Abstract
Gametogenesis, the formation of gametes from gametocytes, an essential step for malaria parasite transmission, is targeted by transmission-blocking drugs and vaccines. We identified a conserved protein (PBANKA_0305900) in Plasmodium berghei, which encodes a protein of 22 kDa (thus named Pb22) and is expressed in both asexual stages and gametocytes. Its homologs are present in all Plasmodium species and its closely related Hepatocystis, but not in other apicomplexans. Pb22 protein was localized in the cytosols of schizonts, as well as male and female gametocytes. During gamete-to-ookinete development, Pb22 became localized on the plasma membranes of gametes and ookinetes. Compared to the wild-type (WT) parasites, P. berghei with pb22 knockout (KO) showed a significant reduction in exflagellation (~89%) of male gametocytes and ookinete number (~97%) during in vitro ookinete culture. Mosquito feeding assays showed that ookinete and oocyst formation of the pb22-KO line in mosquito midguts was almost completely abolished. These defects were rescued in parasites where pb22 was restored. Cross-fertilization experiments with parasite lines defective in either male or female gametes confirmed that the defects in the pb22-KO line were restricted to the male gametes, whereas female gametes in the pb22-KO line were fertile at the WT level. Detailed analysis of male gametogenesis showed that 30% of the male gametocytes in the pb22-KO line failed to assemble the axonemes, whereas ~48.9% of the male gametocytes formed flagella but failed to egress from the host erythrocyte. To explore its transmission-blocking potential, recombinant Pb22 (rPb22) was expressed and used to immunize mice. In vitro assays showed that the rPb22-antisera significantly inhibited exflagellation by ~64.8% and ookinete formation by ~93.4%. Mosquitoes after feeding on rPb22-immunized mice also showed significant decreases in infection prevalence (83.3–93.3%) and oocyst density (93.5–99.6%). Further studies of the Pb22 orthologs in human malaria parasites are warranted.
Keywords: Plasmodium berghei, exflagellation, sexual development, cross fertilization, transmission-blocking vaccine
1. INTRODUCTION
Malaria is caused by unicellular parasites of the Plasmodium genus that are transmitted to humans by Anopheles mosquitoes. In the last decade, a significant decrease in the global morbidity and mortality of malaria has been achieved through expanded funding for malaria interventions such as long-lasting insecticide-treated bed nets and artemisinin combination therapies. However, the control efforts are thwarted by the emergence of insecticide-resistant mosquitoes and drug-resistant parasites (WHO, 2019). Transmission-blocking vaccines (TBVs) are a promising strategy for effectively reducing malaria transmission and achieving the goal of malaria elimination (Nilsson et al., 2015). Currently, only a few parasite-derived proteins such as P25, P28, P48/45, P230, and HAP2/GCS1 are considered lead TBV candidates. Thus, efforts to discover novel TBV antigens are urgently needed.
In the life cycle of the Plasmodium parasites, gametocytes formed in the human blood are the sexual precursor stage needed for transmission to the mosquitoes. Once ingested by susceptible mosquitoes, gametocytes in the blood meal, in response to environmental changes such as a drop in temperature, an increase of pH, and the presence of xanthurenic acid, transform into gametes (Carter et al., 1977, Nijhout, 1979, Billker et al., 2000, Kehrer et al., 2016). Whereas each female gametocyte forms a single immotile macrogamete, a male gametocyte generates up to eight flagella-like microgametes in a process called “exflagellation”. The egress of fully differentiated gametes from the erythrocyte requires the lysis of two membranes surrounding the parasite: the inner parasitophorous vacuole membrane (PVM) and the outer erythrocyte membrane (Sologub et al., 2011, Deligianni et al., 2013). The activated gametocytes emerging from the erythrocyte follow a sequential, inside-out mode, in which rupture of PVM occurs during the first minutes after gametocyte activation, while the rupture of erythrocyte membrane occurs several minutes later (Andreadaki et al., 2018). Subsequently, the micro- and macro-gametes fuse to form a diploid zygote, which transforms into a motile ookinete that penetrates the midgut epithelium to form an oocyst (Paul et al., 2002, Bousema et al., 2011). A better understanding of the molecular mechanisms of sexual development of the malaria parasites will help identify novel targets for blocking transmission.
TBV targets include parasite proteins that are expressed in sexual stages and mosquito midgut proteins (Wu et al., 2015). So far, a handful of proteins expressed during sexual stages have been evaluated for their transmission-blocking (TB) potential. The gamete-specific surface proteins P48/45 and HAP2, required for gamete adhesion and fusion, have been identified for the pre-fertilization phase. HAP2 is localized on the plasma membrane of microgametocytes and microgametes and has demonstrated potent TB efficacy in the rodent malaria parasite P. berghei and human malaria parasite P. falciparum. Anti-P. berghei HAP2 serum significantly inhibits ookinete and oocyst formation but does not affect microgametocyte exflagellation (Liu et al., 2008, Blagborough et al., 2009). P48/45 belongs to the 6-Cys protein family and is expressed on the surface membranes beginning in gametocyte stages and lasting through zygote stages in the mosquito midgut (van Dijk et al., 2001, van Dijk et al., 2010). Sera from mice immunized with the full-length recombinant Pfs48/45 protein display nearly complete inhibition of oocyst formation (Chowdhury et al., 2009). Another 6-Cys protein P230, exclusively expressed on the P. falciparum gametes, physically interacts with Pfs48/45 (Kumar, 1987, Eksi et al., 2002). Antibodies against Pfs230 can reduce oocyst development by lysing gametes in a complement-dependent manner (Read et al., 1994, Healer et al., 1997, Eksi et al., 2006). The P. yoelii microgamete surface protein (PyMiGS), localized to the male osmiophilic bodies (OBs) in male gametocytes and the surface of microgametes, though dispensable for male gametocyte egress from red blood cells (RBCs), plays an important role in the exflagellation process. Antibodies against PyMiGS severely reduced the formation of oocysts (Tachibana et al., 2018). After fertilization, P25 and P28 are both expressed on the surfaces of zygotes and ookinetes, and antibodies against the P. falciparum protein Pfs25 elicit long-lasting TB activity (Tomas et al., 2001). The limited number of TBV candidates in the vaccine development pipeline demands further efforts in antigen discovery.
The advancement in “omics” technology opens an unprecedented opportunity for vaccine discovery. Previous proteomic analysis characterized the proteomes of the P. berghei parasites in mosquito stages. Based on in silico prediction, 506 of 624 proteins have been described for both cellular localization and function in the microgametes, among which 103 are transmembrane proteins and 129 extracellular or membrane-associated (Hall et al., 2005, Wass et al., 2012). Since membrane or surface localization in the sexual stages is a critical parameter for TBV prediction, it is logical to explore these proteins as novel TBV targets. Among these latter proteins, the P. berghei gene PBANKA _0305900 (designated hitherto as Pb22 based on the molecular weight of the encoded protein) is expressed in asexual stages and gametocytes (plasmodb.org), and is dispensable for the asexual intraerythrocytic development in a large-scale gene knockout (KO) study in P. berghei (Fonager et al., 2012). Here, we pursued the functional analysis of this gene during parasite development and assessed its TB potential using both in vitro and in vivo assays.
2. RESULTS
2.1. Pb22 is a conserved in Plasmodium and Hepatocystis
Proteomic analyses of male and female gametocytes in P. berghei (Khan et al., 2005) and P. falciparum (Lasonder et al., 2016, Miao et al., 2017) identified many proteins with putative signal peptides suggestive of secreted proteins. Here, we characterized one such gene in P. berghei, PBANKA_0305900 (Pb22), which is predicted to encode a sexual-stage protein of 218 amino acids (aa) with a molecular weight of 22 kDa. Pb22 has a putative signal peptide (aa 1–18), suggesting that it might be secreted (Figure S1A). Pb22 did not contain any domains of known functions, while the SMART program (http://smart.embl-heidelberg.de/) detected two low-complexity regions spanning aa 63–75 and aa 166–185, which contain compositionally biased amino acids (Wootton, 1994). P22 is highly conserved among Plasmodium species (Figure S1B). BLAST search also identified a homolog of this protein in Hepatocystis, which is considered to be descended from Plasmodium with the loss of blood schizogony (Aunin et al., 2020). Transcriptomic analysis of asexual stages and separated male and female gametocytes showed that pb22 is expressed in both asexual stages and gametocytes, while in gametocytes pb22 expression level is about five times higher in female than male gametocytes (Yeoh et al., 2017). Consistent with pb22 expression in asexual stages, the Pb22 protein was identified in proteomic analysis of proteins in membrane fractions of schizont-infected RBCs (Fonager et al., 2012). Its ortholog in P. falciparum PF3D7_0208800 was also predominantly expressed in gametocytes, but mass spectrometry analysis only identified this protein in female gametocytes (Lasonder et al., 2016, Miao et al., 2017).
We first attempted to confirm these earlier results about Pb22 expression. To generate antibodies against Pb22, we expressed the recombinant Pb22 (rPb22) protein in Escherichia coli Rosetta-gami B (DE3) and purified it by using Ni-NTA affinity chromatography. Analysis on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel showed that the purified rPb22 protein was relatively pure and with an estimated molecular weight of 21.9 kDa, which is in accord with its predicted size (Figure 1A). Pb22 is predicted to contain seven antigenic peptides based on the B cell epitope prediction program (http://imed.med.ucm.es) (Figure S1C). Mice immunized with rPb22 developed a strong immune response with rPb22-specific antibody titer reaching 1:256000 after the final booster (Figure S2). As a control, we also expressed and purified a recombinant glutathione S-transferase (GST) protein (Figure 1A) and immunized mice with GST (Figure S2A, B).
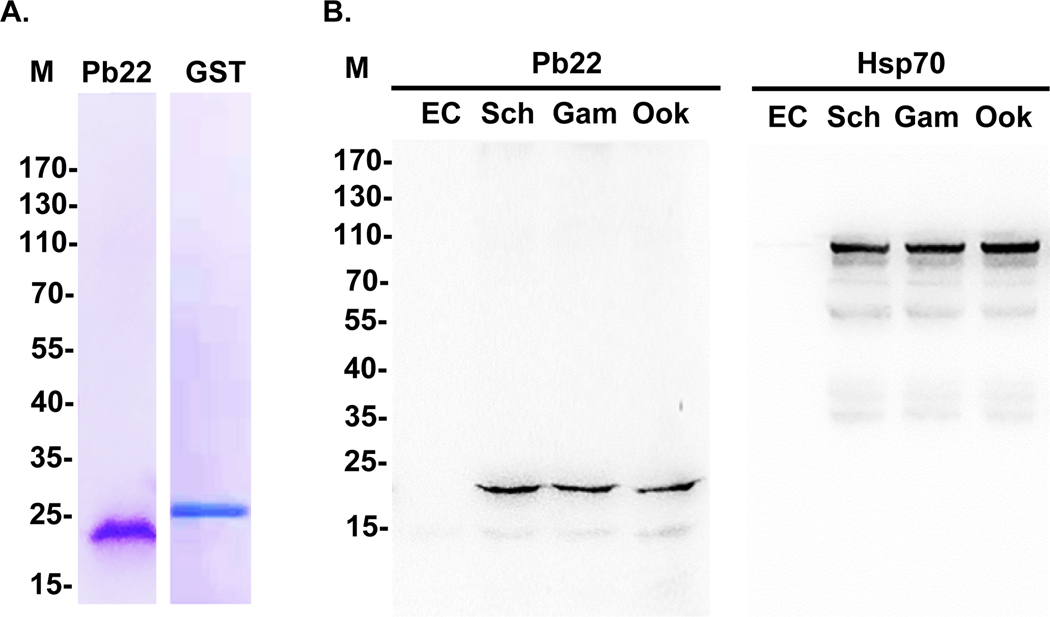
FIGURE 1. Purification of the rPb22 protein and detection of Pb22 expression during development.
A. Purified recombinant Pb22 and GST protein were separated on 10% SDS-PAGE gels and stained with Coomassie brilliant blue. B. Western blot analysis of protein extracts from purified schizonts (Sch), gametocytes (Gam) and ookinetes (Ook) of WT P. berghei parasites and uninfected erythrocytes (EC). The blots were probed with the anti-rPb22 antisera (Pb22, left panel), while protein loading was estimated by the anti-rHsp70 sera (Hsp70, right panel). M, molecular markers in kDa.
To confirm the specificity of anti-rPb22 sera, we purified schizonts, gametocytes, and ookinetes of the wild-type (WT) parasites (Figure S3). Western blotting of lysates from these purified parasites showed that the anti-rPb22 antisera recognized a band of approximately 22 kDa (Figure 1B). No signal was detected in uninfected erythrocyte lysates. Pb22 protein expression levels appeared similar in these three stages.
For the cross-validation purpose, we generated a Pb22-HA-tagged parasite line (Figure S4A). Integration-specific PCR showed the correct tagging of Pb22 in the transgenic line (Figure S4B). Western blot using lysates from purified schizonts, gametocytes, and ookinetes (Figure S3) of the Pb22-HA line probed with anti-HA monoclonal antibody (mAb) again detected the ~22 kDa band in each stage analyzed (Figure S4C). No bands were detected by the anti-HA mAb with lysates from WT gametocytes and uninfected RBCs.
2.2. Pb22 is expressed in both asexual and sexual stages and associated with plasma membranes of gametes and ookinetes
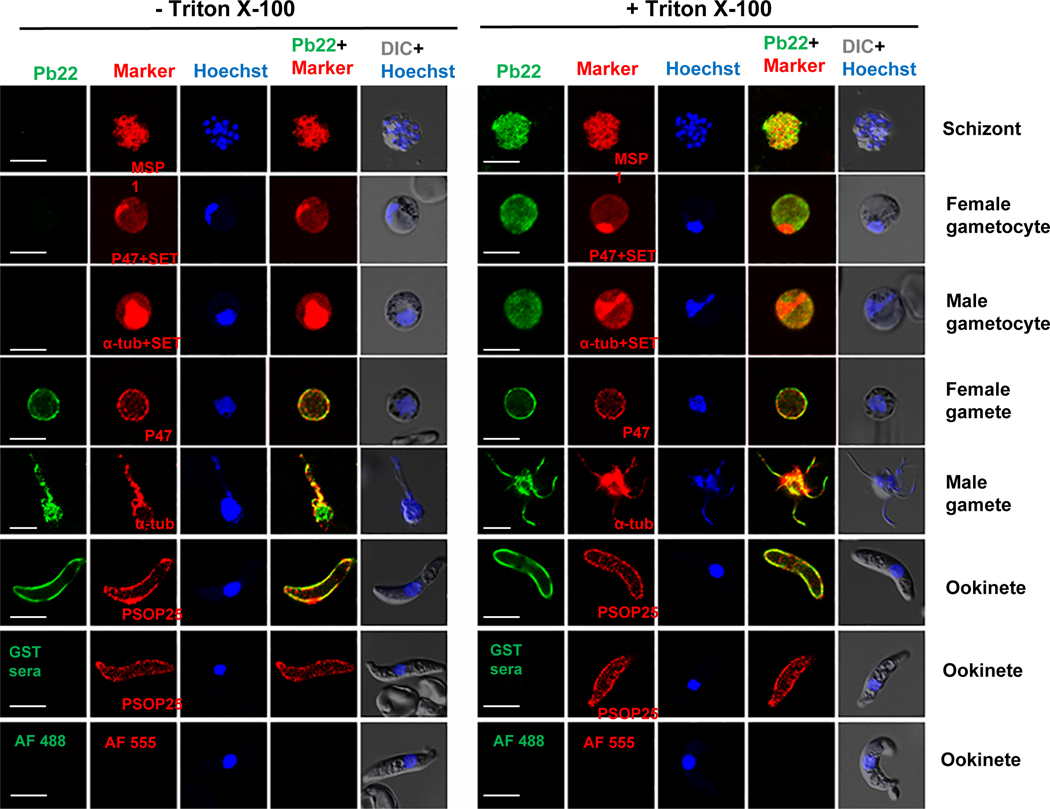
To further characterize Pb22 protein expression, an indirect immunofluorescence assay (IFA) was performed on schizonts, female and male gametocytes, female and male gametes, and ookinetes. The control sera against GST as well as secondary antibodies alone did not produce any detectable staining of ookinetes (Figure 2). In the erythrocytic stages, Pb22 was restricted to the parasites in schizonts and gametocytes, and fluorescence was observed in these stages only after membrane permeabilization (Figure 2). The detection of Pb22 in membrane components of schizonts-infected RBCs in a proteomic study suggests an association of the Pb22 with membrane structure in schizonts (Fonager et al., 2012). Despite that the pb22 mRNA was much more abundant in female than in male gametocytes (Yeoh et al., 2017), IFA revealed abundant expression of the Pb22 protein in both male and female gametocytes. To differentiate male and female parasites, we used a female-specific marker P47 and male-specific marker α-tubulin II to stain gametocytes and gametes (Figure 2). It is noteworthy that anti-α-tubulin antibodies stained both male and female gametocytes, but the fluorescent signal was much stronger in male gametocytes (Figure S5). We also used antibodies against a nuclear marker, the SET protein, which strongly accumulates in male gametocytes, to differentiate the sex of gametocytes (Pace et al., 2006). In the exflagellating male gametes, fluorescence was observed on the residual body as well as the flagella. Consistent with Pb22 as a potentially secreted protein, strong fluorescence was observed on gametes and ookinetes even without membrane permeabilization (Figure 2), indicating that Pb22 was on the plasma membranes of these stages. Transcriptomic and proteomic analyses also detected the orthologs of Pb22 (PVX_003895 in P. vivax and PF3D7_0208800 in P. falciparum) in salivary gland sporozoites (Swearingen et al., 2016, Zanghi et al., 2018, Vivax Sporozoite, 2019).
FIGURE 2. Localization of Pb22 protein by IFA.
Representative photomicrographs of fluorescent staining in WT parasites at different developmental stages. The parasites were incubated with anti-rPb22 (1:500) as the primary antibodies (green) without (left panel, -Triton X-100) or with (right panel, +Triton X-100) membrane permeabilization. The parasites were also labeled with antibodies against the markers for different stages (red) after membrane permeabilization. Makers include PbMSP1 for schizonts, P47 for female gametocytes and gametes, α-tubulin II (α-tub) for male gametocytes and gametes, SET for nuclei of gametocytes, and PSOP25 for ookinetes. Alexa Fluor 488 (AF488)-conjugated goat anti-mouse IgG antibodies and Alexa Fluor 555 (AF555)-conjugated goat-anti-rabbit IgG antibodies were used as the secondary antibodies. WT ookinetes labeled with anti-GST sera and with the secondary antibodies only were used as negative controls. Nucleus was stained with Hoechst-33258 (blue). Images were obtained under the same conditions at a magnification of 1000×. DIC, differential interference contrast microscopy. Scale bar = 5 μm.
To verify these observations on Pb22 expression and localization, we performed IFA using transgenic parasites with HA-tagged Pb22. A similar fluorescent pattern was observed with the Pb22-HA parasites probed with the anti-HA monoclonal antibody (Figure S6). As negative controls, no fluorescence was detected in HA-tagged ookinetes probed with only the Alexa Fluor 488- and 555-labeled secondary antibodies or in WT ookinetes probed with the anti-HA monoclonal antibody (Figure S6). We also used the female-specific marker G377 of the OBs and cytoplasmic marker Hsp70 to further define the localization of Pb22. IFA with these markers confirmed Pb22 localization in the cytoplasm of gametocytes (Figure S7). These data collectively showed that Pb22 was expressed in schizonts, gametocytes, gametes, and ookinetes, and was associated with the plasma membrane of gametes and ookinetes.
2.3. Pb22 deletion results in a significant reduction in infectivity to mosquitoes
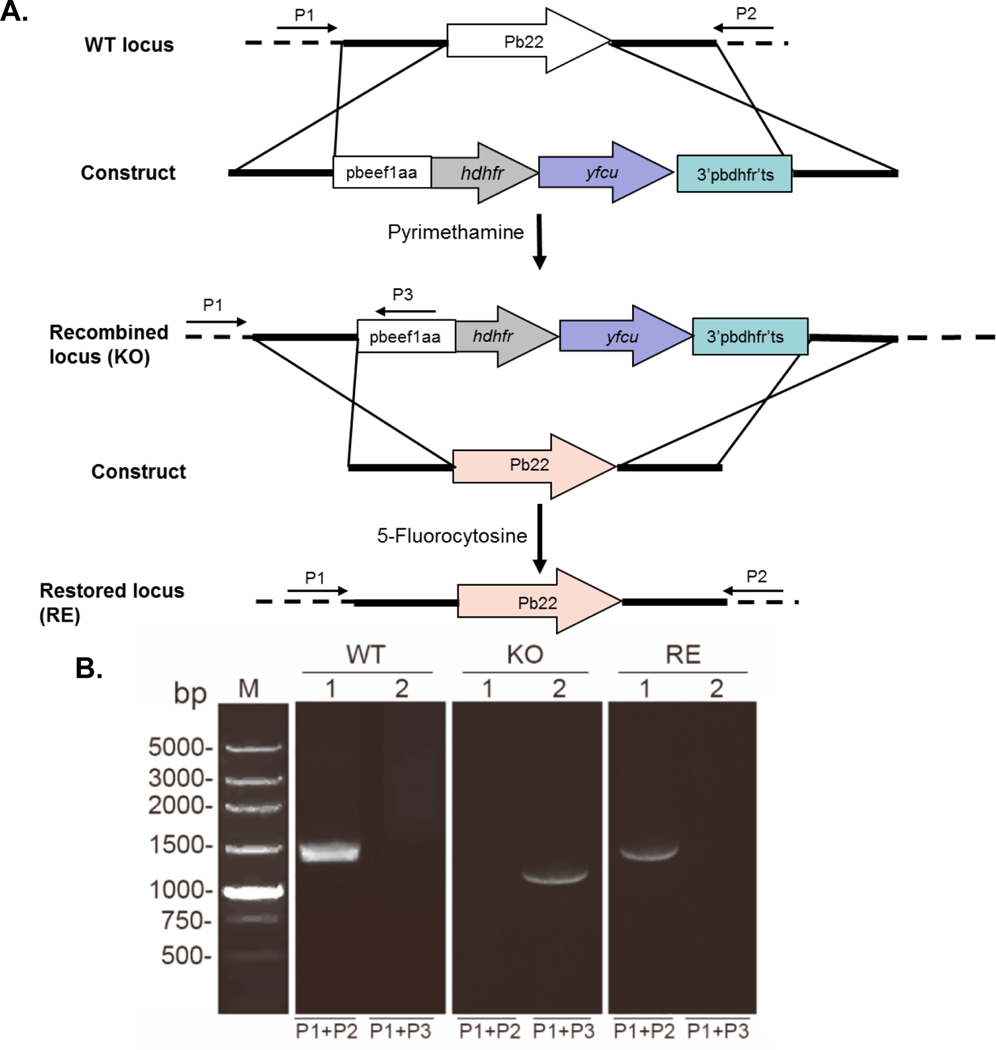
Pb22 was shown to be dispensable for asexual erythrocytic development during a large-scale functional screen (Fonager et al., 2012). To study its functions during P. berghei parasite development, we generated a Pb22 KO line (Figure 3A). To verify that defects in the pb22-KO line were indeed due to pb22 deletion, a pb22-restored (RE) line was then generated using the ‘gene insertion/marker out’ (GIMO) strategy after selection with 5-fluorocytosine (Lin et al., 2011) (Figure 3A). Diagnostic PCR was used to verify independent pb22 deletion and restoration clones (Figure 3A, B). Western blot and IFA analysis using gametocytes purified from different parasite lines further confirmed pb22 deletion in the pb22-KO line and pb22 restoration in the pb22-RE line (Figure S8A, B).
FIGURE 3. Generation of pb22 knockout and restoration parasites.
A. Schematic representation of the WT pb22 locus, the transfection construct, the recombined locus and reconstructed locus. The construct contains a dhfr expression cassette, directed by the pbeef1aa promoter, for selection of the pb22 knockout (KO) parasites with pyrimethamine and yfcu expression cassette for selection of the pb22-restored (RE) parasites with 5-fluorocytosine. Primers used to detect pb22 deletion and restoration are marked. B. Diagnostic PCR of WT, KO and RE parasites. Lane 1, primers 1 + 2 (1449 bp); Lane 2: primer 1 + 3 (1180 bp).
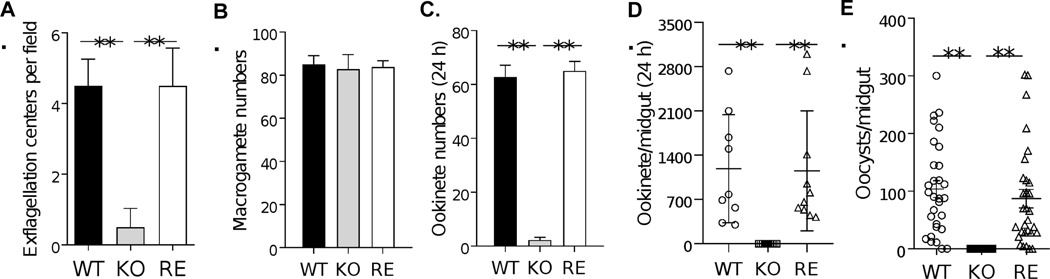
Although Pb22 is expressed in schizonts, pb22 deletion did not result in significant changes in asexual growth of the parasites (Figure S9A), a finding that agrees with the earlier pb22-deletion study (Fonager et al., 2012). In addition, pb22 deletion did not affect the formation of either male or female gametocytes, as gametocytemia and female/male ratio were all comparable among pb22-KO, pb22-RE, and the WT parasites (P > 0.05; Figure S9B, C). To determine whether pb22 KO affects subsequent sexual development, we performed both in vitro ookinete culture and mosquito feeding experiments using WT, pb22-KO, and pb22-RE parasites. In vitro analysis showed that pb22-KO male gametocytes showed a ~89% reduction in the exflagellation centers formed as compared to the WT control (P < 0.0001; Figure 4A). To differentiate the female gametes from gametocytes, we performed labeling of the RBC membrane with the TER-119 antibody, and labeling of female gametocytes and gametes with the anti-P47a antiserum. We found that in the WT, pb22-KO, and pb22-RE parasite lines, macrogamete numbers were similar between the pb22-KO and WT (P > 0.05; Figure 4B), and there was no defect in the formation and egress of female gametes in the pb22-KO line (Figure 5A, B). In vitro ookinete culture assay showed that the ookinete number in pb22-KO line was reduced by ~97% compared to WT control (P < 0.0001; Figure 4C). To determine whether pb22 deletion impacted subsequent development in mosquitoes, the pb22-KO and pb22-RE clones and WT parasites were used to infect mice, and mosquito feeding was conducted. After blood feeding, ookinete formation in the blood boluses of mosquito midguts was assessed at 24 h, while oocyst number was determined at 12 days. Similar to the in vitro assay, ookinete formation was almost completely blocked in the mosquitoes feeding on the pb22-KO parasite-infected mice with only one midgut in 30 midguts dissected containing 5 ookinetes, as compared to 1187–2280 ookinetes/midgut in mosquitoes feeding on WT P. berghei-infected mice (Figure 4D, Table 1). Furthermore, pb22 deletion completely blocked the formation of oocysts in the midguts, and none of the mosquitoes feeding on mice infected with the pb22-KO parasite developed oocysts (P < 0.0001; Figure 4E, Table 1). In comparison, all defects in exflagellation of male gametocytes, ookinete and oocyst formation were effectively restored in the pb22-RE line (Figure 4, Table 1), indicating that these defects were indeed due to pb22 deletion. These results indicate that Pb22 is essential for male gametogenesis, fertilization and possibly subsequent development of the parasites.
FIGURE 4. Phenotypic analyses of pb22-KO and pb22-RE lines.
BALB/c mice were infected by intraperitoneal (i.p.) injection with 1×106 WT, pb22-KO (KO), or pb22-RE (RE) parasites. A. Exflagellation centers per field at 400× magnification. B. Macrogamete numbers in 0.01 μl of infected blood. C. Ookinete numbers in an in vitro culture assay (the same number of mature gametocytes among WT, KO and RE groups). D. Ookinete numbers in midguts 24 h post blood meal. Horizontal bars indicate the mean number (n = 10). E. Oocyst numbers per midgut in mosquitoes 12 days after feeding on WT, KO and RE parasites infected mice. Horizontal bars indicate the mean number (n = 30). D and E are representative graphs of experiment 1 in Table 1. Error bars indicate SD. **, P < 0.01. All experiments included three biological replicates.
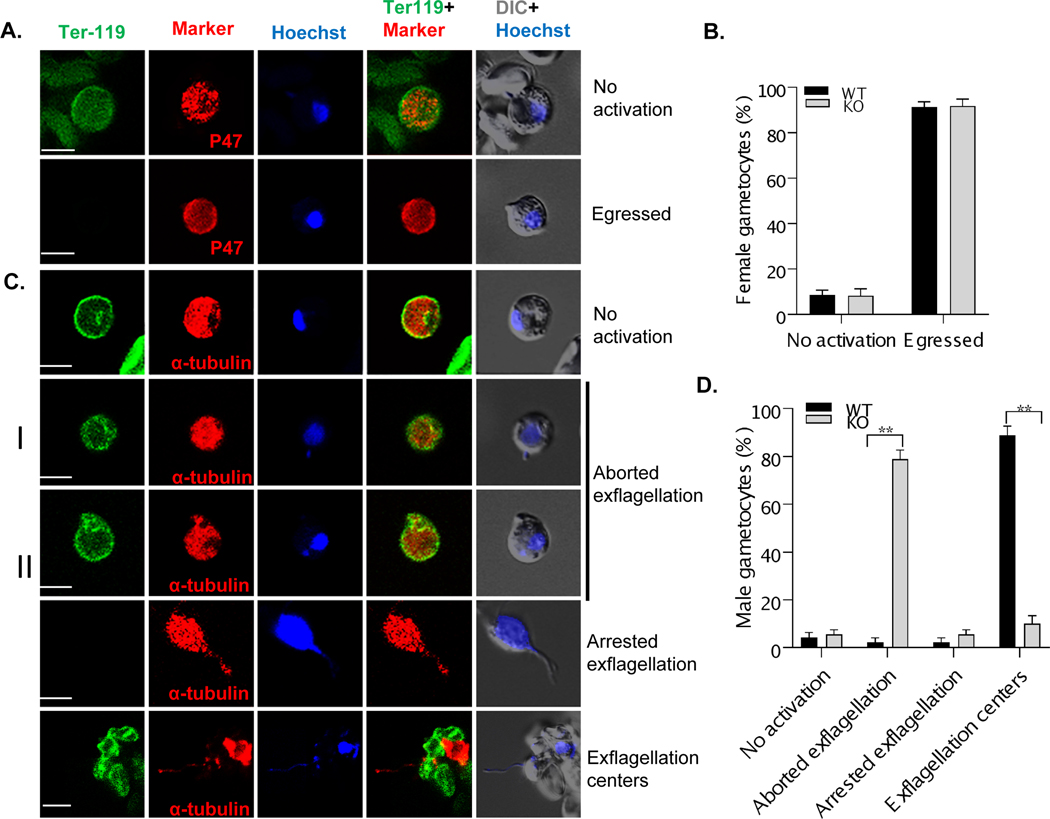
FIGURE 5. Male gametogenesis defects of the pb22-KO line determined by IFA.
Blood with mature gametocytes in WT and pb22-KO parasites induced for gametogenesis for 15 min were stained with antibodies against P47 (red) for female gamete and α-tubulin II (red) for male gamete, TER-119 (green) for mouse RBC membrane, and Hoechst-33258 (blue) for the nucleus. A. Female gametocytes and gametes were stained with P47 and Ter-119. The presence of the RBC membrane indicates ‘No activation’ of the gametocytes, whereas the lack of the RBC membrane indicates ‘egressed’ macrogametes. B. The proportions of female gametocytes showing no signs of activation and egressed macrogametes in the blood of mice infected with the WT and pb22-KO parasites. C. Male gametocytes and gametes were stained with α-tubulin II and Ter-119. Gametocytes were grouped as ‘No activation’ (no DNA replication and RBC membrane present), ‘Aborted exflagellation’ (I – DNA replication occurred, no flagella formed; and II [“bag of worms” phenotype] – flagella formed but RBC membrane present), ‘Arrested exflagellation’ (flagella formed and egressed, but did not interact with RBCs), and ‘Exflagellation centers’. D. The proportions of male gametocytes and gametes in C were compared between the WT (n=30) and pb22-KO (n=30) parasites. Data are presented as mean ± SD. **, P < 0.01. Data is a representative graph of three independent experiments.
Table 1.
Ookinete numbers, oocyst density and prevalence in mosquitoes fed on WT, pb22–KO and pb22–restored (pb22–RE) parasites.
| Exp. | Parasite | Ookinete formation in midgut at 24 h | Oocyst formation at 12 days | |||
|---|---|---|---|---|---|---|
| Ookinete-positive midguts (no. dissected) | No. ookinetes/positive midgut [Mean (range)] | Infection prevalence (no. dissected) a | Reduction in prevalence b | Oocyst density [Mean (range)] c | ||
| 1 | WT | 9 (9) | 1187 (300–2730) | 93.3% (30) | 103.7 (0–300) | |
| pb22–KO | 0 (10) | 0 | 0 (30) | 93.3% | 0 | |
| pb22–RE | 10 (10) | 1154 (420–3000) | 93.3% (30) | 86.9 (0–302) | ||
| 2 | WT | 10 (10) | 2230 (500–4550) | 86.7% (30) | 100.7 (0–319) | |
| pb22–KO | 0 (10) | 0 | 0 (29) | 86.7% | 0 | |
| pb22–RE | 10 (10) | 2065 (750–5300) | 96.6% (29) | 97.5 (0–303) | ||
| 3 | WT | 10 (10) | 2280 (1000–3700) | 93.1% (29) | 110.6 (0–309) | |
| pb22–KO | 1 (10) | 5 (0–5) | 0 (28) | 93.1% | 0 | |
| pb22–RE | 9 (9) | 1985 (600–5050) | 86.7% (30) | 96.8 (0–302) | ||
The infection prevalence was calculated by the number of mosquitoes with oocysts / number of mosquitoes dissected in each group × 100%.
The percent reduction in oocyst prevalence was calculated as % mean prevalence wt – % mean prevalence Pb22-KO.
Mean number and range of oocysts per mosquito midgut.
Exp., Experiment.
2.4. The pb22-KO line is defective in male gametogenesis
To further investigate the exflagellation defect in pb22-KO parasites, we compared WT and pb22-KO parasites by IFA to differentiate defects in flagella formation and egress. At 15 min after induction with xanthurenic acid, male parasites were stained with antibodies against α-tubulin II, nucleus by Hoechst 33258, and RBC membrane by TER-119. DNA replication appeared normal, as DNA contents of microgametocytes in the pb22-KO line and WT were similar (Figure S9D). Also, there were about the same proportions of the microgametocytes showing no signs of activation (Figure 5C, D). The major defects in the pb22-KO line appeared to be at the egress step since 78.9% of the microgametocytes in the pb22-KO line were in the stage of aborted exflagellation with the RBC membrane retained. For microgametocytes with aborted exflagellation, 30% failed to assemble the axonemes and 48.9% were egress-defective (flagella formed but failed to egress from the RBC). Time-lapse recording for 30 min confirmed that most of the activated microgametocytes in the pb22-KO line with flagella (‘bag of worms’ phenotype in Movie S1) or without flagella (Movie S2) failed to egress from the RBC. Eventually, only 15.5% of the microgametocytes in pb22-KO line egressed from the RBC, among which 10% formed exflagellation centers and the remaining male gametes did not interact with the RBCs (arrested exflagellation), as compared to >91.1% male gametes in the WT P. berghei having egressed and 88.9% forming exflagellation centers (P < 0.0001, Figure 5C, D).
2.5. The pb22-KO female gametocytes are competent to form ookinetes
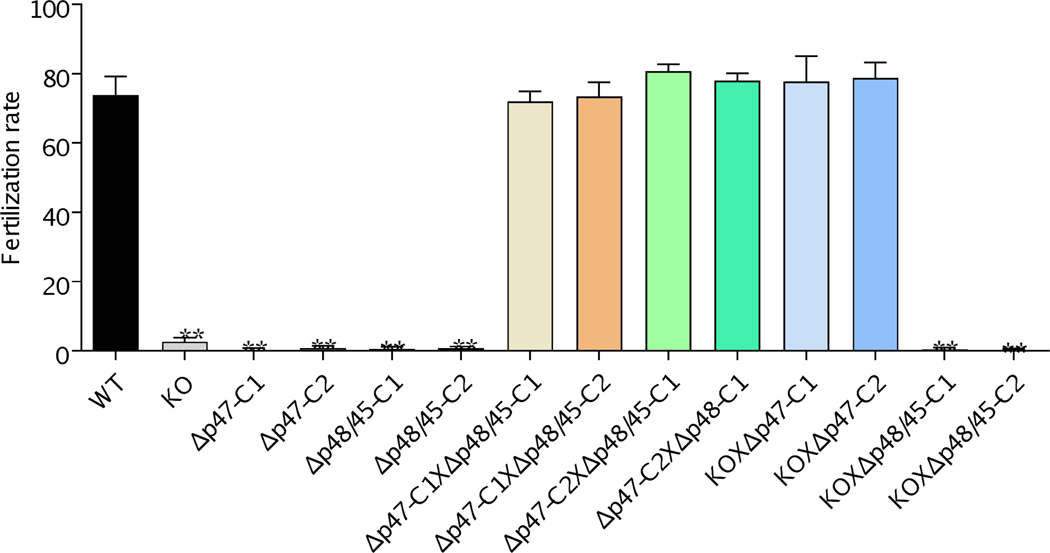
To confirm that pb22-KO line was defective only in male fertility, we performed in vitro cross-fertilization experiments between gametes of different mutants (van Dijk et al., 2010). For this purpose, two clones of Δp47 (male fertile, female infertile) and Δp48/45 (male infertile, female fertile) each were used in cross-fertilization. Both the Δp47 and Δp48/45 lines showed self-fertilization rates that were reduced by more than 99% compared to that in WT (Figure 6). In vitro ookinete cultures demonstrated that both the Δp47 (C1 and C2) and Δp48/45 (C1 and C2) lines contained clusters of unfertilized female gametes, and the pb22-KO line showed a similar defect in in vitro self-fertilization. Consistent with earlier findings (van Dijk et al., 2010), the cross-fertilization rates between Δp47 and Δp48/45 lines were not significantly different from that of the WT (Figure 6), confirming that only one sex in each of these deletion lines was defective. In cross-fertilization experiments, the pb22-KO line and female-defective Δp47 (C1 and C2) lines were able to cross-fertilize at the rates comparable to that of the WT, whereas the pb22-KO line and the male-defective Δp48/45 lines were unable to fertilize to produce ookinetes (Figure 6), confirming that the pb22-KO line was defective only in male fertility.
FIGURE 6. Cross-fertilization of KO line with Δp47 and Δp48/45.
The fertilization rate was calculated as the percentage of female gametes that develop into mature ookinetes. Self-fertilization and cross-fertilization rates of WT, pb22-KO, Δp47and Δp48/45. The pb22-KO female gametes were fertile and fertilized with Δp47 at the level similar to the WT, whereas the pb22-KO male gametes were unable to fertilize with male-defective Δp48/45 (n=100). Data indicate mean ± SD. ** indicates P < 0.01 between the marked group with the WT group. Data are representative of three independent experiments.
2.6. Pb22 possesses obvious transmission-blocking potential
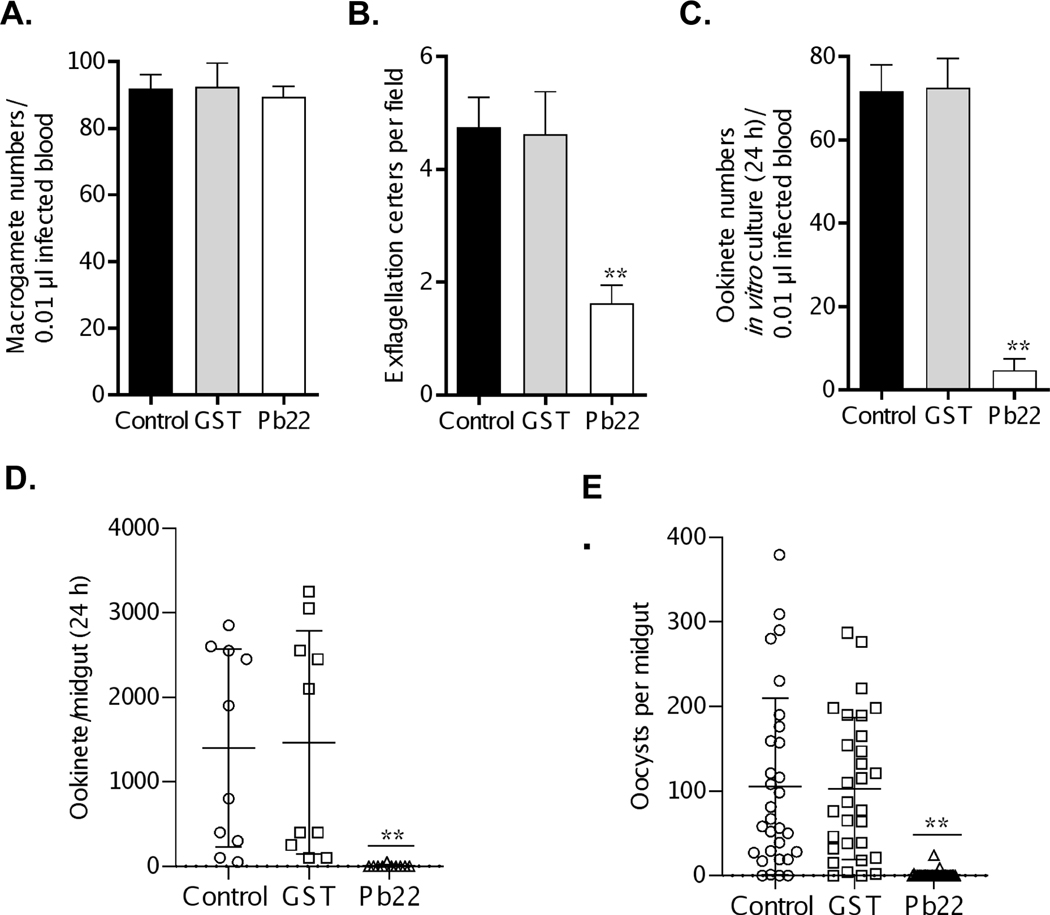
Expression of Pb22 on the surfaces of sexual stages prompted us to evaluate whether this protein could be a TBV candidate. To assess the TB potential of Pb22, mice were immunized with the rPb22 protein or the recombinant GST protein as a control and then infected with the WT P. berghei. Parasitemia (P > 0.05; Figure S10A), gametocytemia (P > 0.05; Figure S10B) and the gametocyte sex ratio (P > 0.05; Figure S10C) were not significantly different among the rPb22 immunization, the GST control, and adjuvant control groups. In addition, in vitro assay showed that rPb22 immunization did not affect macrogamete formation as the macrogamete numbers in the three groups were also similar (P > 0.05; Figure 7A). However, compared with the GST immunization and adjuvant control groups, the number of exflagellation centers in parasitized blood 3 days post-infection was reduced by ~65% in the rPb22 immunization group (P = 0.0026; Figure 7B), which led to a ~93% reduction of ookinetes formed in in vitro culture (P < 0.001; Figure 7C). When immunized mice were used for mosquito feeding, consistent and significant reduction in ookinete formation, oocyst density and infection prevalence was observed in the rPb22-immunization group as compared with the two control groups. Specifically, ookinete numbers in the midguts 24 h after blood feeding showed 83.3–93.3% reduction in the rPb22-immunization group as compared to the GST-immunization group (P < 0.001; Figure 7D, Table 2). The mean oocyst number/midgut in mosquitoes that fed on the GST-immunization group was 102.9–110.4, which was reduced to 0.4–6.7 in those that fed on rPb22-immunized mice, corresponding to 93.5–99.6% reduction in oocyst density (P < 0.0001, Figure 7E, Table 2). Furthermore, compared to an average infection prevalence of 93.1–100% in mosquitoes feeding on the GST control mice, mosquitoes feeding on rPb22-immunized mice had an infection prevalence of 6.7–10%, corresponding to 83.3– 93.3% reduction in infection prevalence (P < 0.0001; Table 2). It is noteworthy that there were no significant differences in these parameters examined between the GST-immunization group and the adjuvant control group (Figure 7 and Table 2).
FIGURE 7. In vivo transmission-blocking activity of rPb22 protein.
Six mice immunized with rPb22 and GST as well as the adjuvant control groups were infected with P. berghei parasites 10 days after the third immunization. A-C. Tail blood was collected at 3 days post-infection, and in vitro ookinete culture assay was used to obtain the macrogamete numbers in 0.01 μl of infected blood (A), exflagellation centers per field (B), and the ookinete numbers in 0.5 μl of culture (roughly equivalent to 0.01 μl of infected blood) (C). D. Representative graph of ookinete numbers in 10 midguts 24 h post blood meal. E. Representative graph of oocyst numbers per midgut 12 days post blood meal in all immunization groups. The D and E are representative graphs of experiment 1 in Table 2. Data are presented as mean ± SD. ** (P < 0.01) represents significance between GST-immunized and rPb22-immunized groups. The results are from three different biological replicates.
Table 2.
Evaluation of transmission-blocking effect of rPb22 immunization in mosquito feeding assays
| Experiment | Group | No. midguts dissected | No. ookinetes/midgut Mean (range) a | Infection prevalence (no. dissected) b | Reduction in prevalence c | Oocyst density Mean (range) d | Reduction in oocyst density e |
|---|---|---|---|---|---|---|---|
| 1 | Control | 9 | 1400 (50–2850) | 90% (30) | 105.3 (0–379) | ||
| GST | 10 | 1465 (100–3250) | 93.3% (30) | 102.9 (0–287) | |||
| rPb22 | 10 | 5 (0–50) | 10% (30) | 83.3% | 1.2 (0–24) | 98.9% | |
| 2 | Control | 10 | 1025 (0–4400) | 89.7% (29) | 103 (0–298) | ||
| GST | 10 | 1055 (0–4700) | 100% (28) | 110.4 (5–330) | |||
| rPb22 | 10 | 30 (0–250) | 6.7% (30) | 93.3% | 6.7 (0–198) | 93.5% | |
| 3 | Control | 10 | 2300 (350–4000) | 96.4% (28) | 114.1 (0–309) | ||
| GST | 10 | 2465 (0–4500) | 93.1% (29) | 106.9 (0–298) | |||
| rPb22 | 9 | 45 (0–400) | 7.1% (28) | 86% | 0.4 (0–9) | 99.6% |
Mean number and range of ookinetes per mosquito midgut inside the blood bolus dissected at 24 h after blood feeding.
The prevalence of infection was calculated by the number of mosquitoes with oocysts / total mosquitoes dissected in each group × 100%.
The percent reduction of prevalence was calculated as % mean prevalence GST – % mean prevalence rPb22.
Mean number and range of oocysts per mosquito midgut.
The percent reduction in oocyst density was calculated as (mean oocyst density control – mean oocyst density Pb22) / mean oocyst density control × 100%.
We performed in vitro assays to further illustrate that the TB activity observed with the rPb22 immunization was due to specific antibodies against Pb22. Among the immune sera collected from the three groups of mice, only the rPb22 immune sera detected the ~22 kDa protein in gametocyte lysates of the WT parasite in Western blot (data not shown) and the rPb22 antisera reacted with the surface of male gametes and ookinetes (Figure S11A). Using the in vitro assay, we showed that the effect of rPb22 immune sera on exflagellation and ookinete formation was concentration-dependent. Regardless of the dilution, the GST control sera showed no differences in the formation of exflagellation centers or ookinetes (Figure S11B, C). At the 1:5 dilution, the rPb22 immune sera conferred a 56.3% decrease in exflagellation centers (Figure S9B) and an 84.8% decrease in ookinete number (Figure S11C) as compared with the GST control sera. However, the inhibitory effects of the rPb22-immune sera were attenuated at the 1:10 dilution (Figure S11B, C).
3. DISCUSSION
In this study, we characterized a protein, Pb22, which is expressed in both asexual and sexual stages of P. berghei but is functionally essential only for male gametogenesis. Pb22 KO did not affect asexual growth or gametocytogenesis, but it led to defective male gametogenesis at multiple steps. As a result, egress, fertilization, ookinete formation and mosquito infectivity were all severely affected. The localization of this protein on the plasma membrane of both male and female gametes and subsequently on ookinetes prompted us to investigate its TB potential. Antibodies against rPb22 demonstrated strong TB activity in both in vitro ookinete culture and mosquito feeding assays.
Gametocytes formed in the vertebrate hosts are obligative for the transmission of malaria parasites to the mosquitoes. Within 15–20 min of blood ingestion, gametogenesis occurs in the mosquito midgut to produce male and female gametes. Whereas a female gametocyte only forms one spherical macrogamete, a male gametocyte undergoes three rounds of DNA replication to produce eight flagella-like gametes, each consisting of an axoneme and a nucleus encased by a flagellar membrane (Kuehn et al., 2010). Egress from the erythrocyte membrane is an important step of gametogenesis and requires multiple factors for the sequential lysis of PVM and the erythrocyte membrane (Sologub et al., 2011, Wirth et al., 2012, Deligianni et al., 2013, Andreadaki et al., 2018). Several factors affect the egress of both male and female gametocytes. MDV1/PEG3 (male development-1/protein of early gametocyte 3) is important for the lysis of PVM; MDV1/PEG3 deletion blocks the egress of both male and female gametes (Ponzi et al., 2009). Similarly, male and female gametocytes lacking the GEST (gamete egress and sporozoite traversal) protein also are not able to lyse the PVM or egress from the RBC (Talman et al., 2011). MTRAP (merozoite-specific thrombospondin-related anonymous protein) is essential for the disruption of the gamete-containing PVM, and MTRAP-deficient male and female gametocytes are wrapped in intact PVM and RBC membrane (Bargieri et al., 2016, Kehrer et al., 2016). In this study, We found that despite Pb22 expression in asexual stages and female gametocytes, pb22 deletion did not affect asexual growth (Fonager et al., 2012), gametocytogenesis or the formation of female gametes. The male-specific defects have been confirmed by IFA to show egress of the macrogametes from the RBCs and by cross-fertilization experiments with male-deficient Δp48/45 and female-deficient Δp47 parasite lines.
Male gametogenesis is a dramatic event and occurs very rapidly within the mosquito midgut. Six minutes after the activation, three rounds of DNA replication are complete (Fang et al., 2017). DNA replication is physically linked to the simultaneous assembly of eight exonemes, followed cytokinesis to form individual male gametes. Gamete escape from erythrocyte is mediated by the secretion of the content of OBs, which contain membranolytic factors, into the parasitophorous vacuole (Olivieri et al., 2015). Several protein kinases and phosphatases have been identified as key regulators of male gametogenesis. Calcium-dependent protein kinase (CDPK) 4 is involved in the initiation of DNA replication, assembly of the mitotic spindle, and finally motility of the male flagellum (Fang et al., 2017). Whereas CDPK1 deletion in P. berghei only delays the exflagellation process (Sebastian et al., 2012), PfCDPK1 KO parasites show no flagellar movement or exflagellation (Bansal et al., 2018). The mitogen-activated protein kinase 2 (MAP-2) affects the final stages of male gamete formation in both P. berghei and P. falciparum (Tewari et al., 2005, Hitz et al., 2020). Recent studies have revealed the participation of some structural and motor proteins in axoneme assembly (Marques et al., 2015, Depoix et al., 2020). Later in male gametogenesis, the egress process requires the release of lytic factors (e.g., the plasmodial perforin-like protein 2 [PPLP2], a ferlin-like protein FLP, the serine protease SUB1, and the gamete egress protein GEP) from secretary vesicles or OBs to lyse the PVM and the RBC membrane (Wirth et al., 2014, Obrova et al., 2019, Pace et al., 2019, Andreadaki et al., 2020). For example, upon PbPPLP2 deletion, male gametes can disrupt the PVM normally but fail to rupture the RBC membrane, thus forming a super-flagellum, where multiple axonemes are sheathed together by the RBC membrane (Deligianni et al., 2013, Wirth et al., 2014). A phospholipase (PfPATPL1) has been found to be important for the re-localization of the PPLP2-containing vesicles, thus affecting gamete egress (Singh et al., 2019). Interestingly, the male OB-associated protein PyMiGS in P. yoelii does not affect egress or formation of flagella, but it affects exflagellation (Tachibana et al., 2018). In this study, the male defects in gametogenesis upon Pb22 deletion seemed to involve both axoneme assembly and egress. While 30% of the microgametocytes have successfully finished DNA replication but failed to form axonemes, ~49% have formed flagella but the microgametes could not egress from the RBC membrane. Its cytoplasmic localization in gametocytes shows a punctate pattern, suggesting it could be present in vesicular structures, OBs, or granules. Consistent with its cytosolic localization, Pb22 does not seem to affect DNA replication as male gametocyte DNA contents after activation were similar between WT and Pb22 KO parasites. Given the involvement of OBs in male gamete egress, those displaying the “bag of worms” phenotype may be defective in the formation of OBs or secretion of the OB content. Like PyMiGS, Pb22 is localized in the cytoplasm of gametocytes but then appeared on the plasma membrane of gametes, suggesting it might be released from the vesicular structure during gametogenesis. Future fine structure analysis and identification of proteins interacting with Pb22 will help to elucidate its subcellular localization and functions.
After fertilization in the midgut, zygote develops into a motile ookinete, which traverses the midgut wall forming an oocyst to establish infection in the mosquito. In addition to the defect in male gamete formation, the pb22-KO parasites may have additional defects in fertilization, ookinete development, and midgut invasion, since mosquitoes feeding on the pb22-KO-infected mice developed no oocysts. Despite ~10% pb22-KO male gametocytes could form exflagellation centers, very few ookinetes were formed with the pb22-KO x Δp48/45 cross. Such a defect indicates that the surface-localized Pb22 may play a direct or indirect role in male-female gamete interaction and fertilization. Furthermore, although residual ookinetes (<3% of the WT level) were formed during in vitro culture of pb22-KO-infected blood, no oocysts were formed during the mosquito feeding assay, suggesting that the ookinetes lacking surface Pb22 protein may have additional defects in midgut traversal and subsequent development.
During gamete formation to ookinete development, Pb22 protein is localized on the plasma membrane of parasites, since IFA without membrane permeabilization still detected Pb22 on the surface of these stages. Pb22 has a predicted signal peptide, but lacks identifiable transmembrane domains, suggesting that surface localization of Pb22 may rely on the interactions with other proteins on the surface of these stages. It has been shown that two male fertility factors P48/45 and P230 form a complex on the surface of the male gametes (Kumar, 1987, Eksi et al., 2006, Cao et al., 2018). It would be interesting to determine how Pb22 is localized on the surface of the parasites, and whether Pb22 deletion affects the surface localization of these male fertility factors.
An important criterion to select TBV candidates is that the target protein is localized on the sexual stages of the parasite (Nunes et al., 2014). TBV development has focused on the pre-fertilization proteins P230 and P48/45, as well as post-fertilization proteins P28/25 (Baton et al., 2005, MacDonald et al., 2016, Lennartz et al., 2018). A TBV candidate expressed during both pre- and post-fertilization, like Pb22, would offer the advantages of blocking parasite development at multiple time points. Although pb22 mRNA expression was only detected in female gametocytes (Yeoh et al., 2017), Pb22 protein was detected in both male and female gametocytes (Khan et al., 2005). Our study further confirmed Pb22 protein expression in asexual stages and in both male and female gametocytes. Its surface localization on male and female gametes, and also during ookinete development led us to investigate its TB potential. In this study, we provided evidence that antibodies against Pb22 could inhibit exflagellation. We reason that the permeabilization of the PVM and RBC membrane during the gamete egress process will likely allow the antibodies to gain access to Pb22 on male gametes and block their release. The in vivo analysis of TB activity showed that mosquitoes that fed on mice immunized with the rPb22 had up to 93.3% reduction in the prevalence of infected mosquitoes and 99.6% reduction in oocyst density. This excellent TB activity of Pb22 in the rodent parasite and conservation of this gene in Plasmodium suggest that it might be a promising TBV candidate and is worth of evaluation in the human malaria parasites.
In conclusion, we have characterized a conserved Plasmodium protein that plays an important role in male gametogenesis and potentially subsequent sexual development. Despite the expression of Pb22 in both male and female gametocytes, the defect of pb22 deletion appears to be restricted to male gametes. Being a surface protein during gamete to ookinete development, Pb22 demonstrated excellent TB activity which supports further exploration of P22 as a TBV candidate antigen in human malaria parasites.
4. EXPERIMENTAL PROCEDURES
4.1. Animals, parasites, and mosquitoes
General maintenance of parasites and mosquitoes was described previously (Zheng et al., 2016). Briefly, P. berghei ANKA 2.34 parasites were maintained in 6–8 week-old female Balb/c mice by serial mechanical passages (up to a maximum of eight passages). Anopheles stephensi mosquitoes (Hor strain) were bred in an insectary under 25°C and 50–80% humidity. Mice and rabbits were kept at the central animal facilities of China Medical University under the care of trained veterinary staff. All animal experiments were approved by the university animal ethics committee.
4.2. Generation of transgenic parasites
Several transgenic parasites were generated using the double-crossover homologous recombination strategy described previously (Janse et al., 2006). To tag the endogenous Pb22 with an HA tag, the 5′ fragment was amplified with the primers HA-5UTR-F and HA-5UTR-R, while the 3′ fragment was amplified with primers HA-3UTR-F and HA-3UTR-R (Table S1). The fragments were cloned into vector PL0035 (containing the human dhfr cassette, yfcu cassette and ha tag). Plasmids (10 μg) were linearized and electroporated into 1×107 purified P. berghei schizonts. After transfection, parasites were mixed with 50 μl complete culture medium and intravenously injected into mice. Transgenic parasites were selected 24 h later with 70 μg/ml pyrimethamine (Sigma Aldrich, St. Louis, MO, USA) added in drinking water for mice. Parasite genomes were extracted from infected blood to examine the transgenic parasite by integration-specific PCR. HA-tagged parasites were cloned using a limiting dilution method (Guttery et al., 2014).
KO lines for pb22 were generated similarly. Primers for the 5’ and 3’ recombination fragments of these genes are listed in Table S1. Purified and digested PCR fragments were cloned into the PL0034 vector. The Δp48/45 and Δp47 lines were generated earlier and used to study sex-specific defects (Zhu et al., 2019). To restore the pb22 gene in the pb22-KO line by GIMO (Lin et al., 2011), the primers KO-5UTR-F and KO-3UTR-R were used to amplify a reconstructed fragment. Purified PCR fragments were digested with HindIII and NotI, then transfected into the pb22-KO schizonts as described above. Parasites with the pb22 gene restored (pb22-RE) were selected by intraperitoneal injection of 0.4 mg/kg/day of 5-fluorocytosine (Sigma) for 4 days, beginning at 24 h after transfection. For the final proof of successful gene KO and restoration, diagnostic PCR and Western blots were performed.
4.3. Expression of rPb22 and other recombinant proteins
The sequence corresponding to Ser19-Ser218 (entire protein minus signal peptide) of Pb22 was amplified by PCR using primers pET32a-Pb22-F and R (Table S1). In addition to the BamHI site, the forward primer contains a sequence (GACGACGACGACAAG) for peptide Asp-Asp-Asp-Asp-Lys, which is the enterokinase cleavage sequence. The PCR product was cloned into the BamHI/NotI sites of the expression vector pET32a, producing a fusion protein under the control of the T7 lac promoter. The plasmids were transformed into E. coli Rosetta-gami B (DE3) cells and the rPb22 protein expression was induced with 1 mM isopropyl-D-1-thiogalactoside at 19°C for 4 h. Recombinant proteins were purified by affinity chromatography on nickel-nitrilotriacetic acid agarose (Ni-NTA, Millipore, Germany) under denaturing conditions. Purified rPb22 was dialyzed using phosphate-buffered saline (PBS, pH 7.4) at 4°C overnight. Eluted fusion protein rPb22 was digested by recombinant enterokinase at 25°C for 16 h in a Tris buffer (0.2 mM Tris-HCI, 100 mM NaCl, pH 8.0). The digested proteins were further purified on a Ni-NTA column, and the released rPb22 protein was analyzed by 10% SDS-PAGE. A recombinant GST protein was similarly expressed in E. coli using the empty vector pGEX-4T-1. It was used as an immunization control since this GST has limited sequence identity (28%) to the P. berghei GST sequence.
We also expressed recombinant P47 and SET for antibody production. The DNA sequences encoding amino acids I23–L154 for P47 (PBANKA_ 1359700) and M1–D267 for SET (PBANKA_0819900) were amplified from P. berghei cDNA using primers listed in Table S1. The PCR products were cloned into pET32a and transformed into DE3. The recombinant proteins were purified from E. coli as described for rPb22. Purified proteins were quantified using a BCA Protein Assay Kit (Beyotime, Haimen, China).
4.4. Production of antisera
Polyclonal antisera against rPb22 were raised in female BALB/c mice (n = 5) by subcutaneously injecting 50 μg purified rPb22 emulsified in complete Freund’s adjuvant (Sigma). Boosting on day 15 and 30 was performed via subcutaneous injection with 30 μg rPb22 in incomplete Freund’s adjuvant. The same immunization procedure was performed for the GST immunization control and the adjuvants only control. Sera were collected and pooled 10 days after the last immunization.
To generate polyclonal antisera against P47 and SET, New Zealand white rabbits were immunized subcutaneously with 250 μg of purified recombinant protein with Freund’s complete adjuvant, followed by two boosters with 250 μg of purified recombinant protein in Freund’s incomplete adjuvant at 3-week intervals. Antisera were collected 14 days after the last immunization. Rabbit anti-α-tubulin II antibodies were custom-made against the peptide CDGEGEDEGYE by Genscript (Nanjing, China).
4.5. Enzyme-linked immunosorbent assay (ELISA)
To estimate the antibody titers of antisera from rPb22 immunized group, ELISA was performed as previously described (Miura et al., 2008). The microtiter plate was coated with purified proteins (5 μg/ml) in 0.05 M sodium carbonate buffer (pH 9.6) at 4°C overnight. Then the plate was washed with PBS-T (0.05% Tween-20 in PBS) three times and blocked with 1% bovine serum albumin (BSA, Sigma) at 37°C for 1 h. To each well of the plate, 100 μl of mouse sera, serially diluted with 1% BSA in PBS from 1:1000 to 1:512000, were added and incubated at 37°C for 2 h. After three washes with PBS-T, 100 μl HRP-conjugated goat anti-mouse secondary antibodies (1:5000, ThermoFisher, 62–6520) were added and incubated at 37°C for 2 h. After additional six washes, 100 μl of substrate solution (tetramethyl benzidine, Amresco, USA) were added and incubated in the dark for 5 min. The reaction was stopped by adding 50 μl of 1 mM H2SO4 and the absorbance at 490 nm was read immediately. The value for the final dilution of the antisera was defined as that above the cut-off value of the control antisera + 3 × standard deviation (SD).
4.6. Western blot
For Western blot analysis, total proteins were prepared from schizonts, gametocytes and ookinetes as previously described (Kou et al., 2016). Briefly, BALB/c mice were intraperitoneally injected with 1×106 WT P. berghei, pb22-KO, pb22-RE and Pb22-HA infected RBCs (iRBCs). Heparinized blood on day 5 post-infection was cultured in a complete schizont culture buffer (RPMI 1640, 50 mg/l penicillin, 50 mg/l streptomycin, 100 mg/l neomycin, 25% [vol/vol] heat-inactivated fetal calf serum, 6 U/ml heparin) at 37°C for 16 h and schizonts were fractionated on a 55% (v/v) Nycodenz (Axis-Shield, Oslo, Norway) gradient. Gametocyte purification was performed based on a previously described protocol (Beetsma et al., 1998). BALB/c mice were pre-treated with 25 mg/ml phenylhydrazine in saline solution to induce hyper-reticulocytosis. Three days later mice were injected intraperitoneally with 1×106 WT iRBCs. Four days post-infection, mice were treated with sulfadiazine (Sigma) at 20 mg/l in drinking water for 48 h to kill other parasite stages. Gametocytes were purified on a 48% (v/v) Nycodenz gradient at 4°C. For ookinetes, purified gametocytes were cultured in a complete ookinete culture medium (RPMI 1640, 50 mg/l penicillin, 50 mg/l streptomycin, 100 mg/l neomycin, 25% [v/v] heat-inactivated fetal calf serum, 6 U/ml heparin, 100 μM xanthurenic acid, pH 8.0) in plastic Petri dishes at 19°C for 24 h and ookinetes were isolated with 62% Nycodenz.
Proteins were extracted with 2% SDS containing protease inhibitors. Equal amounts of parasite lysates (10 μg) were separated by 10% SDS-PAGE under reducing conditions. Proteins were transferred to PVDF membranes (BioRad, Hercules, CA, USA), blocked with 5% non-fat milk in TBST (Tris-buffered saline with 0.1% Tween 20) at room temperature for 2 h, and probed with mouse anti-rPb22 antisera (1:200) or mouse anti-HA monoclonal antibody (1:1000; Invitrogen, Carlsbad, CA, USA). After three washes with TBST, the membrane was incubated with HRP-conjugated anti-mouse secondary antibody. An ECL Western Blot Kit (Pierce, Rockford, IL, USA) was used to visualize protein via chemiluminescence. Protein loading was estimated using the anti-rHsp70 sera (Hsp70, PBANKA_0711900) produced in the laboratory.
4.7. IFA
Pb22 localization was determined by IFA using a modified protocol (Tonkin et al., 2004). WT and Pb22-HA parasites were fixed with 4% paraformaldehyde and 0.0075% glutaraldehyde (Sigma) in PBS for 30 min at room temperature. After washing with PBS, cells were treated with or without 0.1% Triton X-100 (Sigma) in PBS for 10 min and then blocked with 3% BSA/PBS for 60 min. Following another PBS wash, mouse anti-rPb22 and anti-HA mAb (1:500; Invitrogen, 326700) were added, and samples were incubated for 1 h at 37°C. After washing with PBS, the parasites without membrane permeabilization were treated with 0.1% Triton X-100 and then blocked with 3% BSA/PBS for 60 min. Subsequently, parasites were co-incubated with rabbit antisera against PbMSP1 (1:500), P47 (1:500), α-tubulin II (1:500), SET (1:500) and PSOP25 (1:500) as stage-specific markers for schizonts, female gametocytes/gametes, male gametocytes/gametes, nucleus, and ookinetes, respectively (Pace et al., 2006, Molina-Cruz et al., 2017, Zheng et al., 2017, Liu et al., 2018, Liu et al., 2019). After three washes with PBS, Alexa Fluor-488-conjugated goat anti-mouse IgG antibodies (1:500; Invitrogen, A32723) and Alexa Fluor-555-conjugated goat anti-rabbit IgG antibodies (1:500; Abcam, ab50078) were added and incubated for 1 h. Except for the commercial antibodies, antibodies against PbMSP1, P47, Pbg377, SET, Pbs21, and PSOP25 were made in our laboratory. The nucleus was counterstained with Hoechst 33258 (1:1000; Invitrogen). Negative controls for IFA included WT ookinetes incubated with the anti-HA antibody, GST immune sera, and secondary antibodies only, as well as Pb22-HA ookinetes incubated with only the secondary antibodies. Parasites were visualized on a Nikon C2 fluorescence confocal laser scanning microscope (Japan).
4.8. Phenotype analysis of pb22-KO and pb22-RE parasites
To confirm the absence and presence of Pb22 expression in pb22-KO and pb22-RE parasites, respectively, gametocytes of pb22-KO and pb22-RE lines were purified and used for Western blot analysis and IFA using the anti-rPb22 sera and antisera for additional markers. To study the function of Pb22, three groups of mice (3/group) were infected with 1×106 iRBCs of WT, pb22-KO and pb22-RE lines, respectively. Parasitemia was monitored every other day from day 3 post infection. Another three groups of mice (3/group) were used for gametocyte induction as described in 4.6. Five days after infection, gametocytes per 104 RBCs and gametocyte sex ratios from counting 100 gametocytes were estimated in Giemsa-stained thin blood films (Marques et al., 2015). Mature male and female gametocytes were differentiated based on staining with male- and female-specific markers as described above. Exflagellation centers (male gamete interactions with RBCs), macrogamete and ookinete numbers were monitored using in vitro culture (Eksi et al., 2006). Briefly, ~10 μl infected blood containing equal numbers of mature gametocytes were mixed with ookinete culture medium in a total volume of 50 μl at 25°C to induce gamete formation. Fifteen minutes later, 1 μl was placed on a glass slide (Matsunami Glass IND., LTD, Japan) and exflagellation centers (10 view fields per mouse) were enumerated under a phase-contrast microscope at 400×magnification for 10 min. An exflagellation center is defined as an exflagellating male gametocyte with more than four tightly associated RBCs (Templeton et al., 1998). The time-lapse recording of exflagellation centers was captured with a camera mounted on an Olympus microscope. To count macrogametes and ookinetes, ~10 μl infected blood containing equal numbers of mature gametocytes were mixed with the ookinete medium in a total volume of 500 μl at 25°C for 15 min. The total number of macrogametes in 0.5 μl (equivalent to 0.01 μl of infected blood) was counted after labeling with the anti-Pbs21 mAb (1:500) and Alexa-488-conjugated anti-mouse IgG antibodies (1:500) without permeabilization (van Dijk et al., 2001). The culture was continued at 19°C for 24 h to allow ookinete formation. Similarly, the number of ookinetes in 0.5 μl of culture was counted after labeling with the anti-Pbs21 mAb (1:500).
4.9. Mosquito feeding
For direct mosquito-feeding assays, mice were infected with parasites as described above. When asexual parasitemia was approximately 5–7% after 3 days of infection, ~50 4-day-old, pre-starved An. stephensi mosquitoes were allowed to feed on each infected mouse for 1 h. At 24 h after the feeding, approximately 10 engorged mosquitoes per group were ground to enumerate ookinetes in each mosquito using Giemsa-stained films. One mosquito was ground in 50 μl PBS, and 1 μl of the mixture was used to enumerated ookinetes. At 12 days after blood feeding, midguts were dissected from ~30 mosquitoes of each group and stained with 0.5 % mercurochrome for 10 min. Oocysts were counted and expressed as the number of oocysts per positive midgut. Finally, the proportion of infected mosquitoes among all mosquitoes was determined.
4.10. Gametocyte egress assay
WT- and pb22-KO-infected blood samples with gametocytes were mixed with the ookinete culture medium and incubated at 25°C for 15 min. Cells were fixed with 4% paraformaldehyde and 0.0075% glutaraldehyde for 30 min and proceeded for IFA with permeabilization as described above. Samples were labeled with either rabbit anti-P47 antiserum (1:500) or rabbit anti-α-tubulin II antiserum (1:500) as previously described, followed by incubation with Alexa Fluor 555-conjugated goat anti-rabbit IgG antibodies (1:500 dilution). RBC membranes were labeled with rat anti-TER119 antibody conjugated to Alexa Fluor 488 (1:200 dilution, Invitrogen, 4322597). Cell nuclei were counterstained with Hoechst 33258 (1:1000). At least 30 egressed or un-egressed parasites were differentiated under a fluorescence microscope (Bansal et al., 2017, Tachibana et al., 2018). To quantify the nuclear DNA content of male gametocytes, gametocytes were mixed with ookinete culture medium at 25°C, fixed, and permeabilized as described above at 6 min when the male gametocytes should have completed three rounds of genome replication (Fang et al., 2017). The cells were incubated with 3% BSA/PBS for 1 h, washed with PBS, and then incubated with rabbit anti-α-tubulin II serum for 1 h. After washing with 1 ml PBS, they were incubated with Alexa Fluor 555-conjugated goat anti-rabbit IgG antibodies for 1 h, and counterstained with Hoechst 33258 for 10 min. Images of the male gametocytes (Alexa Fluor 555 positive) were captured under a Nikon C2 confocal laser scanning microscope using identical settings. The fluorescent signal intensity of 30 parasites from each group was measured using the Nikon software (Wang et al., 2020).
4.11. Cross-fertilization study
To study whether the pb22-KO parasites was defective in either male or female fertility, in vitro cross-fertilization assay was performed using either the Δp47 line (female defective, male fertile) or the Δp48/45 line (PBANKA_1359600, male defective, female fertile) (van Dijk et al., 2010). Equal numbers of mature gametocytes of each clone (counted by Giemsa staining) were mixed and incubated for 24 h. Ookinetes and unfertilized gametes from a total of 100 parasites were counted separately. Fertilization rate is the percentage of female gametes that develop within 24 h into ookinetes in vitro.
4.12. TB activity
To evaluate the TB activity of anti-rPb22 sera in in vivo assay, 6 mice/group immunized with the rPb22 protein, GST or adjuvants only, respectively. Ten days after the third booster, 3 mice were infected with 1×106 parasites. Parasitemia, gametocytemia, and gametocyte sex ratios were counted on day 3 post infection. For in vitro analysis, exflagellation centers, macrogametes and ookinetes were counted as described above. The other three mice were fed to starved female An. stephensi, and 24 h later, ookinete numbers in mosquitoes were calculated using Giemsa-stained films. The numbers of oocysts and infected mosquitoes were determined at 12 days after feeding.
In vitro assay was further performed using different dilutions of the immune sera. Both the anti-rPb22 sera and the GST sera were diluted at 1:5 and 1:10 with the ookinete culture medium and mixed with 10 μl of infected mouse blood in a total volume of 50 μl. The exflagellation of male gametocytes was quantified as described above. For ookinete culture, serum samples were diluted at 1:5 and 1:10 with the ookinete culture medium and mixed with 10 μl of infected mouse blood in a total volume of 500 μl. The number of ookinetes in 0.5 μl culture was counted at 24 h.
4.13. Statistical analyses
Student’s t-test and ANOVA were used to compare antibody titers, parasitemia, gametocytemia, gametocyte sex ratio, exflagellation and ookinete numbers among groups. Chi-square test was used to compare the proportions. Mann-Whitney U test was used to analyze oocyst density (oocysts number/midgut), while Fisher’s exact test was used to analyze infection prevalence. All data are presented as mean ± SD. Statistical analyses were carried out using the SPSS software, version 21.0 (IBM, North Castle, NY, USA). P < 0.05 was considered statistically significant.
Supplementary Material
Supplemental Movie 1. Exflagellation of male gametocytes in the pb22-KO line. Gametocytes-infected blood collected from the mouse tail was diluted with the ookinete culture medium. The developed flagella were trapped by PVM or erythrocyte membrane over 30 min.
Supplemental Movie 2. Male gametes without flagella development in the pb22-KO line were not able to egress from the red blood cell over 30 min.
Take Away.
We characterized Pb22 that is expressed in schizonts-ookinetes in P. berghei
Pb22 KO showed defects in male gametogenesis and ookinete development
Most Pb22 KO male gametocytes failed in axoneme assembly or egress
Cross-fertilization confirmed defects restricted to male gametes only
Recombinant Pb22 showed obvious transmission-blocking potential
ACKNOWLEDGEMENTS
This study was supported by the National Science Foundation of China (No. 81429004 and 81760367) and by the National Institutes of Health grants R01AI150533 and U19AI089672.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Andreadaki M, Hanssen E, Deligianni E, Claudet C, Wengelnik K, Mollard V, et al. (2018). Sequential Membrane Rupture and Vesiculation during Plasmodium berghei Gametocyte Egress from the Red Blood Cell. Scientific reports 8, 3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadaki M, Pace T, Grasso F, Siden-Kiamos I, Mochi S, Picci L, et al. (2020). Plasmodium berghei Gamete Egress Protein is required for fertility of both genders. Microbiologyopen, e1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aunin E, Bohme U, Sanderson T, Simons ND, Goldberg TL, Ting N, et al. (2020). Genomic and transcriptomic evidence for descent from Plasmodium and loss of blood schizogony in Hepatocystis parasites from naturally infected red colobus monkeys. PLoS pathogens 16, e1008717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Molina-Cruz A, Brzostowski J, Liu P, Luo Y, Gunalan K, et al. (2018). PfCDPK1 is critical for malaria parasite gametogenesis and mosquito infection. Proceedings of the National Academy of Sciences of the United States of America 115, 774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Molina-Cruz A, Brzostowski J, Mu J. and Miller LH (2017). Plasmodium falciparum Calcium-Dependent Protein Kinase 2 Is Critical for Male Gametocyte Exflagellation but Not Essential for Asexual Proliferation. MBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargieri DY, Thiberge S, Tay CL, Carey AF, Rantz A, Hischen F, et al. (2016). Plasmodium Merozoite TRAP Family Protein Is Essential for Vacuole Membrane Disruption and Gamete Egress from Erythrocytes. Cell host & microbe 20, 618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baton LA and Ranford-Cartwright LC (2005). Do malaria ookinete surface proteins P25 and P28 mediate parasite entry into mosquito midgut epithelial cells? Malaria journal 4, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beetsma AL, van de Wiel TJ, Sauerwein RW and Eling WM (1998). Plasmodium berghei ANKA: purification of large numbers of infectious gametocytes. Experimental parasitology 88, 69–72. [DOI] [PubMed] [Google Scholar]
- Billker O, Miller AJ and Sinden RE (2000). Determination of mosquito bloodmeal pH in situ by ion-selective microelectrode measurement: implications for the regulation of malarial gametogenesis. Parasitology 120 ( Pt 6), 547–551. [DOI] [PubMed] [Google Scholar]
- Blagborough AM and Sinden RE (2009). Plasmodium berghei HAP2 induces strong malaria transmission-blocking immunity in vivo and in vitro. Vaccine 27, 5187–5194. [DOI] [PubMed] [Google Scholar]
- Bousema T. and Drakeley C. (2011). Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clinical microbiology reviews 24, 377–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Hart RJ, Bansal GP and Kumar N. (2018). Functional Conservation of P48/45 Proteins in the Transmission Stages of Plasmodium vivax (Human Malaria Parasite) and P. berghei (Murine Malaria Parasite). MBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. and Nijhout MM (1977). Control of gamete formation (exflagellation) in malaria parasites. Science 195, 407–409. [DOI] [PubMed] [Google Scholar]
- Chowdhury DR, Angov E, Kariuki T. and Kumar N. (2009). A potent malaria transmission blocking vaccine based on codon harmonized full length Pfs48/45 expressed in Escherichia coli. PloS one 4, e6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligianni E, Morgan RN, Bertuccini L, Wirth CC, Silmon de Monerri NC, Spanos L, et al. (2013). A perforin-like protein mediates disruption of the erythrocyte membrane during egress of Plasmodium berghei male gametocytes. Cellular microbiology 15, 1438–1455. [DOI] [PubMed] [Google Scholar]
- Depoix D, Marques SR, Ferguson DJ, Chaouch S, Duguet T, Sinden RE, et al. (2020). Vital role for Plasmodium berghei Kinesin8B in axoneme assembly during male gamete formation and mosquito transmission. Cellular microbiology 22, e13121. [DOI] [PubMed] [Google Scholar]
- Eksi S, Czesny B, van Gemert GJ, Sauerwein RW, Eling W. and Williamson KC (2006). Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Molecular microbiology 61, 991–998. [DOI] [PubMed] [Google Scholar]
- Eksi S, Stump A, Fanning SL, Shenouda MI, Fujioka H. and Williamson KC (2002). Targeting and sequestration of truncated Pfs230 in an intraerythrocytic compartment during Plasmodium falciparum gametocytogenesis. Molecular microbiology 44, 1507–1516. [DOI] [PubMed] [Google Scholar]
- Fang H, Klages N, Baechler B, Hillner E, Yu L, Pardo M, et al. (2017). Multiple short windows of calcium-dependent protein kinase 4 activity coordinate distinct cell cycle events during Plasmodium gametogenesis. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonager J, Pasini EM, Braks JA, Klop O, Ramesar J, Remarque EJ, et al. (2012). Reduced CD36-dependent tissue sequestration of Plasmodiuminfected erythrocytes is detrimental to malaria parasite growth in vivo. The Journal of experimental medicine 209, 93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttery DS, Poulin B, Ramaprasad A, Wall RJ, Ferguson DJ, Brady D, et al. (2014). Genome-wide functional analysis of Plasmodium protein phosphatases reveals key regulators of parasite development and differentiation. Cell host & microbe 16, 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, Berriman M, et al. (2005). A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 307, 82–86. [DOI] [PubMed] [Google Scholar]
- Healer J, McGuinness D, Hopcroft P, Haley S, Carter R. and Riley E. (1997). Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infection and immunity 65, 3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitz E, Balestra AC, Brochet M. and Voss TS (2020). PfMAP-2 is essential for male gametogenesis in the malaria parasite Plasmodium falciparum. Scientific reports 10, 11930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse CJ, Ramesar J. and Waters AP (2006). High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nature protocols 1, 346–356. [DOI] [PubMed] [Google Scholar]
- Kehrer J, Frischknecht F. and Mair GR (2016). Proteomic Analysis of the Plasmodium berghei Gametocyte Egressome and Vesicular bioID of Osmiophilic Body Proteins Identifies Merozoite TRAP-like Protein (MTRAP) as an Essential Factor for Parasite Transmission. Molecular & cellular proteomics : MCP 15, 2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Franke-Fayard B, Mair GR, Lasonder E, Janse CJ, Mann M. and Waters AP (2005). Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell 121, 675–687. [DOI] [PubMed] [Google Scholar]
- Kou X, Zheng W, Du F, Liu F, Wang M, Fan Q, et al. (2016). Characterization of a Plasmodium berghei sexual stage antigen PbPH as a new candidate for malaria transmission-blocking vaccine. Parasites & vectors 9, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn A. and Pradel G. (2010). The coming-out of malaria gametocytes. Journal of biomedicine & biotechnology 2010, 976827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. (1987). Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite immunology 9, 321–335. [DOI] [PubMed] [Google Scholar]
- Lasonder E, Rijpma SR, van Schaijk BC, Hoeijmakers WA, Kensche PR, Gresnigt MS, et al. (2016). Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: molecular insight into sex-specific processes and translational repression. Nucleic acids research 44, 6087–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartz F, Brod F, Dabbs R, Miura K, Mekhaiel D, Marini A, et al. (2018). Structural basis for recognition of the malaria vaccine candidate Pfs48/45 by a transmission blocking antibody. Nature communications 9, 3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Annoura T, Sajid M, Chevalley-Maurel S, Ramesar J, Klop O, et al. (2011). A novel ‘gene insertion/marker out’ (GIMO) method for transgene expression and gene complementation in rodent malaria parasites. PloS one 6, e29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Liu Q, Yu C, Zhao Y, Wu Y, Min H, et al. (2019). An MFS-Domain Protein Pb115 Plays a Critical Role in Gamete Fertilization of the Malaria Parasite Plasmodium berghei. Frontiers in microbiology 10, 2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zhao Y, Zheng L, Zhu X, Cui L. and Cao Y. (2018). The Glycosylphosphatidylinositol Transamidase Complex Subunit PbGPI16 of Plasmodium berghei Is Important for Inducing Experimental Cerebral Malaria. Infection and immunity 86, e00929–00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tewari R, Ning J, Blagborough AM, Garbom S, Pei J, et al. (2008). The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes & development 22, 1051–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald NJ, Nguyen V, Shimp R, Reiter K, Herrera R, Burkhardt M, et al. (2016). Structural and Immunological Characterization of Recombinant 6-Cysteine Domains of the Plasmodium falciparum Sexual Stage Protein Pfs230. The Journal of biological chemistry 291, 19913–19922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques SR, Ramakrishnan C, Carzaniga R, Blagborough AM, Delves MJ, Talman AM and Sinden RE (2015). An essential role of the basal body protein SAS-6 in Plasmodium male gamete development and malaria transmission. Cellular microbiology 17, 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Chen Z, Wang Z, Shrestha S, Li X, Li R. and Cui L. (2017). Sex-specific biology of the human malaria parasite revealed from the proteomes of mature male and female gametocytes. Molecular & cellular proteomics : MCP 16, 537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A. and Long CA (2008). Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine 26, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Cruz A, Canepa GE and Barillas-Mury C. (2017). Plasmodium P47: a key gene for malaria transmission by mosquito vectors. Curr Opin Microbiol 40, 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout MM (1979). Plasmodium gallinaceum: exflagellation stimulated by a mosquito factor. Experimental parasitology 48, 75–80. [DOI] [PubMed] [Google Scholar]
- Nilsson SK, Childs LM, Buckee C. and Marti M. (2015). Targeting Human Transmission Biology for Malaria Elimination. PLoS pathogens 11, e1004871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes JK, Woods C, Carter T, Raphael T, Morin MJ, Diallo D, et al. (2014). Development of a transmission-blocking malaria vaccine: progress, challenges, and the path forward. Vaccine 32, 5531–5539. [DOI] [PubMed] [Google Scholar]
- Obrova K, Cyrklaff M, Frank R, Mair GR and Mueller AK (2019). Transmission of the malaria parasite requires ferlin for gamete egress from the red blood cell. Cellular microbiology 21, e12999. [DOI] [PubMed] [Google Scholar]
- Olivieri A, Bertuccini L, Deligianni E, Franke-Fayard B, Curra C, Siden-Kiamos I, et al. (2015). Distinct properties of the egress-related osmiophilic bodies in male and female gametocytes of the rodent malaria parasite Plasmodium berghei. Cellular microbiology 17, 355–368. [DOI] [PubMed] [Google Scholar]
- Pace T, Grasso F, Camarda G, Suarez C, Blackman MJ, Ponzi M. and Olivieri A. (2019). The Plasmodium berghei serine protease PbSUB1 plays an important role in male gamete egress. Cell Microbiol 21, e13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace T, Olivieri A, Sanchez M, Albanesi V, Picci L, Siden Kiamos I, et al. (2006). Set regulation in asexual and sexual Plasmodium parasites reveals a novel mechanism of stage-specific expression. Molecular microbiology 60, 870–882. [DOI] [PubMed] [Google Scholar]
- Paul RE, Brey PT and Robert V. (2002). Plasmodium sex determination and transmission to mosquitoes. Trends in parasitology 18, 32–38. [DOI] [PubMed] [Google Scholar]
- Ponzi M, Siden-Kiamos I, Bertuccini L, Curra C, Kroeze H, Camarda G, et al. (2009). Egress of Plasmodium berghei gametes from their host erythrocyte is mediated by the MDV-1/PEG3 protein. Cellular microbiology 11, 1272–1288. [DOI] [PubMed] [Google Scholar]
- Read D, Lensen AH, Begarnie S, Haley S, Raza A. and Carter R. (1994). Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite immunology 16, 511–519. [DOI] [PubMed] [Google Scholar]
- Sebastian S, Brochet M, Collins MO, Schwach F, Jones ML, Goulding D, et al. (2012). A Plasmodium calcium-dependent protein kinase controls zygote development and transmission by translationally activating repressed mRNAs. Cell host & microbe 12, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Alaganan A, More KR, Lorthiois A, Thiberge S, Gorgette O, et al. (2019). Role of a patatin-like phospholipase in Plasmodium falciparum gametogenesis and malaria transmission. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sologub L, Kuehn A, Kern S, Przyborski J, Schillig R. and Pradel G. (2011). Malaria proteases mediate inside-out egress of gametocytes from red blood cells following parasite transmission to the mosquito. Cellular microbiology 13, 897–912. [DOI] [PubMed] [Google Scholar]
- Swearingen KE, Lindner SE, Shi L, Shears MJ, Harupa A, Hopp CS, et al. (2016). Interrogating the Plasmodium Sporozoite Surface: Identification of Surface-Exposed Proteins and Demonstration of Glycosylation on CSP and TRAP by Mass Spectrometry-Based Proteomics. PLoS pathogens 12, e1005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Ishino T, Takashima E, Tsuboi T. and Torii M. (2018). A male gametocyte osmiophilic body and microgamete surface protein of the rodent malaria parasite Plasmodium yoelii (PyMiGS) plays a critical role in male osmiophilic body formation and exflagellation. Cellular microbiology 20, e12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman AM, Lacroix C, Marques SR, Blagborough AM, Carzaniga R, Menard R. and Sinden RE (2011). PbGEST mediates malaria transmission to both mosquito and vertebrate host. Molecular microbiology 82, 462–474. [DOI] [PubMed] [Google Scholar]
- Templeton TJ, Keister DB, Muratova O, Procter JL and Kaslow DC (1998). Adherence of erythrocytes during exflagellation of Plasmodium falciparum microgametes is dependent on erythrocyte surface sialic acid and glycophorins. The Journal of experimental medicine 187, 1599–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari R, Dorin D, Moon R, Doerig C. and Billker O. (2005). An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Molecular microbiology 58, 1253–1263. [DOI] [PubMed] [Google Scholar]
- Tomas AM, Margos G, Dimopoulos G, van Lin LH, de Koning-Ward TF, Sinha R, et al. (2001). P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. The EMBO journal 20, 3975–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin CJ, van Dooren GG, Spurck TP, Struck NS, Good RT, Handman E, et al. (2004). Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Molecular and biochemical parasitology 137, 13–21. [DOI] [PubMed] [Google Scholar]
- van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JA, Dodemont HJ, et al. (2001). A central role for P48/45 in malaria parasite male gamete fertility. Cell 104, 153–164. [DOI] [PubMed] [Google Scholar]
- van Dijk MR, van Schaijk BC, Khan SM, van Dooren MW, Ramesar J, Kaczanowski S, et al. (2010). Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS pathogens 6, e1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivax Sporozoite C. (2019). Transcriptome and histone epigenome of Plasmodium vivax salivary-gland sporozoites point to tight regulatory control and mechanisms for liver-stage differentiation in relapsing malaria. Int J Parasitol 49, 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Qian P, Cui H, Yao L. and Yuan J. (2020). A protein palmitoylation cascade regulates microtubule cytoskeleton integrity in Plasmodium. The EMBO journal 39, e104168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass MN, Stanway R, Blagborough AM, Lal K, Prieto JH, Raine D, et al. (2012). Proteomic analysis of Plasmodium in the mosquito: progress and pitfalls. Parasitology 139, 1131–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2019) World Malaria Report. [Google Scholar]
- Wirth CC, Glushakova S, Scheuermayer M, Repnik U, Garg S, Schaack D, et al. (2014). Perforin-like protein PPLP2 permeabilizes the red blood cell membrane during egress of Plasmodium falciparum gametocytes. Cellular microbiology 16, 709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth CC and Pradel G. (2012). Molecular mechanisms of host cell egress by malaria parasites. International journal of medical microbiology : IJMM 302, 172–178. [DOI] [PubMed] [Google Scholar]
- Wootton JC (1994). Non-globular domains in protein sequences: automated segmentation using complexity measures. Comput Chem 18, 269–285. [DOI] [PubMed] [Google Scholar]
- Wu Y, Sinden RE, Churcher TS, Tsuboi T. and Yusibov V. (2015). Development of malaria transmission-blocking vaccines: from concept to product. Advances in parasitology 89, 109–152. [DOI] [PubMed] [Google Scholar]
- Yeoh LM, Goodman CD, Mollard V, McFadden GI and Ralph SA (2017). Comparative transcriptomics of female and male gametocytes in Plasmodium berghei and the evolution of sex in alveolates. BMC genomics 18, 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanghi G, Vembar SS, Baumgarten S, Ding S, Guizetti J, Bryant JM, et al. (2018). A Specific PfEMP1 Is Expressed in P. falciparum Sporozoites and Plays a Role in Hepatocyte Infection. Cell reports 22, 2951–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Kou X, Du Y, Liu F, Yu C, Tsuboi T, et al. (2016). Identification of three ookinete-specific genes and evaluation of their transmission-blocking potentials in Plasmodium berghei. Vaccine 34, 2570–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Liu F, He Y, Liu Q, Humphreys GB, Tsuboi T, et al. (2017). Functional characterization of Plasmodium berghei PSOP25 during ookinete development and as a malaria transmission-blocking vaccine candidate. Parasites & vectors 10, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Sun L, He Y, Wei H, Hong M, Liu F, et al. (2019). Plasmodium berghei serine/threonine protein phosphatase PP5 plays a critical role in male gamete fertility. International journal for parasitology 49, 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movie 1. Exflagellation of male gametocytes in the pb22-KO line. Gametocytes-infected blood collected from the mouse tail was diluted with the ookinete culture medium. The developed flagella were trapped by PVM or erythrocyte membrane over 30 min.
Supplemental Movie 2. Male gametes without flagella development in the pb22-KO line were not able to egress from the red blood cell over 30 min.