Abstract
Background
The distribution and nature of symptoms among SARS-CoV-2 infected individuals need to be clarified.
Methods
Between May and August 2020, 11 138 healthcare and administrative personnel from Central Denmark Region were tested for SARS-CoV-2 antibodies and subsequently completed a questionnaire. Symptom prevalence and overall duration for symptoms persisting for more than 30 days were calculated. Logistic regression models were used to estimate adjusted odds ratios (ORs) with 95% CIs.
Results
In total, 447 (4%) of the participants were SARS-CoV-2-seropositive. Loss of sense of smell and taste was reported by 50% of seropositives compared with 3% of seronegatives. Additionally, seropositives more frequently reported fever, dyspnoea, muscle or joint ache, fatigue, cough, headache and sore throat, and they were more likely to report symptoms persisting for more than 30 days. In adjusted models, they had a higher risk of reporting symptoms, with the strongest association observed for loss of sense of taste and smell (OR = 35.6; 95% CI: 28.6–44.3).
Conclusion
In this large study, SARS-CoV-2-seropositive participants reported COVID-19-associated symptoms more frequently than those who were seronegative, especially loss of sense of taste and smell. Overall, their symptoms were also more likely to persist for more than 30 days.
Keywords: SARS-CoV-2, COVID-19, Symptoms, Prevalence, Long-term symptoms, Serosurvey
Introduction
The COVID-19 pandemic caused by SARS-CoV-2 is unprecedented in scale. In spite of the vigorous research in COVID-19, many questions about its symptom prevalence and long-term consequences remain unanswered (Yelin et al., 2020). During recent months, much research has been devoted to characterizing symptoms associated with COVID-19. However, their distribution and nature (infection morbidity rate) remain to be clarified. Studies on hospitalised patients report symptoms such as fever, cough, headache, muscle ache, nausea or vomiting, diarrhoea and shortness of breath (Guan et al., 2020, Docherty et al., 2020). Some symptoms persist for weeks after discharge, especially fatigue, dyspnoea and anosmia (Carfì et al., 2020, Tenforde et al., 2020, Vaira et al., 2020, Arnold et al., 2020). Non-hospitalised patients report similar symptoms (Eyre et al., 2020), and it is suggested that these symptoms may last for weeks or months (Sudre et al., 2021, Nielsen et al., 2021).
The present study aimed to contribute to the growing knowledge of COVID-19 by exploring prevalence of COVID-19-associated symptoms obtained from self-reported questionnaires in a large cohort of 11 138 participants with mild and asymptomatic infections. We included healthcare and administrative employees from Central Denmark Region tested for antibodies against SARS-CoV-2 between May and August 2020.
Methods
The COVID-19 pandemic in Denmark
SARS-CoV-2 has affected most countries in the world over the past year leading to a COVID-19 pandemic. The first known case of COVID-19 in Denmark was diagnosed on 27 February 2020 (The Danish Health Authority, 2020), and the incidence peaked in March. The number of SARS-CoV-2-infected patients admitted to hospital peaked around 1 April 2020. This first wave was followed by low incidence for several months. A second wave affected Denmark from November 2020 through February 2021. During the second wave, the incidence of new infections peaked in December and admissions to hospital peaked at approximately 980 per day by the end of December (The Danish Health Authority, 2021a). Since the beginning of the pandemic, Denmark has used different strategies to control infection spread, such as partial-lock downs, distancing measures, and the use of protective equipment. As of 8 June 2021, 287 325 known COVID-19 cases have been registered in Denmark, in addition to 15 448 admissions and 2520 deaths with COVID-19 (The Danish Health Authority, 2021b).
Study population
All healthcare and administrative personnel at hospitals, prehospital services and specialist practitioners in Central Denmark Region were offered a test for SARS-CoV-2 antibodies. They were also invited to participate in this study by email and, if consent was given, to fill out a questionnaire.
Questionnaire data were collected from 15 May to 19 August 2020, and blood samples for SARS-CoV-2 antibody test were collected from 18 May to 19 June 2020.
Questionnaire data
The questionnaire addressed a wide range of items, including sex, age, current smoking status, body mass index (BMI), certain coexisting chronic diseases and a detailed list of COVID-19-related symptoms and their duration. Participants were asked the following questions: “Have you had any of the following symptoms since March 1? More than one symptom is allowed”. – Answer options: “fever”, “sore throat”, “cough”, “dyspnoea”, “headache”, “muscle or joint ache”, “fatigue”, “loss of sense of taste and smell”. “When did the symptoms begin?” – Answer option: a specific date. “Do you still have symptoms?”. – Answer options: “yes”, “no”. “When did the symptoms stop?” – Answer option: a specific date. Hence, the symptoms were reported as a period prevalence, and the duration of each separate symptom was not available from this questionnaire.
The symptoms presented in this study have previously been associated with COVID-19, and we here report some of the symptoms most often mentioned by the study population.
In sensitivity analyses, we used the following questions regarding chronic diseases: “Do you suffer from (or receive medication in the treatment of) the following chronic diseases? More than one condition is allowed”. – Answer options: “no”, “asthma”, “diabetes”, “chronic lung diseases in addition to asthma (e.g., COPD, emphysema and other respiratory tract conditions)”, “heart disease”, “kidney disease”, “weakened immune system due to medical treatment or chronic conditions such as complete or partial removal of the spleen, organ transplantation, HIV/AIDS, cancer, severe arthritis, etc.”, “high blood pressure (hypertension)”.
The questionnaire items used in this study are further described in the Supplementary material.
Blood sampling and testing
A previous serosurvey has described the demographic characteristics of the participants and given a detailed description of blood sampling, real-time polymerase chain reaction (RT-PCR) testing, serological testing and seroprevalence distribution in the region for this cohort (Jespersen et al., 2020).
After blood samples were collected, experienced staff tested undiluted EDTA plasma for immunoglobulin (Ig)G, IgM and IgA antibodies to the SARS-CoV-2 receptor-binding domain using a commercial SARS-CoV-2 total antibody enzyme-linked immunosorbent assay (ELISA, Wantai Biological Pharmacy Enterprise Co., Ltd., Beijing, China). The method was previously described in detail (Jespersen et al., 2020). The sensitivity of the assay is 96.7%, and its specificity is 99.5% (Harritshøj et al., 2021).
A total of 25 950 employees were invited to participate, and 17 971 reported for antibody testing. Among the 17 971 participants with a SARS-CoV-2 serological test, 11 138 agreed to participate in the survey and completed the questionnaire. Participants with missing responses to the questionnaire, missing blood samples, or poor blood sample quality were excluded. Analyses with adjustment for BMI and current smoking status were performed in 10 776 participants, as a total of 362 participants had missing responses to either BMI or smoking status. Thus, they were excluded from the analyses, see Supplementary material – Figure S1.
From 29 February to 1 August 2020, a sub-group of the study population was tested for SARS-CoV-2 RNA using RT-PCR in case of relevant COVID-19 symptoms or risk of exposure to SARS-CoV-2 transmission (n = 3690). Of these, a substantial group (n = 2954) had been tested for SARS-CoV-2 RNA before being tested for SARS-CoV-2 antibodies.
Statistics
The prevalence of each of the 8 symptoms was reported and compared by SARS-CoV-2 serological status (positive or negative). Furthermore, the overall duration of symptoms persisting for more than 30 days was calculated in two ways: (1) number of participants reporting one or more symptoms persisting after 30 days among participants reporting the specific symptom (example given for seropositives – loss of sense of taste and smell: out of 218 seropositive participants reporting this specific symptom, 111 (51%) reported any symptom persisting for more than 30 days); (2) percentage of participants who reported both the specific symptom and one or more symptoms persisting after 30 days (example given for seropositives – loss of sense of taste and smell: 50% × 51% = 26%).
Prevalences excluding all SARS-CoV-2 RNA-positive participants were used in sensitivity analysis to assess the effect of biased recall of symptoms among participants already aware of their SARS-CoV-2 status.
In the calculation of the overall duration of symptoms persisting for more than 30 days, participants were excluded if their symptoms started within 30 days completing of the questionnaire. Participants were excluded to avoid uncertainty about the duration of symptoms (246 participants were excluded from the main analyses and 243 participants from the sensitivity analyses, which also excluded all SARS-CoV-2 RNA-positive participants). Additionally, participants who reported a start date after the end date of symptoms were excluded (48 participants were excluded from the main analyses and 44 participants from the sensitivity analyses, which also excluded all SARS-CoV-2 RNA-positive participants). In total, 292 participants were excluded from the calculation of overall duration of symptoms persisting for more than 30 days in the main analyses, and the corresponding number of excluded participants in the analyses, excluding all SARS-CoV-2 RNA-positive participants, was 285 participants.
A multivariable logistic regression model was used to investigate the association between SARS-CoV-2 serological status and symptom reporting, with adjustment for potential confounders. The following covariates, obtained from the questionnaire, were considered to adjust for potential confounding: sex (categorical: woman or man), age (categorical: 18–39, 40–59, ≥60), BMI (categorical: non-obese defined as a BMI below 30 kg/m2, obesity defined as a BMI exceeding or equal to 30 kg/m2) and current smoking status (categorical: non-current smoker, current smoker).
Two confounder models were used: A basic model, adjusting for sex and age, and a fully adjusted model with further adjustment for BMI and current smoking status.
Furthermore, sensitivity analyses were performed for participants with or without self-reported chronic diseases based on the basic model. Participants reporting any of the above-mentioned chronic diseases were classified as having a chronic disease.
Interaction terms between SARS-CoV-2 serological status and sex were checked in all analyses.
Results are presented as numbers with percentages, odds ratios (ORs) with 95% CIs or medians with interquartile ranges (IQR). To compare groups, t-tests were used for normally distributed data, and Mann-Whitney U tests were used for non-normally distributed data. Chi-squared tests were used to compare categorical values. A P-value below 0.05 was considered statistically significant.
An a priori decision to stratify by sex was made; however, no statistically significant interaction between sex and outcome was observed, and unstratified results are therefore presented. Results stratified by sex are presented in the Supplementary material.
Analyses were performed using STATA/MP 16.1, RStudio version 1.3, and R software 4.0.3.
Results
The characteristics of the cohort by SARS-CoV-2 serological status are presented in Table 1 .
Table 1.
Characteristics of the cohort by SARS-CoV-2 serological status (n = 11 138).
| Seropositive | Seronegative | P-value | |
|---|---|---|---|
| Participants | 447 (4%) | 10 691 (96%) | |
| Women | 406 (91%) | 9389 (88%) | 0.056 |
| Men | 41 (9%) | 1302 (12%) | |
| Age, years | 44 (35; 54) | 46 (37; 56) | 0.001 |
| 18–39 | 172 (38%) | 3552 (33%) | 0.022 |
| 40–59 | 231 (52%) | 5790 (54%) | |
| ≥60 | 44 (10%) | 1379 (13%) | |
| Smoking status | |||
| Non-current smoker | 410 (92%) | 9525 (89%) | 0.117 |
| Current smoker | 30 (7%) | 941 (9%) | |
| Missing | 7 (1%) | 225 (2%) | |
| BMI, kg/m2 | 24 (22; 28) | 24 (22; 28) | 0.9478 |
| BMI < 30 | 355 (79%) | 8926 (83%) | 0.026 |
| BMI ≥ 30 (obese) | 80 (18%) | 1518 (14%) | |
| Missing | 12 (3%) | 247 (2%) | |
| Chronic disease | |||
| No | 386 (86%) | 8801 (82%) | 0.028 |
| Yes | 61 (14%) | 1890 (18%) | |
| RT-PCR | |||
| SARS-CoV-2 RNA - positive | 235 (53%) | <5 | <0.001 |
| SARS-CoV-2 RNA - negative | 81 (18%) | 3370 (32%) |
Data are presented as numbers with percentages.
Age and body mass index (BMI) are further presented as medians with interquartile ranges (IQR).
To compare groups, t-tests were used for normally distributed data and Mann–Whitney U tests were used for non-normally distributed data. Chi-squared tests were used to compare categorical values.
Women comprised 88% of the participants. SARS-CoV-2-seropositive participants were slightly younger than seronegatives. Furthermore, a higher proportion of seropositive participants were obese. In contrast, a higher proportion of seronegatives reported having a chronic disease. Among seropositive participants, 53% had previously tested positive for SARS-CoV-2 RNA by RT-PCR.
Characteristics of the cohort by sex are presented in Supplementary material, Table S1.
Most participants completed the questionnaire before or at the same date as the antibody test was performed (68%). The median number of days between completing the questionnaire and antibody testing was 0 days (IQR −3 to 3 days, 1st percentile: −16 days; 99th percentile: 16 days). Only 0.5% of the participants responded to the questionnaire after receiving the result from the serological test.
Symptom prevalence by SARS-CoV-2 serological status
The prevalence of each of the 8 symptoms by serological status is presented in Table 2a . SARS-CoV-2-seropositive participants were more likely than seronegatives to report all the symptoms (a P-value below 0.001 was observed for each of the symptoms). Notably, 50% of seropositives reported loss of sense of taste and smell compared with only 3% of seronegatives. Similarly, 53% of seropositives reported fever compared with 11% of seronegatives. Overall, 86% of seropositives reported any symptom compared with 55% of seronegatives. Seropositives who reported any of the specific symptoms were also more likely than seronegatives to have a duration of any symptom for more than 30 days (39% vs 22%). A total of 14% of seropositive participants reported no symptoms.
Table 2a.
Symptom prevalence by SARS-CoV-2 serological status (n = 11 138).
| Seropositive (n = 447) |
Seronegative (n = 10 691) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Reported symptoms |
One or more symptoms persisting ≥ 30 daysa |
Reported symptoms overall persisting ≥ 30 daysb | Reported symptoms |
One or more symptoms persisting ≥ 30 daysa |
Reported symptoms overall persisting ≥ 30 daysb | ||||
| Number | % | Numberc | % | % | Number | % | Numberc | % | % | |
| Loss of sense of taste and smell | 225 | 50 | 111 (218) | 51 | 26 | 293 | 3 | 93 (286) | 33 | 1 |
| Fever | 239 | 53 | 101 (234) | 43 | 23 | 1179 | 11 | 215 (1152) | 19 | 2 |
| Dyspnoea | 133 | 30 | 74 (130) | 57 | 17 | 605 | 6 | 212 (586) | 36 | 2 |
| Muscle or joint ache | 243 | 54 | 113 (237) | 48 | 26 | 1717 | 16 | 450 (1640) | 27 | 4 |
| Fatigue | 287 | 64 | 124 (280) | 44 | 28 | 2598 | 24 | 660 (2486) | 27 | 6 |
| Cough | 228 | 51 | 107 (223) | 48 | 24 | 2351 | 22 | 695 (2267) | 31 | 7 |
| Headache | 300 | 67 | 115 (291) | 40 | 27 | 4060 | 38 | 901 (3907) | 23 | 9 |
| Sore throat | 212 | 47 | 92 (209) | 44 | 20 | 3153 | 29 | 673 (3045) | 22 | 6 |
| Seropositive (n = 447) |
Seronegative (n = 10 691) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Reported any symptom |
Reported any symptom persisting ≥ 30 days |
Reported any symptom |
Reported any symptom persisting ≥ 30 days |
|||||
| Number | % | Numberc | % | Number | % | Numberc | % | |
| 385 | 86 | 151 (376) | 40 | 5924 | 55 | 1331 (5,681) | 23 | |
Data presented as numbers with percentages.
Number of participants reporting one or more symptoms persisting for more than 30 days among participants reporting the specific symptom.
Percentage of participants who both report the specific symptom and report one or more symptoms persisting after 30 days.
Numbers in parentheses represent participants reporting the symptom (corresponding to the number of “Reported symptoms”) but excluding participants whose symptoms started within 30 days completing of the questionnaire (n = 246) in addition to participants with discrepancy between symptom start date and symptom end date (n = 48). In total, 292 participants were excluded.
As described above, 3% (n = 293) reported loss of sense of taste and smell among the seronegatives. Half were tested by RT-PCR and fewer than 5 seronegative participants tested positive for SARS-CoV-2 RNA by RT-PCR. Thus, false-negative serological test results could not be ruled out.
In a sensitivity analysis excluding 239 participants who previously tested positive for SARS-CoV-2 RNA by RT-PCR, we found similar, although attenuated, effects of seropositivity (Table 2b ).
Table 2b.
Symptom prevalence by SARS-CoV-2 serological status after excluding SARS-CoV-2 RNA-positive participants (n = 10 899).
| Seropositive (n = 212) |
Seronegative (n = 10 687) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Reported symptoms |
One or more symptoms persisting ≥ 30 daysa |
Reported symptoms overall persisting ≥ 30 daysb | Reported symptoms |
One or more symptoms persisting ≥ 30 daysa |
Reported symptoms overall persisting ≥ 30 daysb | ||||
| Number | % | Numberc | % | % | Number | % | Numberc | % | % | |
| Loss of sense of taste and smell | 59 | 28 | 18 (58) | 31 | 9 | 292 | 3 | 93 (285) | 33 | 1 |
| Fever | 79 | 37 | 20 (77) | 26 | 10 | 1178 | 11 | 215 (1151) | 19 | 2 |
| Dyspnoea | 32 | 15 | 15 (31) | 48 | 7 | 605 | 6 | 212 (586) | 36 | 2 |
| Muscle or joint ache | 78 | 37 | 22 (75) | 29 | 11 | 1716 | 16 | 450 (1639) | 27 | 4 |
| Fatigue | 95 | 45 | 24 (92) | 26 | 12 | 2596 | 24 | 660 (2484) | 27 | 6 |
| Cough | 73 | 34 | 20 (72) | 28 | 10 | 2349 | 22 | 695 (2265) | 31 | 7 |
| Headache | 112 | 53 | 26 (109) | 24 | 13 | 4056 | 38 | 901 (3904) | 23 | 9 |
| Sore throat | 76 | 36 | 19 | 25 | 9 | 3152 | 29 | 673 (2044) | 22 | 6 |
| Seropositive (n = 212) |
Seronegative (n = 10 687) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Reported any symptom |
Reported any symptom persisting ≥ 30 days |
Reported any symptom |
Reported any symptom persisting ≥ 30 days |
|||||
| Number | % | Numberc | % | Number | % | Numberc | % | |
| 159 | 75 | 40 (156) | 26 | 5920 | 55 | 1331 (5678) | 23 | |
Data presented as numbers with percentages.
Number of participants reporting one or more symptoms persisting for more than 30 days among participants reporting the specific symptom.
Percentage of participants who both report the specific symptom and report one or more symptoms persisting after 30 days.
Numbers in parentheses represent participants reporting the symptom (corresponding to the number of “Reported symptoms”) but excluding participants whose symptoms started within 30 days completing of the questionnaire (n = 243) in addition to participants with discrepancy between symptom start date and symptom end date (n = 44). In total, 285 participants were excluded.
Multivariable logistic regression exploring the association between SARS-CoV-2 serological status and reporting symptoms
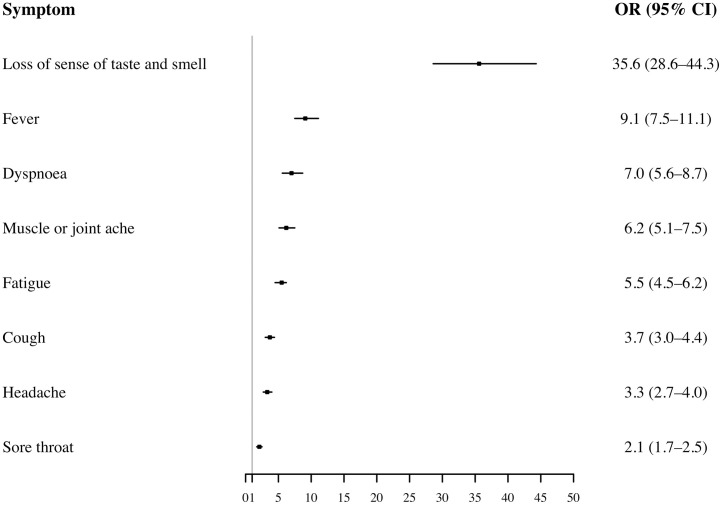
In models adjusted for sex and age, loss of sense of taste and smell was strongly associated with SARS-CoV-2 serological status (OR = 35.6; 95% CI: 28.6–44.3), followed by fever, dyspnoea, muscle or joint ache, fatigue, cough, headache and sore throat (Figure 1 ). Further adjustment for BMI and current smoking status did not alter these results (confounder model 2). Results from confounder model 2 and results from confounder model 1 and 2 stratified by sex are presented in Supplementary material – Tables S2–S4.
Figure 1.
The association between SARS-CoV-2 serological status and reporting symptoms (n = 11 138).
Multivariable logistic regression exploring the association between SARS-CoV-2 serological status and reporting symptoms adjusted for sex and age (categorical: 18–39, 40–59, ≥60) (Confounder model 1).
OR: Odds ratios.
95% CI: 95% confidence intervals.
Boxes with error bars: ORs with 95% CIs.
Results from the sensitivity analyses with participants with or without a chronic disease are presented in Supplementary material – Table S5. The results were almost identical to the results from the main analyses, with slightly broader 95% CIs for participants with chronic disease.
Discussion
To our knowledge, this study is one of the first large studies reporting COVID-19-associated symptoms among SARS-CoV-2-seropositive and seronegative participants. We included 11 138 healthcare and administrative personnel from hospitals, prehospital services and specialist practitioners from Central Denmark Region who were screened for SARS-CoV-2 antibodies and subsequently completed a questionnaire. The study was conducted between May and August 2020 after the first wave and before the second wave of the COVID-19 pandemic in Denmark.
We found that 50% of seropositive participants reported loss of sense of smell and taste, with only very few seronegative participants reporting similar symptoms. More than 50% of seropositive participants reported fever.
The sensitivity of the assay used in this study was 96.7% and is the assay with the highest sensitivity among 16 validated SARS-CoV-2 immunoassays in a Danish national validation study (Harritshøj et al., 2021). In a previous report, we were further able to verify this high sensitivity: 98% of employees who previously tested positive for viral RNA by RT-PCR had a positive test for SARS-CoV-2 antibodies using this test assay (Jespersen et al., 2020). The present study is thus unique as it also includes participants with mild and asymptomatic infections enabling us to estimate the absolute percentage of individuals with specific symptoms. Among the seropositives, 14% reported none of the explored symptoms, while this was the case for 45% of the seronegative participants. The actual percentage of asymptomatic SARS-CoV-2 infection cases may be even larger, and asymptomatic carriers may contribute to infection spread.
In models adjusting for sex and age, seropositives had a distinctly higher risk of reporting symptoms with ORs above 5 for loss of sense of taste and smell, fever, dyspnoea, muscle or joint ache and fatigue. These findings changed inconspicuously after further adjustment for BMI and current smoking status. Even in sensitivity analyses, stratified for self-reported chronic disease, the findings were consistent, indicating that our findings were not affected by these potential confounders. No marked differences between women and men were observed.
The most prominent symptom associated with seropositive status in our study was loss of sense of taste and smell, followed by fever. These findings complement those of a recently published study among 2149 Swedish healthcare workers. However, no measure of symptom duration was available from that study (Rudberg et al., 2020). Other studies similarly showed that alterations in smell or taste were frequently reported by mildly symptomatic patients with SARS-CoV-2 infection and were often the first apparent symptom (Spinato et al., 2020, Menni et al., 2020). We were, however, able to confirm that these effects persisted even after exclusion of SARS-CoV-2 RNA-positive participants. This sensitivity analysis was performed to determine any potential recall bias: participants already aware of having had SARS-CoV-2 infection may report COVID-19-associated symptoms differently from participants who are not aware of having had SARS-CoV-2 infection simply because of the increased focus on COVID-19 symptoms during recent months. Along with this, only 0.5% of the participants responded to the questionnaire after the serological test result was sent to them.
With respect to persisting symptoms, we found that seropositives were more likely than seronegatives to report symptoms lasting more than 30 days. In comparison, a study found that among COVID-19-recovered patients, 87.4% reported persistence of at least one symptom, particularly fatigue and dyspnoea (Carfì et al., 2020). Another study showed that 43.4% of COVID-19 cases had symptoms lasting longer than 30 days, and 24.1% still had at least one symptom after 90 days (Cirulli et al., 2020).
Strengths and limitations
A major strength of this study is our large study population consisting of 11 138 healthcare and administrative personnel screened for SARS-CoV-2 antibodies. It is a limitation that only employees with a current labour market affiliation were included. Should employees have been incapacitated due to severe COVID-19 they were not part of this survey.
The questionnaire, as previously explained, was completed by participants before the result of the antibody test was available among the vast majority of participants. It is a strength of the study that we could perform sensitivity analyses excluding participants with a positive RT-PCR for viral RNA. This analysis seeks to minimize recall bias by assessing effects among participants with no prior positive test.
Most of the explored symptoms are not specific to COVID-19 and could be explained by other conditions and common viruses. Findings among both SARS-CoV-2-seropositive and seronegative participants may therefore be affected by other common infections during the study period. For example, it is well-known, that also other virus infections can cause the same symptoms, especially parainfluenza virus can cause alterations in sense of smell (Wang et al., 2007).
It is possible that some participants developed infection and antibodies after completing the questionnaire, thereby underestimating symptom prevalence. However, 68% of participants completed the questionnaire before, or at the same date as, the antibody test was performed, and 99% of participants were tested within 16 days of responding. When allowing for at least one week from infection to antibody development and that the survey was performed during low incidence of new SARS-CoV-2 infections, the risk of this bias seems low (Sethuraman et al., 2020).
We had information on age, BMI, current smoking status and certain self-reported chronic diseases, which allowed us to consider these potential confounders. However, the interference of other comorbidities and calculations of comorbidities on a weighted scale, for example, by using the Charlson Comorbidity Index as an instrument, could not be ruled out in this study. Moreover, symptoms were self-reported and thus prone to recall bias.
A major limitation is the lack of data on duration for each specific symptom. Still, we observed that seropositives were more likely than seronegatives to report symptoms persisting for more than 30 days.
In the health care system in Central Denmark Region, 80% of the employees are women. In this study, women comprised 88% of the participants. Thus, only a subgroup of the men we could expect to include in this study actually participated.
In analyses stratified by sex, we saw no overt differences between women and men; but with such a low percentage of men in the study, the statistical power to detect differences between sexes is low.
A substantial share (38%) of the study population tested for SARS-CoV-2 antibodies did not complete the questionnaire, contributing to potential selection bias. Symptoms among participants in the serosurvey may have affected their willingness to participate. Several possible biases may exist because symptomatic infections may have affected the chance of participation. However, in the serosurvey with descriptive data on healthcare and administrative personnel from hospitals, prehospital services, and specialist practitioners from Central Denmark Region, including our cohort, the seroprevalence was 3.7% among the 17 917 participants (Jespersen et al., 2020). Compared with the seroprevalence of 4.0% (95% CI: 3.5%–4.4%) among 11 138 participants included in this study, the estimated seroprevalence is 3.3% (95% CI: 2.9%–3.7%) for the 38% of serosurvey subjects not participating in our study.
Conclusion
In this large study of healthcare and administrative employees, loss of sense of smell and taste was reported among 50% of SARS-CoV-2-seropositive participants compared with 3% among seronegative participants. In addition, fever, dyspnoea, muscle or joint ache, fatigue, cough, headache and sore throat were more frequently reported by seropositive participants. The duration of any symptom was more likely to persist for more than 30 days for seropositive participants. This study is unique as it also includes participants with mild and asymptomatic infections, thereby enabling us to estimate the absolute percentage of individuals with specific symptoms. Importantly, we were able to assess symptom prevalence in seropositive participants who had not tested positive for SARS-CoV-2 viral RNA measured by RT-PCR. Thus, the risk of symptom reporting being affected by ongoing media reporting of long-term COVID-19 symptoms was minimized.
Contributions
K. A. K, K. N., S. J., S. M., T. G., M. T., M. K. T., H. J. M., L. Ø., H. A. K. and C. E. planned the study. J.M.V. and T.G. organized data, K.A.K. and C. E. analysed and interpreted the data, and K. A. K. and C. E. drafted the manuscript. All authors were responsible for the questionnaire. S. M., C. E., T. G., M. K. T. and H. J. M. were responsible for the laboratory analyses. All authors were involved in critically revising the manuscript and approved the final version.
Ethics
SARS-CoV-2 antibody screening was performed at the request of the Danish Administrative Regions. Only consenting staff were tested and informed about their result. Furthermore, staff were invited to participate in this research survey. Only consenting staff were included.
This study was approved by the Danish Data Protection Agency (1-16-02-207-20) and by the Central Denmark Region. The Scientific Ethics Committee of the Central Denmark Region concluded that the study required no scientific ethical approval (request no. 127 on ref. no. 1-10-72-1-20). Merging data with information on RT-PCR tests (performed for clinical or screening purposes) was approved by the legal office in the Central Denmark Region (1-45-70-23-20).
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank all staff involved in performing this study. Furthermore, the authors thank all healthcare and administrative personnel at hospitals, prehospital services and specialist practitioners in the Central Denmark Region participating in this study.
This work was supported by The Central Denmark Region. The Wantai tests were donated by The Danish Health Authority requisitioned through Statens Serum Institut.
KAK was supported by BERTHA – the Danish Big Data Centre for Environment and Health funded by the Novo Nordisk Foundation Challenge Programme [grant number NNF17OC0027864].
The funders had no role in performing this study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.06.017.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Arnold D.T., Hamilton F.W., Milne A., Morley A.J., Viner J., Attwood M., et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2020;76:399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfì A., Bernabei R., Landi F., Gemelli Against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli E.T., Barrett K.M.S., Riffle S., Bolze A., Neveaux I., Dabe S., et al. Long-term COVID-19 symptoms in a large unselected population. medRxiv. 2020 Cold Spring Harbor Laboratory Press. 2020.10.07.20208702. [Google Scholar]
- Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D.W., Lumley S.F., O’Donnell D., Campbell M., Sims E., Lawson E., et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. eLife. 2020;9 doi: 10.7554/eLife.60675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harritshøj L.H., Gybel-Brask M., Afzal S., Kamstrup P.R., Jørgensen C.S., Thomsen M.K., et al. Comparison of sixteen serological SARS-CoV-2 immunoassays in sixteen clinical laboratories. J Clin Microbiol. 2021;59(5) doi: 10.1128/JCM.02596-20. e02596-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen S., Mikkelsen S., Greve T., Kaspersen K.A., Tolstrup M., Boldsen J.K., et al. SARS-CoV-2 seroprevalence survey among 17,971 healthcare and administrative personnel at hospitals, pre-hospital services, and specialist practitioners in the Central Denmark Region. Clin Infect Dis. 2020:1–8. doi: 10.1093/cid/ciaa1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A., et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26(7):1037–1040. doi: 10.1038/s41591-020-0916-2. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen K.J., Vestergaard J.M., Schlünssen V., Bonde J.P., Kaspersen K.A., Biering K., et al. Day-by-day symptoms following positive and negative PCR tests for SARS-CoV-2 in non-hospitalized healthcare workers: a 90-day follow-up study. Int J Infect Dis. 2021;108:382–390. doi: 10.1016/j.ijid.2021.05.032. https://www.ijidonline.com/article/S1201-9712(21)00434-3/abstract Elsevier. [Cited 8 June 2021]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudberg A.-S., Havervall S., Månberg A., Falk A.J., Aguilera K., Ng H., et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11(1):5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323(22):2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- Spinato G., Fabbris C., Polesel J., Cazzador D., Borsetto D., Hopkins C., et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323(20):2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer E.C., et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenforde M.W., Kim S.S., Lindsell C.J., Rose E.B., Shapiro N.I., Files D.C., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network — United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Danish Health Authority . Sundhedsstyrelsen; 2020. COVID-19 i Danmark: epidemiens første bølge: status og strategi. Version 23. marts 2020. [Google Scholar]
- The Danish Health Authority . 2021. Coronatal.https://www.sst.dk/da/corona/status-for-epidemien/coronatal [Cited 23 April 2021]. Available from: [Google Scholar]
- The Danish Health Authority . 2021. COVID-19 dashboard.https://experience.arcgis.com/experience/aa41b29149f24e20a4007a0c4e13db1d [Cited 23 April 2021]. Available from: [Google Scholar]
- Vaira L.A., Hopkins C., Petrocelli M., Lechien J.R., Chiesa-Estomba C.M., Salzano G., et al. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J Laryngol Otol. 2020;134(8):703–709. doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.H., Kwon H.J., Jang Y.J. Detection of parainfluenza virus 3 in turbinate epithelial cells of postviral olfactory dysfunction patients. Laryngoscope. 2007;117(8):1445–1449. doi: 10.1097/MLG.0b013e318063e878. [DOI] [PubMed] [Google Scholar]
- Yelin D., Wirtheim E., Vetter P., Kalil A.C., Bruchfeld J., Runold M., et al. Long-term consequences of COVID-19: research needs. Lancet Infect Dis. 2020;20(10):1115–1117. doi: 10.1016/S1473-3099(20)30701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.