Abstract
Background
Determining failure to anti-angiogenic therapy in recurrent glioblastoma (GBM) (rGBM) remains a challenge. The purpose of the study was to assess treatment response to bevacizumab-based therapy in patients with rGBM using MR spectroscopy (MRS).
Methods
We performed longitudinal MRI/MRS in 33 patients with rGBM to investigate whether changes in N-acetylaspartate (NAA)/Choline (Cho) and Lactate (Lac)/NAA from baseline to subsequent time points after treatment can predict early failures to bevacizumab-based therapies.
Results
After stratifying based on 9-month survival, longer-term survivors had increased NAA/Cho and decreased Lac/NAA levels compared to shorter-term survivors. ROC analyses for intratumoral NAA/Cho correlated with survival at 1 day, 2 weeks, 8 weeks, and 16 weeks. Intratumoral Lac/NAA ROC analyses were predictive of survival at all time points tested. At the 8-week time point, 88% of patients with decreased NAA/Cho did not survive 9 months; furthermore, 90% of individuals with an increased Lac/NAA from baseline did not survive at 9 months. No other metabolic ratios tested significantly predicted survival.
Conclusions
Changes in metabolic levels of tumoral NAA/Cho and Lac/NAA can serve as early biomarkers for predicting treatment failure to anti-angiogenic therapy as soon as 1 day after bevacizumab-based therapy. The addition of MRS to conventional MR methods can provide better insight into how anti-angiogenic therapy affects tumor microenvironment and predict patient outcomes.
Keywords: bevacizumab, biomarker, brain tumor, lactate, MR spectroscopy
Key Points.
Anti-VEGF therapy limits conventional MR methods to assess treatment response.
Using MR spectroscopy (MRS), shorter-term survivors had higher tumor Lac/NAA and lower NAA/Cho.
MRS can identify early biomarkers of treatment failure to anti-VEGF therapy.
Importance of the Study.
Patients with recurrent glioblastoma are treated with anti-angiogenic therapy to improve survival and quality of life. Conventional MR techniques have had limited efficacy in predicting treatment response. Here, we perform a longitudinal MR spectroscopy study in patients with recurrent glioblastoma on anti-angiogenic therapy to show that shorter-term survivors have higher levels of Lac/NAA and lower levels of NAA/Cho compared to longer-term survivors at multiple time points. These results suggest that Lac/NAA and NAA/Cho may serve as early biomarkers of treatment response. Future studies may need to consider the impact of differential dosing of anti-angiogenic therapy to alter tumor physiology and improve survival.
Glioblastoma (GBM) is an aggressive primary brain tumor with a grim prognosis.1 With radiographic recurrence characterized by new areas of contrast enhancement or T2-weighted fluid-attenuated inversion recovery (FLAIR) changes on MRI, patients are started on bevacizumab, an anti-vascular endothelial growth factor (VEGF) agent that decreases contrast enhancement via normalizing or pruning tumor vasculature.2,3 This medication has been shown to improve progression-free survival and quality of life compared to historical controls,2,4–6 but its impact on overall survivorship has been less clear. For example, one study in 310 GBM patients suggested that bevacizumab treatment did not improve survival7 while another study in 61 patients suggested that bevacizumab improved survival, with the median overall survival being 10.3 months for the bevacizumab-treated group and 4.2 months for the non-bevacizumab-treated group.8 Due to the absence of a well-established consensus with regards to bevacizumab’s effects on overall survival, there is an unmet clinical need to determine responders to bevacizumab-based therapy.
Much research has focused on advanced MR methods to explore better biomarkers that differentiate pseudo-response from true treatment response after anti-angiogenic therapy.9 Perfusion-weighted imaging assesses tumor hemodynamics such as blood volume (CBV) and flow (CBF); these parameters can become abnormal in aggressive tumors due to malformed vasculature and are affected by anti-VEGF therapy. For example, a few studies that investigated perfusion changes in recurrent GBM (rGBM) after anti-angiogenic therapy found that early changes in CBV were predictive of survival,10–12 although the findings are not consistent.13 The variability among findings could be because perfusion-weighted imaging does not adequately capture the underlying tumor biology after anti-VEGF therapy.
MR spectroscopy (MRS) provides clinically relevant information about the tumor microenvironment after anti-VEGF therapy independent of contrast-enhancement, FLAIR changes, and tumor hemodynamics. Data from a prospective, randomized, phase II multicenter trial evaluating bevacizumab-based therapy suggests that changes in N-acetylaspartate (NAA), a marker for neuronal integrity, and choline (Cho), a marker for tumor proliferation, are useful imaging biomarkers to assess response to anti-angiogenic therapy. In 13 patients, increases in NAA/Cho ratios at 8 weeks post-treatment compared to baseline (BL) levels were predictive for progression-free survival at 6 months (PFS-6) and overall survival at 12 months.14 Lactate (Lac) was not a focus of the study but merits investigation in aggressive tumors that survive in a relatively hypoxic environment.15 Anti-VEGF agents can affect vascular growth, leading to alterations in lactate: higher levels may suggest more aggressive tumor growth while lower levels could suggest treatment response.
To build on our previous work by validating our data findings in a larger patient population, investigating new potential metabolic biomarkers of tumor hypoxia or necrosis (ie, Lac/NAA), and interrogating earlier time points, we performed a prospective, longitudinal study in patients with rGBM receiving bevacizumab-based therapy. We also investigated whether other metabolic biomarkers, especially Lac/NAA, were predictive of treatment outcomes and explored whether earlier time points can predict overall survival in patients with rGBM.
Material and Methods
Recruitment
This study was approved by our center’s institutional review board and was conducted in accordance with the principles of the “World Medical Association Declaration of Helsinki: Research involving human subjects”. Patients with rGBM were recruited in collaboration with their treating neuro-oncologist and provided written informed consent. This study was registered at clinicaltrials.gov (NCT02843230). Eligible participants were at least 18 years old with a BL KPS ≥ 50. Patients had confirmed GBM pathology and recurrence with a treatment plan including bevacizumab monotherapy or combination therapy. The treating neuro-oncologist, in collaboration with a multidisciplinary tumor board, determined patient progression based on increasing tumor size and/or clinical decline. Patients underwent serial MRI scans at the following time points: BL (before bevacizumab treatment), 1–2 days, 2 weeks, 4 weeks, 6–8 weeks, and 12–16 weeks after bevacizumab initiation. The 1–2 days, 6–8 weeks, and 12–16 weeks time points are referenced as 1 day, 8 weeks, and 16 weeks in the manuscript for simplicity.
Imaging Acquisition
MRS was performed on either 3T Siemens (Siemens Healthineers) or 1.5T GE (GE Healthcare) MRI scanners and included the following sequences: FLAIR resolution = 0.85 × 0.85 × 5 mm with 1 mm slice gap (Siemens/GE: repetition time (TR) 9000/9000 ms, echo time (TE) 80/135 ms, inversion time (TI) 2600/2200 ms), T1-weighted imaging res 0.85 × 0.85 × 5 mm with 1 mm slice gap (Siemens/GE: TR 525/475 ms, TE 9/13 ms), and high-resolution 3-dimensional magnetization-prepared rapid acquisition with gradient echo (MPRAGE) and brain volume imaging (BRAVO) post-contrast imaging (Siemens/GE: resolution 1 × 1 × 1 mm/0.5 × 0.5 × 1 mm, TR 2300/8.4 ms, TE 2.3/3.2 ms). Patients were scanned in either GE or Siemens scanners throughout the study to minimize scanner variability.
On the 3T Siemens scanners, 3D MRS data were acquired using a Localization by Adiabatic SElective Refocusing (LASER) pulse sequence with fast SPIRAL k-space acquisition.16,17 Acquisition parameters include: TE = 135 ms, TR = 1700 ms, NA = 4, Matrix = 16 × 16 × 8, FOV = 160 mm, isotropic resolution 1.0 cm3, duration: 7 min. Water suppression was achieved with a modified chemical selective saturation method known as water suppression enhanced through T1 effects (WET), with automatic and subsequent manual shimming of the region of interest (ROI) prior to acquisition to minimize linewidths artifacts. Manual shimming was performed for ~30 s to 1 min.
On the 1.5T GE scanners, 2D MRS data were acquired using a product point-resolved spectroscopy (PRESS) excitation pulse sequence for signal localization with phase encoding. Acquisition parameters included TE 135 ms, TR = 1500 ms, NA = 1, phase encoding = 18 × 18, FOV = 220, resulting in nominal voxel sizes of 1.2 × 1.2 × 1 cm, duration: 8 min. Water suppression was achieved with Chemical Shift Selective (CHESS) methods, with the ROI undergoing automatic and manual shimming to minimize field inhomogeneity. On all MRS acquisitions, scalp lipid contribution was minimized, and the same ROI was selected across time points by using anatomical landmarks (eg, anterior commissure-posterior commissure line) in conjunction with staff annotations for previously selected voxels and automated software such as Auto-Align.18
MRS Data Analysis
The raw data were processed using LCModel 6.3 Software19 to quantify various brain metabolites (NAA, Creatine [Cr], Cho, and Lac). GE data were preprocessed with Spectroscopy Analysis by General Electric (SAGE) software (GE Healthcare). A clinical spectroscopist with over 18 years of experience visually inspected all spectra. Spectra with full width at half maximum (FWHM) < 15 Hz and signal to noise ratio (SNR) ≥ 2 defined as the highest peak divided by the standard deviation of the noise of the residual between the spectrum and the fit (generated by LCModel), were included in the analyses. LCModel has been shown to systematically underestimate the true SNR calculated from the recent MRS consensus paper.20,21 For example, the spectrum from Figure 1F has an LCModel-generated SNR of 8 but a true SNR of 21, suggesting that the true SNR for the data is much higher.
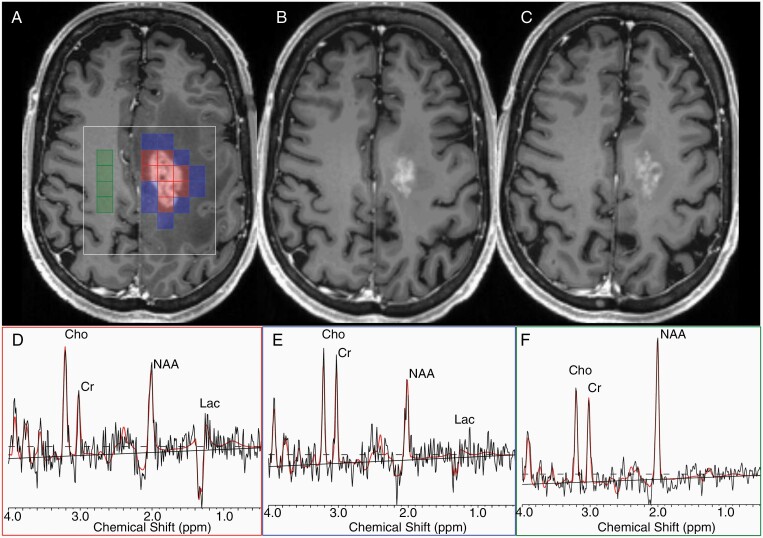
Figure 1.
Representative MRS voxel selection. On an axial T1-weighted post-contrast image (A), intratumor voxels (red), peritumoral (blue), and contralateral normal voxels (green) are selected for analyses. The white square represents the MRS volume of interest. Serial imaging at 4 weeks (B) and 8 weeks (C) after the administration of antiangiogenic therapy is shown for the same patient. Representative spectra for the tumor (D), periphery (E), and contralateral (F) voxels are shown with labeling of NAA, lactate (lac), creatine (Cr), and choline (Cho) peaks where appropriate with the LCModel fit in red.
ROI Selection
The areas of tumor enhancement on the T1-weighted post-contrast image were semi-automatically segmented using 3D Slicer 322,23 as previously described.24 Areas of necrosis and resection cavity were excluded.
The MRS data were overlaid on the post-contrast T1-weighted images. Voxels were classified as contrast-enhancing tumor, nonenhancing tumor periphery, and contralateral normal appearing white matter (Figure 1). Mean metabolite ratios in those regions were calculated as previously described.14 Of note, tumor voxels identified in the BL scan were followed throughout the visits regardless of enhancement. Occasionally, voxel shifting was applied to enable better overlay of baseline scans with follow-up scans.
Statistical Analyses
Based on our previous published work,14 we focused on NAA/Cho at the 8-week time point because it had discriminated between progression-free survival at 6 months and overall survival at 1 year. We also focused on Lac/NAA since other studies have highlighted the importance of hypoxia in GBM.25,26 We also performed exploratory analyses on other metabolites including NAA/contralateral Cr, Cho/contralateral Cr, and Lac/contralateral Cr.
Our previous work chose a 1-year overall survival for classification. However, only 6 patients were alive at this time point, so we chose the 9-month overall survival time point (OS-9). Receiver operating characteristic (ROC) curves for NAA/Cho and Lac/NAA were constructed at OS-9; areas under the ROC curves (AUC), Kaplan–Meier curves, and all analyses, were performed using JMP Pro 15 (SAS, Singapore). A marker was considered effective when the AUC lower 95% CI was greater than 0.50. For demographic data, categorical variables underwent Chi-square testing while continuous variables underwent Mood’s Median Test for between group comparisons.
Results
Subject Demographics
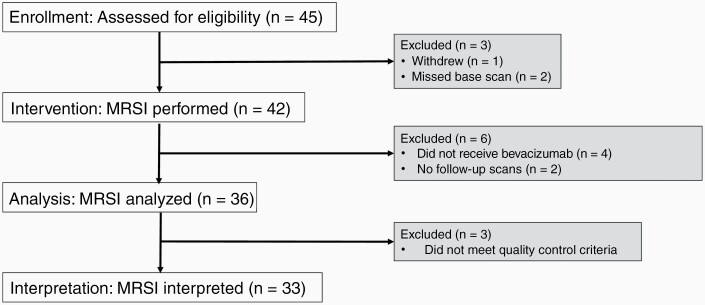
Of the 45 patients enrolled in this study, 3 patients were excluded (1 withdrew, 2 missed the baseline scan). Of the 42 patients who underwent a baseline scan, 4 patients did not receive bevacizumab-based therapy as part of their treatment plan, and 2 patients did not complete serial follow-up scans. Of the remaining patients, 3 subjects were excluded from analysis because their data did not meet quality control criterion due to issues involving voxel placement (too close to skull with lipid contamination, susceptibility artifact resulting in poor SNR with inadequate shimming and water suppression). Thirty-three were included in the final data set (Figure 2). The following patients were scanned at each time point: BL (33), 1 day (13), 2 weeks (10), 4 weeks (25), 8 weeks (21), and 16 weeks (16). There were no group differences when comparing longer-term with shorter-term survivors across age, gender, race, BL KPS, and tumor genetics (Table 1). Patients received bevacizumab monotherapy (10 mg/kg every 2 weeks) or combination therapy with lomustine (90 mg/m2 every 6 weeks), temozolomide (dosing regimen varied; patients either received 50 mg/m2 daily, 150–200 mg/m2 for 5/28 days, or 50 mg-70 mg/m2/day one week on and one week off), and pembrolizumab (2 mg/kg every 3 weeks). Notably, there were no survival differences across the 4 treatment groups.
Figure 2.
Consort flowchart. Forty-five patients are enrolled in the study with 42 patients undergoing MRS imaging. Thirty-six patients have analyzable data, and 33 patients have appropriate quality scans for analyses.
Table 1.
Patient Demographics
| Demographics | Longer-term Survivor | Shorter-term Survivor | Total | P-value |
|---|---|---|---|---|
| Age in years, median ± SD (range) | 62.5 ± 7.0 (54–78) | 63 ± 12.3 (28–81) | 63 ± 10.9 (28–81) | 0.69 |
| Male gender (%) | 5 (15%) | 18 (55%) | 23 (70%) | 0.11 |
| Race (white/Asian/black/other) | 9/0/1/0 | 19/1/2/1 | 28/1/3/1 | 0.68 |
| Baseline KPS, median ± SD (range) | 81 ± 3 (50–90) | 80 ± 2 (50–90) | 81 ± 11 (50–90) | 0.12 |
| IDH1 mutant/wildtype/unk | 1/9/0 | 2/20/1 | 3/29/1 | 0.49 |
| MGMT methylated/unmethylated/unk | 3/6/1 | 3/17/3 | 6/23/4 | 0.53 |
| Treatment type: | 0.43 | |||
| Bev monotherapy | 2 | 5 | 7 | |
| Bev + lomustine | 3 | 11 | 14 | |
| Bev + temozolomide | 5 | 6 | 11 | |
| Bev + pembrolizumab | 0 | 1 | 1 |
There are no group differences between overall longer-term and shorter-term survivors across a variety of demographics including age, gender, tumor genetics, race, BL metabolite level, and treatment type. Unk denotes unknown and Bev denotes bevacizumab.
Longitudinal Metabolic Changes Across Different Treatment Groups
Since patients underwent different bevacizumab-based therapies as prescribed by their neuro-oncologists, we first investigated if there were any group differences in NAA/Cho and Lac/NAA across treatment groups. As there was only one patient treated with bevacizumab and pembrolizumab, this subject was excluded from this analysis. We did not find any effects of treatment on intratumoral or peritumoral metabolites across different time points (P > .05; Supplementary Figure 1), so treatment groups were combined for subsequent analyses.
Tumor Volume
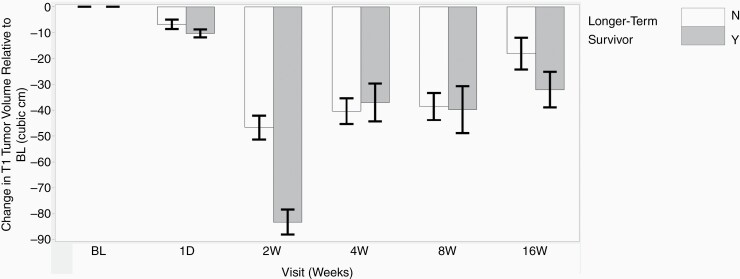
We compared T1-weighted enhancing tumor volume across various time points. Longer-term survivors (OS-9) had an average BL tumor burden volume of 54.81 cm3 while shorter-term survivors (non-OS-9) had an average volume of 61.79 cm3 (P = .65). Regardless of survival, enhancing brain tumor volume significantly decreased from BL at all time points (P < .05) except 1 day. The change in tumor volume discriminated survivorship only at 2 weeks and 16 weeks (Figure 3). ROC analysis for changes in tumor volume showed the following AUCs (confidence interval [CI]: 0.57 (0.43–0.70) for 1 day, 0.93 (0.84–1.0) for 2 weeks, 0.50 (0.39–0.60) at 4 weeks, 0.52 (0.40–0.63) at 8 weeks, and 0.7 (0.58–0.82) at 16 weeks).
Figure 3.
Longitudinal changes in tumor volume compared to BL. Regardless of survival, brain tumor volume significantly decreases from BL to the 2-week, 4-week, 8-week, and 16-week time points. T1-weighted enhancing tumor volume does not discriminate between overall survivorship except at the 2-week and 16-week time points.
NAA/Cho as a Predictor of OS-9
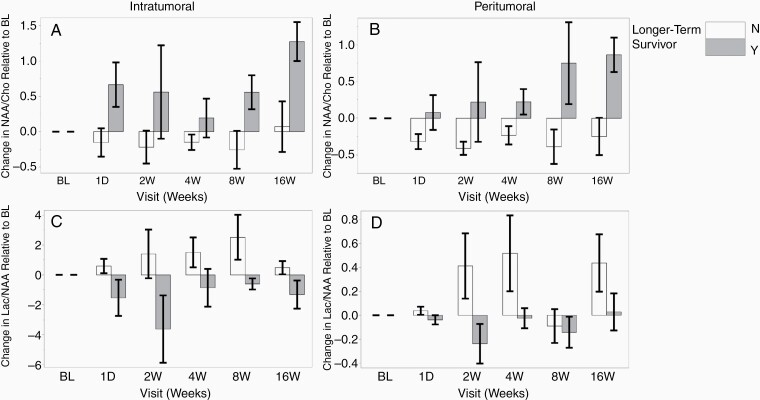
Intratumoral changes in NAA/Cho relative to BL are shown in Figure 4A. Longer-term survivors had a higher level of NAA/Cho compared to shorter-term survivors at 8- and 16-week time points in the tumor and periphery (Figure 4B). ROC analysis for intratumoral NAA/Cho changes compared to BL to assess for stratification based on survival revealed an AUC of 0.71 at 8 weeks and 0.85 at 16 weeks (Table 2). In the tumor periphery, the 8- and 16-week time points were also predictive of survival (AUC 0.75 and 0.88, respectively, Table 2). Contingency analysis at the 8-week time point showed that 88% of the participants with a decrease in NAA/Cho by 0.074 relative to BL did not survive at 9 months.
Figure 4.
Intratumoral and peritumoral changes in NAA/Cho and Lac/NAA relative to BL scan. Longer-term survivors have higher NAA/Cho levels relative to shorter-term survivors in the tumor (A) and peritumoral area (B) across different time points. Lac/NAA is higher in shorter-term survivors compared to longer-term survivors in tumor voxels (C) and in most time points in the peritumoral area except the 8-week time point (D). Error bars represent standard error.
Table 2.
AUCs for Various MRS Metabolites Stratified by 9-Month Survival
| Region | Metabolite | 1 day AUC (95% CI) |
2 week AUC (95% CI) |
4 week AUC (95% CI) |
8 week AUC (95% CI) |
16 week AUC (95% CI) |
|---|---|---|---|---|---|---|
| Tumor | NAA/Cho |
0.92
(0.78,1) |
0.75
(0.54,1) |
0.60 (0.41, 0.78) |
0.71
(0.52, 0.90) |
0.85
(0.68, 1) |
| Lac/NAA |
0.83
(0.64,1) |
1
(1,1) |
0.76
(0.69, 0.92) |
0.76
(0.58, 0.93) |
0.76
(0.56, 0.96) |
|
| Periphery | NAA/Cho |
0.75
(0.53, 0.97) |
0.88
(0.69,1) |
0.75
(0.57, 0.93) |
0.75
(0.57, 0.93) |
0.88
(0.73, 1) |
| Lac/NAA | 0.55 (0.30, 0.79) |
1
(1,1) |
0.68 (0.51, 0.85) |
0.61 (0.41,0.80) |
0.72
(0.51, 0.93) |
The table illustrates AUCs of NAA/Cho and Lac/NAA in the tumor and tumor periphery with 95% CI at different time points. Effective classifications defined by a lower bound of the 95% CI greater than 0.5 are bolded.
We then performed exploratory analyses to determine if NAA/Cho can distinguish longer-term from shorter-term survivors at earlier time points. Longer-term survivors had increased NAA/Cho in the tumor while shorter-term survivors had decreased NAA/Cho compared to BL (Figure 4). NAA/Cho was predictive of survival at 1 day and 2 weeks (AUC 0.92 and 0.75, respectively) but not at 4 weeks (Table 2). In the tumor periphery, changes in NAA/Cho levels compared to BL were associated with longer-term survival at all earlier time points (Table 2, Figure 4B). In sum, changes in intratumoral NAA/Cho were significantly different between longer-term and shorter-term survivors except at the 4-week time point, while changes in peritumoral NAA/Cho were significantly different between shorter-term and longer-term survivors at all time points evaluated.
Survival Analyses
We split our cohort into 2 subgroups, one with a change in NAA/Cho greater than 0.074 and the other with a change in NAA/Cho less than 0.074 compared to BL. After performing a Kaplan-Meier survival analysis, we found that subjects with increased NAA/Cho had a median overall survivorship of 274 days compared to 175 days for subjects with decreased NAA/Cho (Supplementary Figure 2). Generalized Wilcoxon test demonstrated a statistically significant difference between the 2 groups (P = .04).
Lac/NAA as a Predictor of OS-9
Intratumoral changes in Lac/NAA relative to BL are shown in Figure 4C; shorter-term survivors had increased changes in Lac/NAA compared to longer-term survivors at 8 and 16 weeks. ROC analyses revealed an AUC of 0.76 at both time points (Table 2). In the peritumoral voxels, shorter-term survivors were associated with higher Lac/NAA values compared to BL at 16 weeks (AUC 0.72) but not the 8 weeks (Figure 4D, Table 2). Contingency analysis at the 8-week time point revealed that 90% of individuals with an increased Lac/NAA from BL did not survive at 9 months.
Similar to NAA/Cho, we investigated changes in Lac/NAA at earlier time points to see if this could be predictive of survival. ROC analyses within tumor voxels showed that Lac/NAA predicted survival to bevacizumab-based therapy at all earlier time points (Table 2). Within peritumoral voxels, ROC analyses showed an AUC of 1 at the 2-week time point and 0.68 at 4 weeks but did not predict survival at 1 day (Table 2).
Survival Analyses
Similar to before, we generated 2 subgroups, one with increased Lac/NAA and the other with decreased Lac/NAA relative to BL. Kaplan-Meier analysis showed that subjects with increased Lac/NAA had a median overall survivorship of 197 days compared to 292 days for subjects with decreased Lac/NAA (Supplementary Figure 3), although this finding was not significant (P = .11).
Other Metabolic Predictors of OS-9
Finally, we explored whether other metabolites also changed during anti-VEGF treatment. Within the tumor, we analyzed NAA/contralateral Cr, Lac/contralateral Cr, and Cho/contralateral Cr and did not find any group differences across the different time points (Supplementary Figure 4).
Discussion
Our longitudinal MRS study in patients with rGBM illustrates that intratumoral changes in NAA/Cho and Lac/NAA can differentiate longer-term survivors from shorter-term survivors as early as 1 day after initiation of bevacizumab-based therapy and persist at the 2- and 8-week time points. In the tumor periphery, the NAA/Cho was higher in longer-term compared to shorter-term survivors at the early time points, but this was not true for Lac/NAA. Taken together, these results suggest that NAA/Cho and Lac/NAA may serve as timely biomarkers for predicting early treatment failure (or response) to bevacizumab-based therapy.
Our findings that metabolite levels derived from MRS may be robust early biomarkers in assessing patient response to anti-angiogenic therapy are consistent with emerging literature. We previously showed that increased levels of NAA/Cho compared to BL at 8 weeks post-treatment were predictive of overall survival at 12 months,14 and we show that these findings are also present even sooner. In a study by Kim et al., 20 patients with rGBM were treated with cediranib, a tyrosine kinase inhibitor of the VEGF receptor; the authors showed that intratumoral increases in NAA/Cho were higher at 56 days (8 weeks) post-treatment and predictive of 6-month overall survival.27 A longitudinal prospective study in 40 newly diagnosed GBM patients receiving cediranib and comitant chemoradiation showed that Cho/hCr (healthy creatine) at 4 weeks correlated with overall survival and had AUC larger than tumor volumetrics and cerebral blood flow.28 In another study involving 31 patients with GBM receiving anti-angiogenic therapy and radiation, the authors showed that reductions in Cho/NAA and lower Lac/NAA at 16 weeks correlated with progression-free and overall survival.25 Lower Cho/NAA suggests reduced tumor cellularity while decreased Lac/NAA reflects vascular normalization and increased tumor oxygenation. Our data are able to stratify treatment failure sooner than 16 weeks. At 8 weeks, we find that 88% of patients with decreased NAA/Cho from BL and 90% of individuals with an increased Lac/NAA from BL did not survive at 9 months.
Our MRS results illustrate that changes in tumor microenvironment after treatment with anti-angiogenic therapy impact patient survival. GBM has a necrotic core with cells migrating away from the hypoxic environment toward a vascular supply.29 The hypoxic environment helps tumor cells evade the immune system while selecting for more malignant tumor cells.30 This survival advantage is balanced with upregulation of angiogenic factors to maintain adequate perfusion for survival.31,32 Targeting angiogenic factors, such as VEGF, can lead to either pruning of fragile tumor vessels or vascular normalization. Pruning results in increased tumor hypoxia and lactate while normalization has the opposite effect and can improve survival.30 Our results suggest the underlying mechanism of shorter-term survivors could be due to vascular pruning, leading to increased hypoxia or necrosis (and Lac/NAA), and increased tumor cellularity (decreased NAA/Cho levels). Consistent with this,31 phosphorous MRS has shown that shorter-term survivors had higher markers of tumor cell proliferation when measuring phospholipid membrane metabolism.33 Therefore, MRS may be a dynamic biomarker of vascular structure and function and could help tailor bevacizumab dosing. Consistent with this framework, mouse studies have shown dose-dependent effects of bevacizumab on tumor cells and vasculature.34,35 Future studies will need to explore whether shorter-term survivors have fewer VEGFA receptors and would benefit from reduced bevacizumab dosing to shift tumor physiology from vascular pruning to normalization.
Our finding that changes in intratumoral NAA/Cho and Lac/NAA as well as peritumoral NAA/Cho were predictive of survivorship as early as 1 day suggests that anti-angiogenic therapy may have an immediate effect on tumor microenvironment, a finding reported by other groups. A previous study by Gerstner et al. showed that 1 day after BEV treatment, readily detectable changes in contrast-enhancing tumor volume, tumor Ktrans values, and tumor SUV were seen.36 Furthermore, another phase II trial by Sorensen et al. showed that cediranib, a VEGF receptor tyrosine kinase inhibitor, results in extensive normalization of tumor vasculature.37 The extent of this normalization was predictive of PFS and OS.37 Taken together, these results may suggest that anti-angiogenic therapy can result in dramatic changes in tumor microenvironment as early as 1 day after treatment initiation. This is also supported by the rapid decrease in contrast enhancement seen one day after bevacizumab treatment.
We also investigated correlations between tumor volumes and survival and found that enhancing tumor volume decreases at all tested time points regardless of survival. The magnitude of this change was able to distinguish between longer-term and shorter-term survivors at 2 weeks and 16 weeks after bevacizumab-based therapy, which is consistent with reports by other groups who have been able to distinguish between longer-term and shorter-term survivors based on enhancing tumor volume.38 The finding’s clinical relevance is limited by the fact that tumor volume decreased in all subjects regardless of survivorship status and was not significant at other time points. However, our findings regarding Lac/NAA and NAA/Cho are of greater clinical significance because longer-term and shorter-term survivors displayed opposite trajectories. Namely, longer-term survivors display increases in NAA/Cho and decreases in Lac/NAA, the opposite of shorter-term survivors. Furthermore, metabolic changes as detected by MRS were predictive at all time points, further increasing the clinical robustness of these findings by making it easier for clinician evaluation.
Despite its potential to provide meaningful biomarkers to improve diagnosis, better define the natural history of a disease process, or monitor metabolic responses to therapy, there is a wide notion that MRS is difficult to implement within the clinical workflow and lacks reproducibility. Here, we use a non-product 3D MRSI sequence that has been used in several clinical trials,26,39 yields robust findings, and is available to other hospitals and research sites by way of a C2P agreement. Since there is considerable technical expertise that limits methodological implementation, our group has been working with other national and international sites to provide a turn-key package of advanced MRS technology for end users for multi-site/vendor trials and routine clinical use (Siemens, GE, and Philips 3T platforms). To address the variable data quality that may be inherent to standard clinical MRS packages, our group is developing a protocol using a semi-LASER sequence,40 utilizing automated atlas-based voxel positioning selection for the ROI,41 and implementing Automated FASTMAP to improve shimming and line width reduction.42 The goal would be to generate metabolic color maps which would depict increases and decreases in metabolites such as NAA/Cho or Lac/NAA from baseline.
We also acknowledge the limitations common to longitudinal studies. First, there was some patient attrition across scan time points due to changes in goals of care (despite lack of tumor progression) or inability to make some scan time points. This led to a smaller than anticipated sample size during some study visits (ie, 1 day and 2 weeks), making a type 1 error more likely. However, our results still remain significant at later time points with larger samples, highlighting the robustness of the findings. Patients had MRS sequences added onto clinical MRI scans at locations that were convenient for them. We made sure to scan patients using the same type of scanner (GE or Siemens), and we focused on changes in metabolites compared to BL scan to minimize scanner effect and any large systematic confounds. Although only voxels that were clearly defined in the tumor or tumor periphery were included, there could be partial volume effects due to the large voxel size of the data. Given our experience with the method, we feel confident that the voxels selected best represent each of the areas indicated with minimal overlap or shift across time. Furthermore, a diagnosis of recurrence was not determined by histological biopsy but rather a multidisciplinary tumor board in conjunction with the patient’s treating neurooncologist. Although unlikely, pseudoprogression from radiation necrosis may have been a confound when enrolling patients for this study.
Conclusion
Although conventional MR methods can assess for rGBM, they are susceptible to pseudo-response after anti-angiogenic therapy, making them unreliable. Addition of advanced MR methods such as MRS more accurately reflects tumor burden by assessing tumor microenvironment and can work in synergy with conventional MR methods to understand how anti-angiogenic therapy affects the tumor environment, which may lead to better treatment combinations. Our study shows that changes in tumoral and peripheral NAA/Cho and tumoral Lac/NAA can be robust biomarkers for predicting response to anti-angiogenic therapy and are predictive of patient outcomes as early as 1 day and 2 weeks. Incorporating MRS into clinical scans can distinguish between pseudo-response and treatment response early in the course of anti-angiogenic therapy in patients with rGBM.
Supplementary Material
Acknowledgments
This work would not be possible without the patients and their families, participating MGH Neuro-oncologists, MGH Cancer Center and Radiology staff, and Quantitative Tumor Imaging staff. Parts of this data have been presented at the 26th Scientific Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine in 2018 in Paris, France (oral presentation) and the 57th Annual Meeting of the American Society of Neuroradiology (ASNR) in 2019 in Boston, Massachusetts (oral presentation).
Funding
This study was supported by the National Institutes of Health (R01CA190901 to E.M.R., R01CA129371 to T.B., R21AG067562 to Y.Y., and R21GM137227 to Y.Y.).
Conflict of interest statement. T.B.: Genomicare (Scientific Advisory Board) and UpToDate. E.M.R: BrainSpec Inc (Scientific Advisory Board)
Authorship Statement. Experimental design: J.D., M.V., T.B., R.G., E.G., B.R., O.R., and E.M.R. Implementation: D.K., J.D., M.F., M.W., O.R., I.A., O.A., Y.Y., E.G., D.F., and E.M.R. Data analysis/interpretation: D.K., M.E., P.T., J.D., M.F., O.R., J.K., R.G., B.R., E.G., M.W., J.H., S.N., M.V., A.V., and E.M.R. Manuscript draft/revisions: M.E., P.T., D.K., and E.M.R. Approved final manuscript: all authors.
References
- 1. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 2020;22(12 Suppl 2):iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahase S, Rattenni RN, Wesseling P, et al. Hypoxia-mediated mechanisms associated with antiangiogenic treatment resistance in glioblastomas. Am J Pathol. 2017;187(5):940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb Perspect Med. 2012;2(3):a006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 5. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. [DOI] [PubMed] [Google Scholar]
- 7. Gramatzki D, Roth P, Rushing EJ, et al. Bevacizumab may improve quality of life, but not overall survival in glioblastoma: an epidemiological study. Ann Oncol. 2018;29(6):1431–1436. [DOI] [PubMed] [Google Scholar]
- 8. Hofmann S, Schmidt MA, Weissmann T, et al. Evidence for improved survival with bevacizumab treatment in recurrent high-grade gliomas: a retrospective study with (“pseudo-randomized”) treatment allocation by the health insurance provider. J Neurooncol. 2020;148(2):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boxerman JL, Zhang Z, Safriel Y, et al. Early post-bevacizumab progression on contrast-enhanced MRI as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 Central Reader Study. Neuro Oncol. 2013;15(7):945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stecco A, Amatuzzo P, Sponghini AP, et al. Prognostic value of relative cerebral blood volume in patients with recurrent glioblastoma multiforme treated with bevacizumab. J Neurosurg Sci. 2019;63(4):394–401. [DOI] [PubMed] [Google Scholar]
- 11. Kickingereder P, Radbruch A, Burth S, et al. MR Perfusion-derived hemodynamic parametric response mapping of bevacizumab efficacy in recurrent Glioblastoma. Radiology. 2016;279(2):542–552. [DOI] [PubMed] [Google Scholar]
- 12. Schmainda KM, Zhang Z, Prah M, et al. Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 multicenter trial. Neuro Oncol. 2015;17(8):1148–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stadlbauer A, Pichler P, Karl M, et al. Quantification of serial changes in cerebral blood volume and metabolism in patients with recurrent glioblastoma undergoing antiangiogenic therapy. Eur J Radiol. 2015;84(6):1128–1136. [DOI] [PubMed] [Google Scholar]
- 14. Ratai EM, Zhang Z, Snyder BS, et al. Magnetic resonance spectroscopy as an early indicator of response to anti-angiogenic therapy in patients with recurrent glioblastoma: RTOG 0625/ACRIN 6677. Neuro Oncol. 2013;15(7):936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeBerardinis RJ, Chandel NS. We need to talk about the Warburg effect. Nat Metab. 2020;2(2):127–129. [DOI] [PubMed] [Google Scholar]
- 16. Andronesi OC, Ramadan S, Mountford CE, Sorensen AG. Low-power adiabatic sequences for in vivo localized two-dimensional chemical shift correlated MR spectroscopy. Magn Reson Med. 2010;64(6):1542–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andronesi OC, Gagoski BA, Sorensen AG. Neurologic 3D MR spectroscopic imaging with low-power adiabatic pulses and fast spiral acquisition. Radiology. 2012;262(2):647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benner T, Wisco JJ, van der Kouwe AJ, et al. Comparison of manual and automatic section positioning of brain MR images. Radiology. 2006;239(1):246–254. [DOI] [PubMed] [Google Scholar]
- 19. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. [DOI] [PubMed] [Google Scholar]
- 20. Kreis R, Boer V, Choi IY, et al. Terminology and concepts for the characterization of in vivo MR spectroscopy methods and MR spectra: background and experts’ consensus recommendations. NMR Biomed. 2020;34:e4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schirmer T, Werner B, Hancu I, Martin E. SNR Measurements in a single voxel MRS experiment. Paper presented at: 13th Scientific Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine2005; Miami, FL USA. [Google Scholar]
- 22. Pieper S, Halle M, Kikinis R. 3D Slicer. Paper presented at: Proc of the 1st IEEE International Symposium on Biomedical Imaging: From Nano to Macro2004. [Google Scholar]
- 23. Pieper S, Lorensen B, Schroeder W, Kikinis R. The NA-MIC Kit: ITK, VTK, Pipelines, Grids and 3D Slicer as An Open Platform for the Medical Image Computing Community. Paper presented at: 3rd IEEE International Symposium on Biomedical Imaging: Macro to Nano2006. [Google Scholar]
- 24. Chang K, Beers AL, Bai HX, et al. Automatic assessment of glioma burden: a deep learning algorithm for fully automated volumetric and bidimensional measurement. Neuro Oncol. 2019;21(11):1412–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nelson SJ, Li Y, Lupo JM, et al. Serial analysis of 3D H-1 MRSI for patients with newly diagnosed GBM treated with combination therapy that includes bevacizumab. J Neurooncol. 2016;130(1):171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ratai EM, Zhang Z, Fink J, et al. ACRIN 6684: multicenter, phase II assessment of tumor hypoxia in newly diagnosed glioblastoma using magnetic resonance spectroscopy. PLoS One. 2018;13(6):e0198548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim H, Catana C, Ratai EM, et al. Serial magnetic resonance spectroscopy reveals a direct metabolic effect of cediranib in glioblastoma. Cancer Res. 2011;71(11):3745–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andronesi OC, Esmaeili M, Borra RJH, et al. Early changes in glioblastoma metabolism measured by MR spectroscopic imaging during combination of anti-angiogenic cediranib and chemoradiation therapy are associated with survival. NPJ Precis Oncol. 2017;1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brat DJ, Castellano-Sanchez AA, Hunter SB, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64(3):920–927. [DOI] [PubMed] [Google Scholar]
- 30. Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zagzag D, Amirnovin R, Greco MA, et al. Vascular apoptosis and involution in gliomas precede neovascularization: a novel concept for glioma growth and angiogenesis. Lab Invest. 2000;80(6):837–849. [DOI] [PubMed] [Google Scholar]
- 32. Zagzag D, Friedlander DR, Margolis B, et al. Molecular events implicated in brain tumor angiogenesis and invasion. Pediatr Neurosurg. 2000;33(1):49–55. [DOI] [PubMed] [Google Scholar]
- 33. Hattingen E, Bähr O, Rieger J, Blasel S, Steinbach J, Pilatus U. Phospholipid metabolites in recurrent glioblastoma: in vivo markers detect different tumor phenotypes before and under antiangiogenic therapy. PLoS One. 2013;8(3):e56439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. von Baumgarten L, Brucker D, Tirniceru A, et al. Bevacizumab has differential and dose-dependent effects on glioma blood vessels and tumor cells. Clin Cancer Res. 2011;17(19):6192–6205. [DOI] [PubMed] [Google Scholar]
- 35. García-Romero N, Palacín-Aliana I, Madurga R, et al. Bevacizumab dose adjustment to improve clinical outcomes of glioblastoma. BMC Med. 2020;18(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gerstner ER, Emblem KE, Chang K, et al. Bevacizumab reduces permeability and concurrent temozolomide delivery in a subset of patients with recurrent Glioblastoma. Clin Cancer Res. 2020;26(1):206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sorensen AG, Emblem KE, Polaskova P, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72(2):402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ellingson BM, Kim HJ, Woodworth DC, et al. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andronesi OC, Arrillaga-Romany IC, Ly KI, et al. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat Commun. 2018;9(1):1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deelchand DK, Berrington A, Noeske R, et al. Across-vendor standardization of semi-LASER for single-voxel MRS at 3T. NMR Biomed. 2019;34:e4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park YW, Deelchand DK, Joers JM, et al. AutoVOI: real-time automatic prescription of volume-of-interest for single voxel spectroscopy. Magn Reson Med. 2018;80(5):1787–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29(6):804–811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.