Abstract
Introduction
Preeclampsia is a pregnancy specific disorder which affects 2%-8% of all gestations and is associated with high maternal, fetal and neonatal morbidity and mortality worldwide. There is no “cure” for the disease except for early delivery of the fetus and placenta, however leaving preeclampsia a long term health risk both for mothers and infants.
Aim
The aim of the study is to review currently available information linking preclampsia to long-term cardiovascular complications in infants and children.
Results
Currently, there is evidence of predisposition to cardiovascular disease, and a higher incidence of cardiovascular risk factors among children born to preeclamptic mothers. Both in experimental models and human epidemiological studies it is now clear that the infants of pregnancies complicated by preeclampsia have an increased risk of developing high blood pressure and double the risk of stroke in later life. Preeclampsia is consistently associated with higher blood pressure and body mass index as early as 4–10 years of age. Also there is some evidence of higher cardiovascular risk in adults exposed to maternal hypertensive disorders of pregnancy. It seems that preeclampsia has an impact on the cardiovascular system independent of preterm birth and is associated with endothelial dysfunction, increased carotid intima media thickness and reductions in cardiac function that cannot be accounted for by prematurity alone.
Conclusion
Taking into consideration the currently available evidence, it can now be suggested that preeclampsia is linked to adverse effects on the cardiometabolic health of the infant. Understanding the relationship between preeclampsia and cardiovascular disease will allow for implementation of early interventions to prevent or delay the onset of adverse events in this high risk population.
Keywords: Preeclampsia, offspring, cardiovascular disease, blood pressure, prevention, cardiovascular risk factors
1. Introduction
Preeclampsia is a pregnancy specific hypertensive disorder which affects 2%-8% of all gestations and is associated with high maternal, fetal and neonatal morbidity and mortality worldwide [1, 2]. Its incidence and severity has increased over the last 20 years in the United States of America due to the epidemic of obesity [3].
Preeclampsia has traditionally been described as a syndrome characterized by hypertension, proteinuria and edema occurring after 20 to 24 weeks of gestation. In the last decade, the definition of preeclampsia has been revisited, as the identification of the mechanisms underlying the disease begun to clarify [4, 5]. Recent guidelines no longer require the presence of proteinuria to make the diagnosis of preeclampsia, if there is evidence of other maternal organ dysfunction. This reflects the variable clinical presentation of the disorder, which ranges from a mild asymptomatic condition identified on routine screening to fulminant disease with significant short-term consequences for both mother and neonate [6].
According to the Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy, current diagnostic criteria of preeclampsia include blood pressure ≥140 mmHg systolic or ≥90 mmHg diastolic on 2 occasions at least 4 hours apart after 20 weeks of pregnancy in a woman with a previously normal blood pressure and proteinuria ≥300 mg/24hours of urine collection or this amount extrapolated from a timed collection or protein/creatinine ratio≥0.3 or a dipstick reading of 1+ (used if other quantitative methods are not available). In the absence of proteinuria the diagnosis of preeclampsia is set in the presence of new onset hypertension with the above mentioned criteria and any of the following: platelet count <100,000 μL, serum creatinine concentrations >1.1 mg/dl or a doubling in creatinine concentration in the absence of other renal disease, elevation of blood concentrations of liver transaminases to twice normal concentration, pulmonary edema, cerebral or visual symptoms [7]. If blood pressure is ≥160 mmHg systolic or ≥110 mmHg diastolic the measurement should be confirmed within a short interval (minutes) to facilitate timely initiation of antihypertensive therapy [7].
2. Maternal data
There is growing evidence that these effects on end organs persist after pregnancy. Several studies have examined the relationship between preeclampsia and future cardiovascular disease in the mother. A recent meta-analysis showed that after adjusting for potential confounders (age, body mass index, and diabetes mellitus), preeclampsia is associated with an increased risk of heart failure, stroke, coronary heart disease, composite cardiovascular disease, and death because of coronary heart or cardiovascular disease. The increase in the risk for heart failure, stroke, and cardiovascular disease death is higher during the first 10 years after a pregnancy affected by preeclampsia compared with that beyond 10 years [8].
However, further research is required to determine whether women with preeclampsia have an adverse cardiovascular risk profile at baseline, which contributes to their increased risk of cardiovascular diseases in later life [8]. In 2011 the guidelines for the prevention of cardiovascular disease in women of the American Heart Association suggest that a previous history of preeclampsia or gestational diabetes is a major risk factor as part of its risk assessment system. The American Heart Association advises yearly follow-up of blood pressure, lipid profile, and blood glucose concentration for women who had hypertension in pregnancy [9]. The risk of preeclampsia and pregnancy-induced hypertension was not recognized in Europe until the 2016 European Guidelines on cardiovascular disease prevention in clinical practice, suggesting consideration of periodic screening for hypertension and diabetes mellitus (IIa class of recommendation, B level of evidence) [10].
The cause of preeclampsia remains unclear. Some women are genetically predisposed to developing the disease which may run into families. Associations have been reported between preeclampsia and gene variants involved in thrombophilia, inflammation, oxidative stress and the renin angiotensin system [1].
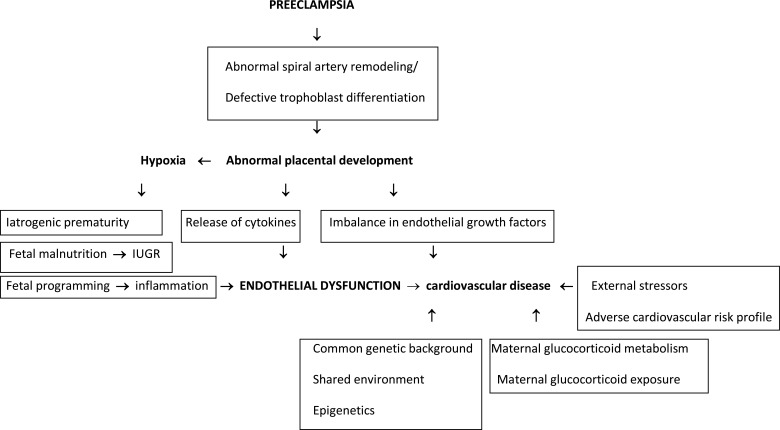
At present, the most accepted theory of the pathogenesis of preeclampsia is abnormal placental development as a result of abnormal spiral artery remodeling and defective trophoblast invasion/differentiation. This process subsequently leads to a hypoperfusion/hypoxemia/ischemia sequence that causes a release of cytokines and an imbalance in vascular endothelial growth factors that induce systemic endothelial dysfunction and the systemic effects of the disease [11] (Fig. 1).
Fig. (1).
Proposed pathogenetic mechanisms involved in cardiovascular disease in the offspring of mothers with preeclampsia.
Early-onset preeclampsia is defined as development before 34 weeks’ gestation, and affects up to 1% of pregnancies. Compared with late-onset disease, early onset disease is associated with increased risk of complications, especially early fetal growth restriction, intensive care, preterm delivery, and a 20-fold increased risk of maternal mortality [11].
3. Aim
There is no “cure” for the disease except for early delivery of the fetus and placenta, however leaving preeclampsia a long term health risk both for mothers and their infants. Regarding infants previous studies have mainly addressed the immediate adverse outcomes such the increased risk of fetal growth restriction, perinatal death and severe neonatal morbidity [12-14]. While there are studies on the long term neurological consequences of preeclampsia on the infants less data exists regarding the long term cardiovascular effects, even though there is evidence of predisposition to cardiovascular disease, and a higher incidence of cardiovascular risk factors among children [15, 16]. Not only does preeclampsia have a long-term effect on the cardiovascular system of the mother later in life, but it also has an effect on the cardiovascular system of the infants. Endothelial dysfunction is a common feature of pregnancies with preeclampsia, atherosclerosis, and cardiovascular disease and thus, endothelial dysfunction could serve as an underlying mechanism in the development of cardiovascular disease in preeclamptic women or their infants [17] (Fig. 1).
4. Methods
We performed a narrative review on all published literature in the English language on preeclampsia and offspring cardiovascular disease from January 2009 to January 2019. All publications were eligible for review, with particular emphasis on observational and interventional studies. The reviews and meta-analyses already published in the field were also assessed for their context and references. We have excluded case reports, letters to the editor, and editorials.
The search was conducted in the MEDLINE and EMBASE library. We used the following key words and combinations of these words for the search: neonat*, child*, preeclampsia, hypertension, cardiovascular, long-term, experiment*, offspring. The two authors independently screened all title/abstracts and hand-searched references of the retrieved articles and reviews for additional studies.
5. Results
Both in experimental models and human epidemiological studies it is now clear that the infants of pregnancies complicated by preeclampsia have an increased risk of developing high blood pressure and double the risk of stroke in later life [18, 19]. In a systematic review, Davis et al found that preeclampsia is consistently associated with higher blood pressure and body mass index as early as 4-10 years of age. Even gestational hypertension, in the absence of proteinuria, elevated liver enzymes, or thrombocytopenia, was associated with higher blood pressure in young children [20].
Less is known about the cardiovascular risk in adults affected by maternal preeclampsia or other maternal hypertensive disorders of pregnancy, although some studies suggest a higher risk of cardiovascular disease.
In a recent meta-analysis in adult subjects there was some evidence of higher cardiovascular risk in adults exposed to maternal hypertensive disorders of pregnancy. In particular, there was evidence of small but significant increases in blood pressure and higher risk of diagnosis of hypertension in adulthood in infants of pregnancies affected by hypertensive disorders. Likewise, the body mass index of the infants of pregnancies affected by a hypertensive disorder was slightly higher, and more subjects were classified as overweight and obese. The results for glucose metabolism and diabetes were mixed [18].
Pinheiro et al. reported in another meta-analysis contradictory outcomes of cardiovascular risk in children after intrauterine exposure to preeclampsia. Most studies report higher systolic blood pressure at the age of 9 to 17 years after exposure to preeclampsia. Other studies found only higher diastolic blood pressure in these children, whereas there are studies reporting increase of both systolic and diastolic blood pressure in children of preeclamptic mothers [21, 22]. Kajantie et al. were the only to consider the severity of preeclampsia and found an increased risk of hypertension in adults exposed to severe maternal disease [19].
Through a series of investigations based around preterm infants whose mothers did, or did not, have a hypertensive disorder of pregnancy, the group of Leeson has demonstrated that preeclampsia has an impact on the cardiovascular system independent of preterm birth. Specifically, as young adults, they exhibit endothelial dysfunction and increased carotid intima media thickness, a subclinical marker of atherosclerosis [19]. In addition, they have reductions in cardiac function that cannot be accounted for by prematurity alone, without additional cardiac structural changes [23]. Their hypothesis is that the associations between preeclampsia and later cardiovascular function relate to the abnormal placental development in preeclampsia and not to premature birth [17] (Fig. 1).
Also, children who were born to a preeclamptic mother demonstrated an approximately 30% higher pulmonary arterial pressure as compared with children born to normotensive mothers. Thus, preeclampsia appears to leave a permanent defect in the systemic and pulmonary circulation of the infants, which, when stressed, may lead to cardiovascular disease later in life [4, 24].
In an extensive relatively old meta-analysis that adjusted for maternal age, hypertension, hyperlipidemia, diabetes or impaired glucose tolerance, family history of cardiovascular disease, and smoking, McDonald et al. calculated the odds or relative risk for cardiac disease among the infants of women who had developed preeclampsia during pregnancy. In their analysis the authors found an odds ratio of 2.47 and a relative risk of 2.33 for the primary outcome of cardiac disease, which included ischemic heart disease, coronary artery disease, myocardial infarction, congestive heart failure, or death from any of the above [25].
A recent study that examined adults born by mothers with gestational hypertension, term and preterm preeclampsia showed that those whose mothers had hypertension in pregnancy had higher systolic blood pressure, higher diastolic blood pressure, higher body mass index, and wider waist circumference, compared with those of normotensive pregnancies. Similar differences were observed for gestational hypertension and term preeclampsia. Term preeclampsia was also associated with higher concentrations of non–high-density lipoprotein cholesterol and triglycerides. However, siblings born after a normotensive pregnancy had nearly identical risk factor levels as siblings born after maternal hypertension. The authors conclude that infants born after maternal hypertension in pregnancy have a more adverse cardiovascular risk profile in young adulthood than infants of normotensive pregnancies (Fig. 1). Their siblings, born after a normotensive pregnancy, have a similar risk profile, suggesting that shared genes or lifestyle may account for the association, rather than an intrauterine effect (Fig. 1). They suggest that all children of mothers who have experienced hypertension in pregnancy may be at increased lifetime risk of cardiovascular disease. However, this study enrolled mainly subjects born from hypertensive mothers or term preeclampsia and therefore the role of preterm preeclampsia which represents the more severe form of the disease could not be thoroughly investigated [26]. Indeed, a study by Lazdam et al showed that at the age 6 to 13 years infants of mothers with early onset preeclampsia had higher systolic blood pressure compared with those born to late-onset preeclampsia. Infants of mothers who developed early onset preeclampsia displayed specific adverse blood pressure characteristics later in life which are not evident in mothers and infants after late-onset preeclampsia or normotensive pregnancy [27].
6. Animal data
Animal studies using models mimicking preeclampsia indicate that exposure to hypoxia related to abnormal placental development results in elevated myocardial collagen [28]. This is consistent with findings in piglets, where short-term exposure to hypoxemia led to sustained reductions in longitudinal peak systolic strain [29]. This is potentially mediated through subendocardial and subepicardial fibres, which are susceptible to ischaemia.
7. Pathophysiology
Several mechanisms may explain the association between pregnancy-associated hypertension and infant blood pressure. The potential mechanisms likely represent a complex interplay of several mechanisms including fetal programming, genetics, and shared environment [30] (Fig. 1).
It has been hypothesized that shallow invasion of the spiral arteries leads to fetal malnutrition. Fetal programming due to hypoxia may also involve a response to inflammation and endothelial dysfunction associated with preeclampsia [31] (Fig. 1).
The associations between hypertensive disorders of pregnancy and subsequent cardiovascular disease in the infants are complex. Hypertensive disorders of pregnancy are associated with prematurity (which may be iatrogenic) and low birth weight even when corrected for gestation. Small for gestational age children have been associated with higher risk of cardiovascular disease [32] (Fig. 1). Physiological explanations that can be underlying the observed associations between preeclampsia and infants’ cardiovascular health may be attributed apart from abnormal placental implantation and inflammatory biomarkers during pregnancy on maternal glucocorticoid metabolism, exogenous glucocorticoid exposure and epigenetic aspects [21]. Furthermore, we cannot exclude a probable common genetic background and environmental exposures that could influence both mother and infant, or the possibility of residual confounders in the studies [21] (Fig. 1).
Also there may be familial aggregation of risk which stresses the complex interactions between the maternal and fetal environments, parental phenotype and inheritance, epigenetic modifications, and the environment of the infant on the long term risk of cardiovascular disease [33] (Fig. 1).
8. Consideration for screening, intervention and monitoring
The differences in blood pressure between infants of preeclamptic and normontensive mothers are of clinical importance, as blood pressure measurements track and may ultimately progress to hypertension. Even by 20-30 years the prevalence of hypertension is much higher in subjects who although normotensive in childhood, had blood pressure measurements in the top quintile [34]. Hypertension is a potent cardiovascular risk factor and its prompt treatment reduces cardiovascular morbidity and mortality [34]. Indeed, American Academy of Pediatrics supports routine annual blood measurement in all healthy children from the age of 3 years and measurement of blood pressure in high risk polulations at younger ages. Maternal preeclampsia is not mentioned as a specific risk factor [35].
Pregnancy is a critical time for long‐term blood pressure regulation in both mother and child. Pregnancies complicated by placental insufficiency, resulting in pre‐eclampsia and intrauterine growth restriction result in an adverse intrauterine environment, which programmes the fetus and the second generation to develop hypertension in adult life. Thus, pregnancy can be viewed as a window of opportunity to improve long-term cardiovascular health of the infant. Research on interventions during pregnancy to prevent long-term development of hypertension may ultimately help us to reduce the future burden of cardiovascular disease [36, 37].
Conclusion
Taking into consideration the currently available evidence, it can now be suggested that preeclampsia is linked to adverse effects on the cardiometabolic health of the infants affecting principally blood pressure. The degree of involvement in the programming of the cardiovascular system is difficult to estimate, as multiple pathophysiological pathways have been implicated in the pathogenesis and the clinical course of preeclampsia and may play separate roles in the development of long term cardiovascular disease [33]. Depending on which mechanism is dominantly involved, and which environmental stressors are present, the function of the cardiometabolic system may be altered in different ways. The role of abnormal placentation as a principal regulator of long term consequences of preeclampsia in the infant may be one of the parameters that need further investigation. Identification of individuals at risk and development of intervention policies or prevention strategies relies on understanding the mechanisms by which preeclampsia alters intrauterine growth and development and intervenes on the programming of the cardiometabolic system [32]. The fact that exposed subjects can be easily identified based on precise clinical criteria in their mother, enables timely, targeted, primary prevention strategies for cardiovascular risk reduction in this population [33]. Understanding the relationship between preeclampsia and cardiovascular disease will allow for early interventions to prevent or delay the onset of adverse events among individuals at risk.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Mol B.W.J., Roberts C.T., Thangaratinam S., Magee L.A., de Groot C.J.M., Hofmeyr G.J. Pre-eclampsia. Lancet. 2016;387(10022):999–1011. doi: 10.1016/S0140-6736(15)00070-7. [DOI] [PubMed] [Google Scholar]
- 2.Jido T.A., Yakasai I.A. Preeclampsia: a review of the evidence. Ann. Afr. Med. 2013;12(2):75–85. doi: 10.4103/1596-3519.112395. [DOI] [PubMed] [Google Scholar]
- 3.Wallis A.B., Saftlas A.F., Hsia J., Atrash H.K. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am. J. Hypertens. 2008;21(5):521–526. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 4.Jim B., Karumanchi S.A. Preeclampsia: Pathogenesis, Prevention, and Long-Term Complications. Semin. Nephrol. 2017;37(4):386–397. doi: 10.1016/j.semnephrol.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Amaral L.M., Wallace K., Owens M., LaMarca B. Pathophysiology and Current Clinical Management of Preeclampsia. Curr. Hypertens. Rep. 2017;19(8):61. doi: 10.1007/s11906-017-0757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goffin S.M., Derraik J.G.B., Groom K.M., Cutfield W.S. Maternal pre-eclampsia and long-term offspring health: Is there a shadow cast? Pregnancy Hypertens. 2018;12:11–15. doi: 10.1016/j.preghy.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy: Executive Summary. Obstet. Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 8.Wu P., Haththotuwa R., Kwok C.S., et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes. 2017;10(2):e003497. doi: 10.1161/CIRCOUTCOMES.116.003497. [DOI] [PubMed] [Google Scholar]
- 9.Mosca L., Benjamin E.J., Berra K., et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation. 2011;123(11):1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piepoli M.F., Hoes A.W., Agewall S., et al. ESC Scientific Document Group 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016;37(29):2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pauli J.M., Repke J.T. Preeclampsia. Short and long-term implications. Obstet. Gynecol. Clin. North Am. 2015;42(2):299–313. doi: 10.1016/j.ogc.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Nahum Sacks K., Friger M., Shoham-Vardi I., et al. Prenatal exposure to preeclampsia as an independent risk factor for long-term cardiovascular morbidity of the offspring. Pregnancy Hypertens. 2018;13:181–186. doi: 10.1016/j.preghy.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Story L., Chappell L.C. Preterm pre-eclampsia: What every neonatologist should know. Early Hum. Dev. 2017;114:26–30. doi: 10.1016/j.earlhumdev.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Backes C.H., Markham K., Moorehead P., Cordero L., Nankervis C.A., Giannone P.J. Maternal preeclampsia and neonatal outcomes. J. Pregnancy. 2011;2011214365 doi: 10.1155/2011/214365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amaral L.M., Cunningham M.W., Jr, Cornelius D.C., LaMarca B. Preeclampsia: long-term consequences for vascular health. Vasc. Health Risk Manag. 2015;11:403–415. doi: 10.2147/VHRM.S64798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueiró-Filho E.A., Mak L.E., Reynolds J.N., et al. Neurological function in children born to preeclamptic and hypertensive mothers - A systematic review. Pregnancy Hypertens. 2017;10:1–6. doi: 10.1016/j.preghy.2017.07.144. [DOI] [PubMed] [Google Scholar]
- 17.Lazdam M., de la Horra A., Pitcher A., et al. Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension. 2010;56(1):159–165. doi: 10.1161/HYPERTENSIONAHA.110.150235. [DOI] [PubMed] [Google Scholar]
- 18.Thoulass J.C., Robertson L., Denadai L., et al. Hypertensive disorders of pregnancy and adult offspring cardiometabolic outcomes: a systematic review of the literature and meta-analysis. J. Epidemiol. Community Health. 2016;70(4):414–422. doi: 10.1136/jech-2015-205483. [DOI] [PubMed] [Google Scholar]
- 19.Kajantie E., Eriksson J.G., Osmond C., Thornburg K., Barker D.J. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40(4):1176–1180. doi: 10.1161/STROKEAHA.108.538025. [DOI] [PubMed] [Google Scholar]
- 20.Davis E.F., Lazdam M., Lewandowski A.J., et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129(6):e1552–e1561. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 21.Pinheiro T.V., Brunetto S., Ramos J.G., Bernardi J.R., Goldani M.Z. Hypertensive disorders during pregnancy and health outcomes in the offspring: a systematic review. J. Dev. Orig. Health Dis. 2016;7(4):391–407. doi: 10.1017/S2040174416000209. [DOI] [PubMed] [Google Scholar]
- 22.Rice M.M., Landon M.B., Varner M.W., et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Pregnancy-Associated Hypertension and Offspring Cardiometabolic Health. Obstet. Gynecol. 2018;131(2):313–321. doi: 10.1097/AOG.0000000000002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewandowski A.J., Augustine D., Lamata P., et al. Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation. 2013;127(2):197–206. doi: 10.1161/CIRCULATIONAHA.112.126920. [DOI] [PubMed] [Google Scholar]
- 24.Lewandowski A.J., Leeson P. Preeclampsia, prematurity and cardiovascular health in adult life. Early Hum. Dev. 2014;90(11):725–729. doi: 10.1016/j.earlhumdev.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 25.McDonald S.D., Malinowski A., Zhou Q., Yusuf S., Devereaux P.J. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am. Heart J. 2008;156(5):918–930. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 26.Lazdam M., de la Horra A., Diesch J., et al. Unique blood pressure characteristics in mother and offspring after early onset preeclampsia. Hypertension. 2012;60(5):1338–1345. doi: 10.1161/HYPERTENSIONAHA.112.198366. [DOI] [PubMed] [Google Scholar]
- 27.Alsnes I.V., Vatten L.J., Fraser A., et al. Hypertension in Pregnancy and Offspring Cardiovascular Risk in Young Adulthood: Prospective and Sibling Studies in the HUNT Study (Nord-Trøndelag Health Study) in Norway. Hypertension. 2017;69(4):591–598. doi: 10.1161/HYPERTENSIONAHA.116.08414. [DOI] [PubMed] [Google Scholar]
- 28.Tong W., Xue Q., Li Y., Zhang L. Maternal hypoxia alters matrix metalloproteinase expression patterns and causes cardiac remodeling in fetal and neonatal rats. Am. J. Physiol. Heart Circ. Physiol. 2011;301(5):H2113–H2121. doi: 10.1152/ajpheart.00356.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Børke W.B., Edvardsen T., Fugelseth D., et al. Reduced left ventricular function in hypoxemic newborn pigs: a strain Doppler echocardiographic study. Pediatr. Res. 2006;59(5):630–635. doi: 10.1203/01.pdr.0000214846.00318.36. [DOI] [PubMed] [Google Scholar]
- 30.de la Calzada D.G., García L.O., Remírez J.M., Lázaro A., Cajal M.D. Study of early detection of cardiovascular risk factors in children born small (SGA) and review of literature. Pediatr. Endocrinol. Rev. 2009;6(Suppl. 3):343–349. [PubMed] [Google Scholar]
- 31.Barker D.J., Larsen G., Osmond C., Thornburg K.L., Kajantie E., Eriksson J.G. The placental origins of sudden cardiac death. Int. J. Epidemiol. 2012;41(5):1394–1399. doi: 10.1093/ije/dys116. [DOI] [PubMed] [Google Scholar]
- 32.Stojanovska V., Scherjon S.A., Plösch T. Preeclampsia as modulator of offspring health. Biol. Reprod. 2016;94(3):53. doi: 10.1095/biolreprod.115.135780. [DOI] [PubMed] [Google Scholar]
- 33.Herrera-Garcia G., Contag S. Maternal preeclampsia and risk for cardiovascular disease in offspring. Curr. Hypertens. Rep. 2014;16(9):475. doi: 10.1007/s11906-014-0475-3. [DOI] [PubMed] [Google Scholar]
- 34.Davis E.F., Newton L., Lewandowski A.J., et al. Pre-eclampsia and offspring cardiovascular health: mechanistic insights from experimental studies. Clin. Sci. (Lond.) 2012;123(2):53–72. doi: 10.1042/CS20110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao W., Threefoot S.A., Srinivasan S.R., Berenson G.S. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. Am. J. Hypertens. 1995;8(7):657–665. doi: 10.1016/0895-7061(95)00116-7. [DOI] [PubMed] [Google Scholar]
- 36.D’Agostino R.B., Sr, Vasan R.S., Pencina M.J., et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 37.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. National Heart, Lung, and Blood Institute Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl. 5):S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]