Abstract
Background
Recent studies have identified that glioblastoma IDH-wildtype (GBM IDH-WT) might be comprised of molecular subgroups with distinct prognoses. Therefore, we investigated the correlation between genetic alterations and survival in 282 GBM IDH-WT patients, to identify subgroups with distinct outcomes.
Methods
We reviewed characteristics of GBM IDH-WT (2009–2019) patients analyzed by next-generation sequencing interrogating 205 genes and 26 rearrangements. Progression-free survival (PFS) and overall survival (OS) were evaluated with the log-rank test and Cox regression models. We validated our results utilizing data from cBioPortal (MSK-IMPACT dataset).
Results
Multivariable analysis of GBM IDH-WT revealed that treatment with chemoradiation and RB1-mutant status correlated with improved PFS (hazard ratio [HR] 0.25, P < .001 and HR 0.47, P = .002) and OS (HR 0.24, P < .001 and HR 0.49, P = .016). In addition, younger age (<55 years) was associated with improved OS. Karnofsky performance status less than 80 (HR 1.44, P = .024) and KDR amplification (HR 2.51, P = .008) were predictors of worse OS. KDR-amplified patients harbored coexisting PDGFRA and KIT amplification (P < .001) and TP53 mutations (P = .04). RB1-mutant patients had less frequent CDKN2A/B and EGFR alterations (P < .001). Conversely, RB1-mutant patients had more frequent TP53 (P < .001) and SETD2 (P = .006) mutations. Analysis of the MSK-IMPACT dataset (n = 551) validated the association between RB1 mutations and improved PFS (11.0 vs 8.7 months, P = .009) and OS (34.7 vs 21.7 months, P = .016).
Conclusions
RB1-mutant GBM IDH-WT is a molecular subgroup with improved PFS and OS. Meanwhile, 4q12 amplification (KDR/PDGFRA/KIT) denoted patients with worse OS. Identifying subgroups of GBM IDH-WT with distinct survival is important for optimal clinical trial design, incorporation of targeted therapies, and personalized neuro-oncological care.
Keywords: glioblastoma, IDH-WT, KDR, RB1, VEGFR2, 4q12
Key Points.
RB1-mutant GBM IDH-WT patients have improved PFS and OS.
RB1-mutant GBM IDH-WT patients have a lower frequency of CDKN2A/B loss and EGFR alterations.
4q12-amplified GBM IDH-WT patients have worse survival.
Importance of the Study.
Glioblastoma IDH-wildtype (GBM IDH-WT) comprises different molecular subgroups; however, the prognostic significance of these subgroups has not been defined. We demonstrated in a large molecularly characterized GBM IDH-WT cohort 2 genetically distinct subgroups with different prognoses. Our findings were validated with large external GBM IDH-WT datasets. GBM IDH-WT with RB1 mutations is a molecular subgroup with improved PFS and OS and decreased frequency of CDKN2A/B loss and EGFR alterations. On the other hand, 4q12 (KDR/PDGFRA/KIT) amplified patients have worse survival. Our data revealed the importance of genetic profiling of GBM IDH-WT to identify subgroups with distinct survival. This is crucial for optimal clinical trial design, targeted therapies, and personalized neuro-oncological care.
Glioblastoma (GBM) is the most common and aggressive central nervous system (CNS) primary malignancy.1 Despite aggressive treatment with maximal safe resection and chemoradiotherapy,2 and multimodal therapy upon recurrence, GBM is associated with a dismal prognosis.1
Over the past decade, molecular characterization of gliomas has revealed the heterogeneous nature of this group of tumors.3–7 These findings led to a reclassification of infiltrating gliomas, in which both tumor histology and genetic alterations are considered.8 Infiltrating gliomas are classified by the presence or absence of mutations in the isocitrate dehydrogenase (IDH) 1 or 2 genes, as IDH-wildtype (WT) or IDH-mutant, with different demographic, clinical, and prognostic characteristics.8 Recent studies have identified that GBM IDH-WT consists of different molecular subgroups, which might have a distinct prognosis.6,7,9,10 However, more studies are needed to understand molecular subgroups of GBM IDH-WT as survival differences between these have not been thoroughly investigated.
Therefore, we examined the correlation between genetic alterations and survival in a cohort of GBM IDH-WT patients, to identify potential subgroups with different behavior, who may potentially benefit from targeted therapies. Our findings were validated using a large publicly available dataset from Memorial Sloan Kettering Cancer Center (MSK-IMPACT).11
Methods
Patients and Tumor Samples
We performed a retrospective review of GBMs in an institutional glioma registry of patients diagnosed between 2009 and 2019. The inclusion criteria for this study were confirmed diagnosis of GBM IDH-WT according to the cIMPACT-NOW Update 312 and availability of sequencing data from a comprehensive next-generation sequencing (NGS) assay. A flow diagram selection of the study population is depicted in Supplementary Figure S1.
Data for this study were collected from Memorial Hermann Hospital’s electronic medical records. Data were managed with REDCap electronic data capture tools hosted at the University of Texas Health Science Center at Houston (UTHealth).13 These included age, sex, race, Karnofsky performance status (KPS), diagnosis, radiographic extent of resection, treatment strategy, and survival. Tumors were classified by a board-certified neuropathologist following the 2016 WHO Classification of Tumors of the CNS8 and cIMPACT-NOW updates. Radiographic extent of resection was classified as gross total resection (GTR), near-total resection (NTR), or subtotal resection as previously described.14 Recurrence and therapeutic strategy were determined by individual revision of cases by a multidisciplinary tumor board as previously described.15
Ethical Statement
This study was approved by the Institutional Review Board (ID: HSC-MS-17-0917) of UTHealth and Memorial Hermann Hospital, Houston, TX.
Targeted Sequencing
Tumor samples were analyzed for genomic alterations by a targeted NGS panel interrogating 205 genes and 26 gene rearrangements including telomerase reverse transcriptase promoter (TERTp) mutations (FoundationOne; Foundation Medicine, Inc.). The FoundationOne assay was performed in a Clinical Laboratory Improvement Amendments certified laboratory, as previously described.16,17TERTp status was not available for 61 patients.
Validation Cohorts
To validate our findings, we utilized the dataset from the MSK-IMPACT available at cBioPortal (https://www.cbioportal.org/), accessed on August 2020.11,18,19 This dataset provided clinical and genetic information including IDH, MGMT, and TERTp status. Additionally, we used a GBM cohort with IDH1 p.R132H status from a publicly available study evaluating the 4q12 amplicon in GBM.20 Tumors classified as GBM IDH-WT according to the cIMPACT-NOW Update 3 criteria were analyzed.12
Statistical Analyses
Descriptive analyses were performed by the Mann–Whitney U test or Fisher’s exact test for continuous or categorical variables, respectively. The endpoints of the study were overall survival (OS) and progression-free survival (PFS). OS was calculated as the time in months from diagnosis to death or the last available follow-up. PFS was calculated as the time in months from diagnosis to progression of the disease. The univariable two-sided log-rank test was used to examine statistical significance in survival, while the Kaplan–Meier method was employed to plot visual survival curves. Univariable and multivariable Cox proportional hazard regression models were utilized to calculate the hazard ratio (HR) estimates with a 95% confidence interval (CI) adjusted for possible confounders. Multivariable Cox proportional hazard regression model analysis for PFS and OS was adjusted for the variables with a P value of .05 or less in univariable analysis, as these might affect survival. Demographic, clinical, and genetic characteristics were evaluated by the genes of interest to identify differences in such traits. The genes of interest were defined as the genes that correlate with survival. P value of .05 or less was considered statistically significant and was two-sided. The differences in genetic characteristics were adjusted for multiple comparisons utilizing the Benjamini–Hochberg FDR correction procedure (q value). Statistical analyses were performed using EZR (1.40)17,21 and Prism v.8.4.3 (GraphPad). The oncoplots were created using cBioPortal OncoPrinter Tool.18,19Figures 4 and 5 were created with BioRender.com.
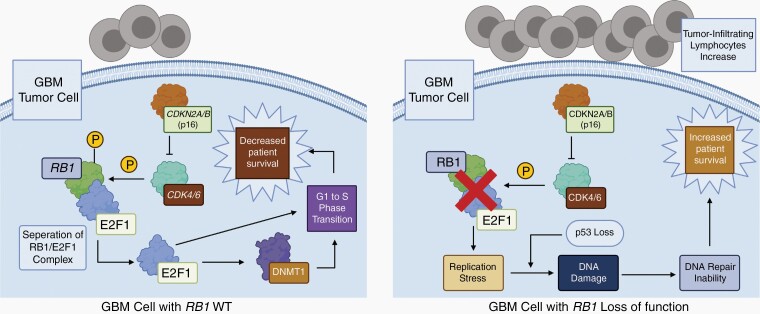
Figure 4.
Proposed mechanism of increased survival of GBM IDH-WT, RB1-mutant patients based on prior studies. GBM IDH-WT patients with RB1 loss-of-function mutations have an increased survival through replication stress and DNA damage, which leads to DNA repair inability in the tumor cells. In addition, RB1 loss of function is correlated with increased tumor-infiltrating lymphocytes (right). These might be the cause of the increased survival compared to RB1-WT, GBM IDH-WT patients (left). RB1 loss of function is represented by the red “X.”
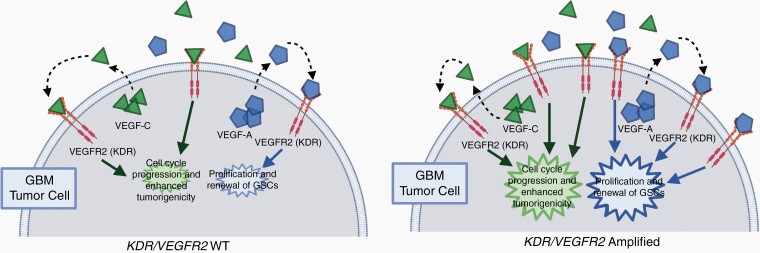
Figure 5.
Proposed mechanism for the proliferative effects of KDR/VEGFR2 amplification in GBM IDH-WT based on prior studies. As seen on the left, binding of VEGF-A and VEGF-C ligands results in increased proliferation of GBM cells. On the right, it is hypothesized that KDR amplification would increase the number of available receptors, thus magnifying these effects. In addition, with KDR amplification, there may also be an additional elevation of certain protein kinase levels, such as that of c-Met and p38, which serve to increase the invasiveness of the tumor.
Results
Cohort Characteristics
A total of 282 patients with GBM IDH-WT met the inclusion criteria (Supplementary Figure S1). The median age of this cohort was 61 years (interquartile range 53–67.8). One hundred seventy patients (60%) were males, 201 patients (71%) were non-Hispanic White, and 107 patients (38%) had KPS of at least 80. Twenty-seven patients (10%) had a biopsy, while 92 patients (33%) received a GTR. Chemoradiotherapy with temozolomide (TMZ) was administered in 258 (91%) patients according to the Stupp protocol.2 Also, 33 (12%) patients were treated with up-front tumor-treating fields (TTFs). Furthermore, 203 patients had documented recurrence of their disease, and out of these patients, 88 (43%) had reoperation for their first recurrence, 94 (46%) received TMZ, 143 (70%) received bevacizumab, 78 (38%) received irinotecan, 17 (8%) lomustine, 63 (31%) TTF, 50 (25%) re-irradiation, and 78 (38%) salvage radiosurgery (Supplementary Table S1).
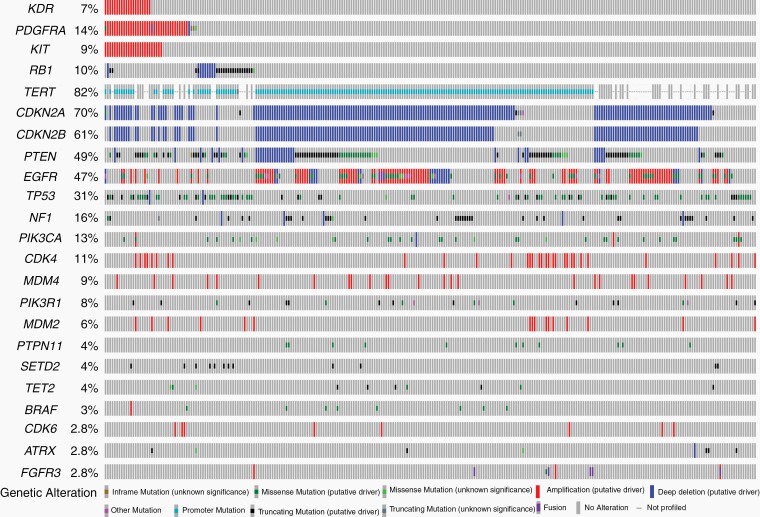
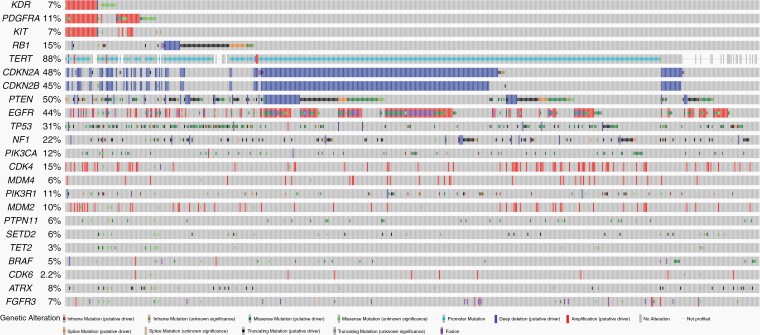
Genes mutated in at least 3% of patients are depicted in Figure 1 and Supplementary Table S1. The most common genomic alterations in the cohort were in the following genes: TERTp—81%, CDKN2A/B—70%, PTEN—48%, EGFR—46%, TP53—30%, NF1—16%, PDGFRA—14%, PIK3CA—13%, CDK4—11%, RB1—10%, MDM4—9%, KIT—9%, and KDR—7%.
Figure 1.
Oncoplot representing the genomic landscape of GBM IDH-WT from UTHealth (n = 282).
Outcomes in GBM IDH-WT
Univariable analysis of PFS showed that patients who received chemoradiotherapy with TMZ according to the Stupp protocol (HR 0.25, P < .001) had a significantly lower risk of death. This finding was further confirmed by multivariable analysis (HR 0.25, P < .001; Table 1). Additionally, it was observed that GBM IDH-WT patients harboring RB1 mutations (n = 28) had an improved PFS compared to RB1-WT (n = 254) patients (11.9 vs 7.5 months, P = .0001, log-rank test; Supplementary Figure S2A). This finding was confirmed by multivariable analysis (HR 0.47, P = .002; Table 1). The PFS of GBM IDH-WT patients harboring a KDR amplification was not significantly different compared to KDR-WT patients (5.8 vs 8.3 months, Supplementary Figure S2B).
Table 1.
Univariable and Multivariable Cox Proportional Hazard Regression Models of Progression-Free Survival and Overall Survival From GBM IDH-WT Patients in the UTHealth Cohort (n = 282)
| Variable | Univariable | P | Multivariable | P |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Progression-free survival | ||||
| Age at diagnosis <55 years | 1.11 (0.83–1.50) | .479 | ||
| Male | 0.82 (0.62–1.09) | .177 | ||
| KPS <80 at diagnosis | 0.83 (0.63–1.10) | .191 | ||
| Extent of resection | ||||
| GTR | 1.07 (0.60–1.90) | .822 | ||
| NTR | 0.96 (0.51–1.80) | .888 | ||
| STR | 1.09 (0.62–1.91) | .778 | ||
| Chemoradiotherapy with TMZ | 0.25 (0.13–0.45) | <.001 | 0.25 (0.14–0.48) | <.001 |
| KDR mutant | 1.03 (0.57–1.84) | .935 | ||
| KIT mutant | 1.13 (0.69–1.86) | .625 | ||
| PDGFRA mutant | 1.17 (0.79–1.72) | .438 | ||
| RB1 | 0.46 (0.29–0.75) | .002 | 0.47 (0.29–0.76) | .002 |
| TP53 mutant | 1.00 (0.74–1.36) | .993 | ||
| Overall survival | ||||
| Age at diagnosis <55 years | 0.59 (0.43–0.80) | <.001 | 0.63 (0.45–0.90) | .010 |
| Male | 0.94 (0.72–1.23) | .670 | ||
| KPS <80 at diagnosis | 1.63 (1.24–2.15) | <.001 | 1.44 (1.04–1.98) | .024 |
| Extent of resection | ||||
| GTR | 0.59 (0.37–0.94) | .028 | 1.17 (0.62–2.22) | .626 |
| NTR | 0.52 (0.31–0.88) | .015 | 1.01 (0.50–2.03) | .975 |
| STR | 0.64 (0.41–1.01) | .056 | 1.13 (0.60–2.13) | .697 |
| Biopsy | Ref. | Ref. | ||
| Chemoradiotherapy with TMZ | 0.32 ((0.19–0.53) | <.001 | 0.24 (0.12–0.50) | <.001 |
| Second surgery | 0.74 (0.54–1.02) | .062 | ||
| Salvage bevacizumab | 0.55 (0.40–0.77) | <.001 | 0.54 (0.38–0.76) | <.001 |
| Salvage tumor-treating fields | 0.83 (0.60–1.15) | .267 | ||
| KDR mutant | 1.97 (1.18–3.28) | .009 | 2.51 (1.27–4.94) | .008 |
| KIT mutant | 1.30 (0.81–2.09) | .271 | ||
| PDGFRA mutant | 1.21 (0.83–1.76) | .323 | ||
| RB1 mutant | 0.60 (0.38–0.95) | .028 | 0.49 (0.27–0.87) | .016 |
| TP53 mutant | 1.28 (0.96–1.70) | .093 |
UTHealth, University of Texas Health Science Center at Houston; GBM, glioblastoma; WT, wildtype; KPS, Karnofsky performance status; HR, hazard ratios; CI, confidence interval; GTR, gross total resection; NTR, near-total resection; STR, subtotal resection; TMZ, temozolomide.
P ≤ .05 was considered statistically significant and is denoted in bold.
Univariable analysis of OS demonstrated that patients younger than 55 years of age (HR 0.59, P < .001), who underwent GTR (HR 0.59, P = .028) and NTR (HR 0.52, P = .015), who received chemoradiotherapy with TMZ (HR 0.32, P < .001), and salvage bevacizumab (HR 0.55, P < .001) had a significantly lower risk of death. Meanwhile, patients with a KPS less than 80 (HR 1.63, P < .001) had a significantly higher risk of death (Table 1).
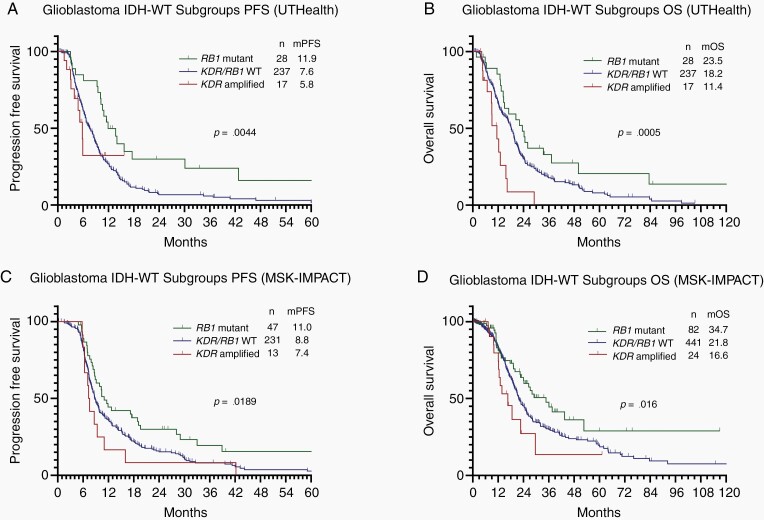
Furthermore, we evaluated the impact of genomic alterations present in at least 3% of the cohort on the OS of GBM IDH-WT patients. The univariable log-rank test showed that GBM IDH-WT patients harboring RB1 mutations (n = 28) had an improved OS compared to RB1-WT (n = 254) patients (23.5 vs 17.7 months, P = .026; Supplementary Figure S2C). Additionally, GBM IDH-WT patients harboring KDR amplification (n = 20) had worse survival compared to KDR-WT (n = 262) patients (11.4 vs 18.2 months, P = .0008; Supplementary Figure S2D). Notably, RB1-mutant and KDR-amplified GBM IDH-WT subgroups harbored a distinct PFS and OS, in which RB1-mutant patients had doubled the PFS (11.9 vs 5.8 months, P = .0691) and OS (23.5 vs 11.4 months, P = .0002) compared to KDR-amplified GBM IDH-WT (Figure 2A and B).
Figure 2.
Kaplan–Meier progression-free survival and overall survival of the different molecular subgroups of glioblastoma IDH-WT. (A and B) It demonstrates that RB1-mutant patients had the best PFS and OS, KDR/RB1-WT patients had an intermediate PFS and OS, and KDR-amplified patients had the worse PFS and OS in the UTHealth cohort (n = 282). (C and D) It demonstrates that RB1-mutant patients had the best PFS and OS, KDR/RB1-WT patients had an intermediate PFS and OS, and KDR-amplified patients had the worse PFS and OS in the MSK-IMPACT cohort (n = 291 – PFS* and n = 551 – OS). PFS, progression-free survival; OS, overall survival; GBM, glioblastoma; WT, wildtype; mOS, median overall survival; mPFS, median progression-free survival; UTHealth, University of Texas Health Science Center at Houston; MSK-IMPACT, Memorial Sloan Kettering. Patients who presented concomitant KDR amplification and RB1 alteration were categorized in the RB1 alteration group. *In the MSK-IMPACT, only 291 patients had PFS available information.
Multivariable analysis of OS demonstrated that patients younger than 55 years of age (HR 0.63, P = 0.010), who received chemoradiotherapy with TMZ (HR 0.24, P < .001), received salvage Bevacizumab after progression (HR 0.54, P < .001), and harbored an RB1 mutation (HR 0.49, P = .016) had a significantly lower risk of death. Conversely, patients who had a KPS less than 80 (HR 1.44, P = .024) and who harbored a KDR amplification (HR 2.51, P = .008) had a significantly higher risk of death (Table 1). Overall, our institutional cohort shows that GBM IDH-WT can be divided into 3 subgroups with different PFS and OS, by KDR and RB1 status (Figure 2).
RB1-Mutated GBM IDH-WT
Further analysis of GBM IDH-WT tumors by RB1 status (RB1 mutant n = 28 and RB1 WT n = 254) demonstrated no demographic or clinical differences between the groups. RB1-mutant patients were defined as those presenting alterations considered as pathogenic according to COSMIC database as previously described,16,17 of which most 27 of 28 (96%) cause loss of function of the gene; meanwhile, the other patient had a p.D697E mutation, which has been previously confirmed as a somatic mutation (COSM7765412). RB1-mutant GBMs less frequently harbored CDKN2A/B (RB1 mutant 14% vs RB1 WT 76%, P < .001, q < 0.001) and EGFR alterations (RB1 mutant 7% vs RB1 WT 51%, P < .001, q < 0.001). Meanwhile, they had increased frequency of SETD2 (RB1 mutant 18% vs RB1 WT 3%, P = .003, q = 0.006) and TP53 mutations (RB1 mutant 75% vs RB1 WT 26%, P < .001, q < 0.001; Supplementary Table S1).
GBM IDH-WT, KDR Amplified
Further analysis of GBM IDH-WT tumors by KDR status (KDR amplified n = 20 and KDR WT n = 262) demonstrated no demographic or clinical differences between the groups. However, KDR-amplified tumors were significantly associated with increased TP53 (KDR amplified 60% vs KDR WT 28%, P = .005, q = 0.04) mutations and amplifications in KIT (KDR amplified 95% vs KDR WT 2%, P < .001, q < 0.001) and PDGFRA (KDR amplified 100% vs KDR WT 8%, P < 0.001, q < .001; Supplementary Table S1).
Validation of Findings With the MSK-IMPACT Dataset
To validate our findings, we evaluated the MSK-IMPACT GBM IDH-WT dataset (n = 551). Eighty (14.5%) patients harbored RB1 mutations in this dataset. Consistent with our findings, RB1 mutation was associated with a significantly longer PFS (11.0 vs 8.7 months, P = .009) and OS (34.7 vs 21.7 months, P = .016; Supplementary Figure S3). Additionally, RB1 mutations concomitantly occurred with TP53 (q < 0.001) and were mutually exclusive with CDKN2A/B (q < 0.001) and EGFR amplification (q = 0.011) in the MSK-IMPACT GBM IDH-WT data (Figure 3). MSK-IMPACT data also demonstrated a mutual exclusivity of CDK4 amplification in RB1-altered patients (q = 0.004) and more frequent NTRK1 mutations (q = 0.0002). In the MSK-IMPACT cohort, 432 of 551 (78%) patients had information regarding the MGMT status. There were no differences in MGMT status between RB1-mutant (36.76% MGMT methylated and 63.24% MGMT unmethylated) and RB1-wildtype (30.49% MGMT methylated and 69.51% MGMT unmethylated) patients (P = .3212).
Figure 3.
Oncoplot representing the genomic landscape of GBM IDH-WT from the MSK-IMPACT study (n = 551).
We also evaluated the KDR amplification in the MSK-IMPACT GBM IDH-WT dataset (n = 551). Twenty-six (5%) patients harbored KDR amplification in this dataset. However, KDR was not significantly associated with PFS (7.4 vs 9.0 months, P = .176) or OS (16.6 vs 22.8 months, P = .118; Supplementary Figure S3). Additionally, KDR amplification concomitantly occurred with KIT (q < 0.001) and PDGFRA (q < 0.001) amplification in the MSK-IMPACT dataset. Although TP53 mutations appeared to co-occur more frequently in patients with KDR amplification (42% vs 28%), it was not significant after multiple comparison adjustments. Additionally, we did not observe differences in MGMT status between KDR-amplified and -WT patients (P = .2180).
Furthermore, we evaluated the relationship of KDR amplification and survival in GBM IDH-WT, utilizing the data of a published study that evaluated KDR amplification through fluorescence in situ hybridization (FISH).20 In this dataset, 19 of 142 (13%) GBM IDH-WT tumors had KDR amplification. Consistent with our study, KDR-amplified patients also had worse survival compared to KDR-WT patients (3.6 vs 9.2 months, P = .009; Supplementary Figure S4). Remarkably, as observed in the UTHealth cohort, the PFS (11.0 vs 7.4 months, P = .0237) and OS (34.7 vs 16.6 months, P = .0168) of RB1-mutant patients differ dramatically from KDR-amplified GBM IDH-WT (Figure 2C and D).
Discussion
In the present study, we sought to identify molecular subgroups of GBM IDH-WT with prognostic significance. We evaluated 282 patients who underwent comprehensive genomic characterization by an NGS assay, interrogating 205 genes and 26 rearrangements. We have identified that GBM IDH-WT patients with KDR amplification had worse survival compared to KDR-WT patients. Furthermore, RB1-mutant patients had improved survival compared to RB1-WT patients. Notably, KDR-amplified and RB1-mutant patients have distinct genetic alterations. These findings suggest that within the group of IDH-WT GBMs there are molecular subgroups with differences in prognosis.
Outcomes in GBM IDH-WT
Several studies have demonstrated that the survival of patients with GBM is affected by age, KPS, extent of resection, and chemoradiotherapy with TMZ.1,2,22,23 The relationship of age in GBM IDH-WT has been confirmed by studies with comprehensive genetic characterization.6,10 Our results further confirmed that younger age and chemoradiotherapy with TMZ improved OS in GBM IDH-WT. In line with prior studies, patients with low preoperative functional status (KPS <80) had worse survival.23 In addition, we observed that patients treated with salvage bevacizumab had an improved outcome. However, this finding deserves further study, as patients treated with salvage bevacizumab would inevitably have a lead-time bias to improve survival compared to patients who died without a documented recurrence and who were unable to benefit from this therapy. Despite the lack of survival benefit of bevacizumab in randomized clinical trials,24,25 these trials were performed prior to the molecular classification of gliomas. Additional studies are needed to evaluate salvage bevacizumab therapy in molecular subgroups of GBM IDH-WT. A recent report has shown that EGFR-amplified and classical GBM subgroups are associated with poor response to bevacizumab in recurrent GBM.26
GBM IDH-WT, RB1 Mutant
The RB1 gene was the first tumor-suppressor gene to be molecularly defined.27 Dysregulations of the RB pathway signaling are a critical event in gliomagenesis.28 In our study, we identified that RB1 loss-of-function mutations are present in 10% of our institutional cohort and 14.6% of MSK-IMPACT GBM IDH-WT. Interestingly, we observed that RB1-mutant GBM IDH-WT patients less frequently harbored EGFR alterations and CDKN2A/B loss. In addition, RB1-mutant cases had a higher frequency of mutations in TP53 in both the UTHealth and MSK-IMPACT cohorts. Our findings demonstrated that RB1-mutant tumors are a subgroup of GBM IDH-WT with a distinct prognosis. TP53 and RB1 mutation co-occurrence has been previously reported in several cancers.7,27 Also, RB1 exclusivity with EGFR amplification has been demonstrated in GBM xenograft models and glioma patients.7,28 Importantly, we identified for the first time that RB1-mutant GBM IDH-WT had improved PFS and OS than RB1-WT patients after multivariable adjustment (Figure 2 and Table 1). Remarkably, our findings were validated by the MSK-IMPACT cohort. Importantly, these results were not explained by differences in MGMT status in the MSK-IMPACT cohort, as RB1-mutant patients’ MGMT status did not differ from RB1-WT patients. Loss of the RB1 gene coupled with a loss in homologous recombination DNA repair pathway genes in other cancers, particularly high-grade ovarian carcinoma, has been associated with increased CD8+ tumor-infiltrating lymphocytes (TILs), PFS, and OS.29 In GBM, an increase in TILs has been associated with RB1 mutations.30 Additionally, it has been suggested that RB1 mutations provoke replication stress in tumor cells, leading to DNA damage and activation of the innate immune system. This has been hypothesized to enhance immune checkpoint blockade in ovarian carcinoma.31 The DNA damage and increased TILs in RB1-mutant patients are plausible explanations for the increased survival in this subtype of GBM IDH-WT (Figure 4). These findings should provoke further studies to identify if RB1-mutant GBM IDH-WT might respond more favorably to immune checkpoint inhibitors, a previously failed therapy in this dismal disease.32
GBM IDH-WT, KDR Amplified
KDR (VEGFR2) is a vascular endothelial growth factor (VEGF) receptor located on the chromosomal 4q11–12 locus, along with KIT and PDGFRA.33,34KDR activation by binding of VEGF ligands leads to activation of several downstream oncogenic signaling pathways such as PI3K/AKT, focal adhesion kinase, and mitogen-activated kinase, all resulting in increased cell survival, migration, and angiogenesis.35KDR alterations are frequently observed in various cancers, including GBM.33KDR is usually expressed within the tumor endothelium and is the primary VEGF signal transducer, which results in increased cell survival, proliferation, and angiogenesis20 (Figure 5). The critical role of VEGF in tumor angiogenesis has been demonstrated in multiple studies. This led to the development of bevacizumab, a monoclonal antibody against the VEGF-A ligand that binds to KDR.36KDR status and its relationship with outcomes have been investigated in GBM.20,37–39 However, these studies were performed in a heterogeneous group of patients (IDH-WT and IDH-mutant), prior to the TMZ era, or in relatively small cohorts (Supplementary Table S2). Moreover, recent studies have demonstrated that KDR activation through sustained VEGF-C promotes GBM maintenance and growth even under bevacizumab therapy, meaning that activation of KDR through VEGF-C is an escape mechanism of GBM to overcome bevacizumab therapy.40 The proliferative effects of KDR activation in GBM occur with the binding of both VEGF-A and VEGF-C ligands, though it should be noted that the effects of these 2 VEGF ligands have been demonstrated to be non-overlapping (Figure 5). In our study, we identified that KDR amplification, which would cause an activation of the KDR signaling pathway, correlated to worse outcomes. These findings were further validated with the results of a published GBM IDH-WT study in patients evaluated for KDR amplification using FISH.20 In contrast to the UTHealth cohort and the published study by Burford et al.,20 both demonstrating a statistically significant association with survival, the MSK-IMPACT cohort demonstrated a trend toward shorter survival (16.6 vs 22.8 months) in KDR-amplified cases which was not statistically significant.
In addition, autocrine VEGF-C/KDR signaling has been shown to regulate cell viability, cell cycle, and in vivo tumor growth in GBM, while autocrine VEGF-A/KDR signaling plays a similarly critical role in the proliferation and self-renewal of GBM stem-like cells.40KDR amplification, accompanied by the presence of more available receptors, would thus serve to exacerbate the effects of such signaling. Along with this, KDR amplification has been previously shown in non-small cell lung carcinoma to be associated with VEGF-induced elevated expression of KDR, p38, and mTOR pathway components, promoting a more invasive phenotype.41 Specifically, it was noted that there was phosphorylation of p38, elevated levels of HIF1α, and increased c-Met activation, correlating with increased angiogenesis and tumorigenesis. Considering these effects together, it would seem to reasonably explain the decreased survival observed in our cohort (Figure 5). Multi-institutional large GBM IDH-WT cohorts are required to further validate the deleterious effect of KDR amplification and help identify targeted therapies for this molecular subgroup of GBM IDH-WT. More importantly, survival differences between these molecular subgroups should be considered for clinical trial enrollment to avoid unintended bias.
Limitations
The limitations of our study include its retrospective design and potential selection bias, as not all GBM IDH-WT patients in our institution underwent NGS. MGMT promoter status for most of the UTHealth cohort was unavailable. However, we did not identify differences in MGMT status by RB1 or KDR mutational status in the MSK-IMPACT cohort. UTHealth and MSK-IMPACT datasets represent different geographic, socioeconomic, and ethnic populations. Moreover, there might be variations in practice patterns between institutions. Additionally, this study did not assess for KDR protein expression levels or TILs. However, we hope that the hypotheses generated by the study results motivate neuro-oncology researchers to identify the mechanisms underlying the survival differences between GBM IDH-WT subgroups. Despite these limitations, our study confirms the association of age, KPS, and chemoradiotherapy (TMZ) with survival in GBM IDH-WT patients. Moreover, we identified molecular subgroups of GBM IDH-WT with prognostic significance in 2 large independent cohorts.
Conclusions
The current study demonstrates that GBM IDH-WT, RB1-mutant represents a different molecular subgroup of GBM with improved PFS and OS. Additionally, we identified that KDR-amplified patients had worse survival, which might be related to increased angiogenesis and potential resistance to bevacizumab. Further studies are needed to determine the best treatment strategy for various GBM IDH-WT subgroups, allowing the incorporation of targeted therapies and personalized neuro-oncological care.
Supplementary Material
Acknowledgments
We thank Dr. Cameron Brennan for his insightful advice and guidance in the revision and preparation of this manuscript.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number K08CA241651 (to L.Y.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement. The authors declared no conflict of interest.
Authorship Statement. Study design: A.D., L.Y.B., and Y.E.; data recollection: A.D.; data analysis: A.D., A.V.R., and E.W.; manuscript writing: A.D., A.V.R., E.W., M.S., and Y.E.; manuscript revision and editing: A.D., N.T., L.Y.B., and Y.E.; study supervision: L.Y.B. and Y.E.; approved final manuscript: all authors.
References
- 1. Thakkar JP, Dolecek TA, Horbinski C, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. McLendon R, Friedman A, Bigner D, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan Y, Takayasu T, Hines G, et al. Landscape of genomic alterations in IDH wild-type glioblastoma identifies PI3K as a favorable prognostic factor. JCO Precis Oncol. 2020;4:575–584. [DOI] [PubMed] [Google Scholar]
- 8. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 9. Diplas BH, He X, Brosnan-Cashman JA, et al. The genomic landscape of TERT promoter wildtype-IDH wildtype glioblastoma. Nat Commun. 2018;9(1):2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galbraith K, Kumar A, Abdullah KG, et al. Molecular correlates of long survival in IDH-wildtype glioblastoma cohorts. J Neuropathol Exp Neurol. 2020;79(8):843–854. [DOI] [PubMed] [Google Scholar]
- 11. Jonsson P, Lin AL, Young RJ, et al. Genomic correlates of disease progression and treatment response in prospectively characterized gliomas. Clin Cancer Res. 2019;25(18):5537–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Esquenazi Y, Friedman E, Liu Z, Zhu JJ, Hsu S, Tandon N. The survival advantage of “Supratotal” resection of glioblastoma using selective cortical mapping and the subpial technique. Neurosurgery. 2017;81(2):275–288. [DOI] [PubMed] [Google Scholar]
- 15. Morris SL, Zhu P, Rao M, et al. Gamma knife stereotactic radiosurgery in combination with bevacizumab for recurrent glioblastoma. World Neurosurg. 2019;127:e523–e533. [DOI] [PubMed] [Google Scholar]
- 16. Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dono A, Wang E, Lopez-Rivera V, et al. Molecular characteristics and clinical features of multifocal glioblastoma. J Neurooncol. 2020;148(2):389–397. [DOI] [PubMed] [Google Scholar]
- 18. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burford A, Little SE, Jury A, et al. Distinct phenotypic differences associated with differential amplification of receptor tyrosine kinase genes at 4q12 in glioblastoma. PLoS One. 2013;8(8):e71777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu P, Du XL, Zhu JJ, Esquenazi Y. Improved survival of glioblastoma patients treated at academic and high-volume facilities: a hospital-based study from the National Cancer Database. J Neurosurg. 2019;132(2):491–502. [DOI] [PubMed] [Google Scholar]
- 23. Mirimanoff RO, Gorlia T, Mason W, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol. 2006;24(16):2563–2569. [DOI] [PubMed] [Google Scholar]
- 24. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 26. Hovinga KE, McCrea HJ, Brennan C, et al. EGFR amplification and classical subtype are associated with a poor response to bevacizumab in recurrent glioblastoma. J Neurooncol. 2019;142(2):337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dyson NJ. RB1: a prototype tumor suppressor and an enigma. Genes Dev. 2016;30(13):1492–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chow LM, Endersby R, Zhu X, et al. Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell. 2011;19(3):305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garsed DW, Alsop K, Fereday S, et al. Homologous recombination DNA repair pathway disruption and retinoblastoma protein loss are associated with exceptional survival in high-grade serous ovarian cancer. Clin Cancer Res. 2018;24(3):569–580. [DOI] [PubMed] [Google Scholar]
- 30. Rutledge WC, Kong J, Gao J, et al. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res. 2013;19(18):4951–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peng G, Mills GB. Surviving ovarian cancer: an affair between defective DNA repair and RB1. Clin Cancer Res. 2018;24(3):508–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the checkMate 143 Phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holtkamp N, Ziegenhagen N, Malzer E, Hartmann C, Giese A, von Deimling A. Characterization of the amplicon on chromosomal segment 4q12 in glioblastoma multiforme. Neuro Oncol. 2007;9(3):291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bleeker FE, Molenaar RJ, Leenstra S. Recent advances in the molecular understanding of glioblastoma. J Neurooncol. 2012;108(1):11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamberg P, Verweij J, Sleijfer S. (Pre-)clinical pharmacology and activity of pazopanib, a novel multikinase angiogenesis inhibitor. Oncologist. 2010;15(6):539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chi AS, Sorensen AG, Jain RK, Batchelor TT. Angiogenesis as a therapeutic target in malignant gliomas. Oncologist. 2009;14(6):621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Joensuu H, Puputti M, Sihto H, Tynninen O, Nupponen NN. Amplification of genes encoding KIT, PDGFRalpha and VEGFR2 receptor tyrosine kinases is frequent in glioblastoma multiforme. J Pathol. 2005;207(2):224–231. [DOI] [PubMed] [Google Scholar]
- 38. Nobusawa S, Stawski R, Kim YH, Nakazato Y, Ohgaki H. Amplification of the PDGFRA, KIT and KDR genes in glioblastoma: a population-based study. Neuropathology. 2011;31(6):583–588. [DOI] [PubMed] [Google Scholar]
- 39. Trevisan P, Graziadio C, Rodrigues DBK, et al. Clinical and molecular characterization of adult glioblastomas in Southern Brazil. J Neuropathol Exp Neurol. 2019;78(4):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michaelsen SR, Staberg M, Pedersen H, et al. VEGF-C sustains VEGFR2 activation under bevacizumab therapy and promotes glioblastoma maintenance. Neuro Oncol. 2018;20(11):1462–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nilsson MB, Giri U, Gudikote J, et al. KDR amplification is associated with VEGF-induced activation of the mTOR and invasion pathways but does not predict clinical benefit to the VEGFR TKI Vandetanib. Clin Cancer Res. 2016;22(8):1940–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.