Abstract
Objective:
To develop a system for accurate staging of patients with locally advanced gastric adenocarcinoma (GA) who undergo neoadjuvant chemotherapy (NC) followed by gastrectomy with D2 lymphadenectomy (LAD).
Background:
NC followed by gastrectomy with D2LAD is commonly used for patients with locally advanced GA. The 8th American Joint Committee on Cancer (AJCC) ypTNM staging system was validated based on patients undergoing more limited LAD.
Methods:
We developed a modified system (m-ypTNM) based on overall survival (OS) of patients receiving NC followed by gastrectomy with D2 LAD at Memorial Sloan Kettering Cancer Center (MSKCC) and validated the system using data from an international cohort of patients that underwent a similar treatment.
Results:
Among 325 patients form the derivation cohort, 33 (10.2%) had ypT0N0/+ tumours, which are not classifiable under the AJCC system. Five-year OS for m-ypTNM stages I, II, IIIA, and IIIB were 89%, 71%, 42%, and 10%, respectively, compared with 82%, 65%, and 29% for AJCC stages I, II, and III, respectively. The concordance index (C-index, 0.730 vs. 0.709), estimated area under the curve (0.765 vs. 0.740), and time-dependent ROC curve throughout the observation period were all superior for m-ypTNM staging. For the validation cohort of 186 patients, the m-ypTNM system was again better at separating patients into prognostic groups for OS.
Conclusion:
The m-ypTNM staging system stages improves the accuracy of OS prediction for patients treated with NC followed by gastrectomy with D2 LAD.
Keywords: Gastric adenocarcinoma, Neoadjuvant chemotherapy, Gastrectomy, Staging, Outcomes
INTRODUCTION
Worldwide, gastric cancer is the fifth most common cancer and the third leading cause of cancer-related death1. In the United States alone, there were an estimated 26,240 new cases and 10,800 deaths related to gastric cancer in 20182. Except in a few Asian countries such as Japan and South Korea where endoscopic screening is widespread, the majority of gastric cancer patients present with locally advanced or metastatic disease3.
There is increasing evidence that neoadjuvant and adjuvant chemotherapy can increase overall survival in patients with gastric adenocarcinoma4–6. In Japan and Korea, the majority of patients undergo gastrectomy and adjuvant chemotherapy decisions are based on surgical specimen pathology7. One major advantage of this strategy is that risk stratification based on surgical pathology is more accurate than clinical staging. In many centers in Europe and the United States, patients with gastric cancer undergo clinical staging and patients with locally advanced disease are given neoadjuvant chemotherapy. One major disadvantage of this approach is that the resection specimen has undergone changes of he primary tumour and lymph nodes affecting accurate staging of the disease.
The American Joint Committee on Cancer (AJCC) staging system is the most widely used staging system for gastric cancer8. The 8th edition of the AJCC Cancer Staging Manual for gastric adenocarcinoma introduced both a clinical staging system (cTNM) and a staging system for those receiving neoadjuvant therapy (ypTNM)9. The AJCC ypTNM staging system was validated based on the United States National Cancer Database (NCDB)10. The mean number of resected nodes among the 40,281 patients with gastric adenocarcinoma treated surgically in the NCDB database from 2004 to 2014 was 16.0 ± 10.92. 11 A mean of 16 nodes examined means that substantial proportion of patients in the NCDB have fewer nodes examined than what the AJCC recommends. As limited lymphadenectomy leads to understaging12, 13, the AJCC system may not be accurate for patients undergoing more extensive D2 lymphadenectomy. At Memorial Sloan Kettering Cancer Center (MSKCC) the mean number of resected nodes following D2 lymphadenctomy is 26.6 ± 11.914. In addition, the NCDB patients used to validate the AJCC ypTNM staging system had both neoadjuvant chemotherapy as well as chemoradiation10. Finally, patients with complete primary tumour regression (ypT0) are not included in the AJCC ypTNM staging system and thus are left without a stage designation.
The objective of this study was to develop an accurate staging system for patients with locally advanced gastric adenocarcinoma who undergo neoadjuvant chemotherapy followed by gastrectomy and D2 lymphadenectomy. Such a system would allow us to give more accurate predictions of survival.
PATIENTS AND METHODS
Study Population
For the training set, the institutional database at MSKCC was reviewed following Institutional Review Board (IRB) approval. Inclusion criteria were: histologically confirmed primary gastric adenocarcinoma or Siewert II or III gastroesophageal junction adenocarcinoma, no distant metastasis; administration of neoadjuvant chemotherapy, and R0 gastrectomy with D2 lymphadenectomy. The exclusion criteria included preoperative chemoradiotherapy or radiation therapy, incomplete histopathological or survival data, gastric remnant carcinoma, death within 30 days of surgery.
The validation set consisted of patients treated between 2000 and 2014 who satisfied the aforementioned inclusion and exclusion criteria. The first group was from Fujian Medical University Union Hospital (FMUUH) in Fujian, China, a tertiary referral center for gastric cancer that performs more than 800 gastrectomies per year for gastric adenocarcinoma. The other group was from the International study group on Minimally Invasive surgery for Gastric Cancer (IMIGASTRIC) trial (registration number NCT02325453)15, which includes centers in Europe, Asia, and North America.
Patient Characteristics and Clinicopathologic Data
Demographic and clinicopathologic characteristics and treatment information for the study population were determined by review of the database and medical records. T status, N status, and ypTNM stage were determined using the 8th edition of the AJCC staging system9. Pathological response to neoadjuvant chemotherapy was assessed by experienced gastric cancer pathologists. In general, this involved both the gross and microscopic examination of the resected surgical specimen. If grossly viable tumour was present, a minimum of 5 representative sections of the tumour were evaluated. If no grossly viable tumour was present, the entire scar-like lesion at the primary tumour site was submitted for histopathologic evaluation. At the microscopic level, treatment-related effects were observed as abolition of the malignant epithelium and replacement by dense fibrosis or fibroinflammation. The pathologic response to treatment was determined by the amount of residual viable carcinoma in relation to the extent of fibrosis or fibroinflammation within the gross lesion. Acellular mucin was regarded as a form of treatment response and not as viable tumour16. The extent of lymphadenectomy (D1 or D2) was classified according to the Japanese Gastric Cancer Association definitions in the 2nd English Edition (1998) and the 3rd English Edition (2010)17, 18. This study included 10 patients in the training cohort who underwent proximal gastrectomy with removal of node stations node stations 1, 2, 3, 4sa, 4sb, 7, 8a, 9, 10, 11p, and 11d. In the 2nd English Edition for an upper third tumour, node stations 5, 6, and 12a were not part of a D2 lymphadenectomy18.
Follow-up and Outcome Data
Follow-up after resection generally consisted of visits to the outpatient department with blood tests (complete blood count, chemistry panel, CEA level, CA19–9 level) and CT scans repeated every 3–6 months for the first 2 years and every 6–12 months for years 3–5. The survival time was measured from the time of surgery to the last date tof follow up or date of death, whichever occurred first. Last date of follow up was July 2017.
Statistics
Statistical analyses were performed using SPSS software (version 22.0, SPSS Inc, Chicago, IL), R ver. 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria), and STATA version 12.0 (StataCorp, College Station, TX). The Kaplan-Meier method and log-rank test were used to compare survival curves. Multivariable analysis on OS was performed with the Cox proportional hazards regression model. Variables associated with overall survival with p<0.05 were included in the multivariable analyses. The prognostic abilities of the two staging systems were compared by generating time-dependent receiver operating characteristic (ROC) curves and by calculating the estimated area under the curve (AUC). Time-dependent ROC curve analysis is an extension of the ROC curve, which assesses the discriminatory power of continuous markers for time-dependent disease outcomes19, 20, the R package “time ROC” was used this analysis. Sequential AUCs were compared between the AJCC ypTNM and modified ypTNM staging systems using independent and identically distributed representations of the AUC estimators. In addition, the relative discriminatory abilities of the two ypTNM staging systems were also assessed using the Akaike Information Criteria (AIC) and Harrell’s concordance index (C-index). In general, a predictive model with a low AIC indicates a better model fit. The Harrell’s concordance index or C-index is a measure of goodness of fit for binary outcomes in a logistic regression. In our analysis, the C-index gives the probability that a randomly selected patient who died had a higher stage than a randomly selected patient who did not die. A high C-index represents better discriminatory ability21-23,24
Differences were assumed at p-values of less than 0.05 in a two-tailed test.
RESULTS
Patients and treatment
In total, 325 patients with neoadjuvant chemotherapy underwent potentially curative gastrectomy with D2 lymphadenectomy between January 2000 and December 2014. The validation set included 186 patients (FMUUH, n=98; IMIGASTRIC, n=88). Patient’s demographics, treatment, and pathology are shown in Table 1. All patients in both the training and validation set were treated with 5-FU and/or cisplatin-based neoadjuvant chemotherapy for 2–6 cycles followed by gastrectomy with D2 lymphadenectomy. There were more distal gastrectomies in the training set compared to the validation set (50% vs. 22%). About half of the patients in both cohorts received post-operative adjuvant chemotherapy or chemoradiotherapy.
Table 1.
Demographic, treatment, and pathologic variables in the training and validation cohorts
| Training cohort (n=325) |
Validation cohort (n=186) |
p-value | |
|---|---|---|---|
| Age, years (mean +/− s.d.) | 61.4 ± 11.6 | 58.6 ± 11.8 | 0.010 |
| ≤ 60 | 139 (48.2%) | 100 (53.8%) | 0.017 |
| > 60 | 186 (57.2%) | 86 (46.2%) | |
| Sex | 0.018 | ||
| Male | 195 (60.0%) | 131 (70.4%) | |
| Female | 130 (40.0%) | 55 (29.6%) | |
| Body mass index, kg/m2 (mean +/− s.d.) | 26.7 ± 4.7 | 23.2 ± 3.8 | <0.001 |
| Race | <0.001 | ||
| Caucasian | 233 (71.7%) | 88 (47.3%) | |
| Asian/other | 92 (28.3%) | 98 (52.7%) | |
| Type of gastectomy | <0.001 | ||
| Distal gastrectomy | 161 (49.5%) | 40 (21.5%) | |
| Total gastrectomy | 154 (47.4%) | 146 (78.5%) | |
| Proximal gastrectomy | 10 (3.1%) | 0 (0%) | |
| Surgical approach | <0.001 | ||
| Open | 283 (87.1%) | 91 (48.9%) | |
| Minimally invasive | 42 (12.9%) | 95 (51.1%) | |
| Post-operative adjuvant therapy | 0.001 | ||
| None | 168 (51.7%) | 92 (49.5%) | |
| Adjuvant chemotherapy | 136 (41.8%) | 94 (50.5%) | |
| Adjuvant chemoradiotherapy | 21 (6.5%) | 0 (0%) | |
| Tumour size, cm (mean +/− s.d.) | 4.0 ± 3.3 | 5.7 ± 2.6 | <0.001 |
| Tumour location | 0.001 | ||
| Lower third | 135 (41.5%) | 46 (24.7%) | |
| Middle third | 75 (23.1%) | 56 (30.1%) | |
| Upper third | 102 (31.4%) | 68 (36.6%) | |
| More than two parts | 13 (4.0%) | 16 (8.6%) | |
| Lauren type | - | - | |
| Intestinal | 156 (48.0%) | - | |
| Diffuse | 107 (32.9%) | - | |
| Mixed/unknown | 62 (19.1%) | - | |
| Differentiation | <0.001 | ||
| Differentiated | 173 (53.2%) | 69 (37.1%) | |
| Undifferentiated/unknown | 152 (46.8%) | 117 (62.9%) | |
| Vascular invasion | - | ||
| Yes | 163 (50.2%) | - | |
| No | 162 (49.8%) | - | |
| Perineural invasion | - | ||
| Yes | 159 (48.9%) | - | |
| No | 166 (51.1%) | - | |
| Treatment effect | - | ||
| 0–49% | 192 (59.1%) | - | |
| 50–89% | 73 (22.5%) | - | |
| 90–100% | 60 (18.5%) | - | |
| ≥16 lymph nodes examined | 301 (92.6%) | 179 (96.2%) | 0.099 |
| Lymph nodes examined (mean +/− s.d.) | 26.6 ± 11.9 | 31.4 ± 11.9 | <0.001 |
| Positive lymph nodes (mean +/− s.d.) | 3.2 ± 5.6 | 5.7 ± 2.6 | <0.001 |
| ypT status | <0.001 | ||
| T0 | 33 (10.2%) | 1 (0.5%) | |
| T1 | 39 (12.0%) | 17 (9.2%) | |
| T2 | 41 (12.6%) | 33 (17.7%) | |
| T3 | 127 (39.0%) | 62 (33.3%) | |
| T4 | 85 (26.2%) | 73 (39.3%) | |
| ypN status | <0.001 | ||
| N0 | 147 (45.2%) | 55 (29.6%) | |
| N1 | 70 (21.5%) | 31 (16.7%) | |
| N2 | 48 (14.8%) | 36 (19.4%) | |
| N3a | 49 (15.1%) | 45 (24.2%) | |
| N3b | 11 (3.4%) | 19 (10.2%) | |
| ypTNM stage | <0.001 | ||
| ypT0N0/+ | 33 (10.2%) | 1 (0.5%) | |
| I | 51 (15.7%) | 26 (14.0%) | |
| II | 125 (38.5%) | 57 (30.7%) | |
| III | 116 (35.7%) | 102 (54.8%) | |
In the training set, tumours were significantly smaller, more often located in the lower third of the stomach, more often well or moderately differentiated and had an earlier disease stage as compared to the validation set (Table 1). Treatment effect data was not available for the validation set.
Demographics, treatment, and pathologic characteristics for the FMUUH and IMIGASTRIC cohorts of the validation cohort are shown in Supplementary Table 1.
Outcomes
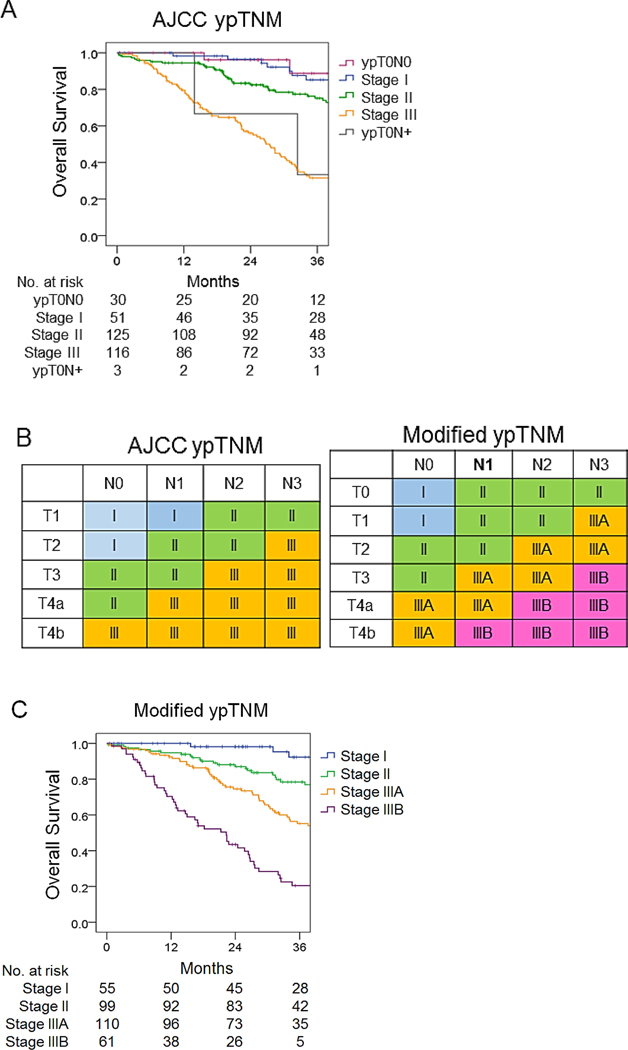
The median follow-up was 86 months in training cohort and 71 months in validation cohort. The 5-year overall survival (OS) rate in the training cohort was 52%. For patients that died during follow-up, the vast majority of deaths were from gastric cancer. For the training cohort, there were 121 deaths during tfollow-up period, 111 of 121 patients (92%) died of disease, and 10/121 patients (8%) died of other causes. For the validation cohort, 80 patients died during the follow-up period, 77 of 80 patients (96%) died of disease, and 3 of 80 (4%) died of other causes. to the 8th edition of the AJCC ypTNM staging system, the 5-year OS rate for patients with stage I disease was 82%; for stage II, 65%; and for stage III, 24% with statistically significant differences among all stages (Fig. 1A; χ2=67.961, p<0.001). Survival for patients with ypT0N0 and stage I was similar as well as for ypT0N+ tumours and stage III (Fig. 1A, Suppl. Table 2).
Figure 1.
Overall survival of the training set based on two staging systems. (A) Kaplan-Meier overall survival for the training set according to 8th edition AJCC ypTNM stage, with the addition of ypT0N0 and ypT0N+. (B) AJCC ypTNM staging system and modified ypTNM staging system. (C) Kaplan-Meier overall survival for the training set according to modified ypTNM stage.
Modified ypTNM staging system
A modified ypTNM staging system using pathologic and overall survival data from the MSKCC training set was constructed. To develop the modified ypTNM staging system, each patient was classified, based on the 8th edition of the AJCC pTNM staging system, as stage IA, IB, IIA, IIB, IIIA, IIIB, or IIIC. This was done even though these patients had received neoadjuvant chemotherapy Patients with a complete pathologic response in their primary tumour were classified as ypT0N0 or ypT0N+. Overall survival was compared between each of pTNM stage groupings. (Suppl. Table 3). It was found that the 5-year survival rate for ypT0N0 patients was 87%, which was similar to that for pTNM IA patients (90%, p > 0.05). Survival was also similar between both pTNM IB and pTNM IIA and ypT0N+; pTNM IIB and pTNM IIIA; and pTNM IIIB and pTNM IIIC. Based on these findings, pTNM groups with similar overall survivals were grouped together to generate the modified ypTNM staging system. The modified system was compared with the 8th edition AJCC system in Fig. 1B. The 5-year OS rate for modified ypTNM stage I patients was 89%, stage II 71%, stage IIIA 42%, and stage IIIB 10%, with significant differences between each modified ypTNM stage (p < 0.01, Fig. 1C, Suppl. Table 4).
On univariable analysis, several clinicopathologic factors were associated with overall survival including tumour location, tumour size, differentiation type, Lauren type, vascular invasion, perineural invasion, treatment effect, AJCC ypTNM stage, and modified ypTNM stage (Table 2). On multivariable analysis including both ypTNM staging systems, modified ypTNM stage was still independently associated with OS (p=0.004) but AJCC ypTNM stage was not (p = 0.127). Two additional multivariable analyses, one excluding AJCC ypTNM stage and the other excluding modified ypTNM stage also showed that AJCC and modified ypTNM stage were independently associated with survival (Suppl. Table 5).
Table 2.
Univariable and multivariable analysis of clinicopathologic factors associated with overall survival.
| Characteristic | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| 5-yr (%) | p-value | OR | 95% CI | p-value | |
| Type of gastrectomy | 0.002 | 0.067 | |||
| Distal | 60.8 | Ref | |||
| Total/proximal | 42.5 | 1.882 | 0.980–3.613 | ||
| Tumour size, cm | 0.001 | 0.666 | |||
| ≤4.0 | 60.2 | Ref | |||
| >4.0 | 41.2 | 0.913 | 0.603–1.381 | ||
| Tumour location | <0.001 | 0.026 | |||
| Lower third | 62.2 | Ref | |||
| Middle third | 48.8 | 0.780 | 0.408–1.493 | 0.453 | |
| Upper third | 44.1 | 0.915 | 0.437–1.917 | 0.814 | |
| More than one part | 0.00 | 2.916 | 1.049–8.104 | 0.040 | |
| Lauren type | <0.001 | 0.812 | |||
| Intestinal | 62.0 | Ref | |||
| Diffuse | 38.2 | 0.910 | 0.446–1.855 | 0.795 | |
| Mixed/unknown | 48.5 | 0.801 | 0.384–1.671 | 0.554 | |
| Differentiation type | <0.001 | 0.076 | |||
| Differentiated | 67.7 | Ref | |||
| Undifferentiated/unknown | 37.2 | 1.803 | 0.940–3.458 | ||
| Vascular invasion | <0.001 | 0.416 | |||
| Yes | 37.0 | Ref | |||
| No | 65.3 | 1.215 | 0.760–1.943 | ||
| Perineural invasion | <0.001 | 0.843 | |||
| Yes | 39.8 | Ref | |||
| No | 63.8 | 0.956 | 0.614–1.489 | ||
| Treatment effect | 0.020 | 0.354 | |||
| 0–49% | 46.4 | Ref | |||
| 50–89% | 56.9 | 1.085 | 0.665–1.769 | 0.744 | |
| 90–100% | 61.7 | 1.899 | 0.793–4.550 | 0.150 | |
| AJCC ypTNM stage | <0.001 | 0.127 | |||
| I | 82.1 | Ref | |||
| II | 62.9 | 1.487 | 0.480–4.605 | 0.491 | |
| III | 23.9 | 2.678 | 0.740–9.693 | 0.133 | |
| Modified ypTNM stage | <0.001 | 0.004 | |||
| I | 86.5 | Ref | |||
| II | 74.1 | 2.349 | 0.420–13.154 | 0.331 | |
| IIIA | 45.3 | 3.506 | 0.542–22.682 | 0.188 | |
| IIIB | 9.7 | 8.585 | 1.224–60.222 | 0.031 | |
Comparison of the AJCC and modified ypTNM staging
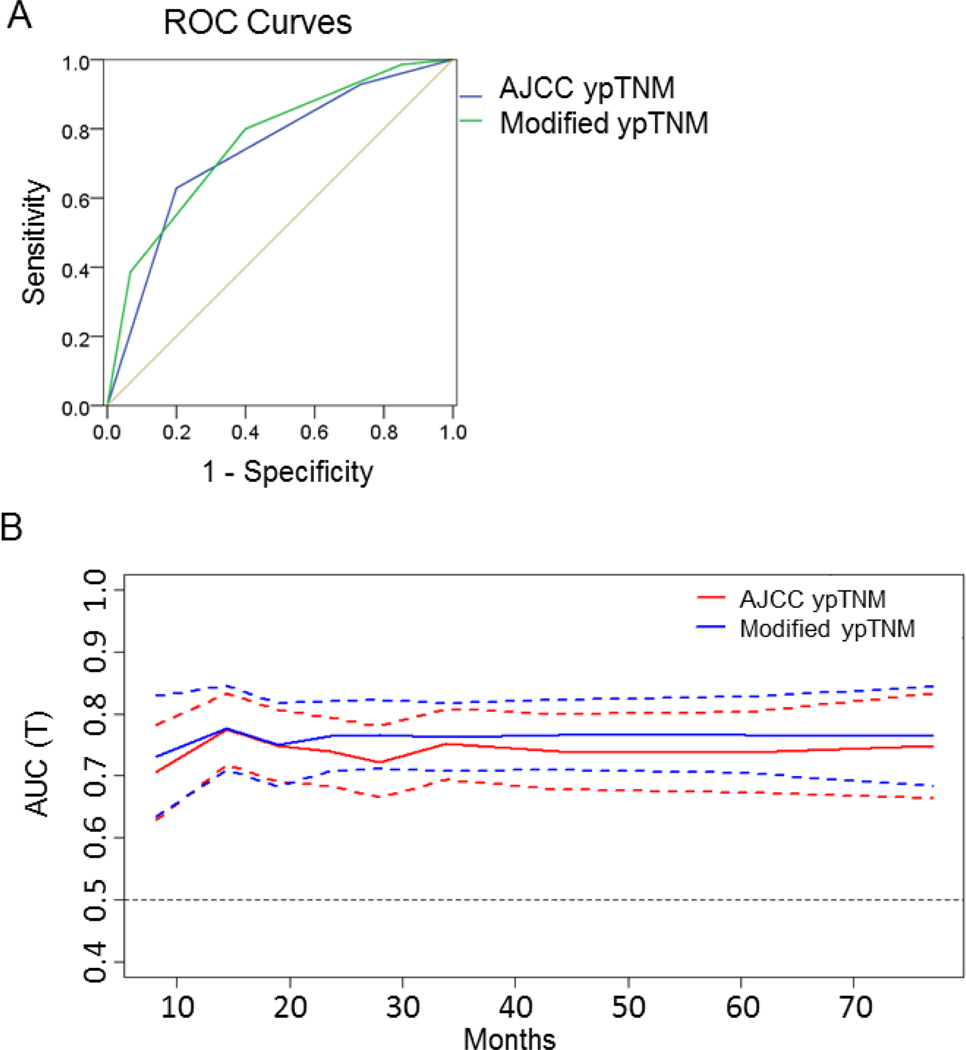
The C-index was 0.730 for the modified ypTNM staging system and 0.709 for the AJCC ypTNM staging system. Integrating the estimated areas under the ROC curves revealed that the modified ypTNM system was superior to the AJCC ypTNM staging system in predicting 5-year overall survival (AUC 0.765 vs. 0.740, Fig. 2A). Similarly, the time-dependent ROC curve of the modified system was continuously superior to that of the AJCC system (Fig. 2B). Moreover, the modified system also had a smaller Akaike Information Criteria (AIC) value (1,428.56 vs. 1,450.38 for the AJCC system), thereby indicating more accurate prognostic stratification.
Figure 2.
Survival discrimination and prognostic accuracy of the 8th edition AJCC and modified ypTNM staging systems for the training set. ROC curves (A) and time-dependent ROC curves (B) for the AJCC ypTNM and m-ypTNM staging systems. Solid lines represent time dependent ROC curves and dotted lines represent 95% confidence intervals.
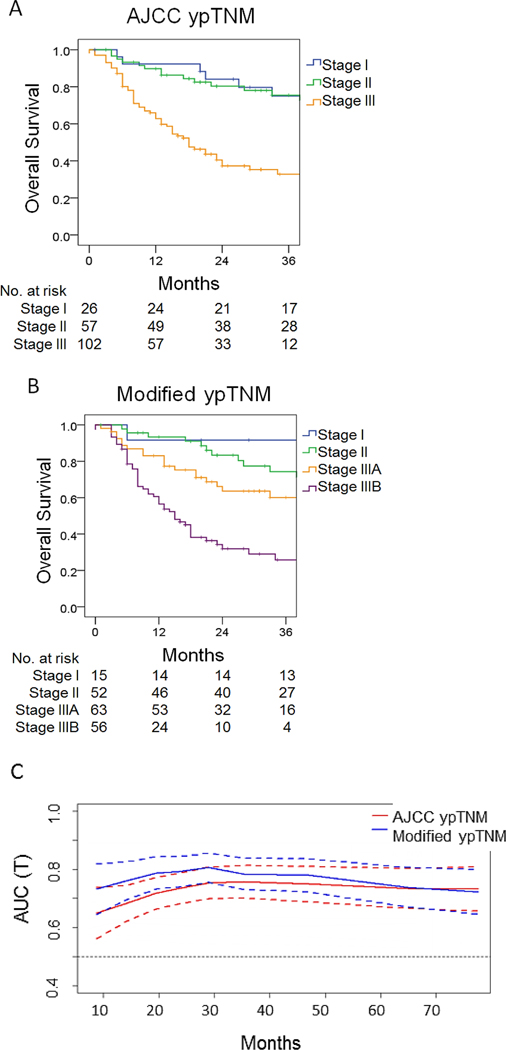
In the validation set, the AJCC ypTNM staging system failed to discriminate the survival of stage I patients from that of stage II patients (5-year overall survival 60% and 62%, respectively), and there was a significant gap in survival between stage I/II and stage III (5-year overall survival 19%) (Fig. 3A). The survival of patients classified using the modified ypTNM system differed significantly among all stages (Fig. 3B). The 5-year overall survival of ypTNM stage I, II, and III was 58.8%, 58.5%, and 22.1%, respectively. The time-dependent ROC curve of the modified ypTNM staging system was superior to that of the AJCC ypTNM system for 5 years postoperatively (Fig. 3C). The modified system also showed a higher C-index (0.688 vs. 0.657), a larger AUC (0.691 vs. 0.652), and smaller AIC (831.45 vs. 842.61) than the AJCC system.
Figure 3.
Kaplan-Meier overall survival in the validation set according to AJCC TNM stage (A) and modified ypTNM stage (B). (C) Time-dependent ROC curves for the 8th edition AJCC and modified ypTNM staging systems.
Effect of pathologic response to neoadjuvant treatment
Overall survival was stratified based on percent treatment effect (0–49%, 50–89%, and 90–100%). In the training cohort, an increased treatment effect was associated with improved overall survival (Suppl. Fig. 1A). However, there was no statistically significant difference in survival based on percent treatment effect when individual modified ypTNM stages were examined (Suppl. Fig. 1B-E).
DISCUSSION
In this study, a modified ypTNM staging system for patients undergoing neoadjuvant chemotherapy followed by gastrectomy with D2 lymphadenectomy tumourbetter predicted survival than the AJCC ypTNM system according to multiple measures of prognostic value. Most notably, time-dependent ROC curves indicated that the modified system was continuously superior to the AJCC system over the 5 years following surgery. Validation of the modified ypTNM system using an external multi-institutional cohort demonstrated its relevance across populations of patients treated with neoadjuvant chemotherapy followed by gastrectomy with D2 lymphadenectomy.
More extensive lymphadenectomy improves the accuracy of staging12, 13 and prognostic prediction in patients with gastric cancer12, 25. There is significant variability in the extent of lymphadenectomy and number of lymph nodes examined among different institutions and regions. As a result, the number of examined lymph nodes can vary greatly among different patient cohorts. Several studies have demonstrated that examination of more than 16 lymph nodes improves the ability to predict prognosis in patients with gastric cancer12, 25. For example, overall survival of patients in one SEER database analysis, with an average of 8 harvested lymph nodes, was significantly inferior, stage for stage, when compared with survival of patients at MSKCC, where 81% of patients underwent D2 lymphadenectomy and a median of 22 lymph nodes were examined14. Thus it is not surprising that the AJCC ypTNM staging system, which is based on patients who generally underwent less than a D2 lymphadenectomy, does not accurately stage patients who all underwent D2 lymphadenectomy.
Neoadjuvant chemotherapy is being increasingly used for patients with locally advanced gastric adenocarcinoma. The perioperative chemotherapy regimen used is this study was based primarily on the MAGIC trial 26. In our training and validation cohorts, patients did not receive postoperative chemotherapy for reasons similar to that of patients in the MAGIC study. The practice at MSKCC for patients who are tolerating preoperative chemotherapy well and who are responding to chemotherapy based on radiologic studies is to try and complete the 6 cycles of chemotherapy in the preoperative setting. This was accomplished in about 15% of MSKCC patients.
The recently reported FLOT4-AIO trial 27 reported pathological complete regression in 6% in the MAGIC group and 16% in the FLOT group28. For those that underwent surgery, there was at least partial tumour regression for the majority of patients in both groups. The major advantage of neoadjuvant chemotherapy is that patients can generally tolerate chemotherapy better before surgery than after surgery29, but a major disadvantage is that downstaging of both T status and N status makes determination of prognosis more difficult. The present study directly addresses this problem of staging patients following neoadjuvant chemotherapy.
The AJCC system was based on patients given either neoadjuvant chemotherapy or chemoradiotherapy before surgery while the modified system was based on neoadjuvant chemotherapy only. The treatment effects of neoadjuvant chemoradiotherapy and chemotherapy may be quite different. The RTOG 9904 study found a pathological complete response to neoadjuvant chemoradiotherapy of 26%30, a much higher rate than that for neoadjuvant chemotherapy alone31–33. Similarly, a phase III study directly comparing the two treatments for patients with locally advanced adenocarcinoma of the lower esophagus or esophagogastric junction showed that treatment with chemoradiotherapy followed by surgery led to a significantly higher pathologic complete response rate (15.6% vs. 2.0%) and was associated with a statistically insignificant survival advantage (3-year survival rate, 47% vs. 28%)5.
TNM staging after chemotherapy is itself influenced by the degree of tumour response. Downstaging of both T status and N status can be inferred from some prospective randomized trials. For example in the MAGIC study, the proportion of patients with T3/T4 status and positive node status was 63.2% and 73.1%, respectively, in the surgery alone group, and 48.3% and 69.9%, respectively, in the neoadjuvant chemotherapy group26. Our system differs from the system proposed by Becker et al., which combined ypT and ypN categories with tumour regression grading. Their system was also more accurate in predicting survival than AJCC staging32. The omission of tumour response from our modified ypTNM should not limit its prognostic power, however, as this factor was not independently associated with survival in our analysis, and patients with more responsive tumours did not survive longer than patients with less responsive tumours when subdivided by stage (Suppl. Fig. 1B-E). In addition, tumour regression grading is not widely used, and its inclusion would result in a more complex staging system that may limit its application and use.
There are several limitations to this study. One major limitation is that various chemotherapy protocols were used for neoadjuvant treatment, and different treatment protocols may have influenced our findings. Second, the modified ypTNM staging system likely is not valid for patients undergoing more limited lymphadenectomy at non-referral institutions. Since the AJCC ypTNM staging system was developed from data from such patients, the AJCC system may be better for these patients. However, as we have published previously, staging gastric adenocarcinoma based on limited lymph node analysis results in less accurate staging34. Third, the number of patients with ypT0N0/+ status (33 in the training set and 1 in the validation set) was limited, so further investigation is needed to determine the accuracy of the modified ypTNM staging system for predicting prognosis in these patients. The relative lack of ypT0 patients in the validation set may be partly explained by global differences in treatment practices for locally advanced gastric adenocarcinoma. For example in China, neoadjuvant chemotherapy is often reserved for patients with more advanced tumours (cT4a/T4b).
Use of the modified staging system may aid oncologists in better predicting survival, making treatment decisions, and comparing cohorts of patients undergoing more extensive lymph node dissections.
Supplementary Material
ACKNOWLEDGMENTS
This research funded in part by the NIH/NCI Cancer Center Support Grant P30 CA008748, the Scientific and Technological Innovation Joint Capital Projects of Fujian Province, China (No.2016Y9031), and the Construction Project of Fujian Province Minimally Invasive Medical Center (No. [2017] 171). We also thank MSKCC senior editor Jessica Moore for editing this manuscript.
Footnotes
Conflicts of interest: The authors declare they have no conflicts of interest.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. Ca-Cancer J Clin 2011;61(2): 69–90. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. Ca-Cancer J Clin 2018;68(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Kim KM, Cheong J-H, Noh SH. Current management and future strategies of gastric cancer. Yonsei medical journal 2012;53(2): 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang LB, Shen JG, Xu CY, Chen WJ, Song XY, Yuan XM. Neoadjuvant chemotherapy versus surgery alone for locally advanced gastric cancer: a retrospective comparative study. Hepatogastroenterology 2008;55(86–87): 1895–1898. [PubMed] [Google Scholar]
- 5.Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, Langer P, Engenhart-Cabillic R, Bitzer M, Konigsrainer A, Budach W, Wilke H. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27(6): 851–856. [DOI] [PubMed] [Google Scholar]
- 6.Ott K, Sendler A, Becker K, Dittler H-J, Helmberger H, Busch R, Kollmannsberger C, Siewert JR, Fink U. Neoadjuvant chemotherapy with cisplatin, 5-FU, and leucovorin (PLF) in locally advanced gastric cancer: a prospective phase II study. Gastric cancer 2003;6(3): 159–167. [DOI] [PubMed] [Google Scholar]
- 7.Shen L, Shan Y-S, Hu H-M, Price TJ, Sirohi B, Yeh K-H, Yang Y-H, Sano T, Yang H-K, Zhang X. Management of gastric cancer in Asia: resource-stratified guidelines. The Lancet Oncology 2013;14(12): e535–e547. [DOI] [PubMed] [Google Scholar]
- 8.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017;67(2): 93–99. [DOI] [PubMed] [Google Scholar]
- 9.Amin MB; Edge SB; Greene FL; et al. e. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017: 203–220. [Google Scholar]
- 10.In H, Ravetch E, Langdon-Embry M, Palis B, Ajani JA, Hofstetter WL, Kelsen DP, Sano T . The newly proposed clinical and post-neoadjuvant treatment staging classifications for gastric adenocarcinoma for the American Joint Committee on Cancer (AJCC) staging. Gastric Cancer 2018;21(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 11.Naffouje SA, Salti GI. Extensive Lymph Node Dissection Improves Survival among American Patients with Gastric Adenocarcinoma Treated Surgically: Analysis of the National Cancer Database. J Gastric Cancer 2017;17(4): 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. Journal of clinical oncology 2005;23(28): 7114–7124. [DOI] [PubMed] [Google Scholar]
- 13.Bouvier AM, Haas O, Piard F, Roignot P, Bonithon‐Kopp C, Faivre J. How many nodes must be examined to accurately stage gastric carcinomas? Cancer 2002;94(11): 2862–2866. [DOI] [PubMed] [Google Scholar]
- 14.D’angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Annals of surgery 2004;240(5): 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desiderio J, Jiang ZW, Nguyen NT, Zhang S, Reim D, Alimoglu O, Azagra JS, Yu PW, Coburn NG, Qi F, Jackson PG, Zang L, Brower ST, Kurokawa Y, Facy O, Tsujimoto H, Coratti A, Annecchiarico M, Bazzocchi F, Avanzolini A, Gagniere J, Pezet D, Cianchi F, Badii B, Novotny A, Eren T, Leblebici M, Goergen M, Zhang B, Zhao YL, Liu T, Al-Refaie W, Ma J, Takiguchi S, Lequeu JB, Trastulli S, Parisi A. Robotic, laparoscopic and open surgery for gastric cancer compared on surgical, clinical and oncological outcomes: a multi-institutional chart review. A study protocol of the International study group on Minimally Invasive surgery for GASTRIc Cancer-IMIGASTRIC. BMJ Open 2015;5(10): e008198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73(11): 2680–2686. [DOI] [PubMed] [Google Scholar]
- 17.Japanese Gastric Cancer A Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer 1998;1(1): 10–24. [DOI] [PubMed] [Google Scholar]
- 18.Japanese Gastric Cancer A Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14(2): 101–112. [DOI] [PubMed] [Google Scholar]
- 19.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56(2): 337–344. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Alvarez MX, Meira-Machado L, Abu-Assi E, Raposeiras-Roubin S. Nonparametric estimation of time-dependent ROC curves conditional on a continuous covariate. Stat Med 2016;35(7): 1090–1102. [DOI] [PubMed] [Google Scholar]
- 21.Han DS, Suh YS, Kong SH, Lee HJ, Choi Y, Aikou S, Sano T, Park BJ, Kim WH, Yang HK. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol 2012;30(31): 3834–3840. [DOI] [PubMed] [Google Scholar]
- 22.Harrell FE Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15(4): 361–387. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE, Jr. Regression Modeling Strategies with Application to Linear Models, Logistic Regression, and Survival Analysis. New York, NY, Springer Verlag; 2001. [Google Scholar]
- 24.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15(4): 361–387. [DOI] [PubMed] [Google Scholar]
- 25.Shen JY, Kim S, Cheong JH, Kim YI, Hyung WJ, Choi WH, Choi SH, Wang LB, Noh SH. The impact of total retrieved lymph nodes on staging and survival of patients with pT3 gastric cancer. Cancer 2007;110(4): 745–751. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. New Engl J Med 2006;355(1): 11–20. [DOI] [PubMed] [Google Scholar]
- 27.Al-Batran S-E, Homann N, Schmalenberg H, Kopp H-G, Haag GM, Luley KB, Schmiegel WH, Folprecht G, Probst S, Prasnikar N. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. In: American Society of Clinical Oncology; 2017. [Google Scholar]
- 28.Al-Batran S-E, Hofheinz RD, Pauligk C, Kopp H-G, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg H. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. The Lancet Oncology 2016;17(12): 1697–1708. [DOI] [PubMed] [Google Scholar]
- 29.Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon J-P, Rougier P, Sakamoto J, Sargent D, Sasako M. Benefit of adjuvant chemotherapy for resectable gastric cancer a meta-analysis. 2010. [DOI] [PubMed] [Google Scholar]
- 30.Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, Greskovich JF, Anne PR, Bradley JD, Willett C, Rich TA. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol 2006;24(24): 3953–3958. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Xiong J, Wang J, Zheng L, Gao Y, Guan Z. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin in Chinese patients with advanced and metastatic gastric cancer: Re-analysis of efficacy and safety data from the ML17032 phase III clinical trial. Asia Pac J Clin Oncol 2018. [DOI] [PubMed] [Google Scholar]
- 32.Becker K, Reim D, Novotny A, zum Buschenfelde CM, Engel J, Friess H, Hofler H, Langer R. Proposal for a Multifactorial Prognostic Score That Accurately Classifies 3 Groups of Gastric Carcinoma Patients With Different Outcomes After Neoadjuvant Chemotherapy and Surgery. Annals of Surgery 2012;256(6): 1002–1007. [DOI] [PubMed] [Google Scholar]
- 33.Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J, Ott K, Hoelscher A, Schneider PM, Bechstein W, Wilke H, Lutz MP, Nordlinger B, Van Cutsem E, Siewert JR, Schlag PM. Neoadjuvant Chemotherapy Compared With Surgery Alone for Locally Advanced Cancer of the Stomach and Cardia: European Organisation for Research and Treatment of Cancer Randomized Trial 40954. Journal of Clinical Oncology 2010;28(35): 5210–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Dang P, Raut CP, Pandalai PK, Maduekwe UN, Rattner DW, Lauwers GY, Yoon SS. Comparison of a lymph node ratio–based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Annals of surgery 2012;255(3): 478–485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.