Dear Editor,
SARS-CoV-2 virus, first described in Wuhan in 2019, has been a culprit of the global pandemic disease, namely COVID-19, associated with flu-like acute and severe respiratory failure. Also, it may cause a wide spectrum of skin rashes, including those similar to chilblain, livedo vasculitis, urticaria, vesicles and maculopapular rash including morbilliform rash, which represents a viral exanthema [[1], [2], [3]]. Recently, Japan Ministry of Health has granted approval to the Pfizer-BioNTech Covid-19 vaccine (BNT162b2) on February 17, 2021. This vaccine comprises of mRNA encoding the SARS-CoV-2 spike protein enveloped in lipid nanoparticles. Recent reports have described that not negligible number of participants in clinical trials with mRNA vaccines, either BNT162b2 or Moderna (mRNA-1273), developed delayed injection site reaction, so called ‘COVID-arm’ [4,5]. However, there has been a quite few reports on maculopapular rash or morbilliform exanthema following mRNA vaccination [6]. Herein, we report a case of morbilliform rash after administration of BNT162b2. Strikingly, the histological view was similar to that in skin involvements associated with SARS-CoV-2 infection.
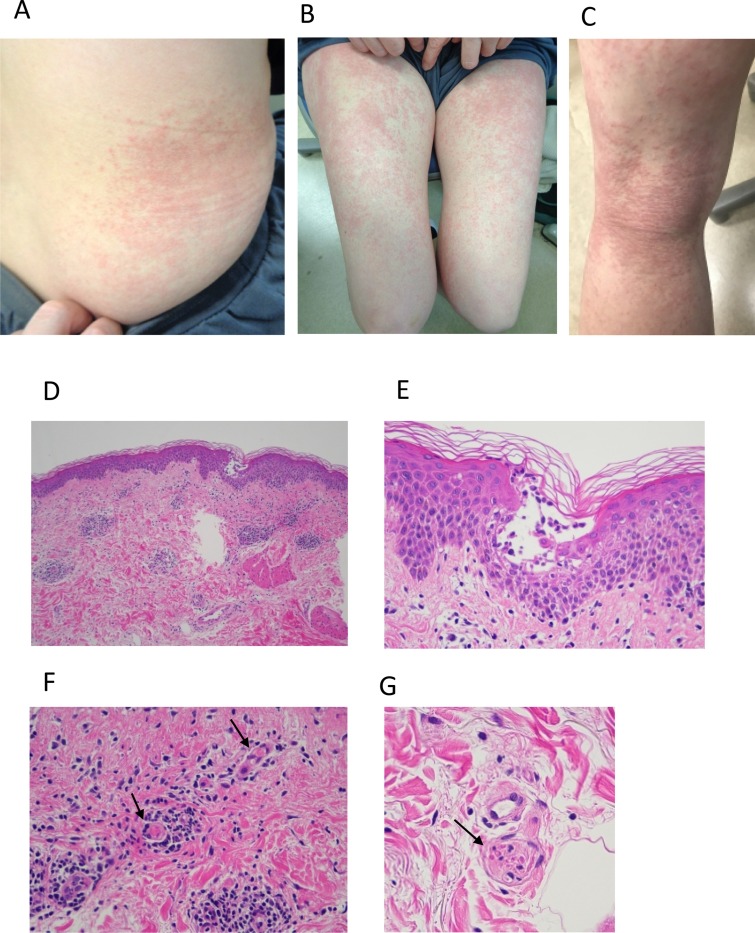
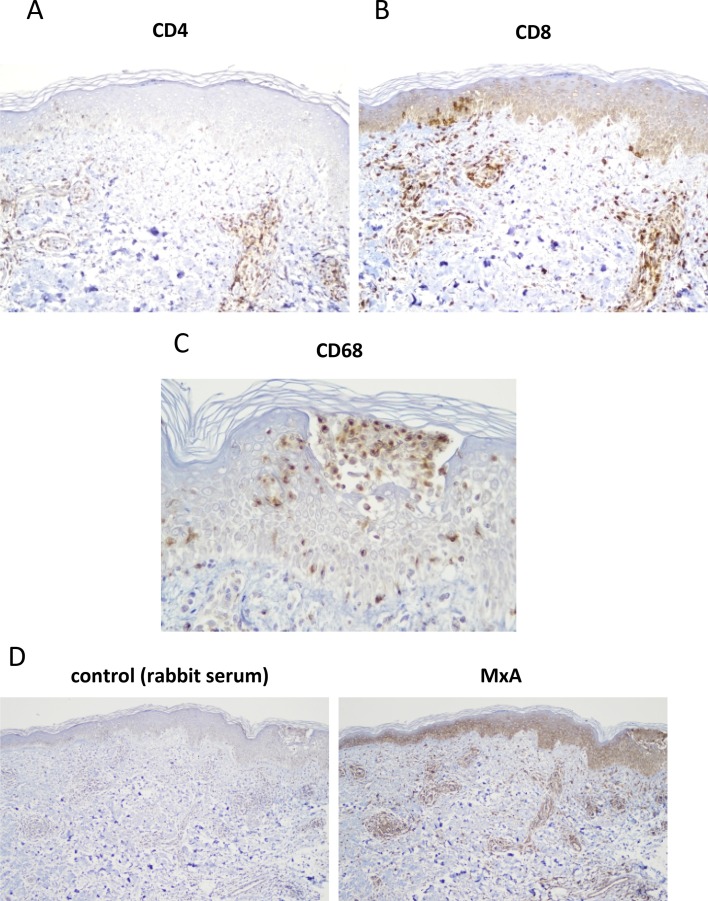
Case: a 55-year-old female with no past medical history received the initial shot of Covid-19 vaccine (BNT162b2) intramuscularly at her left deltoid region. Next day, she was suffered from pain at the injection site without skin lesion or systemic symptom. Two days after the injection, that was 55 h of post-injection, pruritic papules and erythematous lesions developed over the entire body except for the face. She was treated with topical betamethasone dipropionate and oral antihistamine, which were, however, ineffective. She presented herself to our department on the day 6 after the vaccine administration with mild pruritic vesicopapular, erythematous macular and morbilliform eruption on the bilateral flanks and extremities (Fig. 1 A–C). Histopathology of the lesion in the flank revealed perivascular lymphocytic infiltrates (Fig. 1D) basal cell vacuolization and intraepidermal vesicle with mild spongiotic change (Fig. 1E). In the vesicle, there were collections of Langerhans cells (Fig. 2 C) and degenerated acantholysis-like keratinocytes (Fig. 1E), both of which were found in COVID-19-associated skin lesions [2,7]. Strikingly, there were microthrombi in small vessels in the mid- (Fig. 1F, arrows) and deep dermis (Fig. 1G, arrow), as also shown in COVID-19-associated lesions [2,7]. Immunohistochemical study revealed perivascular and intraepidermal lymphocytic infiltrates with the CD8+ predominance over CD4+ cells (Fig. 2A and B), as previously reported in skin lesion in patients with COVID-19 infection [7]. Further, MxA, one of the type I interferon signatures, was highly expressed in the epidermis, cell infiltrates and vascular endothelium (Fig. 2D), as previously shown in COVID-19-associated chilblain-like lesions [8].
Fig. 1.
(A, B, C) Maculopapular and papulovesicular rash (morbilliform) on the trunk and legs. (D) Histopathology of the rash (Hematoxylin-eosin staining). Perivascular lymphocytic infiltrates and intraepidermal vesicle (original x100). (E) High magnification of the intraepidermis vesicle; mononuclear cell and neutrophils infiltrate, acantholysis-like, degradated keratinocyte and mild vecuolation of the basal cells are shown (original x400). Microthrombi in the vessels in the mid-dermis (arrows, F, original x400) and deep dermis (arrow, G, original x400).
Fig. 2.
Immunohistochemical staining of the biopsied lesion for CD4 (A, original x200), CD8 (B, original x200), CD68 (C, original x400), negative control and MxA (D, original x100). Color developed with diaminobenzidine with a counter staining with hematoxylin.
Laboratory testing revealed all normal in blood cell counts, liver, renal function, except for an increase of prothrombin(PT) time (118.4 %), which represents a reported feature of COVID-19-associated coagulation dysfuntion [9]. She was then treated with 15 mg of oral predonisolone, by which all the rash resolved in a week. She did not receive the second dose of the vaccine because of expected recurrence of rash or other unfavorable reaction.
To our knowledge, only one report described a case of morbilliform rash following administration of Pfizer COVID-19 vaccine [6]. Their case of morbilliform rash emerged after the initial and second dose, and the latent time after each vaccination was 48 h, which was similar to our case. Morbilliform rash is a predictable rash of SARS-CoV-2 infection [10], although skin biopsies have never found viral RNA or proteins [3], suggesting an immune activation-mediated skin response. Immunohistochemical features in our case strikingly mimicked those found in COVID-19-assocated skin lesions [2,7,8]. More importantly, our case was similar to SARS-CoV-2-associated conditions of not only skin rash such as microvascular thrombi, but also PT time elongation, the sign of coagulation abnormality [9]. Together, plausible underlying conditions of this case with Pfizer COVID-19 vaccination to develop skin rash are as follows; 1) Viral proteins (spike protein) might be expressed within 2 days of mRNA vaccination. 2) The recipient might have been infected and immunized with previous coronavirus (either conventional or Wuhan-derived coronavirus), whose spike protein might be a cross-react antigen with the one that the mRNA vaccine encoded. 3) The abrupt immune responses raised by the mRNA vaccine mimicked those by SARS-CoV-2 infection and might cause the similar symptoms not only in the skin but also systemically, particular, coagulation dysfunction. Therefore, if the COVID-19-related rash develops after the initial dose of vaccination, we suggest that the second dose is unnecessary, since anti-viral immunity would have been already established, or even should be avoided because of any risk for recurrence of cutaneous disease and emergence of systemic symptom such as coagulation tendency.
As a number of people over the world are receiving COVID-19 vaccines, dermatologists need to be aware of post-dose rashes to verify if they mimic COVID-19-associated cutaneous manifestations.
Funding
There was no funding source for this work.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Acknowledgements
We thank Ms. Reiko Kamijima for technical assistance and Dr. Soichi Nukuzuma for helpful discussion.
References
- 1.Sachdeva M., Gianotti R., Shah M., Bradanini L., Tosi D., Veraldi S., Ziv M., Leshem E., Dodiuk-Gad R.P. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J. Dermatol. Sci. 2020;98:75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gianotti R., Zerbi P., Dodiuk-Gad R.P. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J. Dermatol. Sci. 2020;98:141–143. doi: 10.1016/j.jdermsci.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fattori A., Cribier B., Chenard M.P., Mitcov M., Mayeur S., Weingertner N. Cutaneous manifestations in patients with coronavirus disease 2019: clinical and histological findings. Hum. Pathol. 2021;107:39–45. doi: 10.1016/j.humpath.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston M.S., Galan A., Watsky K.L., Little A.J. Delayed localized hypersensitivity reactions to the moderna COVID-19 vaccine: a case series. JAMA Dermatol. 2021 doi: 10.1001/jamadermatol.2021.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Nieto D., Hammerle J., Fernandez-Escribano M., Moreno-Del Real C.M., Garcia-Abellas P., Carretero-Barrio I., Solano-Solares E., de-la-Hoz-Caballer B., Jimenez-Cauhe J., Ortega-Quijano D., Fernandez-Guarino M. Skin manifestations of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers.’ COVID-arm’: a clinical and histological characterization. J. Eur. Acad. Dermatol. Venereol. 2021 doi: 10.1111/jdv.17250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jedlowski P.M., Jedlowski M.F. Morbilliform rash after administration of Pfizer-BioNTech COVID-19 mRNA vaccine. Dermatol. Online J. 2021;27:1–3. [PubMed] [Google Scholar]
- 7.Gianotti R., Recalcati S., Fantini F., Riva C., Milani M., Dainese E., Boggio F. Histopathological study of a broad spectrum of skin dermatoses in patients affected or highly suspected of infection by COVID-19 in the Northern Part of Italy: analysis of the many faces of the viral-induced skin diseases in previous and new reported cases. Am. J. Dermatopathol. 2020;42:564–570. doi: 10.1097/DAD.0000000000001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aschoff R., Zimmermann N., Beissert S., Günther C. Type I interferon signature in chilblain-like lesions associated with the COVID-19 pandemic. Dermatopathology. 2020;7:57–63. doi: 10.3390/dermatopathology7030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J., Yan H., Chen H., He C., Lin C., He H., Zhang S., Shi S., Lin K. COVID-19 and coagulation dysfunction in adults: a systematic review and meta-analysis. J. Med. Virol. 2021;93:934–944. doi: 10.1002/jmv.26346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni R.B., Lederman Y., Afiari A., Savage J.A., Jacob J., Rash Morbilliform. An uncommon herald of SARS-CoV-2. Cureus. 2020;12:e9321. doi: 10.7759/cureus.9321. [DOI] [PMC free article] [PubMed] [Google Scholar]