Abstract
Background:
Mild physiologic mitral regurgitation (MR) is common in normal individuals. Patients with primary MR due to mitral valve prolapse (MVP) may also exhibit less than moderate MR. We sought to determine whether MVP patients with less than moderate MR displayed early cardiac chamber remodeling or factors related to early remodeling and whether early remodeling predicted MR progression.
Methods:
Consecutive MVP patients with less than moderate MR by proximal isovelocity surface area–derived effective regurgitant orifice < 20 mm2 and regurgitant volume < 30 mL, were matched for age and sex with non-MVP patients (controls) having less than moderate MR. Patients with moderate or greater dysfunctional left- or right-sided valves and left ventricular ejection fraction < 50% were excluded. We evaluated left ventricle (LV) and left atrium (LA) remodeling parameters (LV end-diastolic and end-systolic indexed diameters, LA volume-index, and LV mass-index) as well as determinants of remodeling. The last available transthoracic echocardiography was reviewed to identify progression to moderate-severe MR or more.
Results:
A total of 253 MVP patients with less than moderate MR were matched to 344 controls (P for age and sex, ≥.18) with less than moderate MR. Patients with MVP (mean effective regurgitant orifice and regurgitant volume, 12 ± 4 mm2 and 18 ± 6 mL, respectively) had more premature ventricular contractions (PVCs), larger LV and LA remodeling parameters, and more mild-to-moderate MR (all P < .0001). Multivariate linear regression models showed that larger LV remodeling parameters were independently associated with MVP and female sex but not MR severity (all P < .0001). The LA volume index was independently associated with MVP, age, and E/e’ (all P < .0001). The LV mass index was associated with MVP, age, and hypertension (all P ≤ .002). Presence of PVCs was associated with LV end-systolic diameter ≥ 40 mm and indexed ≥ 22 mm2 (P = .005). Among 323 (54%) patients having subsequent transthoracic echocardiography, 17 patients (all MVP) progressed to moderate-severe MR or more at a median of 4.3 (interquartile range, 1.7–6.4) years. Isolated posterior leaflet prolapse was the single factor associated with MR progression (adjusted hazard ratio, 2.70; 95% CI, 0.99–7.34; P = .048) after adjustment for MR severity. At a median of 5.9 (interquartile range, 4.6–7.2) years of follow-up, female sex and MVP (vs controls) were protective factors for mortality.
Conclusions:
Patients with less than moderate MR due to MVP exhibit early LV and LA remodeling, which does not predict MR progression or mortality. Left ventricle remodeling is associated with MVP, female sex, and presence of PVCs. Early chamber remodeling associated with MVP may be the phenotypical expression of a genetically mediated process and is at least partially related to PVCs.
Keywords: Left ventricular remodeling, Left atrial remodeling, Mitral regurgitation, Mitral valve prolapse
Normal individuals may have a trivial or mild degree of “physiologic” mitral regurgitation (MR). As for pathologic MR caused by primary mitral valve disease (i.e., degenerative MR), the most common etiology is mitral valve prolapse (MVP),1 a genetically mediated condition2 with various phenotypic expressions that has a prevalence of 2.4% in the general population.3 The natural history of MVP is diverse, ranging from slow progression of mild MR degrees, to rapid progression to severe MR requiring cardiac surgery,4 to infective endocarditis,5 to “malignant MVP,” which is associated with sudden cardiac death.6 Indeed, in patients with MVP, premature ventricular contractions (PVCs) are a common finding7 and a high burden of PVCs can be associated with dilated cardiomyopathy.8
Severe MR caused by MVP is associated with eccentric left ventricular (LV) and left atrial (LA) chamber remodeling with LV dilatation, eccentric LV hypertrophy (eLVH), and LA dilatation, which may become surgical triggers in asymptomatic patients by current guidelines.1,9 Interestingly, we have observed LV and LA dilatation in some MVP patients with mild degrees of MR; however, whether there actually exists MVP- associated early chamber remodeling independent of MR severity or PVC-mediated early chamber remodeling in MVP patients with mild MR degrees (i.e., less than moderate MR) is unknown.
Therefore, in MVP patients with less than moderate MR, we sought to assess (1) the presence and severity of LV and LA remodeling, (2) the determinant factors of LV and LA remodeling, and (3) whether early remodeling is a marker of MR progression.
METHODS
Study Population
From January 1, 2007, to April 30, 2015, we retrospectively identified consecutive MVP patients who had MR quantification by proximal isovelocity surface area (PISA); with less than moderate MR defined as PISA-derived effective regurgitant orifice area (EROA) <20 mm2 and regurgitant volume (Rvol) < 30 cm3/beat, as well as by the comprehensive integrated transthoracic echocardiography (TTE) approach.9 We then attempted to match them 1:2 for age and sex with non-MVP patients having less than moderate MR (non-MVP control group) by the comprehensive integrated TTE approach.9 Although both MVP patients and controls had less than moderate MR, we further subclassified MR severity into trivial-mild and mild-to-moderate as per the comprehensive integrated TTE approach, in order to comprehensively assess the significance of MR severity in relation to early chamber remodeling.
Patients with the following conditions were not included: dysfunction of other valves of moderate or greater degree, left ventricular ejection fraction (LVEF) < 50%, renal dysfunction (creatinine ≥ 1.3 mg/dL), hypertrophy cardiomyopathy, constrictive pericarditis, prior mitral valve surgeries, and cyanotic congenital heart disease. Baseline medical history such as hypertension, diabetes, and coronary artery disease (CAD) was obtained from electronic medical records.
Echocardiography
Standard TTE was performed with chamber and diastology assessment according to guidelines.10,11 Maximal two-dimensional LV dimensions were obtained in the parasternal long-axis view by choosing the largest measurements from basal to mid LV. The LA volume was calculated by the disk summation technique, the LV mass was calculated using linear methods, and MVP was defined as recommended.3 All baseline TTEs were reviewed de novo to confirm MVP diagnosis and carefully classify MVP as single-leaflet (either anterior or posterior) or bileaflet prolapse for the MVP group. Additionally, mitral annular disjunction (MAD) was defined as present if there was a wide separation (≥5 mm) between the posterior leaflet insertion into the LA wall and the base of the LV free wall in the parasternal long-axis view in systole.12
PVC Ascertainment
Rhythm, heart rate, systolic, and diastolic blood pressure were recorded at the time of baseline TTE. To ascertain the presence of PVCs, 12-lead electrocardiograms within ±3 months of baseline TTE and rhythm during TTE exam were evaluated. One or more PVCs on these diagnostic studies defined “presence of PVCs.” In a subgroup of patients having Holter exams within 3 months of TTE, PVC counts were recorded and corrected for hours of monitoring to match a 24-hour exam.
Chamber Remodeling Parameters
The LV remodeling parameters studied were LV end-systolic dimension index (LVESDi), LV end-diastolic dimension index (LVEDDi), LV mass index (LVMi), presence of eLVH (relative wall thickness ≤ 0.42 and LVMi > 95 g/m2 in women and >115 g/m2 in men), and LA volume index (LAVi).
MR Progression
All last available follow-up TTE reports, if available, were reviewed to assess the progression of MR in both MVP and non-MVP patients. For those having more than one subsequent TTE, the most recent TTE (i.e., before MV surgery, if applicable) was used. “Progression” of MR was defined as attainment of moderate-severe MR or more at last available follow-up TTE.
Survival
The survival endpoint was defined as all-cause mortality. The follow-up interval was between baseline TTE and mitral valve surgery (mitral surgery eliminates excess mortality),4 last clinical follow-up, or death. The survival status was retrieved using Accurint (LexisNexis, RELX Group, New York, NY), a proprietary resource combining multiple national sources (queried September 2019).
Statistical Analysis
Continuous variables, expressed as mean (SD) or median (interquartile range [IQR]), were compared using the Student’s t test or Wilcoxon rank-sum test as appropriate. Categorical data, presented as percentages, were compared using χ2 and Fisher’s exact test. Multivariable generalized linear and logistic regression models were used to identify factors associated with remodeling parameters (LVEDDi, LVESDi, LVMi, eLVH, LAVi). Variables selected for univariate linear regression models were based on clinical and pathophysiologic relevance as potential determinants for early remodeling, as per the authors’ clinical judgment. Variables with P value < .05 were chosen for multivariable linear regression analyses. When systolic blood pressure (SBP) and hypertension were both significant, the one with the smaller P value was chosen for analysis. Determinants of MR progression were analyzed using Cox proportional hazard regression models censoring patients who had at least one subsequent TTE at the last available TTE. Results were presented as odds ratio (OR) or hazard ratios (HR) with corresponding 95% CIs. Mortality and its determinants were analyzed using the Cox proportional hazards model. Statistical analyses were performed using commercially available software (JMP 11 and SAS 9.4, SAS Institute, Cary, NC). A P value <.05 was considered statistically significant.
RESULTS
A total of 253 consecutive MVP patients who met the criteria were matched for age and sex to 344 consecutive controls who met the criteria (not all MVP patients could be assigned a second match, and postmatching quality control prompted further patient exclusions). The baseline characteristics of patients with MVP and the non-MVP control group are presented in Table 1; indications for TTE in the control group are shown in the Supplementary Materials. Compared with the control group, MVP patients had similar SBP, heart rate, LVEF, and presence of CAD. The MVP patients also had more PVCs, larger LVEDDi, LVESDi, LVMi, and LAVi, more eLVH, and more mild-to-moderate (vs trivial/mild) MR by the comprehensive integrated TTE approach, albeit very small EROA and Rvol.
Table 1.
Demographic and echocardiographic parameters in study population (N = 597)
| MVP (n = 253) | Control (n = 344) | P value | |
|---|---|---|---|
| Age, years | 63 ± 16 | 64 ± 13 | .18 |
| Sex, male | 91 (36) | 139 (40) | .27 |
| Hypertension | 83 (33) | 140 (41) | .05 |
| Diabetes | 17 (7) | 26 (8) | .71 |
| CAD | 42 (17) | 64 (19) | .55 |
| Creatinine, mg/dL | 0.89 ± 0.17 | 0.88 ± 0.19 | .76 |
| Body surface area, m2 | 1.80 ± 0.22 | 1.84 ± 0.23 | .06 |
| Body mass index, kg/m2 | 24 ± 5 | 25 ± 5 | .03 |
| Atrial fibrillation | 16 (6) | 20 (6) | .76 |
| Presence of PVCs | 60 (24) | 37 (11) | <.0001 |
| SBP, mm Hg | 122 ± 28 | 123 ± 19 | .87 |
| Diastolic blood pressure, mm Hg | 71 ± 10 | 71 ± 11 | .82 |
| Heart rate, beats/minute | 68 ± 13 | 68 ± 14 | .57 |
| Single-leaflet prolapse | 105 (42) | — | — |
| Bileaflet prolapse | 148 (58) | — | — |
| MAD | 92 (36) | — | — |
| MR severity | |||
| MR EROA, mm2 | 12 ± 4 | Less than moderate MR | — |
| MR Rvol, mL/beat | 18 ± 6 | Less than moderate MR | — |
| Trivial or mild MR | 120 (47) | 337 (98) | <.0001 |
| Mild to moderate MR | 133 (53) | 7 (2) | <.0001 |
| LVEF (%) | 62 ± 5 | 63 ± 5 | .10 |
| LVEDD, mm (n = 591) | 49 ± 5 | 47 ± 5 | <.0001 |
| LVEDD index, mm/m2 (n = 591) | 28 ± 3 | 26 ± 3 | <.0001 |
| LVESD, mm (n = 586) | 31 ± 4 | 30 ± 4 | <.0001 |
| LVESD index, mm/m2 (n = 586) | 18 ± 2 | 16 ± 2 | <.0001 |
| LVMi, g/m2 (n = 580) | 94 ± 20 | 87 ± 19 | <.0001 |
| Relative wall thickness (n = 569) | 0.39 ± 0.07 | 0.40 ± 0.06 | .016 |
| eLVH | 41 (16) | 28 (8) | .002 |
| LA volume index, mL/m2 (n = 567) | 38 ± 16 | 33 ± 10 | <.0001 |
| E/A ratio (n = 550) | 1.13 ± 0.45 | 1.16 ± 0.48 | .47 |
| E/e’ ratio (n = 584) | 11 ± 5 | 11 ± 5 | .65 |
| Mild AR* | 31 (12) | 33 (10) | .30 |
| Mild-to-moderate TR† | 32 (13) | 19 (6) | .002 |
| AV peak velocity, m/sec | 1.3 ± 0.2 | 1.3 ± 0.3 | .05 |
AR, Aortic regurgitation; AV, aortic valve; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic dimension; TR, tricuspid regurgitation.
Data are expressed as mean ± SD or n (%).
The remainder had trivial or no AR.
The remainder had no, trivial, or mild TR (none/trivial TR in 348 [58%] and mild in 198 [33%]).
MVP Group
Baseline characteristics of MVP patients according to single or bileaflet phenotype are presented in Table 2. Within the MVP group (n = 253), there were 148 (58%) patients with bileaflet prolapse and 105 (42%) with single-leaflet prolapse, of which 75 (71%) and 30 (29%) had posterior and anterior leaflet prolapse, respectively. Mitral annular disjunction was present in 91 (36%) MVP patients. Compared with single-leaflet prolapse, patients with bileaflet prolapse were younger (60 ± 16 vs 66 ± 15, P = .003) and had more MAD (47% vs 21%, P < .0001) but similar presence of PVCs (24%, P = .97). Notably, all chamber remodeling parameters were similar between single and bileaflet MVP patients, except LVMi, which was larger in single-leaflet MVP (Table 2).
Table 2.
Demographic and echocardiographic parameters between bileaflet and single-leaflet prolapse (N = 253)
| Single-leaflet prolapse (n = 105) | Bileaflet prolapse (n = 148) | P value | |
|---|---|---|---|
| Age, years | 66 ± 15 | 60 ± 16 | .003 |
| Sex, male | 39 (37) | 52 (35) | .74 |
| Hypertension | 41 (39) | 42 (29) | .07 |
| Diabetes | 7 (7) | 10 (7) | .98 |
| CAD | 20 (19) | 23 (16) | .45 |
| Creatinine, mg/dL | 0.90 ± 0.16 | 0.87 ± 0.18 | .15 |
| Body surface area, m2 | 1.81 ± 0.20 | 1.79 ± 0.22 | .35 |
| Body mass index, kg/ m2 | 24.8 ± 4.5 | 24.0 ± 4.6 | .16 |
| Atrial fibrillation | 4 (4) | 12 (8) | .15 |
| Presence of PVCs | 25 (24) | 35 (24) | .97 |
| SBP, mm Hg | 123 ± 19 | 119 ± 18 | .06 |
| Diastolic blood pressure, mm Hg | 72 ± 10 | 69 ± 9 | .019 |
| Heart rate beats/minute | 67 ± 12 | 68 ± 13 | .54 |
| MAD | 22 (21) | 69 (47) | <.0001 |
| MR EROA, mm2 | 11.5 ± 3.5 | 12.6 ± 3.7 | .023 |
| MR Rvol, mL/beat | 18.9 ± 5.8 | 17.1 ± 6.0 | .016 |
| LVEF, % | 62 ± 6 | 62 ± 5 | .82 |
| LVEDD, mm | 49 ± 5 | 49 ± 4 | .68 |
| LVEDD index, mm/m2 | 27.3 ± 3.2 | 27.6 ± 2.9 | .51 |
| LVESD, mm | 31 ± 5 | 31 ± 4 | .99 |
| LVESD index, mm/m2 | 17.4 ± 2.7 | 17.7 ± 2.3 | .44 |
| LVMi, g/m2 | 97 ± 21 | 91 ± 19 | .028 |
| Relative wall thickness | 0.39 ± 0.07 | 0.38 ± 0.06 | .10 |
| eLVH | 22 (21) | 19 (13) | .08 |
| LA volume index, mL/ m2 (n = 567) | 38 ± 20 | 38 ± 13 | .80 |
| E/A ratio (n = 550) | 1.08 ± 0.42 | 1.17 ± 0.46 | .16 |
| E/e’ ratio (n = 584) | 11 ± 5 | 11 ± 4 | .43 |
| Mild AR* | 15 (14) | 15 (10) | .37 |
| Mild-to-moderate TR† | 16 (15) | 16 (11) | .29 |
| AV peak velocity, m/sec | 1.38 ± 0.24 | 1.28 ± 0.24 | .001 |
AR, Aortic regurgitation; AV, aortic valve; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic dimension; TR, tricuspid regurgitation.
Data are expressed as mean ± SD or n (%).
The remainder has trivial or no AR.
The remainder has no, trivial, or mild TR.
Within the MVP group, compared with men, women had similar age, similar prevalence of MAD and presence of PVCs, similar prevalence of mild-to-moderate MR (51% vs 55%), and similar EROA and Rvol (P ≥.30). Notwithstanding, women had higher LVEDDi (29 ± 3 vs 26 ± 3 mm), higher LVESDi (18 ± 2 vs 17 ± 2 mm), all P ≤.03, but similar LAVi. In addition, compared with men, women had less CAD (13% vs 24%), smaller LVMi (91 ± 18 vs 99 ± 23 g/m2—however, closer to abnormal [95 g/m2] in women compared with men [115 g/m2])—and higher LVEF (62.9% ± 5.1% vs 61.5% ± 4.7%), all P ≤ .03. Figure 1 shows a 44-year-old woman with bileaflet prolapse, MAD, late-systolic MR, and PVCs.
Figure 1.
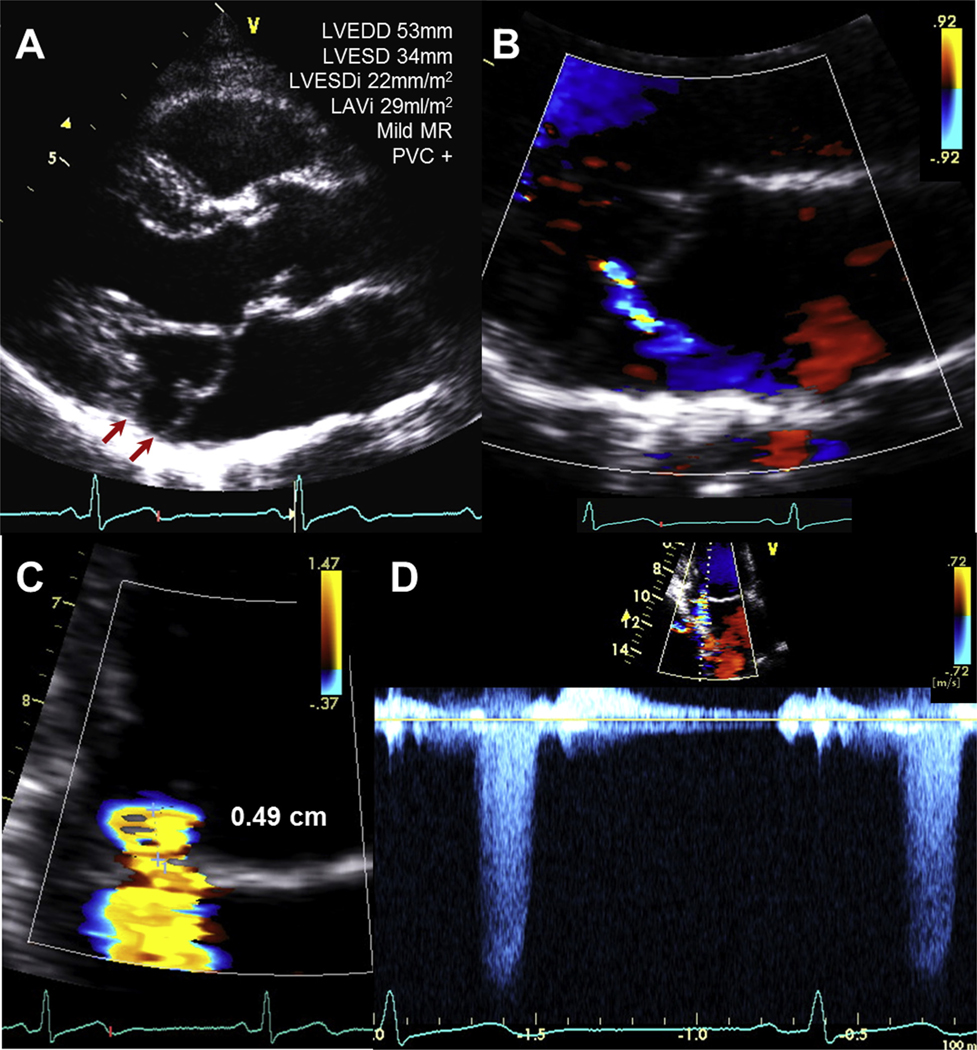
(A) A 44-year-old woman with bileaflet prolapse, mildly enlarged LVEDD, and LVESDi. A MAD (arrows) was present. (B) Mild MR was noted exclusively at end systole. (C) Radius of flow convergence was 0.49 cm; EROA and Rvol were 0.1 cm2 and 11 mL/beat, respectively. (D) MR velocity-time integral was only evident in end systole, supporting the mild nature of MR. See Supplemental Video 1, available at www.onlinejase.com.
Determinants of Chamber Remodeling in the Total Cohort (MVP and Controls)
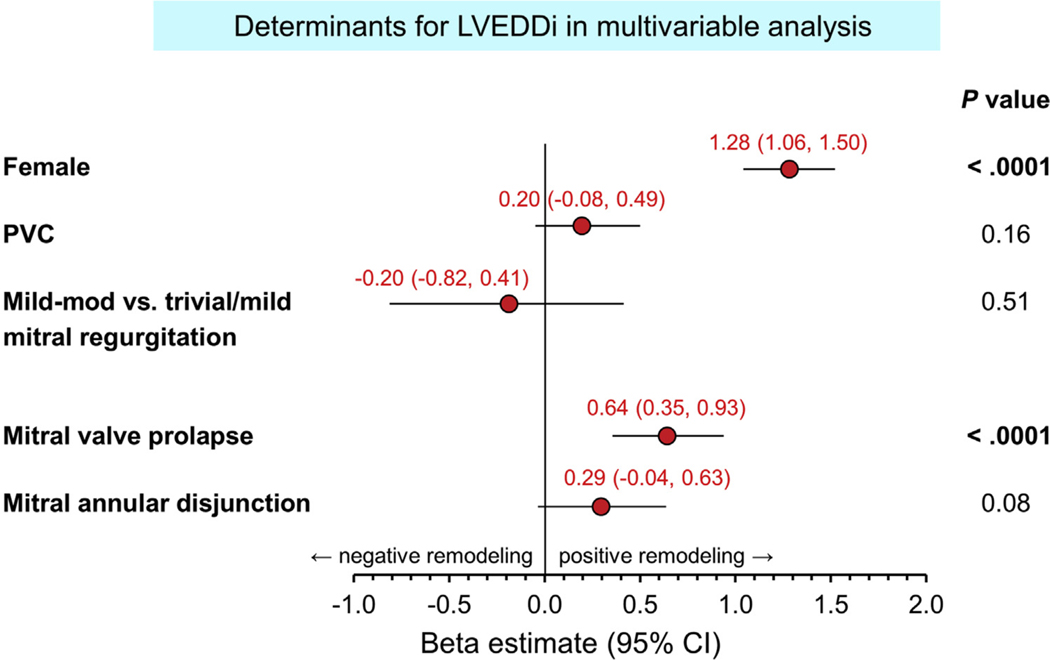
Table 3 shows variables associated with increased LVEDDi (also see Figure 2), LVESDi, LVMi, and LAVi for the total cohort (n = 597). Independent multivariable factors associated with increased LVEDDi were female sex, MVP, and a trend for MAD, but not MR severity. For LVESDi, younger age, female sex, and MVP were independent determinants with a trend for MAD. As for LAVi, older age, male sex, MVP, and elevated early mitral inflow velocity over mitral annular early diastolic velocity (E/e’) were independent associated factors. Regarding LVMi, older age, male sex, hypertension, and MVP were independently associated factors (Table 3).
Table 3.
Predictors of LVEDDi, LVESDi, LAVi, and LVMi
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Beta estimate | 95% CI | P value | Beta estimate | 95% CI | P value | |
| LVEDDi | ||||||
| Age, per 10-year increase | −0.08 | −0.25, 0.08 | .31 | |||
| Sex, female | 1.31 | 1.08, 1.53 | <.0001 | 1.28 | 1.06, 1.50 | <.0001 |
| CAD | −0.14 | −0.46, 0.17 | .37 | |||
| Hypertension | −0.11 | −0.36, 0.13 | .37 | |||
| SBP, per 10 mm Hg | −0.02 | −0.13, 0.07 | .59 | |||
| PVC | 0.33 | 0.00, 0.65 | .047 | 0.20 | −0.08, 0.49 | .16 |
| Mild-moderate vs trivial/mild MR | 0.96 | 0.40, 1.53 | .0009 | −0.20 | −0.82, 0.41 | .51 |
| MVP | 0.78 | 0.54, 1.02 | <.0001 | 0.64 | 0.35, 0.93 | <.0001 |
| MAD | 0.74 | 0.41, 1.07 | <.0001 | 0.29 | −0.04, 0.63 | .08 |
| LVESDi | ||||||
| Age, per 10 years | −0.15 | −0.29, −0.02 | .02 | −0.15 | −0.28, −0.02 | .017 |
| Sex, female | 0.71 | 0.51, 0.90 | <.0001 | 0.70 | 0.51, 0.88 | <.0001 |
| CAD | −0.12 | −0.37, 0.13 | .35 | |||
| Hypertension | −0.09 | −0.29, 0.10 | .35 | |||
| SBP, per 10 mm Hg | −0.06 | −0.14, 0.02 | .13 | |||
| PVC | 0.28 | 0.02, 0.55 | .034 | 0.21 | −0.03, 0.46 | .08 |
| Mild-moderate vs trivial/mild MR | 0.60 | 0.14, 1.06 | .009 | −0.29 | −0.81, 0.23 | .28 |
| MVP | 0.60 | 0.41, 0.79 | <.0001 | 0.51 | 0.26, 0.76 | <.0001 |
| MAD | 0.62 | 0.35, 0.88 | <.0001 | 0.25 | −0.03, 0.54 | .08 |
| LAVi | ||||||
| Age, per 10 years | 2.55 | 1.85, 3.25 | <.0001 | 1.58 | 0.82, 2.35 | <.0001 |
| Sex, female | −1.20 | −2.29, −0.12 | .028 | −2.08 | −3.08, −1.08 | <.0001 |
| CAD | 1.11 | −0.26, 2.49 | .11 | |||
| Hypertension | 1.76 | 0.67, 2.85 | .001 | |||
| SBP, per 10 mm Hg | 0.79 | 0.35, 1.24 | .0005 | 0.39 | −0.14, 0.93 | .15 |
| PVC | 1.04 | −0.40, 2.48 | .15 | |||
| Mild-moderate vs trivial/mild MR | 4.48 | 2.03, 6.94 | .0004 | 0.82 | −1.94, 3.59 | .55 |
| MVP | 2.33 | 1.28, 3.38 | <.0001 | 2.55 | 1.36, 3.75 | <.0001 |
| MAD | 0.57 | −0.88, 2.03 | .43 | |||
| E/e’ | 0.84 | 0.64, 1.05 | <.0001 | 0.68 | 0.45, 0.90 | <.0001 |
| LVMi | ||||||
| Age, per 10 years | 2.40 | 1.32, 3.48 | <.0001 | 1.91 | 0.83, 2.99 | .0005 |
| Sex, female | −4.58 | −6.18, −2.98 | <.0001 | −4.76 | −6.30, −3.22 | <.0001 |
| CAD | 2.94 | 0.87, 5.01 | .005 | 0.73 | −1.28, 2.75 | .47 |
| Hypertension* | 3.43 | 1.80, 5.06 | <.0001 | 2.59 | 0.95, 4.24 | .002 |
| SBP, per 10 mm Hg | 2.24 | 1.41, 3.07 | <.0001 | |||
| PVC | 2.86 | 0.71, 5.00 | .009 | 1.21 | −0.85, 3.27 | .24 |
| Mild-moderate vs trivial/mild MR | 8.45 | 4.77, 12.1 | <.0001 | 4.15 | −0.19, 8.49 | .06 |
| MVP | 3.28 | 1.68, 4.87 | <.0001 | 2.57 | 0.68, 4.46 | .007 |
| MAD | 1.80 | −0.40, 4.01 | .10 | |||
Hypertension was chosen for analysis because it has a higher beta estimate than SBP in this model.
Figure 2.
Forest plot of determinants of LVEDDi. Female sex and MVP were associated with larger LVEDDi.
Regarding eccentric LV remodeling, eLVH (n = 69), in a multivariable model adjusted for age, sex, and mild-to-moderate MR, MVP (adjusted OR = 2.05; 95% CI, 1.09–3.86; P = .02) and increased SBP (adjusted OR per 10 mm Hg, 1.18; 95% CI, 1.03–1.35; P = .018) were associated with eLVH. Regarding LVESD ≥ 40 mm and/or LVESDi ≥ 22 mm2 (n = 26), in univariate analysis PVCs were the only associated factor (OR = 3.44; 95% CI, 1.51–7.84; P = .005).
Determinants of Chamber Remodeling in Patients with Trivial/Mild MR Exclusively (MVP and Controls)
Supplemental Table 1 shows patients with trivial/mild MR exclusively, comparing patients with and without MVP (n = 457). The MVP patients were younger, had less hypertension, had more PVCs, had larger LV and LA chamber sizes, and had more eLVH, all P ≤ .03, similar to the entire cohort (Table 1). Independent multivariable factors associated with increased LVEDDi were female sex, PVCs, and MVP (all P ≤ .02; Supplemental Table 2). Similarly, younger age, female sex, PVCs, and MVP were associated with increased LVESDi (all P ≤ .04). Conversely, older age, male sex, MVP, and increased E/e’ were associated with increased LAVi, while increased SBP was associated with increased LVMi (all P ≤ .02; (Supplemental Table 2).
Notably, in regards to factors associated with chamber remodeling, the main difference between the whole cohort (Table 3) and patients with exclusive trivial/mild MR (Supplemental Table 2) was that PVCs became independently associated with LV remodeling in patients with exclusive trivial/mild MR. Nevertheless, female sex and MVP continued to be significantly associated with LV and LA remodeling.
Determinants of Chamber Remodeling in Patients with Holter Exams
In our cohort, 141 (76 controls and 65 MVP group; P = .3) patients had Holter exams within 3 months of baseline TTE (median time, 2 [IQR, 0–11] days to TTE). Those with Holter exams versus those without were more likely to have baseline PVCs on electrocardiogram (ECG; 29% vs 12%, P < .0001).
Of 141 patients, 130 (92%) had PVCs on Holter (range from 1 to 52,087; eight had >15,000 PVC counts [four in the MVP group], and 41/130 [32%] had PVCs on ECG), and 10 patients without PVCs on Holter also had no PVCs on ECG (Fisher’s P = .03). The median 24-hour PVC count on Holter was 156 (IQR, 13–1,075) and was used in the analysis (Table 4). The results were similar to those in Table 3, but now PVC counts were associated with larger LVEDDi, LVESDi, and LVMi after adjustment for other factors.
Table 4.
Predictors of LVEDDi, LVESDi, LAVi, and LVMi in patients with Holter exam (N = 141)
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Beta estimate | 95% CI | P value | Beta estimate | 95% CI | P value | |
| LVEDDi | ||||||
| Age, per 10-year increase | −0.32 | −0.63, −0.01 | .043 | −0.14 | −0.41, 0.12 | .29 |
| Sex, female | 1.25 | 0.79, 1.70 | <.0001 | 1.12 | 0.71, 1.53 | <.0001 |
| CAD | −0.31 | −0.95, 0.32 | .33 | |||
| Hypertension | −0.81 | −1.32, −0.30 | .001 | −0.39 | −0.84, 0.04 | .08 |
| SBP, per 10 mm Hg | −0.27 | −0.54, −0.01 | .043 | |||
| PVC counts ≥ 156 | 0.63 | 0.14, 1.11 | .010 | 0.58 | 0.16, 1.00 | .006 |
| Mild-moderate vs trivial/mild MR | 1.35 | 0.21, 2.49 | .020 | −0.64 | −1.79, 0.51 | .27 |
| MVP | 1.23 | 0.78, 1.68 | <.0001 | 1.05 | 0.52, 1.59 | .0001 |
| MAD | 1.04 | 0.45, 1.63 | .0007 | 0.06 | −0.53, 0.66 | .83 |
| LVESDi | ||||||
| Age, per 10 years | −0.33 | −0.59, −0.08 | .009 | −0.23 | −0.47, −0.01 | .045 |
| Sex, female | 0.71 | 0.31, 1.10 | .0005 | 0.58 | 0.23, 0.94 | .001 |
| CAD | −0.41 | −0.93, 0.11 | .12 | |||
| Hypertension | −0.61 | −1.02, −0.19 | .004 | −0.30 | −0.69, 0.08 | .12 |
| SBP, per 10 mm Hg | −0.21 | −0.43, 0.01 | .06 | |||
| PVC counts ≥ 156 | 0.59 | 0.20, 0.98 | .003 | 0.58 | 0.21, 0.94 | .001 |
| Mild-moderate vs trivial/mild MR | 0.91 | −0.02, 1.85 | .056 | −0.56 | −1.55, 0.43 | .26 |
| MVP | 0.95 | 0.58, 1.32 | <.0001 | 0.85 | 0.39, 1.31 | .0004 |
| MAD | 0.72 | 0.24, 1.21 | .003 | −0.08 | −0.60, 0.42 | .73 |
| LAVi | ||||||
| Age, per 10 years | 3.69 | 1.81, 5.56 | .0002 | 2.20 | 0.19, 4.20 | .031 |
| Sex, female | −3.76 | −6.76, −0.76 | .014 | −2.99 | −5.83, −0.15 | .038 |
| CAD | −0.84 | −4.85, 3.17 | .67 | |||
| Hypertension | 3.16 | −0.01, 6.33 | .050 | |||
| SBP, per 10 mm Hg | 2.68 | 1.07, 4.29 | .001 | 1.39 | −0.17, 2.96 | .08 |
| PVC counts ≥ 156 | 2.53 | −0.44, 5.51 | .09 | |||
| Mild-moderate vs trivial/mild MR | 2.83 | 4.24, 9.91 | .43 | |||
| MVP | 4.38 | 1.45, 7.30 | .003 | 4.33 | 1.58, 7.07 | .002 |
| MAD | −0.89 | −4.56, 2.77 | .62 | |||
| E/e’ | 1.11 | 0.51, 1.70 | .0003 | 0.66 | 0.06, 1.27 | .030 |
| LVMi | ||||||
| Age, per 10 years | 2.50 | 0.36, 4.64 | .022 | 1.44 | −0.61, 3.50 | .16 |
| Sex, female | −4.44 | −7.80, −1.09 | .009 | −3.90 | −6.98, −0.83 | .013 |
| CAD | 2.06 | −2.33, 6.47 | .35 | |||
| Hypertension | 2.07 | −1.50, 5.66 | .25 | |||
| SBP, per 10 mm Hg | 2.46 | 0.61, 4.30 | .009 | 1.38 | −0.34, 3.12 | .11 |
| PVC counts ≥ 156 | 6.16 | 2.93, 9.39 | .0002 | 3.77 | 0.61, 6.92 | .019 |
| Mild-moderate vs trivial/mild MR | 11.5 | 3.76, 19.25 | .003 | 0.82 | −7.74, 9.38 | .85 |
| MVP | 6.82 | 3.62, 10.01 | <.0001 | 5.89 | 2.21, 9.57 | .001 |
| MAD | 3.66 | −0.46, 7.79 | .08 | |||
Factors Associated with PVCs
Compared with patients without PVCs (n = 500), PVCs (n = 97) were associated with older age (68 ± 15 vs 63 ± 14 years, P = .003), more mild-to-moderate MR (37% vs 21%, P = .0009), more MVP (62% vs 39%, P < .0001), more MAD (24% vs 14%, P = .015), and a trend toward lower ejection fraction (61.9 ± 5.2 vs 62.9 ± 5.1, P = .07). However, in a multivariable logistic model, independent factors related to PVC were only older age (OR = 1.32 per 10 years; 95% CI, 1.12–1.56; P = .0006) and MVP (OR = 2.21; 95% CI, 1.23–3.98; P = .009) in a model adjusted for MR severity, MAD, and LVEF.
MR Progression and Survival
Up to August 2019, 323 (54%; 147 [58%] from the MVP group and 176 [51%] from the control group) patients had a follow-up TTE at a median interval of 4.3 (IQR, 1.7–6.4) years from baseline TTE (5.5 [IQR, 3.0–8.6] years in MVP patients versus 3.2 [IQR, 1.2–5.3] years in controls; P < .0001). Development of MVP in the control group occurred in three patients (bileaflet, anterior, and posterior in each) at an average of 1.5 ± 0.6 years. Progression of MR to moderate-severe or more occurred in 17 patients (baseline anterior leaflet prolapse in one, posterior leaflet prolapse in 10, bileaflet prolapse in six), all from the MVP group. Quantification of MR was available in 43 patients (13%; MVP in 40 and control in three), including 28 without progression (EROA 19 ± 6 mm2, Rvol 33 ± 9 mL) and 15 (out of 17) with progression (EROA 33 ± 9 mm2, Rvol 63 ± 12 mL; both P < .0001 compared with those without progression). The time lag between baseline and last available TTE in 17 patients with progression versus those without was significantly longer (75 ± 32 vs 52 ± 36 months, P = .008). Cox proportional hazard model for determinants of progression within the MVP group showed that univariately, single-leaflet prolapse (posterior plus anterior prolapse vs bileaflet prolapse) showed a trend toward MR progression (HR = 2.54; 95% CI, 0.93–6.93; P = .06). Isolated posterior leaflet prolapse was associated with MR progression compared with bileaflet prolapse (HR = 3.10; 95% CI, 1.10–8.68; P = .03), while age, sex, baseline EROA, Rvol, PVCs, and MAD were not; qualitative MR severity (mild-to-moderate vs trivial/mild MR) showed a trend of association (P = .056). After adjusted for mild-to-moderate MR, isolated posterior leaflet prolapse was the single factor associated with MR progression to moderate-severe or more (adjusted HR = 2.70; 95% CI, 1.0–7.34; P = .048). Interestingly, LVEDDi (P = .30), LVESDi (P = .32), and LAVi (P = .47) were not predictors of MR progression.
At a median of 5.9 (IQR, 4.6–7.2) years of follow-up under medical treatment (i.e., three patients having MV surgery were censored at time of surgery), 131 patients died (55 in the MVP group and 76 controls). Compared with expected survival from the age- and sex-matched U.S. population, the MVP group had similar survival, while the control group had less than expected survival, supporting that MVP patients in our cohort had indeed only mild forms of MR (Figure 3). Table 5 shows univariable and multivariable analyses for determinants of all-cause death. In multivariable analysis, older age, male sex, increased E/e’, and increased LVMi and LAVi were independently associated with mortality. Female sex and MVP (vs control group) were protective factors in our cohort.
Figure 3.
Cohort expected vs observed survival. Survival in MVP patients (blue line) and control group (red line) was similar and inferior to the age- and sex-matched U.S. population (green dotted line), respectively. The 6-year survival was 77% ± 2% in the control group and 85% ± 2% in the MVP group.
Table 5.
Cox proportional model for predictors of all-cause mortality (N = 597; 131 deaths)
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, per 10 years | 1.59 (1.38–1.85) | <.0001 | 1.62 (1.35–1.96) | <.0001 |
| Sex, female | 0.70 (0.49–0.99) | .04 | 0.57 (0.39–0.85) | .005 |
| CAD | 1.42 (0.94–2.14) | .09 | ||
| Hypertension | 1.44 (1.02–2.04) | .03 | 1.18 (0.79–1.75) | .39 |
| SBP, per 10 mm Hg | 1.04 (0.95–1.14) | .30 | ||
| MVP | 0.59 (0.40–0.87) | .006 | 0.63 (0.41–0.96) | .031 |
| Trivial/mild vs mid-moderate MR | 0.86 (0.57–1.29) | .47 | ||
| PVC | 1.24 (0.81–1.91) | .32 | ||
| LVEF | 1.00 (0.96–1.03) | .98 | ||
| LVEDDi | 1.00 (0.94–1.06) | .88 | ||
| LVESDi | 1.00 (0.93–1.08) | .86 | ||
| Eccentric remodeling | 1.52 (0.95–2.42) | .09 | ||
| LVMi | 1.01 (1.00–1.02) | .005 | 1.01 (0.99–1.01) | .26 |
| LAVi | 1.01 (1.01–1.02) | <.0001 | 1.01 (0.99–1.01) | .24 |
| E/e’ | 1.07 (1.04–1.10) | <.0001 | 1.03 (1.00–1.07) | .03 |
DISCUSSION
Our study is the first to systematically assess patterns and determinants of early chamber remodeling in MVP patients with mild degrees of MR. Our principal findings are the following: In patients with less than moderate MR (1) the presence of MVP was associated with both LV and LA chamber remodeling, including LVEDDi, LVESDi, LAVi, LVMi, and eLVH. (2) Female sex and MVP were significantly associated with LV chamber remodeling, including LVEDDi and LVESDi. (3) In patients with exclusive trivial/mild MR, female sex, MVP and presence of PVCs were robust determinants of LV chamber remodeling, including LVEDDi and LVESDi. (4) Early chamber remodeling was not a predictor of MR progression; instead, isolated posterior leaflet prolapse was the single factor associated with progression to moderate-severe MR. And (5) female sex and presence of MVP (as compared with controls) were protective factors for mortality.
Early Chamber Remodeling in MVP with Less than Moderate MR: Independent of MR Severity
In order to guarantee less than moderate MR severity in the MVP group, we chose consecutive patients with PISA-derived EROA <20 mm2 and Rvol < 30 cm3/beat, plus less than moderate estimation by the comprehensive integrated TTE approach.9 For the control group, very few patients had PISA quantification because it is rare in clinical practice to further quantify MR deemed trivial/mild (98% of the control group) or mild-to-moderate by the TTE approach in patients with normal mitral valves. Interestingly, despite very small EROA and Rvol in the MVP group, half of the patients were classified as mild-moderate MR by the comprehensive integrated TTE approach (Table 1), and indeed, univariately, mild-tomoderate MR was highly associated with higher LVEDDi, LVESDi, LAVi, LVMi, and eLVH. However, in all multivariate analyses (Table 3), mild-moderate MR lost all association, and the presence of MVP and female sex were associated with early chamber remodeling. Because MVP was related with more mild-moderate MR and the effect of comprehensive integrated MR severity could have been “diluted” in multivariate analyses, we analyzed all patients with trivial/mild MR exclusively as a separate cohort (Supplemental Tables 1 and 2) and found the exact same associations where MVP and female sex were highly associated with larger LVEDDi and LVESDi and MVP was associated with larger LAVi (all P < .0001). In a previous study by our group, Mantovani et al.13 demonstrated higher LVEDDi, LVESDi, and LA diameter index (all P < .0001) in women with MVP compared with men with MVP and severe MR. Therefore, for both mild and severe grades of MVP-related MR, women exhibit a higher degree of LV and LA chamber remodeling. The reason for this pervasive finding is unknown, and we speculate it could be part of the phenotypic expression of MVP. Certainly, MVP exhibits a strong heritable component,14 hence, MVP-associated early chamber remodeling may be the phenotypical expression of a genetically mediated process. These hypotheses require further study.
Early Chamber Remodeling in MVP with Less than Moderate MR: PVC Induced?
Premature ventricular contractions were associated independently with age and MVP. Interestingly, in the trivial/mild MR subgroup analysis, the presence of PVCs—which were the only univariate determinants of higher LVEDDi and LVESDi in the entire cohort—became an independent determinant of LV chamber remodeling (Supplemental Table 2). Furthermore, the presence of PVCs was the only univariate factor associated with LVESD ≥ 40 mm and/or LVESDi ≥ 22 mm2, P = .005, cutoffs that are considered “surgical triggers” in severe MR. Although in the present study LVEF was preserved and clinically similar in those patients with and without PVCs, the presence of PVCs could cause repeated dyssynchrony, triggering adverse LV remodeling.15 Therefore, the most robust determinants of early chamber remodeling in this study were the presence of MVP, female sex, and the presence of PVCs. Another possible explanation for PVC-related LV remodeling could be due to mechanical induction of myocardial replacement fibrosis,16 which drives remodeling in the absence of significant volume load.
The prevalence of MAD in the single-leaflet group and bileaflet subgroup was 21% and 47%, respectively (P < .0001), consistent with prior findings that MAD is not uncommon in MVP and more prevalent in those with bileaflet prolapse.12 Interestingly, however, MAD was not an independent determinant of chamber remodeling and was not independently associated with the presence of PVCs either. Indeed, the clinical significance of MAD remains controversial,17 and it is possible that follow-up was too short and the sample too small to evaluate its association with mortality.
MR Progression and Mortality
Early chamber remodeling and MR severity (trivial-mild vs mild-moderate) were not associated with MR progression or mortality, suggesting that, indeed, the MR was less than moderate and that the LV/LA remodeling were not due to significant volume overload, which would have likely resulted in a survival penalty. On the contrary, female sex was protective for death and MVP patients had similar survival as expected, suggesting that early remodeling in MVP patients with less than moderate MR is benign and not related to volume overload. In addition, patients in the control group, albeit “normal” in terms of valvular function, had other comorbidities warranting a TTE exam in our center (Supplementary Results). Notwithstanding, the MVP group was associated with progression to hemodynamically significant MR, which is consistent with the notion that MVP is the most common cause for chronic primary MR in developed countries.1 In addition, isolated posterior leaflet prolapse was associated with progression within the MVP group, particularly as compared with bileaflet prolapse. This finding is consistent with prior reports that posterior prolapse has the highest prevalence in patients with severe MR undergoing surgery.18 We also speculate that patients with bileaflet prolapse could have larger MV coaptation area (which explains late systolic MR), resulting in slower or less MR progression compared with isolated leaflet prolapse.
Clinical and Research Implications
Chamber remodeling of the LV and LA are expected with significant MR, and the presence of significant MR (i.e., 3–4+) has to be put in doubt in the absence of LV/LA remodeling.1,9 Hence, it may be confusing to the clinician to find mild forms of MR associated with chamber remodeling, which could result in subjective overestimation of MR severity and even consideration of surgery where none is needed. Indeed, some patients exhibited surgical cutoff chamber measurements (i.e., LVESD ≥ 40 mm and/or LVESDi ≥ 22 mm2) while having less than moderate MR, an observation highly related to the presence of PVCs. While routine 24-hour Holter monitoring in all patients with MVP is not feasible, our study suggests it should be considered for those who display signs of LV remodeling and PVCs on ECG and/or during echocardiography, in order to quantify PVC burden. Whether early chamber remodeling in MVP with mild forms of MR is a genetically determined phenotypic expression, to what extent do PVCs contribute to early remodeling, and why female patients exhibit worse remodeling than male patients are currently unknown and require further study. Lastly, a limited number of patients had MR quantification at follow-up TTE because the majority of patients did not have progression of MR. Due to the small number of patients progressing to moderate-severe MR or more, determinants for progression need to be tested in future larger studies.
Limitations
Due to the cross-setional and retrospective nature of our work, we could not compare the continuous trend or linear progression of chamber remodeling in these patients. There were no MR quantitative data for the control group (i.e., EROA and Rvol) because it is generally not indicated to quantify mild forms of MR arising from normal mitral valves. In our laboratory, we tend to PISA quantify MR if there is organic leaflet disease (i.e., MVP) regardless of suspected severity, yet we cannot exclude that the PISA quantification of less than moderate MR in the MVP group could have been related to the physician suspecting more than mild MR in some patients, such that the only way to objectively guarantee that MR was indeed less than moderate in our study’s MVP group was to choose consecutive patients with PISA quantification. Although mild-to-moderate MR was more prevalent in the MVP group, the average EROA and Rvol were clearly in the mild range (13 ± 3 mm2 and 19 ± 6 mL, respectively), and MVP was still associated with chamber remodeling after adjusting for MR severity. Moreover, the results were identical when comparing MVP with controls with trivial/mild MR exclusively. Nonetheless, MVP, MAD, and MR severity are closely correlated, which could create confounding in the multivariate analysis.
There were no data regarding exercise-hemodynamic echocardiography to evaluate dynamic changes of MR severity, which is generally not warranted in asymptomatic patients with mild forms of MR,1 yet it could be possible that early chamber remodeling was associated with worsening MR on exertion. Lastly, total PVC burden cannot be assessed without Holter monitoring, which we did not have in a majority of patients.
CONCLUSION
Patients with less than moderate degenerative MR due to MVP exhibit “benign” early LV and LA remodeling that appears to be not related to MR severity and not associated with MR progression or mortality. Robust determinants for LV remodeling included MVP, female sex, and presence of PVCs. Progression to moderate-severe MR or more was associated with the presence of isolated posterior leaflet prolapse. MVP-associated early chamber remodeling may be the phenotypical expression of a genetically mediated process and is at least partially related to the presence of PVCs.
Supplementary Material
HIGHLIGHTS.
Patients with MVP and less than moderate MR exhibit early chamber remodeling.
LV remodeling in these patients is strongly associated to MVP, female sex, and PVCs.
Early LV and LA remodeling does not predict MR progression or associate with mortality.
Posterior leaflet prolapse was the only independent predictor of MR progression.
Abbreviations
- CAD
Coronary artery disease
- EDD
End-diastolic diameter
- ECG
Electrocardiogram
- ESD
End-systolic diameter
- eLVH
Eccentric left ventricular hypertrophy
- EROA
Effective regurgitant orifice area
- HR
Hazard ratio
- IQR
Interquartile range
- LA
Left atrium, atrial
- LAVi
Left atrial volume index
- LV
Left ventricle, ventricular
- LVEF
Left ventricular ejection fraction
- LVEDDi
Left ventricular end-diastolic dimension index
- LVESDi
Left ventricular end-systolic dimension index
- LVMi
Left ventricular mass index
- MAD
Mitral annular disjunction
- MR
Mitral regurgitation
- MVP
Mitral valve prolapse
- OR
Odds ratio
- PISA
Proximal isovelocity surface area
- PVC
Premature ventricular contraction
- Rvol
Regurgitant volume
- SBP
Systolic blood pressure
- TTE
Transthoracic Echocardiography
Footnotes
Paul A. Grayburn, MD, FASE, served as guest editor for this report.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at https://doi.org/10.1016/j.echo.2020.01.016.
Conflicts of Interest: None.
REFERENCES
- 1.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57–185. [DOI] [PubMed] [Google Scholar]
- 2.Le Tourneau T, Merot J, Rimbert A, Le Scouarnec S, Probst V, Le Marec H, et al. Genetics of syndromic and non-syndromic mitral valve prolapse. Heart (Br Card Soc) 2018;104:978–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999; 341:1–7. [DOI] [PubMed] [Google Scholar]
- 4.Suri RM, Vanoverschelde JL, Grigioni F, Schaff HV, Tribouilloy C, Avierinos JF, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013;310:609–16. [DOI] [PubMed] [Google Scholar]
- 5.Katan O, Michelena HI, Avierinos JF, Mahoney DW, DeSimone DC, Baddour LM, et al. Incidence and predictors of infective endocarditis in mitral valve prolapse: a population-based study. Mayo Clin Proc 2016;91:336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garbi M, Lancellotti P, Sheppard MN. Mitral valve and left ventricular features in malignant mitral valve prolapse. Open Heart 2018;5:e000925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller MA, Dukkipati SR, Turagam M, Liao SL, Adams DH, Reddy VY. Arrhythmic mitral valve prolapse: JACC review topic of the week. J Am Coll Cardiol 2018;72:2904–14. [DOI] [PubMed] [Google Scholar]
- 8.Latchamsetty R, Bogun F. Premature ventricular complexes and premature ventricular complex induced cardiomyopathy. Curr Prob Cardiol 2015;40:379–422. [DOI] [PubMed] [Google Scholar]
- 9.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30: 303–71. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 11.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107–33. [DOI] [PubMed] [Google Scholar]
- 12.Lee AP, Jin CN, Fan Y, Wong RHL, Underwood MJ, Wan S. Functional implicationofmitralannulardisjunctioninmitralvalveprolapse:aquantitativedynamic 3D echocardiographic study. JACC Cardiovasc Imaging 2017;10:1424–33. [DOI] [PubMed] [Google Scholar]
- 13.Mantovani F, Clavel MA, Michelena HI, Suri RM, Schaff HV, Enriquez-Sarano M. Comprehensive imaging in women with organic mitral regurgitation: implications for clinical outcome. JACC Cardiovasc Imaging 2016; 9:388–96. [DOI] [PubMed] [Google Scholar]
- 14.Dina C, Bouatia-Naji N, Tucker N, Delling FN, Toomer K, Durst R, et al. Genetic association analyses highlight biological pathways underlying mitral valve prolapse. Nat Genet 2015;47:1206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, et al. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol 2015;66:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 2011;57:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enriquez-Sarano M. Mitral annular disjunction: the forgotten component of myxomatousmitralvalvedisease. JACCCardiovascImaging 2017;10:1434–6. [DOI] [PubMed] [Google Scholar]
- 18.Lazam S, Vanoverschelde JL, Tribouilloy C, Grigioni F, Suri RM, Avierinos JF, et al. Twenty-year outcome after mitral repair versus replacement for severe degenerative mitral regurgitation: analysis of a large, prospective, multicenter, international registry. Circulation 2017;135:410–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.