Abstract
Background
Metabolic reprogramming is a common phenomenon in tumorigenesis and tumor progression. Amino acids are important mediators in cancer metabolism, and their kinetics in tumor tissue are far from being understood completely. Mass spectrometry imaging is capable to spatiotemporally trace important endogenous metabolites in biological tissue specimens. In this research, we studied L-[ring-13C6]-labeled phenylalanine and tyrosine kinetics in a human non-small cell lung carcinoma (NSCLC) xenografted mouse model using matrix-assisted laser desorption/ionization Fourier-transform ion cyclotron resonance mass spectrometry imaging (MALDI-FTICR-MSI).
Methods
We investigated the L-[ring-13C6]-Phenylalanine (13C6-Phe) and L-[ring-13C6]-Tyrosine (13C6-Tyr) kinetics at 10 min (n = 4), 30 min (n = 3), and 60 min (n = 4) after tracer injection and sham-treated group (n = 3) at 10 min in mouse-xenograft lung tumor tissues by MALDI-FTICR-MSI.
Results
The dynamic changes in the spatial distributions of 19 out of 20 standard amino acids are observed in the tumor tissue. The highest abundance of 13C6-Phe was detected in tumor tissue at 10 min after tracer injection and decreased progressively over time. The overall enrichment of 13C6-Tyr showed a delayed temporal trend compared to 13C6-Phe in tumor caused by the Phe-to-Tyr conversion process. Specifically, 13C6-Phe and 13C6-Tyr showed higher abundances in viable tumor regions compared to non-viable regions.
Conclusions
We demonstrated the spatiotemporal intra-tumoral distribution of the essential aromatic amino acid 13C6-Phe and its de-novo synthesized metabolite 13C6-Tyr by MALDI-FTICR-MSI. Our results explore for the first time local phenylalanine metabolism in the context of cancer tissue morphology. This opens a new way to understand amino acid metabolism within the tumor and its microenvironment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40170-021-00262-9.
Keywords: L-[ring-13C6]-Phenylalanine, L-[ring-13C6]-Tyrosine, Amino acids, Isotope labeling, Tumor, Mass spectrometry imaging
Introduction
Cancer cells are known to exhibit unusual metabolic activity to sustain their proliferation [1]. A well-known example is the non-essential amino acid glutamine, which is associated with neoplastic proliferation [2]. It is also known that phenylalanine consumption correlates with the growth of tumor cell lines and negative patient outcomes [3–12]. Plasma phenylalanine concentrations are elevated in patients with cancer [12]. Phenylalanine is an indispensable amino acid and phenylalanine flux in the body is entirely derived from the diet and cellular protein turnover. It is also needed to synthesize the amino acid tyrosine (Tyr) by the phenylalanine hydroxylase enzyme mainly in the liver and kidney [13]. Isotopically labeled phenylalanine has been used in tracer studies as a measure of protein synthesis [14, 15] and liver function [16, 17]. Phenylalanine metabolism, however, in relation to the morphology of cancer tissue has not been explored yet.
The molecular and cellular heterogeneity in a tumor plays a crucial role in cancer treatment efficacy and outcome [18, 19]. Current strategies to personalize treatment response use genomics data [20] which is not able to reflect the dynamics of metabolic processes in the context of the spatial intratumor heterogeneity. Mass spectrometry imaging (MSI) enables the in situ visualization of metabolites in biological tissue specimens. Adding isotopically labeled versions of target compounds allows the quantitative study of spatiotemporal metabolic dynamics in these tissues [17]. Multi-isotope imaging mass spectrometry (MIMS) has been applied to study the heterogeneity of glucose and glutamine utilization in murine tumors recently [21]. However, matrix-assisted laser desorption/ionization (MALDI), a softer ionization technique without suffering from extensive fragmentation and complexity of interpretation of mass spectra, is increasingly applied in biomedical research [22]. We have recently used this method to track the hepatocellular incorporation of L-[ring-13C6]-Phenylalanine (13C6-Phe) and its metabolite L-[ring-13C6]-Tyrosine (13C6-Tyr) [17].
In this study, we used our previously developed MALDI-MSI method of 13C6-Phe [17] to construct spatial and dynamic metabolic flux maps in relation with spatial tumor heterogeneity in a human non-small cell lung carcinoma xenograft model.
Materials and methods
Animal experiments
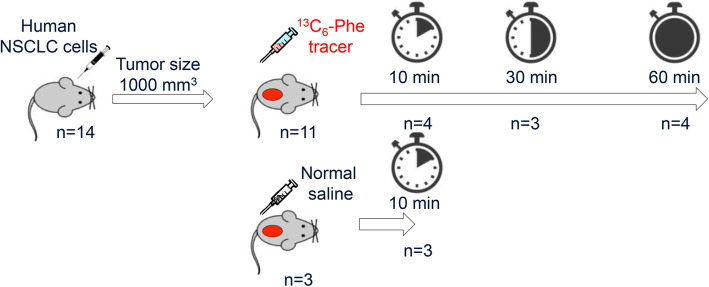
A total of 14 adult female immune-compromised Crl:NU-Foxn1nu nu/nu nude mice (Charles River, Den Bosch, The Netherlands) were used. Human NCI-H460 non-small cell lung carcinoma (NSCLC) cells suspended in matrigel (BD Biosciences, Breda, The Netherlands) were injected subcutaneously into the flank region of each mouse. Tumor volume was monitored 3 times per week using a Vernier caliper. All animals had unrestricted access to food and water before injection. They received a regular chow diet, which contains 12% fat, 27% protein, and 61% carbohydrate based on calories. Eleven mice were injected with 13C6-Phe (Cambridge Isotope Laboratories, Andover, MA, USA) at a dose of 1.0 micromole/g body weight, and 3 additional mice were injected with normal saline into the lateral tail vein when the tumors reached 1000 mm3. The tracer-infused mice were subsequently sacrificed at 10 min (n = 4), 30 min (n = 3), and 60 min (n = 4) after injection, and sham-treated mice (n = 3) were sacrificed after 10 min. Tumors were rapidly dissected, snap-frozen with liquid nitrogen, and stored at − 80 °C until cryo-sectioning. All experimental procedures were approved by the Animal Ethical Committee of the Maastricht University.
Tissue sectioning
Tumor tissues were sectioned at 10 μm using a cryotome (Leica, Rijswijk, The Netherlands) at − 20 °C, thaw-mounted onto indium−tin oxide coated glass slides (CG-40IN-S115, Delta Technologies, Loveland, CO, USA), and stored at − 80 °C until further measurement.
On-tissue derivatization and matrix application
P-N,N,N-trimethylamonioanilyl N-hydroxysuccinimidylcarbamate iodide (TAHS) on-tissue derivatization was applied to tissue sections prior to matrix application as described by Arts et al. [17] with slight modifications: fresh frozen tissue sections were dried in a vacuum desiccator for 15 min. Subsequently, a TAHS solution of 1.25 mg/mL in acetonitrile was sprayed onto the sections using an automated, temperature-controlled spraying system (TM-sprayer, HTX Technologies, Chapel Hill, NC, USA). Six layers were sprayed at 55 °C with a constant flow rate of 0.1 mL/min and at a speed of 1200 mm/min. Next, all tumor sections were incubated at 55 °C in a humid environment (methanol to water = 1:1, v/v) for 24 h.
A 30 mg/mL 2,5-dihydroxybenzoic acid (DHB, Sigma-Aldrich, St. Louis, MO, USA) matrix solution in methanol/water (7:3, v/v) containing 0.2% trifluoroacetic acid was applied in six layers with the HTX sprayer at 85 °C with a fixed flow rate of 0.1 mL/min, followed by immediate mass spectrometry imaging measurements.
Mass spectrometry imaging experiments
High mass resolution (R = 1.5E5 at m/z 200) matrix-assisted laser desorption/ionization Fourier-transform ion cyclotron resonance mass spectrometry imaging (MALDI-FTICR-MSI) experiments were performed with a Solarix 9.4 T (Bruker Daltonics, Bremen, Germany). MSI data were acquired within a mass range of m/z 100–1200 (1E6 data points) in positive ionization mode and in magnitude mode with a 75-μm spatial raster width. The laser operated at a laser power of 18% and a frequency of 2000 Hz with 50 shots accumulated per pixel. Data acquisition was controlled using ftmsControl and FlexImaging 4.1 (Bruker Daltonik, Bremen, Germany).
LC-MS and GC-C-IRMS measurements
The enrichment of free amino acids (13C6-Phe and 13C6-Tyr) and protein-bound 13C6-Phe were measured in tumor tissue homogenates with liquid chromatography–mass spectrometry (LC-MS) [23] and gas chromatography combustion isotope ratio mass spectrometry (GC-C-IRMS) [24] using the same protocols as described by Van et al. and Arts et al., respectively to complement and validate the MSI data.
Histological staining
After MSI measurement, all tissue sections were washed with 70% ethanol for 30 s to remove the matrix prior to hematoxylin and eosin staining (H&E). The samples were rehydrated in MilliQ water, followed by 3 min in hematoxylin (Merck, Darmstadt, Germany), 1 min in distilled water, and 30 s in eosin (Merck, Darmstadt, Germany). Then, all sections were dehydrated in a graded ethanol series and followed by clearance for 2 min in xylene. Coverslips were mounted onto the slides with Entellan mounting medium (Merck, Darmstadt, Germany), and all sections were air-dried overnight at room temperature. The H&E stained slides were scanned using a digital slide scanner (Mirax Desk, Zeiss, Jena, Germany) and a pathologist annotated viable tumor and non-viable tumor regions digitally in the scanned images. Next, the digitalized H&E images were manually co-registered to the MSI data using FlexImaging 4.1 (Bruker Daltonics, Bremen, Germany).
Data analysis
The tracer-to-tracee ratio (TTR) and the molar percentage excess (MPE) values of 13C6-Phe and 13C6-Tyr, and ratios of MPE (Tyr) to MPE (Phe) were calculated for every pixel individually using a custom MATLAB script (MATLAB R2014b, Mathworks, Natick, MA, USA) as described by Arts et al. [17]. This resulted in tabular ASCII files that can be imported for heatmap reconstructions in FlexImaging 4.1 (Bruker Daltonics, Bremen, Germany).
In parallel, all MSI data, their co-registered H&E images combined with the tumor annotations, were imported to SCiLS Lab 2020a (Bruker Daltonics). There, the peak interval width was set to 5 mDa and each pixel was normalized to its root mean square value. The average intensity (“maximum mean value”) data of annotated regions were exported.
Metabolite identification
The human metabolome database (www.hmdb.ca) was used for assignment of identities to m/z values with a maximum mass tolerance of 2 ppm. Underivatized molecules were identified assuming single protonation (M = m/z - H+), while derivatized molecules ([M + TAHS]+) were identified based on subtracting the monoisotopic mass value of TAHS to obtain the neutral molecular weight (M = m/z - 177.1022394).
Results
We performed MALDI-FTICR-MSI analysis of mouse-xenograft lung tumor tissues 10 min (n = 4), 30 min (n = 3), and 60 min (n = 4) after tracer injection and after 10 min in a sham group (n = 3) to study 13C6-Phe and 13C6-Tyr kinetics (Fig. 1).
Fig. 1.
Eleven mice were injected with ring-13C6-Phe and three additional mice were injected with normal saline into the lateral tail vein when the tumors reached 1000 mm3. The tracer-infused mice were subsequently sacrificed at 10 min (n = 4), 30 min (n = 3), and 60 min (n = 4) after injection, and sham-treated mice were sacrificed after 10 min
Standard amino acids
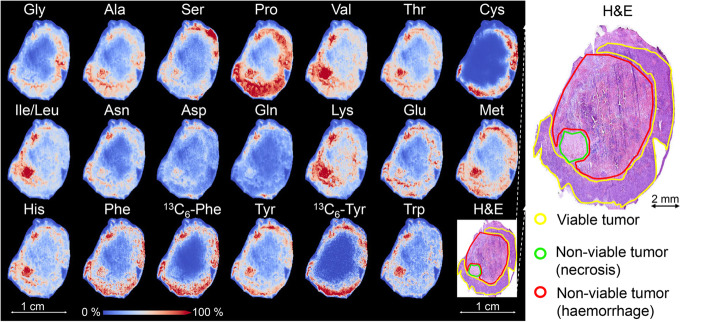
Nineteen out of 20 standard amino acids were detected in all tumor sections and 13C6-Phe and 13C6-Tyr were exclusively detected in tracer-injected mice samples. MSI images of labeled and unlabeled amino acids in a representative tumor tissue at 30 min after 13C-Phe injection are shown in Fig. 2. All amino acids exhibit heterogeneous distributions, which correlate with the different morphological components of the tumor (mainly viable tumor and non-viable tumor). Most of unlabeled amino acids as well as 13C6-Phe and 13C6-Tyr showed similar spatial distributions with a higher abundance in viable tumor compared to the non-viable regions in the core of the tumor.
Fig. 2.
Distributions of 19 detected standard amino acids in a representative mouse-xenograft lung tumor tissue at 30 min after 13C6-Phe injection. All pixels were normalized to their root mean square value. The co-registered, hematoxylin and eosin stained (H&E) image shows the different histomorphological components of the tissue: viable tumor (yellow), non-viable tumor fraction (necrosis, green), and non-viable tumor region (hemorrhage, red)
13C6-labeled Phe and Tyr
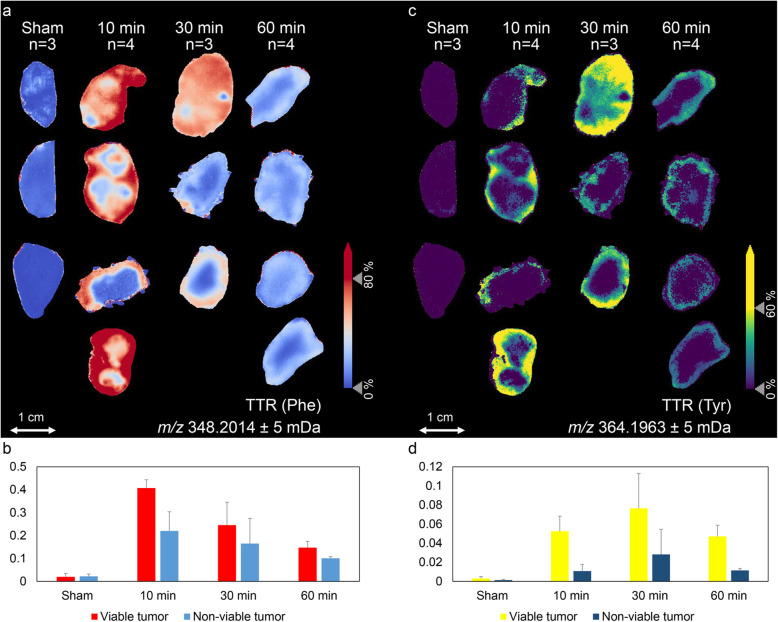
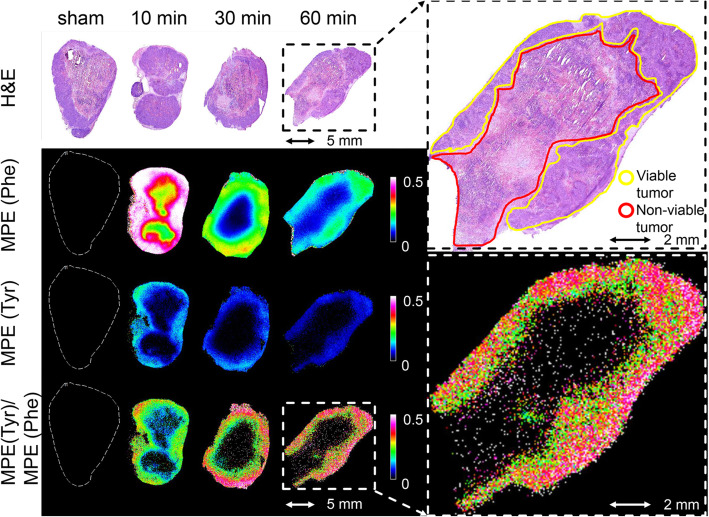
TTR and MPE values were used to assess the spatial enrichment of both 13C6-Phe and 13C6-Tyr in the tumor samples. The visualizations of TTR (Phe) and TTR (Tyr) across all tumor sections are shown in Fig. 3a and c, respectively. A differentiated quantitative analysis of TTR (Phe) and TTR (Tyr) in the annotated viable tumor and non-viable tumor fractions at every time point are shown in Fig. 3b and d, respectively. MPE (Phe), MPE (Tyr), ratio of MPE (Tyr) to MPE (Phe) in representative tumor tissues at 10, 30, and 60 min after tracer injection and at 10 min of control group are shown in Fig. 4. MPE values of Phe and Tyr in plasma samples from the same mice over the same time course were measured by GC-C-IRMS and are shown in Supplementary Figure 1.
Fig. 3.
Distributions of 13C6-Phe (a) and 13C6-Tyr (c) in mouse-xenograft lung tumor tissues (ntotal = 11) at 10, 30, and 60 min after tracer injection and at 10 min of control group (n = 3). Tracer-to-tracee (TTR) images for phenylalanine (a) and tyrosine (c) were calculated by normalizing the labeled amino acid signals to the intensities of their respective unlabeled versions. TTR values of Phe (b) and Tyr (d) were then differentiated annotated viable tumor and non-viable tumor regions for every time point
Fig. 4.
Visualizations of the molar percentage excess (MPE) for 13C6-Phe and 13C6-Tyr and the ratio of MPE (Tyr) to MPE (Phe) in representative tumor tissues at 10, 30, and 60 min after tracer injection and at 10 min of sham group. Their co-registered, hematoxylin and eosin stained (H&E) images are shown on the top. A magnification of the ratio of MPE (Tyr) to MPE (Phe) in one representative tissue section at 60 min and its co-registered H&E image is shown on the right
The highest enrichment for 13C6-Phe was detected at 10 min after bolus injection followed by a decreasing trend over time. The overall enrichment of 13C6-Tyr was substantially lower than 13C6-Phe, and it had a delayed temporal trend compared with 13C6-Phe with its peak at 30 min. Additional experiments on plasma of the same mice show that both, 13C6-Phe and 13C6-Tyr, reached their highest level at 10 min after bolus injection followed by a decreasing trend over time (Supplementary Figure 1).
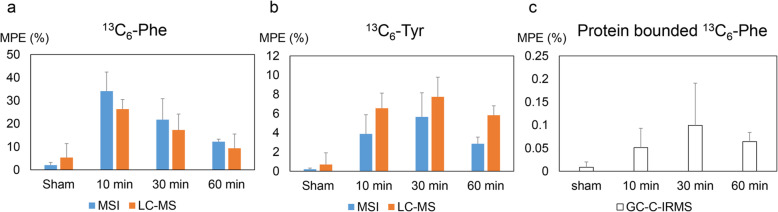
The MSI enrichments of 13C6-Phe and 13C6-Tyr were further complemented by LC-MS data acquired from homogenized tumor tissues, which showed a similar trend over time as the spatially convolved MSI data (Fig. 5a and b). Furthermore, the incorporation of 13C6-Phe into proteins in tumor tissue homogenates was investigated with GC-C-IRMS. The highest protein bounded 13C6-Phe was detected at 30 min and decreasing over time (Fig. 5c).
Fig. 5.
Overall enrichment of tissue free 13C6-Phe (a) and 13C6-Tyr (b) by MSI (blue) and LC-MS (orange), and 13C6-Phe protein enrichments (c) in homogenized tumor tissues by GC-C-IRMS. Enrichment is shown as mean MPE ± SE (%)
Discussion
In this study, we used MALDI-FTICR mass spectrometry imaging (MSI) to study 13C6-Phe and 13C6-Tyr kinetics in mouse-xenograft lung tumor tissues at four different time points after injection. The use of MSI allowed us to detect all unlabeled and labeled amino acids simultaneously.
All observed amino acids exhibit heterogeneous distributions, which correlate with the different morphological components of the tumor (Fig. 2). For example, branched-chain amino acids (leucine, isoleucine, and valine) are most pronounced in the necrotic region, and all amino acids are much less abundant in the hemorrhagic region. This indicates the necessity to investigate amino acid kinetics in a spatially differentiated fashion using imaging technologies.
Looking at the labeled amino acids of interest, 13C6-Phe and 13C6-Tyr were found exclusively localized in the viable tumor region in contrast to their non-labeled equivalents, which are additionally present in necrotic regions.
When calculating the TTR and MPE values for both labeled amino acids for viable and non-viable tumor regions, the highest enrichments for 13C6-Phe and 13C6-Tyr were detected at 10 min and at 30 min after bolus injection, respectively, followed by a decreasing trend over time. Additional experiments on plasma of the same mice show that both, 13C6-Phe and 13C6-Tyr, reached their highest level at 10 min after bolus injection followed by a decreasing trend over time (Supplementary Figure 1). This together with the observation that the highest enrichment for 13C6-Tyr in the tumor was delayed indicates that 13C6-Tyr was subsequently transported to the tumor tissue after a Phe-to-Tyr conversion process, which is assumed to predominantly occur in the liver [13]. Moreover, viable and non-viable tumor fractions showed similar enrichment trends over time, but the viable tumor exhibited greater enrichment of both labeled amino acids than non-viable tumor at every time point (Fig. 3). This might be related to the higher metabolic activity of viable tumor cells and their higher perfusion over non-viable tumor tissue. Interestingly, the ratio of MPE (Tyr) to MPE (Phe), representing the Phe-to-Tyr turnover (in the tumor or elsewhere in the organism) was significantly higher in the outer rim of the viable tumor region (Fig. 4). This is a reflection of the intra-tumor heterogeneity within the viable tumor fraction, which might be attributed to spatial variation in perfusion, vascularization, cell growth, viability, or differences in metabolic activity [25].
These MSI enrichments of 13C6-Phe and 13C6-Tyr were further complemented by LC-MS and GC-C-IRMS data acquired from homogenized tumor tissues. The LC-MS data showed a similar trend over time as the spatially convolved MSI data, and thereby validated the accuracy of the MSI approach (Fig. 5a and b). Interestingly, the GC-C-IRMS data indicated a delayed incorporation of 13C6-Phe in the tumor protein synthesis as compared to the unbound labeled amino acids (Fig. 5), which might correlate with a lower clearance rate of 13C6-Phe in the tumor as compared to the liver where both 13C6-Phe and 13C6-Tyr were already cleared at 30 min and 60 min post-inoculation [17]. MALDI-MSI showed that clearance of both amino acids in the tumor was delayed compared to the liver, consequently delaying the incorporation of 13C6-Phe into the proteins synthetized in the tumor (Fig. 5c). Based on this, we can hypothesize that the incorporation of 13C6-Phe in the tumor protein synthesis presents a different kinetic compared to the liver progressive incorporation from 10 to 60 min. Nevertheless, while the liver tissue is fully viable and reasonably homogeneous, the heterogeneous tumor tissues were composed of both viable and non-viable parts. Only the viable tumor cells contribute to metabolic activities and so to protein synthesis in the tumor. Therefore, as the tumor tissue homogenates do not benefit from an estimation of the proportion of viable and non-viable parts, the protein incorporation results from homogenates should be interpreted with caution.
This again underlines the necessity to study kinetics in a spatially resolved manner. In that sense, this study demonstrates the usefulness of MSI to investigate spatial 13C6-Phe and 13C6-Tyr kinetics in tumor and also reflects inter-organ amino acid shifts. The translation to human samples will offer new insights in diagnostic molecular markers and tumor treatment.
Conclusions
In this work, we demonstrated for the first time the spatiotemporal intra-tumoral distribution of the aromatic amino acid L-phenylalanine and its derivative L-tyrosine by MSI in tumor tissue. Furthermore, we showed the distribution of these molecular targets in relation to the tumor morphology, allowing us to monitor altered local amino acid metabolism in tumor cells and their microenvironment. Our approach can enhance our understanding on inter-organ amino acid metabolism and provide further insights to improve and develop novel strategies for cancer therapy.
Supplementary Information
Additional file 1: Supplementary Figure 1. The molar percentage excess (MPE) for 13C6-Phe (left) and 13C6-Tyr (right) in plasma samples from the same mice over the same time course and measured by GC-C-IRMS.
Acknowledgements
Not applicable.
Abbreviations
- 13C6-Phe
L-[ring-13C6]-phenylalanine
- 13C6-Tyr
L-[ring-13C6]-tyrosine
- Phe
Phenylalanine
- Tyr
Tyrosine
- NSCLC
Non-small cell lung carcinoma
- TTR
Tracer-to-tracee ratio
- MPE
Molar percentage excess
- MALDI
Matrix-assisted laser desorption/ionization
- MSI
Mass spectrometry imaging
- MALDI-FTICR-MSI
Matrix-assisted laser desorption/ionization Fourier-transform ion cyclotron resonance mass spectrometry imaging
- MIMS
Multi-isotope imaging mass spectrometry
- LC-MS
Liquid chromatography–mass spectrometry
- GC-C-IRMS
Gas chromatography combustion isotope ratio mass spectrometry
- TAHS
P-N,N,N-trimethylamonioanilyl N-hydroxysuccinimidylcarbamate iodide
- DHB
2,5-Dihydroxybenzoic acid
- H&E
Hematoxylin and eosin staining
Authors’ contributions
J.C. was a major contributor in writing the manuscript. B.B. performed the data analysis and revised the manuscript. M.A. provided the MSI data. L.J. performed the animal experiments. L.V. performed the GC-C-IRMS experiments. T.H. provided the THAS derivatization compound. H.V performed the LC-MS experiments. G.E. performed data curation. L.H. performed the pathological annotation of the H&E images. Z.S., S.O., and R.H provided conceptualization, methodology and revised the manuscript. The authors read and approved the final manuscript.
Funding
This work was supported by the China Scholarship Council (No. 201706040068) and by the Dutch Province of Limburg through the LINK program. BB acknowledges the financial support by the Dutch Cancer Foundation KWF (ERA-NET TRANSCAN 2; Grant No. 643638).
Availability of data and materials
The data is available upon request from the corresponding author.
Declarations
Ethics approval and consent to participate
All experimental procedures were approved by the Animal Ethical Committee of the Maastricht University.
Consent for publication
All authors have read and approved the final manuscript.
Competing interests
The authors have no conflict of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer. 2017;3(3):169–180. doi: 10.1016/j.trecan.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitruka M, Gore CR, Kumar A, Sarode SC, Sharma NK. Undetectable free aromatic amino acids in nails of breast carcinoma: biomarker discovery by a novel metabolite purification VTGE system. Front Oncol. 2020;10. 10.3389/fonc.2020.00908. [DOI] [PMC free article] [PubMed]
- 4.Cai Y, Rattray NJW, Zhang Q, Mironova V, Santos-Neto A, Muca E, Vollmar AKR, Hsu KS, Rattray Z, Cross JR, et al. Tumor tissue-specific biomarkers of colorectal cancer by anatomic location and stage. Metabolites. 2020;10(6):257. doi: 10.3390/metabo10060257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai CK, Lin CY, Kang CJ, Liao CT, Wang WL, Chiang MH, Yen TC, Lin G. Nuclear magnetic resonance metabolomics biomarkers for identifying high risk patients with extranodal extension in oral squamous cell carcinoma. J Clin Med. 2020;9(4):951. doi: 10.3390/jcm9040951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang CY, Chiao CC, Phan NN, Li CY, Sun ZD, Jiang JZ, Hung JH, Chen YL, Yen MC, Weng TY, Chen WC, Hsu HP, Lai MD. Gene signatures and potential therapeutic targets of amino acid metabolism in estrogen receptor-positive breast cancer. Am J Cancer Res. 2020;10(1):95–113. [PMC free article] [PubMed] [Google Scholar]
- 7.Ijare OB, Holan C, Hebert J, Sharpe MA, Baskin DS, Pichumani K. Elevated levels of circulating betahydroxybutyrate in pituitary tumor patients may differentiate prolactinomas from other immunohistochemical subtypes. Sci Rep-Uk. 2020;10(1):1334. doi: 10.1038/s41598-020-58244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang MX, Liu XY, Liu X, Li HZ, Sun W, Zhang YS. A pilot investigation of a urinary metabolic biomarker discovery in renal cell carcinoma. Int Urol Nephrol. 2020;52(3):437–446. doi: 10.1007/s11255-019-02332-w. [DOI] [PubMed] [Google Scholar]
- 9.Lautaoja JH, Lalowski M, Nissinen TA, Hentila J, Shi Y, Ritvos O, Cheng SL, Hulmi JJ. Muscle and serum metabolomes are dysregulated in colon-26 tumor-bearing mice despite amelioration of cachexia with activin receptor type 2B ligand blockade. Am J Physiol-Endoc M. 2019;316(5):E852–E865. doi: 10.1152/ajpendo.00526.2018. [DOI] [PubMed] [Google Scholar]
- 10.Loras A, Trassierra M, Sanjuan-Herraez D, Martinez-Bisbal MC, Castell JV, Quintas G, et al. Bladder cancer recurrence surveillance by urine metabolomics analysis. Scientific Reports. 2018;8:9172. [DOI] [PMC free article] [PubMed]
- 11.Duskova K, Vesely S, Silva JD, Cernei N, Zitka O, Heger Z, Adam V, Havlova K, Babjuk M. Differences in urinary amino acid patterns in individuals with different types of urological tumor urinary amino acid patterns as markers of urological tumors. In Vivo. 2018;32(2):425–429. doi: 10.21873/invivo.11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neurauter G, Grahmann AV, Klieber M, Zeimet A, Ledochowski M, Sperner-Unterweger B, Fuchs D. Serum phenylalanine concentrations in patients with ovarian carcinoma correlate with concentrations of immune activation markers and of isoprostane-8. Cancer Lett. 2008;272(1):141–147. doi: 10.1016/j.canlet.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Matthews DE. An overview of phenylalanine and tyrosine kinetics in humans. J Nutr. 2007;137(6 Suppl 1):1549S–1555S; discussion 1573S-1575S. doi: 10.1093/jn/137.6.1549S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olde Damink SW, Jalan R, Deutz NE, Dejong CH, Redhead DN, Hynd P, Hayes PC, Soeters PB. Isoleucine infusion during “simulated” upper gastrointestinal bleeding improves liver and muscle protein synthesis in cirrhotic patients. Hepatology. 2007;45(3):560–568. doi: 10.1002/hep.21463. [DOI] [PubMed] [Google Scholar]
- 15.Groen BBL, Horstman AM, Hamer HM, de Haan M, van Kranenburg J, Bierau J, Poeze M, Wodzig WKWH, Rasmussen BB, van Loon LJC. Post-prandial protein handling: you are what you just ate. PLoS One. 2015;10(11):e0141582. doi: 10.1371/journal.pone.0141582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safaei A, Arefi Oskouie A, Mohebbi SR, Rezaei-Tavirani M, Mahboubi M, Peyvandi M, Okhovatian F, Zamanian-Azodi M. Metabolomic analysis of human cirrhosis, hepatocellular carcinoma, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis diseases. Gastroenterol Hepatol Bed Bench. 2016;9(3):158–173. [PMC free article] [PubMed] [Google Scholar]
- 17.Arts M, Soons Z, Ellis SR, Pierzchalski KA, Balluff B, Eijkel GB, Dubois LJ, Lieuwes NG, Agten SM, Hackeng TM, et al. Detection of localized hepatocellular amino acid kinetics by using mass spectrometry imaging of stable isotopes. Angew Chem Int Edit. 2017;56(25):7146–7150. doi: 10.1002/anie.201702669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501(7467):355–364. doi: 10.1038/nature12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 20.Berger MF, Mardis ER. The emerging clinical relevance of genomics in cancer medicine. Nat Rev Clin Oncol. 2018;15(6):353–365. doi: 10.1038/s41571-018-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Guillermier C, De Raedt T, Cox AG, Maertens O, Yimlamai D, Lun MY, Whitney A, Maas RL, Goessling W, et al. Imaging mass spectrometry reveals tumor metabolic heterogeneity. Iscience. 2020;23(8):101355. doi: 10.1016/j.isci.2020.101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chughtai K, Heeren RM. Mass spectrometric imaging for biomedical tissue analysis. Chem Rev. 2010;110(5):3237–3277. doi: 10.1021/cr100012c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Eijk HM, Wijnands KA, Bessems BA, Olde Damink SW, Dejong CH, Poeze M. High sensitivity measurement of amino acid isotope enrichment using liquid chromatography-mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2012;905:31–36. doi: 10.1016/j.jchromb.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 24.Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, Lemosquet S, Saris WHM, Boirie Y, van Loon LJC. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr. 2009;90(1):106–115. doi: 10.3945/ajcn.2009.27474. [DOI] [PubMed] [Google Scholar]
- 25.Furuya M, Nishiyama M, Kasuya Y, Kimura S, Ishikura H. Pathophysiology of tumor neovascularization. Vasc Health Risk Manag. 2005;1(4):277–290. doi: 10.2147/vhrm.2005.1.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Figure 1. The molar percentage excess (MPE) for 13C6-Phe (left) and 13C6-Tyr (right) in plasma samples from the same mice over the same time course and measured by GC-C-IRMS.
Data Availability Statement
The data is available upon request from the corresponding author.