Abstract
Aim: We aimed to examine the association between the maximum intima-media thickness of the carotid artery (Max IMT) and renal prognosis, considering their potential interaction with age.
Methods: Survival analyses were performed in 112 patients with chronic kidney disease (CKD), to assess renal prognosis, with the endpoint defined as a ≥ 30% decline in estimated glomerular filtration rate (eGFR) or end-stage renal disease.
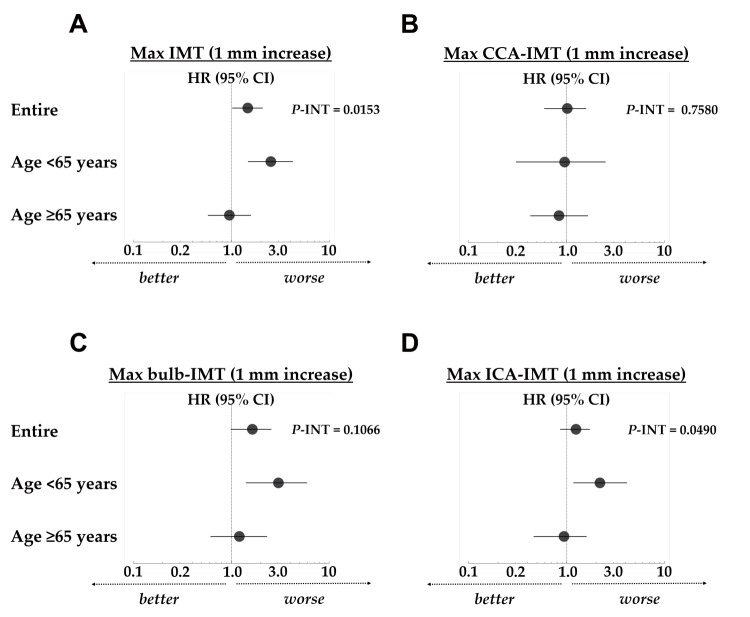
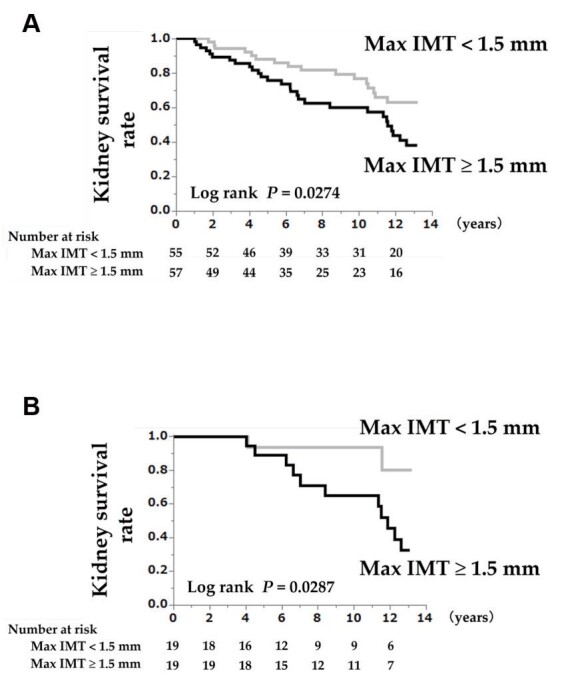
Results: During a median follow-up of 12.5 years, 44 participants reached the study endpoint. The major determinant of Max IMT was the maximum IMT of the internal carotid artery (Max ICA-IMT), which was the distribution ratio of 50.0% of Max IMT. Kaplan–Meier analyses showed that Max IMT ≥ 1.5 mm was significantly associated with renal prognosis when age and eGFR were matched. On multivariate Cox regression analysis, Max IMT was significantly associated with the renal outcomes and had a significant interaction with the age categories (≥ 65 years or <65 years) ( P =0.0153 for interaction). A 1-mm increase in Max IMT was significantly associated with disease progression in the sub-cohort <65 years age-category, but not in the ≥ 65 years age-category; similarly the hazard ratio (HR) in the <65 years age-category was higher than in the ≥ 65 years age-category (HR: 2.52 vs. 0.95). Comparable results were obtained for Max ICA-IMT, Max bulb-IMT, but not for Max common carotid artery-IMT.
Conclusions: A higher Max IMT was a significant renal prognosis factor in patients with CKD aged <65 years. Our results may provide new insights into treating CKD.
Keywords: Maximum carotid intima-media thickness, Chronic kidney disease, Prognosis, Interactions, Elderly
See editorial vol. 28: 471-473
Introduction
Carotid intima-media thickness (IMT), provided by innocuous and repeatable B-mode ultrasound, has associations with cardiovascular diseases (CVDs) 1 - 4) , and measuring carotid IMT is thought to be useful to refine risk assessment for patients at intermediate CVD risk 5 , 6) . In the Framingham Offspring Study 1) , a maximum IMT of the internal carotid artery (Max ICA-IMT) >1.5 mm significantly added predictive value to the Framingham risk score. In a Japanese study of 1,358 men aged 60 to 74 years 2) , those with Max IMT ≥ 1.5 mm had a 3-fold higher risk of stroke than those with Max IMT <1.5 mm. Furthermore, among patients with coronary artery disease with a maximum IMT of the common carotid artery (Max CCA-IMT) ≥ 1.1 mm, progression of the Max CCA-IMT was associated with future coronary events 4) . Recently, Polak et al. 6) made the assertion that ICA-IMT and CCA-IMT represent different phenotypes, because of the different characteristics of their association with risk factors 7 , 8) .
On the other hand, in the field of nephrology, atherosclerotic vascular damage is now considered a common pathway for cardiac failure and renal dysfunction 9 - 11) . It has been reported that an increased carotid IMT is associated with albuminuria progression 12) and incident chronic kidney disease (CKD) 13) . However, until recently, longitudinal studies evaluating carotid IMT as a predictor of kidney function decline in patients with CKD were lacking, and whether the carotid IMT is useful for predicting kidney disease progression remained unclarified 14) . More recently, a few studies reported that an increased carotid IMT is associated with CKD progression in diabetic patients 15 , 16) and end-stage renal disease (ESRD) in the general population 17) . However, these previous studies included patients without CKD; no study has exclusively focused on patients with CKD. Furthermore, in these former studies, renal function was lower in the group with increased carotid IMT than in other groups. Considering the fact that age 16 , 17) and decreased renal function 16 - 18) , which are also risk factors of CKD progression, were associated with increased carotid IMT, a study that eliminates the effects of age and renal function is desired.
This study aimed firstly to examine the association between Max IMT and renal outcomes in patients with CKD, with consideration of a potential interaction with age, and secondly, to confirm the association between renal prognosis and Max IMT ≥ 1.5 mm using a cohort matched for age, sex, glycemic control status, and estimated glomerular filtration rate (eGFR).
Patients and Methods
Study Population
We screened 2012 outpatients with CKD who visited the Kidney Center at Tokyo Women’s Medical University Hospital in Japan between August 2006 and August 2007. Among these, 113 patients underwent B-mode ultrasound. After excluding one patient with nephrotic syndrome, 112 patients were ultimately enrolled and prospectively evaluated in the present study (Supplementary Fig.1) . CKD was diagnosed according to previously described criteria 19) . The subjects’ human rights and methods for protecting their personal information were considered in detail. All the relevant and responsible staff adhered to the principles of the Helsinki Declaration (amended October 2013) and the Ethical Guidelines for Clinical Studies (revised February 28, 2017; referred to hereafter as the Clinical Studies Ethical Guidelines) in the execution of this study. This cohort study was approved by the Medical Ethics Committee of Tokyo Women’s Medical University (#4599). All participants gave their written informed consent at the time of entry.
Supplementary Fig. 1. Patient selection flow chart .
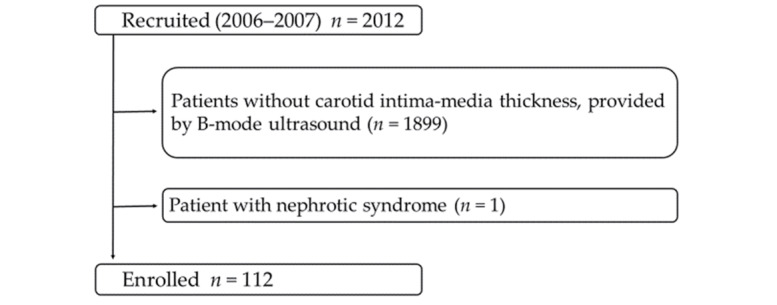
The patient selection flowchart is shown. Among 2012 screened patients, 1899 patients without B-mode ultrasound data, and 1 patient with nephrotic syndrome were excluded from the study. The remaining 112 patients were enrolled.
Covariate Assessments
Anthropometric and physical examinations for baseline characteristics, including blood pressure components, body height, body weight, body mass index, and Max IMTs, were conducted during a regular outpatient clinic visit. Blood pressure was measured in triplicate using a mercury sphygmomanometer, and the average value was used in analysis. Max IMTs were measured using B-mode ultrasound. All biochemical analyses were performed on samples obtained after overnight fasting. Serum creatinine levels were enzymatically measured. The eGFR was calculated using a previously described formula for Japanese patients 20) . Concomitant drug use (antihypertensive drugs, diuretics, and medications for hyperuricemia, dyslipidemia, and diabetes mellitus) and comorbidities at the time of entry were assessed 21) . Definitions of comorbidities and primary causes of CKD are presented in the Supplementary Methods. Patients were followed up until December 31, 2019.
Carotid Ultrasonography
All carotid ultrasound examinations were performed by experienced and centrally-trained vascular sonographer specialists blinded to patient data using ultrasonographic B-mode imaging of the carotid artery via a high-resolution real-time ultrasonograph with a 12-MHz in-line Sectascanner (SSA-390A; Aloka, Tokyo, Japan). Carotid ultrasonic examinations were based on previous reports 5 , 22) . Briefly, for this examination, after the patients rested in the supine position for at least 10 min, their neck was placed in a slightly hyperextended position and optimal bilateral visualization of the carotid arteries was performed. The scan was performed with image acquisition at the near and far walls of the right and left CCAs, carotid bulbs, and ICAs. Max IMT was defined as the maximum measurable IMT in the entire scanned CCAs, carotid bulbs, and ICAs. We also evaluated the component of Max IMT: Max CCA-IMT, Max bulb-IMT, and Max ICA-IMT. All patients received carotid ultrasonography at the baseline visit or before and after the baseline visit.
Study Endpoint
The endpoint of the study was kidney disease progression, which was defined as a ≥ 30% decline in eGFR from baseline (≥ 30% eGFR decline) or the development of ESRD requiring dialysis.
Statistical Analysis
Continuous variables are expressed as means and standard deviations (SDs) or as medians (quartile 1, quartile 3). Categorical variables are expressed as percentages unless otherwise stated. Group differences were evaluated using the unpaired t -test, Mann-Whitney U test, Chi-square test, or Fisher’s exact test, as appropriate. Univariate and multivariate linear regression analyses were performed to determine the factors associated with the baseline Max IMT level. Odds ratios were estimated by using logistic regression analysis. Univariate and multivariate Cox proportional hazards analyses were performed to determine the variables associated with renal outcomes. Variables with P <0.1 on the univariate analysis, as well as age, sex, eGFR, and interaction terms (all variables * age ≥ 65 years), were included in the primary multivariate analyses. Subgroup analyses were performed by dividing patients into two pre-specified subgroups, namely a high Max IMT group (Max IMT ≥ 1.5 mm) and a low Max IMT group (Max IMT <1.5 mm). Survival curves were plotted using the Kaplan–Meier method and evaluated using the log-rank test. To reduce confounding biases, in patients with stage 3 CKD, we fitted propensity score-matched models that included potentially modifying variables, namely, age, sex, hemoglobin A1c, and eGFR. The caliper-matching method was used, with a maximum tolerance level of 0.2. P -values of <0.05 were considered statistically significant. All statistical analyses were performed using JMP Pro software, Windows v15.0.0 (SAS Institute, North Carolina, USA).
Results
Patient Characteristics
Table 1 presents the baseline characteristics according to the Max IMT category. The 112 participants comprised 55 patients with Max IMT <1.5 mm and 57 patients with Max IMT ≥ 1.5 mm, with a mean age at baseline of 63.0±10.3 years (range: 24–83 years). Distribution ratio of Max-IMT were CCA/ bulb /ICA=16.1%/ 33.9%/ 50.0%, and the major determinant of Max-IMT was the Max ICA-IMT. The median follow-up duration was 12.5 years (interquartile range: 4.1–12.9 years), and 44 patients showed disease progression (i.e., a ≥ 30% eGFR decline or the development of ESRD) during the follow-up period. Comparative analyses revealed that the patients with Max IMT ≥ 1.5 mm had an older age, a higher percentage of men, higher levels of triglycerides, and higher Max IMTs, and lower levels of serum albumin and high-density lipoprotein (HDL) cholesterol than the patients with Max IMT <1.5 mm. Regarding concomitant drugs and comorbidity rates, anti-dyslipidemic agent use, hypertriglyceridemia, and low HDL cholesterol were more frequent in patients with Max IMT ≥ 1.5 mm than in patients with Max IMT <1.5 mm (Table 1) . Supplementary Table 1 provides the results of the propensity score-matched models based on age, sex, hemoglobin A1c, and eGFR. There were no significant differences between the propensity score-matched groups in any of the parameters except anti-hyperuricemic agent use (Max IMT ≥ 1.5 mm vs. Max IMT <1.5 mm: 26.3% vs. 63.2%, P =0.0489), and Max IMTs (Supplementary Table 1) . Supplementary Table 2 provides the baseline characteristics according to age category (age ≥ 65 years or <65 years).
Table 1. Patient characteristics according to the levels of the maximum intima–media thickness of the carotid artery (Entire cohort: n = 112) .
| Variables | Entire Cohort | Max IMT <1.5 mm | Max IMT ≥ 1.5 mm | P -Value | SD |
|---|---|---|---|---|---|
| n = 112 | n = 55 | n = 57 | |||
| Clinical Findings | |||||
| Age (years) | 63.0±10.3 [112] | 58.7±9.9 | 67.1±9.1 | <0.0001 | 0.883 |
| Gender (Men; %) | 66 (58.9) [112] | 24 (43.6) | 42 (73.7) | 0.0012 | 0.642 |
| SBP (mmHg) | 126.2±6.7 [112] | 126.2±6.7 | 126.2±6.7 | 0.9805 | 0.000 |
| DBP (mmHg) | 77.4±5.0 [112] | 77.6±4.8 | 77.2±5.3 | 0.6953 | 0.079 |
| MBP (mmHg) | 93.6±5.4 [112] | 93.8±5.3 | 93.5±5.4 | 0.7977 | 0.056 |
| BMI (kg/m 2 ) | 24.5±3.4 [112] | 24.3±3.3 | 24.6±3.6 | 0.6845 | 0.087 |
| Max CCA-IMT (mm) | 0.9 (0.8–1.3) [112] | 0.8 (0.7–0.9) | 1.2 (0.8–1.7) | <0.0001 | 0.966 |
| Max bulb-IMT (mm) | 1.2 (0.9–1.7) [88] | 0.9 (0.8–1.2) | 1.7 (1.3–2.3) | <0.0001 | 1.490 |
| Max ICA-IMT (mm) | 1.6 (1.2–2.4) [82] | 1.1 (0.9–1.2) | 2.0 (1.6–3.3) | <0.0001 | 1.665 |
| Max IMT (mm) | 1.5 (1.2–2.2) [112] | 1.2 (0.9–1.2) | 2.1 (1.8–3.2) | <0.0001 | 2.069 |
| Distribution ratio of Max-IMT: CCA/ bulb /ICA | 18 (16.1)/ 38 (33.9)/ 56 (50.0) [112] | 11 (20.0)/ 24 (43.6)/ 20 (36.4) | 7 (12.3)/ 14 (24.6)/ 36 (63.2) | 0.0178 | NA |
| Laboratory Findings | |||||
| Serum Albumin (g/dL) | 4.22±0.28 [112] | 4.29±0.27 | 4.16±0.28 | 0.0216 | 0.473 |
| Hemoglobin (g/dL) | 13.5±1.7 [112] | 13.7±1.7 | 13.3±1.8 | 0.2575 | 0.228 |
| Serum Creatinine (mg/dL) | 1.18±0.82 [112] | 1.15±0.92 | 1.20±0.72 | 0.7359 | 0.061 |
| eGFR (mL/min/1.73 m 2 ) | 56.0±20.6 [112] | 59.0±22.1 | 53.1±18.9 | 0.1327 | 0.287 |
| CKD stage 1/ 2/ 3a/3b/4/5 (%) |
5 (4.5)/ 40 (35.7)/ 37 (33.0)/ 16 (14.3)/ 10 (8.9)/ 4 (3.6) [112] |
2 (3.6)/ 25 (45.5)/ 16 (29.1)/ 4 (7.3)/ 5 (9.1)/ 3 (5.5) |
3 (5.3)/ 15 (26.3)/ 21 (36.8)/ 12 (21.1)/ 5 (8.8)/ 1 (1.8) |
0.1383 | NA |
| Uric Acid (mg/dL) | 5.82±1.42 [112] | 5.58±1.65 | 6.05±1.13 | 0.0751 | 0.332 |
| Triglyceride (mg/dL) | 145.6±71.9 [112] | 127.9±62.9 | 162.7±76.4 | 0.0098 | 0.497 |
| Total Cholesterol (mg/dL) | 202.4±37.8 [112] | 208.7±38.0 | 196.4±36.8 | 0.0838 | 0.329 |
| LDL Cholesterol (mg/dL) | 118.0±34.5 [112] | 124.3±35.5 | 112.0±32.7 | 0.0585 | 0.360 |
| HDL Cholesterol (mg/dL) | 55.4±15.6 [112] | 58.8±14.6 | 52.1±15.9 | 0.0221 | 0.439 |
| Glucose (mg/dL) | 104.5±22.5 [111] | 102.2±19.2 | 106.8±25.3 | 0.2893 | 0.205 |
| Hemoglobin A1c (NGSP) (%) | 6.03±0.83 [91] | 5.89±0.63 | 6.17±0.97 | 0.1078 | 0.342 |
| Hs-CRP (ng/mL) | 473.0 (263.8–735.8) [110] | 468.0 (199.0–702.0) | 552.0 (281.5–950.5) | 0.1965 | 0.408 |
| UACR (mg/g Cre) | 59.2 (19.3–191.35) [112] | 42.1 (18.2–130.6) | 82.0 (22.8–459.7) | 0.0551 | 0.449 |
| Primary cause of CKD | |||||
| Diabetic nephropathy (%) | 11 (9.8) [112] | 3 (5.5) | 8 (14.0) | 0.2036 | 0.290 |
| Chronic glomerulonephritis (%) | 49 (43.8) [112] | 26 (47.3) | 23 (40.4) | 0.4604 | 0.139 |
| Nephrosclerosis (%) | 31 (27.7) [112] | 14 (25.5) | 17 (29.8) | 0.6053 | 0.096 |
| Others (%) | 21 (18.8) [112] | 12 (21.8) | 9 (15.8) | 0.4138 | 0.154 |
| Concomitant drugs | |||||
| Antihypertensive agents (%) | 78 (69.6) [112] | 36 (65.5) | 42 (73.7) | 0.3437 | 0.179 |
| ARB and or ACEI | 59 (52.7) [112] | 26 (47.3) | 33 (57.9) | 0.2603 | 0.213 |
| CCB | 34 (30.4) [112] | 16 (29.1) | 18 (31.6) | 0.7747 | 0.054 |
| Antidyslipidemic agents ( n (%)) | 48 (42.9) [112] | 16 (29.1) | 32 (56.1) | 0.0038 | 0.568 |
| Antihyperuricemic agents (%) | 45 (40.2) [112] | 24 (43.6) | 21 (36.8) | 0.4634 | 0.139 |
| Antidiabetic agents (%) | 12 (10.7) [112] | 3 (5.5) | 9 (15.8) | 0.1247 | 0.339 |
| Corticosteroids (%) | 8 (7.1) [112] | 4 (7.3) | 4 (7.0) | 1.0000 | 0.012 |
| Immunosuppressants (%) | 6 (5.4) [112] | 3 (5.5) | 3 (5.3) | 1.0000 | 0.009 |
| Diuretics (%) | 25 (22.3) [112] | 16 (29.1) | 9 (15.8) | 0.0910 | 0.323 |
| Comorbidities | |||||
| Hypertension (%) | 77 (68.8) [112] | 36 (65.5) | 41 (71.9) | 0.4598 | 0.138 |
| Hyperuricemia (%) | 55 (49.1) [112] | 29 (52.7) | 26 (45.6) | 0.4516 | 0.142 |
| Hypertriglyceridemia (%) | 67 (59.8) [112] | 26 (47.3) | 41 (71.9) | 0.0078 | 0.518 |
| Hypercholesterolemia (%) | 76 (67.9) [112] | 35 (63.6) | 41 (71.9) | 0.3475 | 0.178 |
| Low HDL cholesterol (%) | 52 (46.4) [112] | 16 (29.1) | 36 (63.2) | 0.0003 | 0.728 |
| Hyperglycemia (%) | 37 (33.0) [112] | 15 (27.3) | 22 (38.6) | 0.2027 | 0.242 |
| Diabetes mellitus (%) | 21 (18.8) [112] | 7 (12.7) | 14 (24.6) | 0.1087 | 0.309 |
* Continuous variables are expressed as means and standard deviations. Categorical variables are expressed as n (%). Values of nonmissing data are shown in [ ]. Abbreviations: Max IMT, maximum measurable intima-media thickness in the entire scanned common carotid arteries, carotid bulbs, and internal carotid arteries; n , number; P , calculated probability; SD, standardized differences; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; BMI, body mass index; Max CCA-IMT, maximum intima–media thickness of the common carotid artery; Max bulb-IMT, maximum intima–media thickness of the carotid bulb; Max ICA-IMT, maximum intima–media thickness of the internal carotid artery; NA, not applicable; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; LDL, low-density lipoprotein; HDL, high- density lipoprotein; Hs-CRP, high sensitivity C-reactive protein; UACR, urine albumin-to-creatinine ratio; ARB, angiotensin Ⅱ receptor blocker; ACEI, angiotensin converting enzyme inhibitor; CCB, calcium-channel blocker.
Supplementary Table 1. Patient characteristics according to the levels of the maximum intima–media thickness of the carotid artery (Propensity score matched cohort: n = 38) .
| Variables | Entire Cohort | Max IMT <1.5 mm | Max IMT ≥ 1.5 mm | P -Value | SD |
|---|---|---|---|---|---|
| n = 38 | n = 19 | n = 19 | |||
| Clinical Findings | |||||
| Age (years) | 62.2±9.2 [38] | 61.8±10.4 | 62.5±8.2 | 0.8098 | 0.075 |
| Gender (Men; %) | 28 (73.7) [38] | 14 (73.7) | 14 (73.7) | 1.0000 | 0.000 |
| SBP (mmHg) | 126.0±7.1 [38] | 125.6±6.9 | 126.3±7.4 | 0.7821 | 0.098 |
| DBP (mmHg) | 77.6±5.4 [38] | 77.6±4.3 | 77.5±6.5 | 0.9454 | 0.018 |
| MBP (mmHg) | 93.7±5.8 [38] | 93.6±5.1 | 93.8±6.5 | 0.9438 | 0.034 |
| BMI (kg/m 2 ) | 25.1±3.6 [38] | 25.0±3.3 | 25.3±3.9 | 0.8210 | 0.083 |
| Max CCA-IMT (mm) | 0.9 (0.7–1.3) [38] | 0.8 (0.7–1.1) | 0.9 (0.8–1.8) | 0.0378 | 0.819 |
| Max bulb-IMT (mm) | 1.2 (0.9–1.9) [30] | 1.0 (0.9–1.3) | 1.8 (1.1–2.4) | 0.0028 | 1.176 |
| Max ICA-IMT (mm) | 1.8 (1.2–2.5) [25] | 1.2 (1.1–1.3) | 2.1 (1.7–3.4) | 0.0024 | 1.415 |
| Max IMT (mm) | 1.5 (1.2–2.4) [38] | 1.2 (0.9–1.3) | 2.4 (1.9–3.3) | <0.0001 | 2.026 |
| Distribution ratio of Max-IMT: CCA/ bulb /ICA | 6 (15.8)/ 13 (34.2)/ 19 (50.0) | 4 (21.1)/ 9 (47.4)/ 6 (31.6) | 2 (10.5)/ 4 (21.1)/ 13 (68.4) | 0.0754 | NA |
| Laboratory Findings | |||||
| Serum Albumin (g/dL) | 4.23±0.30 [38] | 4.17±0.33 | 4.29±0.26 | 0.2174 | 0.404 |
| Hemoglobin (g/dL) | 14.1±1.5 [38] | 14.1±1.7 | 14.2±1.3 | 0.9573 | 0.066 |
| Serum Creatinine (mg/dL) | 0.97±0.24 [38] | 0.97±0.25 | 0.97±0.24 | 0.9372 | 0.000 |
| eGFR (mL/min/1.73 m 2 ) | 61.0±16.7 [38] | 61.3±16.5 | 60.7±17.3 | 0.9133 | 0.035 |
| CKD stage 1/ 2/ 3a/3b/4/5 (%) |
2 (5.3)/ 15 (39.5)/ 16 (42.1)/ 5 (13.2)/ 0 (0.0)/ 0 (0.0) |
1 (5.3)/ 7 (36.8)/ 9 (47.4)/ 2 (10.5)/ 0 (0.0)/ 0 (0.0) |
1 (5.3)/ 8 (42.1)/ 7 (36.8)/ 3 (15.8)/ 0 (0.0)/ 0 (0.0) |
0.9152 | NA |
| Uric Acid (mg/dL) | 6.17±1.28 [38] | 6.22±1.48 | 6.11±1.08 | 0.7935 | 0.085 |
| Triglyceride (mg/dL) | 141.3±64.0 [38] | 125.1±55.2 | 157.5±69.4 | 0.1203 | 0.517 |
| Total Cholesterol (mg/dL) | 207.3±33.9 [38] | 202.6±31.7 | 211.9±36.2 | 0.4069 | 0.273 |
| LDL Cholesterol (mg/dL) | 123.8±33.8 [38] | 120.6±35.0 | 127.0±33.2 | 0.5631 | 0.188 |
| HDL Cholesterol (mg/dL) | 55.6±12.9 [38] | 57.1±12.5 | 54.2±13.5 | 0.4979 | 0.223 |
| Glucose (mg/dL) | 108.6±27.7 [38] | 112.1±24.6 | 105.0±30.7 | 0.4366 | 0.255 |
| Hemoglobin A1c (NGSP) (%) | 6.05±0.69 [38] | 6.11±0.78 | 5.99±0.59 | 0.6265 | 0.174 |
| Hs-CRP (ng/mL) | 412.0 (246.0–680.5) [37] | 473.0 (301.3–714.0) | 402.0 (219.0–620.0) | 0.5740 | 0.166 |
| UACR (mg/g Cre) | 40.3 (18.0–148.3) [38] | 42.1 (18.6–150.4) | 34.8 (17.5–147.0) | 0.8040 | 0.253 |
| Primary cause of CKD | |||||
| Diabetic nephropathy (%) | 2 (5.3) [38] | 1 (5.3) | 1 (5.3) | 1.0000 | 0.000 |
| Chronic glomerulonephritis (%) | 18 (47.4) [38] | 7 (36.8) | 11 (57.9) | 0.3300 | 0.432 |
| Nephrosclerosis (%) | 12 (31.6) [38] | 8 (42.1) | 4 (21.1) | 0.2953 | 0.464 |
| Others (%) | 6 (15.8) [38] | 3 (15.8) | 3 (15.8) | 1.0000 | 0.000 |
| Concomitant drugs | |||||
| Antihypertensive agents (%) | 26 (68.4) [38] | 12 (63.2) | 14 (73.7) | 0.7281 | 0.227 |
| ARB and or ACEI | 18 (47.4) [38] | 8 (42.1) | 10 (52.6) | 0.7459 | 0.211 |
| CCB | 9 (23.7) [38] | 4 (21.1) | 5 (26.3) | 1.0000 | 0.123 |
| Antidyslipidemic agents ( n (%)) | 16 (42.1) [38] | 7 (36.8) | 9 (47.4) | 0.7431 | 0.216 |
| Antihyperuricemic agents (%) | 17 (44.7) [38] | 12 (63.2) | 5 (26.3) | 0.0489 | 0.799 |
| Antidiabetic agents (%) | 5 (13.2) [38] | 2 (10.5) | 3 (15.8) | 1.0000 | 0.157 |
| Corticosteroids (%) | 5 (13.2) [38] | 3 (15.8) | 2 (10.5) | 1.0000 | 0.157 |
| Immunosuppressants (%) | 3 (7.9) [38] | 2 (10.5) | 1 (5.3) | 1.0000 | 0.194 |
| Diuretics (%) | 9 (23.7) [38] | 5 (26.3) | 4 (21.1) | 1.0000 | 0.123 |
| Comorbidities | |||||
| Hypertension (%) | 26 (68.4) [38] | 12 (63.2) | 14 (73.7) | 0.7281 | 0.227 |
| Hyperuricemia (%) | 22 (57.9) [38] | 14 (73.7) | 8 (42.1) | 0.0991 | 0.676 |
| Hypertriglyceridemia (%) | 22 (57.9) [38] | 9 (47.4) | 13 (68.4) | 0.3245 | 0.435 |
| Hypercholesterolemia (%) | 25 (65.8) [38] | 10 (52.6) | 15 (79.0) | 0.1704 | 0.579 |
| Low HDL cholesterol (%) | 18 (47.4) [38] | 7 (36.8) | 11 (57.9) | 0.3300 | 0.432 |
| Hyperglycemia (%) | 22 (57.9) [38] | 14 (73.7) | 8 (42.1) | 0.0991 | 0.676 |
| Diabetes mellitus (%) | 8 (21.1) [38] | 4 (21.1) | 4 (21.1) | 1.0000 | 0.000 |
* Continuous variables are expressed as means and standard deviations. Categorical variables are expressed as n (%). Values of nonmissing data are shown in [ ]. Abbreviations: Max IMT, maximum measurable intima-media thickness in the entire scanned common carotid arteries, carotid bulbs, and internal carotid arteries; n , number; P , calculated probability; SD, standardized differences; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; BMI, body mass index; Max CCA-IMT, maximum intima–media thickness of the common carotid artery; Max bulb-IMT, maximum intima–media thickness of the carotid bulb; Max ICA-IMT, maximum intima–media thickness of the internal carotid artery; NA, not applicable; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; LDL, low-density lipoprotein; HDL, high- density lipoprotein; Hs-CRP, high sensitivity C-reactive protein; UACR, urine albumin-to-creatinine ratio; ARB, angiotensin Ⅱ receptor blocker; ACEI, angiotensin converting enzyme inhibitor; CCB, calcium-channel blocker.
Supplementary Table 2. Patient characteristics of the entire cohort and of subcohorts stratified by age.
| Variables | Entire Cohort | Age <65 | Age ≥ 65 | P -Value |
|---|---|---|---|---|
| n = 112 | n = 64 | n = 48 | ||
| Clinical Findings | ||||
| Age (years) | 63.0±10.3 [112] | 55.6±6.4 | 72.9±4.7 | <0.0001 |
| Gender (Men; %) | 66 (58.9) [112] | 36 (56.3) | 30 (62.5) | 0.5058 |
| SBP (mmHg) | 126.2±6.7 [112] | 126.1±7.2 | 126.3±5.9 | 0.9105 |
| DBP (mmHg) | 77.4±5.0 [112] | 77.7±5.4 | 76.9±4.5 | 0.4244 |
| MBP (mmHg) | 93.6±5.4 [112] | 93.8±6.0 | 93.4±4.5 | 0.6492 |
| BMI (kg/m 2 ) | 24.5±3.4 [112] | 24.9±3.6 | 23.9±3.1 | 0.1076 |
| Max CCA-IMT (mm) | 0.9 (0.8–1.3) [112] | 0.9 (0.7–1.2) | 1.1 (0.8–1.4) | 0.0093 |
| Max bulb-IMT (mm) | 1.2 (0.9–1.7) [88] | 1.1 (0.9–1.6) | 1.4 (1.0–2.1) | 0.0242 |
| Max ICA-IMT (mm) | 1.6 (1.2–2.4) [82] | 1.3 (1.1–2.0) | 1.8 (1.3–2.9) | 0.0330 |
| Max IMT (mm) | 1.5 (1.2–2.2) [112] | 1.2 (0.9–2.0) | 1.8 (1.4–2.9) | 0.0007 |
| Distribution ratio of Max-IMT: CCA/ bulb /ICA | 18 (16.1)/ 38 (33.9)/ 56 (50.0) [112] | 12 (18.8)/ 23 (35.9)/ 29 (45.3) | 6 (12.5)/ 15 (31.3)/ 27 (56.3) | 0.4722 |
| Laboratory Findings | ||||
| Serum Albumin (g/dL) | 4.22±0.28 [112] | 4.32±0.26 | 4.10±0.27 | <0.0001 |
| Hemoglobin (g/dL) | 13.5±1.7 [112] | 14.1±1.6 | 12.6±1.6 | <0.0001 |
| Serum Creatinine (mg/dL) | 1.18±0.82 [112] | 1.07±0.60 | 1.32±1.03 | 0.1016 |
| eGFR (mL/min/1.73 m 2 ) | 56.0±20.6 [112] | 59.4±18.9 | 51.5±22.1 | 0.0429 |
| CKD stage 1/ 2/ 3a/3b/4/5 (%) |
5 (4.5)/ 40 (35.7)/ 37 (33.0)/ 16 (14.3)/ 10 (8.9)/ 4 (3.6) [112] |
3 (4.7)/ 28 (43.8)/ 20 (31.3)/ 7 (10.9)/ 5 (7.8)/ 1 (1.6) |
2 (4.2)/ 12 (25.0)/ 17 (35.4)/ 9 (18.8)/ 5 (10.4)/ 3 (6.3) |
0.3132 |
| Uric Acid (mg/dL) | 5.82±1.42 [112] | 5.77±1.50 | 5.89±1.33 | 0.6735 |
| Triglyceride (mg/dL) | 145.6±71.9 [112] | 147.7±79.4 | 142.9±61.2 | 0.7301 |
| Total Cholesterol (mg/dL) | 202.4±37.8 [112] | 209.0±36.9 | 193.7±37.5 | 0.0333 |
| LDL Cholesterol (mg/dL) | 118.0±34.5 [112] | 123.5±34.4 | 110.8±33.7 | 0.0529 |
| HDL Cholesterol (mg/dL) | 55.4±15.6 [112] | 6.2±13.8 | 54.4±17.8 | 0.5417 |
| Glucose (mg/dL) | 104.5±22.5 [111] | 105.1±24.8 | 103.7±19.3 | 0.7585 |
| Hemoglobin A1c (NGSP) (%) | 6.03±0.83 [91] | 6.01±0.74 | 6.06±0.96 | 0.7638 |
| Hs-CRP (ng/mL) | 473.0 (263.8–735.8) [110] | 385.5 (197.0–626.8) | 630.5 (398.5–933.8) | 0.0021 |
| UACR (mg/g Cre) | 59.2 (19.3–191.3) [112] | 56.6 (18.5–182.2) | 61.2 (21.6–239.9) | 0.5805 |
| Primary cause of CKD | ||||
| Diabetic nephropathy (%) | 11 (9.8) [112] | 5 (7.8) | 6 (12.5) | 0.5250 |
| Chronic glomerulonephritis (%) | 49 (43.8) [112] | 28 (43.8) | 21 (43.8) | 1.0000 |
| Nephrosclerosis (%) | 31 (27.7) [112] | 17 (26.6) | 14 (29.2) | 0.7605 |
| Others (%) | 21 (18.8) [112] | 14 (21.9) | 7 (14.6) | 0.4637 |
| Concomitant drugs | ||||
| Antihypertensive agents (%) | 78 (69.6) [112] | 44 (68.8) | 34 (70.8) | 0.8124 |
| ARB and or ACEI | 59 (52.7) [112] | 34 (53.1) | 25 (52.1) | 0.9130 |
| CCB | 34 (30.4) [112] | 17 (26.6) | 17 (35.4) | 0.3132 |
| Antidyslipidemic agents ( n (%)) | 48 (42.9) [112] | 24 (37.5) | 24 (50.0) | 0.1859 |
| Antihyperuricemic agents (%) | 45 (40.2) [112] | 28 (43.8) | 17 (35.4) | 0.3734 |
| Antidiabetic agents (%) | 12 (10.7) [112] | 6 (9.4) | 6 (12.5) | 0.7592 |
| Corticosteroids (%) | 8 (7.1) [112] | 4 (6.3) | 4 (8.3) | 0.7227 |
| Immunosuppressants (%) | 6 (5.4) [112] | 3 (4.7) | 3 (6.3) | 1.0000 |
| Diuretics (%) | 25 (22.3) [112] | 18 (28.1) | 7 (14.6) | 0.0885 |
| Comorbidities | ||||
| Hypertension (%) | 77 (68.8) [112] | 44 (68.8) | 33 (68.8) | 1.0000 |
| Hyperuricemia (%) | 55 (49.1) [112] | 33 (51.6) | 22 (45.8) | 0.5484 |
| Hypertriglyceridemia (%) | 67 (59.8) [112] | 36 (56.3) | 31 (64.6) | 0.3734 |
| Hypercholesterolemia (%) | 76 (67.9) [112] | 44 (68.8) | 32 (66.7) | 0.8153 |
| Low HDL cholesterol (%) | 53 (47.3) [112] | 27 (42.2) | 26 (54.2) | 0.2089 |
| Hyperglycemia (%) | 58 (51.8) [112] | 31 (48.4) | 27 (56.3) | 0.4129 |
| Diabetes mellitus (%) | 21 (18.8) [112] | 11 (17.2) | 10 (20.8) | 0.6339 |
* Continuous variables are expressed as means and standard deviations. Categorical variables are expressed as n (%). Values of nonmissing data are shown in [ ]. Abbreviations: Max IMT, maximum measurable intima-media thickness in the entire scanned common carotid arteries, carotid bulbs, and internal carotid arteries; n , number; P , calculated probability; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; BMI, body mass index; Max CCA-IMT, maximum intima–media thickness of the common carotid artery; Max bulb-IMT, maximum intima–media thickness of the carotid bulb; Max ICA-IMT, maximum intima–media thickness of the internal carotid artery; NA, not applicable; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Hs-CRP, high sensitivity C-reactive protein; UACR, urine albumin-to-creatinine ratio; ARB, angiotensin Ⅱ receptor blocker; ACEI, angiotensin converting enzyme inhibitor; CCB, calcium-channel blocker.
3.2. Correlations between the Maximum Carotid Intima-Media Thickness and other Parameters
Since Max IMT values may be affected by confounders, baseline Max IMT values were tested for any correlations with clinical and laboratory parameters at baseline (Supplementary Table 3) . On univariate analysis, Max IMT was significantly correlated with age (β=0.35; P =0.0002), male sex (β=0.36; P =0.0001), HDL cholesterol (β=−0.19; P =0.0483), and hs-CRP (β=0.36; P =0.0001). On multivariate linear regression analysis, Max IMT was correlated with age (β=0.32; P =0.0003), male sex (β=0.30; P =0.0013), and hs-CRP (β=0.23; P =0.0075).
Supplementary Table 3. Results of the univariate and the multivariate linear regression analyses for the factors associ- ated with baseline maximum carotid intima-media thickness among the entire cohort ( n = 122) .
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| β | P -Value | β | P -Value | |
| Age (years) | 0.35 | 0.0002 | 0.32 | 0.0003 |
| Men (vs Women) | 0.36 | 0.0001 | 0.30 | 0.0013 |
| MBP (mmHg) | 0.09 | 0.3229 | ||
| BMI (kg/m 2 ) | 0.01 | 0.8766 | ||
| eGFR (mL/min/1.73 m 2 ) | -0.07 | 0.4936 | 0.10 | 0.2721 |
| Uric acid (mg/dL) | 0.11 | 0.2626 | ||
| Triglyceride (mg/dL) | 0.10 | 0.2932 | ||
| HDL cholesterol (mg/dL) | -0.19 | 0.0483 | 0.09 | 0.2910 |
| Glucose (mg/dL) | 0.02 | 0.8455 | ||
| Hemoglobin A1c (NGSP) (%) | -0.01 | 0.8951 | ||
| Hs-CRP (ng/mL) | 0.36 | 0.0001 | 0.23 | 0.0075 |
| UACR (mg/g Cre) | 0.16 | 0.0824 | 0.06 | 0.5047 |
* Variables with a P -value <0.1 in the univariate model, as well as age, sex, and eGFR, were included in the multivariate model. Abbreviations: n , number; β, standardized partial regression coefficient; P , calculated probability; MBP, mean blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; Hs-CRP, high sensitivity C-reactive protein; UACR, urine albumin-to-creatinine ratio; Cre, creatinine.
Discussion
Recently, patient-centered medicine has been advocated 23 , 24) . For patient-centered medicine application, it is necessary to treat individual patients according to their heterogeneous characteristics 25) . The development of patient-centered medicine requires disaggregation of data and analysis of differences in subgroups 26 , 27) .
There are two main findings in the present study. Firstly, an association between Max IMT and renal outcomes was confirmed for the first time in a cohort exclusively consisting of patients with CKD, as well as in a cohort matched for age, sex, hemoglobin A1c, and eGFR. Secondly, Max IMT showed a significant interaction with age category (age ≥ 65 years) with regard to the renal prognosis. As a result, there were differences in the renal prognostic factors between the two age-based sub-cohorts (age ≥ 65 years and age <65 years). One of the reasons we were able to detect an association between Max IMT and renal outcomes in the present study may be the follow-up duration. Previous studies that reported an association between carotid IMT and renal function decline in diabetic patients (two studies) 15 , 16) , and the general population 17) , had median follow-up durations of 6.0 years, 10.8 years, and 22.7 years, respectively. The median follow-up period of 12.5 years in the present study was comparable to these previous studies. The independent association between Max IMT and CKD progression observed in long-term follow-up studies may be because of the stability of Max IMT as a renal risk factor over time.
The measurement of the arterial wall using B-mode ultrasound has improved the early identification of patients with CKD who are prone to developing atherosclerosis. However, until recently, whether carotid IMT is useful in predicting the progression of kidney disease was not clarified 14) . Generally, CKD is multifactorial 27) , and its progression is associated with various risk factors linked to atherosclerosis, such as hypertension 28) , diabetes mellitus, dyslipidemia 29) , obesity, and metabolic syndrome 30) . Interpreting the results of clinical studies can sometimes be challenging, as these factors complicatedly affect one another. Indeed, as we recently reported 27) , renal prognosis analyses are strongly influenced by the factors examined and the cohort analyzed. Therefore, the usefulness of IMT measurements for renal prognosis may change depending on the variable examined (ICA-IMT or CCA-IMT) and the cohort evaluated (young or elderly).
The results of the present study provide meaningful information, as the association between Max IMT and CKD progression remained significant after propensity matching for age, sex, hemoglobin A1c, and eGFR, suggesting an independent contribution of Max IMT to the progression of CKD. In contrast, Max CCA-IMT was not associated with renal prognosis. ICA-IMT and CCA-IMT are the two major IMT types commonly evaluated. Polak et al. 6) suggested that these two IMT types likely represent separate phenotypes, since their patterns of associations with risk factors are different 1 , 7 , 8 , 31) . Thus, further evaluation of the role of ICA-IMT is required. Previous research showed that the ICA-IMT has a qualitatively stronger association with low-density lipoprotein cholesterol 7) , as well as stronger associations with coronary heart disease events 1 , 8 , 31) , than does CCA-IMT. Nevertheless, attempts to determine the diagnostic significance of the IMT formerly focused on CCA-IMT and not on ICA-IMT 32) . Additionally, the American College of Cardiology/American Heart Association Work Group recommended against measuring carotid IMT as routine clinical practice for risk assessment in a first atherosclerotic CVD event, based on the evidence regarding CCA-IMT provided by Ruijter et al. 33 - 35) . However, there is still a possibility that ICA-IMT or the combination of CCA-IMT and ICA-IMT may add incremental value to traditional risk factors for predicting CVD 6) or CKD progression. Although the present study is observational in nature, we found an association between CKD progression and Max IMT which may imply a close relationship between CKD progression and coronary heart disease 36) .
Considering that increased IMT is associated with age 16 , 17) , studies that evaluate its interaction with age (young or elderly) are desired. In the present study, we found an interaction between Max IMT and age category (age ≥ 65 years), and the HR of Max IMT (1-mm increase) was higher in the sub-cohort with age <65 years than in the sub-cohort with age ≥ 65 years (HR: 2.52 vs. 0.95). In a previous study on CVD, O’Leary et al. cross-sectionally measured ICA-IMT in elderly patients (age ≥ 65 years) and found that ICA-IMT was associated with existing coronary heart disease 8) . O’Leary and colleagues also reported that increased ICA-IMT is directly associated with an increased risk of myocardial infarction and stroke in individuals age ≥ 65 years and without a history of CVD 31) . Generally, as reported in these studies, elderly people develop advanced arteriosclerosis and are more prone to cardiovascular events 37) .
On the other hand, although the results of studies on elderly status as a renal prognostic factor in patients with CKD are inconsistent 38) , the Chronic Renal Insufficiency Cohort Study in the United States reported that older age (age ≥ 65 years) is associated with a lower rate of ESRD (age >65 vs. <45 years: HR, 0.55) 39) . The finding that Max IMT significantly interacted with age category (age ≥ 65 years or <65 years) with regard to renal prognosis is consistent with previous reports on pathophysiological differences between elderly and younger adult patients with CKD 40 , 41) . Furthermore, the finding of a higher HR of Max IMT (1-mm increase) in terms of renal prognosis in patients aged <65 years than in patients aged ≥ 65 years is meaningful from a preventive perspective. Considering that elderly patients with CKD have complex pathophysiology, IMT as a screening tool may be suitable for younger patients with CKD.
This study has several limitations. First, although data of IMTs were obtained by experienced and centrally-trained vascular sonographer specialists, we did not assess the intra-observer and inter-observer variability. Therefore, the presence of some systematic measurement error cannot be ruled out. Second, the impact of subsequent Max IMT changes on outcomes could not be demonstrated because only baseline laboratory data were analyzed. Third, the serum creatinine level was based on a single assessment at baseline, which may have been influenced by existing comorbidities at the time of the assessment. Fourth, a potential selection bias was unavoidable because patients voluntarily enrolled in this study. Fifth, since all the participants were Japanese patients with CKD, the association between Max IMTs and renal outcomes may not be generalizable to other populations. Sixth, the study was observational in nature; thus, the observed associations do not prove causality, and residual confounding due to unmeasured or unknown factors cannot be ruled out. On the other hand, the strengths of the present study include its well-characterized population of Japanese patients with CKD who were treated by nephrologists at a single center using standard CKD care guidelines and the detailed analyses that were designed to disaggregate the data based on Max IMTs, which is significant for achieving patient-centered medicine 23 , 24) .
5. Conclusions
Max IMT ≥ 1.5 mm was significantly associated with renal prognosis in patients with CKD, confirmed by age- and eGFR-matched cohort analysis. Importantly, a higher Max IMT/Max bulb-IMT/Max ICA-IMT was a significant factor for renal prognosis in patients with CKD aged <65 years. Our results may provide new insights into treating CKD.
Declaration of Competing Interest
None.
Financial Support
None.
Supplementary Methods
Definitions of Comorbidities and Primary Causes of CKD
Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, or taking an antihypertensive agent. Hyperuricemia was defined as serum UA level ≥ 7.0 mg/dL or taking an antihyperuricemic agent. Hyperglycemia was defined as blood glucose ≥ 100 mg/dL. DM was defined as glycated hemoglobin level ≥ 6.5%, diagnosis of DM, or intake of an antidiabetic agent. Hypertriglyceridemia was defined as a serum TG level ≥ 150 mg/dL or intake of an oral lipid-lowering agent. Hypercholesterolemia was defined as serum total cholesterol level ≥ 220 mg/dL, serum LDL cholesterol level ≥ 140 mg/dL, or intake of an oral lipid-lowering agent. Low HDL cholesterol was defined as a serum HDL cholesterol level ≤ 40 mg/dL among men and ≤ 50 mg/dL among women. Diabetic kidney disease, chronic glomerulonephritis, and nephrosclerosis were diagnosed either from biopsies or clinically by the doctor in charge.
References
- 1).Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA and D’Agostino RB, Sr.: Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med, 2011; 365(3): 213-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Kitamura A, Iso H, Imano H, Ohira T, Okada T, Sato S, Kiyama M, Tanigawa T, Yamagishi K and Shimamoto T: Carotid intima-media thickness and plaque characteristics as a risk factor for stroke in Japanese elderly men. Stroke, 2004; 35(12): 2788-2794 [DOI] [PubMed] [Google Scholar]
- 3).Kokubo Y, Watanabe M, Higashiyama A, Nakao YM, Nakamura F and Miyamoto Y: Impact of Intima-Media Thickness Progression in the Common Carotid Arteries on the Risk of Incident Cardiovascular Disease in the Suita Study. J Am Heart Assoc, 2018; 7(11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Hirano M, Nakamura T, Kitta Y, Takishima I, Deyama J, Kobayashi T, Fujioka D, Saito Y, Watanabe K, Watanabe Y, Kawabata K, Obata JE and Kugiyama K: Short-term progression of maximum intima-media thickness of carotid plaque is associated with future coronary events in patients with coronary artery disease. Atherosclerosis, 2011; 215(2): 507-512 [DOI] [PubMed] [Google Scholar]
- 5).Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS and American Society of Echocardiography Carotid Intima-Media Thickness Task F: Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Heart Assoc, 2017; 6(1) [DOI] [PubMed] [Google Scholar]
- 6).Polak JF, Szklo M and O’Leary DH: Carotid Intima-Media Thickness Score, Positive Coronary Artery Calcium Score, and Incident Coronary Heart Disease: The Multi-Ethnic Study of Atherosclerosis. Journal of the American Heart Association, 2017; 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Polak JF, Person SD, Wei GS, Godreau A, Jacobs DR, Jr., Harrington A, Sidney S and O’Leary DH: Segment-specific associations of carotid intima-media thickness with cardiovascular risk factors: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Stroke, 2010; 41(1): 9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).O’Leary DH, Polak JF, Kronmal RA, Savage PJ, Borhani NO, Kittner SJ, Tracy R, Gardin JM, Price TR and Furberg CD: Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular Health Study Collaborative Research Group. Stroke, 1996; 27(2): 224-231 [DOI] [PubMed] [Google Scholar]
- 9).Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP and Kalantar-Zadeh K: Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol, 2013; 9(2): 99-111 [DOI] [PubMed] [Google Scholar]
- 10).Satirapoj B, Triwatana W and Supasyndh O: Arterial Stiffness Predicts Rapid Decline in Glomerular Filtration Rate Among Patients with High Cardiovascular Risks. J Atheroscler Thromb, 2020; 27(6): 611-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Sonoda H, Nakamura K and Tamakoshi A: Ankle-Brachial Index is a Predictor of Future Incident Chronic Kidney Disease in a General Japanese Population. J Atheroscler Thromb, 2019; 26(12): 1054-1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Yu Z, Schneck M, Jacobs DR, Jr., Liu K, Allison M, O’Leary D, Durazo R, Darwin C and Kramer H: Association of carotid intima-media thickness with progression of urine albumin-creatinine ratios in The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis, 2011; 57(1): 62-70 [DOI] [PubMed] [Google Scholar]
- 13).Shimizu M, Furusyo N, Mitsumoto F, Takayama K, Ura K, Hiramine S, Ikezaki H, Ihara T, Mukae H, Ogawa E, Toyoda K, Kainuma M, Murata M and Hayashi J: Subclinical carotid atherosclerosis and triglycerides predict the incidence of chronic kidney disease in the Japanese general population: results from the Kyushu and Okinawa Population Study (KOPS). Atherosclerosis, 2015; 238(2): 207-212 [DOI] [PubMed] [Google Scholar]
- 14).Tomiyama H and Yamashina A: Clinical Considerations for the Association between Vascular Damage and Chronic Kidney Disease. Pulse (Basel), 2014; 2(1-4): 81-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Takenouchi A, Tsuboi A, Kurata M, Fukuo K and Kazumi T: Carotid Intima-Media Thickness and Visit-to-Visit HbA1c Variability Predict Progression of Chronic Kidney Disease in Type 2 Diabetic Patients with Preserved Kidney Function. J Diabetes Res, 2016; 2016: 3295747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Cardoso CRL, Salles GC, Leite NC and Salles GF: Prognostic impact of carotid intima-media thickness and carotid plaques on the development of micro- and macrovascular complications in individuals with type 2 diabetes: the Rio de Janeiro type 2 diabetes cohort study. Cardiovasc Diabetol, 2019; 18(1): 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Pang Y, Sang Y, Ballew SH, Grams ME, Heiss G, Coresh J and Matsushita K: Carotid Intima-Media Thickness and Incident ESRD: The Atherosclerosis Risk in Communities (ARIC) Study. Clinical journal of the American Society of Nephrology: Clin J Am Soc Nephrol, 2016; 11(7): 1197-1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Desbien AM, Chonchol M, Gnahn H and Sander D: Kidney function and progression of carotid intima-media thickness in a community study. Am J Kidney Dis, 2008; 51(4): 584-593 [DOI] [PubMed] [Google Scholar]
- 19).Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL and Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. In: Kidney Int: United States; 2011, 17-28 [DOI] [PubMed] [Google Scholar]
- 20).Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H and Hishida A: Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis, 2009; 53(6): 982-992 [DOI] [PubMed] [Google Scholar]
- 21).Ording AG and Sorensen HT: Concepts of comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin Epidemiol, 2013; 5: 199-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Pignoli P, Tremoli E, Poli A, Oreste P and Paoletti R: Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation, 1986; 74(6): 1399-1406 [DOI] [PubMed] [Google Scholar]
- 23).Sacristan JA: Patient-centered medicine and patient-oriented research: improving health outcomes for individual patients. BMC Med Inform Decis Mak, 2013; 13: 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Bardes CL: Defining “patient-centered medicine”. N Engl J Med, 2012; 366(9): 782-783 [DOI] [PubMed] [Google Scholar]
- 25).Kravitz RL, Duan N and Braslow J: Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q, 2004; 82(4): 661-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Sacristan JA: Clinical research and medical care: towards effective and complete integration. BMC Med Res Methodol, 2015; 15: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Kataoka H, Ono K, Mochizuki T, Hanafusa N, Imai E, Hishida A and Nitta K: A Body Mass Index-Based Cross-Classification Approach for the Assessment of Prognostic Factors in Chronic. Kidney Blood Press Res, 2019; 44(3): 362-383 [DOI] [PubMed] [Google Scholar]
- 28).Kataoka H, Sawara Y, Kawachi K, Manabe S, Mochizuki T and Nitta K: Impacts of Sex Differences in Pulse Pressure among Patients with Chronic Kidney Disease. J Pers Med, 2019; 9(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Kawachi K, Kataoka H, Manabe S, Mochizuki T and Nitta K: Low HDL cholesterol as a predictor of chronic kidney disease progression: a cross-classification approach and matched cohort analysis. Heart Vessels, 2019; 34(9): 1440-1455 [DOI] [PubMed] [Google Scholar]
- 30).Sawara Y, Takei T, Uchida K, Tsuchiya K and Nitta K: Metabolic syndrome and anthropometric factors in Japanese patients with chronic kidney disease. Heart Vessels, 2009; 24(3): 199-203 [DOI] [PubMed] [Google Scholar]
- 31).O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL and Wolfson SK, Jr.: Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med, 1999; 340(1): 14-22 [DOI] [PubMed] [Google Scholar]
- 32).Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S and Reference Values for Arterial Measurements C: Reference intervals for common carotid intima-media thickness measured with echotracking: relation with risk factors. Eur Heart J, 2013; 34(30): 2368-2380 [DOI] [PubMed] [Google Scholar]
- 33).Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, Engstrom G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Koffijberg H, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O’Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Witteman JC, Moons KG and Bots ML: Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA, 2012; 308(8): 796-803 [DOI] [PubMed] [Google Scholar]
- 34).Goff DC, Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr., Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr., Sorlie P, Stone NJ and Wilson PWF: 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 2014; 63(25 Pt B): 2935-2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC, Jr., Virani SS, Williams KA, Sr., Yeboah J and Ziaeian B: 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol, 2019; 74(10): 1376-1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Sarnak MJ, Amann K, Bangalore S, Cavalcante JL, Charytan DM, Craig JC, Gill JS, Hlatky MA, Jardine AG, Landmesser U, Newby LK, Herzog CA, Cheung M, Wheeler DC, Winkelmayer WC, Marwick TH and Conference P: Chronic Kidney Disease and Coronary Artery Disease: JACC State-of-the-Art Review. J Am Coll Cardiol, 2019; 74(14): 1823-1838 [DOI] [PubMed] [Google Scholar]
- 37).Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, Karia K and Panguluri SK: Cardiovascular Risks Associated with Gender and Aging. J Cardiovasc Dev Dis, 2019; 6(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Tsai WC, Wu HY, Peng YS, Ko MJ, Wu MS, Hung KY, Wu KD, Chu TS and Chien KL: Risk Factors for Development and Progression of Chronic Kidney Disease: A Systematic Review and Exploratory Meta-Analysis. Medicine (Baltimore), 2016; 95(11): e3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Yang W, Xie D, Anderson AH, Joffe MM, Greene T, Teal V, Hsu CY, Fink JC, He J, Lash JP, Ojo A, Rahman M, Nessel L, Kusek JW and Feldman HI: Association of kidney disease outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis, 2014; 63(2): 236-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Uhlig K and Boyd C: Guidelines for the older adult with CKD. Am J Kidney Dis, 2011; 58(2): 162-165 [DOI] [PubMed] [Google Scholar]
- 41).O’Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM and Landefeld CS: Age affects outcomes in chronic kidney disease. Journal of the American Society of Nephrology: J Am Soc Nephrol, 2007; 18(10): 2758-2765 [DOI] [PubMed] [Google Scholar]