Abstract
Introduction:
Melatonin levels are partially driven by the parenchyma volume of the pineal gland. Low urinary levels of 6-sulfatoxymelatonin have been associated with increased risk of advanced prostate cancer, but the relationship between pineal gland volume and composition and prostate cancer risk has not been examined.
Materials and Methods:
We utilized data from 864 men from the AGES-Reykjavik Study with complete pineal gland volumes and urinary 6-sulfatoxymelatonin measurements. Pineal parenchyma, calcification, and cyst volumes were calculated from brain magnetic resonance imaging (MRI). Levels of 6-sulfatoxymelatonin were assayed from prediagnostic urine samples. We calculated Pearson correlation coefficients between parenchyma volume and urinary 6-sulfatoxymelatonin levels. We used Cox proportional hazards regression to calculate multivariable hazard ratios (HRs) and 95% confidence intervals (95% CIs) comparing prostate cancer risk across parenchyma volume tertiles and across categories factoring in parenchyma volume, gland composition, and urinary 6-sulfatoxymelatonin level.
Results:
Parenchyma volume was moderately correlated with urinary 6-sulfatoxymelatonin level (r = 0.24, p <0.01). There was no statistically significant association between parenchyma volume tertile and prostate cancer risk. Men with high parenchyma volume, pineal cysts and calcifications, and low urinary 6-sulfatoxymelatonin levels had almost twice the risk of total prostate cancer as men with low parenchyma volume, no pineal calcifications or cysts, and low urinary 6-sulfatoxymelatonin levels (HR: 1.98, 95% CI: 1.02, 3.84, p: 0.04).
Conclusions:
Although parenchyma volume is not associated with prostate cancer risk, pineal gland composition and other circadian dynamics may influence risk for prostate cancer. Additional studies are needed to examine the interplay of pineal gland volume, composition, and melatonin levels on prostate cancer risk.
Keywords: prostate cancer, pineal gland, melatonin, circadian rhythm
Introduction
The pineal gland is a neuroendocrine gland in the brain that produces melatonin, a hormone that signals biochemical darkness, at night1. Variation in melatonin levels is driven in part by pineal gland volume. Pineal calcification has been reported both to have no effect on melatonin levels and to have been associated with lower melatonin levels2,3, and the effect of pineal cysts on melatonin levels has only been reported for small case reports (N<5), which differ in their findings4,5. Our group previously found a positive association between pineal parenchyma volume and 6-sulfatoxymelatonin levels in a sample of 122 men from the Age, Gene/Environment Susceptibility-Reykjavik (AGES-Reykjavik) Study, an Icelandic cohort6. Other studies with small sample sizes have also reported positive correlations between pineal gland volume and melatonin levels, as measured in urine, plasma, and saliva7–9.
Existing literature on the relationship between melatonin levels and prostate cancer risk is sparse. Studies examining the association between proxies for circadian disruption—such as shift work, sleep duration, and disrupted sleep—and prostate cancer risk have found both positive association and no association10–14. A small study (n=24) found men with prostate cancer had lower levels of serum melatonin than those with benign prostatic hyperplasia15. A prospective study by our group in an Icelandic cohort showed men with low levels of urinary 6-sulfatoxymelatonin, the primary metabolite of melatonin, had an increased risk of advanced prostate cancer compared to men with high levels16.
No previous studies to date have examined the relationship between pineal parenchyma volume, gland composition, and prostate cancer risk. In this novel study, we sought to confirm our previous finding of a positive association between pineal parenchyma volume and 6-sulfatoxymelatonin levels6 and investigate the hypothesis that larger pineal parenchyma volume is associated with lower prostate cancer risk. We also assessed the effect of pineal calcification and cyst on prostate cancer risk, hypothesizing that participants with pineal calcification and cysts will have lower levels of melatonin and a higher risk of prostate cancer. We assessed these relationships in a sample of Icelandic men (N=864) from the AGES-Reykjavik Study.
Materials and Methods
Study Population
Our study was nested within the AGES-Reykjavik Study, a cohort study that aims to assess risk factors for disability and disease of aging populations that has been described in detail elsewhere17. Participants in the AGES-Reykjavik Study underwent a clinical assessment between September 2002 and February 2006 at the Icelandic Heart Association. The assessment was completed in three clinic visits, consisting of biospecimen collection (including a first morning urinary void sample), brain magnetic resonance imaging (MRI), and a detailed questionnaire covering health history and diet and lifestyle habits. The study included 2,425 men living in Reykjavik, Iceland, aged 67 to 96 years. We limited this analysis to men who had complete pineal gland volume and urinary 6-sulfatoxymelatonin measurements and did not have a history of cancer at baseline for a final subsample of 864 men.
The protocol for the AGES-Reykjavik Study is approved by the Icelandic Data Protection Authority, the National Bioethics Committee in Iceland, and the National Institute on Aging Institutional Review Board. Informed consent was obtained from all participants. The protocol for this study was approved by the Institutional Review Boards at the Harvard T.H. Chan School of Public Health and Michigan Public Health.
Urinary 6-Sulfatoxymelatonin Levels
Participants obtained a first morning urinary void sample at baseline and on the same day of collection returned it to the Icelandic Heart Association. Laboratory personnel, who were blinded to case status of the participants, then assayed these samples for 6-sulfatoxymelatonin using the Melatonin-Sulfate ELISA (IBL International, Germany), as described in detail elsewhere16. For this assay, the minimal detectable concentration is 1.0 ng/mL. The samples were measured in duplicate, and urinary 6-sulfatoxymelatonin levels were measured in 32 batches and were batch-adjusted using a quantile normalization approach18. The range of the coefficient of variation across batches was 5.4% to 8.5%. Because urinary volume was unavailable, we adjusted for creatinine when melatonin was included in the models. Creatinine concerntration was obtained using the Creatinine Jaffe method on Hitachi 912 (Roche Diagnostic, Switzerland).
Pineal Gland Volume
Ascertainment of pineal gland volume has been published in detail previously6. Participants underwent MRI of the brain using a 1.5 T Signa Twinspeed EXCITE system (General Electric Medical System, Waukesha, WI) during the second day of the clinical assessment. MRI measurements were all in the axial plane. Pineal gland volumes in mm3 were obtained from this MRI through manual calculation of each voxel, which was equal to 1.33 mm3, and multiplying the number of voxels by 1.33 mm3/voxel. The composition of the pineal glands (parenchyma, calcification, and cyst) was determined by consensus between assessments of the image by a physician and a radiographer, who were blinded to clinical information. Because labeling of the components was subjective, reliability of pineal measurements was assessed using intra-class correlation and the Bland-Altman method to determine the level of agreement between two separate readings of the pineal gland in a sample of 29 participants. This method had high reliability, as evidenced by a high level of agreement between the first and second readings6.
Prostate Cancer Cases and Deaths
Prostate cancer cases were identified by linking unique identification numbers assigned to all Icelandic residents to the Icelandic Cancer Registry, and cause of death was obtained by linking participants to Statistics Iceland. Details on how the Icelandic Cancer Registry identifies cancer cases are described in one of our previous studies16. TNM stage was available for 90.3% of the prostate cancer cases in this study. Advanced prostate cancer is defined as T3b or higher, N1, or M1 stage at diagnosis, or prostate cancer-related death during follow-up. Gleason grade was available for 94.4% of the prostate cancer cases. High-grade prostate cancer is defined as Gleason grades 8 to 10. Follow-up for prostate cancer cases and deaths was available through December 31, 2016.
Statistical Analysis
We calculated the mean, standard deviation, median, and range for total pineal gland volume and for pineal parenchyma, cyst, and calcification volumes individually. The relationship between pineal parenchyma volume and urinary 6-sulfatoxymelatonin was assessed using Pearson’s correlation coefficient.
Pineal parenchyma volume was used to assess the relationship between pineal gland volume and prostate cancer risk. We divided parenchyma volume into tertiles and used Cox proportional hazards regression to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for each tertile, setting the lowest tertile as the reference group. We also conducted a sensitivity analysis that excluded cases diagnosed less than a year from baseline.
We defined risk time as the time from study baseline to time of prostate cancer diagnosis, death from a non-prostate cancer related cause, or end of last follow-up. We report both the age-adjusted and multivariable-adjusted HRs and 95% CIs for total prostate cancer, advanced prostate cancer, and high-grade prostate cancer separately. The multivariable-adjusted models controlled for age, family history of prostate cancer, smoking status, physical activity, history of depression, current beta-blocker use, and annual doctor’s exam. Because sleep problems could be considered either a mediator or a confounder in this model, we ran models both with and without adjusting for sleep problems. Because our previous study of this cohort found no difference in urinary 6-sulfatoxymelatonin levels by season of collection,16 we did not adjust for this in our models. Annual doctor’s exam was used as a proxy for prostate cancer screening. We utilized the missing indicator approach to handle missing data for physical activity (N=39), history of depression (N=52), and current beta-blocker use (N=87).
We examined the interplay between parenchyma volume, melatonin level, and presence of pineal calcification and cyst among the 861 participants in the pineal gland composition analysis. We created eight categories based on a combination of pineal parenchyma volume (high or low), both cyst and calcification present (yes or no), and melatonin level (high or low). The high and low categories were determined by grouping measurements above the median (high) and below the median (low) for pineal parenchyma volume (median = 122.6 mm3) and melatonin (median = 17.0 ng/mL) respectively. We calculated age-and creatinine-adjusted and multivariable-adjusted HRs and 95% CIs for the association between each category and total prostate cancer, setting low parenchyma, no calcifications or cysts, and low melatonin as the reference group.
Finally, we divided pineal secretion capacity (defined as 6-sulfatoxymelatonin (ng/mL) per parenchyma volume (mm3)) into tertiles and calculated the age-and creatinine-adjusted and multivariable-adjusted HRs and 95% CIs for total prostate cancer risk for each tertile to assess the potential role of pineal gland productivity, settling the lowest tertile as the reference group.
All analyses were conducted using SAS Version 9.4 software (SAS Institute, Cary, NC). The alpha level of significance was set at 0.05, and all p-values reported are two-sided.
Results
Pineal parenchyma, calcified, and cystic volume all varied (Table 1). We found a moderate, positive association between parenchyma volume and melatonin levels (Figure 1, r = 0.24, p-value <0.01).
Table 1:
Descriptive statistics for pineal gland components among men in the AGES-Reykjavik Study (n = 864)
| Volume (mm3) | Number (%) | Mean (SD) | Median | Range |
|---|---|---|---|---|
| Total volume | 864 (100.0) | 166.1 (93.4) | 146.9 | 32.9–1314.3 |
| Parenchyma volume | 864 (100.0) | 138.0 (64.6) | 122.6 | 32.9–646.8 |
| Calcified volume† | 247 (28.6) | 12.0 (11.4) | 8.3 | 0.7–81.6 |
| Cystic volume‡ | 629 (72.8) | 33.9 (42.4) | 25.5 | 1.6–665.6 |
247 men had calcifications present in the pineal gland.
629 men had cysts present in the pineal gland.
Figure 1:
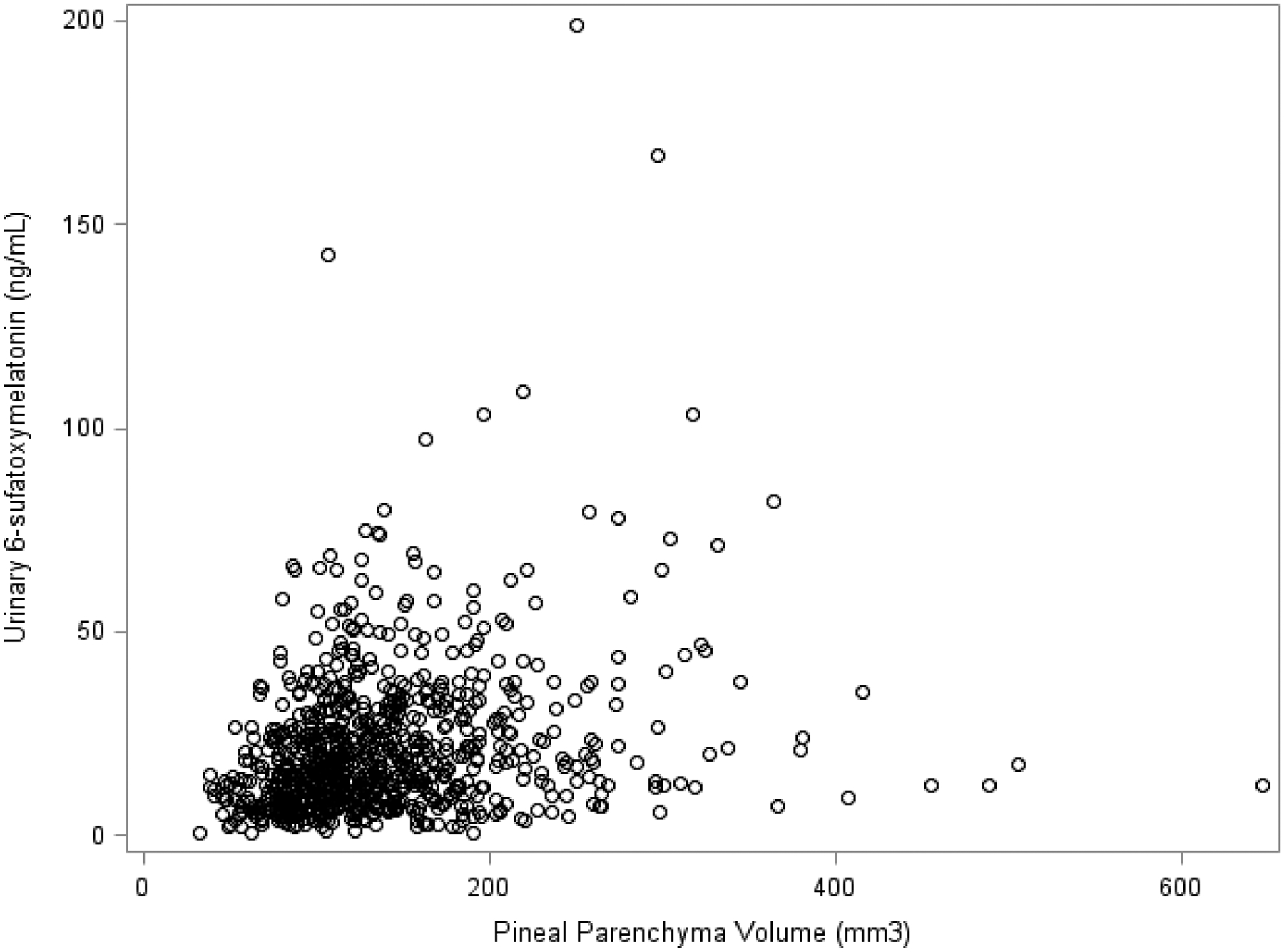
Correlation between pineal parenchyma volume and melatonin of men in the AGES-Reykjavik Study (n = 864)
Most participants had some combination of calcification and/or cysts (Table 2): While only 3 participants had calcifications, 28.2% had both calcifications and cysts and 44.6% had cysts. Pineal gland composition also varied across parenchyma volume tertile: participants in the lower tertile had lower frequency of glands with both calcifications and cysts (25.3%) than participants in the middle (30.2%) and upper (29.2%) tertiles. Participants in the lower tertile also had lower frequency of glands with just cysts (33.0%) compared to participants in the middle (46.2%) and upper (54.5%) tertiles. There was a positive association between parenchyma tertile and melatonin level. Participants in the lower tertile had a higher frequency of history of depression (9.0%) and benign prostate hyperplasia (BPH) (36.8%) compared to participants in the middle and upper tertiles. Men with pineal cyst and calcification and cyst had higher mean urinary 6-sulfatoxymelatonin levels compared to men without either pineal calcification or cyst (Supplemental Table 1).
Table 2:
Baseline characteristics of men in the AGES-Reykjavik Study by pineal parenchyma volume tertile (n = 864)
| Variable | Total (n = 864) |
T1†
32.9–105.2 mm3 (n = 288) |
T2†
105.3–146.5 mm3 (n = 288) |
T3†
146.6–646.8 mm3 (n = 288) |
|---|---|---|---|---|
| Mean age, years (SD) | 76.3 (5.3) | 76.6 (5.5) | 76.3 (4.9) | 75.9 (5.4) |
| Mean height, m (SD) | 1.7 (0.06) | 1.7 (0.06) | 1.7 (0.06) | 1.8 (0.06) |
| Mean body mass index, kg/m2 (SD) | 26.8 (3.8) | 26.8 (3.8) | 27.0 (3.7) | 26.6 (3.8) |
| Mean 6-sulfatoxymelatonin, ng/mL (SD) | 21.3 (18.0) | 15.7 (11.4) | 22.0 (16.4) | 26.4 (22.8) |
| Mean creatinine, mg/mL (SD) | 1.0 (0.4) | 1.0 (0.4) | 1.0 (0.4) | 1.0 (0.4) |
| Mean pineal secretion capacity‡, (SD) | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) | 0.1 (0.1) |
| Pineal gland composition | ||||
| Has no cyst or calcification, N (%) | 232 (26.8) | 119 (41.3) | 66 (22.9) | 47 (16.3) |
| Has calcification and cyst, N (%) | 244 (28.2) | 73 (25.3) | 87 (30.2) | 84 (29.2) |
| Has cyst only, N (%) | 385 (44.6) | 95 (33.0) | 133 (46.2) | 157 (54.5) |
| Has calcification only, N (%) | 3 (0.35) | 1 (0.35) | 2 (0.69) | 0 (0.0) |
| Family history of prostate cancer, N (%) | 98 (11.3) | 37 (12.8) | 27 (9.4) | 34 (11.8) |
| Benign prostate hyperplasia, N (%) | 286 (33.1) | 106 (36.8) | 82 (28.5) | 98 (34.0) |
| Missing | 14 (1.6) | 3 (1.0) | 6 (2.1) | 5 (1.7) |
| Smoking, N (%) | ||||
| Never | 233 (27.0) | 76 (26.4) | 81 (28.1) | 76 (26.4) |
| Former | 535 (61.9) | 182 (63.2) | 179 (62.1) | 174 (60.4) |
| Current | 96 (11.1) | 30 (10.4) | 28 (9.7) | 38 (13.9) |
| History of Diabetes, N (%) | 133 (15.4) | 46 (16.0) | 43 (14.9) | 44 (15.3) |
| Physical activity, N (%) | ||||
| Never | 189 (21.9) | 68 (23.6) | 64 (22.2) | 57 (19.8) |
| Rarely or occasionally | 334 (38.7) | 104 (36.1) | 111 (38.5) | 119 (41.3) |
| Moderate or high | 302 (34.9) | 107 (37.1) | 94 (32.6) | 101 (35.1) |
| Missing | 39 (4.5) | 9 (3.1) | 19 (6.6) | 11 (3.8) |
| Annual doctor’s checkup, N (%) | 713 (82.5) | 245 (85.1) | 237 (82.3) | 231 (80.2) |
| Education level completed, N (%) | ||||
| Primary school | 138 (16.0) | 41 (14.2) | 49 (17.0) | 48 (16.7) |
| Secondary school | 460 (53.2) | 158 (54.9) | 154 (53.5) | 148 (51.4) |
| College | 117 (13.5) | 35 (12.1) | 53 (18.4) | 29 (10.1) |
| University | 148 (17.1) | 53 (18.4) | 32 (11.1) | 63 (21.9) |
| Missing | 1 (0.1) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| History of depression, N (%) | 59 (6.8) | 26 (9.0) | 19 (6.6) | 14 (4.9) |
| Missing | 52 (6.0) | 19 (6.6) | 12 (4.2) | 21 (7.3) |
| Current beta-blocker use, N (%) | 292 (33.8) | 101 (35.1) | 99 (34.4) | 92 (31.9) |
| Missing | 87 (10.1) | 28 (9.7) | 36 (12.5) | 23 (8.0) |
| Sleep problems, N (%) | ||||
| Any sleep problems | 556 (64.3) | 190 (66.0) | 182 (63.2) | 184 (63.9) |
| Taking medications for sleep | 258 (29.9) | 92 (31.9) | 82 (28.5) | 84 (29.2) |
| Waking up at night | 338 (39.1) | 124 (43.1) | 108 (37.5) | 106 (36.8) |
| Problems falling asleep | 253 (29.3) | 77 (26.7) | 79 (27.4) | 97 (33.7) |
| Problems staying asleep | 264 (30.6) | 99 (34.4) | 80 (27.8) | 85 (29.5) |
| Characteristics of the prostate cancer cases (n = 144) | ||||
| Variable | Total Cases (n = 144) |
T1† 32.9–105.2 mm3 (n = 54) |
T2† 105.3–146.5 mm3 (n = 38) |
T3† 146.6–646.8 mm3 (n = 52) |
| Mean time to diagnosis, years (SD) | 3.5 (2.7) | 3.3 (2.3) | 3.5 (2.9) | 3.8 (2.8) |
| Stage at diagnosis, N (%) | ||||
| T1, T2 (N0, M0) | 108 (75.0) | 43 (79.6) | 27 (71.0) | 38 (73.1) |
| T3a (N0, M0) | 7 (4.9) | 2 (3.7) | 4 (10.5) | 1 (1.9) |
| T3b (N0, M0) | 3 (2.1) | 1 (1.8) | 0 (0.0) | 2 (3.8) |
| N1/M1 | 12 (8.3) | 3 (5.6) | 3 (7.9) | 6 (11.5) |
| Missing | 14 (9.7) | 5 (9.3) | 4 (10.5) | 5 (9.6) |
| Gleason Score, N (%) | ||||
| Grades 5–6 | 69 (47.9) | 29 (53.7) | 17 (44.7) | 23 (44.2) |
| Grade 7 (3+4) | 15 (10.4) | 7 (13.0) | 4 (10.5) | 4 (7.7) |
| Grade 7 (4+3) | 16 (11.1) | 7 (13.0) | 1 (2.6) | 8 (15.4) |
| Grades 8–10 | 36 (25.0) | 10 (18.5) | 13 (34.2) | 13 (25.0) |
| Missing | 8 (5.6) | 1 (1.8) | 3 (7.9) | 4 (7.7) |
| Prostate cancer death, N (%) | 27 (18.7) | 9 (16.7) | 7 (18.4) | 11 (21.2) |
T1 = lower tertile; T2 = middle tertile; T3= upper tertile
Pineal secretion capacity: 6-sulfatoxymelatonin (ng/mL) per parenchyma volume (mm3)
During follow-up, 144 men developed prostate cancer (Table 2). Most cases (75.0%) presented with T1 or T2 stage disease at diagnosis. Nearly half of the cases were low grade Gleason score 5–6 (47.9%). Mean time to diagnosis was 3.5 years (range: 0.03–11.5 years). Twenty-seven men died of prostate cancer during the follow-up period.
There were no significant associations between pineal parenchyma volume tertile and total, advanced, or high-grade prostate cancer (Table 3). Adjusting for sleep problems did not change the estimates, so we report the models excluding sleep problems for more parsimonious models. When we restricted the analysis to exclude cases diagnosed before one year of follow-up, we found a significant inverse association for the middle tertile compared to the lower tertile for total prostate cancer (HR: 0.55, 95% CI: 0.34, 0.89, p: 0.02; Supplemental Table 2). Associations were not significant for total pineal gland volume and prostate cancer risk (results not shown).
Table 3:
Hazard ratios and 95% confidence intervals of the association between pineal parenchyma volume and prostate cancer risk by parenchyma volume tertile (n = 864), AGES-Reykjavik Study
| Tertiles for pineal parenchyma volume | |||
|---|---|---|---|
| T1†
32.9–105.2 mm3 |
T2† 105.3–146.5 mm3 |
T3† 146.6–646.8 mm3 |
|
| Total prostate cancer | |||
| Prostate cancer cases/Participants | 54/288 | 38/288 | 52/288 |
| HR (95% CI) | |||
| Age-adjusted | Ref. | 0.67 (0.44, 1.02) | 0.94 (0.64, 1.38) |
| Multivariable adjusted‡ | Ref. | 0.67 (0.44, 1.02) | 0.96 (0.65, 1.42) |
| Advanced prostate cancer | |||
| Prostate cancer cases/Participants | 12/288 | 8/288 | 16/288 |
| HR (95% CI) | |||
| Age-adjusted | Ref. | 0.64 (0.26, 1.57) | 1.33 (0.63, 2.83) |
| Multivariable adjusted‡ | Ref. | 0.65 (0.26, 1.61) | 1.47 (0.69, 3.17) |
| High grade (Gleason 8–10) prostate cancer | |||
| Prostate cancer cases/Participants | 10/288 | 13/288 | 13/288 |
| HR (95% CI) | |||
| Age-adjusted | Ref. | 1.25 (0.55, 2.84) | 1.29 (0.56, 2.94) |
| Multivariable adjusted‡ | Ref. | 1.29 (0.56, 2.98) | 1.38 (0.60, 3.19) |
HR = hazard ratio; 95% CI = 95% confidence interval
T1 = lower tertile; T2 = middle tertile; T3= upper tertile
Multivariable-adjusted model adjusted for age, family history of prostate cancer, smoking status, physical activity (never, rarely/occasionally, moderate/high), history of depression, current beta-blocker use, and annual doctor’s exam.
p<0.05
Men with both pineal calcification and cysts, however, were 65% less likely to develop advanced prostate cancer compared to men who did not have cysts or calcifications (Supplemental Table 3: HR: 0.35, 95% CI: 0.12, 0.98, p: 0.05), with a similar but not statistically significant association for high-grade disease. We did not find significant associations for risk of advanced or high-grade prostate cancer comparing men with pineal cysts to men without pineal calcifications or cysts. Men with high parenchyma volume, both pineal cysts and calcifications, and low urinary 6-sulfatoxymelatonin levels had almost twice the risk of total prostate cancer compared to men with low parenchyma volume, no pineal calcifications or cysts, and low melatonin levels (Table 4: HR: 1.98, 95% CI: 1.02, 3.84, p: 0.04). These men also had the highest proportion of prostate cancer cases. There was no significant association found between pineal secretion capacity and risk of total prostate cancer when examining potential effect of pineal gland productivity on total prostate cancer risk, however (results not shown).
Table 4:
Hazard ratios and 95% confidence intervals of the association between pineal parenchyma volume, pineal gland composition, melatonin, and total prostate cancer risk (n = 861), AGES-Reykjavik Study
| Parenchyma volume† | Pineal calcifications and cysts | Melatonin level‡ | Total prostate cancer cases/participants | HR (95% CI) Age-and creatinine-adjusted |
HR (95% CI) Multivariable-adjusted§ |
|---|---|---|---|---|---|
| Low | No | Low | 30/193 | Ref. | Ref. |
| Low | No | High | 25/124 | 1.15 (0.66, 2.00) | 1.02 (0.58, 1.80) |
| Low | Yes | Low | 7/60 | 0.77 (0.34, 1.76) | 0.83 (0.36, 1.90) |
| Low | Yes | High | 7/54 | 0.74 (0.32, 1.70) | 0.67 (0.29, 1.54) |
| High | No | Low | 23/127 | 1.22 (0.71, 2.11) | 1.21 (0.70, 2.10) |
| High | No | High | 26/173 | 0.84 (0.49, 1.45) | 0.79 (0.45, 1.38) |
| High | Yes | Low | 13/50 | 1.97 (1.03, 3.80)* | 1.98 (1.02, 3.84)* |
| High | Yes | High | 11/80 | 0.79 (0.39, 1.59) | 0.79 (0.39, 1.61) |
HR = hazard ratio; 95% CI = 95% confidence interval
Pineal parenchyma volumes were dichotomized by the median for the subsample of 122.6 mm3: low is below or equal to the median and high is above the median.
Urinary 6-sulfatoxymelatonin levels were dichotomized by the median for the subsample 17.0 ng/mL: low is below or equal to the median and high is above the median.
Multivariable-adjusted model adjusted for age, family history of prostate cancer, smoking status, physical activity (never, rarely/occasionally, moderate/high), history of depression, current beta-blocker use, annual doctor’s exam, and creatinine (ng/mL).
p<0.05
Discussion
In this cohort of Icelandic men, we found a moderate, positive correlation between pineal parenchyma volume and melatonin level, confirming the association found in our previous study6. We did not find an overall association between parenchyma volume and risk of prostate cancer, but we did find a significant inverse association between the middle tertile and total prostate cancer risk in a sensitivity analysis excluding prostate cancer cases diagnosed less than year from baseline, contradicting our a priori hypothesis. When examining the effects of pineal parenchyma volume, presence of cysts and calcifications, and melatonin levels, we found men with high parenchyma volume, both pineal cysts and calcifications, and low urinary 6-sulfatoxymelatonin had nearly twice the risk of developing prostate cancer compared to men with low parenchyma volume, no calcifications or cysts, and low urinary 6-sulfatoxymelatonin.
To our knowledge, this is the first study examining the role of the pineal gland specifically in prostate cancer risk. Literature has indicated circadian disruption10–12 and melatonin levels15,16 may be important in the development of prostate cancer, suggesting the potential importance of the pineal gland in relation to melatonin production. Experimental models also have found a dose-dependent association between melatonin suppression due to pinealectomy and tumor growth19,20, and the pineal gland has been shown to be important in mammary tumor risk in rodents because of its role in melatonin production21–23. We did not find a significant association between parenchyma volume and prostate cancer, but we did find a significant inverse association for the middle parenchyma tertile when early cases were excluded, suggesting unmeasured circadian factors may be influencing prostate cancer risk. However, we did not find an association between pineal gland productivity and total prostate cancer risk. Because of the novelty of our study, the interpretation of these results is unclear. While a functional pineal gland is important to maintain melatonin production, volume of the pineal gland may be less important in prostate cancer risk than hypothesized. Additional studies are necessary to discern the role of pineal parenchyma volume in prostate cancer risk.
Other individual-level factors may be important in influencing melatonin levels in addition to parenchyma volume, which is supported by our finding of only a moderate correlation between pineal parenchyma volume and urinary melatonin levels. Light exposure late in the evening and at night directly suppresses melatonin24, and light exposure the night before the sample may have affected overall level in the morning void.
It is difficult to interpret our results on the role of pineal calcification and cyst in prostate cancer risk because of limited information available in the literature. No studies to date have found a clear relationship between pineal calcification and melatonin production2,3. Furthermore, the small number of participants with only pineal calcification (N=3) in our study prevented us from assessing the effect of pineal calcification alone on prostate cancer risk. Pineal cysts potentially influence melatonin production; however, this has only been observed in a case report and in another small patient sample4,5. In support of this, we found men with pineal cysts and/or calcifications had higher mean melatonin than men without pineal calcification or cyst. We also observed men with calcification and cyst had lower risk for advanced prostate cancer, compared to men with no calcifications or cysts. However, in our analysis examining the combined association of pineal gland volume, composition, and melatonin level on prostate cancer risk, we did not find a significant inverse association for participants with pineal calcifications and cysts and high levels of melatonin. Because this relationship has not been examined before, the significance of these findings is unclear. Additionally, the number of prostate cancer cases for each category in this analysis were small (range: 7–30 cases), so we were likely underpowered to detect differences in risk between each group. Adequately powered studies assessing the relationship between pineal cyst and calcification and melatonin production are needed to determine the role of these components on melatonin production and prostate cancer risk.
A strength of our study is the prospective nature of the design, reducing the chance of reverse causation. Furthermore, in the sensitivity analysis excluding cases diagnosed within a year of pineal gland assessment, we found a significant inverse association between the middle tertile of parenchyma volume and prostate cancer risk, compared with the lower, although this was based on small numbers. The AGES-Reykjavik Study cohort includes detailed information on a variety of sociodemographic factors, dietary and lifestyle factors, and biospecimen measurements17, allowing adjustment for most possible confounders. Our main outcomes, prostate cancer diagnosis and cause of death, obtained from the Icelandic Cancer Registry and Statistics Iceland respectively, had a high degree of reliability24, and most of the prostate cancer cases had available information on stage and Gleason grade.
Our study has several limitations to consider: Pineal gland components were determined through physician and radiologist assessment, which may raise concern about the objectivity of the component measurements. As mentioned previously, the inter-rater reliability of pineal measurements was assessed and determined to be reliable and was conducted blinded to case status6. The cohort also has several limitations: We did not have information for occupation and shift work for this cohort, so we were unable to adjust for the effect of these factors in our models. The sample size of the cohort was also small, with a limited number of prostate cancer cases (N=144) and deaths (N=27), with only 44 of the prostate cases occurring after 1 year of follow-up. Because of the size of the cohort and the small number of prostate cancer cases, our study may have lacked adequate power to sufficiently detect an association between prostate cancer risk and pineal gland characteristics. Finally, because this cohort included mostly white Icelandic men, the results observed may not be generalizable to all populations.
Conclusions
Although our results suggest pineal parenchyma volume may not be crucial in the development of prostate cancer, pineal gland composition and melatonin levels together may influence prostate cancer risk through mechanisms not yet comprehensively assessed. To our knowledge, this is the first study to examine pineal parenchyma volume and prostate cancer risk; therefore, more studies examining the relationship between pineal parenchyma volume, pineal gland composition, and melatonin production are needed to better understand the role of the pineal gland in prostate cancer risk.
Supplementary Material
Funding:
This work was supported by funding from the National Cancer Institute of the National Institutes of Health (P50 CA 090381-15 to SCM, LAM; R01 CA202690 to SCM, LAM, UA), and the Prostate Cancer Foundation (LAM). The AGES-Reykjavik study is supported by the Intramural Research Program of the National Institute on Aging (contract N01 AG 12100), the Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). In addition, this study was supported by funding from the Harvard Catalyst Award and the Icelandic Cancer Society.
Footnotes
Data Availability: All data are from the AGES-Reykjavik study and can be obtained upon application
Conflict of interest: The authors have no proprietary or commercial interest in any materials discussed in this article.
Ethical approval The protocol for the AGES-Reykjavik Study is approved by the Icelandic Data Protection Authority, the National Bioethics Committee in Iceland, and the National Institute on Aging Institutional Review Board. The protocol for this study was approved by the Institutional Review Boards at the Harvard T.H. Chan School of Public Health and Michigan Public Health.
Informed consent Informed consent was obtained from all participants.
Consent to publish The study is approved by the AGES-Reykjavik steering committee.
References
- 1.Sapède D, Cau E. The pineal gland from development to function. Curr Top Dev Biol. 2013;106:171–215. doi: 10.1016/B978-0-12-416021-7.00005-5 [DOI] [PubMed] [Google Scholar]
- 2.Tan DX, Xu B, Zhou X, Reiter RJ. Pineal Calcification, Melatonin Production, Aging, Associated Health Consequences and Rejuvenation of the Pineal Gland. Mol J Synth Chem Nat Prod Chem. 2018;23(2). doi: 10.3390/molecules23020301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Commentz JC, Fischer P, Stegner H, Winkler P, Helmake K, Willig RP. Pineal calcification does not affect melatonin production. J Neural Transm. 1986;21:481–502. [Google Scholar]
- 4.Ferri L, Filardi M, Moresco M, et al. Non-24-Hour Sleep-Wake Rhythm Disorder and Melatonin Secretion Impairment in a Patient With Pineal Cyst. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2017;13(11):1355–1357. doi: 10.5664/jcsm.6816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Májovský M, Řezáčová L, Sumová A, et al. Melatonin and cortisol secretion profile in patients with pineal cyst before and after pineal cyst resection. J Clin Neurosci Off J Neurosurg Soc Australas. 2017;39:155–163. doi: 10.1016/j.jocn.2017.01.022 [DOI] [PubMed] [Google Scholar]
- 6.Sigurdardottir LG, Markt SC, Sigurdsson S, et al. Pineal Gland Volume Assessed by MRI and Its Correlation with 6-Sulfatoxymelatonin Levels among Older Men. J Biol Rhythms. 2016;31(5):461–469. doi: 10.1177/0748730416656948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunz D, Schmitz S, Mahlberg R, et al. A new concept for melatonin deficit: on pineal calcification and melatonin excretion. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 1999;21(6):765–772. doi: 10.1016/S0893-133X(99)00069-X [DOI] [PubMed] [Google Scholar]
- 8.Liebrich L-S, Schredl M, Findeisen P, Groden C, Bumb JM, Nölte IS. Morphology and function: MR pineal volume and melatonin level in human saliva are correlated. J Magn Reson Imaging JMRI. 2014;40(4):966–971. doi: 10.1002/jmri.24449 [DOI] [PubMed] [Google Scholar]
- 9.Nölte I, Lütkhoff A-T, Stuck BA, et al. Pineal volume and circadian melatonin profile in healthy volunteers: an interdisciplinary approach. J Magn Reson Imaging JMRI. 2009;30(3):499–505. doi: 10.1002/jmri.21872 [DOI] [PubMed] [Google Scholar]
- 10.Wendeu-Foyet MG, Menegaux F. Circadian Disruption and Prostate Cancer Risk: An Updated Review of Epidemiological Evidences. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2017;26(7):985–991. doi: 10.1158/1055-9965.EPI-16-1030 [DOI] [PubMed] [Google Scholar]
- 11.Sigurdardottir LG, Valdimarsdottir UA, Fall K, et al. Circadian disruption, sleep loss, and prostate cancer risk: a systematic review of epidemiologic studies. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2012;21(7):1002–1011. doi: 10.1158/1055-9965.EPI-12-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigurdardottir LG, Valdimarsdottir UA, Mucci LA, et al. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2013;22(5):872–879. doi: 10.1158/1055-9965.EPI-12-1227-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickerman BA, Markt SC, Koskenvuo M, et al. Sleep disruption, chronotype, shift work, and prostate cancer risk and mortality: a 30-year prospective cohort study of Finnish twins. Cancer Causes Control CCC. 2016;27(11):1361–1370. doi: 10.1007/s10552-016-0815-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markt SC, Grotta A, Nyren O, et al. Insufficient Sleep and Risk of Prostate Cancer in a Large Swedish Cohort. Sleep. 2015;38(9):1405–1410. doi: 10.5665/sleep.4978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartsch C, Bartsch H, Schmidt A, Ilg S, Bichler KH, Flüchter SH. Melatonin and 6-sulfatoxymelatonin circadian rhythms in serum and urine of primary prostate cancer patients: evidence for reduced pineal activity and relevance of urinary determinations. Clin Chim Acta Int J Clin Chem. 1992;209(3):153–167. doi: 10.1016/0009-8981(92)90164-l [DOI] [PubMed] [Google Scholar]
- 16.Sigurdardottir LG, Markt SC, Rider JR, et al. Urinary melatonin levels, sleep disruption, and risk of prostate cancer in elderly men. Eur Urol. 2015;67(2):191–194. doi: 10.1016/j.eururo.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165(9):1076–1087. doi: 10.1093/aje/kwk115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinforma Oxf Engl. 2003;19(2):185–193. doi: 10.1093/bioinformatics/19.2.185 [DOI] [PubMed] [Google Scholar]
- 19.Toma JG, Amerongen HM, Hennes SC, O’Brien MG, McBlain WA, Buzzell GR. Effects of olfactory bulbectomy, melatonin, and/or pinealectomy on three sublines of the Dunning R3327 rat prostatic adenocarcinoma. J Pineal Res. 1987;4(3):321–338. doi: 10.1111/j.1600-079x.1987.tb00870.x [DOI] [PubMed] [Google Scholar]
- 20.Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13(4):257–264. doi: 10.1016/j.smrv.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 21.Blask DE, Dauchy RT, Sauer LA. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine. 2005;27(2):179–188. doi: 10.1385/ENDO:27:2:179 [DOI] [PubMed] [Google Scholar]
- 22.Tamarkin L, Cohen M, Roselle D, Reichert C, Lippman M, Chabner B. Melatonin inhibition and pinealectomy enhancement of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in the rat. Cancer Res. 1981;41(11 Pt 1):4432–4436. [PubMed] [Google Scholar]
- 23.Aubert C, Janiaud P, Lecalvez J. Effect of pinealectomy and melatonin on mammary tumor growth in Sprague-Dawley rats under different conditions of lighting. J Neural Transm. 1980;47(2):121–130. doi: 10.1007/BF01670163 [DOI] [PubMed] [Google Scholar]
- 24.Gooley JJ, Chamberlain K, Smith KA, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96(3):E463–472. doi: 10.1210/jc.2010-2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sigurdardottir LG, Jonasson JG, Stefansdottir S, et al. Data quality at the Icelandic Cancer Registry: Comparability, validity, timeliness and completeness. Acta Oncol. 2012;51(7):880–889. doi: 10.3109/0284186X.2012.698751 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


