Abstract
Exhaled breath aerosol (EBA) is an important non-invasive biological medium for detecting exogenous environmental contaminants and endogenous metabolites present in the pulmonary tract. Currently, EBA is typically captured as a constituent of the mainstream clinical tool referred to as exhaled breath condensate (EBC). This article describes a simpler, completely non-invasive method for collecting EBA directly from different forms of hard-surface plastic respirator masks and disposable hospital paper breathing masks without first collecting EBC. The new EBA methodology bypasses the complex EBC procedures that require specialized collection gear, dry ice or other coolant, in-field sample processing, and refrigerated transport to the laboratory. Herein, mask samples collected from different types of plastic respirators and paper hospital masks worn by volunteers in the laboratory were analyzed using high resolution-liquid chromatography-mass spectrometry (HR-LC-MS) and immunochemistry. The results of immunochemistry analysis revealed that cytokines were collected above background on both plastic respirator surfaces and paper hospital masks, confirming the presence of human biological constituents. Non-targeted HR-LC-MS analyses demonstrated that larger exogenous molecules such as plasticizers, pesticides, and consumer product chemicals as well as endogenous biochemicals, including cytokines and fatty acids were also detected on mask surfaces. These results suggest that mask sampling is a viable technique for EBA collection to assess potential inhalation exposures and endogenous indicators of health state.
Keywords: Exhaled breath aerosol, respiratory masks, hospital masks, immunochemistry, liquid chromatography-mass spectrometry, non-invasive
1. INTRODUCTION
1.1. Breath sampling, gas-phase and condensates
Breath-based testing has a long history focused on analyzing gas-phase compounds for environmental exposures or for indications of health-state via preclinical biomarkers (Pleil, 2016; Wallace & Pleil, 2018). More recently, the concept of condensed-phase breath collection, referred to as exhaled breath condensate (EBC), has extended the analyte range beyond organic gases to include water-soluble inorganic constituents, as well as a wide range of compounds that are otherwise difficult to collect due to polarity and/or low volatility (Ahmadzai et al., 2013; Kubáň & Foret, 2013; Zamuruyev et al., 2018). EBC has been collected to assess airway pH for tracking asthma status (Accordino et al., 2008; Morton, Henry, & Thomas, 2006; Ojoo, Mulrennan, Kastelik, Morice, & Redington, 2005). A wide variety of compounds are also part of the EBC fluid that could complement, or even replace some blood or urine assays (Stiegel, Pleil, Sobus, Morgan, & Madden, 2015; Wallace, Kormos, & Pleil, 2016). EBC became a robust biological medium used for a wide variety of environmental and clinical biomonitoring applications (Barbara et al., 2018; Marie-Desvergne et al., 2016; Peralbo-Molina, Calderón-Santiago, Priego-Capote, Jurado-Gámez, & Castro, 2016a; Sauvain, Hohl, Wild, Pralong, & Riediker, 2014).
Certainly, EBC has some logistical advantages over blood sampling in that there is little, if any, infectious waste generated, and that no specialized medical personnel are required. However, there are some challenges for collecting EBC in the field that revolve around infrastructure and subject/patient time. EBC sample collection requires specialized equipment, dry ice or other coolant, and a clinical/laboratory space to process frozen condensate into liquid samples (Wallace & Pleil, 2018; Zamuruyev et al., 2018). Subjects are required to perform breathing maneuvers generally for 10 minutes, each with previously sterilized sampling gear. Although the resulting EBC samples are of low-volume (1–2 mL of liquid), like blood, they need to be shipped to the laboratory at sub-ambient temperature to maintain integrity (Ahmadzai et al., 2013; Wallace & Pleil, 2018).
1.2. Exhaled breath aerosol (EBA)
The proposed solution to streamlining EBC sampling is based on the observation that many of the analytes of interest are likely entrained in liquid aerosols formed during exhalation, and thus could be expected to form films on surfaces or become entrained within filters. Termed exhaled breath aerosol (EBA), it represents a small fraction (< 0.1 %) of the EBC containing larger organic constituents (Hayes et al., 2016). Aerosols have been classified as particles ≤5 μm, while respiratory droplets are those >5 μm (Duguid, 1946; Siegel, 2007; Zhang, Leung, Cowling, & Yang, 2018). So, even when dried, it was presumed that EBA films and deposits would retain the semi- and non-volatile fraction of EBC. Certainly, polar volatile organic compounds (PVOCS) and robust measures of pH would be lost in this method, but the strategic advantages could outweigh the loss. The philosophical aspects of EBC and EBA sampling have been described in the literature (Pleil & Wallace, 2018; Pleil, Wallace, & Madden, 2018; Wallace & Pleil, 2018). Traditional methods for EBC sampling typically involve breathing through a long, chilled tube to condense the aqueous fraction of exhaled breath (Ahmadzai et al., 2013; Davis, Fowler, & Montpetit, 2018; Kubáň & Foret, 2013; Mutlu, Garey, Robbins, Danziger, & Rubinstein, 2001; Wallace & Pleil, 2018; Winters et al., 2017). Methods for EBA sampling include collection on filters, use of commercial sampling devices, and particle impaction (Beck, Olin, & Mirgorodskaya, 2016; Kintz, Mathiaux, Villéger, & Gaulier, 2016; Mikheev & Morozov, 2018; Seferaj et al., 2018; Wallace & Pleil, 2018). Figure 1 is a diagram of how all breath constituents relate.
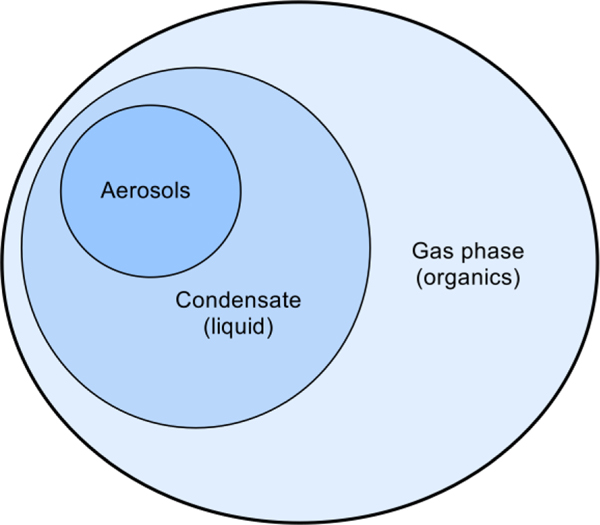
Figure 1.
Diagram of whole exhaled breath (not to scale) showing the relative overlap of the different constituent phases. Exhaled breath includes gases such as N2, O2, CO2, and a humidified liquid phase (condensate). The aerosols are completely captured within the condensate phase.
1.3. Analytes expected in EBA
Based on previous research using EBC, it is expected that certain groups of analytes would be preserved in EBA samples. These non-volatile particles are contained within the fraction of EBC, as shown in Figure 1. The most likely compounds are the heavier (non-volatile) organic molecules representing exogenous exposures to pesticides, consumer products (fragrances, cosmetics), cooking, drugs/medications, combustion sources (fuel, cigarettes), and the various endogenous metabolic compounds representative of human biology, including fatty acids, proteins, surfactants, and cytokines in part derived from lung epithelial lining fluid (Beck, Stephanson, Sandqvist, & Franck, 2013; Davis et al., 2018; Ljungkvist et al., 2015; Ljungkvist et al., 2017; Oldham et al., 2017; Phares, Collier, Zheng, & Jung, 2017; Soares et al., 2018; Tinglev et al., 2016; Trefz et al., 2017; Ullah, Sandqvist, & Beck, 2018). In addition, EBA contains protein and DNA remnants of organisms (e.g., bacteria, viruses, fungal spores) that could be indicative of infection (Heo, Lim, Kim, & Lee, 2017; Morozov et al., 2018; Patrucco et al., 2018; Yao, 2018; Zhang et al., 2018; Zheng, Chen, Yao, & Li, 2018). There is also the possibility that semi-volatile compounds may be collected on mask surfaces, such as aldehydes, alcohols, and other organic compounds that have been previously observed in EBC (Peralbo-Molina et al., 2016a; Peralbo-Molina, Calderón-Santiago, Priego-Capote, Jurado-Gámez, & De Castro, 2016b; Pleil, Hubbard, Sobus, Sawyer, & Madden, 2008).
1.4. Evolution of EBA sampling using assorted mask materials
In our laboratory, the original concept for exploiting EBA evolved from solving practical and logistical problems in biomonitoring. The initial work along these lines came from the observation that disposable filters from pulmonary function instruments (spirometers) might be a valuable resource for assessing infections. As these filters are routinely discarded in a clinical setting, they appeared to be a pragmatic source of interesting biological specimens. A second application was derived from the evaluation of exposures and health states from occupational settings wherein standard biomonitoring could not be done easily. For example, it is not practical to intrude on firefighters or military pilots during their activities, but one could easily sample the internal surfaces of their masks afterwards. Finally, we found that standard disposable paper masks worn in hospitals could be used as a non-invasive sampling method for the general public. The masks only need to be worn for 10 minutes to collect EBA and do not affect normal activities. An overview of these applications has been published recently (Pleil et al., 2018).
2. Materials and Methods
2.1. Sample collection
This exploratory analytical research is based on a series of biological samples collected from randomly selected hard-surface plastic respiratory masks and disposable paper hospital masks. The following plastic masks were included in mask sampling: half-face respirator (3M, North), full-face respirator (3M), supplied air (Navy), face shield (US Safety, Crews), and the following disposable hospital-style paper/polypropylene masks were included: Stanley Safe-T-fit N95 and 3M1818. Hard-surface plastic respirators and disposable paper hospital masks were donated by the U.S. EPA health and safety office. Hard-surface plastic masks and paper hospital masks were both worn by volunteers for 10 min in a laboratory setting, and volunteers breathed normally while wearing the masks. This work was performed as part of an ongoing development effort for assessing breath-sampling methods; it was approved under Human Studies Research Review request HSR-001023 by the University of North Carolina Institutional Review Board and by the U.S. EPA.
For the purposes of this article, we have grouped all biological samples as either hard-surface samples from respirators, supplied air, and face shields (plastic masks) or as disposable hospital-style paper masks (paper masks). The intent is to demonstrate what kinds of compounds could be recovered from a variety of masks and surfaces, not to assess the meaning of these compounds with respect to different mask designs or the health state of the individuals who wore the masks.
2.1.1. Sampling of plastic mask surfaces
After sample collection, interior surfaces of the hard-surface plastic masks listed above (one control and five plastic mask samples) were wiped using cellulose filters (42.5 mm diameter) wetted in phosphate buffered saline (PBS). The control sample consisted of an unused plastic mask that was wiped using the same procedure as the samples. Different sections of the masks were wiped to collect duplicate samples for LC-MS and immunochemistry analysis. Filters were placed in 15 mL sterile polypropylene tubes and stored at 4 °C until extraction.
2.1.2. Sampling of disposable paper hospital masks
Four disposable paper hospital mask samples were collected, and a control paper hospital mask of the same material that was not worn or pre-treated was also included in the study. Equal sections (approximately 2 × 2 in) of the paper mask control and samples were cut out and placed in 15 mL sterile polypropylene tubes and stored at 4 °C. Duplicate samples from each mask were prepared for LC-MS and immunochemistry analysis.
2.2. Sample preparation
A volume of 4.5 mL methanol was added to the 15 mL polypropylene tubes containing the cellulose filters and paper hospital mask materials that were collected. The samples were sonicated for 30 min and then vortexed for 1 min each. The samples were centrifuged at 10,000 rpm for 10 min and the liquid was decanted into new tubes. The samples were placed on a nitrogen concentrator to reduce the sample volume to approximately 300–500 μL. The samples were centrifuged at 10,000 rpm for 10 min and the liquid samples were decanted into new vials. A volume of 100 μL sample was transferred into an amber glass vial and diluted with 300 μL of 0.4 mM ammonium formate buffer. A volume of 10 μL non-targeted analysis tracer mix was added to each sample. The non-targeted analysis tracer mix contained the following standards for negative ionization mode (13C6 methyl paraben, 13C6 butyl paraben, 13C4 perfluorooctanoic acid (PFOA), 13C5 perfluorononanoic acid, 13C415N2 fipronil, 13C4 perfluorooctanesulfonic acid (PFOS), 13C415N2 fipronil sulfone, 13C2 perfluorodecanoic acid) and the following standards for positive ionization mode (D3 thiamethoxam, 13C3 atrazine, D3 pyriproxyfen) to assess mass accuracy during sample analyses. The samples were stored at 4 °C until HR-LC-MS analysis.
2.3. High resolution-liquid chromatography-mass spectrometry analysis
The samples were analyzed by HR-LC-MS using an Agilent 6530 quadrupole-time-of-flight (QTOF) mass spectrometer. A 50 mm Agilent Zorbax extend-C18 column (part no. 727700–902) with 2.1 mm ID and 1.8 μm particle size was used for chromatographic separation. Solvent A was 5% methanol/water with 0.4 mM ammonium formate, and solvent B was 95% methanol/water with 0.4 mM ammonium formate. The gradient started at 75% solvent A, changed to 20% A at 12.14 min, went to 0% A at 19.43 min and held until 21.86 min, then changed to 75% A at 21.87 min and held until 24 min. Spectra were collected from 100–1000 m/z in MS mode and 1 spectra/s. The fragmentor, skimmer, and Vcap were set at 80, 65, and 3500 V, respectively. The samples were run in triplicate in both positive and negative modes.
2.4. Immunochemistry analysis
A volume of 2 mL of 1× Dulbecco’s PBS with + 0.05% Tween 20 buffer was added to each sample designated for immunochemistry analysis; a similar extraction methodology of cytokines has been performed from blood spots on Whatman cellulose protein saver cards (Hejl et al., 2013). Samples were vortexed for 30 s and placed on a rotator at 4 °C overnight (17 h). The samples were centrifuged at 500 × g for 10 min at 10 °C, and the sample liquid was decanted into new tubes. Samples were analyzed using the MesoScale Discovery (MSD, LLC, Rockville MD, USA) V-plex kit human proinflammatory panel 1 containing the following ten cytokines: IFN-γ, IL-10, IL-12p70, IL-13, IL-1β, IL-2, IL-4, IL-5, IL-8, TNF-α (MSD). Standards and samples were analyzed in duplicate using an MSD Quickplex 120 instrument.
2.5. Data reduction and statistical analysis
For the LC-MS analyses, all resulting features in the chromatograms were processed with onboard vendor supplied software to achieve preliminary assignments of chemical formula. All quantitative results were considered relative to each other based on peak area, as non-targeted compounds do not have external standards. Agilent Profinder software was used for deconvolution, peak picking and feature alignment, and Agilent Mass Profiler Professional software was used for database searching to assign compound formulae. Data were further processed in RStudio for data reduction. The data for the paper and plastic masks were analyzed separately, and the abundances for the control samples for each type of mask were used for background subtraction.
The unique chemical formulae for the paper and plastic masks were searched against the U.S. EPA CompTox Chemicals Dashboard (McEachran, Sobus, & Williams, 2017) to obtain the top five most likely compound identifications for each molecular formula. The following information was exported into Microsoft Excel for each identification: DSSTox Substance Identifier (DTXSID), preferred name, CASRN, IUPAC name, molecular formula, monoisotopic mass, number of data sources, Toxcast parameters, Expocast parameters, and whether or not the compound is present in the NHANES database. The data were then sorted from low to high based on the number of data sources the compound has been included in. The chemical categories and uses were obtained for the top 20% of the retrieved compounds for both the paper and plastic masks. This accounted for the top 148 compounds for the paper masks (compounds with 15 or more data sources) and the top 126 compounds for the plastic masks (compounds with 10 or more data sources). Data repositories, including U.S. EPA CompTox Chemicals Dashboard, PubChem, DrugBank, European Chemicals Agency (ECHA), and Environmental Working Group (EWG)’s Skin Deep Cosmetics Database were searched to determine chemical uses. The compounds were placed into one of seven categories: 1) industrial (e.g., plasticizers, solvents, adhesives, lubricants, detergents), 2) endogenous (e.g., fatty acids, fatty acid esters, wax esters, metabolites), 3) medication (e.g., prescription drugs, biologically active compounds), 4) food (e.g., flavoring agents, food additives), 5) pesticides (e.g., herbicides, insecticides, repellants), 6) personal care products (PCPs; e.g., cosmetics, hair care, skin care, topical), and 7) non-specific chemicals (e.g., multiple uses or no specific use could be determined).
The immunochemistry analyses were targeted for a suite of 10 human cytokines (IL-1β, IL-2, IL-8, IL-13, IL-6, IL-12p70, IFN-γ, IL-4, IL-10, and TNF-α). The quantitative results were expressed as pg/mL within the sample based on external standards. One-tailed t-tests (α=0.05) were used to identify significant differences between the cytokine concentrations in the paper and plastic mask extracts compared to the MSD instrument blanks. Statistical analyses were simplified in that all samples were originally anonymous without any meta-data. The important factors were frequency of occurrence, distribution, and variability of different compounds. The differences between paper and plastic mask media were also explored with summary statistics. Microsoft Excel and GraphPad Prism version 7 were used for graphing and statistical analyses.
3. Results and Discussion
3.1. Method development for mask sampling and extraction
A basic procedure for sampling and extraction of disposable paper hospital mask materials/cellulose filter wipes was developed in this study to determine the feasibility of using masks to capture and analyze EBA. An advantage of this protocol is its simplicity and ease of use. Other than instrumentation, few specialized materials are needed for sampling, and no health professionals are required to collect samples, unlike with blood or urine sampling. This completely non-invasive sampling technique can be applied in the field with minimal supervision. The sampling and analysis workflow for non-targeted investigation of mask materials is provided in Figure 2.
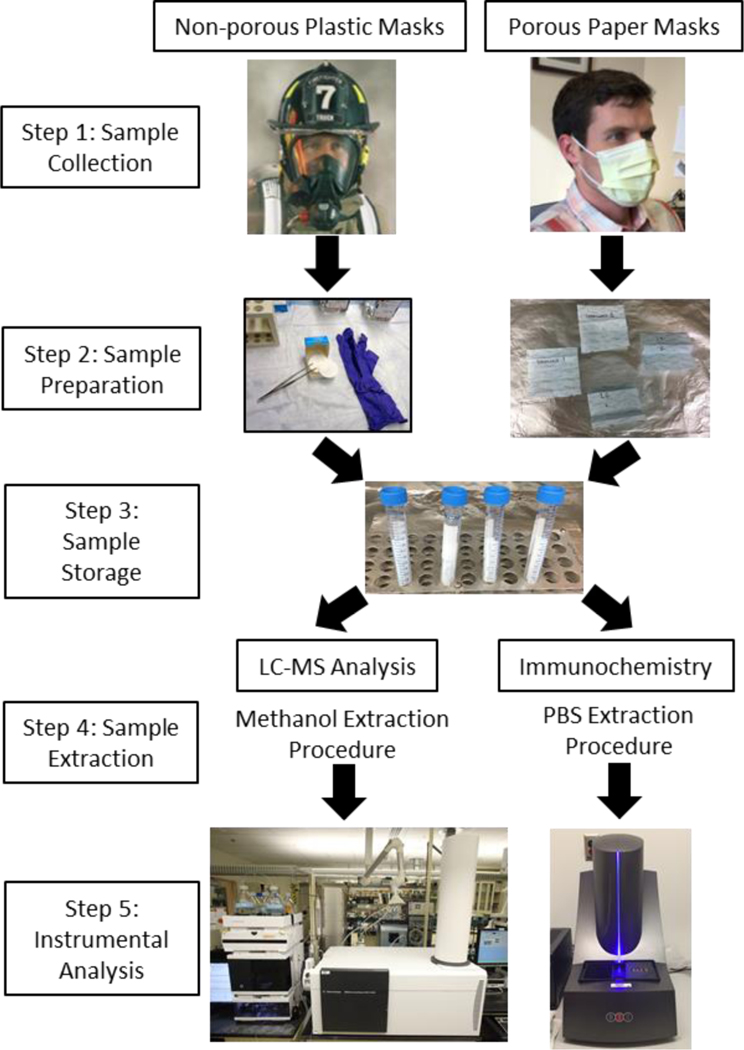
Figure 2:
Workflow diagram for EBA sampling and analysis of hard-surface plastic masks and disposable paper hospital masks using LC-MS and immunochemistry analyses. Provided photos are examples and are not representative of the masks used for the experiments conducted in the current study.
Two types of mask materials were investigated in this study: non-porous hard-surface plastic masks (including respirators) and standard porous paper hospital masks. To collect the EBA samples, subjects wore the masks for 10 min while breathing normally (step 1). Hard-surface plastic masks will mostly contain materials that are exhaled and deposited onto the mask surface, while the paper masks may contain particles that are both inhaled and exhaled through or around the mask. To sample the plastic masks, cellulose filters were immersed in either methanol or PBS, and the entire interior surface of the mask was wiped to collect deposited particles. The paper masks are cut into four equal portions (step 2). The wetted cellulose filter papers from sampling the hard-surface plastic masks and the cut out portions of the paper masks are then placed into polypropylene tubes for sample storage (step 3). For field work, hard-surface plastic masks would ideally be sampled immediately after use and cellulose filter papers placed into clean polypropylene tubes, while paper masks would be sealed into plastic envelopes and shipped to the lab, where the paper masks would be cut into squares and sealed in vials in a more sterile environment.
For HR-LC-MS preparation, compounds were extracted using methanol, and samples were vortexed and centrifuged to separate the liquid sample from the cellulose filter wipe/paper mask material for concentration and subsequent analysis (LC-MS step 4). For immunochemistry analysis, a similar procedure was followed except that the masks were submerged in PBS and incubated overnight prior to the vortex/centrifugation steps (immunochemistry step 4). Ultimately, the samples were analyzed using either HR-LC-MS or immunochemistry instrumentation (step 5). The choice of instrumentation in this last step will affect the quality and quantity of the acquired results, as some instruments are faster and more sensitive than others.
3.2. Immunochemistry investigation of mask extracts
The first goal of the study was to demonstrate that the collected samples were indeed representative of aerosol and contained human compounds. Cytokines were evaluated to assess this aim because these compounds are not found in the environment and will indicate whether the masks contain detectable human compounds. The samples were analyzed using a 10-plex human proinflammatory cytokine kit on the MSD instrument. The distributions of concentrations (pg/mL) of five selected cytokines from the panel (IL-1β, IL-2, IL-8, IL-13, and TNF-α) in the instrument blank, disposable paper mask samples, and hard-surface plastic mask samples are shown in Figure 3.
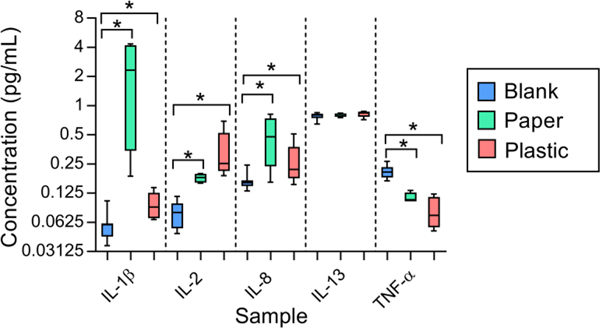
Figure 3.
Selected cytokines investigated in disposable paper hospital masks and hard-surface plastic mask extracts. Three cytokines (IL-1β, IL-2, and IL-8) were significantly elevated in mask extracts compared to instrument blanks (one-tailed t-tests, p<0.05), while other cytokines were not observed at higher levels than the blanks in mask extracts. Data are plotted from min-max in log-scale for visualization.
Three of the ten cytokines were significantly elevated above the instrument blank concentrations in both the disposable paper and hard-surface plastic mask samples (IL-1β, IL-2, and IL-8). The remaining seven cytokines either showed no significant differences in concentrations (such as IL-13) or displayed a significantly decreased concentration (such as TNF-α). These results indicate that some human cytokines were successfully collected and extracted from both the paper and plastic masks. The cytokines that did not show significantly increased concentrations in the mask samples may not have been very abundant in the mask samples or may have a poor extraction efficiency. This is hypothesized to be the case for TNF-α, which showed extremely low concentrations in both the paper and plastic mask samples. TNF-α has shown similar behavior in other studies of mask materials in the laboratory and does not appear to be well recovered in this assay.
As the goal of this experiment was to check for human material, the immunochemistry experiment was considered successful. The presence of some cytokines in both the paper and plastic mask extracts revealed that human material was collected and extracted from the masks, and the cytokines are hypothesized to have originated from EBA that impacted onto the mask during sampling. This was a confirmatory step, and it is unclear at this point whether mask sampling is an advantageous method for analysis of cytokines in general. Instead, at the present time blood or EBC sampling is recommended for thorough evaluation of these compounds (Stiegel et al., 2015).
3.3. Non-targeted HR-LC-MS investigation of mask extracts
The second goal of the study was to investigate what types of compounds could be detected in the mask extracts using HR-LC-MS analysis. The numbers of chemical features (defined based on an exact mass and retention time) and unique chemical formulae that were identified for paper and plastic mask extracts are shown in Table 1. While more chemical features were observed in the paper masks, the paper and plastic masks contained similar numbers of compounds with unique chemical formulae. The number of unique chemical formulae is much lower than the number of chemical features because many features, despite having the same chemical formula, represent compounds with different chemical structures that eluted at distinct retention times.
Table 1.
Chemical features and unique chemical formulae identified in mask sample extracts using HR-LC-MS. The top five compound matches for the unique chemical formulae were curated from the EPA CompTox Chemicals Dashboard.
| Paper Masks | Plastic Masks | |
|---|---|---|
| Chemical features | 3461 | 1112 |
| Unique chemical formulae | 248 | 236 |
| EPA CompTox Chemicals Dashboard compound identifications (top 5 matches) | 734 | 620 |
| Top 20% of compounds searched for classifications | 148 | 126 |
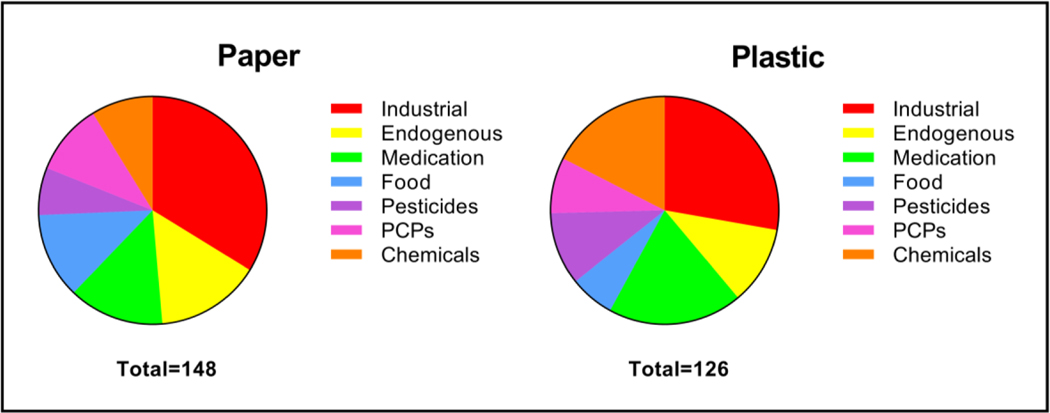
The distributions of the compound classes identified in the top 20% of the paper and plastic masks are shown in Figure 4. Compounds placed into the industrial class included those used as plasticizers, solvents, adhesives, lubricants, sealants, and detergents. Endogenous compounds are those related to biological functioning, such as fatty acids, fatty acid esters, wax esters, metabolites and hormones. Compounds in the medication category include specific drugs as well as biologically active compounds and those used in formulations. The food category includes compounds directly derived from foods and those used as flavoring agents and food additives. The pesticide category includes herbicides, insecticides, and chemicals used as repellants. The PCP category compounds include those used in cosmetics, hair care, skin care, and other topical products. The non-specific chemical category includes those that have multiple uses or for which no specific application could be determined in the literature. The full list of chemicals and their determined categories can be found in the Supplemental Information. Table S1 contains the identifications for the paper masks, and Table S2 contains the identifications for the hard-surface plastic masks. The distributions of the compound classes exhibited only slight differences between the paper and plastic masks (Figure 4). Overall, the presence of similar compounds in the masks shows that both types of mask surfaces can be used for EBA collection and analysis.
Figure 4.
Pie charts depicting distributions of types of chemicals tentatively identified in the paper and plastic masks. Paper masks: approximately 34.5% industrial, 14.9% endogenous, 14.2% medication, 12.2% food, 6.8% pesticides, 10.1% PCPs, and 7.4% chemicals. Plastic masks: approximately 30.1% industrial, 11.1% endogenous, 20.6% medication, 7.1% food, 10.3% pesticides, 9.5% PCPs, and 11.1% chemicals.
In this study, the identification of endogenous human compounds was of interest. Most of the compounds present in the endogenous group were of direct human origin and included compounds such as fatty acids, fatty acid esters, wax esters, metabolites, sterols, and ribonucleosides. Examples of some of these compounds that were identified in the paper and plastic masks are listed in Table 2. Interestingly, there was little overlap in the chemicals identified in the paper and plastic masks. Only the fatty acid tetracosanoic acid was identified in both types of masks, although similar compound classes were observed in each. However, this does not mean that the paper and plastic mask materials are not capable of capturing the same compounds. The compounds that are identified in the mask samples depend upon the origins of the sample, as individuals may have different compounds in exhaled breath due to their lifestyles and recent chemical exposures. While many of the compounds in Table 2 are predicted to be of endogenous origins, it is important to note that there is overlap between some endogenous and exogenous compounds (Winters et al., 2018). Pentadecanoic acid is primarily derived from dairy products and meat, but there is some evidence for endogenous production (Pfeuffer & Jaudszus, 2016). However, the presence of so many types of endogenous compounds in both types of masks supports the concept that the samples contain human materials that were likely from exhaled breath aerosol.
Table 2.
Examples of endogenous compounds tentatively identified in paper and plastic mask extracts. The table indicates the compound class, name, chemical formula, DSSTox Substance Identifier (DTXSID) and whether the compound was identified in a paper or plastic mask sample.
| Class | Compound Name | Chemical Formula | DTXSID | Paper | Plastic |
|---|---|---|---|---|---|
| Fatty aids | Pentadecanoic acid | C15H30O2 | DTXSID2021652 | X | |
| Octacosanoic acid | C28H56O2 | DTXSID2075051 | X | ||
| Tetracosanoic acid | C24H48O2 | DTXSID6021664 | X | X | |
| Fatty Acid Esters | Methyl hexadecanoate | C17H34O2 | DTXSID4029149 | X | |
| Diethyl hexanedioate | C10H18O4 | DTXSID2021999 | X | ||
| Wax Esters | Dodecyl hexadecanoate | C28H56O2 | DTXSID4068375 | X | |
| Tetradecyl tetradecanoate | C28H56O2 | DTXSID6066658 | X | ||
| Metabolites | 6-Hydroxymelatonin glucuronide | C19H24N2O9 | DTXSID60241639 | X | |
| Decanedioic Acid | C10H18O4 | DTXSID7026867 | X | ||
| Linoleamide | C18H33NO | DTXSID4042098 | X | ||
| 7-Hydroxyflavone | C15H10O3 | DTXSID3022328 | X | ||
| Sterol | 7-Dehydrositosterol | C29H48O | DTXSID50200093 | X | |
| Ribonucleoside | 6-Methylmercaptopurine ribonucleoside | C11H14N4O4S | DTXSID5030454 | X | |
The non-endogenous compounds identified in the mask samples may have originated from a variety of sources, although in this case they are presumed to be predominantly from human EBA. Some endogenous compounds may also be due to saliva contamination. Although saliva contamination was not tested in the current study, this can be accomplished in future work. Many of the compounds in the non-endogenous categories provide information likely representative of human lifestyles. For example, chemicals from food may be indicative of meals and drinks consumed by subjects, and medications/drugs may have been taken by the subjects. According to the National Conference of State Legislatures (NCSL), more than 50% of Americans take at least one prescription drug daily. Previous studies have detected both pharmaceutical medications and non-volatile drugs of abuse in breath samples (Beck et al., 2013; Trefz et al., 2017; Ullah et al., 2018).
As shown in Figure 4, some of the compounds in classes such as industrial chemicals may have come from the mask materials themselves (i.e., plasticizers). While it is very difficult to fully separate the compounds originating from human EBA and those from the masks, this shows how careful experimental preparation and data analysis steps are crucial. In this experiment, a background subtraction of the blank samples that were analyzed for both the paper and plastic mask materials was conducted. This step was performed to eliminate compounds that represent background material from the masks. It is still possible that some compounds remain in the final analysis that did not originate from EBA but may still be indicative of human interaction. For example, PCP chemicals may have been deposited on the masks in EBA or may be indicative of skin contact with the mask material, as chemicals from cosmetics, lotions, and haircare were also in contact with the mask materials during sampling. Identification of the specific compounds and classes of compounds can be utilized in future optimization of mask sampling study design to decrease background components and/or interferences. Additionally, masks could be pre-treated by exposure to humidity for 10 min prior to sampling to more accurately mimic the conditions of human breath. Mask controls pre-treated under humid conditions may contain more surface plasticizers or other contaminants inherent to the mask material that are not prevalent under dry conditions.
It is also important to note that these compounds only represent those that were most likely to have been present in the mask extracts, but no further steps other than the EPA CompTox Chemicals Dashboard searches were taken to confirm identifications. The purpose of this preliminary study was to determine if compounds could be extracted from the two types of mask materials, and if so, what types of compounds were represented. In a larger study using these procedures, further data post-processing steps could be performed to confirm identifications of chemicals of interest. Additionally, many large biomolecules may be present in the mask extract but not identified in this study due to the restrictions of the mass spectrometry technique utilized and the current size of the chemical library.
3.4. Mask preparation for human studies
This preliminary investigation into collecting EBA using disposable paper and hard-surface plastic masks has revealed some important considerations for future human studies. The observation of PCPs, including perfumes and cosmetics, shows that some interfering compounds were present on the mask materials. To reduce sample contamination, subjects should be instructed to remove cosmetics and avoid perfume and other scented PCPs on the day of sampling. Plastic masks should be thoroughly wiped with a damp cloth and an alcohol swab and allowed to dry completely prior to sampling. The masks should also be cleaned immediately following sampling if they are to be used in subsequent studies. Disposable paper masks should be kept in plastic packaging until right before sampling to avoid contamination with dust and other particulates in the air. Disposable paper hospital masks should only be used once. Additionally, to avoid contamination with compounds from the skin, human subjects and scientists should wear gloves at all times while wearing and analyzing the masks. Adhering to these basic guidelines will help eliminate confounding compounds during the analysis. Researchers suggest storing mask samples at 4 °C prior to extraction. Mask extracts may be stored at −20 °C if a delay in instrumental analysis is expected. At this time, a detailed investigation of mask sample stability has not been performed.
3.5. Applications for mask sampling
This completely non-invasive procedure for EBA sampling using mask materials has been demonstrated in this preliminary study using a small set of samples from volunteers in a laboratory setting. However, the technique has many perceived applications, especially for future investigations of occupational exposure. Hard-surface plastic masks and respirators are used routinely for a variety of jobs. For example, firefighters, military pilots, and hazmat removal workers use respirators for oxygen supply while performing job duties. Scientists and individuals working with dangerous materials may also wear plastic face shields for protection. These surfaces can be easily sampled for EBA using cellulose filter wipes after the individual has completed work. Similarly, disposable paper masks are routinely used in hospitals and dentists’ offices for respiratory protection. Individuals may voluntarily don masks when ill to protect others while contagious. In locations with poor air quality, individuals often wear paper hospital masks to protect against inhaling particulate matter. Paper hospital masks are for single use only, and instead of discarding them, they can easily be retained for EBA analysis. For disposable paper masks, analyzed compounds may include those that have collected on the outside of the mask surface as well as exhaled compounds, as these masks are porous, and the entire mask is submerged for extraction. Therefore, during sampling paper masks may collect additional contaminants from the environment that may be representative of potential exposure but do not originate from EBA. The extent to which this confounding issue may affect experimental results remains to be investigated. Hard-surface plastic masks are non-porous and thus should not be affected by the presence of environmental constituents during sampling. Several of the mentioned applications are currently being investigated in our laboratory.
4. Conclusions
In this initial study, we have reported a novel experimental protocol by which human endogenous and exogenous compounds were extracted from both disposable paper hospital masks and hard-surface plastic masks. This simple procedure can be used for non-targeted discovery analysis to evaluate compounds in EBA that are captured on the mask surfaces. A variety of endogenous and exogenous compounds were identified in the mask sample extracts, including compounds such as cytokines that confirmed the presence of human material in these samples, as well as exogenous compounds from sources such as food, PCPs, medication, and industry. In future, additional optimization strategies, such as altering the duration of exposure and improving the extraction procedures can be employed to yield different types of compounds, and a variety of instrumentation could be utilized to expand the scope and sensitivity of the technique. Additional steps can be taken to confirm the identifications of compounds of interest. This non-invasive technique can be easily used in the field to assess occupational exposure and health state.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank colleagues at the U.S. EPA health and safety office for providing the anonymous mask samples, including hard-surface plastic respirators and disposable hospital paper masks. The authors acknowledge Lisa Dailey for assisting with immunochemistry analyses. This article was reviewed by the U.S. EPA and approved for publication. The conclusions are those of the authors and do not reflect EPA policy. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Bios:

Dr. M. Ariel Geer Wallace is a Chemist at U.S. EPA in the Office of Research and Development. Dr. Wallace’s research at EPA focuses on analytical methods development and understanding the extent of human exposure to environmental contaminants through biomarkers research. Dr. Wallace specializes in techniques including gas- and liquid-chromatography/mass spectrometry and immunochemistry. She holds a B.S. in Biochemistry from Indiana Wesleyan University (Marion, IN) and a Ph.D. in Chemistry from Duke University (Durham, NC).

Dr. Joachim Pleil is a Research Scientist at U.S. EPA in the Office of Research and Development. Dr. Pleil’s research at EPA focuses on developing analytical methods for environmental pollutants, human systems biology, and biomarkers. He serves as Professor at the University of North Carolina School of Public Health and is assigned to NASA working on the Pilot Breathing Assessment project. He holds B.S. degrees in Mathematics and Physics, M.S. in Physics (SIU, Carbondale, IL), and Ph.D. in Environmental Science and Engineering (UNC, Chapel Hill, NC). He has served as Editor-in-Chief of Journal of Breath Research (JBR) since 2014.

Dr. Michael Madden is a Research Biologist at U.S. EPA in Chapel Hill, NC. He examines biological responses and health effects in people exposed to air pollution, including ozone and diesel engine exhaust. His work involves validating biomarkers of exposure and identifying susceptible populations. His research strategies use an array of approaches from in vitro exposures of cells to controlled and field exposures of volunteers. Dr. Madden is adjunct faculty at the University of North Carolina-Chapel Hill (UNC; PhD 1987) in the Department of Environmental Sciences and Engineering. He serves on the Editorial Board for Journal of Breath Research.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Accordino R, Visentin A, Bordin A, Ferrazzoni S, Marian E, Rizzato F, … Maestrelli P. (2008). Long-term repeatability of exhaled breath condensate pH in asthma. Respiratory Medicine, 102(3), 377–381. doi: 10.1016/j.rmed.2007.10.014 [DOI] [PubMed] [Google Scholar]
- Ahmadzai H, Huang S, Hettiarachchi R, Lin J-L, Thomas PS, & Zhang Q. (2013). Exhaled breath condensate: a comprehensive update. Clinical chemistry and laboratory medicine, 51(7), 1343–1361. [DOI] [PubMed] [Google Scholar]
- Barbara R, Carina S, Sabina G, Attila K, Thomas G, Georg Martin F, … Manuela F-C (2018). Exhaled breath condensate as a potential biomarker tool for idiopathic pulmonary fibrosis—a pilot study. Journal of Breath Research, 12(1), 016003. [DOI] [PubMed] [Google Scholar]
- Beck O, Olin A-C, & Mirgorodskaya E. (2016). Potential of mass spectrometry in developing clinical laboratory biomarkers of nonvolatiles in exhaled breath. Clinical chemistry, 62(1), 84–91. [DOI] [PubMed] [Google Scholar]
- Beck O, Stephanson N, Sandqvist S, & Franck J. (2013). Detection of drugs of abuse in exhaled breath using a device for rapid collection: comparison with plasma, urine and self-reporting in 47 drug users. Journal of breath research, 7(2), 026006. [DOI] [PubMed] [Google Scholar]
- Davis MD, Fowler SJ, & Montpetit AJ (2018). Exhaled breath testing–A tool for the clinician and researcher. Paediatric Respiratory Reviews. [DOI] [PubMed] [Google Scholar]
- Duguid J. (1946). The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. Epidemiology & Infection, 44(6), 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SA, Haefliger S, Harris B, Pavlakis N, Clarke SJ, Molloy MP, & Howell VM (2016). Exhaled breath condensate for lung cancer protein analysis: a review of methods and biomarkers. Journal of breath research, 10(3), 034001. [DOI] [PubMed] [Google Scholar]
- Hejl AM, Adetona O, Diaz-Sanchez D, Carter JD, Commodore AA, Rathbun SL, & Naeher LP (2013). Inflammatory effects of woodsmoke exposure among wildland firefighters working at prescribed burns at the Savannah River Site, SC. Journal of occupational and environmental hygiene, 10(4), 173–180. [DOI] [PubMed] [Google Scholar]
- Heo KJ, Lim CE, Kim HB, & Lee BU (2017). Effects of human activities on concentrations of culturable bioaerosols in indoor air environments. Journal of Aerosol Science, 104, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintz P, Mathiaux F, Villéger P, & Gaulier J. m. (2016). Testing for Drugs in Exhaled Breath Collected With ExaBreath in a Drug Dependence Population: Comparison With Data Obtained in Urine After Liquid Chromatographic-Tandem Mass Spectrometric Analyses. Therapeutic drug monitoring, 38(1), 135–139. [DOI] [PubMed] [Google Scholar]
- Kubáň P, & Foret F. (2013). Exhaled breath condensate: Determination of non-volatile compounds and their potential for clinical diagnosis and monitoring. A review. Analytica Chimica Acta, 805, 1–18. doi: 10.1016/j.aca.2013.07.049 [DOI] [PubMed] [Google Scholar]
- Ljungkvist G, Beck O, Stein K, Danielsson Å, Agge A, Friberg H, … Sandqvist S. (2015). Study on the origin and collection of exogenous compounds in exhaled breath aerosol particles: Eur Respiratory Soc. [Google Scholar]
- Ljungkvist G, Ullah S, Tinglev Å, Stein K, Bake B, Larsson P, … Sandqvist S. (2017). Two techniques to sample non-volatiles in breath—exemplified by methadone. Journal of breath research, 12(1), 016011. [DOI] [PubMed] [Google Scholar]
- Marie-Desvergne C, Dubosson M, Touri L, Zimmermann E, Gaude-Môme M, Leclerc L, … Vachier I. (2016). Assessment of nanoparticles and metal exposure of airport workers using exhaled breath condensate. Journal of breath research, 10(3), 036006. [DOI] [PubMed] [Google Scholar]
- McEachran AD, Sobus JR, & Williams AJ (2017). Identifying known unknowns using the US EPA’s CompTox Chemistry Dashboard. Analytical and bioanalytical chemistry, 409(7), 1729–1735. [DOI] [PubMed] [Google Scholar]
- Mikheev AY, & Morozov VN (2018). AFM imaging of exhaled microdroplets and dry residues collected by impactor. Journal of Aerosol Science, 123, 131–140. [Google Scholar]
- Morozov VN, Nikolaev AA, Shlyapnikov YM, Mikheev AY, Shlyapnikova EA, Bagdasaryan TR, … Larionova EE (2018). Non-invasive approach to diagnosis of pulmonary tuberculosis using microdroplets collected from exhaled air. Journal of breath research, 12(3), 036010. [DOI] [PubMed] [Google Scholar]
- Morton J, Henry RL, & Thomas PS (2006). Exhaled breath condensate nitrite/nitrate and pH in relation to pediatric asthma control and exhaled nitric oxide. Pediatric pulmonology, 41(10), 929–936. [DOI] [PubMed] [Google Scholar]
- Mutlu GM, Garey KW, Robbins RA, Danziger LH, & Rubinstein I. (2001). Collection and analysis of exhaled breath condensate in humans. American journal of respiratory and critical care medicine, 164(5), 731–737. [DOI] [PubMed] [Google Scholar]
- Ojoo J, Mulrennan S, Kastelik J, Morice A, & Redington A. (2005). Exhaled breath condensate pH and exhaled nitric oxide in allergic asthma and in cystic fibrosis. Thorax, 60(1), 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham MJ, Wagner KA, Gene Gilman I, Beach JB, Liu J, Rostami AA, & Sarkar MA (2017). Development/verification of methods for measurement of exhaled breath and environmental e-vapor product aerosol. Regulatory Toxicology and Pharmacology, 85, 55–63. doi: 10.1016/j.yrtph.2017.01.006 [DOI] [PubMed] [Google Scholar]
- Patrucco F, Gavelli F, Ravanini P, Daverio M, Statti G, Castello LM, … Balbo PE (2018). Use of an innovative and non-invasive device for virologic sampling of cough aerosols in patients with community and hospital acquired pneumonia: a pilot study. Journal of breath research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralbo-Molina A, Calderón-Santiago M, Priego-Capote F, Jurado-Gámez B, & Castro M. D. L. d. (2016a). Metabolomics analysis of exhaled breath condensate for discrimination between lung cancer patients and risk factor individuals. Journal of Breath Research, 10(1), 016011. [DOI] [PubMed] [Google Scholar]
- Peralbo-Molina A, Calderón-Santiago M, Priego-Capote F, Jurado-Gámez B, & De Castro ML (2016b). Identification of metabolomics panels for potential lung cancer screening by analysis of exhaled breath condensate. Journal of breath research, 10(2), 026002. [DOI] [PubMed] [Google Scholar]
- Pfeuffer M, & Jaudszus A. (2016). Pentadecanoic and heptadecanoic acids: multifaceted odd-chain fatty acids. Advances in Nutrition, 7(4), 730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares DJ, Collier S, Zheng Z, & Jung HS (2017). In-situ analysis of the gas-and particle-phase in cigarette smoke by chemical ionization TOF-MS. Journal of Aerosol Science, 106, 132–141. [Google Scholar]
- Pleil JD (2016). Breath biomarkers in toxicology. Archives of toxicology, 90(11), 2669–2682. [DOI] [PubMed] [Google Scholar]
- Pleil JD, Hubbard HF, Sobus JR, Sawyer K, & Madden MC (2008). Volatile polar metabolites in exhaled breath condensate (EBC): collection and analysis. Journal of breath research, 2(2), 026001. [DOI] [PubMed] [Google Scholar]
- Pleil JD, & Wallace MAG (2018). New breath related topics: sample collection for exhaled breath condensate and aerosol, development of real-time medical alerts, measurement of artificial atmospheres, and analysis of legalized cannabis product. Journal of breath research, 12(3), 039001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil JD, Wallace MAG, & Madden MC (2018). Exhaled breath aerosol (EBA): the simplest non-invasive medium for public health and occupational exposure biomonitoring. Journal of breath research, 12(2), 027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvain J-J, Hohl MSS, Wild P, Pralong JA, & Riediker M. (2014). Exhaled breath condensate as a matrix for combustion-based nanoparticle exposure and health effect evaluation. Journal of aerosol medicine and pulmonary drug delivery, 27(6), 449–458. [DOI] [PubMed] [Google Scholar]
- Seferaj S, Ullah S, Tinglev Å, Carlsson S, Winberg J, Stambeck P, & Beck O. (2018). Evaluation of a new simple collection device for sampling of microparticles in exhaled breath. Journal of breath research, 12(3), 036005. [DOI] [PubMed] [Google Scholar]
- Siegel J. (2007). Healthcare Infection Control Practices Advisory Committee 2007 Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. http://www.cdc.gov/ncidod/dhqp/gl_isolation.html. [DOI] [PMC free article] [PubMed]
- Soares M, Mirgorodskaya E, Koca H, Viklund E, Richardson M, Gustafsson P, … Siddiqui S. (2018). Particles in exhaled air (PExA): non-invasive phenotyping of small airways disease in adult asthma. Journal of breath research, 12(4), 046012. [DOI] [PubMed] [Google Scholar]
- Stiegel MA, Pleil JD, Sobus JR, Morgan MK, & Madden MC (2015). Analysis of inflammatory cytokines in human blood, breath condensate, and urine using a multiplex immunoassay platform. Biomarkers, 20(1), 35–46. [DOI] [PubMed] [Google Scholar]
- Tinglev ÅD, Ullah S, Ljungkvist G, Viklund E, Olin A-C, & Beck O. (2016). Characterization of exhaled breath particles collected by an electret filter technique. Journal of breath research, 10(2), 026001. [DOI] [PubMed] [Google Scholar]
- Trefz P, Kamysek S, Fuchs P, Sukul P, Schubert JK, & Miekisch W. (2017). Drug detection in breath: non-invasive assessment of illicit or pharmaceutical drugs. Journal of breath research, 11(2), 024001. [DOI] [PubMed] [Google Scholar]
- Ullah S, Sandqvist S, & Beck O. (2018). A liquid chromatography and tandem mass spectrometry method to determine 28 non-volatile drugs of abuse in exhaled breath. Journal of pharmaceutical and biomedical analysis, 148, 251–258. [DOI] [PubMed] [Google Scholar]
- Wallace MAG, Kormos TM, & Pleil JD (2016). Blood-borne biomarkers and bioindicators for linking exposure to health effects in environmental health science. Journal of Toxicology and Environmental Health, Part B, 19(8), 380–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MAG, & Pleil JD (2018). Evolution of clinical and environmental health applications of exhaled breath research: Review of methods and instrumentation for gas-phase, condensate, and aerosols. Analytica Chimica Acta, 1024, 18–38. doi: 10.1016/j.aca.2018.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BR, Pleil JD, Angrish MM, Stiegel MA, Risby TH, & Madden MC (2017). Standardization of the collection of exhaled breath condensate and exhaled breath aerosol using a feedback regulated sampling device. Journal of Breath Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BR, Pleil JD, Boyer JC, Nylander-French LA, Wallace MAG, & Madden MC (2018). Endogenously Produced Volatiles for In Vitro Toxicity Testing Using Cell Lines. Applied In Vitro Toxicology, 4(2), 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. (2018). Reprint of bioaerosol: A bridge and opportunity for many scientific research fields: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamuruyev KO, Borras E, Pettit DR, Aksenov AA, Simmons JD, Weimer BC, … Davis CE(2018). Effect of temperature control on the metabolite content in exhaled breath condensate. Analytica chimica acta, 1006, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-H, Leung NH, Cowling BJ, & Yang Z-F (2018). Role of viral bioaerosols in nosocomial infections and measures for prevention and control. Journal of Aerosol Science, 117, 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Chen H, Yao M, & Li X. (2018). Bacterial pathogens were detected from human exhaled breath using a novel protocol. Journal of Aerosol Science, 117, 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.