of IL-18 in experimental AAAs. In Ang II/β-aminopropionitrile (lysyl oxidase inhibitor)-induced AAA model 79) , genetic deficiency of IL-18 suppressed aneurysmal aortic expansion and lowered AAA incidence in association with reduced aortic CD68-positive macrophage infiltration, pro- and active MMP levels, and osteopontin. IL-18 deficiency also resulted in a switch from proinflammatory M1 macrophage activation toward anti-inflammatory M2 macrophage activation as indicated by reduced ratio of CD11c-positive to CD206-positive macrophages in aneurysmal aorta. Similarly, deficiency of either receptor for IL-18, IL-18 receptor or Na-Cl cotransporter, also suppressed Ang II-induced AAAs in ApoE-deficient hyperlipidemic mice as evidenced by reduced aortic diameter expansion and active MMP2 and MMP9 73) . In these experimental settings, adipocytes have been shown to promote IL-18 function by inducing the expression of leptin and fatty acid-binding protein 4. Together, accumulating evidence from recently published studies supports the importance of both mediators in AAA disease using various AAA modeling systems.
Two studies, however, have failed to demonstrate the contribution of IL-1 to experimental AAAs. In an early study, treatment with anti-IL-1 receptor antagonist was not effective in suppressing elastase-induced AAAs in rats 86) . In experimental AAAs created by topical application of calcium chloride, deficiency of IL-1β or IL-1 receptor had no remarkable impact on AAA formation, although it decreased MMP expression levels 75) .
Influence of Genetic NLRP3 Inflammasome Component Deficiency on Experimental AAAs
Processing pro-IL-1β and pro-IL-18 to their active forms can also be mediated by other inflammasomes rather than NLRP3. As shown in Table 2 , mice with genetic deficiency of individual NLRP3 inflammasome components have contributed to our understanding of NLRP3 inflammasome in AAA pathogenesis. In Ang II infusion/ApoE-deficient AAA model, whole-body ablation of NLRP3, ASC, or caspase 1 reduced aneurysm incidence, attenuated aneurysmal aortic enlargement, and preserved medial elastin and smooth muscle cells 74) . Similarly, AAA suppression has also been confirmed in high fat diet fed NLRP3- or caspase 1-deficient mice following chronic Ang II infusion 87) . In Kawasaki disease-associated AAAs, deficiency of NLRP3 or caspase 1 also led to AAA suppression 77) . In addition, topical application of lentivirus siRNA to aortic wall abrogated AAAs in alternative calcium AAA model 78) . Altogether, these genetic studies provide solid evidence for NLRP3 inflammasome in AAA pathogenesis.
Table 2. Effect of genetic deficiency of individual NLRP3 inflammasome components on experimental AAAs.
| Mouse strain | AAA model | Gene knockout | Influence on experimental AAAs | References |
|---|---|---|---|---|
| ApoE -/- | Ang II infusion | NLRP3 -/- , ASC -/- , or caspase-1-/- |
•Reduce AAA severity, aortic inflammatory cell infiltration and the serum levels of IL-1β, MMP2, MMP9 |
[74] Usui F et al. Arterioscler Thromb Vasc Biol 35: 127-136, 2015. |
| ApoE -/- | Ang II infusion and homocysteine diet | Lentivirus-mediated NLRP3 silencing |
•Reduced aneurysmal aortic diameter and severity •Downregulated the expression levels of aortic inducible nitric oxide synthase and myofibroblast markers •Reduced aortic MMP9 activity |
[78] Sun W et al. J Mole Cell Cardiol 81: 96-106, 2015. |
| C57BL/6 | Lactobacillus casei cell-wall extract injection | Caspase-1 -/- or NLRP3 -/- | •Prevented AAA formation | [77] Wakita D et al. Arterioscler Thromb Vasc Biol 36: 886-897, 2016. |
| C57BL/6 | Ang II infusion and high fat diet | Caspase-1 -/- or NLRP3 -/- |
•Reduced aneurysm incidence and maximal diameter •Degraded aortic contractile proteins |
[87] Wu D et al. Arterioscler Thromb Vasc Biol 37: 694-706, 2017. |
AAA: Abdominal aortic aneurysm. Ang II: Angiotensin II. ApoE: Apolipoprotein E. ASC: Apoptosis-associated speck-like protein containing a caspase recruitment domain. IL: Interleukin. MMP: Matrix metalloproteinase. -/-: Deficient mice
Influence of Pharmacological NLRP3 Inflammasome Inhibition on Experimental AAAs
Table 3 summarizes published studies on the influence of various NLRP3 inflammasome inhibitors on experimental AAAs. Yamanouchi D et al. have shown that treatment with Q-Vd-OPh, a pan-caspase inhibitor, inhibited Ang II infusion-induced aneurysmal aortic enlargement and reduced AAA incidence in ApoE-deficient mice. The AAA suppression was accompanied by attenuation of aortic macrophage infiltration, smooth muscle cell apoptosis, and CCL2-mediated macrophage migration 88) . Administration of tranilast has been shown to suppress calcium chloride-induced AAAs in association with preservation of medial elastin, attenuation of mural mast cells and lymphocytes, reduced neoangiogenesis, and reduced MMP9 activity 89) . Treatment with MCC950 also inhibited the development of aortic aneurysms at various aortic segments, including abdominal aorta, in conjunction with reduced expression levels of NLRP3, caspase 1, and MMPs and preserved contractible proteins of smooth muscle cells 84) . Treatment with glyburide also reduced AAA formation induced by Ang II 87) , potentially associated with AAA inhibition in diabetic patients 90 - 92) . Treatment with glucagon-like peptide receptor agonist suppressed elastase infusion-induced AAAs in rats by reducing ROS production and aortic inflammatory response 93) . Sitagliptin, a dipeptidyl peptidase-4 inhibitor, prevented the formation and progression of AAAs by increasing glucagon-like peptide 94 - 97) . Resveratrol has also been reported to suppress AAA formation in Ang II, elastase, and/or calcium models 98 - 100) .
Table 3. Influence of pharmacological NLRP3 inflammasome inhibition on experimental AAAs.
| Animal species and strain | AAA model | Intervention | Influence on experimental AAAs | References |
|---|---|---|---|---|
| Wild type Sprague- Dawley rats | Calcium chloride | NLRP3 inhibitor (Tranilast) |
•Reduced aneurysmal aortic dilation and preserved medial elastin •Attenuated aortic infiltration of mast cells and T cells •Reduced aortic MMP activity |
[89] Tsuruda T et al. Circ Res 102: 1368-1377, 2008. |
| ApoE -/- mice | Ang II infusion | Pan-caspase inhibitor (Quinoline- Val-Asp difluorophenoxymethylketone) |
•Reduced aneurysm diameter and incidence •Inhibited cell apoptosis and reduced macrophage infiltration |
[88] Yamanouchi D et al. Arterioscler Thromb Vasc Biol 30: 702-707, 2010. |
| Wild type C57BL/6 mice | Calcium chloride | ROS scavenger (Resveratrol) |
•Inhibited AAA formation •Reduced inflammatory cell infiltration and aortic expression of MMPs, MCP-1 and TNF-α |
[99] Kaneko H et al. Atherosclerosis 217: 350- 367, 2011. |
| Wild type Sprague- Dawley rats | Elastase infusion | ROS scavenger (Resveratrol) |
•Reduced aneurysmal size •Reduced systemic and aortic levels of TNF-α and MMP9 •Reduced circulating L-selectin-expressing monocytes and aortic macrophages |
[100] Palmieri D et al. J Surg Res 171: e237-e246, 2011. |
| ApoE -/- mice | Ang II infusion | Dipeptidyl peptidase-4 inhibitor (sitagliptin) | •Inhibited AAA formation, reduced aortic inflammation and preserved elastin lamina | [94] Lu HY et al. Plos One 10: e0121077, 2015. |
| ApoE -/- mice | Ang II infusion and high fat diet | Dipeptidyl peptidase-4 inhibitor (MK0626) |
•Reduced aneurysm diameter and incidence •Reduced aortic IL-1β mRNA levels but increased aortic TIMP-2 mRNA levels |
[96] Kohashi K et al. J Atheroscler Thromb 23: 441- 454, 2016. |
| Wild type Sprague- Dawley rats | Intra-aortic Elastase infusion and abluminal calcium chloride application | Glucagon-like peptide 1 receptor analog (lixisenatide) |
•Attenuated aneurysmal aortic dilation and macrophage infiltration but increased aortic elastin contents •Reduced aortic ROS production, oxidative DNA damage and ERK phosphorylation •Reduced the aortic mRNA levels of MMP9 and TNF-α |
[93] Yu J et al. Surgery Today 46: 1099-1107, 2016. |
| Wild type C57BL/6 mice | Ang II infusion and high fat diet | NLRP3 inhibitor (Glyburide) | •Reduce aneurysmal aortic diameter, aneurysm incidence and elastin destruction |
[87] Wu D et al. Arterioscler Thromb Vasc Biol 37: 694- 706, 2017. |
| ApoE -/- mice | Ang II infusion and high fat diet | ROS scavenger (Resveratrol) |
•Reduced aneurysmal aortic diameter •Increased the expression levels of aortic ACE2 and SIRT1 •Reduced the phosphorylation of AKT1 and ERK1/2 as well as the expression levels of Ang II type-1 receptor, MMP2 and MMP9 |
[98] Moran CS et al. Arterioscler Thromb Vasc Biol 37: 2195-2203, 2017. |
| ApoE -/- mice | Ang II infusion and high fat diet | Dipeptidyl peptidase-4 inhibitor (teneligliptin) |
•Suppressed aneurysmal aortic expansion, reduced aneurysm severity, and lowered aneurysm incidence •Attenuated medial elastin degradation and macrophage infiltration •Reduced MMP activity, the phosphorylation of AKT and ERK and IL-6 mRNA levels in aneurysmal aorta |
[97] Takahara Y et al. J Atherioscler Thromb 25: 698-708, 2018. |
| Wild type C57BL/6 mice | Ang II infusion and high fat diet | NLRP3 inhibitor (MCC950) |
•Reduced aortic diameter, aneurysm/ dissection rate and macrophage accumulation •Reduced aortic expression of active IL-1β and caspase 1 as well as MMP activity |
[84] Ren P et al. J am Heart Assoc 9: e014044, 2020. |
AAA: Abdominal aortic aneurysm. ACE2: Angiotensin-converting enzyme 2. AKT1: Rac-alpha serine/threonine-protein kinase or protein kinase B. Ang II: Angiotensin II. ApoE: Apolipoprotein E. ERK: Extracellular-signal-regulated kinase. IL: Interleukin. MMPs: Matrix metalloproteinases. MCP: Monocyte chemotactic protein. ROS: Reactive oxygen species. SIRT1: Silent mating type information regulation 2 homolog 1. TNF: Tumor necrosis factor. -/-: Deficient mice
All published studies were to evaluate the preventive effect of pharmacological NLRP3 inhibitor on AAAs by administering inhibitors prior to AAA induction. It has not been investigated whether NLRP3 inhibition is effective in limiting the progression of existing small experimental AAAs, which is more clinically relevant. In addition, most studies have also been performed in Ang II-induced AAAs in hyperlipidemic animals, which is primarily initiated and driven by aortic dissection. Thus, it warrants more validating studies in non-dissection AAA models such as intra-aortic elastase infusion model or alternatives.
Conclusions
In summary, clinical and experimental evidence supports the critical importance of NLRP3 inflammasome in AAA pathogenesis. Thus, pharmacologically targeting NLRP3 inflammasome activity may provide a novel translational application for clinically treating small AAA disease.
Conflicts of Interest
All authors declare no conflicts of interest to this work.
Financial Support
This work was in part supported by grants from the National Natural Science Foundation of China (grant number 81600256), the Natural Science Foundation of Shanxi Province (grant number 2014021038-2), the Postdoctoral Research Startup Fund of Shanxi Medical University First Hospital.
Abbreviations
AAA: Abdominal aortic aneurysm
Ang: Angiotensin
ASC: Apoptosis-associated speck-like protein containing a caspase recruitment domain
IL: Interleukin
NF-kB: Nuclear factor kappa B
NLRP: Nucleotide-binding oligomerization domain-like receptor family protein
ROS: Reactive oxygen species
COVID: Coronavirus disease
References
- 1).Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, Krupski WC, Barone GW, Acher CW, Ballard DJ: Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med, 1997; 126: 441-449 [DOI] [PubMed] [Google Scholar]
- 2).Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, Gelijns AC, Greco G: Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg, 2010; 52: 539-548 [DOI] [PubMed] [Google Scholar]
- 3).Carter JL, Morris DR, Sherliker P, Clack R, Lam KBH, Halliday A, Clarke R, Lewington S, Bulbulia R: Sex-Specific Associations of Vascular Risk Factors With Abdominal Aortic Aneurysm: Findings From 1.5 Million Women and 0.8 Million Men in the United States and United Kingdom. J Am Heart Assoc, 2020; 9: e014748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Li X, Zhao G, Zhang J, Duan Z, Xin S: Prevalence and trends of the abdominal aortic aneurysms epidemic in general population--a meta-analysis. PLoS One, 2013; 8: e81260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Chan WK, Yong E, Hong Q, Zhang L, Lingam P, Tan GWL, Chandrasekar S, Lo ZJ: Systematic review and meta-analysis of the prevalence of abdominal aortic aneurysm in Asian populations. J Vasc Surg, 2020; 26: S0741-5214(20)32120-0, DOI.10.1016/j.jvs.2020.08.140 [DOI] [PubMed] [Google Scholar]
- 6).Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, Oderich GS, Patel MS, Schermerhorn ML, Starnes BW: The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg, 2018; 67: 2-77.e72 [DOI] [PubMed] [Google Scholar]
- 7).Liu Z, Fitzgerald M, Meisinger T, Batra R, Suh M, Greene H, Penrice AJ, Sun L, Baxter BT, Xiong W: CD95-ligand contributes to abdominal aortic aneurysm progression by modulating inflammation. Cardiovasc Res, 2019; 115: 807-818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).López-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW: Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol, 1997; 150: 993-1007 [PMC free article] [PubMed] [Google Scholar]
- 9).Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW: Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest, 2000; 105: 1641-1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT: Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest, 2002; 110: 625-632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Allaire E, Forough R, Clowes M, Starcher B, Clowes AW: Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J Clin Invest, 1998; 102: 1413-1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Iida Y, Xu B, Schultz GM, Chow V, White JJ, Sulaimon S, Hezi-Yamit A, Peterson SR, Dalman RL: Efficacy and mechanism of angiotensin II receptor blocker treatment in experimental abdominal aortic aneurysms. PLoS One, 2012; 7: e49642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Xuan H, Xu B, Wang W, Tanaka H, Fujimura N, Miyata M, Michie SA, Dalman RL: Inhibition or deletion of angiotensin II type 1 receptor suppresses elastase-induced experimental abdominal aortic aneurysms. J Vasc Surg, 2018; 67: 573-584.e572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Xu B, Xuan H, Iida Y, Miyata M, Dalman RL: Pathogenic and Therapeutic Significance of Angiotensin II Type I Receptor in Abdominal Aortic Aneurysms. Curr Drug Targets, 2018; 19: 1318-1326 [DOI] [PubMed] [Google Scholar]
- 15).Thatcher SE, Zhang X, Howatt DA, Yiannikouris F, Gurley SB, Ennis T, Curci JA, Daugherty A, Cassis LA: Angiotensin-converting enzyme 2 decreases formation and severity of angiotensin II-induced abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol, 2014; 34: 2617-2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Cassis LA, Rateri DL, Lu H, Daugherty A: Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol, 2007; 27: 380-386 [DOI] [PubMed] [Google Scholar]
- 17).Emeto TI, Moxon JV, Au M, Golledge J: Oxidative stress and abdominal aortic aneurysm: potential treatment targets. Clin Sci (Lond), 2016; 130: 301-315 [DOI] [PubMed] [Google Scholar]
- 18).Yang P, Schmit BM, Fu C, DeSart K, Oh SP, Berceli SA, Jiang Z: Smooth muscle cell-specific Tgfbr1 deficiency promotes aortic aneurysm formation by stimulating multiple signaling events. Sci Rep, 2016; 6: 35444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadière C, Rénia L, Johnson JL, Tharaux PL, Tedgui A, Mallat Z: TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest, 2010; 120: 422-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Chen PY, Qin L, Li G, Malagon-Lopez J, Wang Z, Bergaya S, Gujja S, Caulk AW, Murtada SI, Zhang X, Zhuang ZW, Rao DA, Wang G, Tobiasova Z, Jiang B, Montgomery RR, Sun L, Sun H, Fisher EA, Gulcher JR, Fernandez-Hernando C, Humphrey JD, Tellides G, Chittenden TW, Simons M: Smooth Muscle Cell Reprogramming in Aortic Aneurysms. Cell Stem Cell, 2020; 26: 542-557.e511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Zhang J, Chen H, Liu L, Sun J, Shi MA, Sukhova GK, Shi GP: Chemokine (C-C motif) receptor 2 mediates mast cell migration to abdominal aortic aneurysm lesions in mice. Cardiovasc Res, 2012; 96: 543-551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Ishibashi M, Egashira K, Zhao Q, Hiasa K, Ohtani K, Ihara Y, Charo IF, Kura S, Tsuzuki T, Takeshita A, Sunagawa K: Bone marrow-derived monocyte chemoattractant protein-1 receptor CCR2 is critical in angiotensin II-induced acceleration of atherosclerosis and aneurysm formation in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol, 2004; 24: e174-178 [DOI] [PubMed] [Google Scholar]
- 23).Iida Y, Xu B, Xuan H, Glover KJ, Tanaka H, Hu X, Fujimura N, Wang W, Schultz JR, Turner CR, Dalman RL: Peptide inhibitor of CXCL4-CCL5 heterodimer formation, MKEY, inhibits experimental aortic aneurysm initiation and progression. Arterioscler Thromb Vasc Biol, 2013; 33: 718-726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Wang W, Xu B, Xuan H, Ge Y, Wang Y, Wang L, Huang J, Fu W, Michie SA, Dalman RL: Hypoxia-inducible factor 1 in clinical and experimental aortic aneurysm disease. J Vasc Surg, 2018; 68: 1538-1550.e1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Choke E, Cockerill GW, Dawson J, Howe F, Wilson WR, Loftus IM, Thompson MM: Vascular endothelial growth factor enhances angiotensin II-induced aneurysm formation in apolipoprotein E-deficient mice. J Vasc Surg, 2010; 52: 159-166.e151 [DOI] [PubMed] [Google Scholar]
- 26).Rouer M, Xu BH, Xuan HJ, Tanaka H, Fujimura N, Glover KJ, Furusho Y, Gerritsen M, Dalman RL: Rapamycin limits the growth of established experimental abdominal aortic aneurysms. Eur J Vasc Endovasc Surg, 2014; 47: 493-500 [DOI] [PubMed] [Google Scholar]
- 27).Kaneko H, Anzai T, Takahashi T, Kohno T, Shimoda M, Sasaki A, Shimizu H, Nagai T, Maekawa Y, Yoshimura K, Aoki H, Yoshikawa T, Okada Y, Yozu R, Ogawa S, Fukuda K: Role of vascular endothelial growth factor-A in development of abdominal aortic aneurysm. Cardiovasc Res, 2011; 91: 358-367 [DOI] [PubMed] [Google Scholar]
- 28).Xu B, Iida Y, Glover KJ, Ge Y, Wang Y, Xuan H, Hu X, Tanaka H, Wang W, Fujimura N, Miyata M, Shoji T, Guo J, Zheng X, Gerritsen M, Kuo C, Michie SA, Dalman RL: Inhibition of VEGF (Vascular Endothelial Growth Factor)-A or its Receptor Activity Suppresses Experimental Aneurysm Progression in the Aortic Elastase Infusion Model. Arterioscler Thromb Vasc Biol, 2019; 39: 1652-1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Tedesco MM, Terashima M, Blankenberg FG, Levashova Z, Spin JM, Backer MV, Backer JM, Sho M, Sho E, McConnell MV, Dalman RL: Analysis of in situ and ex vivo vascular endothelial growth factor receptor expression during experimental aortic aneurysm progression. Arterioscler Thromb Vasc Biol, 2009; 29: 1452-1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Sho E, Sho M, Nanjo H, Kawamura K, Masuda H, Dalman RL: Hemodynamic regulation of CD34+ cell localization and differentiation in experimental aneurysms. Arterioscler Thromb Vasc Biol, 2004; 24: 1916-1921 [DOI] [PubMed] [Google Scholar]
- 31).Hoshina K, Sho E, Sho M, Nakahashi TK, Dalman RL: Wall shear stress and strain modulate experimental aneurysm cellularity. J Vasc Surg, 2003; 37: 1067-1074 [DOI] [PubMed] [Google Scholar]
- 32).Sho E, Sho M, Hoshina K, Kimura H, Nakahashi TK, Dalman RL: Hemodynamic forces regulate mural macrophage infiltration in experimental aortic aneurysms. Exp Mol Pathol, 2004; 76: 108-116 [DOI] [PubMed] [Google Scholar]
- 33).Schultz G, Tedesco MM, Sho E, Nishimura T, Sharif S, Du X, Myles T, Morser J, Dalman RL, Leung LL: Enhanced abdominal aortic aneurysm formation in thrombin-activatable procarboxypeptidase B-deficient mice. Arterioscler Thromb Vasc Biol, 2010; 30: 1363-1370 [DOI] [PubMed] [Google Scholar]
- 34).Swanson KV, Deng M, Ting JP: The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol, 2019; 19: 477-489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Weber ANR, Bittner ZA, Shankar S, Liu X, Chang TH, Jin T, Tapia-Abellán A: Recent insights into the regulatory networks of NLRP3 inflammasome activation. J Cell Sci, 2020; 133: jcs248344.DOI.10.1242/jcs.248444 [DOI] [PubMed] [Google Scholar]
- 36).Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA: Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ Res, 2020; 126: 1260-1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Schett G, Dayer JM, Manger B: Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol, 2016; 12: 14-24 [DOI] [PubMed] [Google Scholar]
- 38).Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA: A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med, 2015; 21: 248-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Karasawa T, Takahashi M: Role of NLRP3 Inflammasomes in Atherosclerosis. J Atheroscler Thromb, 2017; 24: 443-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J: Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell death and differentiation, 2007; 14: 1583-1589 [DOI] [PubMed] [Google Scholar]
- 41).Compan V, Baroja-Mazo A, López-Castejón G, Gomez AI, Martínez CM, Angosto D, Montero MT, Herranz AS, Bazán E, Reimers D, Mulero V, Pelegrín P: Cell Volume Regulation Modulates NLRP3 Inflammasome Activation. Immunity, 2012; 37: 487-500 [DOI] [PubMed] [Google Scholar]
- 42).Annett H, Veit H, C PG, R SC, G MB, Thomas R, A FK, Eicke L, J MK, T GD: The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol, 2008; 9: 857-865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Chu J, Thomas LM, Watkins SC, Franchi L, Núñez G, Salter RD: Cholesterol-dependent cytolysins induce rapid release of mature IL-1beta from murine macrophages in a NLRP3 inflammasome and cathepsin B-dependent manner. J Leukoc Biol, 2009; 86: 1227-1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Zhong Z, Zhai Y, Liang S, Mori Y, Han R, Sutterwala FS, Qiao L: TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat Commun, 2013; 4: 1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T: Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A, 2012; 109: 11282-11287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E: Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov, 2018; 17: 588-606 [DOI] [PubMed] [Google Scholar]
- 47).Zahid A, Li B, Kombe AJK, Jin T, Tao J: Pharmacological Inhibitors of the NLRP3 Inflammasome. Front Immunol, 2019; 10: 2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Jiang H, Gong T, Zhou R: The strategies of targeting the NLRP3 inflammasome to treat inflammatory diseases. Adv Immunol, 2020; 145: 55-93 [DOI] [PubMed] [Google Scholar]
- 49).Bertinaria M, Gastaldi S, Marini E, Giorgis M: Development of covalent NLRP3 inflammasome inhibitors: Chemistry and biological activity. Arch Biochem Biophys, 2019; 670: 116-139 [DOI] [PubMed] [Google Scholar]
- 50).Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, Meng R, Quong AA, Latz E, Scott CP, Alnemri ES: Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem, 2010; 285: 9792-9802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Fulp J, He L, Toldo S, Jiang Y, Boice A, Guo C, Li X, Rolfe A, Sun D, Abbate A, Wang XY, Zhang S: Structural Insights of Benzenesulfonamide Analogues as NLRP3 Inflammasome Inhibitors: Design, Synthesis, and Biological Characterization. J Med Chem, 2018; 61: 5412-5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD: The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med, 2015; 21: 263-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Goldberg EL, Asher JL, Molony RD, Shaw AC, Zeiss CJ, Wang C, Morozova-Roche LA, Herzog RI, Iwasaki A, Dixit VD: β-Hydroxybutyrate Deactivates Neutrophil NLRP3 Inflammasome to Relieve Gout Flares. Cell Rep, 2017; 18: 2077-2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Coll RC, Hill JR, Day CJ, Zamoshnikova A, Boucher D, Massey NL, Chitty JL, Fraser JA, Jennings MP, Robertson AAB, Schroder K: MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol, 2019; 15: 556-559 [DOI] [PubMed] [Google Scholar]
- 55).Coll RC, Hill JR, Day CJ, Zamoshnikova A, Boucher D, Massey NL, Chitty JL, Fraser JA, Jennings MP, Robertson AAB, Schroder K: MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol, 2019; 15: 556-559 [DOI] [PubMed] [Google Scholar]
- 56).Jiang H, He H, Chen Y, Huang W, Cheng J, Ye J, Wang A, Tao J, Wang C, Liu Q, Jin T, Jiang W, Deng X, Zhou R: Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med, 2017; 214: 3219-3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Marchetti C, Swartzwelter B, Gamboni F, Neff CP, Richter K, Azam T, Carta S, Tengesdal I, Nemkov T, D’Alessandro A, Henry C, Jones GS, Goodrich SA, St Laurent JP, Jones TM, Scribner CL, Barrow RB, Altman RD, Skouras DB, Gattorno M, Grau V, Janciauskiene S, Rubartelli A, Joosten LAB, Dinarello CA: OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc Natl Acad Sci U S A, 2018; 115: E1530-e1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).He H, Jiang H, Chen Y, Ye J, Wang A, Wang C, Liu Q, Liang G, Deng X, Jiang W, Zhou R: Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun, 2018; 9: 2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM: Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol, 2009; 187: 61-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Misawa T, Saitoh T, Kozaki T, Park S, Takahama M, Akira S: Resveratrol inhibits the acetylated α-tubulin-mediated assembly of the NLRP3-inflammasome. Int Immunol, 2015; 27: 425-434 [DOI] [PubMed] [Google Scholar]
- 61).Klück V, Jansen T, Janssen M, Comarniceanu A, Efdé M, Tengesdal IW, Schraa K, Cleophas MCP, Scribner CL, Skouras DB, Marchetti C, Dinarello CA, Joosten LAB: Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol, 2020; 2: e270-e280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjälä H, Airaksinen J, Leinonen M, Saikku P, Juvonen T: Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol, 1997; 17: 2843-2847 [DOI] [PubMed] [Google Scholar]
- 63).Roberts RL, Van Rij AM, Phillips LV, Young S, McCormick SP, Merriman TR, Jones GT: Interaction of the inflammasome genes CARD8 and NLRP3 in abdominal aortic aneurysms. Atherosclerosis, 2011; 218: 123-126 [DOI] [PubMed] [Google Scholar]
- 64).Marculescu R, Sodeck G, Domanovits H, Hobusch G, Exner M, Heinzl H, Huber K, Mannhalter C, Minar E, Wagner O, Schillinger M: Interleukin-1 gene cluster variants and abdominal aortic aneurysms. Thromb Haemost, 2005; 94: 646-650 [PubMed] [Google Scholar]
- 65).Johnston WF, Salmon M, Su G, Lu G, Stone ML, Zhao Y, Owens GK, Upchurch GR, Ailawadi G: Genetic and pharmacologic disruption of interleukin-1β signaling inhibits experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol, 2013; 33: 294-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Newman KM, Jean-Claude J, Li H, Ramey WG, Tilson MD: Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation, 1994; 90: Ii224-227 [PubMed] [Google Scholar]
- 67).Shteinberg D, Halak M, Shapiro S, Kinarty A, Sobol E, Lahat N, Karmeli R: Abdominal aortic aneurysm and aortic occlusive disease: a comparison of risk factors and inflammatory response. Eur J Vasc Endovasc Surg, 2000; 20: 462-465 [DOI] [PubMed] [Google Scholar]
- 68).Wu X, Cakmak S, Wortmann M, Hakimi M, Zhang J, Böckler D, Dihlmann S: Sex- and disease-specific inflammasome signatures in circulating blood leukocytes of patients with abdominal aortic aneurysm. Mol Med, 2016; 22: 505-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Meher AK, Spinosa M, Davis JP, Pope N, Laubach VE, Su G, Serbulea V, Leitinger N, Ailawadi G, Upchurch GR, Jr.: Novel Role of IL (Interleukin)-1β in Neutrophil Extracellular Trap Formation and Abdominal Aortic Aneurysms. Arterioscler Thromb Vasc Biol, 2018; 38: 843-853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70).Franklin IJ, Walton LJ, Greenhalgh RM, Powell JT: The influence of indomethacin on the metabolism and cytokine secretion of human aneurysmal aorta. Eur J Vasc Endovasc Surg, 1999; 18: 35-42 [DOI] [PubMed] [Google Scholar]
- 71).Pearce WH, Sweis I, Yao JS, McCarthy WJ, Koch AE: Interleukin-1 beta and tumor necrosis factor-alpha release in normal and diseased human infrarenal aortas. J Vasc Surg, 1992; 16: 784-789 [PubMed] [Google Scholar]
- 72).Schönbeck U, Sukhova GK, Gerdes N, Libby P: T(H)2 predominant immune responses prevail in human abdominal aortic aneurysm. Am J Pathol, 2002; 161: 499-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Liu CL, Ren J, Wang Y, Zhang X, Sukhova GK, Liao M, Santos M, Luo S, Yang D, Xia M, Inouye K, Hotamisligil GS, Lu G, Upchurch GR, Libby P, Guo J, Zhang J, Shi GP: Adipocytes promote interleukin-18 binding to its receptors during abdominal aortic aneurysm formation in mice. Eur Heart J, 2020; 41: 2456-2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74).Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, Yoshimura K, Aoki H, Tsutsui H, Noda T, Sagara J, Taniguchi S, Takahashi M: Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm. Arterioscler Thromb Vasc Biol, 2015; 35: 127-136 [DOI] [PubMed] [Google Scholar]
- 75).Batra R, Suh MK, Carson JS, Dale MA, Meisinger TM, Fitzgerald M, Opperman PJ, Luo J, Pipinos, II, Xiong W, Baxter BT: IL-1β (Interleukin-1β) and TNF-α (Tumor Necrosis Factor-α) Impact Abdominal Aortic Aneurysm Formation by Differential Effects on Macrophage Polarization. Arterioscler Thromb Vasc Biol, 2018; 38: 457-463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Van Vickle-Chavez SJ, Tung WS, Absi TS, Ennis TL, Mao D, Cobb JP, Thompson RW: Temporal changes in mouse aortic wall gene expression during the development of elastase-induced abdominal aortic aneurysms. J Vasc Surg, 2006; 43: 1010-1020 [DOI] [PubMed] [Google Scholar]
- 77).Wakita D, Kurashima Y, Crother TR, Noval Rivas M, Lee Y, Chen S, Fury W, Bai Y, Wagner S, Li D, Lehman T, Fishbein MC, Hoffman HM, Shah PK, Shimada K, Arditi M: Role of Interleukin-1 Signaling in a Mouse Model of Kawasaki Disease-Associated Abdominal Aortic Aneurysm. Arterioscler Thromb Vasc Biol, 2016; 36: 886-897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Sun W, Pang Y, Liu Z, Sun L, Liu B, Xu M, Dong Y, Feng J, Jiang C, Kong W, Wang X: Macrophage inflammasome mediates hyperhomocysteinemia-aggravated abdominal aortic aneurysm. J Mol Cell Cardiol, 2015; 81: 96-106 [DOI] [PubMed] [Google Scholar]
- 79).Suehiro C, Suzuki J, Hamaguchi M, Takahashi K, Nagao T, Sakaue T, Uetani T, Aono J, Ikeda S, Okura T, Okamura H, Yamaguchi O: Deletion of interleukin-18 attenuates abdominal aortic aneurysm formation. Atherosclerosis, 2019; 289: 14-20 [DOI] [PubMed] [Google Scholar]
- 80).Wortmann M, Xiao X, Wabnitz G, Samstag Y, Hakimi M, Böckler D, Dihlmann S: AIM2 levels and DNA-triggered inflammasome response are increased in peripheral leukocytes of patients with abdominal aortic aneurysm. Inflamm Res, 2019; 68: 337-345 [DOI] [PubMed] [Google Scholar]
- 81).Carmen G-H, Joaquin DH, Silvia B, Cristina C, Ignacio M, Francisco A: Differential mRNA expression of inflammasome genes NLRP1 and NLRP3 in abdominal aneurysmal and occlusive aortic disease. The Adv Cardiovasc Dis, 2018; 12: 123-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Erhart P, Cakmak S, Grond-Ginsbach C, Hakimi M, Böckler D, Dihlmann S: Inflammasome activity in leucocytes decreases with abdominal aortic aneurysm progression. Int J Mol Med, 2019; 44: 1299-1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Wortmann M, Skorubskaya E, Peters AS, Hakimi M, Böckler D, Dihlmann S: Necrotic cell debris induces a NF-κB-driven inflammasome response in vascular smooth muscle cells derived from abdominal aortic aneurysms (AAA-SMC). Biochem Biophys Res Commun, 2019; 511: 343-349 [DOI] [PubMed] [Google Scholar]
- 84).Ren P, Wu D, Appel R, Zhang L, Zhang C, Luo W, Robertson AAB, Cooper MA, Coselli JS, Milewicz DM, Shen YH, LeMaire SA: Targeting the NLRP3 Inflammasome With Inhibitor MCC950 Prevents Aortic Aneurysms and Dissections in Mice. J Am Heart Assoc, 2020; 9: e014044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85).Isoda K, Akita K, Kitamura K, Sato-Okabayashi Y, Kadoguchi T, Isobe S, Ohtomo F, Sano M, Shimada K, Iwakura Y, Daida H: Inhibition of interleukin-1 suppresses angiotensin II-induced aortic inflammation and aneurysm formation. Int J Cardiol, 2018; 270: 221-227 [DOI] [PubMed] [Google Scholar]
- 86).Keen RR, Nolan KD, Cipollone M, Scott E, Shively VP, Yao JS, Pearce WH: Interleukin-1 beta induces differential gene expression in aortic smooth muscle cells. J Vasc Surg, 1994; 20: 774-784 [DOI] [PubMed] [Google Scholar]
- 87).Wu D, Ren P, Zheng Y, Zhang L, Xu G, Xie W, Lloyd EE, Zhang S, Zhang Q, Curci JA, Coselli JS, Milewicz DM, Shen YH, LeMaire SA: NLRP3 (Nucleotide Oligomerization Domain-Like Receptor Family, Pyrin Domain Containing 3)-Caspase-1 Inflammasome Degrades Contractile Proteins: Implications for Aortic Biomechanical Dysfunction and Aneurysm and Dissection Formation. Arterioscler Thromb Vasc Biol, 2017; 37: 694-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).Yamanouchi D, Morgan S, Kato K, Lengfeld J, Zhang F, Liu B: Effects of caspase inhibitor on angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol, 2010; 30: 702-707 [DOI] [PubMed] [Google Scholar]
- 89).Tsuruda T, Kato J, Hatakeyama K, Kojima K, Yano M, Yano Y, Nakamura K, Nakamura-Uchiyama F, Matsushima Y, Imamura T, Onitsuka T, Asada Y, Nawa Y, Eto T, Kitamura K: Adventitial mast cells contribute to pathogenesis in the progression of abdominal aortic aneurysm. Circ Res, 2008; 102: 1368-1377 [DOI] [PubMed] [Google Scholar]
- 90).Fujimura N, Xiong J, Kettler EB, Xuan H, Glover KJ, Mell MW, Xu B, Dalman RL: Metformin treatment status and abdominal aortic aneurysm disease progression. J Vasc Surg, 2016; 64: 46-54.e48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91).Hsu CY, Su YW, Chen YT, Tsai SH, Chang CC, Li SY, Huang PH, Chen JW, Lin SJ: Association between use of oral-antidiabetic drugs and the risk of aortic aneurysm: a nested case-control analysis. Cardiovasc Diabetol, 2016; 15: 125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Itoga NK, Rothenberg KA, Suarez P, Ho TV, Mell MW, Xu B, Curtin CM, Dalman RL: Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg, 2019; 69: 710-716.e713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93).Yu J, Morimoto K, Bao W, Yu Z, Okita Y, Okada K: Glucagon-like peptide-1 prevented abdominal aortic aneurysm development in rats. Surg Today, 2016; 46: 1099-1107 [DOI] [PubMed] [Google Scholar]
- 94).Lu HY, Huang CY, Shih CM, Chang WH, Tsai CS, Lin FY, Shih CC: Dipeptidyl peptidase-4 inhibitor decreases abdominal aortic aneurysm formation through GLP-1-dependent monocytic activity in mice. PLoS One, 2015; 10: e0121077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95).Lu HY, Huang CY, Shih CM, Lin YW, Tsai CS, Lin FY, Shih CC: A potential contribution of dipeptidyl peptidase-4 by the mediation of monocyte differentiation in the development and progression of abdominal aortic aneurysms. J Vasc Surg, 2017; 66: 1217-1226.e1211 [DOI] [PubMed] [Google Scholar]
- 96).Kohashi K, Hiromura M, Mori Y, Terasaki M, Watanabe T, Kushima H, Shinmura K, Tomoyasu M, Nagashima M, Hirano T: A Dipeptidyl Peptidase-4 Inhibitor but not Incretins Suppresses Abdominal Aortic Aneurysms in Angiotensin II-Infused Apolipoprotein E-Null Mice. J Atheroscler Thromb, 2016; 23: 441-454 [DOI] [PubMed] [Google Scholar]
- 97).Takahara Y, Tokunou T, Ichiki T: Suppression of Abdominal Aortic Aneurysm Formation in Mice by Teneligliptin, a Dipeptidyl Peptidase-4 Inhibitor. J Atheroscler Thromb, 2018; 25: 698-708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98).Moran CS, Biros E, Krishna SM, Wang Y, Tikellis C, Morton SK, Moxon JV, Cooper ME, Norman PE, Burrell LM, Thomas MC, Golledge J: Resveratrol Inhibits Growth of Experimental Abdominal Aortic Aneurysm Associated With Upregulation of Angiotensin-Converting Enzyme 2. Arterioscler Thromb Vasc Biol, 2017; 37: 2195-2203 [DOI] [PubMed] [Google Scholar]
- 99).Kaneko H, Anzai T, Morisawa M, Kohno T, Nagai T, Anzai A, Takahashi T, Shimoda M, Sasaki A, Maekawa Y, Yoshimura K, Aoki H, Tsubota K, Yoshikawa T, Okada Y, Ogawa S, Fukuda K: Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis, 2011; 217: 350-367 [DOI] [PubMed] [Google Scholar]
- 100).Palmieri D, Pane B, Barisione C, Spinella G, Garibaldi S, Ghigliotti G, Brunelli C, Fulcheri E, Palombo D: Resveratrol Counteracts Systemic and Local Inflammation Involved in Early Abdominal Aortic Aneurysm Development. J Surg Res, 2011; 171: e237-e246 [DOI] [PubMed] [Google Scholar]