Abstract
Alginate-based composites have been extensively studied for applications in energy and environmental sectors due to their biocompatible, nontoxic, and cost-effective properties. This review is designed to provide an overview of the synthesis and application of alginate-based composites. In addition to an overview of current understanding of alginate biopolymer, gelation process, and cross-linking mechanisms, this work focuses on adsorption mechanisms and performance of different alginate-based composites for the removal of various pollutants including dyes, heavy metals, and antibiotics in water and wastewater. While encapsulation in alginate gel beads confers protective benefits to engineered nanoparticles, carbonaceous materials, cells and microbes, alginate-based composites typically exhibit enhanced adsorption performance. The physical and chemical properties of alginate-based composites determine the effectiveness under different application conditions. A series of alginate-based composites and their physicochemical and sorptive properties have been summarized. This critical review not only summarizes recent advances in alginate-based composites but also presents a perspective of future work for their environmental applications.
Keywords: Alginate, hydrogel, nanocomposites, dyes, heavy metals, antibiotics
1. Introduction
Achieving environmental goals while supporting robust economic growth demands innovative technologies for water and wastewater treatment (Gupta and Ali, 2012; Lim and Aris, 2014; Michael et al., 2013; Yagub et al., 2014). Adsorption technology has been considered as one of the most effective and environmentally sound methods for remediating contaminants that are difficult to degrade in the environment (Ali, 2012; Ali and Gupta, 2006; Fu and Wang, 2011; Lim and Aris, 2014; Wan et al., 2018). Recently, various biomaterials have been developed for improving adsorption capacities, increasing environmental compatibility and operation efficiency as alternatives for conventional activated carbon (Burakov et al., 2018; Gupta et al., 2009). As a low cost and highly efficient absorbent, alginate-based composites have been extensively studied for the removal of heavy metals, industrial dyes, pesticides, antibiotics, and other pollutants in water and wastewater (Fomina and Gadd, 2014; Wan Ngah et al., 2011; Wang et al., 2018b; Wang et al., 2018c; Wang et al., 2018e; Yagub et al., 2014).
Alginate is an anionic polysaccharide found in the outer cell wall of brown algae, such as kelps. The major component of alginate is alginic acid while sodium alginate (SA) is Na-salt of alginic acid, which is a polymer with abundant free hydroxyl and carboxyl groups distributed along the backbone chain of the polymer (Fig. 1). The linear, anionic polysaccharide consists of two kinds of 1,4-linked hexuronic acid residues, namely β-d-mannuronopyranosyl (M) and α-l-guluronopyranosyl (G) residues, arranged in blocks of repeating M residues (MM blocks), blocks of repeating G residues (GG blocks), and blocks of mixed M and G residues (MG-blocks) (Yang et al., 2011). Sodium alginate itself is non-toxic, stable in the environment, with strong gelation, film-forming, and complexing abilities. The sodium alginate gel is soft and soluble in alkaline solution. It can go through an irreversible chemical process with polyvalent cations (except magnesium) to form a crosslinking bond, and finally the formation of a thermo-irreversible gel. For example, when Ca2+ is added to the SA solution, Ca2+ displaces part of H+ and Na+ to form a calcium alginate (CA) gel. Due to its non-toxicity, biocompatibility, and the ability to form crosslinks with cations, alginate has been utilized for encapsulation of chemical and biological compounds with a wide range of application in agriculture, food technologies, pharmaceutical cosmetics, chemical engineering, environmental engineering, paper and textile industry, and many other areas.
Figure 1.
The molecular structure of sodium alginate (SA)
Environmental applications of alginate hinge partly on the fact that the rich surface functional groups (e.g., carboxyl and hydroxyl) in alginate could capture metallic or cationic ions via ion exchange between the crosslinking cations and target pollutants such as heavy metals or dyes. However, alginate gel has disadvantages such as high rigidity and fragility with poor elasticity and mechanical properties (Thakur et al., 2016). Organic and inorganic alginate-based composites have been synthesized to enhance mechanical and thermal stability, and swelling properties of pure alginate gels (Thakur et al., 2016). These composites possess unique physicochemical properties and excellent biocompatibility. Over the past decade, alginate-based composites combining alginate gels and other polymers, natural and engineered nanoparticles, and microorganisms are extensively studied for the removal of pollutants from aqueous solution (Ali et al., 2016a; Ali et al., 2016b; Ali et al., 2015; Wang et al., 2018a; Wang et al., 2018b; Wang et al., 2018c; Zhao et al., 2016). However, these studies are scattered, aiming to report the adsorption performance of specific composites. No comprehensive literature reviews on alginate-based composites as adsorbents for environmental applications are currently available.
The objective of this paper is to provide a systematic synthesis of the existing literature over the past two decades regarding environmental applications of alginate-based composites with respect to their adsorption capacities and experimental conditions. Most of these studies focus on the removal of dyes and heavy metals, as well as dozens of studies on antibiotics and other pollutants. This review starts with an examination of the synthesis of alginate-based composites and their special functionalities resulting from various materials encapsulated in alginate. Subsequently, the adsorption mechanisms and performance of different alginate-based composites for the removal of dyes, heavy metals, and antibiotics from aqueous solutions are reviewed. Future perspectives on application of alginate-based composites for environmental remediation is presented.
2. Synthesis of alginate-based composites as adsorbents
Properties and potential applications of alginate-based composites depend largely on their synthesis, i.e. physical and chemical crosslinking methods (Ching et al., 2017; Idris et al., 2012). Four common methods including ionic crosslinking, emulsification, electrostatic complexation, and self-assembly have been used for synthesis of alginate-based composites (Akhtar et al., 2016; Mane et al., 2015; Paques et al., 2014). Physically crosslinked hydrogels are synthesized by ionic interaction, crystallization, stereocomplex formation, hydrophobized polysaccharides, protein interaction and hydrogen bond. In contrast, chemically crosslinked hydrogels are synthesized by chain growth polymerization, addition and condensation polymerization and gamma and electron beam polymerization (Maitra and Shukla, 2014). These synthesis methods have their own advantages and disadvantages. The synthesis of physically crosslinked sodium alginate hydrogel is simple, and the conditions are gentle, but the gel strength is poor. The structural regularity of chemically crosslinked sodium alginate hydrogel is better, and the preparation conditions are more complicated, requiring complete removal of the unreacted cross-linking agents for post-treatment.
Sodium alginate contains a large number of functional groups, such as active hydroxyl group and carboxyl group along with its backbone chain and can be chemically modified by chemical crosslinking, esterification and etherification. The fundamental process involving gel formation is the interaction between sodium alginate and divalent cations (such as calcium ions) or cationic polymers. Sodium alginate has a -COO- group in the molecule. When a divalent cation is added to the sodium alginate solution, sodium alginate undergoes a cross-linking reaction, Na+ from the guluronic acid (G) blocks is exchanged with these divalent cations to form a water-insoluble gel with a characteristic “egg-box” structure. Different cations show different affinity for alginate, the ability of sodium alginate to bind to multivalent cations follows the sequence of Pb2+> Cu2+> Cd2+> Ba2+> Sr2+> Ca2+> Co2+> Ni2+> Zn2+> Mn2+ (Russo et al., 2007). In this ionic cross-linking process of sol-gel reaction, the solution concentration, pH value, and metal ion intensity all affect the stability, mechanical strength, shape and structure of the gel beads (Chan et al., 2002).
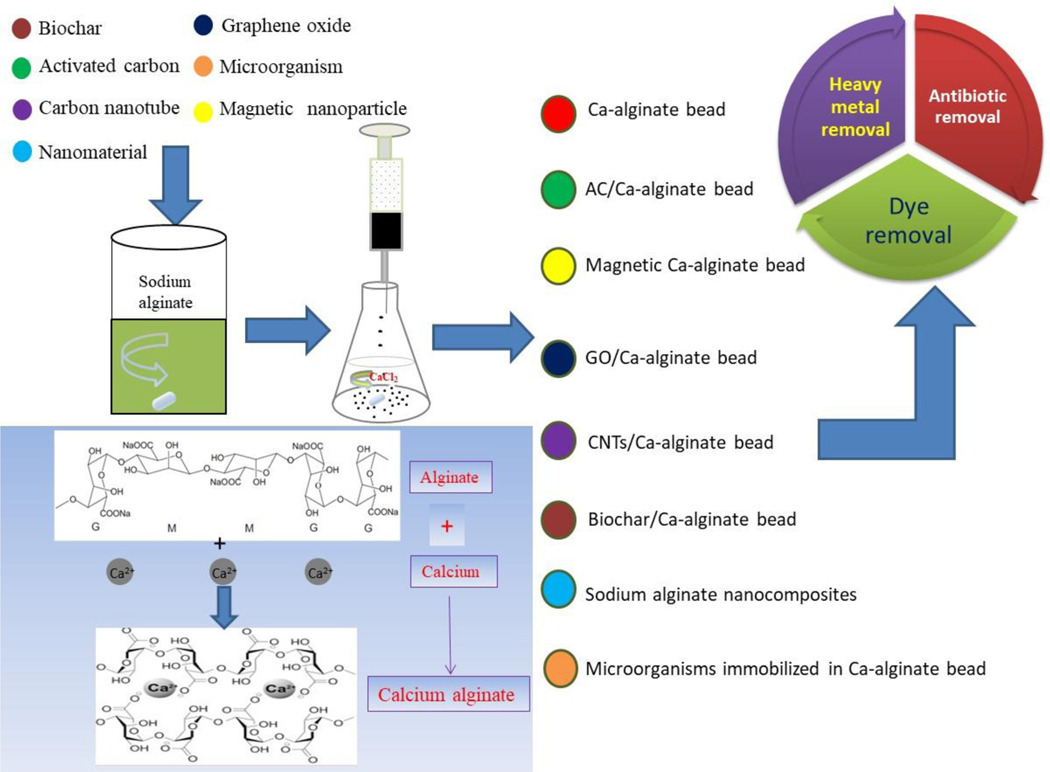
In order to improve the performance and stability of alginate for environmental applications, various materials have been incorporated into alginate hydrogel (microspheres) (de-Bashan et al., 2002; Hong et al., 2017; Rezaei et al., 2017; Wang et al., 2018d; Zhuang et al., 2016a). Synthesis of these composites typically starts with mixing the material with sodium alginate solution before the gelation of calcium alginate (Fig. 2). A comprehensive review of the literature indicates that the materials encapsulated in alginate for environmental applications include activated carbon (AC), biochar, carbon nanotube (CNT), graphene oxide (GO), nanoparticle, magnetic materials and microorganism (Fig. 2). Selection of the materials to be encapsulated depends on the functionality of the material and the intended application so that synergetic benefits can be attained by the composite. While alginate-based composites typically exhibit enhanced physical/mechanical properties for bioengineering applications, a few other benefits achieved through fabrication are worth mentioning here.
Figure 2.
Fabrication of different alginate-based composites
First, alginate beards may serve as a stable matrix for other types of absorbents that are too fine in particle size and too difficult to separate from aqueous solution. These absorbents are typically carbon-based, such as AC, biochar, CNTs, and GO (Gupta et al., 2014; Mohammadi et al., 2011; Robati et al., 2016). AC has been widely used for wastewater treatment (Maneerung et al., 2016). However, AC is mostly used as a fine powder, and the difficulty in separation and regeneration from the effluent may result in significant loss of the adsorbent. Biochar has been recently used as a cost-effective alternative of AC in water/wastewater treatment (Ahmad et al., 2014; Fang et al., 2018; Inyang et al., 2016; Mohan et al., 2014; Wang et al., 2017). Biochar can be ball milled to increase its surface areas (Lyu et al., 2017; Lyu et al., 2018b; Lyu et al., 2018c). Like AC powders, ball milled biochar is difficult to separate from water due to its small particle size (Wang et al., 2018b; Wang et al., 2018e). CNTs and GO both have been intensively studied for removal of organic and inorganic pollutants because of their unique structural features and large specific areas (Chen et al., 2015; Gupta et al., 2013). The facts that GO disperses extremely well in water and CNTs are very small and form aggregates make it difficult to separate them from aqueous solution (Ding et al., 2014; Inyang et al., 2014; Tian et al., 2012; Wang et al., 2010). Encapsulation of these carbon-based materials into alginate hydrogels or beads offer ease of separation and regeneration for water/wastewater treatment (Wang et al., 2018a; Wang et al., 2018b; Wang et al., 2018c; Wang et al., 2018e).
Second, fabricating magnetic materials and nanoparticles into alginate brings in nano-effects and magnetic technology into the composites while attaining excellent absorption performance and reducing the potential environmental risk of nanoparticles. Nanotechnology and magnetic technology have been increasingly used in water and wastewater treatment (Qu et al., 2013; Theron et al., 2008). Alginate/nanomaterial composites are blends of alginate and nanomaterials with enhanced adsorption capacity (Fig. 3). Furthermore, a magnetic adsorbent (called magsorbent) can be developed by encapsulating magnetic functionalized nanoparticles in alginate beads along with different cross-linking agents (Lee et al., 2000; Russo et al., 2007). For example, incorporating maghemite with the alginate in bead form is very useful in isolation or recovery process (Idris et al., 2012). Magnetic technology has the advantage of simple operation and easy separation.
Figure 3.
Photographs of different nanomaterial-alginate hybrid beads. Ca-alginate beads(A); Zero-valent iron nanoparticle-alginate composite beads(B); Silver nanoparticle-alginate composite beads(C); Fe2O3 nanoparticle-alginate composite beads(D); MgO nanoparticle-alginate composite beads(E)
Third, alginate can serve a carrier of microorganisms to optimize the microbial processes for environmental and agricultural applications (Cohen, 2001; Covarrubias et al., 2012; Martins et al., 2013). Compared with the conventional suspension system, alginate microorganism composites offer a multitude of advantages, such as high biomass, high metabolic activity and strong resistance to toxic chemicals (An et al., 2008; Cai et al., 2011; Junter and Jouenne, 2004; Liu et al., 2012a). Moreover, immobilized microorganisms can be used several times without significant loss of activity (Rhee et al., 1996). Therefore, alginate immobilized microorganism technology has received substantial attention for wastewater treatment (An et al., 2008).
3. Alginate-based composites as adsorbents for environmental applications
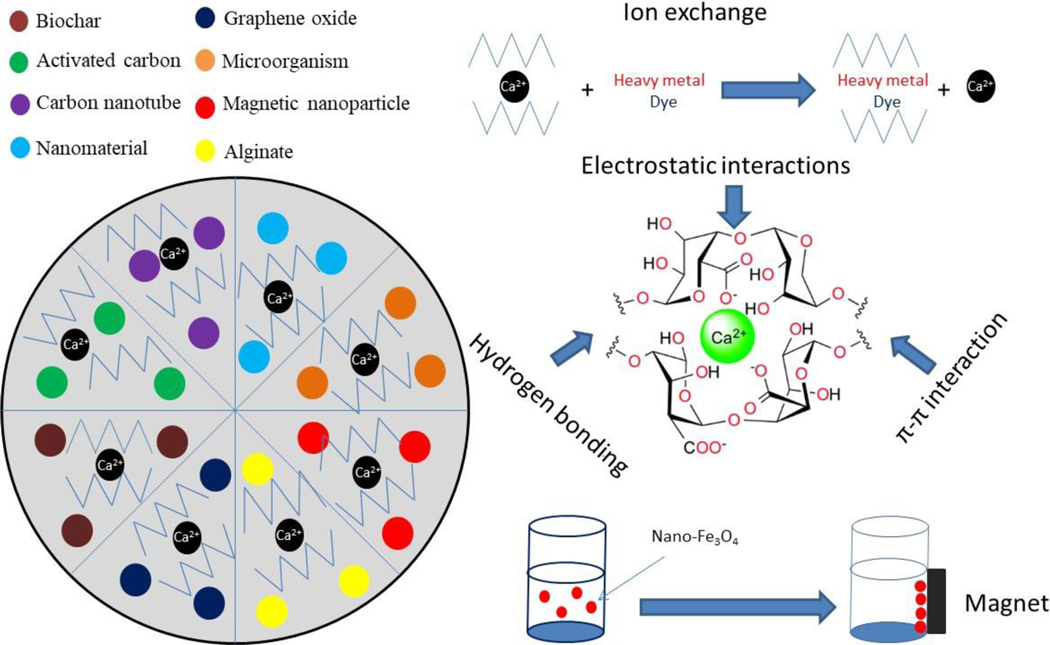
Alginate-based composites are fabricated as absorbents for both inorganic and organic contaminants. The adsorption mechanisms typically involve ion exchange and electrostatic interactions (Fig. 4). Special functionalities of encapsulated materials may bring in other benefits depending on the needs and applications. This section is intended to discuss the performance and mechanisms of various types of composites for the removal of dyes, heavy metals, and antibiotics.
Figure 4.
Adsorption mechanisms of different alginate-based composites on organic and inorganic contaminants in water.
3.1. Dyes
Dyes are intensely colored complex organic compounds which have been heavily used in the textile industry. Release of processed dye wastes into the aquatic environment may result in harmful impacts on human health and the environment. The most obvious impact is the reduction of light penetration, thereby affecting the primary productivity of aquatic ecosystems. Some dyes and their derivatives are toxic to aquatic plants, fish, and shell fish. The removal of dye from wastewater is largely based on the adsorption technique whereby the dissolved dye is adsorbed by the sorbent (Fang et al., 2017; Fang et al., 2016; Gupta and Suhas, 2009; Robinson et al., 2001; Zhang and Gao, 2013). Alginate itself is an excellent sorbent for dye removal. The potential use of pure calcium alginate beads for removal of black dyes was studied in a dynamic batch mode by Aravindhan et al. (Aravindhan et al., 2007). The adsorption isotherm suggested a Langmuir adsorption capacity of 57.70 mg g−1. The performance of alginate-based composites for dye removal is summarized in Table 1 for a subset of studies. Each type of the composites is highlighted below.
Table 1.
Alginate-based composites for the removal of synthetic dyes from aqueous solution
| Adsorbent | Adsorbate | Adsorption capacity (mg g−1) | pH | Temperature (°C) | Ref |
|---|---|---|---|---|---|
| GO/CA fibers | Methylene blue | 181.81 | 5.4 | 25 | (Li et al., 2013) |
| Calcium alginate beads | Basic black dye | 57.70 | 4.0 | 30 | (Aravindhan et al., 2007) |
| Graphene/alginate nanocomposite | Methylene blue | 2300 | 8.0 | 25 | (Zhuang et al., 2016b) |
| Alginate-halloysite nanotube | Methylene blue | 250 | - | 35 | (Liu et al., 2012b) |
| CA/MWCNTs | Methylene blue | 606.1 | - | 25 | (Sui et al., 2012) |
| CA/MWCNTs | Methyl orange | 12.5 | - | 25 | (Sui et al., 2012) |
| CA/AC beads | Methylene blue | 892 | - | 20 | (Hassan et al., 2014b) |
| CA/AC beads | Methylene blue | 730 | - | 40 | (Hassan et al., 2014b) |
| AC–bentonite–alginate beads | Methylene blue | 756.97 | - | 30 | (Benhouria et al., 2015) |
| AC–bentonite–alginate beads | Methylene blue | 982.47 | - | 40 | (Benhouria et al., 2015) |
| AC–bentonite–alginate beads | Methylene blue | 994.06 | - | 50 | (Benhouria et al., 2015) |
| Sodium alginate- Fe3O4 | Malachite green | 47.84 | 7.0 | 25 | (Mohammadi et al., 2014) |
| AC/CA beads | Rhodamine 6G | - | - | - | (Annadurai et al., 2002) |
| Alginate/polyaspartate beads | Methylene blue | 700 | - | 25 | (Jeon et al., 2008) |
| Alginate/polyaspartate beads | Malachite green | 350 | - | 25 | (Jeon et al., 2008) |
| Magnetic alginate beads | Methylene blue | 22.06 | 6.7 | - | (Rocher et al., 2008) |
| Magnetic alginate beads | Methyl orange | 0.65 | 6.7 | - | (Rocher et al., 2008) |
| Magnetic alginate beads crosslinked with epichlorohydrin | Methylene blue | 261.73 | - | - | (Rocher et al., 2010) |
| Magnetic alginate beads crosslinked with epichlorohydrin | Methyl orange | 6.55 | - | - | (Rocher et al., 2010) |
| CABI nano-goethite | Congo red | 181.1 | 3.0 | 25 | (Munagapati and Kim, 2017) |
| SA/n-TiO2 | Direct Red 80 | 163.9 | 2.0 | 25 | (Mahmoodi et al., 2011) |
| SA/n-TiO2 | Acid Green 25 | 151.5 | 2.0 | 25 | (Mahmoodi et al., 2011) |
| Activated organo-bentonite/SA | Methylene blue | 414 | - | 23 | (Belhouchat et al., 2017) |
| Activated organo-bentonite/SA | Methylene orange | 116 | - | 23 | (Belhouchat et al., 2017) |
| Methylcellulose/CA beads | Methylene blue | 336.7 | - | 20 | (Li et al., 2016) |
| Silver nanocomposite hydrogel | Methylene blue | 213.7 | - | Room T | (Devi et al., 2016) |
| Montmorillonite/CA composite | basic red 46 (BR46) | 35 | - | - | (Hassani et al., 2015) |
| SA/poly(N-vinyl-2-pyrrolidone) beads | Reactive red-120 (RR), | 116.82 | - | 25 | (Inal and Erduran, 2015) |
| Magnetic ferrite nanoparticle–alginate composite | Basic Blue 9 (BB9) | 106 | - | 25 | (Mahmoodi, 2013) |
| Magnetic ferrite nanoparticle–alginate composite | Basic Blue 41 (BB41) | 25 | - | 25 | (Mahmoodi, 2013) |
| Magnetic ferrite nanoparticle–alginate composite | Basic Red 18 (BR18) | 56 | - | 25 | (Mahmoodi, 2013) |
| SA/poly(N-vinyl-2-pyrrolidone) beads | cibacron brilliant red 3B-A | 73.3 | - | 25 | (Inal and Erduran, 2015) |
| SA/poly(N-vinyl-2-pyrrolidone) beads | Remazol brilliant blue R | 55.28 | - | 25 | (Inal and Erduran, 2015) |
| Alginate–montmorillonite composite beads | Methylene blue | 181.8 | - | 40 | (Uyar et al., 2016) |
3.1.1. Activated carbon/alginate beads
Recent studies have shown that AC immobilized into calcium alginate removed a significant amount of dyes from wastewater (Hassan et al., 2014b). Benhouria et al. prepared bentonite-alginate beads, activated carbon-alginate beads, and activated carbon-bentonite-alginate beads via a simple fabrication method to remove methylene blue (MB). The results showed that maximum monolayer adsorption capacity of activated carbon-bentonite-alginate beads was 756.97 mg g−1 at 30 °C with high resilience on adsorption efficiency after six regeneration cycles (Benhouria et al., 2015). Annadurai et al. studied batch adsorption equilibrium of Rhodamine 6G using activated carbon incorporated into calcium alginate beads and obtained high percentages of adsorption of Rhodamine 6G (Annadurai et al., 2002).
Although the activated carbon and alginate composite processes excellent adsorption properties for dyes, the cost and reuse of activated carbon is still of concern. In order to make the activated carbon-alginate beads a magnetically separable adsorbent, the magnetic beads were prepared with a high magnetic sensitivity under an external magnetic field. This provides an easy and efficient way to separate the beads from aqueous solution.
3.1.2. Graphene oxide/alginate composites
The GO and alginate biopolymer composites offer great potential for dye removal from wastewaters. For example, Yin et al. successfully fabricated graphene oxide (GO)/sodium alginate (SA)/polyacrylamide (PAM) (GO/SA/PAM) composite hydrogels for adsorption of cationic dyes (R6G, MB, MG, and BG) and anionic dyes (CA, MO, BR and RB) (Fan et al., 2013). In addition, Fan et al. fabricated a novel graphene oxide (GO)/sodium alginate (SA)/polyacrylamide (PAM) ternary nanocomposite hydrogel through free-radical polymerization of acrylamide (AAm) and SA in the presence of GO in an aqueous system followed with ionically crosslinking of calcium ions. The GO/SA/PAM ternary nanocomposite hydrogel exhibited excellent adsorption properties for water-soluble dyes. After introducing GO, the dye adsorption capacities of the hydrogel were significantly improved (Fan et al., 2013). Li et al. prepared the calcium alginate-GO composites and found that the maximum MB adsorption capacity obtained from Langmuir isotherm equation was 181.81 mg g−1. The adsorption reaction was exothermic and spontaneous in nature, occurring on the homogenous surface of GO/CA by monolayer adsorption (Li et al., 2013).
3.1.3. Carbon nanotubes (CNTs)/Ca-alginate beads
Carbon nanotubes (CNTs) have been intensively studied as a potential material to be used in a variety of applications based on their specific physical and chemical properties (Gupta et al., 2013; Gupta and Saleh, 2013; Wang et al., 2018c). Sui et al. investigated the adsorption of methylene blue (MB) and methyl orange (MO) ionic dyes onto calcium alginate/multi-walled carbon nanotubes (CA/MWCNT) composite fibers with varying MWCNTs content and pH values. The results showed that an introduction of MWCNTs increased the adsorption capacity of MO by 3 times, and enhanced the adsorption rate for MB compared to that of native CA (Sui et al., 2012). Li et al. prepared the CA/MWCNTs composite fiber to remove MO anionic dyes and the results illustrated that the introduction of MWCNTs obviously increased the adsorption capacity of MO, reaching about 14.13 mg g−1 (Li et al., 2012). Although the adsorption capacity increased using CNTs, the tedious centrifugation separation process might be a limiting factor and thus introducing magnetic properties into multi-wall carbon nanotube system will help with the separation process (Gong et al., 2009).
3.1.4. Other alginate nanocomposites
In addition to GO and CNTs, alginate has been blended with other natural and engineered nanoparticles to form nanocomposites to enhance the adsorption capacity (Tesh and Scott, 2014; Wang et al., 2018a; Wang et al., 2018b; Wang et al., 2018e). Mohammadi et al. prepared superparamagnetic sodium alginate-coated Fe3O4 nanoparticles for removal of malachite green (MG) from aqueous solutions. The maximum adsorption capacity obtained from Langmuir isotherm equation was 47.84 mg g−1 (Mohammadi et al., 2014). Liu et al. prepared a new kind of porous beads by immobilizing halloysite nanotubes with alginate and found that the maximum MB adsorption capacity of about 250 mg g−1. After 10 successive adsorption-desorption cycles, the removal efficiency of MB could be kept above 90% (Liu et al., 2012b). The alginate gel beads populated by halloysite nanotubes improved their ability to capture the dye so that they can have important implications for the enhancement of controlled adsorption. Compared to the unloaded gel beads, the hybrid gel beads are very effective and efficient for removing positively charged dye from the aqueous phase with enhanced properties (Cavallaro et al., 2013).
Some novel synthesized composites showed great promise in removal of dyes. Nano-sized montmorillonite (MMT)/calcium alginate (CA) composite synthesized by Hassani et al. removed basic red 46 (BR46) in aqueous solution with the maximum adsorption capacity of about 35 mg g−1 (Hassani et al., 2015). Wang et al. prepared a series of NaAlg-g-p(AA-co-St)/organo-I/S nanocomposite absorbents to remove methylene blue (MB) and found that the nanocomposite can rapidly adsorb MB with an adsorption capacity of 1843.46 mg g−1 (Wang et al., 2013a). TiO2 immobilized in a Ca-alginate bead retained its photoactivity during all of the experiments and the TiO2-gel beads presented good stability in water for maintaining its shape after several uses (Albarelli et al., 2009). Impregnated calcium alginate beads with nano-goethite (CABI nano-goethite) removed congo red (CR) from an aqueous solution, the maximum monolayer adsorption capacity was 181.1 mg g−1 at pH 3.0 and the adsorption process was endothermic and favored at high temperature (Munagapati and Kim, 2017).
3.1.5. Magnetic Ca-alginate beads
A series of studies have reported that alginate beads containing magnetic nanoparticles and activated carbon (AC-MAB) can selectively remove two dyes with different charges: positively charged MB and negatively charged methyl orange (MO). The adsorption capacity of beads was found to be higher than non encapsulated AC for MB and of the same order of magnitude for MO. The AC-MAB system selectively and strongly adsorbs MB due to the presence of carboxylate functions of both alginate and magnetic nanoparticles through ionic exchange with calcium ions (Rocher et al., 2008).
Rocher et al. prepared another magnetic alginate beads by an extrusion technique and crosslinked with epichlorohydrin which contains both magnetic nanoparticles and activated carbon (Rocher et al., 2010). With the addition of magnetic properties, the beads can be easily recovered or manipulated by an external magnetic field. Two mechanisms can explained the adsorption process: (1) a hydrophobic adsorption onto encapsulated activated carbon which depends neither on the electrical charge of the dye, nor of the solution pH; (2) an ionic exchange between the positively charged dye and calcium ions and sodium ions, the counter ions of the carboxylate functions of both alginate and citrate-coated magnetic nanoparticles (Rocher et al., 2010).
Rosales et al. studied the decolorization of dyes under electro-Fenton process using Fe alginate gel beads and found that around 98–100% of dye decolorization was obtained for both dyes by an electro-Fenton process in successive batches (Rosales et al., 2012). Mahmoodi synthesized magnetic ferrite nanoparticle-alginate composite used to remove dyes from the binary system. The maximum dye adsorption capacity of MFN-alginate was 106 mg g−1, 25 mg g−1, and 56 mg g−1 for BB9, BB41, and BR18, respectively (Mahmoodi, 2013).
3.1.6. Microalgae immobilized in Ca-alginate beads
Immobilization of microbial cells in alginate beads has received increasing interest for dye removal. Chen et al. developed an efficient sol-gel method for fabricating alginate-silicate organic-inorganic gel beads for immobilization of P. luteola cells and demonstrated the usefulness of such immobilized cells system in azo dye decolorization. The results indicated that the alginate-silicate matrix showed improvement over other synthetic or natural polymer gel matrices for immobilizing P. luteola in decolorization of Reactive Red 22 (Chen and Lin, 2007). Enayatzamir et al. studied the ability of white-rot fungus Phanerochaete chrysosporium immobilized on Ca-alginate beads to decolorize different recalcitrant azo dyes. The results showed that the MnP secreted by the fungus played the main role while adsorption was found to be negligible except for the dye BB in this decoloration process (Enayatzamir et al., 2010). Daâssi et al. found that the immobilization of P. laccase into Ca-alginate beads improved its thermal and storage stabilities and the immobilized P. laccase exhibited efficient textile dye decolorization in several successive batches (Daâssi et al., 2014).
3.2. Heavy metals
Heavy metals in wastewater of industry and mining enterprises are of great environmental concerns due to their low toxicity thresholds and cumulative biological effects (Inyang et al., 2012; Xue et al., 2012; Zhou et al., 2013). It is extremely expensive to remove heavy metals from wastewater and reduce their toxicity in the environment. Several low-cost adsorbents and biopolymers, such as alginate and chitosan extracted from microalgae, shrimp, crab, and fungi are known to bind metal ions and could be used for heavy metal removal from wastewater (Babel and Kurniawan, 2003; Zhou et al., 2014a; Zhou et al., 2014b; Zhou et al., 2013). Alginate is rich in carboxyl, hydroxyl and other active functional groups, which can react with heavy metals through ion exchange or complex reaction. Therefore, it can be used as adsorption material to remove heavy metals. The performance of alginate-based composites for heavy metal removal is summarized in Table 2. Each type of the composites is highlighted below.
Table 2.
Alginate-based composites for the removal of heavy metals from aqueous solution
| Adsorbent | Adsorbate | Adsorption capacity (mg g−1) | pH | Temperature (°C) | Ref. |
|---|---|---|---|---|---|
| Biochar-alginate beads | Cd(II) | 9.73 | 6.0 | - | (Roh et al., 2015) |
| Graphite nano carbon beads | Co(II) | 11.63 | 5.0 | Ambient T | (Jung et al., 2015) |
| Graphite nano carbon beads | Ni(II) | 11.48 | 5.0 | Ambient T | (Jung et al., 2015) |
| Chitosan coated calcium alginate | Ni(II) | 222.2 | 5.0 | Room T | (Vijaya et al., 2008) |
| Alginate–chitosan hybrid gel beads | Cu(II) | 8.38 | 3.5 | 25 | (Gotoh et al., 2004b) |
| Alginate–chitosan hybrid gel beads | Cd(II) | 6.63 | 3.5 | 25 | (Gotoh et al., 2004b) |
| Alginate–chitosan hybrid gel beads | Co(II) | 3.18 | 3.5 | 25 | (Gotoh et al., 2004b) |
| Iron oxide loaded alginate beads | As(V) | 0.0226 | 7.0 | - | (Zouboulis and Katsoyiannis, 2002) |
| Waste metal (hydr)oxide in CA beads | As(III) | 126.5 | 8.0 | 20 | (Escudero et al., 2009) |
| Waste metal (hydr)oxide in CA beads | As(V) | 41.6 | 8.0 | 20 | (Escudero et al., 2009) |
| CA/GO beads | Cu(II) | 60.2 | - | - | (Algothmi et al., 2013) |
| Biochar-alginate capsule | Pb(II) | 263.158 | 5.0 | 27 | (Do and Lee, 2013) |
| CA beads from Laminaria digitata | Cu(II) | 88.95 | 4.5 | 25 | (Papageorgiou et al., 2006) |
| CA beads from Laminaria digitata | Cd(II) | 130.77 | 4.5 | 25 | (Papageorgiou et al., 2006) |
| CA beads from Laminaria digitata | Pb(II) | 374.67 | 4.5 | 25 | (Papageorgiou et al., 2006) |
| Orange peel cellulose immobilized CA beads | Cu(II) | 166.7 | - | 28 | (Lai et al., 2010) |
| Orange peel cellulose immobilized CA beads | Pb(II) | 128.2 | - | 28 | (Lai et al., 2010) |
| Orange peel cellulose immobilized CA beads | Zn(II) | 156.25 | - | 28 | (Lai et al., 2010) |
| Banana peel cellulose immobilized CA beads | Cu(II) | 163.93 | - | 28 | (Lai et al., 2010) |
| Banana peel cellulose immobilized CA beads | Pb(II) | 121.95 | - | 28 | (Lai et al., 2010) |
| Banana peel cellulose immobilized CA beads | Zn(II) | 183.85 | - | 28 | (Lai et al., 2010) |
| Ca-alginate beads | Cu(II), | 84.39 | 4.5 | 25 | (Papageorgiou et al., 2009) |
| Ca-alginate beads | Cd(II) | 141.97 | 4.5 | 25 | (Papageorgiou et al., 2009) |
| Ca-alginate beads | Pb(II) | 360.11 | 4.5 | 25 | (Papageorgiou et al., 2009) |
| Sodium alginate | Cu(II) | 167.1 | 4.5 | - | (Wang et al., 2016a) |
| Sodium alginate | Cd(II) | 179.0 | 4.5 | - | (Wang et al., 2016a) |
| Sodium alginate | Pb(II) | 435.3 | 4.5 | - | (Wang et al., 2016a) |
| Polyvinyl alcohol (PVA)- SA beads | Cu(II) | 0.69 | - | - | (Cai et al., 2016) |
| Halloysite/alginate nanocomposite beads | Pb(II) | 325 | 5.0 | 25 | (Chiew et al., 2016) |
| Polyvinyl alcohol (PVA)- SA beads | Cd(II) | 0.52 | - | - | (Cai et al., 2016) |
| Polyvinyl alcohol (PVA)- SA beads | Pb(II) | 0.60 | - | - | (Cai et al., 2016) |
| Ca-Fe beads | As(V) | 352 | - | - | (Min and Hering, 1998) |
| SA-hydroxyapatite-CNT beads | Co(II) | 347.8 | 6.8 | 21 | (Karkeh-abadi et al., 2016) |
| Fe0-Fe3O4 nanocomposites embedded polyvinyl alcohol (PVA)/sodium alginate | Cr(VI) | 5.0 | 30 | (Lv et al., 2013) | |
| Magnetic alginate beads | Pb(II) | 100 | 4.7 | Room T | (Bée et al., 2011) |
| Magnetic alginate beads | Pb(II) | 50 | 7.0 | 30 | (Idris et al., 2012) |
| Alginate-montmorillonite/polyaniline nanocomposite | Cr(VI) | 29.89 | - | - | (Olad and Farshi Azhar, 2014) |
| Bio-polymeric beads | Cr(VI) | 0.833 | 6.0 | - | (Bajpai et al., 2004) |
| Fe3O4@Alg-Ce magnetic beads | Cr(VI) | 9.166 | 5.0 | 30 | (Gopalakannan and Viswanathan, 2015) |
| Fe3O4@Alg-Ce magnetic beads | Cr(VI) | 11.069 | 5.0 | 40 | (Gopalakannan and Viswanathan, 2015) |
| Fe3O4@Alg-Ce magnetic beads | Cr(VI) | 12.503 | 5.0 | 50 | (Gopalakannan and Viswanathan, 2015) |
| Chitosan–alginate beads | Cu (II) | 67.66 | 4.5 | Room T | (Ngah and Fatinathan, 2008) |
| Microgel/SA composite | Cu(II) | 46.39 | - | - | (Zhao et al., 2016) |
| Alginate activated carbon beads | Phenol | 96.0 | 3 | 25 | (Kim et al., 2008) |
| Alginate activated carbon beads | Phenol | 85.6 | 7 | 25 | (Kim et al., 2008) |
| Alginate activated carbon beads | Phenol | 69.6 | 10 | 25 | (Kim et al., 2008) |
| Nanochitosan/SA/microcrystalline cellulose beads | Cu(II) | 43.32 | - | - | (Vijayalakshmi et al., 2016) |
| Magnetic nanocomposite beads | Cu(II) | 72.99 | 6.0 | 25 | (Bakr et al., 2014) |
| Graphene/alginate double-network nanocomposite | Cu(II) | 169.5 | 4.0 | - | (Zhuang et al., 2016a) |
| Graphene/alginate double-network nanocomposite | Cr(VI) | 72.5 | 4.0 | - | (Zhuang et al., 2016a) |
| Sodium alginate/graphene oxide aerogel | Cu(II) | 98.0 | 5.0 | 30 | (Jiao et al., 2016) |
| Sodium alginate/graphene oxide aerogel | Pb(II) | 267.4 | 5.5 | 30 | (Jiao et al., 2016) |
| Nanohydroxyapatite–alginate composite | Pb(II) | 270.3 | 5.0 | Ambient T | (Googerdchian et al., 2012) |
| Halloysite nanotube–alginate hybrid beads | Cu(II) | 74.13 | - | - | (Wang et al., 2013b) |
| Silica nanopowders/alginate composite | Pb(II) | 83.33 | 5.0 | - | (Soltani et al., 2014) |
| Alginate activated carbon bead | Zn(II) | 135 | 6.8 | 32 | (Choi et al., 2009) |
| Alginate activated carbon bead | Toluene | 215 | 6.8 | 32 | (Choi et al., 2009) |
| Alginate Pleurotus ostreatus | Pb(II) | 121.21 | 6.5 | 25 | (Xiangliang et al., 2005) |
| White-rot fungus Trametes versicolor in CA bead | Cd(II) | 120.6 | 6.0 | 25 | (Arıca et al., 2001) |
| Lentinus sajor-caju immobilized Ca-alginate | Cd(II) | 123.5 | 6.0 | 25 | (Bayramoglu et al., 2002) |
| Alginate-Ayous wood sawdust (Triplochiton scleroxylon) | Cd(II) | 6.21 | - | - | (Njimou et al., 2016) |
| CA immobilized Phanerochaete chrysosporium | Cd(II) | 85.4 | 6.0 | 25 | (Kaçar et al., 2002) |
| CA immobilized Phanerochaete chrysosporium | Hg(II) | 112.6 | 6.0 | 25 | (Kaçar et al., 2002) |
| Fe3O4 nanoparticles embedded SA | Cd(II) | 97.8 | 6.2 | (Jiao et al., 2015) | |
| CA immobilized Phanerochaete chrysosporium | Pb(II) | 355 | 7.0 | 25 | (Yakup Arıca et al., 2003) |
| CA immobilized Phanerochaete chrysosporium | Zn(II) | 48 | 7.0 | 25 | (Yakup Arıca et al., 2003) |
| Spirulina platensis TISTR 8217 immobilized in alginate | Cd(II) | 70.92 | 7.0 | 26 | (Rangsayatorn et al., 2004) |
| Ca-alginate immobilized wood-rotting fungus | Hg(II) | 403.2 | 6.0 | 20 | (Arıca et al., 2004) |
| Ca-alginate immobilized wood-rotting fungus | Zn(II) | 54.0 | 6.0 | 20 | (Arıca et al., 2004) |
| Ca-alginate immobilized wood-rotting fungus | Cd(II) | 191.6 | 6.0 | 20 | (Arıca et al., 2004) |
| Ca-alginate immobilized-algal beads | Hg(II) | 116.8 | 5.0 | 25 | (Bayramoğlu et al., 2006) |
| Ca-alginate immobilized-algal beads | Cd(II) | 88.6 | 5.0 | 25 | (Bayramoğlu et al., 2006) |
| Ca-alginate immobilized-algal beads | Pb(II) | 384.4 | 5.0 | 25 | (Bayramoğlu et al., 2006) |
| Bacterial consortia immobilized in alginate beads | Cr(VI) | 657 | 3.0 | 30 | (Samuel et al., 2013) |
| Alginate–goethite beads | Cr(Ⅲ) | 20.67 | 3.0 | 20 | (Lazaridis and Charalambous, 2005) |
| Alginate–goethite beads | Cr(VI) | 23.38 | 3.0 | 20 | (Lazaridis and Charalambous, 2005) |
| Scenedesmus quadricauda immobilized Ca-alginate beads | Cu(II) | 75.6 | 5.0 | 25 | (Bayramoğlu and Yakup Arıca, 2009) |
| Scenedesmus quadricauda immobilized Ca-alginate beads | Zn(II) | 55.2 | 5.0 | 25 | (Bayramoğlu and Yakup Arıca, 2009) |
| Scenedesmus quadricauda immobilized Ca-alginate beads | Ni(II) | 30.4 | 5.0 | 25 | (Bayramoğlu and Yakup Arıca, 2009) |
| SA-polyaniline nanofibers | Cr(VI) | 73.34 | 4.2 | 30 | (Karthik and Meenakshi, 2015) |
| SA-polyaniline nanofibers | Cr(VI) | 74.46 | 4.2 | 40 | (Karthik and Meenakshi, 2015) |
| SA-polyaniline nanofibers | Cr(VI) | 75.82 | 4.2 | 50 | (Karthik and Meenakshi, 2015) |
| Ca-alginate immobilized sericite bead | Ni(II) | 10.743 | 7.5 | (Jeon and Cha, 2015) | |
| Goethite impregnated calcium alginate beads | As(V) | 30.44 | 5.0 | 25 | (Basu et al., 2015) |
| Phosphate-embedded calcium alginate beads | Pb(II) | 263.16 | 4.0 | 25 | (Wang et al., 2016b) |
| Phosphate-embedded calcium alginate beads | Cd(II) | 82.64 | 5.5 | 25 | (Wang et al., 2016b) |
| SA-graft-poly(methyl methacrylate) beads | Pb(II) | 526 | - | - | (Salisu et al., 2016a) |
| Alginate graft polyacrylonitrile beads | Pb(II) | 454 | - | - | (Salisu et al., 2016b) |
| SA–carboxymethyl cellulose gel beads | Pb(II) | 1727 | 5.0 | 37 | (Ren et al., 2016) |
| Quercetin loaded nanoparticles based on alginate | Pb(II) | 140.37 | 7.0 | 25 | (Qi et al., 2015) |
| Functional CNTs-SA | U(II) | 6.01 | 6.0 | Ambient T | (Allaboun et al., 2016) |
| CNTs/CA | Cu(II) | 84.88 | 5.0 | 20 | (Li et al., 2010) |
3.2.1. Ca-alginate beads
Alginate with a high M/G ratio, extracted from Laminaria digitata, was evaluated for Cu(II), Cd(II) and Pb(II) sorption in acidic solutions, in the form of calcium cross-linked beads. The high M/G ratio of alginate extracted from this algal species is most likely the determining factor for the increased adsorption capacity of the investigated metals, and the mannuronic acid in particular is responsible for the ion exchange mechanism. The presence of carboxyl groups in the alginate structure enhances the adsorption of many metal ions compared with other adsorbents. There are pronounced differences between sorption capacities of the alginate beads for different metals examined, with a general order of Pb(II) > Cu(II) > Cd(II) (Papageorgiou et al., 2006). Alginate gel beads showed a high affinity for heavy metal ions of Cu(II) and Mn(II), especially in a low concentration region. After covalently cross-linked with 1,6-diaminohexane bridges, the matrix of alginate gel beads was expected to improve the mechanical strength and resistance to chemical and microbial degradation of the beads, without the change in adsorption property. Ca-alginate beads also were applied to remove U(VI) ions from the solution and the results indicated that the interaction between uranium ions and Ca-alginate beads is endothermic in nature. Values of entropy and Gibbs free energy change suggested that the adsorption of uranium on Ca-alginate is a spontaneous process (Gok and Aytas, 2009). That is, the covalently cross-linked alginate gel beads are expected to be a good candidate for adsorbents to remove heavy metal ions from low heavy metal concentration wastewater (Gotoh et al., 2004a).
3.2.2. Activated carbon/Ca-alginate beads
While AC has used widely to remove organic substances, AC immobilized in alginate beads has been studied for the removal of heavy metals in water and wastewater (Hassan et al., 2014a). Hassan et al. investigated three different adsorbent materials namely; KOH-activated carbon-based apricot stone (C), calcium alginate beads (G) and calcium alginate/activated carbon composite beads (GC) for the As removal. The results indicated that GC exhibited the maximum As(V) adsorption (66.7 mg g−1 at 30 °C) (Hassan et al., 2014a). Kim et al. studied adsorption equilibrium characteristics of Cu and phenol onto powdered AC, alginate bead and alginate-activated carbon (AAC) bead. The adsorption capacity of Cu(II) onto different adsorbents was in the following order: alginate bead > AAC bead > AC. That of phenol was: AC > AAC bead > alginate bead (Kim et al., 2008). Choi et al. produced a novel alginate complex by impregnating synthetic zeolite and powdered activated carbon (PAC) into alginate gel bead and found that the composite could simultaneously remove zinc and toluene from aqueous solution. The maximum adsorption capacity of alginate complex for zinc and toluene obtained from Langmuir adsorption isotherm was 4.3 g kg−1 and 13.0 g kg−1, respectively (Choi et al., 2009).
3.2.3. Biochar/Ca-alginate composites
Previous studies have indicated that engineered biochar serves as a low-cost AC alternative for adsorption of heavy metals (Ding et al., 2016; Lyu et al., 2018a; Wan et al., 2016; Wan et al., 2017; Wang et al., 2018f). Adsorption of Cd(II) by biochar-alginate bead was studied using batch systems and continuous fixed bed columns and the results indicated that biochar-alginate beads, Ambrosia trifida L. var. Trifida biochar-alginate beads (ATLB-AB) can be applied as an eco-friendly and potential adsorbent for the removal of Cd(II) from groundwaters (Roh et al., 2015; Wang et al., 2018b). Do and Lee also synthesized a biochar-alginate capsule to remove lead ions Pb(II) from an aqueous solution. The maximum adsorption capacity for Pb(II) was found to be 263.158 mg g−1 at pH of 5.0 (Do and Lee, 2013).
3.2.4. Graphene oxide/Ca-alginate beads
In the last decade, GO has been studied for the removal of heavy metals, synthetic dyes, and other organic compounds (Bai et al., 2016; Chen et al., 2014; Chen et al., 2015; Zhang et al., 2013a). However, regeneration and separation of GO from aqueous media are difficult because it disperses so well in water. To solve this problem, several attempts were made to couple magnetic nanoparticles with fabrication of GO composites (Chandra et al., 2010; Liu et al., 2016; Shen et al., 2010; Zhang et al., 2013b; Zhang et al., 2012). Vu et al. fabricated magnetite GO encapsulated in calcium alginate beads (mGO/beads) to absorb Cr(VI) and As(V) from wastewater (Vu et al., 2017). They found that the mGO/bead maintained its activity in wastewater and exhibited greater adsorption efficiency for both Cr(VI) and As(V) than activated carbon and carbon nanotube. Lv et al. introduced graphene oxide (GO) into alginate gel before mixing with zero-valent iron nanoparticles (Fe0 NPs) to create Fe0 NPs embedded graphene oxide alginate beads (Fe@GOA beads), which were further reduced to Fe0 NPs embedded reduced graphene oxide-alginate beads (Fe@GA beads) (Lv et al., 2017). The Fe@GA beads were examined for Cr(VI) removal. The result showed that 1% of alginate and 1.5–2.0% of Fe0 by weight performed the best with a maximum adsorption capacity of about 34 mg g−1.
3.2.5. Carbon nanotubes (CNTs)/Ca-alginate beads
CNTs-alginate beads show synergistic effects on removal of heavy metals (Wang et al., 2018c). Studies have shown that the introduction of carbon nanotubes into alginate can improve the physicochemical properties of alginate-based composites, thereby enhancing its ability to adsorb heavy metals (Wang et al., 2018c). Li et al. mixed CNTs and SA and added to the CaCl2 solution to prepare CNTs-CA composites. The results show that the specific surface area and pore size of CA gel is 28 m2 g−1 and 0.06 cm3 g−1, respectively. When combined with CNTs, the high specific surface area and pore size of CNTs can form microchannels in the composites. The specific surface area and pore size of CNTs-CA composites were 76 m2 g−1 and 0.37 cm3 g−1, respectively. Under the same conditions, the adsorption capacity of Cu (II) on CA gel was better than that of CNTs. When the equilibrium concentration was 5 mg L−1, the adsorption capacity was 52.1mg g−1 for CA gel and increased to 67.9 mg g−1 for CNTs-CA (Li et al., 2010). Under the same conditions, the adsorption performance of CNTs-CA composites to Cu(II) was significantly higher than that of CNTs.
3.2.6. Other alginate nanocomposites
Alginate nanocomposites have excellent functional properties, biocompatibility, and special nano-effects for heavy metal remediation. Googerdchian et al. prepared the natural hydroxyapatite nanoparticles by mechanical activation method, and then compounded the particles with sodium alginate to prepare the granular and film SA/nano-hydroxyapatite composites for adsorption of Pb(II). The SA/nanohydroxyapatite composite membrane exhibited strong Pb(II) adsorption ability (Googerdchian et al., 2012). Soltani et al. entrapped silica nanopowders within calcium alginate and reported that an optimal initial pH of 5.0 was good for Pb(II) adsorption with the maximum adsorption capacity of 83.33 mg g−1 (Soltani et al., 2014). The potential of Hal/alginate nanocomposite beads for the removal of Pb(II) in aqueous solutions was investigated, and the Hal/alginate beads removed Pb(II) through ion exchange with Ca(II) followed by coordination with carboxylate groups of alginate, in addition to physisorption on Hal nanotubes (Chiew et al., 2016). Wang et al. examined the adsorption behavior of Cu(II) onto the halloysite nanotube-alginate hybrid bead by a continuous fixed bed column adsorption experiment and demonstrated that the adsorption capacity reached 74.13 mg g−1 (Wang et al., 2013b). Lazaridis et al. developed a composite alginate-goethite sorbent material for the removal of trivalent and hexavalent chromium ions from binary aqueous solutions. The sorption capacities for Cr(VI) and Cr(III) increased from 20.5 to 29.5 mg g−1 and 20.7 to 25.3 mg g−1, respectively, when temperature increased from 20 to 60 °C (Lazaridis and Charalambous, 2005).
3.2.7. Magnetic alginate beads
Several reports documented that magnetic materials fabricated in alginate had excellent performance for the removal of Co(II), Pb(II), Ni(II), Cu(II), Cr(VI), Au(III) (Bakr et al., 2014; Bée et al., 2011; Gopalakannan and Viswanathan, 2015). Bée et al. developed a magsorbent by encapsulation of magnetic functionalized nanoparticles in calcium-alginate beads and reported that it was easily collected from aqueous media by using an external magnetic field. The authors concluded that magnetic alginate beads could be efficiently used to remove heavy metals in a water treatment process (Bée et al., 2011). Synthesis of magnetic alginate hybrid beads was also tested for efficient removal of chromium (VI) (Gopalakannan and Viswanathan, 2015). The removal of nickel ions from aqueous solution using magnetic alginate microcapsules was studied and the result indicated that the sorption capacity of nickel increases with increasing pH (Ngomsik et al., 2006). Metal uptake capacity at low pH is attributed to an ionic exchange between protons and nickel ions. At higher pH, the adsorption of Ni is pH-dependent and corresponds to a competition between nickel and calcium ions. A new calcium-alginate magnetic sorbent was prepared by an electrostatic extrusion technique with a maximum adsorption capacities of arsenic and copper ions of 6.75 and 60.24 mg g−1, respectively, much higher than those of commercial adsorbents (Lim and Chen, 2007). The introduction of magnetic properties into calcium-alginate beads system combines the high adsorption capacity of calcium-alginate beads and the separation convenience of magnetic materials, offering a viable technique for future applications.
3.2.8. Microorganisms immobilized in Ca-alginate beads
Alginate can be used as an immobilizing carrier to maintain the biological activity of microorganisms and enzymes for the removal of heavy metal ions. A large number of studies have shown that microbial immobilization is effective for treatment of wastewaters with low concentrations of heavy metals to meet discharge standards. Natural polymers, such as cellulose derivatives, alginate, chitosan, and chitin have been used as the matrix for immobilization of microbial cells. These polymers are also known to bind metal ions (Zargar et al., 2015). Arica et al. used calcium alginate to immobilize white rot fungi to adsorb different metal ions in wastewater. The maximum experimental biosorption capacities for entrapped live and dead fungal mycelia of T. versicolor were 102.3 mg g−1 and 120.6 mg g−1, respectively (Arıca et al., 2001). Then Arica et al. immobilized the basidio spores of Phanerochaete chryosporium in alginate gel beads to remove Pb(II) and Zn(II) ions from artificial wastewater. The results indicated that the maximum biosorption capacity of alginate beads and both immobilized live and heat inactivated fungus were 230, 282 and 355 mg for Pb(II) and 30, 37 and 48 mg for Zn(II) per gram of dry biosorbents, respectively (Yakup Arıca et al., 2003). Ariea et al. also immobilized Funalia trogii biomass in Ca-alginate gel beads to adsorb Hg(II), Cd(II) and Zn(II) ions. The results indicated that the metal biosorption capacities of the heat-inactivated immobilized F. trogii for Hg(II), Cd(II) and Zn(II) were 403.2, 191.6, and 54.0 mg g−1, respectively, while Hg(II), Cd(II) and Zn(II) biosorption capacities of the immobilized live form were 333.0, 164.8 and 42.1 mg g−1, respectively (Arıca et al., 2004).
Bayramoglu et al. entrapped a white rot fungus species (Lentinus sajor-caju) biomass into alginate gel via a liquid curing method in the presence of Ca(II) to remove Cd(II) in a batch system. The maximum experimental biosorption capacities for entrapped live and dead fungal mycelia of L. sajur-caju were found to be 104.8 and 123.5 mg g−1, respectively. The kinetics of cadmium biosorption were fast, with approximately 85% of biosorption taking place within 30 min (Bayramoglu et al., 2002). Kacar et al. immobilized basidiospores of Phanerochaete chryosporium into Ca-alginate beads via entrapment, and the beads incubated for vegetation at 30 °C for 5 days. The alginate beads and both entrapped live and heat inactivated fungal mycelia of P. chryosporium were used for the removal of Hg(II) and Cd(II) ions from aqueous solution in the concentrations range of 30–500 mg L−1. The adsorption capacities of the immobilized live and heat inactivated fungal biomass reached 66.1 and 112.6 mg g−1 for mercury and 50.0 and 85.4 mg g−1 for cadmium, respectively (Kaçar et al., 2002).
A large body of evidence shows that algae can effectively absorb and enrich heavy metals in sewage (Prakasham et al., 1999; Rangsayatorn et al., 2004). The enrichment factor can reach several thousand times with an enrichment capacity up to 10% of its dry weight. Immobilization can increase the resistance of algal cells to heavy metal toxicity. Some scholars studied the removal rate of the immobilization system and compared heavy metal adsorption with dead and live algae. Prakasham et al. indicated that immobilized microbial on sodium alginate effectively removed hexavalent chromium at pH = 2. The adsorbed metal ions can be desorbed by dilute sulfuric acid (Prakasham et al., 1999). Rangsayatorn et al. studied biosorption of cadmium by immobilized Spirulina platensis on alginate gel and silica gel and found that the maximum biosorption capacities for alginate immobilized cells and silica immobilized cells were 70.92 and 36.63 mg g−1 biomass, respectively (Rangsayatorn et al., 2004).
3.3. Antibiotics
As an emerging pollutant, antibiotics pose a great threat to human health and the environment in spite of their low concentrations in the aquatic environment (Kümmerer, 2009). Traditional wastewater treatment processes do not normally work well with most antibiotics. Therefore, alginate-based composites have been investigated as a new adsorbent to remediate antibiotic pollution (Table 3).
Table 3.
Alginate-based composites for the removal of antibiotics from aqueous solution
| Adsorbent | Adsorbate | Adsorption capacity (mg g−1) | pH | Temperature (°C) | Ref. |
|---|---|---|---|---|---|
| EPCs@CMCS gel beads | Tetracycline | 136.9 | 6.0 | 25 | (He et al., 2016) |
| CMCS gel beads | Tetracycline | 9.47 | 6.0 | 25 | (He et al., 2016) |
| SA/graphene oxide beads | Ciprofloxacin | 86.12 | - | 25 | (Fei et al., 2016) |
| GO/CA fibers | Ciprofloxacin | 39.06 | 5.9 | Room T | (Wu et al., 2013) |
| GO/CA fibers | Tetracycline | 131.6 | 6.0 | 25 | (Zhu et al., 2018) |
| Alginate/graphene double network hydrogel | Tetracycline | 290.70 | 8.0 | 25 | (Zhuang et al., 2017) |
| Alginate/graphene double network hydrogel | Ciprofloxacin | 344.83 | 8.0 | 25 | (Zhuang et al., 2017) |
3.3.1. Magnetic alginate beads
There are a few reports about removal of antibiotics in water using magnetic alginate beads. Kim et al. found that nZVI-immobilized alginate beads removed trichloroethylene (TCE) from aqueous solution by >99.8% (Kim et al.). Konwar et al. prepared magnetic alginate-Fe3O4 hydrogel fibers using a simple laboratory micropipette and found that the magnetic alginate-Fe3O4 hydrogel fibers were effective in adsorption of ciprofloxacin hydrochloride, while the blank alginate hydrogel fiber did not show any significant adsorption. Anion exchange mechanism mainly controlled the adsorption of antibiotic and the formation of hydrogen bonding between the antibiotic and magnetic alginate beads can also result in the increase of adsorption capacity (Konwar et al., 2015). Such magnetic alginate-Fe3O4 hydrogel fibers can serve as a simple and cost-effective probe for adsorption/separation of antibiotics, with additional advantages of being easy to fabricate and having high thermal stability and mechanical strength (Konwar et al., 2015).
3.3.2. Graphene oxide/Ca-alginate beads
Wu et al. prepared a new biocomposite fibers by a wet spinning method using graphene oxide doped calcium alginate (GO/CA) (Wu et al., 2013). The comparative study indicated that the addition of GO could significantly improve the adsorption capacities of ciprofloxacin onto GO/CA fibers. The encapsulation of GO into SA made the materials more porous, provided π-π electron donor-acceptor interactions between graphene oxide and ciprofloxacin, and introduced C=O bonds into the composite (Fei et al., 2016). Zhu et al. prepared graphene oxide/calcium alginate (GO/CA) composite fibers via a freeze-drying method using calcium chloride as a cross-linking reagent between graphene oxide and sodium alginate. The maximum tetracycline adsorption capacity of the GO/CA composite fibers predicted by the Langmuir model reached 131.6 mg g−1. The mechanism of adsorption was the hydrogen bonding and π-π interaction which serve as predominant contributions to the significantly enhanced adsorption capability (Zhu et al., 2018). To improve the adsorption capacity of double network hydrogel, physical and chemical modifications were made on alginate/graphene double network hydrogel. The modified hydrogel featured a more porous structure and more functional groups than that before modification. The maximum adsorption capacities of tetracycline and ciprofloxacin on GAD were 290.70 and 344.83 mg g−1, respectively (Zhuang et al., 2017).
3.4. Other environmental applications
In addition to dyes, heavy metals and antibiotics, alginate-based composites have also been used for remediation of other pollutants (Table 4). For example, MnO2-alginate beads and alginate/Fe3O4 composite were used to remove Sr(II) from seawater (Hong et al., 2016; Hong et al., 2017). Removal of some rare earth elements and radionuclides from water was reported using different alginate-based composites (Elwakeel et al., 2017; Khotimchenko et al., 2015; Wu et al., 2010; Ye et al., 2009). Besides removal of cations, alginate-based nanomaterial composites were also studied for removal of some anions in water (Pandi and Viswanathan, 2015; Qiusheng et al., 2015; Siwek et al., 2016; Sujana et al., 2013). Electrochemically modified biochar calcium-alginate beads was also applied to remove phosphate under batch and continuous fixed-bed column conditions (Jung et al., 2017). With continued research and development, alginate-based nanocomposites will be increasingly applied to various fields of environmental remediation in the future.
Table 4.
Alginate-based composites for the removal of other pollutants from aqueous solution
| Adsorbent | Adsorbate | Adsorption capacity (mg g−1) | pH | Temperature (°C) | Ref. |
|---|---|---|---|---|---|
| MnO2-alginate beads | Sr(II) | 102.0 | - | 25 | (Hong et al., 2017) |
| Alginate/Fe3O4 composite | Sr(II) | 12.5 | 6.0 | 25 | (Hong et al., 2016) |
| Calcium alginates | Yi (III) | 97.087 | 6.0 | 24 | (Khotimchenko et al., 2015) |
| Sodium alginates | Yi (III) | 181.818 | 6.0 | 24 | (Khotimchenko et al., 2015) |
| Zirconium alginate beads | Fluoride | 28.05 | 2.0 | 30 | (Qiusheng et al., 2015) |
| Hydrous ferric oxide doped alginate beads | Fluoride | 8.90 | 7.0 | Ambient T | (Sujana et al., 2013) |
| n-HApAlgLa Composite Beads | Fluoride | 4.536 | - | Room T | (Pandi and Viswanathan, 2015) |
| n-HApAlgLa Composite Beads | Fluoride | 4.916 | - | Room T | (Pandi and Viswanathan, 2015) |
| n-HApAlgLa Composite Beads | Fluoride | 5.271 | - | Room T | (Pandi and Viswanathan, 2015) |
| Iron oxide loaded CA beads | La(III) | 123.5 | 5.0 | 25 | (Wu et al., 2010) |
| Magnetic alginate beads | La(III) | 250 | 4.0 | 25 | (Elwakeel et al., 2017) |
| nZnO-entrapped alginate (alginate-nZnO) beads | H2S | - | - | - | (Gautam et al., 2016; Gautam et al., 2017) |
| nZnO-entrapped alginate (alginate-nZnO) beads | Greenhouse gases’ | - | - | - | (Gautam et al., 2016; Gautam et al., 2017) |
| Silver nanoparticle-alginate composite beads | Disinfecting bacteria | - | - | - | (Lin et al., 2013) |
| Ammonium molybdophosphate–CA composite | Rb(Ⅰ) | 49.57 | 3.5–4.5 | 25 | (Ye et al., 2009) |
| Ammonium molybdophosphate–CA composite | Cs(Ⅰ) | 91.70 | 3.5–4.5 | 25 | (Ye et al., 2009) |
| Alginate/Iron (III) Chloride Capsules | Phosphate | - | - | 20 | (Siwek et al., 2016) |
| Electrochemically modified biochar CA beads | Phosphate | 214.2 | 4.0 | 10 | (Jung et al., 2017) |
| Electrochemically modified biochar CA beads | Phosphate | 292.98 | 4.0 | 20 | (Jung et al., 2017) |
| Electrochemically modified biochar CA beads | Phosphate | 342.67 | 4.0 | 30 | (Jung et al., 2017) |
4. Conclusions and future perspectives
Alginate-based composites have been fabricated by encapsulating various materials, such as AC, biochar, GO, CNT, magnetic and nanomaterials, as well as microorganisms into alginate hydrogels/beads with demonstrated utility as a biosorbent for environmental application. These composites offer great potential for real world applications for the removal of dyes, heavy metals, antibiotics, and other pollutants from water and wastewater. While alginate-based composites typically exhibit enhanced physical/mechanical properties over pure alginate gels or beads, the biocompatibility of alginate coupled with new properties of the encapsulated materials often lend synergetic functionalities of the new derivatives. Among these are ease of separation and regeneration of the biosorbent for wastewater treatment, reduced environmental risk of the encapsulated materials such as nanomaterials, and optimized bioprocesses of microbial immobilization technology.
Future environmental applications of alginate-based composites, which will likely evolve considerably, require further research on the mechanisms involved in pollutant uptakes by various alginate-based composites should be emphasized. Comparative studies among the composites under controlled laboratory settings can be conducted. Another research need is to optimize existing and engineer new alginate-based composites with distinct properties and novel functionalities for targeted applications. While new techniques, such as genetic engineering will likely advance the design and creation of new composite, more effective combination of materials to improve the adsorption capacity and mechanical, chemical and thermal stability when crosslinking with alginate beads can still be explored in a systematic fashion. Although not a focus in this paper, encapsulation strategies can be directly relevant to the production of new alginate-based composites to meet different applications. Parallel to this research need is the investigation into what chemically modifies alginate, which will benefit alginate-based composites. Because alginate contains abundant free hydroxyl and carboxyl groups distributed along the polymer chain backbone, chemical modifications of these two types of functional groups that alter the characteristics of alginate can be a future research area to fabricate new alginate-based composites for targeted environmental applications.
Most of the reported studies described in this review were conducted in a laboratory setting. Scaling up for real world applications in an uncontrolled environment requires further testing as the characteristics and mechanical/thermal stability of alginate-based composites may change. For example, the dynamic swelling of alginate-based composites in soil would be influenced by varying soil physical and chemical properties in the field. Therefore, the performance of alginate-based composites under field conditions can be explored further. Such work can also be realized in studies involving multicomponent solutions and/or complex effluents under dynamic conditions to mimic the field conditions. Potential risks associated with nanomaterials or metals in encapsulated in alginate should also be evaluated when considering applications in ambient soil and water environments.
In attempts to test alginate-based composites in large-scale applications, cost and effectiveness are important factors to be evaluated. Because microbial treatment is potentially less harmful to the environment and more cost-effective than chemical treatment or physical removal of soil or water to an off-site location, encapsulation of microorganisms in alginate beads as a carrier will be cost effective. Investigating the further effectiveness of immobilization technology, particularly in the areas of microbial survival, binding, and transport, as well as in-situ bioremediation of contaminated soil or groundwater will evolve alginate based technology and make it more competitive for use in remediation applications.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2016YFC0502602), the Key Agriculture R & D Program of Guizhou Province (NZ [2013]3012), the International Scientific and Technological Cooperation Project of Guizhou Province (G[2012]7050), the “Dawn of West China” Talent Training Program of the Chinese Academy of Sciences ( [2012]179) and the Opening Fund of State Key Laboratory of Environmental Geochemistry (SKLEG2018907). The authors would also like to thank Dr. Elizabeth George and two anonymous reviewers for their comments and suggestions. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the funding agencies or the U.S. Environmental Protection Agency.
References
- 1.Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, et al. 2014. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 99: 19–33. [DOI] [PubMed] [Google Scholar]
- 2.Akhtar MF, Hanif M, Ranjha NM 2016. Methods of synthesis of hydrogels … a review. Saudi Pharmaceutical Journal 24: 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albarelli JQ, Santos DT, Murphy S, Oelgemöller M. 2009. Use of ca–alginate as a novel support for tio2 immobilization in methylene blue decolorisation. Water Science and Technology 60: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 4.Algothmi WM, Bandaru NM, Yu Y, Shapter JG, Ellis AV 2013. Alginate–graphene oxide hybrid gel beads: An efficient copper adsorbent material. Journal of Colloid and Interface Science 397: 32–38. [DOI] [PubMed] [Google Scholar]
- 5.Ali I. 2012. New generation adsorbents for water treatment. Chemical reviews 112: 5073–5091. [DOI] [PubMed] [Google Scholar]
- 6.Ali I, Al-Othman ZA, Al-Warthan A. 2016a. Molecular uptake of congo red dye from water on iron composite nano particles. Journal of Molecular Liquids 224: 171–176. [Google Scholar]
- 7.Ali I, Al-Othman ZA, Al-Warthan A. 2016b. Removal of secbumeton herbicide from water on composite nanoadsorbent. Desalination and Water Treatment 57: 10409–10421. [Google Scholar]
- 8.Ali I, Al-Othman ZA, Sanagi MM 2015. Green synthesis of iron nano-impregnated adsorbent for fast removal of fluoride from water. Journal of Molecular Liquids 211: 457–465. [Google Scholar]
- 9.Ali I, Gupta V. 2006. Advances in water treatment by adsorption technology. Nature protocols 1: 2661–2667. [DOI] [PubMed] [Google Scholar]
- 10.Allaboun H, Fares MM, Abu Al-Rub FA 2016. Removal of uranium and associated contaminants from aqueous solutions using functional carbon nanotubes-sodium alginate conjugates. Minerals 6: 9. [Google Scholar]
- 11.An T, Zhou L, Li G, Fu J, Sheng G. 2008. Recent patents on immobilized microorganism technology and its engineering application in wastewater treatment. Recent Patents on Engineering 2: 28–35. [Google Scholar]
- 12.Annadurai G, Juang R-S, Lee D-J 2002. Factorial design analysis for adsorption of dye on activated carbon beads incorporated with calcium alginate. Advances in Environmental Research 6: 191–198. [Google Scholar]
- 13.Aravindhan R, Fathima NN, Rao JR, Nair BU 2007. Equilibrium and thermodynamic studies on the removal of basic black dye using calcium alginate beads. Colloids and Surfaces A: Physicochemical and Engineering Aspects 299: 232–238. [Google Scholar]
- 14.Arıca MY, Bayramoǧlu G, Yılmaz M, Bektaş S, Genç Ö. 2004. Biosorption of hg2+, cd2+, and zn2+ by ca-alginate and immobilized wood-rotting fungus funalia trogii. Journal of Hazardous Materials 109: 191–199. [DOI] [PubMed] [Google Scholar]
- 15.Arıca MY, Kaçar Y, Genç Ö. 2001. Entrapment of white-rot fungus trametes versicolor in ca-alginate beads: Preparation and biosorption kinetic analysis for cadmium removal from an aqueous solution. Bioresource Technology 80: 121–129. [DOI] [PubMed] [Google Scholar]
- 16.Babel S, Kurniawan TA 2003. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. Journal of Hazardous Materials 97: 219–243. [DOI] [PubMed] [Google Scholar]
- 17.Bai J, Sun HM, Yin XJ, Yin XQ, Wang SS, Creamer AE, et al. 2016. Oxygen-content-controllable graphene oxide from electron-beam-irradiated graphite: Synthesis, characterization, and removal of aqueous lead [pb(ii)]. Acs Applied Materials & Interfaces 8: 25289–25296. [DOI] [PubMed] [Google Scholar]
- 18.Bajpai J, Shrivastava R, Bajpai AK 2004. Dynamic and equilibrium studies on adsorption of cr(vi) ions onto binary bio-polymeric beads of cross linked alginate and gelatin. Colloids and Surfaces A: Physicochemical and Engineering Aspects 236: 81–90. [Google Scholar]
- 19.Bakr A-SA, Moustafa YM, Khalil MM, Yehia MM, Motawea EA 2014. Magnetic nanocomposite beads: Synthesis and uptake of cu (ii) ions from aqueous solutions. Canadian Journal of Chemistry 93: 289–296. [Google Scholar]
- 20.Basu H, Singhal R, Pimple M, Reddy A. 2015. Arsenic removal from groundwater by goethite impregnated calcium alginate beads. Water, Air, & Soil Pollution 226: 22. [Google Scholar]
- 21.Bayramoglu G, Denizli A, Bektas S, Yakup Arica M. 2002. Entrapment of lentinus sajor-caju into ca-alginate gel beads for removal of cd(ii) ions from aqueous solution: Preparation and biosorption kinetics analysis. Microchemical Journal 72: 63–76. [Google Scholar]
- 22.Bayramoğlu G, Tuzun I, Celik G, Yilmaz M, Arica MY 2006. Biosorption of mercury(ii), cadmium(ii) and lead(ii) ions from aqueous system by microalgae chlamydomonas reinhardtii immobilized in alginate beads. International Journal of Mineral Processing 81: 35–43. [Google Scholar]
- 23.Bayramoğlu G, Yakup Arıca M. 2009. Construction a hybrid biosorbent using scenedesmus quadricauda and ca-alginate for biosorption of cu(ii), zn(ii) and ni(ii): Kinetics and equilibrium studies. Bioresource Technology 100: 186–193. [DOI] [PubMed] [Google Scholar]
- 24.Bée A, Talbot D, Abramson S, Dupuis V. 2011. Magnetic alginate beads for pb (ii) ions removal from wastewater. Journal of Colloid and Interface Science 362: 486–492. [DOI] [PubMed] [Google Scholar]
- 25.Belhouchat N, Zaghouane-Boudiaf H, Viseras C. 2017. Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads. Applied Clay Science 135: 9–15. [Google Scholar]
- 26.Benhouria A, Islam MA, Zaghouane-Boudiaf H, Boutahala M, Hameed B. 2015. Calcium alginate–bentonite–activated carbon composite beads as highly effective adsorbent for methylene blue. Chemical Engineering Journal 270: 621–630. [Google Scholar]
- 27.Burakov AE, Galunin EV, Burakova IV, Kucherova AE, Agarwal S, Tkachev AG, et al. 2018. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicology and Environmental Safety 148: 702–712. [DOI] [PubMed] [Google Scholar]
- 28.Cai C-X, Xu J, Deng N-F, Dong X-W, Tang H, Liang Y, et al. 2016. A novel approach of utilization of the fungal conidia biomass to remove heavy metals from the aqueous solution through immobilization. Scientific Reports 6: 36546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai T, Chen L, Ren Q, Cai S, Zhang J. 2011. The biodegradation pathway of triethylamine and its biodegradation by immobilized arthrobacter protophormiae cells. Journal of Hazardous Materials 186: 59–66. [DOI] [PubMed] [Google Scholar]
- 30.Cavallaro G, Gianguzza A, Lazzara G, Milioto S, Piazzese D. 2013. Alginate gel beads filled with halloysite nanotubes. Applied Clay Science 72: 132–137. [Google Scholar]
- 31.Chan LW, Jin Y, Heng PWS 2002. Cross-linking mechanisms of calcium and zinc in production of alginate microspheres. International Journal of Pharmaceutics 242: 255–258. [DOI] [PubMed] [Google Scholar]
- 32.Chandra V, Park J, Chun Y, Lee JW, Hwang I-C, Kim KS 2010. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS nano 4: 3979–3986. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, Gao B, Li H. 2014. Functionalization, ph, and ionic strength influenced sorption of sulfamethoxazole on graphene. Journal of Environmental Chemical Engineering 2: 310–315. [Google Scholar]
- 34.Chen H, Gao B, Li H. 2015. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. Journal of Hazardous Materials 282: 201–207. [DOI] [PubMed] [Google Scholar]
- 35.Chen J-P, Lin Y-S 2007. Decolorization of azo dye by immobilized pseudomonas luteola entrapped in alginate–silicate sol–gel beads. Process Biochemistry 42: 934–942. [Google Scholar]
- 36.Chiew CSC, Yeoh HK, Pasbakhsh P, Krishnaiah K, Poh PE, Tey BT, et al. 2016. Halloysite/alginate nanocomposite beads: Kinetics, equilibrium and mechanism for lead adsorption. Applied Clay Science 119: 301–310. [Google Scholar]
- 37.Ching SH, Bansal N, Bhandari B. 2017. Alginate gel particles–a review of production techniques and physical properties. Critical reviews in food science and nutrition 57: 1133–1152. [DOI] [PubMed] [Google Scholar]
- 38.Choi J-W, Yang K-S, Kim D-J, Lee CE 2009. Adsorption of zinc and toluene by alginate complex impregnated with zeolite and activated carbon. Current Applied Physics 9: 694–697. [Google Scholar]
- 39.Cohen Y. 2001. Biofiltration-the treatment of fluids by microorganisms immobilized into the filter bedding material: A review. Bioresource Technology 77: 257–274. [DOI] [PubMed] [Google Scholar]
- 40.Covarrubias SA, De-Bashan LE, Moreno M, Bashan Y. 2012. Alginate beads provide a beneficial physical barrier against native microorganisms in wastewater treated with immobilized bacteria and microalgae. Applied Microbiology and Biotechnology 93: 2669–2680. [DOI] [PubMed] [Google Scholar]
- 41.Daâssi D, Rodríguez-Couto S, Nasri M, Mechichi T. 2014. Biodegradation of textile dyes by immobilized laccase from coriolopsis gallica into ca-alginate beads. International Biodeterioration & Biodegradation 90: 71–78. [Google Scholar]
- 42.De-Bashan LE, Moreno M, Hernandez J-P, Bashan Y. 2002. Removal of ammonium and phosphorus ions from synthetic wastewater by the microalgae chlorella vulgaris coimmobilized in alginate beads with the microalgae growth-promoting bacterium azospirillum brasilense. Water Research 36: 2941–2948. [DOI] [PubMed] [Google Scholar]
- 43.Devi GK, Kumar PS, Kumar KS 2016. Green synthesis of novel silver nanocomposite hydrogel based on sodium alginate as an efficient biosorbent for the dye wastewater treatment: Prediction of isotherm and kinetic parameters. Desalination and Water Treatment 57: 27686–27699. [Google Scholar]
- 44.Ding ZH, Hu X, Morales VL, Gao B. 2014. Filtration and transport of heavy metals in graphene oxide enabled sand columns. Chemical Engineering Journal 257: 248–252. [Google Scholar]
- 45.Ding ZH, Hu X, Wan YS, Wang SS, Gao B. 2016. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests. Journal of Industrial and Engineering Chemistry 33: 239–245. [Google Scholar]
- 46.Do XH, Lee BK 2013. Removal of pb2+ using a biochar-alginate capsule in aqueous solution and capsule regeneration. Journal of Environmental Management 131: 375–382. [DOI] [PubMed] [Google Scholar]
- 47.Elwakeel KZ, Daher A, Abd El-Fatah A, Abd El Monem H, Khalil MM 2017. Biosorption of lanthanum from aqueous solutions using magnetic alginate beads. Journal of Dispersion Science and Technology 38: 145–151. [Google Scholar]
- 48.Enayatzamir K, Alikhani H, Yakhchali B, Tabandeh F, Rodríguez-Couto S. 2010. Decolouration of azo dyes by phanerochaete chrysosporium immobilised into alginate beads. Environmental Science and Pollution Research 17: 145–153. [DOI] [PubMed] [Google Scholar]
- 49.Escudero C, Fiol N, Villaescusa I, Bollinger J-C 2009. Arsenic removal by a waste metal (hydr) oxide entrapped into calcium alginate beads. Journal of Hazardous Materials 164: 533–541. [DOI] [PubMed] [Google Scholar]
- 50.Fan J, Shi Z, Lian M, Li H, Yin J. 2013. Mechanically strong graphene oxide/sodium alginate/polyacrylamide nanocomposite hydrogel with improved dye adsorption capacity. Journal of Materials Chemistry A 1: 7433–7443. [Google Scholar]
- 51.Fang J, Gao B, Mosa A, Zhan L. 2017. Chemical activation of hickory and peanut hull hydrochars for removal of lead and methylene blue from aqueous solutions. Chemical Speciation and Bioavailability 29: 197–204. [Google Scholar]
- 52.Fang J, Gao B, Zimmerman AR, Ro KS, Chen JJ 2016. Physically (co2) activated hydrochars from hickory and peanut hull: Preparation, characterization, and sorption of methylene blue, lead, copper, and cadmium. Rsc Advances 6: 24906–24911. [Google Scholar]
- 53.Fang J, Zhan L, Ok YS, Gao B. 2018. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. Journal of Industrial and Engineering Chemistry 57: 15–21. [Google Scholar]
- 54.Fei Y, Li Y, Han S, Ma J. 2016. Adsorptive removal of ciprofloxacin by sodium alginate/graphene oxide composite beads from aqueous solution. Journal of Colloid and Interface Science 484: 196–204. [DOI] [PubMed] [Google Scholar]
- 55.Fomina M, Gadd GM 2014. Biosorption: Current perspectives on concept, definition and application. Bioresource Technology 160: 3–14. [DOI] [PubMed] [Google Scholar]
- 56.Fu F, Wang Q. 2011. Removal of heavy metal ions from wastewaters: A review. Journal of Environmental Management 92: 407–418. [DOI] [PubMed] [Google Scholar]
- 57.Gautam DP, Rahman S, Bezbaruah AN, Borhan MS 2016. Evaluation of calcium alginate entrapped nano zinc oxide to reduce gaseous emissions from liquid dairy manure. Applied Engineering in Agriculture 32: 89–102. [Google Scholar]
- 58.Gautam DP, Rahman S, Fortuna A-M, Borhan MS, Saini-Eidukat B, Bezbaruah AN 2017. Characterization of zinc oxide nanoparticle (nzno) alginate beads in reducing gaseous emission from swine manure. Environmental Technology 38: 1061–1074. [DOI] [PubMed] [Google Scholar]
- 59.Gok C, Aytas S. 2009. Biosorption of uranium (vi) from aqueous solution using calcium alginate beads. Journal of Hazardous Materials 168: 369–375. [DOI] [PubMed] [Google Scholar]
- 60.Gong J-L, Wang B, Zeng G-M, Yang C-P, Niu C-G, Niu Q-Y, et al. 2009. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. Journal of Hazardous Materials 164: 1517–1522. [DOI] [PubMed] [Google Scholar]
- 61.Googerdchian F, Moheb A, Emadi R. 2012. Lead sorption properties of nanohydroxyapatite–alginate composite adsorbents. Chemical Engineering Journal 200: 471–479. [Google Scholar]
- 62.Gopalakannan V, Viswanathan N. 2015. Synthesis of magnetic alginate hybrid beads for efficient chromium (vi) removal. International Journal of Biological Macromolecules 72: 862–867. [DOI] [PubMed] [Google Scholar]
- 63.Gotoh T, Matsushima K, Kikuchi K-I 2004a. Adsorption of cu and mn on covalently cross-linked alginate gel beads. Chemosphere 55: 57–64. [DOI] [PubMed] [Google Scholar]
- 64.Gotoh T, Matsushima K, Kikuchi K-I 2004b. Preparation of alginate–chitosan hybrid gel beads and adsorption of divalent metal ions. Chemosphere 55: 135–140. [DOI] [PubMed] [Google Scholar]
- 65.Gupta VK, Ali I. Environmental water: Advances in treatment, remediation and recycling: Newnes, 2012. [Google Scholar]
- 66.Gupta VK, Carrott PJM, Ribeiro Carrott MML, Suhas 2009. Low-cost adsorbents: Growing approach to wastewater treatment - a review. Critical Reviews in Environmental Science and Technology 39: 783–842. [Google Scholar]
- 67.Gupta VK, Kumar R, Nayak A, Saleh TA, Barakat MA 2013. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: A review. Advances in Colloid and Interface Science 193–194: 24–34. [DOI] [PubMed] [Google Scholar]
- 68.Gupta VK, Nayak A, Agarwal S, Tyagi I. 2014. Potential of activated carbon from waste rubber tire for the adsorption of phenolics: Effect of pre-treatment conditions. Journal of Colloid and Interface Science 417: 420–430. [DOI] [PubMed] [Google Scholar]
- 69.Gupta VK, Saleh TA 2013. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene-an overview. Environmental Science and Pollution Research 20: 2828–2843. [DOI] [PubMed] [Google Scholar]
- 70.Gupta VK, Suhas 2009. Application of low-cost adsorbents for dye removal – a review. Journal of Environmental Management 90: 2313–2342. [DOI] [PubMed] [Google Scholar]
- 71.Hassan A, Abdel-Mohsen A, Elhadidy H. 2014a. Adsorption of arsenic by activated carbon, calcium alginate and their composite beads. International journal of biological macromolecules 68: 125–130. [DOI] [PubMed] [Google Scholar]
- 72.Hassan A, Abdel-Mohsen A, Fouda MM 2014b. Comparative study of calcium alginate, activated carbon, and their composite beads on methylene blue adsorption. Carbohydrate Polymers 102: 192–198. [DOI] [PubMed] [Google Scholar]
- 73.Hassani A, Soltani RDC, Karaca S, Khataee A. 2015. Preparation of montmorillonite–alginate nanobiocomposite for adsorption of a textile dye in aqueous phase: Isotherm, kinetic and experimental design approaches. Journal of Industrial and Engineering Chemistry 21: 1197–1207. [Google Scholar]
- 74.He J, Dai J, Xie A, Tian S, Chang Z, Yan Y, et al. 2016. Preparation of macroscopic spherical porous carbons@ carboxymethylcellulose sodium gel beads and application for removal of tetracycline. RSC Advances 6: 84536–84546. [Google Scholar]
- 75.Hong H-J, Jeong HS, Kim B-G, Hong J, Park I-S, Ryu T, et al. 2016. Highly stable and magnetically separable alginate/fe3o4 composite for the removal of strontium (sr) from seawater. Chemosphere 165: 231–238. [DOI] [PubMed] [Google Scholar]
- 76.Hong H-J, Kim B-G, Hong J, Ryu J, Ryu T, Chung K-S, et al. 2017. Enhanced sr adsorption performance of mno2-alginate beads in seawater and evaluation of its mechanism. Chemical Engineering Journal 319: 163–169. [Google Scholar]
- 77.Idris A, Ismail NSM, Hassan N, Misran E, Ngomsik A-F 2012. Synthesis of magnetic alginate beads based on maghemite nanoparticles for pb(ii) removal in aqueous solution. Journal of Industrial and Engineering Chemistry 18: 1582–1589. [Google Scholar]
- 78.Inal M, Erduran N. 2015. Removal of various anionic dyes using sodium alginate/poly(n-vinyl-2-pyrrolidone) blend hydrogel beads. Polymer Bulletin 72: 1735–1752. [Google Scholar]
- 79.Inyang M, Gao B, Yao Y, Xue Y, Zimmerman AR, Pullammanappallil P, et al. 2012. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresource Technology 110: 50–56. [DOI] [PubMed] [Google Scholar]
- 80.Inyang M, Gao B, Zimmerman A, Zhang M, Chen H. 2014. Synthesis, characterization, and dye sorption ability of carbon nanotube–biochar nanocomposites. Chemical Engineering Journal 236: 39–46. [Google Scholar]
- 81.Inyang MI, Gao B, Yao Y, Xue YW, Zimmerman A, Mosa A, et al. 2016. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Critical Reviews in Environmental Science and Technology 46: 406–433. [Google Scholar]
- 82.Jeon C, Cha J-H 2015. Removal of nickel ions from industrial wastewater using immobilized sericite beads. Journal of Industrial and Engineering Chemistry 24: 107–112. [Google Scholar]
- 83.Jeon YS, Lei J, Kim J-H 2008. Dye adsorption characteristics of alginate/polyaspartate hydrogels. Journal of Industrial and Engineering Chemistry 14: 726–731. [Google Scholar]
- 84.Jiao C, Xiong J, Tao J, Xu S, Zhang D, Lin H, et al. 2016. Sodium alginate/graphene oxide aerogel with enhanced strength–toughness and its heavy metal adsorption study. International Journal of Biological Macromolecules 83: 133–141. [DOI] [PubMed] [Google Scholar]
- 85.Jiao L, Qi P, Liu Y, Wang B, Shan L. 2015. Fe3o4 nanoparticles embedded sodium alginate/pvp/calcium gel composite for removal of cd 2+. Journal of Nanomaterials 16: 257. [Google Scholar]
- 86.Jung K-W, Jeong T-U, Choi J-W, Ahn K-H, Lee S-H 2017. Adsorption of phosphate from aqueous solution using electrochemically modified biochar calcium-alginate beads: Batch and fixed-bed column performance. Bioresource Technology 244: 23–32. [DOI] [PubMed] [Google Scholar]
- 87.Jung W, Jeon B-H, Cho D-W, Roh H-S, Cho Y, Kim S-J, et al. 2015. Sorptive removal of heavy metals with nano-sized carbon immobilized alginate beads. Journal of Industrial and Engineering Chemistry 26: 364–369. [Google Scholar]
- 88.Junter G-A, Jouenne T. 2004. Immobilized viable microbial cells: From the process to the proteome… or the cart before the horse. Biotechnology Advances 22: 633–658. [DOI] [PubMed] [Google Scholar]
- 89.Kaçar Y, Arpa Ç, Tan S, Denizli A, Genç Ö, Arıca MY 2002. Biosorption of hg(ii) and cd(ii) from aqueous solutions: Comparison of biosorptive capacity of alginate and immobilized live and heat inactivated phanerochaete chrysosporium. Process Biochemistry 37: 601–610. [Google Scholar]
- 90.Karkeh-Abadi F, Saber-Samandari S, Saber-Samandari S. 2016. The impact of functionalized cnt in the network of sodium alginate-based nanocomposite beads on the removal of co(ii) ions from aqueous solutions. Journal of Hazardous Materials 312: 224–233. [DOI] [PubMed] [Google Scholar]
- 91.Karthik R, Meenakshi S. 2015. Removal of cr(vi) ions by adsorption onto sodium alginate-polyaniline nanofibers. International Journal of Biological Macromolecules 72: 711–717. [DOI] [PubMed] [Google Scholar]
- 92.Khotimchenko M, Kovalev V, Khozhaenko E, Khotimchenko R. 2015. Removal of yttrium (iii) ions from water solutions by alginate compounds. International Journal of Environmental Science and Technology 12: 3107–3116. [Google Scholar]
- 93.Kim H, Hong H-J, Jung J, Kim S-H, Yang J-W 2010. Degradation of trichloroethylene (tce) by nanoscale zero-valent iron (nzvi) immobilized in alginate bead. Journal of Hazardous Materials 176: 1038–1043. [DOI] [PubMed] [Google Scholar]
- 94.Kim TY, Jin HJ, Park SS, Kim SJ, Cho SY 2008. Adsorption equilibrium of copper ion and phenol by powdered activated carbon, alginate bead and alginate-activated carbon bead. Journal of Industrial and Engineering Chemistry 14: 714–719. [Google Scholar]
- 95.Konwar A, Gogoi A, Chowdhury D. 2015. Magnetic alginate-fe3o4 hydrogel fiber capable of ciprofloxacin hydrochloride adsorption/separation in aqueous solution. RSC Advances 5: 81573–81582. [Google Scholar]
- 96.Kümmerer K. 2009. Antibiotics in the aquatic environment – a review – part i. Chemosphere 75: 417–434. [DOI] [PubMed] [Google Scholar]
- 97.Lai Y-L, Thirumavalavan M, Lee J-F 2010. Effective adsorption of heavy metal ions (cu2+, pb2+, zn2+) from aqueous solution by immobilization of adsorbents on ca-alginate beads. Toxicological and Environ Chemistry 92: 697–705. [Google Scholar]
- 98.Lazaridis NK, Charalambous C. 2005. Sorptive removal of trivalent and hexavalent chromium from binary aqueous solutions by composite alginate–goethite beads. Water Research 39: 4385–4396. [DOI] [PubMed] [Google Scholar]
- 99.Lee KY, Rowley JA, Eiselt P, Moy EM, Bouhadir KH, Mooney DJ 2000. Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density. Macromolecules 33: 4291–4294. [Google Scholar]
- 100.Li Y, Du Q, Liu T, Sun J, Wang Y, Wu S, et al. 2013. Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohydrate Polymers 95: 501–507. [DOI] [PubMed] [Google Scholar]
- 101.Li Y, Liu F, Xia B, Du Q, Zhang P, Wang D, et al. 2010. Removal of copper from aqueous solution by carbon nanotube/calcium alginate composites. Journal of Hazardous Materials 177: 876–880. [DOI] [PubMed] [Google Scholar]
- 102.Li Y, Sui K, Liu R, Zhao X, Zhang Y, Liang H, et al. 2012. Removal of methyl orange from aqueous solution by calcium alginate/multi-walled carbon nanotubes composite fibers. Energy Procedia 16: 863–868. [Google Scholar]
- 103.Li ZM, Yao Y, Wei GT, Jiang WY, Wang YZ, Zhang LY 2016. Adsorption and heat-energy-aid desorption of cationic dye on a new thermo-sensitive adsorbent: Methyl cellulose/calcium alginate beads. Polymer Engineering and Science 56: 1382–1389. [Google Scholar]
- 104.Lim AP, Aris AZ 2014. A review on economically adsorbents on heavy metals removal in water and wastewater. Reviews in Environmental Science and Bio/Technology 13: 163–181. [Google Scholar]
- 105.Lim SF, Chen JP 2007. Synthesis of an innovative calcium-alginate magnetic sorbent for removal of multiple contaminants. Applied Surface Science 253: 5772–5775. [Google Scholar]
- 106.Lin S, Huang R, Cheng Y, Liu J, Lau BLT, Wiesner MR 2013. Silver nanoparticle-alginate composite beads for point-of-use drinking water disinfection. Water Research 47: 3959–3965. [DOI] [PubMed] [Google Scholar]
- 107.Liu H, Guo L, Liao S, Wang G. 2012a. Reutilization of immobilized fungus rhizopus sp. Lg04 to reduce toxic chromate. Journal of applied microbiology 112: 651–659. [DOI] [PubMed] [Google Scholar]
- 108.Liu L, Wan Y, Xie Y, Zhai R, Zhang B, Liu J. 2012b. The removal of dye from aqueous solution using alginate-halloysite nanotube beads. Chemical Engineering Journal 187: 210–216. [Google Scholar]
- 109.Liu TZ, Gao B, Fang JN, Wang B, Cao XD 2016. Biochar-supported carbon nanotube and graphene oxide nanocomposites for pb(ii) and cd(ii) removal. Rsc Advances 6: 24314–24319. [Google Scholar]
- 110.Lv X, Jiang G, Xue X, Wu D, Sheng T, Sun C, et al. 2013. Fe0-fe3o4 nanocomposites embedded polyvinyl alcohol/sodium alginate beads for chromium (vi) removal. Journal of Hazardous Materials 262: 748–758. [DOI] [PubMed] [Google Scholar]
- 111.Lv X, Zhang Y, Fu W, Cao J, Zhang J, Ma H, et al. 2017. Zero-valent iron nanoparticles embedded into reduced graphene oxide-alginate beads for efficient chromium (vi) removal. Journal of Colloid and Interface Science 506: 633–643. [DOI] [PubMed] [Google Scholar]
- 112.Lyu H, Gao B, He F, Zimmerman AR, Ding C, Huang H, et al. 2018a. Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environmental Pollution 233: 54–63. [DOI] [PubMed] [Google Scholar]
- 113.Lyu HH, Gao B, He F, Ding C, Tang JC, Crittenden JC 2017. Ball-milled carbon nanomaterials for energy and environmental applications. Acs Sustainable Chemistry & Engineering 5: 9568–9585. [Google Scholar]
- 114.Lyu HH, Gao B, He F, Zimmerman AR, Ding C, Huang H, et al. 2018b. Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environmental Pollution 233: 54–63. [DOI] [PubMed] [Google Scholar]
- 115.Lyu HH, Gao B, He F, Zimmerman AR, Ding C, Tang JC, et al. 2018c. Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chemical Engineering Journal 335: 110–119. [Google Scholar]
- 116.Mahmoodi NM 2013. Magnetic ferrite nanoparticle–alginate composite: Synthesis, characterization and binary system dye removal. Journal of the Taiwan Institute of Chemical Engineers 44: 322–330. [Google Scholar]
- 117.Mahmoodi NM, Hayati B, Arami M, Bahrami H. 2011. Preparation, characterization and dye adsorption properties of biocompatible composite (alginate/titania nanoparticle). Desalination 275: 93–101. [Google Scholar]
- 118.Maitra J, Shukla VK 2014. Cross-linking in hydrogels-a review. American Journal of Polymer Science 4: 25–31. [Google Scholar]
- 119.Mane S, Ponrathnam S, Chavan N. 2015. Effect of chemical cross-linking on properties of polymer microbeads: A review. Can. Chem. Trans 3: 473–485. [Google Scholar]
- 120.Maneerung T, Liew J, Dai Y, Kawi S, Chong C, Wang C-H 2016. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies. Bioresource Technology 200: 350–359. [DOI] [PubMed] [Google Scholar]
- 121.Martins SCS, Martins CM, Fiúza LMCG, Santaella ST 2013. Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial wastewater. African journal of biotechnology 12. [Google Scholar]
- 122.Michael I, Rizzo L, Mcardell CS, Manaia CM, Merlin C, Schwartz T, et al. 2013. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Research 47: 957–995. [DOI] [PubMed] [Google Scholar]
- 123.Min JH, Hering JG 1998. Arsenate sorption by fe(iii)-doped alginate gels. Water Research 32: 1544–1552. [Google Scholar]
- 124.Mohammadi A, Daemi H, Barikani M. 2014. Fast removal of malachite green dye using novel superparamagnetic sodium alginate-coated fe3o4 nanoparticles. International Journal of Biological Macromolecules 69: 447–455. [DOI] [PubMed] [Google Scholar]
- 125.Mohammadi N, Khani H, Gupta VK, Amereh E, Agarwal S. 2011. Adsorption process of methyl orange dye onto mesoporous carbon material–kinetic and thermodynamic studies. Journal of Colloid and Interface Science 362: 457–462. [DOI] [PubMed] [Google Scholar]
- 126.Mohan D, Sarswat A, Ok YS, Pittman CU 2014. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent - a critical review. Bioresource Technology 160: 191–202. [DOI] [PubMed] [Google Scholar]
- 127.Munagapati VS, Kim DS 2017. Equilibrium isotherms, kinetics, and thermodynamics studies for congo red adsorption using calcium alginate beads impregnated with nano-goethite. Ecotoxicology and Environmental Safety 141: 226–234. [DOI] [PubMed] [Google Scholar]
- 128.Ngah WSW, Fatinathan S. 2008. Adsorption of cu(ii) ions in aqueous solution using chitosan beads, chitosan–gla beads and chitosan–alginate beads. Chemical Engineering Journal 143: 62–72. [Google Scholar]
- 129.Ngomsik A-F, Bee A, Siaugue J-M, Cabuil V, Cote G. 2006. Nickel adsorption by magnetic alginate microcapsules containing an extractant. Water Research 40: 1848–1856. [DOI] [PubMed] [Google Scholar]
- 130.Njimou JR, Maicaneanu A, Indolean C, Nanseu-Njiki CP, Ngameni E. 2016. Removal of cd (ii) from synthetic wastewater by alginate-ayous wood sawdust (triplochiton scleroxylon) composite material. Environmental Technology 37: 1369–1381. [DOI] [PubMed] [Google Scholar]
- 131.Olad A, Farshi Azhar F. 2014. A study on the adsorption of chromium (vi) from aqueous solutions on the alginate-montmorillonite/polyaniline nanocomposite. Desalination and Water Treatment 52: 2548–2559. [Google Scholar]
- 132.Pandi K, Viswanathan N. 2015. Synthesis of alginate beads filled with nanohydroxyapatite: An efficient approach for fluoride sorption. Journal of Applied Polymer Science 132. [Google Scholar]
- 133.Papageorgiou SK, Katsaros F, Kouvelos E, Kanellopoulos N. 2009. Prediction of binary adsorption isotherms of cu 2+, cd 2+ and pb 2+ on calcium alginate beads from single adsorption data. Journal of Hazardous Materials 162: 1347–1354. [DOI] [PubMed] [Google Scholar]
- 134.Papageorgiou SK, Katsaros FK, Kouvelos EP, Nolan JW, Le Deit H, Kanellopoulos NK 2006. Heavy metal sorption by calcium alginate beads from laminaria digitata. Journal of Hazardous Materials 137: 1765–1772. [DOI] [PubMed] [Google Scholar]
- 135.Paques JP, Van Der Linden E, Van Rijn CJM, Sagis LMC 2014. Preparation methods of alginate nanoparticles. Advances in Colloid and Interface Science 209: 163–171. [DOI] [PubMed] [Google Scholar]
- 136.Prakasham RS, Merrie JS, Sheela R, Saswathi N, Ramakrishna SV 1999. Biosorption of chromium vi by free and immobilized rhizopus arrhizus. Environmental Pollution 104: 421–427. [Google Scholar]
- 137.Qi Y, Jiang M, Cui Y-L, Zhao L, Zhou X. 2015. Synthesis of quercetin loaded nanoparticles based on alginate for pb (ii) adsorption in aqueous solution. Nanoscale research letters 10: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qiusheng Z, Xiaoyan L, Jin Q, Jing W, Xuegang L. 2015. Porous zirconium alginate beads adsorbent for fluoride adsorption from aqueous solutions. RSC Advances 5: 2100–2112. [Google Scholar]
- 139.Qu X, Alvarez PJ, Li Q. 2013. Applications of nanotechnology in water and wastewater treatment. Water Research 47: 3931–3946. [DOI] [PubMed] [Google Scholar]
- 140.Rangsayatorn N, Pokethitiyook P, Upatham ES, Lanza GR 2004. Cadmium biosorption by cells of spirulina platensis tistr 8217 immobilized in alginate and silica gel. Environment International 30: 57–63. [DOI] [PubMed] [Google Scholar]
- 141.Ren H, Gao Z, Wu D, Jiang J, Sun Y, Luo C. 2016. Efficient pb(ii) removal using sodium alginate–carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydrate Polymers 137: 402–409. [DOI] [PubMed] [Google Scholar]
- 142.Rezaei H, Haghshenasfard M, Moheb A. 2017. Optimization of dye adsorption using fe3o4 nanoparticles encapsulated with alginate beads by taguchi method. Adsorption Science & Technology 35: 55–71. [Google Scholar]
- 143.Rhee S-K, Lee G, Lee S-T 1996. Influence of a supplementary carbon source on biodegradation of pyridine by freely suspended and immobilized pimelobacter sp. Applied Microbiology and Biotechnology 44: 816–822. [DOI] [PubMed] [Google Scholar]
- 144.Robati D, Mirza B, Rajabi M, Moradi O, Tyagi I, Agarwal S, et al. 2016. Removal of hazardous dyes-br 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chemical Engineering Journal 284: 687–697. [Google Scholar]
- 145.Robinson T, Mcmullan G, Marchant R, Nigam P. 2001. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresource Technology 77: 247–255. [DOI] [PubMed] [Google Scholar]
- 146.Rocher V, Bee A, Siaugue J-M, Cabuil V. 2010. Dye removal from aqueous solution by magnetic alginate beads crosslinked with epichlorohydrin. Journal of hazardous materials 178: 434–439. [DOI] [PubMed] [Google Scholar]
- 147.Rocher V, Siaugue J-M, Cabuil V, Bee A. 2008. Removal of organic dyes by magnetic alginate beads. Water Research 42: 1290–1298. [DOI] [PubMed] [Google Scholar]
- 148.Roh H, Yu M-R, Yakkala K, Koduru JR, Yang J-K, Chang Y-Y 2015. Removal studies of cd (ii) and explosive compounds using buffalo weed biochar-alginate beads. Journal of Industrial and Engineering Chemistry 26: 226–233. [Google Scholar]
- 149.Rosales E, Iglesias O, Pazos M, Sanromán MA 2012. Decolourisation of dyes under electro-fenton process using fe alginate gel beads. Journal of Hazardous Materials 213: 369–377. [DOI] [PubMed] [Google Scholar]
- 150.Russo R, Malinconico M, Santagata G. 2007. Effect of cross-linking with calcium ions on the physical properties of alginate films. Biomacromolecules 8: 3193–3197. [DOI] [PubMed] [Google Scholar]
- 151.Salisu A, Sanagi MM, Abu Naim A, Wan Ibrahim WA, Abd Karim KJ 2016a. Removal of lead ions from aqueous solutions using sodium alginate-graft-poly (methyl methacrylate) beads. Desalination and Water Treatment 57: 15353–15361. [Google Scholar]
- 152.Salisu A, Sanagi MM, Naim AA, Karim KJA, Ibrahim W. a. W., Abdulganiyu U. 2016b. Alginate graft polyacrylonitrile beads for the removal of lead from aqueous solutions. Polymer Bulletin 73: 519–537. [Google Scholar]
- 153.Samuel J, Pulimi M, Paul ML, Maurya A, Chandrasekaran N, Mukherjee A. 2013. Batch and continuous flow studies of adsorptive removal of cr(vi) by adapted bacterial consortia immobilized in alginate beads. Bioresource Technology 128: 423–430. [DOI] [PubMed] [Google Scholar]
- 154.Shen J, Hu Y, Shi M, Li N, Ma H, Ye M. 2010. One step synthesis of graphene oxide− magnetic nanoparticle composite. The Journal of Physical Chemistry C 114: 1498–1503. [Google Scholar]
- 155.Siwek H, Bartkowiak A, Włodarczyk M, Sobecka K. 2016. Removal of phosphate from aqueous solution using alginate/iron (iii) chloride capsules: A laboratory study. Water, Air, & Soil Pollution 227: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Soltani RDC, Khorramabadi GS, Khataee AR, Jorfi S. 2014. Silica nanopowders/alginate composite for adsorption of lead (ii) ions in aqueous solutions. Journal of the Taiwan Institute of Chemical Engineers 45: 973–980. [Google Scholar]
- 157.Sui K, Li Y, Liu R, Zhang Y, Zhao X, Liang H, et al. 2012. Biocomposite fiber of calcium alginate/multi-walled carbon nanotubes with enhanced adsorption properties for ionic dyes. Carbohydrate Polymers 90: 399–406. [DOI] [PubMed] [Google Scholar]
- 158.Sujana MG, Mishra A, Acharya BC 2013. Hydrous ferric oxide doped alginate beads for fluoride removal: Adsorption kinetics and equilibrium studies. Applied Surface Science 270: 767–776. [Google Scholar]
- 159.Tesh SJ, Scott TB 2014. Nano-composites for water remediation: A review. Advanced Materials 26: 6056–6068. [DOI] [PubMed] [Google Scholar]
- 160.Thakur S, Pandey S, Arotiba OA 2016. Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydrate Polymers 153: 34–46. [DOI] [PubMed] [Google Scholar]
- 161.Theron J, Walker J, Cloete T. 2008. Nanotechnology and water treatment: Applications and emerging opportunities. Critical Reviews in Microbiology 34: 43–69. [DOI] [PubMed] [Google Scholar]
- 162.Tian Y, Gao B, Morales VL, Wu L, Wang Y, Munoz-Carpena R, et al. 2012. Methods of using carbon nanotubes as filter media to remove aqueous heavy metals. Chemical Engineering Journal 210: 557–563. [Google Scholar]
- 163.Uyar G, Kaygusuz H, Erim FB 2016. Methylene blue removal by alginate–clay quasi-cryogel beads. Reactive and Functional Polymers 106: 1–7. [Google Scholar]
- 164.Vijaya Y, Popuri SR, Boddu VM, Krishnaiah A. 2008. Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel (ii) through adsorption. Carbohydrate Polymers 72: 261–271. [Google Scholar]
- 165.Vijayalakshmi K, Gomathi T, Latha S, Hajeeth T, Sudha P. 2016. Removal of copper (ii) from aqueous solution using nanochitosan/sodium alginate/microcrystalline cellulose beads. International Journal of Biological Macromolecules 82: 440–452. [DOI] [PubMed] [Google Scholar]
- 166.Vu HC, Dwivedi AD, Le TT, Seo S-H, Kim E-J, Chang Y-S 2017. Magnetite graphene oxide encapsulated in alginate beads for enhanced adsorption of cr(vi) and as(v) from aqueous solutions: Role of crosslinking metal cations in ph control. Chemical Engineering Journal 307: 220–229. [Google Scholar]
- 167.Wan Ngah WS, Teong LC, Hanafiah M. a. K. M. 2011. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydrate Polymers 83: 1446–1456. [Google Scholar]
- 168.Wan S, Wu J, Zhou S, Wang R, Gao B, He F. 2018. Enhanced lead and cadmium removal using biochar-supported hydrated manganese oxide (hmo) nanoparticles: Behavior and mechanism. Science of the Total Environment 616: 1298–1306. [DOI] [PubMed] [Google Scholar]
- 169.Wan SL, He F, Wu JY, Wan WB, Gu YW, Gao B. 2016. Rapid and highly selective removal of lead from water using graphene oxide-hydrated manganese oxide nanocomposites. Journal of Hazardous Materials 314: 32–40. [DOI] [PubMed] [Google Scholar]
- 170.Wan SL, Wu JY, He F, Zhou SS, Wang R, Bin G, et al. 2017. Phosphate removal by lead-exhausted bioadsorbents simultaneously achieving lead stabilization. Chemosphere 168: 748–755. [DOI] [PubMed] [Google Scholar]
- 171.Wang B, Gao B, Fang J. 2017. Recent advances in engineered biochar productions and applications. Critical Reviews in Environmental Science and Technology 47: 2158–2207. [Google Scholar]
- 172.Wang B, Gao B, Wan Y. 2018a. Comparative study of calcium alginate, ball-milled biochar, and their composites on methylene blue adsorption from aqueous solution. Environmental Science and Pollution Research doi: 10.1007/s11356-018-1497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang B, Gao B, Wan YS 2018b. Entrapment of ball-milled biochar in ca-alginate beads for the removal of aqueous cd(ii). Journal of Industrial and Engineering Chemistry 61: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang B, Gao B, Zimmerman A, Lee X. 2018c. Impregnation of multiwall carbon nanotubes in alginate beads dramatically enhances their adsorptive ability to aqueous methylene blue. Chemical Engineering Research and Design 133: 235–242. [Google Scholar]
- 175.Wang B, Gao B, Zimmerman AR, Zheng Y, Lyu H. 2018d. Novel biochar-impregnated calcium alginate beads with improved water holding and nutrient retention properties. Journal of Environmental Management 209: 105–111. [DOI] [PubMed] [Google Scholar]
- 176.Wang B, Gao B, Zimmerman AR, Zheng YL, Lyu HH 2018e. Novel biochar-impregnated calcium alginate beads with improved water holding and nutrient retention properties. Journal of Environmental Management 209: 105–111. [DOI] [PubMed] [Google Scholar]
- 177.Wang F, Lu X, Li X-Y 2016a. Selective removals of heavy metals (pb2+, cu2+, and cd2+) from wastewater by gelation with alginate for effective metal recovery. Journal of Hazardous Materials 308: 75–83. [DOI] [PubMed] [Google Scholar]
- 178.Wang J-P, Yang H-C, Hsieh C-T 2010. Adsorption of phenol and basic dye on carbon nanotubes/carbon fabric composites from aqueous solution. Separation Science and Technology 46: 340–348. [Google Scholar]
- 179.Wang Q, Wang B, Lee X, Lehmann J, Gao B. 2018f. Sorption and desorption of pb(ii) to biochar as affected by oxidation and ph. Science of the Total Environment 634: 188–194. [DOI] [PubMed] [Google Scholar]
- 180.Wang Y-Y, Yao W-B, Wang Q-W, Yang Z-H, Liang L-F, Chai L-Y 2016b. Synthesis of phosphate-embedded calcium alginate beads for pb(ii) and cd(ii) sorption and immobilization in aqueous solutions. Transactions of Nonferrous Metals Society of China 26: 2230–2237. [Google Scholar]
- 181.Wang Y, Wang W, Wang A. 2013a. Efficient adsorption of methylene blue on an alginate-based nanocomposite hydrogel enhanced by organo-illite/smectite clay. Chemical Engineering Journal 228: 132–139. [Google Scholar]
- 182.Wang Y, Zhang X, Wang Q, Zhang B, Liu J. 2013b. Continuous fixed bed adsorption of cu (ii) by halloysite nanotube-alginate hybrid beads: An experimental and modelling study. Water Science and Technology 70: 192–199. [DOI] [PubMed] [Google Scholar]
- 183.Wu D, Zhao J, Zhang L, Wu Q, Yang Y. 2010. Lanthanum adsorption using iron oxide loaded calcium alginate beads. Hydrometallurgy 101: 76–83. [Google Scholar]
- 184.Wu S, Zhao X, Li Y, Zhao C, Du Q, Sun J, et al. 2013. Adsorption of ciprofloxacin onto biocomposite fibers of graphene oxide/calcium alginate. Chemical Engineering Journal 230: 389–395. [Google Scholar]
- 185.Xiangliang P, Jianlong W, Daoyong Z. 2005. Biosorption of pb(ii) by pleurotus ostreatus immobilized in calcium alginate gel. Process Biochemistry 40: 2799–2803. [Google Scholar]
- 186.Xue Y, Gao B, Yao Y, Inyang M, Zhang M, Zimmerman AR, et al. 2012. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chemical Engineering Journal 200–202: 673–680. [Google Scholar]
- 187.Yagub MT, Sen TK, Afroze S, Ang HM 2014. Dye and its removal from aqueous solution by adsorption: A review. Advances in Colloid and Interface Science 209: 172–184. [DOI] [PubMed] [Google Scholar]
- 188.Yakup Arıca M, Arpa Ç, Ergene A, Bayramoğlu G, Genç Ö. 2003. Ca-alginate as a support for pb(ii) and zn(ii) biosorption with immobilized phanerochaete chrysosporium. Carbohydrate Polymers 52: 167–174. [Google Scholar]
- 189.Yang J-S, Xie Y-J, He W. 2011. Research progress on chemical modification of alginate: A review. Carbohydrate Polymers 84: 33–39. [Google Scholar]
- 190.Ye X, Wu Z, Li W, Liu H, Li Q, Qing B, et al. 2009. Rubidium and cesium ion adsorption by an ammonium molybdophosphate–calcium alginate composite adsorbent. Colloids and Surfaces A: Physicochemical and Engineering Aspects 342: 76–83. [Google Scholar]
- 191.Zargar V, Asghari M, Dashti A. 2015. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng Reviews 2: 204–226. [Google Scholar]
- 192.Zhang M, Gao B. 2013. Removal of arsenic, methylene blue, and phosphate by biochar/alooh nanocomposite. Chemical Engineering Journal 226: 286–292. [Google Scholar]
- 193.Zhang M, Gao B, Cao XD, Yang LY 2013a. Synthesis of a multifunctional graphene-carbon nanotube aerogel and its strong adsorption of lead from aqueous solution. RSC Advances 3: 21099–21105. [Google Scholar]
- 194.Zhang M, Gao B, Li Y, Zhang XW, Hardin IR 2013b. Graphene-coated pyrogenic carbon as an anode material for lithium battery. Chemical Engineering Journal 229: 399–403. [Google Scholar]
- 195.Zhang M, Gao B, Yao Y, Xue YW, Inyang M. 2012. Synthesis, characterization, and environmental implications of graphene-coated biochar. Science of the Total Environment 435: 567–572. [DOI] [PubMed] [Google Scholar]
- 196.Zhao F, Qin X, Feng S. 2016. Preparation of microgel/sodium alginate composite granular hydrogels and their cu 2+ adsorption properties. RSC Advances 6: 100511–100518. [Google Scholar]
- 197.Zhou Y, Gao B, Zimmerman AR, Chen H, Zhang M, Cao XD 2014a. Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Bioresource Technology 152: 538–542. [DOI] [PubMed] [Google Scholar]
- 198.Zhou YM, Gao B, Zimmerman AR, Cao XD 2014b. Biochar-supported zerovalent iron reclaims silver from aqueous solution to form antimicrobial nanocomposite. Chemosphere 117: 801–805. [DOI] [PubMed] [Google Scholar]
- 199.Zhou YM, Gao B, Zimmerman AR, Fang J, Sun YN, Cao XD 2013. Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chemical Engineering Journal 231: 512–518. [Google Scholar]
- 200.Zhu H, Chen T, Liu J, Li D. 2018. Adsorption of tetracycline antibiotics from an aqueous solution onto graphene oxide/calcium alginate composite fibers. RSC advances 8: 2616–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhuang Y, Yu F, Chen H, Zheng J, Ma J, Chen J. 2016a. Alginate/graphene double-network nanocomposite hydrogel beads with low-swelling, enhanced mechanical properties, and enhanced adsorption capacity. Journal of Materials Chemistry A 4: 10885–10892. [Google Scholar]
- 202.Zhuang Y, Yu F, Chen J, Ma J. 2016b. Batch and column adsorption of methylene blue by graphene/alginate nanocomposite: Comparison of single-network and double-network hydrogels. Journal of Environmental Chemical Engineering 4: 147–156. [Google Scholar]
- 203.Zhuang Y, Yu F, Ma J, Chen J. 2017. Enhanced adsorption removal of antibiotics from aqueous solutions by modified alginate/graphene double network porous hydrogel. Journal of Colloid and Interface Science 507: 250–259. [DOI] [PubMed] [Google Scholar]
- 204.Zouboulis AI, Katsoyiannis IA 2002. Arsenic removal using iron oxide loaded alginate beads. Industrial & Engineering Chemistry Research 41: 6149–6155. [Google Scholar]