Abstract
Introduction.
Until recently, cholangiocarcinoma (CCA) was a largely overlooked disease, and among CCAs, extrahepatic CCA (eCCA) was even more neglected. Despite the growing impact of molecularly targeted therapies and immunotherapy, prognosis of eCCA is dismal. Therefore, unravelling the complex molecular landscape of eCCA has become an urgent need. Deep phenotyping studies have revealed that eCCA is a heterogeneous tumor, harboring specific alterations categorizable into four classes, “Mesenchymal”, “Proliferation”, “Immune”, and “Metabolic”. Molecular alterations convey the activation of several pro-oncogenic pathways, where either actionable drivers or outcome predictors can be identified.
Areas covered.
We offer insights on perturbed pathways, molecular profiling and actionable targets in eCCA and present a perspective on the potential stepping-stones to future progress. A systematic literature search in PubMed/ClinicalTrials.gov websites was performed by authors from different disciplines according to their specific topic knowledge to identify the newest and most relevant advances in precision medicine of eCCA.
Expert opinion.
eCCA is a distinct entity with unique features in terms of molecular classes, oncogenic drivers, and tumor microenvironment. Since more prevalent mutations are currently undruggable, and immunotherapy can be offered only to a minority of patients, international collaborations are instrumental to improve the understanding of the molecular underpins of this disease.
Keywords: cholangiocyte, cholangiocarcinoma, extrahepatic cholangiocarcinoma, epithelial-to-mesenchymal transition, immunotherapy, molecularly targeted therapies, oncogenic drivers, tumor microenvironment
1. INTRODUCTION
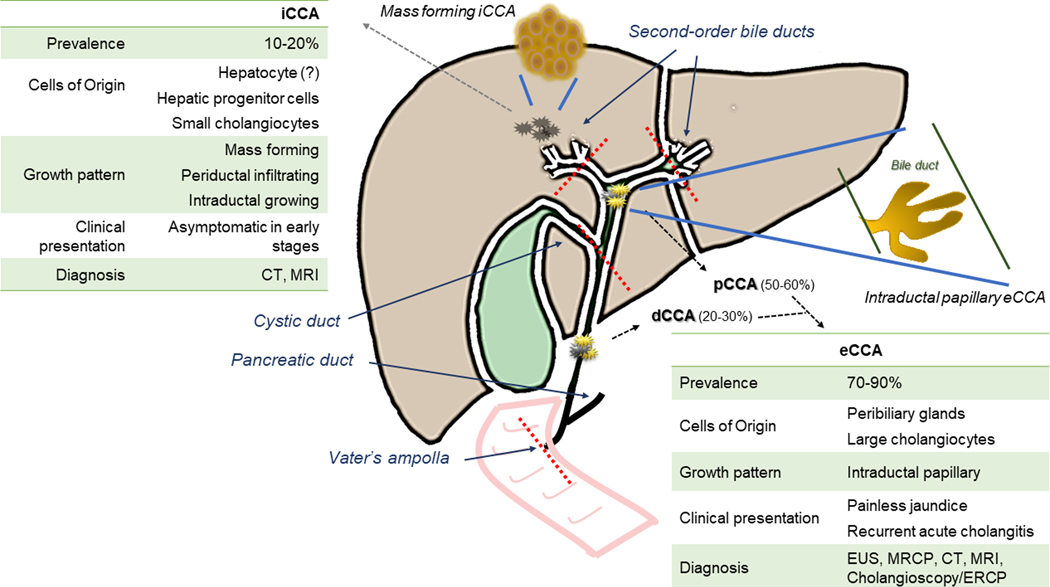
Cholangiocarcinomas (CCA) encompass a heterogeneous group of epithelial cancers with features of diverse degree of cholangiocyte differentiation that can arise from any segment of the biliary tree, except from the gallbladder, which represents a distinct type of cancer [1]. Currently, CCAs are classified as intrahepatic (iCCA), perihilar (pCCA), and distal (dCCA), based on the different anatomical location, each characterized by specific epidemiological and clinical features [1–3]. As shown in Figure 1, the point of demarcation between iCCA and pCCA is the confluence of the second order bile ducts, whereas the insertion of the cystic duct represents the border between pCCA and dCCA, which extends to the Vater’s ampulla. Generally, pCCA and dCCA are grouped in a single extrahepatic CCA (eCCA) entity, although they represent subtypes with distinct clinicopathological features, prognosis and therapeutic options. Unfortunately, most clinical trials have been conducted considering all CCAs as a single disease, sometimes including even gallbladder cancer, thereby hindering the development of tailored therapies aimed at the specific type of biliary tract cancer [4].
Figure 1. Criteria classification and main clinical features of eCCA compared with iCCA.
Cholangiocarcinomas (CCAs) are classified as intrahepatic (iCCA), and extrahepatic (eCCA), which includes the perihilar (pCCA) and distal (dCCA) variants. The central diagram illustrates the lines of demarcation (red dotted lines) along with the pattern of growth of each subtypes, mass forming, periductal infiltrating, intraductal growing (iCCA) and intraductal papillary (eCCA). Mass forming and intraductal papillary patterns are shown in enlarged cartoons (connected with the biliary system by blue lines). The boxes right- and left-sided highlight the main clinical differences between eCCA and iCCA, with respect to prevalence, cells of origin, pattern of growth, presentation and diagnosis. Of note, painless jaundice and recurrent acute cholangitis caused by biliary obstruction may favour a diagnosis earlier in eCCA than in iCCA. Moreover, diagnosis of eCCA harnesses imaging techniques, as endoscopy ultrasonography (EUS) and magnetic resonance cholangiopancreatography (MRCP), which allow a better visualization of the extrahepatic bile ducts, though computed tomography (CT) and magnetic resonance imaging (MRI) show the highest accuracy for both pCCA and iCCA, especially in the pre-operative setting. In the diagnostic work-up of eCCA, beside endoscopic retrograde cholangiopancreatography (ERCP), cholangioscopy is now a fundamental procedure, particularly in pCCA, as it can increase sensitivity up to 80–90% by providing visual inspection and direct sampling of tumoral lesions.
Typically, eCCAs are mucin-producing adenocarcinomas presenting as poorly defined sclerosing nodules or, less frequently, papillary tumours with periductular and/or intraductal growth (Figure 2) [5]. As cell origin, they may derive from transformation of columnar mucous cholangiocytes and/or of progenitor cells located in the peribiliary glands [6–8]. Biologically, eCCA is a highly aggressive: dedifferentiated tumor cells, a strong desmoplastic nature, with activation of cell survival and chemoresistance pathways, and a high genetic variability, are all factors contributing to early invasion and resistance to therapy [1,3,9]. Among all gastrointestinal cancers, eCCA accounts for 2–3% [3,10], with an apparent decrease in age-standardized incidence and mortality rates in the last decades. However, this trend must be interpreted with caution [11], since the nomenclature of eCCA has changed over time, sometimes including pCCAs and sometime not. This resulted in changes of incidence according to the inclusion or not of pCCA as eCCA. More recent studies indicate that indeed the worldwide incidence of both pCCA and dCCA is increasing in the last years [4]. CCA is considered a malignancy of the elderly population, however in the presence of risk factors, like chronic biliary inflammation [12], the disease may affect a younger population of patients [13,14] (Table 1). Diagnosis of eCCA is challenging; though painless jaundice and recurrent acute cholangitis may occur in the early phase, symptoms may be non-specific [15]. The diagnostic work-up includes laboratory, imaging, endoscopy, and pathology [3,4]. Contrast-enhanced computed tomography (CT) scan and magnetic resonance cholangiopancreatography (MRCP) are essential to select patients for curative surgery [16]. Endoscopic procedures provide both diagnostic and therapeutic potential [17], and the role of endoscopic ultrasound and cholangioscopy has expanded in the last decade [18] (Figure 1). Currently, diagnostic research aims to unveil tumor biomarkers enabling early detection of eCCA [19–26], since surgical resection with histologically negative margins, which remains the only potential curative options, can be performed in 35% of patients [27,28]. However, 5-years survival rates remain disappointing, ranging from 20 to 40% due to disease recurrence or metastasis [29,30]. Likewise, liver transplantation is an option only for a small subset of selected pCCA patients [31–35]. In advanced disease, systemic treatment has a limited impact on prognosis, with survival generally shorter than 12 months [36,37]. Thus, enrolling eCCA patients in clinical trials to test efficacy of new treatments is an urgent need [1,4]. Recent studies performed at different genetic, epigenetic, proteomic and microRNA levels have started to unravel the intricate molecular landscape underpinning the pathogenesis of CCA in relation to specific subtypes, and possible therapeutic targets are emerging, though none have been approved yet [38–40].
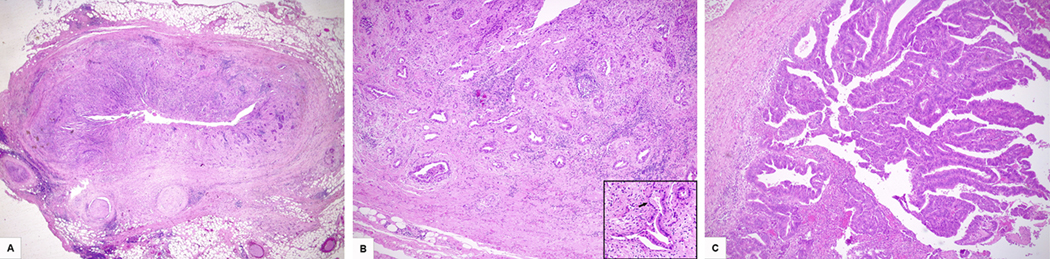
Figure 2. Histological features of eCCA.
A) Distal moderately differentiated cholangiocarcinoma arisen in the choledochus. The tumor extends into periductal soft tissues and shows diffuse peri-neural invasion (HE; original magnification 2x). B) Perihilar well differentiated cholangiocarcinoma (HE; original magnification 5x). The magnification in the lower right corner shows neoplastic glands lined by tall mucin-secreting cells (arrow) and immersed in a desmoplastic stroma (HE; original magnification 20x). C) Perihilar cholangiocarcinoma, papillary type (HE; original magnification 5x).
Table 1.
Risk factors for eCCA categorized according to odds ratio (OR).
| Risk factor | Disease |
|---|---|
| OR > 10 | Choledochal cyst [114] |
| Choledocholithiasis [114] | |
| Primary sclerosing cholangitis [115] | |
| Caroli disease [115] | |
| 3 < OR < 10 | Chronic pancreatitis [115] |
| Cholelithiasis [114] | |
| Liver fluke infection (Opisthorchis viverrini, Clonorchis sinensis) [116] | |
| Cirrhosis [114] | |
| OR < 3 | Nonalcoholic fatty liver disease [117] |
| Cholecystolithiasis [114] | |
| Alcohol-related disorders [114] | |
| Autoimmune hepatitis [114] | |
| Inflammatory bowel disease [114] | |
| HBV [114] | |
| HCV [114] | |
| Smoking [114] | |
| Type 2 diabetes [118] | |
| Hypertension [114] |
2. SIGNALING PERTURBATIONS, MOLECULAR PROFILING AND CLASSIFICATION IN eCCA
The understanding of the molecular landscape of eCCA is limited by the high heterogeneity of most studies conducted so far without distinction of the specific tumor subtypes. Recent studies based on next generation sequencing (NGS) have reported several genetic alterations in CCAs, including gene fusions, variations in copy number and point mutations [4]. Overall, these genetic alterations lead to profound changes in cell metabolism and functions, including Warburg effect, derangement of cell proliferation/survival ratio, overactivation of intracellular signals downstream of cell surface receptors including tyrosine kinase receptors (RTK), and epigenomic perturbations [4]. Table 2 summarizes the genetic changes detected in eCCA as compared with the other types of biliary tract cancers (BTCs). The differences in genetic mutations across the different CCA subtypes was first highlighted by Nakamura et al. [41] who investigated a cohort of 239 BTC, composed by 137 iCCA, 74 eCCA, and 28 gallbladder cancers. Notably, 40% of BTC showed mutations behaving as putative targets for intervention. In this study, eCCA harbored specific genetic mutations, in particular gene fusions of protein kinase cAMP-activated catalytic subunit (PRKAC)A and PRKACB, two serin/threonine protein kinases acting as catalytic subunits of the protein kinase A. The authors also described two novel fusions, ATPase, Na + /K + transporting, b polypeptide (ATPB1B)-PRKACA and ATPB1B-PRKACB, the fusions PRKACA-DNAJB1 (DnaJ heat shock protein family member B1) and PRKACB-C7orf50, and nonsense mutations in PRKACA, all involved in the deregulation of cAMP-mediated pathways and potentially representing actionable targets. Compared with other BTCs, the presence of mutations occurring with higher frequency in but not exclusive of eCCA, such as AT-rich interaction domain (ARID) 1A and 1B, TP53, Kirsten rat sarcoma (KRAS), BRAF, Guanine nucleotide binding protein, alpha stimulating activity polypeptide (GNAS), small mother against decapentaplegic homolog 4 (SMAD4), Serine/Threonine kinase 11 (STK11), and E74 like ETS transcription factor 3 (ELF3), which is prevalent in ampullary carcinoma (a subtype of dCCA), was confirmed in other studies with smaller cohorts of patients [42,43]. Among the morphogenetic pathways, the overexpression of Notch receptor, initially described in iCCA, has been reported also in eCCA, including pCCA and dCCA, where it associated with gain of cancer stem cell-like properties [44]. A seminal paper by Montal and col. has been the first to focus solely on eCCA. By performing an integrative genomic profiling in a large cohort of 184 eCCA, 38 of which with paired non-neoplastic tissue, the authors found a differential expression of 174 genes that allowed to categorize eCCA into four classes: i) Metabolic; ii) Proliferation; iii) Mesenchymal; and iv) Immune [39]. The “Metabolic” class (18.7% of all cases) was characterized by alterations of genes involved in the metabolism of bile acids and fatty acids, as the peroxisome regulating glucose and lipid metabolism. A distinctive phenotypic trait of this class was the up-regulation of hepatocyte markers, in particular HNF4A, the master regulator of hepatocyte differentiation signals, and HDAC6, a tubulin deacetylase influencing the activity of bile acid receptors and the levels of acetylated alpha-tubulin in the primary cilium, leading to primary cilium alterations in CCA cells [45]. The “Proliferation” class (22.5%) was characterized by the activation of cell cycle checkpoints, cyclin-dependent kinase, and DNA repair pathways, with enrichment of erb-b2 receptor Tyrosine kinase 2 (ERBB2) mutations and amplifications. This type of eCCA showed a strong up-regulation of biliary epithelial genes (EpCAM and cytokeratins), and histologically, papillary lesions were prevalent. The “Mesenchymal” class (47.3%) displayed features of epithelial-to-mesenchymal transition (EMT), associated with activation of morphogenetic pathways, such as Hedgehog and transforming growth factor-β (TGF-β), a prototypal activator of EMT in cancer. An abundant stromal reaction enriched in cancer-associated fibroblasts (CAFs) and in the matrix component periostin, with a negligible contribution of macrophages was the histological hallmark of this class. The least frequent “Immune” class (11.5%) was dominated by the overexpression of genes regulating the adaptive immune responses, with pronounced lymphocytic infiltration and interferon-γ activation at the histological and biochemical levels, respectively. Of note, expression of immune checkpoints (PD-1/PD-L1) was also increased in the Immune class compared with other eCCAs. Interestingly, dCCA showed prevalence of the “Proliferation” and “Immune” classes, whereas the “Mesenchymal” class was preponderant in pCCA. “Metabolic” tumors were more frequent moving closer to the intrahepatic biliary system. However, no specific patterns of genetic mutations could be related to the known risk factors of eCCA, likely because of the limited clinical information available, though it can be reasoned that several environmental and disease-related factors may cooperate to generate distinct molecular profiles. This integrative molecular classification seems to have strong prognostic significance, since the Mesenchymal class showed the poorest overall survival (OS), differing from the Immune class, which instead correlated with the best OS. Besides expanding the knowledge on the mutations of eCCA, with discovery of novel chromosomal amplifications (YEATS domain-containing 4 (YETS4), E3 ubiquitin-protein ligase Mdm2 (MDM2), Cyclin E1 (CCNE1), Cyclin dependent kinase 4 (CDK4), and ERBB2), this study wired genomic alterations to structural aberrations of four fundamental pro-oncogenic pathways, namely RTK-RAS-phosphatidylinositol 3-kinase (PI3K), TGF-β, histone modification, and TP53-RB, each harboring at least one putative actionable driver. Mutations activating the RTK-RAS-PI3K pathway were detected in 53% of eCCA and were associated to modifications in cell survival and proliferation. Aberrant epidermal growth factor receptor (EGFR), ERBB2 and MET RTK expression were described in the Proliferation class and correlated with advanced stage and poor prognosis. RTK modification triggers RAS/MAPK and PI3K/AKT/mammalian target of rapamycin (mTOR) cascade, to stimulate a hyperproliferative response and to inhibit cell apoptosis [4,46,47]. EGFR mutations and amplification could be found in eCCA with a slightly lower prevalence as respect to iCCA (5–19% vs 11–27%, respectively) [48–50]. Conversely, mutations in human epidermal growth factor 2 (HER2)/ERBB2 is a common signature in 11–20% of eCCA, being instead rarely found in iCCA [51]. In patients with eCCA, ex-novo expression of c-ErbB-2 correlated with higher histological grade (III and IV), perineural invasion, and metastatization [52]. Other clusters of signals commonly found to be deregulated in eCCA (47%) are those supervised by TP53-RB. Mutations in these pathways affect cell survival and apoptosis, as well as the progression through the cell cycle. In eCCA, alterations in the TGF-β pathway are less frequent (18%) and are typically enriched in the Mesenchymal class. Perturbations of the histone acetylation pathways (22%) is a feature typical of tumors classified in the Metabolic and Proliferation classes. They result in the modification of chromatin remodeling processes inducing the aberrant activation of a number of transcription factors, including yes-associated protein (YAP) [53], the main effector of the Hippo pathway.
Table 2.
Differences in genetic mutations in eCCA with respect to other biliary tract cancers (BTCs).
| Genetic mutations | ||
|---|---|---|
| eCCA > BTCs | eCCA = BTCs | eCCA < BTCs |
| ARID1B | ARID1A | ARID2 |
| ELF3 | BRAF | BAP1 |
| PRKACA fusions (ATPB1B-PRKACA; PRKACA-DNAJB1) | BRCA1 | CDK4 amplifications |
| PRKACB fusions (ATPB1B-PRKACB; PRKACB-C7orf50) | BRCA2 | EGFR |
| GNAS | EPHA2 | |
| KRAS | ERBB3 | |
| MDM2 | FGFR2 fusion | |
| PIK3CA | IDH1/2 | |
| SMAD4 | MLL2 | |
| TP53 | MLL3 | |
| PTEN | ||
| TERT | ||
3. ACTIONABLE DRIVERS AND OUTCOME PREDICTORS IN PERTURBED PATHWAYS OF eCCA
According to OncoKB [54], actionable drivers have been found in about 50–60% of cases of iCCA, whereas in eCCA they are present in only 25% of tumors [39] or even less [55]. Besides providing druggable targets, these pathways might be relevant also as outcome predictors, as currently, only parameters obtained by analysis of surgical samples (perineural invasion, margin clearing, node involvement, and advanced TNM stage) are used. In this context, a number of phenotypic changes have been identified as tool to discriminate tumor aggressiveness and to allocate patients to the more appropriate curative approach (surgical resection, liver transplant, chemotherapy, and targeted therapies). In a large meta-analysis of 54 cohorts, that included 4454 patients, Wiggers and coll. [56] evaluated the differential expression of 102 immunohistochemical biomarkers, among which 18 with different and 16 with comparable expression between iCCA and eCCA. Specifically, Akt2 [57], Annexin II [57], Annexin 10 [58], c-ErbB-2 [52], CD10, CDX2, K20, mucin (MUC)1, MUC5A5, p53 [59], and S100P were found to be significantly more expressed in eCCA. In particular, when overexpressed, Annexin 10 behaved as a promising indicator of worst prognosis in patients with pCCA and dCCA, but not iCCA, and its up-regulation resulted in an over-activation of the PLA2G4A/PGE2/COX2/STAT3 pathway, a potentially druggable signaling [58]. A panel of tissue biomarkers, encompassing MUC2, MUC5AC, MUC6, CD10, CDX2 and K20, has been proposed to stratify patients undergoing resection for pCCA and dCCA in two subgroups, where subgroup 1, characterized by a lower expression of MUC5AC and MUC6, showed an impaired survival rate in pCCA, but not in dCCA patients [60]. Recent studies have pinpointed the strong pathobiological significance of S100A4 when expressed at the nuclear level. S100A4 is a cytoskeleton-associated calcium-binding protein that regulates several physiological and pathological processes, such as cell proliferation, migration and invasion, and it is usually expressed in the cytoplasm of mesenchymal lineage-derived cells [61]. In a cohort of 86 patients with surgical resection, the aberrant nuclear translocation of S100A4 in malignant biliary cells was an independent predictor of worst OS and metastatization in either iCCA or eCCA, similarly to what reported for other desmoplastic tumors of epithelial origin (breast, colorectal) [61]. Furthermore, in a xenograft mouse model generated by intraportal injection of human eCCA cells (EGI-1), the low-dose metronomic (LDM) treatment with paclitaxel reduced the hematogenous metastatization to the lungs, by interfering with SUMOylation mechanisms that directs the nuclear shuttling of S100A4 [62,63]. Overexpression of mesothelin (MSLN), a cell surface protein commonly present in mesothelial cells which expression is increased in several malignant tumors, associated with poor OS and relapse-free survival in patients with eCCA [64]. Table 3 summarizes the phenotypic biomarkers expressed at the tissue level in eCCA compared with iCCA. Studies devoted to circulating biomarkers, where eCCAs were kept distinct from iCCAs, are limited. In a mixed cohort of 45 CCAs, including 32 pCCA/dCCA, a panel of miRNA contained in extracellular vesicles (EVs) was used to discriminate CCA from non-tumoral controls, represented by primary sclerosing cholangitis (PSC), biliary obstructions and biliary leaks [65]. A recent study profiled miRNA expression, using nanostring technology, in a highly selected and clean cohort of 12 dCCAs. In particular, miR-451a and miR-144–3p were downregulated and, in vitro experiments demonstrated their ability to suppress both migration and invasion of CCA cells, and these effects were mediated by targeting activating transcription factor 2 (ATF2) and A disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) [66]. Moreover, in a cohort of 32 eCCAs and 55 iCCAs, investigated by a combined approach of immunohistochemistry and high sensitive in situ hybridization (RNAscope), expression of IL-6 and IL-33 was increased in eCCA and also in large, but not in small ducts of iCCA. Among them, overexpression of IL-33 was a predictor of better OS in patients with eCCA [67]. Within the multifaceted tumor cell population, even selective subsets may bear prognostic information and provide targets for therapeutic intervention. An interesting in-vitro study conducted in a very small cohort of eCCA, showed that organoids derived from CD47+/CD147+/EPCAM+/CD45- circulating tumor cells (CTC) immunopurified from portal venous blood, were able to recruit fibroblasts and to proliferate, indicating that CTCs may behave as vehicle for distant tumor spreading and recurrence [68]. Another study showed that CTCs isolated from blood samples using CellSearch System predicted a 10fold-reduced survival in patients with pCCA/dCCA [69]. Serum metabolites abnormally generated by CCA cells are the rational of metabolomics-based approaches that have been used to distinguish iCCA from PSC and hepatocellular carcinoma (HCC) [70] and to differentiate eCCA from iCCA. Thus, in a large cohort of Asian CCAs, 34 serum metabolites showed altered levels compared with healthy subjects. Among them, four molecules (21-deoxycortisol, bilirubin, lysoPC(14:0), and lysoPC(15:0)) were found to be deregulated in eCCA vs iCCA, though their prognostic impact was not assessed [71].
Table 3.
Differential expression of immunohistochemical biomarkers in eCCA with respect to iCCA.
| eCCA > iCCA | eCCA = iCCA | eCCA < iCCA |
|---|---|---|
| Akt2 | Aurora-A | Aurora-B |
| Annexin II | β-catenin | bcl-2 |
| c-erbB-2 | Bax | EGFR |
| CD10 | CK7 | Filamin A |
| CDX2 | CK8 | Galectin-1 |
| CK20 | CK18 | p16 |
| MUC1 | CK19 | p27 |
| MUC5AC | Cyclin D1 | SMAD-4 |
| p53 | COX-2 | VEGF-A |
| S100P | D10 | |
| E-cadherin | ||
| Fas | ||
| hENT1 | ||
| Mdm-2 | ||
| Metallothionein | ||
| MUC6 | ||
| p21 | ||
| S100A4 | ||
| TNF-α | ||
| VEGF | ||
| Vimentin |
4. PROSPECTS FOR THE CLINIC: OPPORTUNITIES FOR ACTIVE DRUGS IN eCCA
Molecular classifications provide unique opportunities to devise novel therapeutic strategies, as recently shown also for eCCA, where multiple targetable mutations are being identified [39]. However, as a rare tumor, designing target-specific single trials in eCCAs is extremely challenging, and therefore, oncologists may focus on umbrella and basket trials to avoid the risk of including target-negative patients and to increase the number of patient cohorts.
Currently, there are 256 ongoing clinical trials including patients with eCCA, aimed at 131 different compounds belonging to the molecular targeted class (Supplemental Table 1) [72]. These studies are mainly designed as basket trials and the most specific ones include not only eCCA, but also gallbladder or iCCA. Targeted DNA-sequencing and whole-genome expression-based studies have pinpointed KRAS, TP53, ARID1A and SMAD4 as the most frequent genetic alterations in eCCA [39]. As aforementioned, in about a quarter of eCCAs, these mutations result in at least a putative actionable driver (BRCA1–2, EGFR, ERBB2, CDK4, IDH1–2, BRAF, NRAS, PI3K, MDM2). Nevertheless, the only currently approved (Level 1) targeted therapy for use in eCCA is the PD-1 monoclonal antibody pembrolizumab, which show an improved outcome in tissue-agnostic DNA MMR deficient tumors including CCA [73], whereas it showed only a 7–13% objective response rate in BTCs [74]. Compared with iCCA, actionable molecular aberrations, with proven clinical benefit in different tumor types, are expressed in a minority of eCCA, such as BRCA1/2 (3%), EGFR (1%), IDH2 mutations (3%), ERBB2 overexpression (5%), CDK4 amplifications (1%), BRAF mutant (2%) [39]. However, the deep molecular phenotyping may open up interesting perspective on new targets amenable of intervention, with a certain specificity for each class (Supplemental Table 2).
The Metabolic class can be linked with treatment strategies aimed at targeting bile acid metabolism with nuclear receptor modulators (the farnesoid X receptor (FXR) agonist obeticholic acid (OCA)), or with inhibitors of the enzyme sphingosine kinase-2 (SphK-2) (ABC-294640, Opaganib) and of the histone deacetylase-6 (HDAC-6) (KA-2507). It is worth mentioning that in bile duct ligated (BDL) rats, the intrahepatic accumulation of bile acids is not sufficient per se to induce the development of CCA, which is instead stimulated by challenging BDL rats with thioacetamide. Neoplastic transformation is then sustained by biliary cell proliferation, portal inflammation and reduction in FXR expression [75]. Interestingly, ability to inhibit CCA progression has been demonstrated for FXR agonists, though limited to in-vitro/in-vivo experimental models [76,77] (NCT03377179, NCT04186156).
The Mesenchymal class, the most prevalent in the Montal’s study, is unfortunately, that with the worst prognosis and nowadays, with the fewest treatment chances [39]. Possible current approaches are hyaluronidase (PEGPH20), TGFβ inhibitors (M-7824) and Hedgehog antagonists (cyclopamine, 5E1, BMS-833923, BI6727). However, the development of PEGPH20 (NCT03267940) was prematurely stopped after negative data obtained in metastatic pancreatic cancer [78]. The bifunctional protein M-7824 targeting TGFβ along with PD-L1 has been testing in a phase II trial as second-line monotherapy in advanced BTCs (NCT03833661), whereas antagonism of the Hedgehog pathway showed encouraging results, but only in in-vivo/in-vitro studies [79–81].
On the other hand, the Proliferation and Immune classes provide the most promising targets for current clinical practice. In the Proliferation class, a number of druggable pathways can be envisaged, by inhibiting ERBB2/HER2 (with the monoclonal antibodies, trastuzumab and pertuzumab, or with the pan-ERBB inhibitors, lapatinib and varlitinib), or its downstream effectors CDK (abenaciclib, alvocidib), CK (silmitasertib) and mTOR (everolimus), with the potential to interfere in an early phase of tumorigenesis [82]. Multiple ongoing phase II clinical trials are studying ERBB2/HER2 inhibitors. Particularly, a phase II umbrella-trial of trastuzumab/lapatinib/everolimus/nivolumab plus GEMOX (NCT02836847) is conducted in eCCA and gallbladder cancer, and a phase II-III trial testing varlitinib plus Gem-Cis (NCT02992340) or capecitabine (NCT03093870, NCT03231176) is being conducted in advanced BTC. Lapatinib, as monotherapy, did not meet clinical outcomes in two phase II trials in the subgroup with advanced CCA (NCT00101036, NCT0107536). By acting on different pathways, an ongoing phase II basket trial (NCT03339843) and a more specific in BTC (NCT04003896) are testing abemaciclib and silmitasertib plus Gem/Cis in CCA (NCT02128282). Targeting BRAF is also emerging as possible therapeutic strategy in CCA, including eCCA. A very recent phase 2 multicenter basket trial (ROAR) has evaluated the effect of the combined treatment with dabrafenib and trametinib in 43 BRAFV600E-mutated BTCs. Results hold promise, with 51% of patients showing partial response, 17% stabilization of the disease, and no treatment-related deaths (NCT2034110) [83].
Finally, the Immune class may benefit from anti-PD-1/PD-L1 inhibitors, and indeed, promising data come from pembrolizumab and nivolumab. In a phase II trial (KEYNOTE-016), 86 patients with mismatch-repair (MMR)-deficient tumors were treated with pembrolizumab, with disease control in all the 4 CCA included [84]. In the KEYNOTE-028 basket trial, 24 patients with BTC expressing PD-L1 (≥1% of tumor cells by immunohistochemistry) underwent pembrolizumab, with 8 patients (34%) showing partial response or stable disease [85]. The KEYNOTE-158, a phase II basket trial of pembrolizumab, conducted in patients with advanced solid tumors including CCAs with disease progression on standard-of-care therapy, is currently ongoing (NCT02628067). Moreover, in a cohort of 46 patients with refractory BTC [86], nivolumab showed modest efficacy with durable response in 2 out of 5 patients (40%) with eCCA (NCT02829918). Multiple phase II-III clinical trials are now testing the effect of immune checkpoint inhibitors added to chemotherapy in advanced BTCs (nivolumab (NCT03101566, NCT04172402), pembrolizumab (NCT03111732, NCT03260712, NCT04003636), toripalimab (NCT03982680, NCT04027764, NCT04191343, NCT04217954, NCT03796429), durvalumab (NCT03046862, NCT03875235, NCT04308174), KN-035 (NCT03478488)). Furthermore, similar clinical trials to evaluate combination of targeted therapies as second-line treatment in advanced BTCs (nivolumab (NCT02866383, NCT03250273, NCT03639935), pembrolizumab (NCT03797326, NCT03895970, 04550624), toripalimab (NCT04010071, NCT04211168)) are under way.
5. CONCLUSIONS
Dissecting the specific molecular alterations harbored by the tumor of a single patient is the pre-requisite to pursue a precision medicine-based approach [87]. This need is becoming even more relevant for those types of cancer where prognosis remains gloomy despite the growing impact of targeted therapies and immunotherapy. Compared with HCC and other more common tumors, CCA has been largely overlooked until recently, and among CCAs, eCCA has been yet neglected, at least if considering the recent interest garnered by iCCA. Moreover, the understanding of the intricate tumor biology of eCCA has remained scarce, essentially because most studies have focused on iCCA, for which identification of clinically relevant targetable findings are more frequently identified. In this context, the comprehensive multi-platform molecular characterization performed in a very recent study [39] has laid the basis to delve into the multifaceted phenotype of eCCA, to identify the distinctive oncogenic fingerprints and possibly, to unveil some areas for targeted interventions. However, this is only the dawn of a new era, where multiple aspects, only partially addressed by the ongoing clinical trials, must be urgently addressed. To develop molecular diagnostic testing, possibly ascertaining the role of liquid biopsy, to implement specific phase II/III clinical trials with enrichment designs to expedite the approval, to dissect the intrinsic/acquired chemoresistance mechanisms, to test the efficacy of combination therapies, are among the issues to put next on the agenda.
6. EXPERT OPINION
Compared with other types of cancer, CCAs seem to miss a predominant and specific driver molecular alteration able to support the clinical decision-making process, as RAS/BRAF for colorectal cancer (CRC) [88], or EGFR for lung, breast and gastric cancers [89–91]. However, eCCA is gaining interest and steps forward are being made in looking for targetable alterations able to be utilized for “precision medicine” approaches in this setting. It has become clear that among primary liver malignancies, eCCA is indeed a “troublesome client”. Despite the progress made in the identification of an increasing number of genetic mutations and perturbed signal pathways, enabling a first molecular classification of eCCA (that we will be discussing below), there are several gray areas and challenges faced by the scientific and medical community that impinge on the current clinical management of this rare cancer. The conventional chemotherapeutic protocols are largely unsatisfactory in eCCA patients, in terms of both survival and quality of life. Of note, first-line treatments are limited to the combined use of gemcitabine and cisplatin, while data regarding second-line treatments are not strong enough. This reflects the limited understanding of the molecular mechanisms that sustain tumorigenesis in eCCA. A small percentage of eCCA (<20%) derives from diseases known to be prodromal of CCA, while the vast majority develop on a healthy biliary tree. Moreover, according to OncoKB targets [54], actionable drivers can be detected in only 25% of cases of eCCA, less than half with respect to iCCA (50–60%), making the application of targeted therapies more difficult and less impactful in eCCA,. In fact, at the time of this writing, just one targeted therapy using pembrolizumab has been approved for the treatment of eCCA. Use of immunotherapy is currently limited to the context of MMR deficiency or MSI high tumours, which represent only around 2% of eCCA [39]. Another important observation is the low prevalence in eCCA of the potentially targetable FGFR aberrations (fusions/mutations/amplifications) and IDH mutant-enriched subtypes, (1% and 4.7%, respectively). These molecular traits are more common in iCCA (20% for FGFR, 15% for IDH) [92,93], further confirming that oncogenic drivers of eCCA and iCCA are different, and actually only iCCA are included in clinical trials assessing efficacy of FGFR and IDH inhibitors [94]. Among FGFR inhibitors, pemigatinib, an oral inhibitor of FGFR2, has recently received the approval of Food and Drug Administration (FDA), for the treatment of iCCA harboring FGFR2 fusions or rearrangements [95–97]. Furthermore, a multicenter, phase III clinical trial (ClarIDHy) [NCT02989857] showed encouraging results in the treatment of iCCA characterized by IDH-1 mutations, using ivosidenib, a small molecule inhibiting IDH-1 [98]. To evaluate the response to therapy, the identification of easily achievable and assessable circulating biomarkers, indicating the disease progression stage and predicting the patient prognosis, would also be of great value, and recent studies dealing with this goal have been less informative in eCCA than in iCCA [4]. Finally, a further issue limiting the study of eCCA is the lack of suitable animal models. Although different rodent models of CCA are in use, generated by toxic or fluke infestation, genetically engineered, and transplantation approaches [99], they recapitulate only in part the complex genetic landscape and the intricate interactions between the different cell types populating the tumor microenvironment (TME), as observed in human eCCA.
According to Montal [39], eCCA can be categorized into four distinctive molecular classes (Mesenchymal, Proliferation, Immune, and Metabolic) converging into four main signaling perturbations (RTK-RAS-PI3K, TGF-β, histone modification, and TP53-RB). Unfortunately, despite the high frequency of KRAS mutations in eCCA (36.7%), there are no approved drugs that target mutated KRAS proteins directly, and also targeting KRAS indirectly by blocking its downstream effectors has been an ineffective strategy in other cancer such as pancreatic ductal adenocarcinoma (PDAC), the prototypical cancer harboring KRAS activation [100,101].
Among the four eCCA molecular classes, the Mesenchymal type is by far, not only the most prevalent (47.3%), but also the most aggressive, with the poorest response to conventional chemotherapy, similar to what reported for other desmoplastic cancers, as PDAC and CRC. The Mesenchymal eCCA is characterized by an intense fibrotic reaction, enrichment in EMT features, and abundant recruitment of CAF, which prevail on tumor-associated macrophages (TAM), which anyway are rarely observed in all eCCA classes, at variance with what observed in the TME of iCCA. However, given the extent and the likely impact of the TME on the marked aggressiveness of this class, we could imagine some alternative strategies. A first possibility is the use of BH3 mimetics (small compounds that antagonize anti-apoptotic BCL-2 family proteins, as navitoclax) based on their ability to induce selectively apoptosis in CAF, with concomitant effects on tumor growth and metastatization in CCA [102,103]. Second, hedgehog (Hh) antagonists (i.e. cyclopamine) might revert the EMT program and potentially reduce tumor growth, as shown in experimental models of CCA. [104]. Third, it has been shown that CAF recruitment is associated with tumor lymphangiogenesis and lymph node dissemination [103], and thus, use of angiogenesis inhibitors is worth pursuing in CAF-enriched Mesenchymal eCCA. Although angiogenesis inhibitors have been often unsuccessful, also in different oncology settings, this should be dependent on the fact that the route mainly responsible for CCA dissemination (lymphatic vascular system) has not been selectively targeted yet. Angiogenesis inhibition may be useful in combination therapy, as shown recently in HCC [105].
The Proliferation class, the second most prevalent in eCCAs (22.5%), harbors activation of two common proliferative pathways, the Ras/MAPK and AKT/mTOR, with chances of therapeutic interference at multiple levels. ERBB2/HER2 overexpression (mutations and amplifications) is a defining feature of this molecular class, and actually, is higher in eCCA (17.4%) compared with iCCA (4.8%) [106]. Unfortunately, the results obtained so far in CCA, with the pan-ERBB inhibitor lapatinib, a dual-targeted small molecule, which blocks RTK phosphorylation by binding to the cytoplasmic ATP-binding sites of EGFR/HER1 and HER2 receptors (NCT00101036, NCT0107536) are disappointing. However, in principle, eCCAs belonging to the proliferation class may benefit also from the use of conventional cytotoxic drugs, potentially also in combination therapies, to add DNA damaging to anti-proliferative effects.
Extrahepatic CCAs belonging to metabolic class (18.7%) displays a hepatocyte-like phenotype pathophysiologically underpinned by an aberrant bile acid metabolism. It provides an intriguing set of molecular alterations that can be translated into actionable targets. For instance, harnessing bile acid metabolism deregulation thorough nuclear receptor modulators (OCA among others) may represent in theory a strategy of translational interest, as suggested by first experimental data [76].
The stratification of patients in molecular classes as proposed by Montal’s study [39] could lead, in the next few years, to the implementation of clinical trials already ongoing or to new eCCA-specific clinical studies where such stratifications are planned from the beginning. This may increase the use of targeted therapies, at least in the quarter of eCCAs with putative actionable targets. With regard to the majority of eCCA patients, without current evidence of driver mutations or druggable pathways, it is desirable that the costs of cutting-edge techniques relevant for target discovery, such as single cell analysis, may become more affordable in the near future to encourage more translational studies. In fact, besides evaluating the presence of genetic mutations, single cell analysis perfectly suits to dissect, within the huge heterogeneity of TME, the intimate relationships between the various cell types herein hosted. Furthermore, it is conceivable that a new generation of experimental models, such as the humanized murine models or the tumor spheroids and organoids, may favor a more comprehensive and thorough study of cell-cell and cell-matrix interactions in eCCA. This approach is of paramount importance to unveil new therapeutic targets in the large subgroup of patients that remain allocated to palliative care because of the lack of actionable alterations. The crosstalk involving tumor, TME cells (CAF, TAM, endothelial cells, along with innate and adaptive immune cells) and the extracellular matrix, is indeed a niche area deserving more consideration, especially by proposals devoted to cancer types featuring a rich desmoplasia. In fact, studies performed in breast and pancreatic cancer [107,108] have shown that these interactions are essential for both tumor development and metastatisation, though the effects exerted by the TME can be ambivalent [109,110]. In eCCA, another knowledge gap in the field of TME relates to tumor chemoresistance. Similar to iCCA and HCC, eCCA shows a poor response to pharmacological treatments, but at present mechanisms of chemoresistance in eCCA are poorly eludicated [111]. Therefore, developing novel strategies to overcome eCCA chemoresistance, by increasing selectivity of drug delivery or by enhancing the amounts of active agents inside the tumor cells, is an urgent need. In this context, it will take more efforts to uncover the biology of cancer stem cells, given their fundamental role in promoting chemoresistance, as well as tumor recurrence and metastasis to distant organs [112].
As we start to unravel the complexity of CCA, it has become clear that eCCA must be regarded as a distinct entity, with unique features in terms of molecular classes, oncogenic drivers, and TME. These concepts must be borne in mind by future studies aimed at developing treatment algorithms for eCCA. It is expected that the treatment opportunities will be improved through close international collaborations, as the European Network Study for CCA (ENS-CCA) has been boosting during the last few years [113].
Supplementary Material
Article Highlights.
Extrahepatic cholangiocarcinoma (eCCA) is a biliary epithelial malignancy defined by well-established anatomical criteria with unique phenotypic traits.
eCCA is characterized by marked aggressiveness and poor prognosis, with scarce therapeutic options.
Within the wide eCCA heterogeneity, four molecular classes, “Mesenchymal”, “Proliferation”, “Immune”, and “Metabolic”, have been recently identified by whole-genome analysis-based studies.
Molecular alterations converge into stimulation of four main pro-oncogenic signalling, RTK-RAS-PI3K, TGF-β, histone modification, and TP53-RB, collectively resulting in the presence of actionable drivers in about 25% of eCCA.
Compared with the intrahepatic variant, FGFR and IDH mutant-enriched subtypes are less common in eCCA.
Phenotypic features associated with immunotherapy response are present in only 2% of eCCA.
Among 256 clinical trials ongoing, the only one actually approved for eCCA treatment, involves the use of pembrolizumab, a PD-1 monoclonal antibody.
Funding
AL received funding from The Christie Charity and the European Union’s Horizon 2020 Research and Innovation Programme [grant number 825510, ESCALON]; she is a memeber of the COST Action European Cholangiocarcinoma Network, supported by COST (European Cooperation in Science and Technology; www.cost.eu), a funding agency for research and innovation networks.
LaFo received funding from ANR (ANR-17-CE14–0013-01), Les Entreprises contre le Cancer Paris – Ile-de-France (GEFLUC) (2019), Cancéropôle Ile-de-France (2019).
MS received funding by the National Institutes of Health (RO1DK096096 and DK101528), by Silvio O. Conte Digestive Diseases Research Core Center (DK034989).
JJGM received funding by the CIBERehd (EHD15PI05/2016) and “Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III”, Spain (PI16/00598 and PI19/00819, co-funded by European Regional Development Fund/European Social Fund); “Junta de Castilla y Leon” (SA063P17); AECC Scientific Foundation (2017/2020), Spain; “Centro Internacional sobre el Envejecimiento” (OLD-HEPAMARKER, 0348_CIE_6_E), Spain; Fundació Marato TV3 (Ref. 201916–31).
JMB received funding from Spanish Carlos III Health Institute (ISCIII) [(FIS PI15/01132, PI18/01075 and Miguel Servet Program CON14/00129 and CPII19/00008) cofinanced by “Fondo Europeo de Desarrollo Regional” (FEDER); “Instituto de Salud Carlos III” (CIBERehd); Department of Health of the Basque Country (2017111010); “Euskadi RIS3” (2019222054, 2020333010); BIOEF (Basque Foundation for Innovation and Health Research: EiTB Maratoia BIO15/CA/016/BD); Department of Industry of the Basque Country (Elkartek: KK-2020/00008); La Caixa Scientific Foundation (HR17–00601); “Fundación Científica de la Asociación Española Contra el Cáncer” (AECC Scientific Foundation); European Union’s Horizon 2020 Research and Innovation Programme [grant number 825510, ESCALON]; COST Action European Cholangiocarcinoma Network, supported by COST (European Cooperation in Science and Technology; www.cost.eu), a funding agency for research and innovation networks.
Abbreviations
- ADAM10
A disintegrin and metalloproteinase domain-containing protein 10
- ATF2
activating transcription factor 2
- ATPB1B
ATPase, Na+ /K+ transporting, b polypeptide
- ARID
AT-Rich Interaction Domain
- BTC
biliary tract cancer
- CAF
cancer-associated fibroblasts
- CDX2
Caudal Type Homeobox 2
- CCA
cholangiocarcinoma
- CTC
circulating tumor cells
- CRC
colorectal cancer
- CT
computed tomography
- CDK4
cyclin dependent kinase 4
- CCNE1
cyclin E1
- dCCA
distal CCA
- DNAJB1
DnaJ heat shock protein family member B1
- ELF3
E74 like ETS transcription factor 3
- EGFR
epidermal growth factor receptor
- EpCAM
epithelial cell adhesion molecule
- EMT
epithelial-to-mesenchymal transition
- ERBB2
erb-b2 receptor tyrosine kinase 2
- EVs
extracellular vesicles
- eCCA
extrahepatic CCA
- FXR
farnesoid X receptor
- OCA
agonist obeticholic acid
- FGFR
Fibroblast Growth Factor Receptor
- GNAS
Guanine Nucleotide binding protein, Alpha Stimulating activity polypeptide
- HCC
hepatocellular carcinoma
- HNF4A
hepatocyte nuclear factor 4 alpha
- HDAC6
histone deacetylase 6
- IL
interleukin
- iCCA
intrahepatic CCA
- IDH
Isocitrate Dehydrogenase (NADP(+))
- KRAS
Kirsten rat sarcoma
- MDM2
E3 Ubiquitin-Protein Ligase Mdm2
- MMR
mismatch-repair
- mTOR
mammalian target of rapamycin
- MRI
magnetic resonance imaging
- MSLN
mesothelin
- MUC
mucin
- NGS
next generation sequencing
- OS
overall survival
- PDAC
pancreatic ductal adenocarcinoma
- PI3K
phosphatidylinositol 3-kinases
- pCCA
perihilar CCA
- PSC
primary sclerosing cholangitis, PSC
- PRKAC
Protein Kinase CAMP-Activated Catalytic Subunit
- RTK
tyrosine kinase receptors, RTK
- S100
S100 Calcium Binding Protein
- SMAD4
small mother against decapentaplegic homolog 4
- STK11
Serine/threonine kinase 11
- SphK-2
sphingosine kinase-2
- TGF-β
transforming growth factor-β
- TAM
tumor-associated macrophages
- TME
tumor microenvironment
- YAP
yes-associated protein
- YETS4
YEATS domain-containing 4
Footnotes
Declaration of interest
AL received travel and educational support from Ipsen, Pfizer, Bayer, AAA, SirtEx, Novartis, Mylan and Delcath; speaker honoraria from Merck, Pfizer, Ipsen, Incyte and AAA; advisory honoraria from EISAI, Nutricia Ipsen, QED and Roche; she is a member of the Knowledge Network and NETConnect Initiatives funded by Ipsen.
MS is member of the advisory board of Engitix, outside the submitted work.
JMB reports grants from INCYTE and ROCHE, personal fees for lecturer from BAYER and INTERCEPT, and consulting for QED and OWL METABOLOMICS, outside the submitted work.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banales JM, Cardinale V, Carpino G, et al. Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261–80. [DOI] [PubMed] [Google Scholar]
- 4.Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–88.• This comprehensive review updates the most recent evidence on epidemiology, pathogenesis, treatment and future directions of cholangiocarcinoma, keeping distinct the intra- and extra-hepatic variants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakanuma Y, Kakuda Y. Pathologic classification of cholangiocarcinoma: New concepts. Best Pract Res Clin Gastroenterol. 2015;29:277–93. [DOI] [PubMed] [Google Scholar]
- 6.Cardinale V, Wang Y, Carpino G, et al. Mucin-producing cholangiocarcinoma might derive from biliary tree stem/progenitor cells located in peribiliary glands. Hepatology. 2012;55:2041–2. [DOI] [PubMed] [Google Scholar]
- 7.Carpino G, Cardinale V, Folseraas T, et al. Neoplastic Transformation of the Peribiliary Stem Cell Niche in Cholangiocarcinoma Arisen in Primary Sclerosing Cholangitis. Hepatology. 2019;69:622–38. [DOI] [PubMed] [Google Scholar]
- 8.Komuta M, Spee B, Vander Borght S, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544–56. [DOI] [PubMed] [Google Scholar]
- 9.Marin JJG, Lozano E, Briz O, et al. Molecular Bases of Chemoresistance in Cholangiocarcinoma. Curr Drug Targets. 2017;18:889–900. [DOI] [PubMed] [Google Scholar]
- 10.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, Abbasi N, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman MH, Webster GJM, Bannoo S, et al. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis: a 25-year single-centre experience. Eur J Gastroenterol Hepatol. 2012;24:1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He X-D, Wang L, Liu W, et al. The risk of carcinogenesis in congenital choledochal cyst patients: an analysis of 214 cases. Ann Hepatol. 2014;13:819–26. [PubMed] [Google Scholar]
- 15.Forner A, Vidili G, Rengo M, et al. Clinical presentation, diagnosis, and staging of cholangiocarcinoma. Liver Int. 2019;39:98–107. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira IS, Kilcoyne A, Everett JM, et al. Cholangiocarcinoma: classification, diagnosis, staging, imaging features, and management. Abdom Radiol. 2017;42:1637–49. [DOI] [PubMed] [Google Scholar]
- 17.Tamada K, Ushio J, Sugano K. Endoscopic diagnosis of extrahepatic bile duct carcinoma: Advances and current limitations. World J Clin Oncol. 2011;2:203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizvi S, Eaton J, Yang JD, et al. Emerging Technologies for the Diagnosis of Perihilar Cholangiocarcinoma. Semin Liver Dis. 2018;38:160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wannhoff A, Gotthardt DN. Recent developments in the research on biomarkers of cholangiocarcinoma in primary sclerosing cholangitis. Clin Res Hepatol Gastroenterol. 2019;43:236–43. [DOI] [PubMed] [Google Scholar]
- 20.Arbelaiz A, Azkargorta M, Krawczyk M, et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2017;66:1125–43. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Yan I, Wen H-J, et al. microRNAs in liver disease: from diagnostics to therapeutics. Clin Biochem. 2013;46:946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudley JC, Zheng Z, McDonald T, et al. Next-Generation Sequencing and Fluorescence in Situ Hybridization Have Comparable Performance Characteristics in the Analysis of Pancreaticobiliary Brushings for Malignancy. J Mol Diagn. 2016;18:124–30. [DOI] [PubMed] [Google Scholar]
- 23.Severino V, Dumonceau J-M, Delhaye M, et al. Extracellular Vesicles in Bile as Markers of Malignant Biliary Stenoses. Gastroenterology. 2017;153:495–504.e8. [DOI] [PubMed] [Google Scholar]
- 24.Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223–38. [DOI] [PubMed] [Google Scholar]
- 25.Yang JD, Yab TC, Taylor WR, et al. Detection of Cholangiocarcinoma by Assay of Methylated DNA Markers in Plasma. Gastroenterology. 2017;152:S1041–2. [Google Scholar]
- 26.Macias RIR, Muñoz-Bellvís L, Sánchez-Martín A, et al. A Novel Serum Metabolomic Profile for the Differential Diagnosis of Distal Cholangiocarcinoma and Pancreatic Ductal Adenocarcinoma. Cancers. 2020;12:1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poruk KE, Pawlik TM, Weiss MJ. Perioperative Management of Hilar Cholangiocarcinoma. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2015;19:1889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansour JC, Aloia TA, Crane CH, et al. Hilar cholangiocarcinoma: expert consensus statement. HPB. 2015;17:691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuzzo G, Giuliante F, Ardito F, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147:26–34. [DOI] [PubMed] [Google Scholar]
- 30.Kwon HJ, Kim SG, Chun JM, et al. Prognostic factors in patients with middle and distal bile duct cancers. World J Gastroenterol. 2014;20:6658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan AS, Dageforde LA. Cholangiocarcinoma. Surg Clin North Am. 2019;99:315–35. [DOI] [PubMed] [Google Scholar]
- 32.Goldaracena N, Gorgen A, Sapisochin G. Current status of liver transplantation for cholangiocarcinoma. Liver Transpl. 2018;24:294–303. [DOI] [PubMed] [Google Scholar]
- 33.Heimbach JK, Haddock MG, Alberts SR, et al. Transplantation for hilar cholangiocarcinoma. Liver Transpl. 2004;10:S65–68. [DOI] [PubMed] [Google Scholar]
- 34.Gores GJ, Darwish Murad S, Heimbach JK et al. Liver transplantation for perihilar cholangiocarcinoma. Dig Dis. 2013;31:126–9. [DOI] [PubMed] [Google Scholar]
- 35.Zamora-Valdes D, Heimbach JK. Liver Transplant for Cholangiocarcinoma. Gastroenterol Clin North Am. 2018;47:267–80. [DOI] [PubMed] [Google Scholar]
- 36.Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25:391–8. [DOI] [PubMed] [Google Scholar]
- 37.Marin JJG, Prete MG, Lamarca A, et al. Current and novel therapeutic opportunities for systemic therapy in biliary cancer. Br J Cancer. 2020;123:1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen JB, Thorgeirsson SS. Genetic profiling of intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol. 2012;28:266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montal R, Sia D, Montironi C, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. 2020;73:315–27.•• This seminal paper provides the first integrative molecular characterization of extrahepatic cholangiocarcinoma, based on a deep genomic profiling in a large cohort of patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fouassier L, Marzioni M, Afonso MB, et al. Signalling networks in cholangiocarcinoma: Molecular pathogenesis, targeted therapies and drug resistance. Liver Int. 2019;39:43–62. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–10. [DOI] [PubMed] [Google Scholar]
- 42.Putra J, de Abreu FB, Peterson JD, et al. Molecular profiling of intrahepatic and extrahepatic cholangiocarcinoma using next generation sequencing. Exp Mol Pathol. 2015;99:240–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin Cancer Res. 2018;24:4154–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aoki S, Mizuma M, Takahashi Y, et al. Aberrant activation of Notch signaling in extrahepatic cholangiocarcinoma: clinicopathological features and therapeutic potential for cancer stem cell-like properties. BMC Cancer. 2016. 07;16:854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gradilone SA, Radtke BN, Bogert PS, et al. HDAC6 Inhibition Restores Ciliary Expression and Decreases Tumor Growth. Cancer Res. 2013;73:2259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valle JW, Lamarca A, Goyal L, et al. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017;7:943–62.• This review thoroughly addresses genetic drivers and molecular signatures of biliary tract cancers, including extrahepatic cholangiocarcinoma, and highlights future challenges of research, taking a translational approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–1031.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakazawa K, Dobashi Y, Suzuki S, et al. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J Pathol. 2005;206:356–65. [DOI] [PubMed] [Google Scholar]
- 49.Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clapéron A, Mergey M, Nguyen Ho-Bouldoires TH, et al. EGF/EGFR axis contributes to the progression of cholangiocarcinoma through the induction of an epithelial-mesenchymal transition. J Hepatol. 2014;61:325–32. [DOI] [PubMed] [Google Scholar]
- 51.Pignochino Y, Sarotto I, Peraldo-Neia C, et al. Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas. BMC Cancer. 2010;10:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng J, Zhu Y-M. Expression of c-erbB-2 proto-oncogene in extrahepatic cholangiocarcinoma and its clinical significance. Hepatobiliary Pancreat Dis Int. 2007;6:412–5. [PubMed] [Google Scholar]
- 53.Sugihara T, Isomoto H, Gores G, et al. YAP and the Hippo pathway in cholangiocarcinoma. J Gastroenterol. 2019;54:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol. 2017;2017:PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lamarca A, Kapacee Z, Breeze M, et al. Molecular Profiling in Daily Clinical Practice: Practicalities in Advanced Cholangiocarcinoma and Other Biliary Tract Cancers. J Clin Med. 2020;9:2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiggers JK, Ruys AT, Groot Koerkamp B, et al. Differences in immunohistochemical biomarkers between intra- and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:1582–94. [DOI] [PubMed] [Google Scholar]
- 57.Guedj N, Zhan Q, Perigny M, et al. Comparative protein expression profiles of hilar and peripheral hepatic cholangiocarcinomas. J Hepatol. 2009;51:93–101. [DOI] [PubMed] [Google Scholar]
- 58.Sun R, Liu Z, Qiu B, et al. Annexin10 promotes extrahepatic cholangiocarcinoma metastasis by facilitating EMT via PLA2G4A/PGE2/STAT3 pathway. EBioMedicine. 2019;47:142–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J, Wang X, Xie S, et al. p53 status and its prognostic role in extrahepatic bile duct cancer: a meta-analysis of published studies. Dig Dis Sci. 2011;56:655–62. [DOI] [PubMed] [Google Scholar]
- 60.Ishida K, Osakabe M, Eizuka M, et al. The expression of gastrointestinal differentiation markers in extrahepatic cholangiocarcinoma: clinicopathological significance based on tumor location. Hum Pathol. 2019;92:91–100. [DOI] [PubMed] [Google Scholar]
- 61.Fabris L, Cadamuro M, Moserle L, et al. Nuclear expression of S100A4 calcium-binding protein increases cholangiocarcinoma invasiveness and metastasization. Hepatol Baltim Md. 2011;54:890–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cadamuro M, Spagnuolo G, Sambado L, et al. Low-Dose Paclitaxel Reduces S100A4 Nuclear Import to Inhibit Invasion and Hematogenous Metastasis of Cholangiocarcinoma. Cancer Res. 2016;76:4775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JS, Choi HJ, Baek SH. Sumoylation and Its Contribution to Cancer. Adv Exp Med Biol. 2017;963:283–98. [DOI] [PubMed] [Google Scholar]
- 64.Kawamata F, Kamachi H, Einama T, et al. Intracellular localization of mesothelin predicts patient prognosis of extrahepatic bile duct cancer. Int J Oncol. 2012;41:2109–18. [DOI] [PubMed] [Google Scholar]
- 65.Li L, Masica D, Ishida M, et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology. 2014;60:896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loeffler MA, Hu J, Kirchner M, et al. miRNA profiling of biliary intraepithelial neoplasia reveals stepwise tumorigenesis in distal cholangiocarcinoma via the miR-451a/ATF2 axis. J Pathol. 2020;252:239–51. [DOI] [PubMed] [Google Scholar]
- 67.Sawada R, Ku Y, Akita M, et al. Interleukin-33 overexpression reflects less aggressive tumour features in large-duct type cholangiocarcinomas. Histopathology. 2018;73:259–72. [DOI] [PubMed] [Google Scholar]
- 68.Arnoletti JP, Fanaian N, Reza J, et al. Pancreatic and bile duct cancer circulating tumor cells (CTC) form immune-resistant multi-cell type clusters in the portal venous circulation. Cancer Biol Ther. 2018;19:887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang JD, Campion MB, Liu MC, et al. Circulating tumor cells are associated with poor overall survival in patients with cholangiocarcinoma. Hepatology. 2016;63:148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banales JM, Iñarrairaegui M, Arbelaiz A, et al. Serum Metabolites as Diagnostic Biomarkers for Cholangiocarcinoma, Hepatocellular Carcinoma, and Primary Sclerosing Cholangitis. Hepatology. 2019;70:547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang Q, Liu H, Zhang T, Jiang Y, et al. Serum metabolomics uncovering specific metabolite signatures of intra- and extrahepatic cholangiocarcinoma. Mol Biosyst. 2016;12:334–40. [DOI] [PubMed] [Google Scholar]
- 72.Home - ClinicalTrials.gov [Internet]. [cited 2020 Oct 16]. Available at: https://clinicaltrials.gov/
- 73.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piha-Paul SA, Oh D-Y, Ueno M, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147:2190–8. [DOI] [PubMed] [Google Scholar]
- 75.Lozano E, Sanchez-Vicente L, Monte MJ, et al. Cocarcinogenic effects of intrahepatic bile acid accumulation in cholangiocarcinoma development. Mol Cancer Res. 2014;12:91–100. [DOI] [PubMed] [Google Scholar]
- 76.Erice O, Labiano I, Arbelaiz A, et al. Differential effects of FXR or TGR5 activation in cholangiocarcinoma progression. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1335–44. [DOI] [PubMed] [Google Scholar]
- 77.Britten CD, Garrett-Mayer E, Chin SH, et al. A Phase I Study of ABC294640, a First-in-Class Sphingosine Kinase-2 Inhibitor, in Patients with Advanced Solid Tumors. Clin Cancer Res. 2017;23:4642–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Cutsem E, Tempero MA, Sigal D, et al. Randomized Phase III Trial of Pegvorhyaluronidase Alfa With Nab-Paclitaxel Plus Gemcitabine for Patients With Hyaluronan-High Metastatic Pancreatic Adenocarcinoma. J Clin Oncol. 2020;38:3185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El Khatib M, Kalnytska A, Palagani V, et al. Inhibition of hedgehog signaling attenuates carcinogenesis in vitro and increases necrosis of cholangiocellular carcinoma. Hepatology. 2013;57:1035–45. [DOI] [PubMed] [Google Scholar]
- 80.Riedlinger D, Bahra M, Boas-Knoop S, et al. Hedgehog pathway as a potential treatment target in human cholangiocarcinoma. J Hepato-Biliary-Pancreat Sci. 2014;21:607–15. [DOI] [PubMed] [Google Scholar]
- 81.Fingas CD, Mertens JC, Razumilava N, et al. Polo-like kinase 2 is a mediator of hedgehog survival signaling in cholangiocarcinoma. Hepatology. 2013;58:1362–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yarlagadda B, Kamatham V, Ritter A, et al. Trastuzumab and pertuzumab in circulating tumor DNA ERBB2-amplified HER2-positive refractory cholangiocarcinoma. NPJ Precis Oncol. 2019;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Subbiah V, Lassen U, Élez E, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21:1234–43. [DOI] [PubMed] [Google Scholar]
- 84.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Neill BH, Wallmark J, Lorente D, et al. Pembrolizumab (MK-3475) for patients (pts) with advanced colorectal carcinoma (CRC): Preliminary results from KEYNOTE-028. EJC. 2015;51:S103. [Google Scholar]
- 86.Kim RD, Chung V, Alese OB, et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020;6:888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pellino A, Loupakis F, Cadamuro M, et al. Precision medicine in cholangiocarcinoma. Transl Gastroenterol Hepatol. 2018;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee MKC, Loree JM. Current and emerging biomarkers in metastatic colorectal cancer. Curr Oncol. 2019;26:S7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Mello RA, Neves NM, Tadokoro H, et al. New Target Therapies in Advanced Non-Small Cell Lung Cancer: A Review of the Literature and Future Perspectives. J Clin Med. 2020;9:E3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakai K, Hung M-C, Yamaguchi H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am J Cancer Res. 2016;6:1609–23. [PMC free article] [PubMed] [Google Scholar]
- 91.Adashek JJ, Arroyo-Martinez Y, Menta AK, et al. Therapeutic Implications of Epidermal Growth Factor Receptor (EGFR) in the Treatment of Metastatic Gastric/GEJ Cancer. Front Oncol. 2020;10:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sia D, Losic B, Moeini A, et al. Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat Commun. 2015;6:6087. [DOI] [PubMed] [Google Scholar]
- 93.Farshidfar F, Zheng S, Gingras M-C, et al. Integrative Genomic Analysis of Cholangiocarcinoma Identifies Distinct IDH-Mutant Molecular Profiles. Cell Rep. 2017;18:2780–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lamarca A, Barriuso J, McNamara MG, et al. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J Hepatol. 2020;73:170–85. [DOI] [PubMed] [Google Scholar]
- 95.Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020. May;21(5):671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Romero D. Benefit from pemigatinib in cholangiocarcinoma. Nat Rev Clin Oncol. 2020. Jun;17(6):337. [DOI] [PubMed] [Google Scholar]
- 97.Merz V, Zecchetto C, Melisi D. Pemigatinib, a potent inhibitor of FGFRs for the treatment of cholangiocarcinoma. Future Oncol. 2020. Oct 9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 98.Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020. Jun;21(6):796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cadamuro M, Brivio S, Stecca T, et al. Animal models of cholangiocarcinoma: What they teach us about the human disease. Clin Res Hepatol Gastroenterol. 2018. October;42(5):403–415. [DOI] [PubMed] [Google Scholar]
- 100.Aung KL, Fischer SE, Denroche RE, et al. Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial. Clin Cancer Res. 2018;24:1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leone F, Marino D, Cereda S, et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: A randomized phase 2 trial (Vecti-BIL study). Cancer. 2016;122:574–81. [DOI] [PubMed] [Google Scholar]
- 102.Mertens JC, Fingas CD, Christensen JD, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cadamuro M, Brivio S, Mertens J, et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J Hepatol. 2019;70:700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fingas CD, Bronk SF, Werneburg NW, et al. Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54:2076–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–1905 [DOI] [PubMed] [Google Scholar]
- 106.Galdy S, Lamarca A, McNamara MG, et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastasis Rev. 2017;36:141–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Piersma B, Hayward MK, Weaver VM. Fibrosis and cancer: A strained relationship. Biochim Biophys Acta Rev Cancer. 2020. Apr;1873(2):188356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pandol S, Edderkaoui M, Gukovsky I, et al. Desmoplasia of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2009. November;7(11 Suppl):S44–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014. June 16;25(6):735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014. June 16;25(6):719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marin JJG, Lozano E, Herraez E, et al. Chemoresistance and chemosensitization in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018. Apr;1864(4 Pt B):1444–1453. [DOI] [PubMed] [Google Scholar]
- 112.Wu HJ, Chu PY. Role of Cancer Stem Cells in Cholangiocarcinoma and Therapeutic Implications. Int J Mol Sci. 2019. August 25;20(17):4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.ENS-CCA [Internet]. [cited 2020 Nov 16]. Available at: http://www.enscca.org/.
- 114.Clements O, Eliahoo J, Kim JU, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol. 2020;72:95–103. [DOI] [PubMed] [Google Scholar]
- 115.Petrick JL, Yang B, Altekruse SF, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PloS One. 2017;12:e0186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shin H-R, Oh J-K, Masuyer E, et al. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wongjarupong N, Assavapongpaiboon B, Susantitaphong P, et al. Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2017;17:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jing W, Jin G, Zhou X, et al. Diabetes mellitus and increased risk of cholangiocarcinoma: a meta-analysis. Eur J Cancer Prev. 2012;21:24–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.